Informing apex predator management: population viability analysis of dingoes under different management scenarios
Emily K. Henderson A * , Benjamin J. Pitcher
A * , Benjamin J. Pitcher  B C and Neil R. Jordan
B C and Neil R. Jordan  A B
A B
A
B
C
Abstract
Along with other large carnivores, dingoes (variously Canis dingo, C. lupus dingo, and C. familiaris) can come into conflict with humans, especially when they are habituated to people and associate them with food. Management actions can range from no response, through removal of target individuals, to indiscriminate culls.
To model a known dingo population where lethal management occurs and to predict how different approaches to lethal management might impact the population over the next 20 years.
We used a software package to model a baseline scenario with no lethal management, and then several current, past or plausible lethal management scenarios for the population, including removing different age-sex classes at various levels and frequencies.
Modelled lethal management decreased the probability of population survival in all scenarios tested, particularly when individuals above the age of 2 years were targeted, and when lethal control was modelled to increase in frequency. Models indicated that targeted lethal management of subadult (age 1–2) males resulted in the highest probability of population persistence, which contrasted most markedly with pack removal through indiscriminate culls.
Modelling identified that targeting problem individuals for lethal control was more sustainable for the population than indiscriminate culls. Research indicates that ‘problem’ animals are often subadult males. Modelling the removal of subadult (age 1–2) males affects the population less than removal of other age-sex classes. This implies that targeted lethal management poses less risk to population persistence than indiscriminate culls, since culling is more likely to remove older animals and females. Modelled increases in the number of animals controlled and the frequency of that control decreases the population’s likelihood of persistence.
Lethal management strategies may have serious impacts on the persistence of carnivore populations, particularly when strategies involve broadscale indiscriminate culls. Adoption of best-practice targeted individual management requires investment in monitoring and identification. While the uptake of individual-focused management is not feasible in many scenarios, more widespread uptake would improve wildlife management outcomes.
Keywords: Canis dingo, carnivore, human–wildlife conflict, lethal control, problem animal profiling, PVA, Vortex, wildlife management.
Introduction
Carnivores are some of the most charismatic and well-known taxa globally, and frequently come into direct conflict with human populations (Sergio et al. 2006; Dickman 2010; König et al. 2020). This human–wildlife conflict contributes to ecological degradation through species declines, extinctions and changes in ecosystem function, and it damages human relationships with their natural surroundings (Nyhus 2016). Humans persecute carnivores due to threats to physical safety, property, and livestock, particularly in urban, peri-urban, and agricultural areas (König et al. 2020). Carnivores influence ecosystems through their role as apex predators, maintaining the diversity and balance of ecosystems, so it is vital that these conflicts be managed sustainably to ensure coexistence and maintain key ecological roles and functions (Morehouse and Boyce 2017; van Eeden et al. 2018).
In response to human–wildlife conflict with carnivores, practitioners often use lethal control (Swan et al. 2017; Lorand et al. 2022). Lethal control can reduce conflict, at least in the short term, in contexts where carnivores pose a threat to livestock (van Eeden et al. 2018), although in some cases it can intensify conflicts (Lorand et al. 2022). However, there are few studies on how lethal control affects the long-term viability of carnivore populations (Allen et al. 2015; Lennox et al. 2018). To ensure effective coexistence, it is vital to monitor how different carnivore management techniques will affect the viability of wildlife populations, to assist managers in applying evidence-based management interventions (Morehouse and Boyce 2017).
Dingoes (Canis dingo, C. lupus dingo, or C. familiaris), the largest terrestrial carnivore in Australia, are frequently in conflict with human populations, creating public concern over livestock predation and attacks on humans and domestic pets (Allen et al. 2013a; Alting et al. 2024). Dingoes are particularly controversial due to their contested status: depending on who you ask, they may be native trophic regulators, hybrid feral pests, cultural kin, or most infamously, baby-killers (Glen et al. 2007; Smith and Litchfield 2009; Purcell 2010; Letnic et al. 2012; Allen et al. 2013b; van Eeden et al. 2021). Dingoes are predominantly managed through lethal techniques, although conservation practitioners are increasingly exploring non-lethal options (Appleby et al. 2017; Smith et al. 2021a, 2021b). Across most of their range, dingoes are managed by lethal control to reduce conflict over livestock. In some urban and peri-urban areas, such as the coast of New South Wales and K’gari (formerly Fraser Island), concerns over public safety prompt management action (van Eeden et al. 2018). Targeted lethal control, in which problem individuals who are involved in conflict with humans are identified and destroyed, is becoming a viable alternative to more indiscriminate culls or baiting programs in some contexts (Allen et al. 2015; O’Neill et al. 2016; Swan et al. 2017). Targeted control of dingoes potentially better protects Aboriginal cultural values than broadscale culls, since fewer individuals are killed and dingoes often have cultural importance for Traditional Custodians (Dickman et al. 2021). Targeted control relies on the identification of dingoes involved in conflicts, which is only possible where dingoes are identified and monitored as individuals. The majority of animals targeted for lethal control are young or subadult males, since they are overrepresented in dingo–human conflicts (Allen et al. 2015; Appleby et al. 2018). Literature is scarce comparing the impact of targeted lethal management to the impact of widespread culls on dingo populations. Understanding the impact of lethal management on dingo population persistence can help conservation practitioners make informed decisions about the best strategy for reducing dingo–human conflict.
In this study, we modelled the dingo population of the Myall Lakes region through a population viability analysis (PVA). PVA is a process of assessing the likelihood that a population will become extinct within a specified time frame, usually modelled using computer simulations, and the process can be useful for comparing potential management approaches (Possingham et al. 1994). We primarily used existing demographic data from the study site to simulate the population, supplemented with data from mainland Australia and K’gari. PVAs can illuminate population trajectories under different intervention scenarios (Slotta-Bachmayr et al. 2004; Heinsohn et al. 2022). They are useful for projecting populations under different hypothetical scenarios over time, and can therefore be useful in evaluating and focusing management interventions. In contrast to costly and challenging experimental studies (Grente et al. 2024), simulations are a cost-efficient and ethical alternative. Here we used the software package Vortex (Lacy and Pollak 2023) to model a dingo population under different lethal management scenarios, and in doing so provide the first PVA of a mainland Australian dingo population. We aimed to identify how different levels and frequencies of lethal management might impact the dingo population over the next 20 years. Specifically, we modelled whether targeting specific problem individuals for lethal management substantially alters the population trajectory compared to non-targeted culls, such as removing entire packs. We predicted that the current dingo population in eastern Myall Lakes is relatively stable in size and is not at risk of extinction under current management practices, and that lethal management strategies which target problematic individuals – usually young male dingoes in the study area – would have the least impact on population viability.
Materials and methods
Study site
