Aerial shooting is unlikely to cause dispersal or consistent changes in the movements of feral pigs (Sus scrofa)
Andrew J. Bengsen A * , Sebastien Comte
A * , Sebastien Comte  B C , Troy Crittle D , Suzie Holbery E , Darren Marshall F , Lachlan Marshall F , Lee Parker A and David M. Forsyth
B C , Troy Crittle D , Suzie Holbery E , Darren Marshall F , Lachlan Marshall F , Lee Parker A and David M. Forsyth  B C
B C
A
B
C
D
E
F
Abstract
Aerial shooting is an important tool for managing feral pig damage to agricultural and biodiversity assets because it can rapidly reduce population densities over large areas. It should also be valuable for reducing host population densities in the event of an emergency animal disease incursion. However, recent tracking studies have not alleviated concerns that the intense disturbance caused by aerial shooting might cause pigs to disperse from target areas.
We investigated the responses of feral pigs to nine aerial shooting operations conducted at five large and divergent sites in south-eastern Australia.
We fitted 71 pigs with GPS tracking collars and monitored changes in their behaviour following exposure to aerial shooting operations that lasted between 1 and 11 days. Repeated exposure of some individuals provided 105 distinct samples. We examined the following three key traits: the location and size of activity ranges, daily activity and movement rates, and daily activity cycles.
We found inconsistent results between sexes and among operations. However, only one pig left the target area after shooting began. This pig did not return.
The fine-scale behaviour of pigs subjected to aerial shooting is likely to vary because of a complex interplay of social, environmental, and operational factors. Behaviour changes observed in this study were unlikely to cause the dispersal of feral pigs or their impacts.
Given our results, and those of previous studies, we believe that aerial shooting should continue to be used as a key method for managing feral pig populations and should also be considered for emergency animal disease response operations.
Keywords: aerial culling, behavioural responses, depopulation, feral swine, GPS tracking, movement ecology, population control, transboundary animal disease, wild boar, wildlife management.
Introduction
Feral pigs (Sus scrofa) are one of the world’s most widely distributed invasive mammal species (Long 2003) and are considered a major pest of agricultural and biodiversity resources in most areas where they occur (Massei et al. 2011; Bevins et al. 2014; Pedrosa et al. 2015; Bengsen et al. 2017). Feral pigs can also be important hosts, amplifiers and spillover sources of many pathogens that cause major human health or economic impacts, such as influenza, Japanese encephalitis, and African swine fever viruses (Dalziel et al. 2016; Gentle et al. 2022).
The management of feral pig impacts often relies on lethal control to reduce population densities over large areas. In the case of agricultural and biodiversity protection, it is generally expected that this will lead to a reduction in a wide range of impacts, such as crop damage, pasture degradation, ecosystem restructuring, and predation on livestock or wildlife (Bengsen et al. 2014; Bevins et al. 2014). When lethal control is used for disease management in wild animal populations, the intent is to reduce population densities below thresholds required for disease persistence and spread (Artois et al. 2001).
Helicopter-based aerial shooting (hereinafter ‘aerial shooting’) can be an effective method for rapidly reducing feral pig population densities over large areas (Choquenot et al. 1999). Aerial shooting has been widely used to manage feral pig population densities and mitigate damage to agriculture and biodiversity assets in Australia and the United States (Bengsen et al. 2014; Davis et al. 2018). It could also provide a valuable tool for managing incursions or outbreaks of emergency animal diseases (Saunders and Bryant 1988; Campbell et al. 2010; Cowled et al. 2012). However, findings from studies involving terrestrial hunters have raised concerns that intense disturbance might cause pigs to disperse from target areas, inadvertently spreading damage or pathogens to new locations or host populations (e.g. African Swine Fever Feral Pig Task Group 2020).
Like that of many ungulates, the behaviour of feral pigs is often sensitive to disturbance by predators, including human hunters. When disturbed by terrestrial hunters, pigs can modify their behaviour in ways that reduce their exposure to diurnal predators (Morelle et al. 2015; Keuling and Massei 2021), including the following: reducing the distance travelled among resting sites (e.g. Fernández-Llario 2004; Fischer et al. 2016); increasing the distance travelled around pre-disturbance activity ranges (e.g. Maillard and Fournier 1995; Scillitani et al. 2010); or fleeing the hunted area (e.g. Sodeikat and Pohlmeyer 2003; Scillitani et al. 2010; Thurfjell et al. 2013). Thus, hunting disturbance can cause pigs to decrease or increase their movement rates and the distances they travel. Results of observational studies suggest that hunting might also cause pigs to reduce diurnal activity (van Doormaal et al. 2015; Reinke et al. 2021), which could make them less available to diurnally-active hunters. Adult females and their offspring may be more sensitive to predation risk than are adult males, leading to more pronounced responses in matrilineal groups (Saïd et al. 2012). The extent to which hunting disturbance influences movement patterns is likely to depend on a complex interplay of factors, including the duration and intensity of disturbance, the availability of refuge and other resources within existing activity ranges and the surrounding area, population density, and the sex–age classes of the pigs exposed to hunters (Keuling et al. 2008; Saïd et al. 2012; Keuling and Massei 2021). These factors can vary greatly among the diverse range of landscapes inhabited by pigs.
In contrast to terrestrial hunting, aerial shooting operations typically present a more intense disturbance over a large geographic area for several consecutive days (Bengsen et al. 2024). The few published studies that have described behavioural responses of feral pigs to this form of disturbance have concluded that aerial shooting did not cause pigs to move out of their normal ranges or to greatly increase the size of the area over which they travelled (Saunders and Bryant 1988; Dexter 1996; Campbell et al. 2010, 2012). An early study using VHF telemetry found no evidence that pigs increased their movement rates during aerial shooting operations (Dexter 1996). However, a more recent GPS tracking study at two sites in the United States found that movement rates increased during shooting (Campbell et al. 2010). That study also reported notable differences in pig behaviour among sites, suggesting that responses of pigs to aerial shooting are influenced by site-specific factors such as the availability of refuge habitat.
To understand when and how aerial shooting might cause feral pigs to disperse or otherwise change their behaviour, it is necessary to understand how pigs respond to this unique form of disturbance across different environmental and management contexts. Inferences from early studies have been limited by small sample sizes and the limitations of VHF telemetry methods. More recent studies have used GPS technology to examine operations of short duration (≤3 days) covering small areas (<200 km2). However, disease response operations will probably need to be conducted over larger areas for longer periods (Pepin et al. 2022; Snow et al. 2024). The sole study that examined more than one site underscores the challenges of generalising to different sites (Campbell et al. 2010). In the present study, we describe the behavioural responses of feral pigs fitted with GPS tracking collars to aerial shooting operations at five large (≥600 km2) sites in south-eastern Australia characterised by distinct biophysical traits and different shooting intensities. We estimate sex-specific changes in (1) the location and size of activity ranges before and after shooting, (2) daily activity and movement rates before, during and after shooting, and (3) daily activity patterns before, during and after shooting. Understanding these responses is vital for understanding how aerial shooting can best be used to manage feral pig impacts, including disease spread within wild host populations and potential spillover to domestic animals (Campbell et al. 2010; Ham et al. 2019; Bengsen et al. 2024).
Materials and methods
Study areas
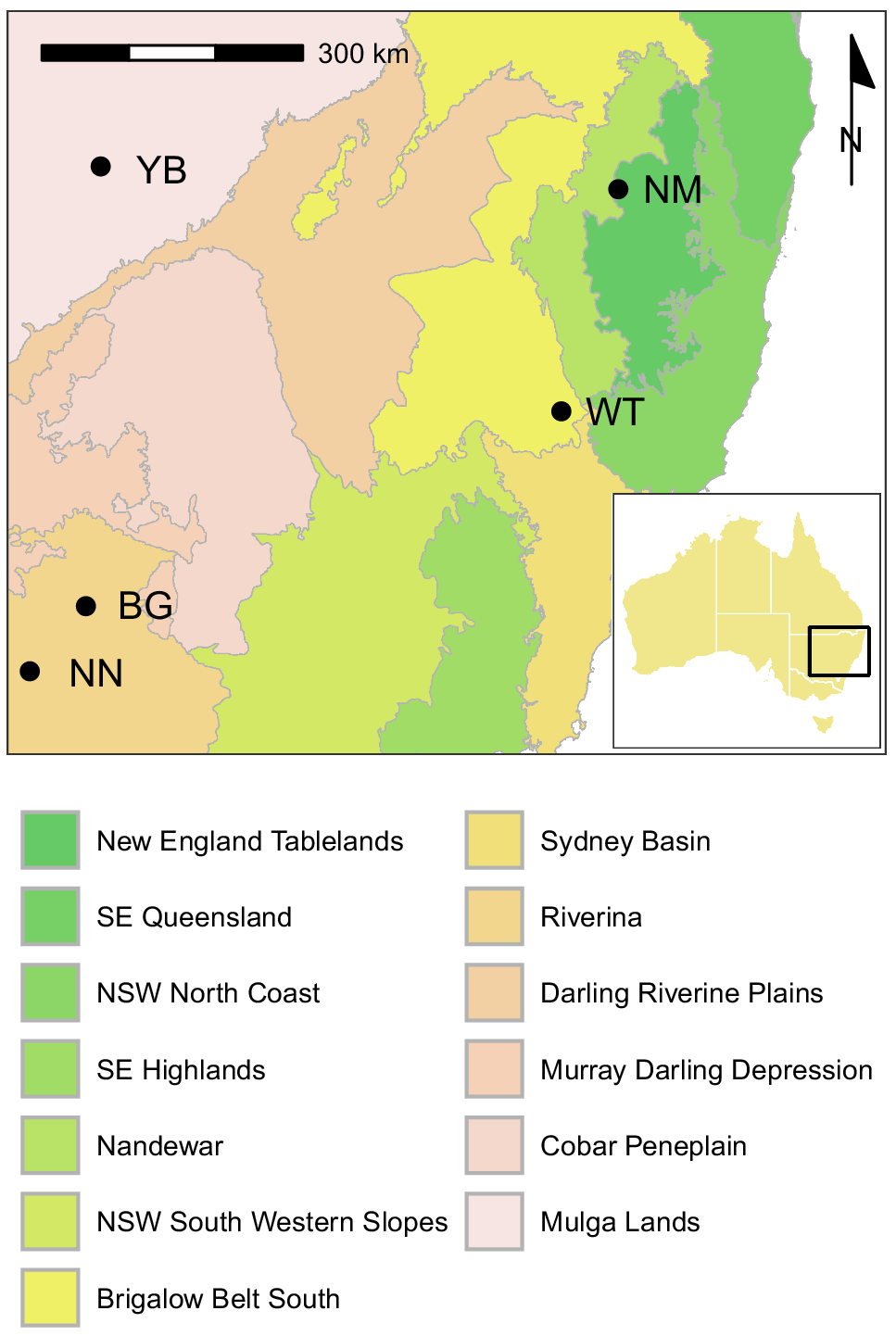
We conducted our study at five sites in New South Wales, Australia, between March 2021 and September 2023 (Fig. 1). These sites represented five distinct bioregions (Commonwealth of Australia, Department of Climate Change, Energy, the Environment and Water 2024 2016). Each site comprised several private properties, most of which were used for livestock production. Some properties were managed for biodiversity or water conservation. Feral pig population density was contemporaneously estimated at some sites, independently of the present study. Densities were estimated from helicopter-based surveys using either a single thermal sensor with distance sampling (O’Dwyer-Hall and Cox 2021) or multiple observers with mark–recapture distance sampling (Pavanato et al. 2025, D. Forsyth and A. Bengsen, unpubl. data). Density estimates ranged from 2.3 to 23.1 pigs km−2 (Table 1).
Location of five study sites in south-eastern Australia at which 58 GPS-collared wild pigs were exposed to aerial shooting. Shading indicates different bioregions. There were three aerial shooting operations at NN and NM, and one at each of the other sites.
| Operation | Area A (km2) | Pigs km−2 (s.d.) | Pigs collared | Shoot days | Shoot month | Pigs killed | Location fixes | Location fix success rate (%) | |
|---|---|---|---|---|---|---|---|---|---|
| NN2 | 1057 | 4.2 (3.9) B | 9 female (F), 3 male (M) | 5 | Jun 2021 | 585 | 11,848 | 66.1 | |
| NN3 | 138 | 4.2 (3.9) B | 6 F, 0 M | 1 | Nov 2021 | 222 | 6385 | 73.4 | |
| BG | 2622 | 2.3 (1.1) B | 3 F, 4 M | 1 | Nov 2021 | 714 | 6678 | 68.2 | |
| NN4 | 1971 | NA | 6 F, 0 M | 1 | Apr 2022 | 848 | 6177 | 71.5 | |
| WT | 603 | 13.7 (2.4) C | 3 F, 7 M | 11 | Feb 2023 | 2007 | 13,666 | 81.3 | |
| YB | 1253 | 23.1 (2.3) D | 5 F, 22 M | 5 | Oct 2023 | 4217 | 38,781 | 93.5 | |
| NM1 | 857 | NA | 5 F, 6 M | 7 | Feb 2023 | 833 | 6081 | 84.5 | |
| NM2 | 857 | NA | 5 F, 8 M | 7 | Mar 2023 | 460 | 11,676 | 80.1 | |
| NM3 | 857 | NA | 5 F 8 M | 7 | Mar 2023 | 334 | 14,547 | 83.5 |
Sites NN and BG were in the Riverina bioregion and were characterised by riparian eucalypt forest and lignum (Muehlenbeckia florulenta) shrublands on the Lowbidgee floodplain, experiencing a cold semiarid climate (Köppen climate zone BSk). Site WT was located on the Liverpool Range in the Brigalow Belt South bioregion and was characterised by eucalypt woodlands on foothills adjoining pasture and cropping country on the plains (Davis et al. 2023), experiencing a humid subtropical climate (Köppen climate zone Cfb). Site YB was in the Cuttaburra Basin, representing the Mulga Lands bioregion. The site was dominated by lignum shrublands on clay floodplains and channels in a matrix of low shrublands on sandy soils experiencing a hot semiarid climate (Köppen climate zone BSh). Water and food were diminishing rapidly during this shooting operation, and a high proportion of pigs encountered were in very poor condition. Conditions were alleviated slightly by heavy rainfall 1 week after the conclusion of the shoot. Site NM was near the locality of Nullamanna, spanning the Nandewar and New England Tablelands bioregions, and was characterised by a mosaic of eucalypt woodland and pasture on undulating terrain, experiencing a humid subtropical climate (Köppen climate zone Cfb). Sites NN and NM were subjected to repeated shooting. Operations at NN were separated by >3 months, but NM was subjected to three operations over the course of an intensive 2-month control program that included trapping and poison baiting. All sites had been subjected to aerial shooting and other pig control works in preceding years.
Pig capture and collaring
Pigs were captured using pen traps baited with grain (Waudby et al. 2022). To avoid strangulation resulting from pigs outgrowing their collars, only adult pigs estimated to be >40 kg in mass were collared. Multiple pigs were collared at the same trap site, although not necessarily on the same day, on nine occasions. Cohort size ranged from two to four pigs.
At NM, pigs were immobilised using zolazepam hydrochloride (2 mL Zoletil 100; Virbac) administered intramuscularly. All other pigs were physically restrained by experienced handlers while GPS tracking collars (Litetrack Iridium 750 PB+, Lotek, Newmarket Ontario, Canada) and ear tags were fitted. Collars were programmed to attempt one location fix every 30 min (NM) or 60 min (all other sites). The physical condition of captured pigs was assessed using a five-point scale (Coffey et al. 1999) and rectal temperature was measured with a digital thermometer.
Aerial shooting
Collared pigs were exposed to aerial shooting operations on nine occasions over 29 months. All operations except NN2 used a Bell 206 Jet Ranger helicopter carrying a navigator and a shooter armed with a .308 semi-automatic rifle. Shooting procedures followed standards established in the Feral Animal Aerial Shooting Team (FAAST) manual (FAAST 2020). Operation NN2 used a Eurocopter AS350 B3 Squirrel helicopter carrying a shooter, navigator and thermal camera operator (Cox et al. 2023). Shooting operations lasted between 1 and 11 days. At sites for which population density estimates were available, shooting operations killed between 12% and 38% of the population that was estimated to occur within the target area (Table 1). Aerial shooting teams typically approximate a Lévy walk search pattern, searching individual habitat patches intensively until kill rates decline, and often revisiting patches over the course of the operation (Bengsen et al. 2024). Consequently, many pigs exposed to multi-day operations were likely to have experienced repeated harassment.
This research was conducted under animal research authorities granted by the NSW Department of Primary Industries’ Orange Animal Ethics Committee (ORA 21-24-3) or the University of New England Animal Ethics Committee (site NM: AEC 20-023).
Data cleaning
As the frequency of relocations varied among datasets, we standardised the tracking data by keeping only locations taken at hourly intervals. We discarded all locations with a horizontal dilution of precision (HDOP) of >5. Following Bjørneraas et al. (2010), we screened each pig’s tracking data to remove unrealistic spikes between location fixes, defined by two consecutive steps of more than 10 km per hour and a turning angle <30°. These criteria were selected because they describe a movement pattern that was considered physically impossible for feral pigs to achieve and could therefore only result from excessive location error. We chose conservative exclusion criteria to avoid the risk of removing data arising from extreme behaviour by pigs responding to harassment by aerial shooting teams.
For each aerial shoot, we considered a 2-month window with location data assigned to one of the following three sampling periods: before (≤30 days before the first day of the shoot), during (each day during the shoot), and after (≤30 days after the last day of the shoot). This time-window allowed us to retain enough locations for robust before–after comparisons of pig ranging behaviours and movements, while minimizing the influence of seasonal factors. At Site NM, the time intervals between aerial shooting operations were shorter than 30 days. We therefore considered the time from the day after the shooting stopped to the day before the next operation started (i.e. 22 and 20 days). For the subsequent analyses, we used only pigs that had tracking data for the whole time-window (Table 1).
Data analysis
We used the movement-based kernel density estimation (Benhamou and Cornélis 2010) to characterise pig activity range areas before and after the aerial shooting operations. We considered the 99% level of utilisation distribution (UD 99%) based on the biased random bridge method (Benhamou 2011) as the closest representation of the pigs’ space use during each period. We first evaluated the effect of the shooting operations by measuring the overlap (0 < θ > 1) between the post-shoot range and the pre-shoot range. We resolved that any pig with an overlap of <0.1 had effectively left its pre-shoot range. We used logistic regression to estimate the probability of a pig leaving its range for each operation. We then calculated the relative change in range area as the area post-shoot divided by the area pre-shoot. We considered evidence of increased ranging behaviour when mean relative change of >1 and 95% confidence intervals (95% CI) excluded 1, change of <1 with 95% CI excluding 1 was considered as evidence of a reduction in ranging behaviour. For both measures of disturbance (range overlap and change in area), we modelled the effects of sex, shoot operation (K = 9) and their interaction by using generalised linear models (GLMs). We fitted the GLMs with a beta family for the overlap and a gamma family for the relative change in area. All spatial analyses were performed with the adehabitatHR package (ver. 0.4.22, https://cran.r-project.org/package=adehabitatHR; Calenge 2006). Range overlap and change in area GLMs were fitted with glmmTMB (ver. 1.1.10, https://cran.r-project.org/package=glmmTMB; Brooks et al. 2017) in R (ver. 4.4.1, https://cran.r-project.org/; R Core Team 2024). The logistic regression was fitted using JAGS (Plummer 2003), called via the runjags package (ver. 2.2.2-1.1, https://cran.r-project.org/package=runjags; Denwood 2016) for R. We checked goodness of fit of our models using standard residual plots.
Daily movements were characterised using two variables, namely, mean distance between hourly location fixes (MHD) and maximum distance between any two location fixes (MxD). MHD reflects the scale of movements during routine activities such as foraging and resting, whereas MxD represents the maximum straight line distance moved per day (Kay et al. 2017). The expected MHD and MxD for each combination of shooting operation and sex were estimated using linear mixed effects models, by using day as a fixed effect and individual pig as a random effect. The time series for each operation except the three shoots at NM started 30 days prior to shooting and finished 30 days after the end of shooting. At NM, the time series started 6 days prior to the first shoot and ended 30 days after the third shoot. Models were fitted using the lme4 package (ver. 1.1-35.1, https://cran.r-project.org/package=lme4; Bates et al. 2015) in R. Trend stationarity of the resulting 24 time series was assessed using the Kwiatkowski–Phillips–Schmidt–Shin (KPSS) unit root test (Kwiatkowski et al. 1992). For each time series, we used an intervention analysis to identify breakpoints at which the expected value of MHD or MxD changed from one stable value to another (following Bengsen et al. 2024). We used the strucchange package (ver. 1.5-2, https://cran.r-project.org/package=strucchange; Zeileis et al. 2002) for R to fit models with different numbers of breakpoints (0:10) and selected the optimal number and location of breakpoints from the model that minimised the Bayesian information criterion. We then examined breakpoint locations to assess whether changes in the MHD or MxD regression coefficients corresponded to the start or finish of shooting operations.
As a response to intense and repeated pressure during aerial shoot operations, we expected pigs to increase their movement during the night when helicopters did not fly. We modelled the diel cycles during each shooting operation by using the linear distance moved by pigs between two consecutive locations (step length, m) taken one hour apart. For each shooting operation, we fitted a gaussian generalised additive mixed model (GAMM) with sex (males and females) and period specific splines (before, during and after aerial shoot) to the step length (m) data. We used cubic cyclic structure for the splines to account for the circular nature of the hour of the day and included animal ID as a random effect (means and smooth terms). The GAMMs were fitted using the mgcv package (ver. 1.8.42, https://cran.r-project.org/package=mgcv; Wood 2011) in R and visually checked for an absence of pattern in the model residuals and a k-index close to 1. No collared males were available for operations NN3 and NN4. The data from one female in operation NN3 was discarded because of missing locations during the shooting operation causing a poor model fit.
Results
We fitted 89 adult feral pigs with GPS tracking collars across the five sites. The sample at NN was female-biased, whereas YB had a strong male bias because few females were assessed as being in robust health at capture (Table 1). Eighteen pigs were not used for analysis because they failed to record locations before and after a shoot operation. The full dataset after processing comprised 132,473 location fixes from 45 male and 26 females exposed to at least one aerial shooting operation (Supplementary Table S1). Thirteen pigs at Site NM and six pigs at Site NN were exposed to multiple operations (four pigs to two operations, 15 pigs to three operations), providing totals of 47 and 58 operation-level samples from females and males respectively. Across all operations, the mean success rate of the GPS collars was 80.2% (s.e. 1.3%).
Space use
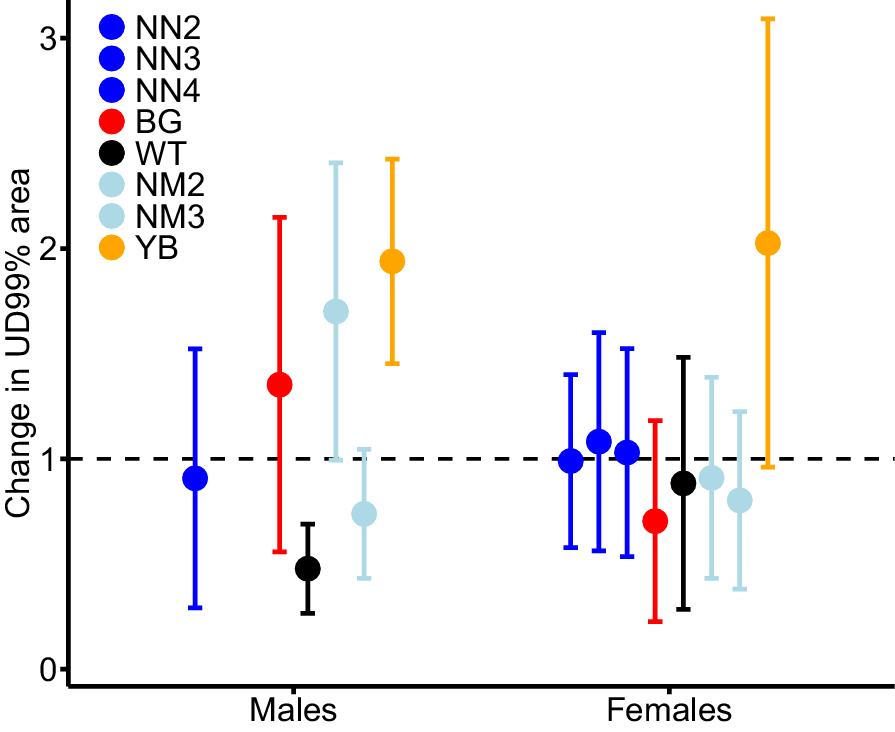
Male activity range areas prior to shooting operations (mean = 1160.0 ha; 95% CI: 880.0–1440.0 ha) were 2.7 times larger than those of females (mean = 513.9 ha; 95% CI: 374.2–653.5 ha). Expected male activity range area remained stable (i.e. confidence intervals including 1) after shooting operations NN2, BG and NM3 (Fig. 2), decreased by 52% after operation WT, and increased by 70% after operation NM2 and 94% after operation YB (Fig. 2). Female activity range area did not change after any operation, except for a 103% increase after YB, but with greater variance than the effect size (i.e. 95% CI including one).
Expected mean (and 95% confidence interval) relative change in activity range area (UD 99%) for male and female pigs after being exposed to one of seven aerial shooting operations. Horizontal dashed line (y = 1) indicates no change, i.e. values above the line show increases in ranging areas and values below the line show decreases in ranging areas after the shoot operations. Note no collared males were exposed to operations NN3 and NN4. For operation locations and timings, see Fig. 1 and Table 1 respectively.
Except for operation YB, post-shoot activity ranges of all pigs overlapped with their pre-shoot range, with a similar degree of overlap between sex and operations (mean overlap = 79.9%; 95% CI: 74.8–84.9%; range: 12.3%−99.9%; Fig. 3). After operation YB, post-shoot activity ranges showed lower overlap (mean = 50.6%; 95% CI: 38.8–62.3%) than for the other operations. There, two males showed overlaps of 3.2% and 3.9%, while one male and one female had overlaps of <1%. The estimated probability of a pig leaving its pre-shoot range was 0.11 (95% CrI = 0.02, 0.20) during operation YB and <0.01 for all other operations. Most cohorts of pigs that were captured at the same trap showed little activity range overlap with each other ( overlap of <0.5). However, one cohort of three pigs captured at Site BG showed high overlap before and after shooting (mean overlap = 96%, 87% respectively) and activity ranges of a pair of pigs captured at the same trap at Site NN had 81% overlap prior to shooting (Supplementary Fig. S1).
Expected mean (and 95% confidence interval) overlap of activity range areas (UD 99%) of male and female pigs subject to one of seven aerial shooting operations. The overlap represents the proportion of the activity range post-shoot that is shared with the activity range pre-shoot. Note that there was no male collared during operation NN3 and NN4. For operation locations and timings, see Fig. 1 and Table 1 respectively.
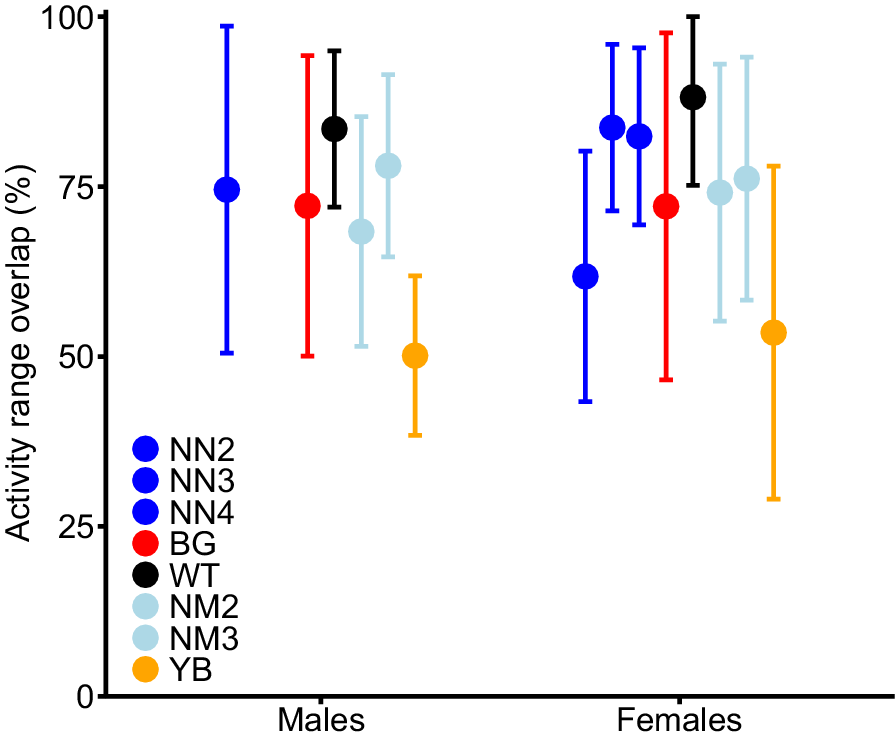
For one male at YB, the observed shift in space use after the shooting (<4% overlap) was mostly an artifact of the 30-day time window. This pig showed bimodal space-use, moving back and forth between two clusters of locations separated by ~5 km. Post-release, this pig alternated between the two clusters for 2 weeks and eventually settled in the east until the start of the shooting operation (2 weeks later). When shooting started, it moved to the western cluster and stayed there for 4 months before alternating between the two clusters again for the next 2 months. The last known position of the animal was less than 1 km from its capture location, both located between the two clusters.
The male and female at YB with <1% range overlap before and after shooting showed similar responses. Both pigs were using well-defined ranges before the shoot (less than 1 km from each other). At the start of the shooting operation, both animals moved out of their range and kept moving for the 4 days of shooting. At the end of the shooting, the male immediately settled in a new area 2 km north-west of its pre-shoot range. The female continued moving for a week after the end of the shooting before settling down in the same area as the male ~5 km north of her pre-shoot range. Both pigs remained in the operational area during shooting and for at least 30 days after.
The last male with <4% overlap at YB showed a different response to the shooting. During the first 2 days of shooting, it restricted its movements to a small area of its pre-shoot range. It then left its pre-shoot range and over a week moved 10 km east where it settled for 4 days before moving 20 km north in 3 days. It stayed in the same area for 3 weeks and shifted again to an adjacent area further north for the next month before the collar failed. This was the only collared pig that moved away from an operational area.
Hourly step length and maximum daily distance
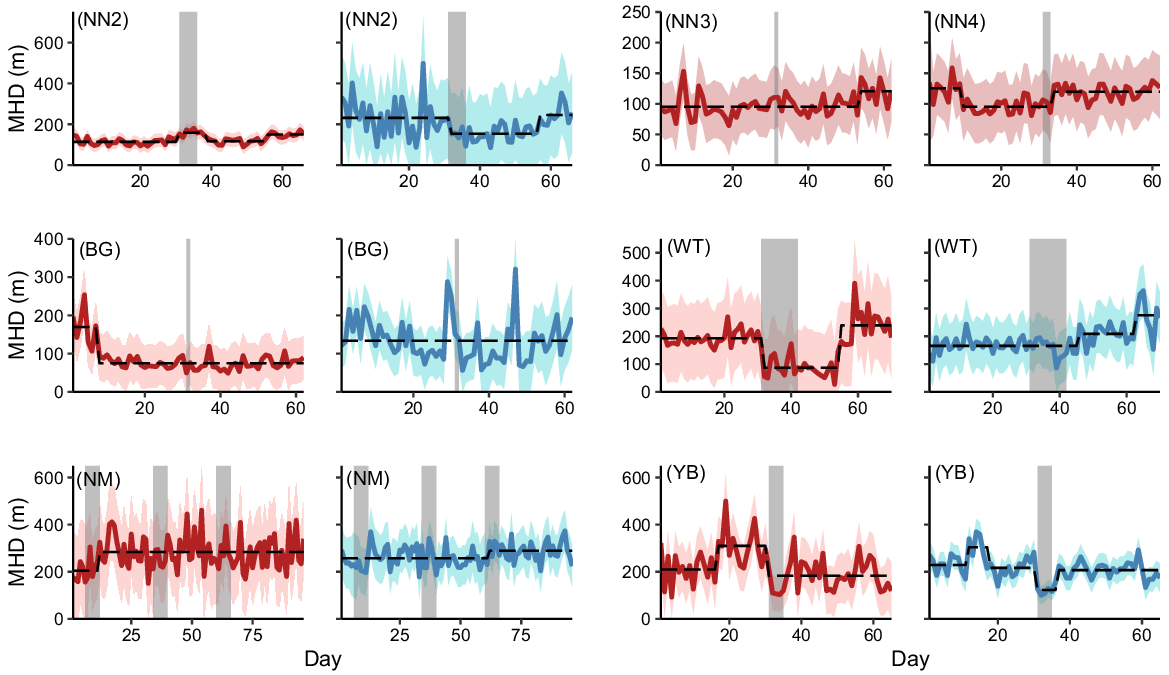
Initial mean hourly distance between location fixes ranged from 95 m (95% CI = 91, 100 m) for female pigs exposed to operation NN3 to 257 m (95% CI = 247, 267 m) for males exposed to the three operations at Site NM. The 12 MHD time series showed inconsistent responses to aerial shooting (Fig. 4). Female pigs exposed to operation NN2 increased their mean hourly distance between location fixes by 39% for 8 days after the start of shooting (Fig. 4). The same pigs increased MHD by 25% 3 days after operation NN4 for the remainder of the monitoring period, but showed no change in MHD during operation NN3. Conversely, the MHD of male pigs exposed to operation NN2 decreased by 33% when shooting started and remained low for 25 days before returning to pre-shoot levels. MHD also decreased for sows exposed to operation WT (55% decrease for 23 days) and for both sexes in operation YB (females 41% for the remainder of the time series, males 43% for 6 days). At Site NM, female MHD increased by 39% at the conclusion of the first operation and remained ~283 m (95% CI = 270, 298 m) for the remainder of the time series. Male MHD at site NM increased by 12% 2 days after the start of the third operation. No other breakpoints coincided with the start or end of shooting.
Time series of mean hourly distance moved (MHD) by female (red) and male (blue) feral pigs exposed to 1 of 12 aerial shooting operations. Red and blue ribbons show 95% confidence intervals for each time series and dashed lines represent the fitted values from a model with the optimal number of breakpoints. Grey vertical bars indicate the timing of shooting operations.
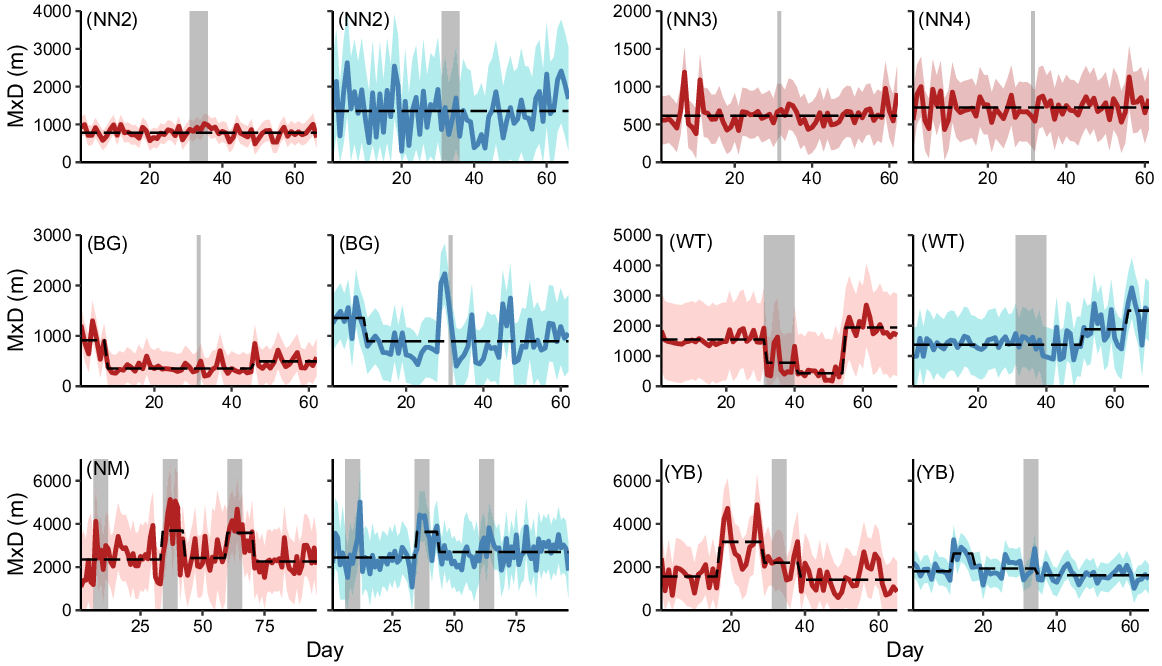
From the 12 maximum daily distance travelled time series, the four time series at NN were best described by a level fit with no breakpoints. The MxD of female pigs exposed to operation WT decreased by 50% 1 day after the start of operation WT and decreased by a further 45% when shooting ceased, remaining low for a further 14 days before returning to a similar level as before shooting. At site NM, MxD of male and female pigs increased by 57% and 49% respectively, during the second shoot and returned to pre-shoot levels 2 days after the shoot ended. Female MxD also increased during the third shoot at NM, by 48%, and returned to pre-shoot levels 4 days after shooting ceased. No other breakpoints coincided with the start or end of shooting (Fig. 5).
Time series of maximum daily distance between location fixes (MxD) for female (red) and male (blue) feral pigs exposed to 1 of 12 aerial shooting operations. Red and blue ribbons show 95% confidence intervals for each time series and dashed lines represent the fitted values from a model with the optimal number of breakpoints. Grey vertical bars indicate the timing of shooting operations.
Diel cycle
The diel cycles of feral pigs before the shooting operations showed strong variability across the five sites and some variability between sexes within sites. At NN, males were more active from sunset to midnight and reduced their movements for the second half of the night and during the day (Fig. 6). Conversely, females were mostly active during the day. During operation NN2, males shifted towards a more crepuscular pattern. After shooting, males reverted to their pre-shoot pattern but maintained longer movements around sunset. Females maintained their daytime movements during and after operation NN2 (Fig. 6). Operation NN3 had little influence on female pig movements, but females showed much larger movements during operation NN4, especially around sunset (2.3 times longer step lengths), before returning to their pre-shoot diel cycle.
Diel movement activity cycle (hourly step length, m) of female (red) and male (blue) feral pigs exposed to one of three aerial shooting operations at site NN. Red and blue ribbons show 95% confidence intervals for each time series and vertical dashed lines represent sunrise and sunset. There were no collared males during operations NN3 and NN4.

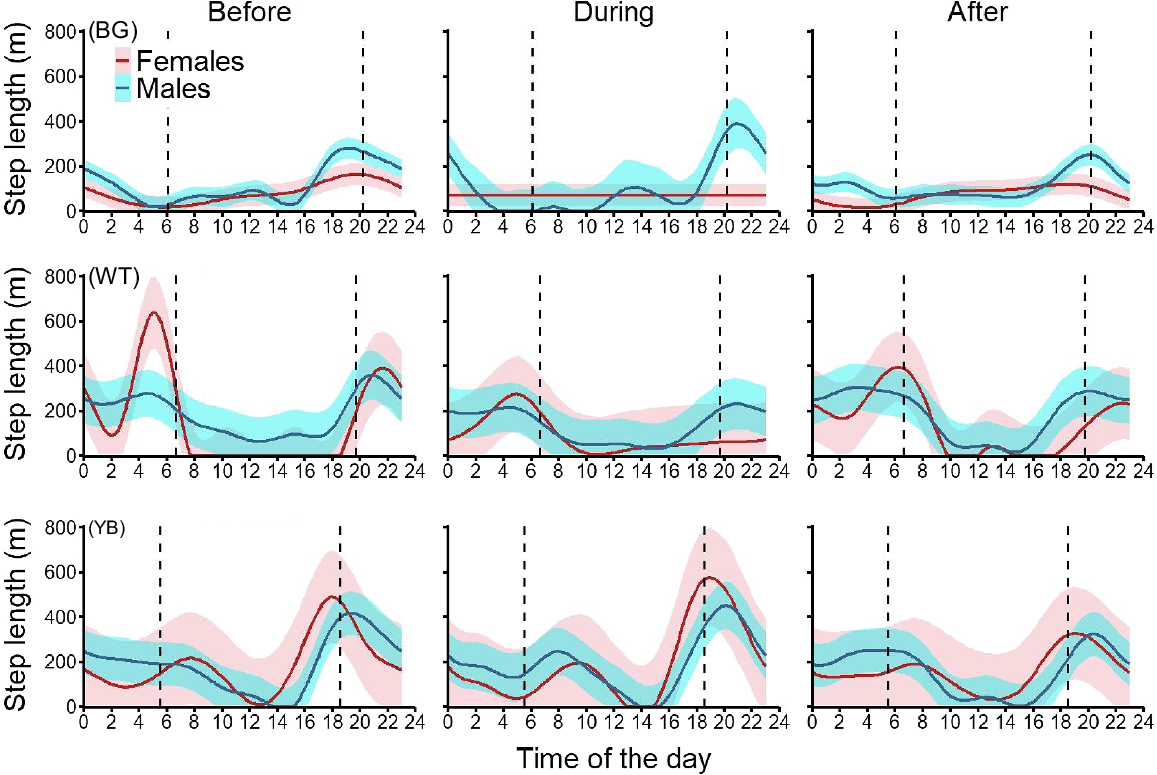
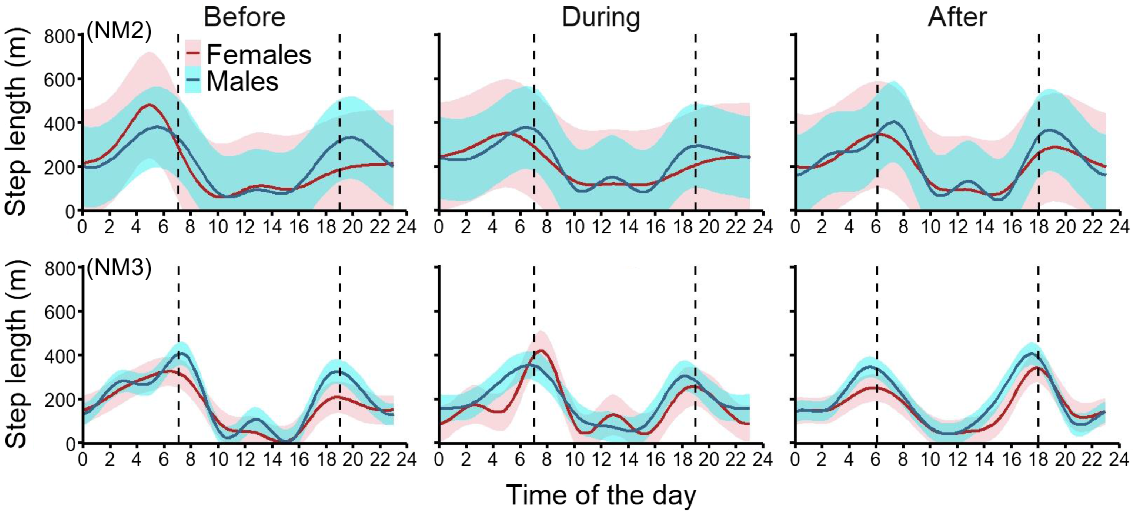
At BG, male pigs showed similar diel cycles as at NN, but females were most active around sunset. During the shooting operation at BG, males concentrated their movements at sunset before returning to pre-shoot activity at the end of the shooting. Female activity during that operation was almost non-existent (very limited movement at any time of the day), but returned to the pre-shoot pattern at the end of the shooting (Fig. 7). At WT, both male and female pigs were crepuscular, with longer movements at sunset and sunrise and limited diurnal compared with nocturnal movements. Males maintained their movement activity during and after the shooting operation, whereas females strongly reduced their movements during the shoot, especially at sunrise and sunset (57% and 83% reduction in step length respectively), before returning to movements closer to their pre-shoot pattern (Fig. 7). At YB, pigs were most active at sunset and during the night. Both males and females maintained the pre-shoot activity during the shooting operation but reduced the distance moved at sunset after the end of the shooting (Fig. 7). Females at this site showed the strongest variability in hourly step lengths (i.e. largest confidence intervals). At NM, both male and female pigs showed a typical crepuscular diel cycle. Both sexes maintained their movement activity during and after the two shooting operations (Fig. 8), with greater variability in hourly step lengths during operation NM2.
Discussion
Several studies have investigated the effects of aerial shooting on the behaviour of surviving feral pigs (Saunders and Bryant 1988; Dexter 1996; Campbell et al. 2010, 2012), but the question of whether such exposure might trigger behaviour changes that cause pigs to disperse remains a concern (e.g. African Swine Fever Feral Pig Task Group 2020; Animal Health Australia 2023). In this study, we examined nine shooting operations across five sites, providing the most comprehensive and varied analysis to date of feral pig responses to aerial shooting. Echoing the findings of the only other study that investigated multiple operations (n = 2, Campbell et al. 2010), we found inconsistent spatial behaviour changes in feral pigs during and immediately after aerial shooting. Specifically, male and female pigs exposed to different shooting operations showed variability in their range sizes before and after shooting, the overlap between pre- and post-shoot ranges, the probability of leaving their pre-shoot ranges, the mean hourly distance between location fixes post-shooting, and the distribution of their activity between daylight and night-time hours before, during, and after the shooting events. The inconsistencies observed in this study align with previous research reporting substantial variability in feral or wild pig behaviour among populations and among individuals within populations (Morelle et al. 2015).
Activity range areas tended to remain consistent or reduced in size after shooting, except for males exposed to operation NM2 and both sexes at operation YB where the average areas used by both sexes more than doubled. Most operations also showed a high overlap between pre- and post-shoot activity ranges, except for both sexes at YB. Interestingly, operation YB was the only one in which any pigs (two males and one female) moved out of their pre-shoot activity ranges. As a result, eight of the nine operations we studied showed no evidence of pigs leaving or expanding their activity ranges in a way that could increase the risk of spreading damage or disease to new areas.
The five study sites represented a diverse collection of vegetation associations, climate zones, and land uses, as characterised by the five different bioregions, but YB provided the most dynamic and challenging conditions for feral pigs around shooting operations. Many pigs exposed to this shoot were probably approaching the limits of their resilience. Prior to and during the shoot, the large (~400 km2) swamp that dominated the site was drying up. The main watercourse that feeds the swamp had not flowed in over 8 months (Water NSW 2024), and standing water was restricted to a small number of rapidly diminishing ponds and channels as temperatures increased towards summer. An abundance of pig carcasses observed in and around the few remaining waterbodies 1 month prior to the shoot suggested high natural mortality, and most pigs encountered at this time were in poor or very poor condition. Previous studies have shown that pigs tend to reduce their movement in hot weather and when food and water are scarce, most likely to conserve energy and water (Massei et al. 1997; Dexter 1999). Further, the presence of extensive low shrublands and woodlands, characteristic of much of Site YB, should provide easily accessible refuge that would negate the need for pigs to move far to avoid helicopter shooting teams (Dexter 1996). Indeed, the mean hourly step length of both sexes decreased during the shoot, as would be expected of pigs seeking local shelter. Under these conditions, pigs at Site YB might be expected to be the least likely to leave their activity ranges during aerial shooting. However, small disturbances can trigger abrupt changes in the behaviour of complex systems, such as wildlife populations, under extreme environmental stress (Scheffer et al. 2009). It is possible that the unexpected behaviour of pigs at YB was at least partially due to the environmental conditions they were experiencing, rather than in spite of them.
The large increases in range size after shooting at YB, and the low overlap of pre- and post-shoot ranges, were similarly unexpected. The data were slightly skewed by five pigs that more than tripled their range sizes after shooting, but all five sows and most males (15/22) increased their range areas after shooting. It is possible that pigs responded to the rain that fell 1 week after shooting concluded, which may have relaxed the need to concentrate their activity around sparsely distributed water points. However, similar range increases after culling have been observed in badgers (Meles meles, Riordan et al. 2011; Ham et al. 2019), white-tailed deer (Odocoileus virginianus, Williams et al. 2008) and fallow deer (Dama dama, Bengsen et al. 2024). These results are consistent with the perturbation hypothesis, which posits that population reductions caused by culling can cause social disruption that drives animals to seek or establish new social networks (Overend 1980). Range expansion and the resulting increased interactions with neighbouring social groups have been associated with an increase in bovine tuberculosis transmission risk following small-scale culling of infected badger populations (Macdonald et al. 2006; Ham et al. 2019). However, pigs exposed to operation YB already showed very high connectivity relative to other sites in eastern Australia, including most sites examined in the present study (Proboste et al. 2024). This was likely to be due to pigs needing to share the small number of rapidly-drying water points there. Moreover, the adverse impacts of population disruption on pathogen diffusion are expected to be negligible when culling is conducted over large spatial scales with few gaps or refugia (Macdonald et al. 2006; Prentice et al. 2019), as occurred in the present study.
The most common response to aerial shooting was a change in movement intensity, with both increases and decreases observed during or immediately after shooting, varying between sexes and among operations. Both sexes increased and decreased their mean hourly step length in different operations so, contrary to expectations that sows should be more risk averse (Saïd et al. 2012), there was no consistent sex-based response. The only consistent site- or operation-based response was the decrease in mean hourly step length for both sexes during shooting in operation YB, where the widespread lignum scrub provided plentiful shelter. Increases in expected step length for female pigs during two operations at Site NN did not correspond with increased maximum distance between daily location fixes, indicating that even though these pigs tended to be more active during shooting, they did not cover a larger area. A similar response was observed in female fallow deer exposed to operation WT (operation C in Bengsen et al. 2024). However, female pigs exposed to operation WT decreased both their step length and their maximum distance between location fixes during shooting, indicating that they reduced their activity and had a smaller spatial footprint. The only occasions in which the maximum distance between fixes increased during shooting was for both sexes during the second operation at NM and for females during the third operation at NM. These pigs covered a wider area per day, on average, during the shoots, but resumed regular behaviour immediately or shortly after shooting ended. The increase in female step length during shooting in operation NN2 is consistent with some previous studies that reported increased movement during actual or simulated aerial shooting (Campbell et al. 2010; Fischer et al. 2016).
The dominance of crepuscular activity among sites prior to shooting aligned with recent findings from other sites in eastern Australia (Wilson et al. 2023). However, pig activity tended to peak most strongly around sunrise at some sites and sunset at others. Whether this variability was due to site-specific factors or seasonal variability is unclear, although pre-shoot diel cycles of sows were largely consistent across three seasons at Site NN. Given the variability in pre-shoot diel cycles among sites, the inconsistent changes in temporal activity patterns during shooting are unsurprising. In contrast to some studies of behavioural responses to terrestrial hunters (van Doormaal et al. 2015; Reinke et al. 2021), there were no cases in which pigs greatly reduced their diurnal activity. This difference might be because previous studies of terrestrial hunting have typically involved cryptic hunters operating within predictable hunting grounds and seasons (Cromsigt et al. 2013), whereas aerial shooting is characterised by the unpredictable appearance of an active predator with distinct, obvious, and uncommon auditory cues (Bengsen et al. 2024). Similar differences have been observed in African ungulates that showed persistent spatial behaviour changes in response to stealthy ambush predators but not to chase hunters (Thaker et al. 2011). The most common change in diel cycles with the onset of shooting in the present study was a slight increase in sunset activity peaks for both sexes at different sites. However, the opposite pattern was observed during operation WT, which was the longest operation. Here, the sunset activity peak of males diminished during shooting, and the sunset peak of sows was completely extinguished. The reduced crepuscular activity of sows during operation WT resulted in reduced overall activity, as indicated by mean hourly step length. In most cases, pigs resumed their pre-shoot activity patterns after shooting ended, as recently observed with fallow deer subjected to aerial shooting (Bengsen et al. 2024).
Our study had several limitations that affect our inferences about the effects of aerial shooting on the behaviour of feral pigs in south-eastern Australia. First, to minimise the risk of strangulation in growing animals, only adult pigs were collared and monitored in this study. Juvenile pigs (i.e. <25 kg) are harder to shoot from the air and can be more prone to escaping when a large group of pigs is targeted (Snow et al. 2024; Chalkowski et al. 2025). This age class is also more susceptible to infection with some viruses (van der Linden et al. 2003). If juveniles are more prone to leave their normal activity ranges than are adult pigs, they may pose a greater disease spread risk than is evident from the results of this study. Future studies could reduce this potential bias by fitting juvenile pigs with a tracking unit that does not require a collar or harness that would injure a growing animal. Second, only female pigs were subjected to operations NN3 and NN4 because no collared males survived up to these shoots, and the sample for operation YB was male-biased because most sows captured were in too poor condition to be collared. However, males were exposed to shooting during the first operation at NN, and both sexes showed similar responses to shooting at YB, so we do not expect these operation-specific sex weightings to have a great impact on our results. Third, all sites had been subjected to aerial shooting in previous years or months, so few of our collared pigs would have been naïve to this form of disturbance. Helicopter shooting teams represent an unusual form of intense, episodic disturbance associated with a high risk of being killed (Bengsen et al. 2024). This combination of cues should induce strong, immediate anti-predator behaviour (Lima and Bednekoff 1999), and previous work has suggested that pigs can rapidly learn to avoid helicopter shooting teams (Saunders and Bryant 1988). Naïve populations might, therefore, display slightly different responses to the early phases of aerial shooting operations. Finally, an emergency animal disease response is likely to require extended and repeated operations to bring the target population below the necessary density threshold (Snow et al. 2024). Sites NN and NM were subjected to repeated operations in this study, and most operations lasted for at least 5 days. The exceptions were operations NN3, NN4 and BG, which were single-day operations that are unlikely to be representative of an emergency animal disease response but do reflect common management practice for programs aiming to reduce agricultural and environmental damage caused by pigs.
Management implications
Feral pigs subjected to harassment by aerial shooting teams in this study showed a range of behavioural responses that varied among shooting operations. This underscores the complexity of managing feral pig populations and impacts, given the diverse environments they inhabit and their inherent behavioural flexibility (Bengsen et al. 2014). Nonetheless, most operations examined in this study showed no evidence to support the concern that aerial shooting might cause pigs to disperse out of targeted areas or inadvertently increase the risk of disease spread. The one pig that left the target area during operation YB is the only collared animal in this study (n = 105 operation level samples) or previous studies (n = 38 animals in four shooting operations; Saunders and Bryant 1988; Dexter 1996; Campbell et al. 2010) known to have done so. Previous studies have shown that pigs sometimes make ad hoc long-distance movements without being exposed to aerial shooting, and possibly in response to disturbance by terrestrial hunters (Saunders and Bryant 1988; Dexter 1996). Any feral pig management program must, therefore, consider the possibility that a small number of pigs might disperse from a target area regardless of the intensity or type of harassment to which they are subjected. Our results from operation YB suggest that pig populations experiencing extreme stress might be more prone to unexpected behaviour than those experiencing more benign conditions, but further data are needed to test this hypothesis.
Given the unique ability of aerial shooting to rapidly reduce feral pig populations over large areas in a short time relative to other available control tools (Saunders and Bryant 1988; Snow et al. 2024), we recommend that it be retained as a primary population control tool. Aerial shooting might not always lead to large-scale dispersal of feral pigs, but our results show that it can trigger changes in their movements and space use patterns, which could have implications for pig and disease management strategies. Typically, models simulating disease transmission and management actions assume constant contact rates within and among animal groups during control operations (Keuling and Massei 2021). However, the varied movements observed in this study suggest that this assumption may be unrealistic. Conducting a dynamic network analysis to estimate changes in contact rates is a significant undertaking (Silk et al. 2019), and was beyond the immediate scope of this study, but such work should improve the reliability and usefulness of disease management simulations.
Conclusions
This study has confirmed that the behavioural responses of feral pigs to aerial shooting can be expected to vary substantially among and within different sites. Factors such as the availability of refuge habitats and resources, the sex of pigs, previous exposure to aerial shooting, environmental stress, and individual behaviour were likely to contribute to the variability observed in this study. Our findings align with expectations given the species’ behavioural flexibility and the diverse environments they inhabit. Whereas some pigs increased their movements during or after shooting, others decreased their movements or showed no change at all. Notably, only one pig in this study left the operational area during shooting. Our study considered only adult pigs, and future research would benefit from examining the responses of juvenile pigs, which could differ from those of adults. Despite this, aerial shooting remains an effective tool for reducing feral pig populations over large contiguous areas in a short amount of time. Given our results, and those of previous studies, we believe that aerial shooting should continue to be used as a key method for managing feral pig populations and should also be considered for emergency animal disease response operations.
Data availability
Fieldwork for this study was conducted on private properties. The geographic data that support this study cannot be publicly shared because of ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate.
Declaration of funding
Collaring and aerial shooting at Site NN was conducted with operational funding by Riverina Local Land Services (LLS), as was shooting at Site BG. Collaring at Sites BG, WT and YB was funded by the NSW Department of Primary Industries and Regional Development (NSW DPIRD). Shooting at Sites WT and YB were conducted under operational funding by North West LLS and Western LLS respectively. Shooting and collaring at NM was funded by NSW LLS through the FMD cloven-hoofed vertebrate pest management project.
Acknowledgements
Mark Lamb (Pest Lures), Chris Brausch (NSW DPIRD), Pip Taylor (NSW DPIRD), and Isobella McGrath (Riverina LLS) assisted with pig capture and collaring. Bridget Roberts and Jack Liersch (Bush Heritage Australia) helped with trapping at site YB. Grant Davis and Brooke Anderson (Western LLS) assisted with operations and collar recovery at site YB. Mitch Bowden (Riverina LLS) assisted with data management for shooting operations at NN and BG. Deane Smith (NSW DPIRD) assisted with data management for site NM.
References
Artois M, Delahay R, Guberti V, Cheeseman C (2001) Control of infectious diseases of wildlife in Europe. The Veterinary Journal 162, 141-152.
| Crossref | Google Scholar | PubMed |
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1-48.
| Crossref | Google Scholar |
Bengsen AJ, Gentle MN, Mitchell JL, Pearson HE, Saunders GR (2014) Impacts and management of wild pigs Sus scrofa in Australia. Mammal Review 44, 135-147.
| Crossref | Google Scholar |
Bengsen AJ, West P, Krull CR (2017) Feral pigs in Australia and New Zealand: range, trend, management and impacts of an invasive species. In ‘Ecology, evolution and management of wild pigs and peccaries. Implications for conservation’. (Eds M Melletti, E Meijaard) pp. 325–338. (Cambridge University Press: Cambridge)
Bengsen AJ, Comte S, Parker L, Forsyth DM, Hampton JO (2024) Site fidelity trumps disturbance: aerial shooting does not cause surviving fallow deer (Dama dama) to disperse. Wildlife Research 51, WR24098.
| Crossref | Google Scholar |
Benhamou S (2011) Dynamic approach to space and habitat use based on biased random bridges. PLoS ONE 6, e14592.
| Crossref | Google Scholar |
Benhamou S, Cornélis D (2010) Incorporating movement behavior and barriers to improve kernel home range space use estimates. The Journal of Wildlife Management 74, 1353-1360.
| Crossref | Google Scholar |
Bevins SN, Pedersen K, Lutman MW, Gidlewski T, Deliberto TJ (2014) Consequences associated with the recent range expansion of nonnative feral swine. BioScience 64, 291-299.
| Crossref | Google Scholar |
Bjørneraas K, Van Moorter B, Rolandsen CM, Herfindal I (2010) Screening global positioning system location data for errors using animal movement characteristics. Journal of Wildlife Management 74, 1361-1366.
| Crossref | Google Scholar |
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9, 378-400.
| Crossref | Google Scholar |
Calenge C (2006) The package ‘adehabitat’ for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling 197, 516-519.
| Crossref | Google Scholar |
Campbell TA, Long DB, Leland BR (2010) Feral swine behavior relative to aerial gunning in southern Texas. The Journal of Wildlife Management 74, 337-341.
| Crossref | Google Scholar |
Campbell TA, Long DB, Lavelle MJ, Leland BR, Blankenship TL, VerCauteren KC (2012) Impact of baiting on feral swine behavior in the presence of culling activities. Preventive Veterinary Medicine 104, 249-257.
| Crossref | Google Scholar | PubMed |
Chalkowski K, Pepin KM, Lavelle MJ, Miller RS, Fischer J, Brown VR, Glow M, Smith B, Cook S, Kohen K, Sherburne S, Smith H, Leland B, VerCauteren KC, Snow NP (2025) Operational lessons learned from simulating an elimination response to a transboundary animal disease in wild animals. Preventive Veterinary Medicine 234, 106365.
| Crossref | Google Scholar |
Choquenot D, Hone J, Saunders G (1999) Using aspects of predator–prey theory to evaluate helicopter shooting for feral pig control. Wildlife Research 26, 251-261.
| Crossref | Google Scholar |
Cowled BD, Garner MG, Negus K, Ward MP (2012) Controlling disease outbreaks in wildlife using limited culling: modelling classical swine fever incursions in wild pigs in Australia. Veterinary Research 43, 3.
| Crossref | Google Scholar |
Cox TE, Paine D, O’Dwyer-Hall E, Matthews R, Blumson T, Florance B, Fielder K, Tarran M, Korcz M, Wiebkin A, Hamnett PW, Bradshaw CJA, Page B (2023) Thermal aerial culling for the control of vertebrate pests populations. Scientific Reports 13, 10063.
| Crossref | Google Scholar |
Cromsigt JPGM, Kuijper DPJ, Adam M, Beschta RL, Churski M, Eycott A, Kerley GIH, Mysterud A, Schmidt K, West K (2013) Hunting for fear: innovating management of human–wildlife conflicts. Journal of Applied Ecology 50, 544-549.
| Crossref | Google Scholar |
Dalziel AE, Peck HA, Hurt AC, Cooke J, Cassey P (2016) Proposed surveillance for influenza A in feral pigs. EcoHealth 13, 410-414.
| Crossref | Google Scholar | PubMed |
Davis AJ, Leland B, Bodenchuk M, VerCauteren KC, Pepin KM (2018) Costs and effectiveness of damage management of an overabundant species (Sus scrofa) using aerial gunning. Wildlife Research 45, 696-705.
| Crossref | Google Scholar |
Davis NE, Forsyth DM, Bengsen AJ (2023) Diet and impacts of non-native fallow deer (Dama dama) on pastoral properties during severe drought. Wildlife Research 50, 701-715.
| Crossref | Google Scholar |
Denwood MJ (2016) runjags: an R package providing interface utilities, model templates, parallel computing methods and additional distributions for MCMC models in JAGS. Journal of Statistical Software 71, 1-25.
| Crossref | Google Scholar |
Dexter N (1996) The effect of an intensive shooting exercise from a helicopter on the behaviour of surviving feral pigs. Wildlife Research 23, 435-441.
| Crossref | Google Scholar |
Dexter N (1999) The influence of pasture distribution, temperature and sex on home-range size of feral pigs in a semi-arid environment. Wildlife Research 26, 755-762.
| Crossref | Google Scholar |
Fernández-Llario P (2004) Environmental correlates of nest site selection by wild boar Sus scrofa. Acta Theriologica 49, 383-392.
| Crossref | Google Scholar |
Fischer JW, McMurtry D, Blass CR, Walter WD, Beringer J, VerCauteren KC (2016) Effects of simulated removal activities on movements and space use of feral swine. European Journal of Wildlife Research 62, 285-292.
| Crossref | Google Scholar |
Gentle M, Wilson C, Cuskelly J (2022) Feral pig management in Australia: implications for disease control. Australian Veterinary Journal 100, 492-495.
| Crossref | Google Scholar | PubMed |
Ham C, Donnelly CA, Astley KL, Jackson SYB, Woodroffe R (2019) Effect of culling on individual badger Meles meles behaviour: potential implications for bovine tuberculosis transmission. Journal of Applied Ecology 56, 2390-2399.
| Crossref | Google Scholar | PubMed |
Kay SL, Fischer JW, Monaghan AJ, Beasley JC, Boughton R, Campbell TA, Cooper SM, Ditchkoff SS, Hartley SB, Kilgo JC, Wisely SM, Wyckoff AC, VerCauteren KC, Pepin KM (2017) Quantifying drivers of wild pig movement across multiple spatial and temporal scales. Movement Ecology 5, 14.
| Crossref | Google Scholar |
Keuling O, Massei G (2021) Does hunting affect the behavior of wild pigs? Human–Wildlife Interactions 15, 11.
| Crossref | Google Scholar |
Keuling O, Stier N, Roth M (2008) How does hunting influence activity and spatial usage in wild boar Sus scrofa L.? European Journal of Wildlife Research 54, 729-737.
| Crossref | Google Scholar |
Kwiatkowski D, Phillips PCB, Schmidt P, Shin Y (1992) Testing the null hypothesis of stationarity against the alternative of a unit root: how sure are we that economic time series have a unit root? Journal of Econometrics 54, 159-178.
| Crossref | Google Scholar |
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. The American Naturalist 153, 649-659.
| Crossref | Google Scholar | PubMed |
Macdonald DW, Riordan P, Mathews F (2006) Biological hurdles to the control of TB in cattle: a test of two hypotheses concerning wildlife to explain the failure of control. Biological Conservation 131, 268-286.
| Crossref | Google Scholar |
Maillard D, Fournier P (1995) Effects of shooting with hounds on size of resting range of wild boar (Sus scrofa L.) groups in Mediterranean habitat. Journal of Mountain Ecology 3, e107.
| Google Scholar |
Massei G, Genov PV, Staines BW, Gorman ML (1997) Factors influencing home range and activity of wild boar (Sus scrofa) in a Mediterranean coastal area. Journal of Zoology 242, 411-423.
| Crossref | Google Scholar |
Massei G, Roy S, Bunting R (2011) Too many hogs? A review of methods to mitigate impact by wild boar and feral hogs. Human-Wildlife Interactions 5, 10.
| Crossref | Google Scholar |
Morelle K, Podgórski T, Prévot C, Keuling O, Lehaire F, Lejeune P (2015) Towards understanding wild boar Sus scrofa movement: a synthetic movement ecology approach. Mammal Review 45, 15-29.
| Crossref | Google Scholar |
Overend ED (1980) Badgers and TB – does gassing spread the disease? Oryx 15, 338-340.
| Crossref | Google Scholar |
Pavanato H, Fewster R, Redmond A, Bengsen AJ, Amos M, Forsyth DM, MacKenzie D (2025) Multi-species abundance estimates using three-observer mark–recapture distance sampling surveys in Cuttaburra Basin, New South Wales. Report for Department of Primary Industries, NSW, Australia, Proteus Client Report: 196. Proteus, Outram, New Zealand.
Pedrosa F, Salerno R, Padilha FVB, Galetti M (2015) Current distribution of invasive feral pigs in Brazil: economic impacts and ecological uncertainty. Natureza & Conservação 13, 84-87.
| Crossref | Google Scholar |
Pepin KM, Brown VR, Yang A, Beasley JC, Boughton R, VerCauteren KC, Miller RS, Bevins SN (2022) Optimising response to an introduction of African swine fever in wild pigs. Transboundary and Emerging Diseases 69, e3111-e3127.
| Crossref | Google Scholar | PubMed |
Prentice JC, Fox NJ, Hutchings MR, White PCL, Davidson RS, Marion G (2019) When to kill a cull: factors affecting the success of culling wildlife for disease control. Journal of The Royal Society Interface 16, 20180901.
| Crossref | Google Scholar |
Proboste T, Turnlund A, Bengsen AJ, Gentle M, Wilson C, Harriott L, Fuller RA, Marshall D, Soares Magalhães RJ (2024) Quantifying feral pig interactions to inform disease transmission networks. eLife 13, RP102643.
| Crossref | Google Scholar |
Reinke H, König HJ, Keuling O, Kuemmerle T, Kiffner C (2021) Zoning has little impact on the seasonal diel activity and distribution patterns of wild boar (Sus scrofa) in an UNESCO Biosphere Reserve. Ecology and Evolution 11, 17091-17105.
| Crossref | Google Scholar | PubMed |
Riordan P, Delahay RJ, Cheeseman C, Johnson PJ, Macdonald DW (2011) Culling-induced changes in badger (Meles meles) behaviour, social organisation and the epidemiology of bovine tuberculosis. PLoS ONE 6, e28904.
| Crossref | Google Scholar |
Saïd S, Tolon V, Brandt S, Baubet E (2012) Sex effect on habitat selection in response to hunting disturbance: the study of wild boar. European Journal of Wildlife Research 58, 107-115.
| Crossref | Google Scholar |
Saunders G, Bryant H (1988) The evaluation of a feral pig eradication program during a simulated exotic disease outbreak. Australian Wildlife Research 15, 73-81.
| Crossref | Google Scholar |
Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Held H, van Nes EH, Rietkerk M, Sugihara G (2009) Early-warning signals for critical transitions. Nature 461, 53-59.
| Crossref | Google Scholar | PubMed |
Scillitani L, Monaco A, Toso S (2010) Do intensive drive hunts affect wild boar (Sus scrofa) spatial behaviour in Italy? Some evidences and management implications. European Journal of Wildlife Research 56, 307-318.
| Crossref | Google Scholar |
Silk MJ, Hodgson DJ, Rozins C, Croft DP, Delahay RJ, Boots M, McDonald RA (2019) Integrating social behaviour, demography and disease dynamics in network models: applications to disease management in declining wildlife populations. Philosophical Transactions of the Royal Society B: Biological Sciences 374, 20180211.
| Crossref | Google Scholar |
Snow NP, Smith B, Lavelle MJ, Glow MP, Chalkowski K, Leland BR, Sherburne S, Fischer JW, Kohen KJ, Cook SM, Smith H, VerCauteren KC, Miller RS, Pepin KM (2024) Comparing efficiencies of population control methods for responding to introductions of transboundary animal diseases in wild pigs. Preventive Veterinary Medicine 233, 106347.
| Crossref | Google Scholar |
Sodeikat G, Pohlmeyer K (2003) Escape movements of family groups of wild boar Sus scrofa influenced by drive hunts in Lower Saxony, Germany. Wildlife Biology 9, 43-49.
| Crossref | Google Scholar |
Thaker M, Vanak AT, Owen CR, Ogden MB, Niemann SM, Slotow R (2011) Minimizing predation risk in a landscape of multiple predators: effects on the spatial distribution of African ungulates. Ecology 92, 398-407.
| Crossref | Google Scholar | PubMed |
Thurfjell H, Spong G, Ericsson G (2013) Effects of hunting on wild boar Sus scrofa behaviour. Wildlife Biology 19, 87-93.
| Crossref | Google Scholar |
van der Linden IFA, Voermans JJM, van der Linde-Bril EM, Bianchi ATJ, Steverink PJGM (2003) Virological kinetics and immunological responses to a porcine reproductive and respiratory syndrome virus infection of pigs at different ages. Vaccine 21, 1952-1957.
| Crossref | Google Scholar | PubMed |
van Doormaal N, Ohashi H, Koike S, Kaji K (2015) Influence of human activities on the activity patterns of Japanese sika deer (Cervus nippon) and wild boar (Sus scrofa) in central Japan. European Journal of Wildlife Research 61, 517-527.
| Crossref | Google Scholar |
Waudby HP, Turner JM, Coulson G, Taggart D, Watson D, Bengsen AJ, Meek PD, Bower DS, Thompson S, Lumsden L, Hampton JO, Death C, Thompson G, Finlayson G, Hamilton DG, Petit S, Dunlop J, Bentley J, Vanderduys E, Ballard GA, Morrant DS (2022) Wildlife capture methods. In ‘Wildlife research in Australia – a practical guide’. (Eds B Smith, HP Waudby, JO Hampton) pp. 108–149. (CSIRO Publishing: Melbourne, Vic, Australia)
Williams SC, DeNicola AJ, Ortega IM (2008) Behavioral responses of white-tailed deer subjected to lethal management. Canadian Journal of Zoology 86, 1358-1366.
| Crossref | Google Scholar |
Wilson C, Gentle M, Marshall D (2023) Feral pig (Sus scrofa) activity and landscape feature revisitation across four sites in eastern Australia. Australian Mammalogy 45, 305-316.
| Crossref | Google Scholar |
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society Series B: Statistical Methodology 73, 3-36.
| Crossref | Google Scholar |
Zeileis A, Leisch F, Hornik K, Kleiber C (2002) strucchange: an R package for testing for structural change in linear regression models. Journal of Statistical Software 7, 1-38.
| Crossref | Google Scholar |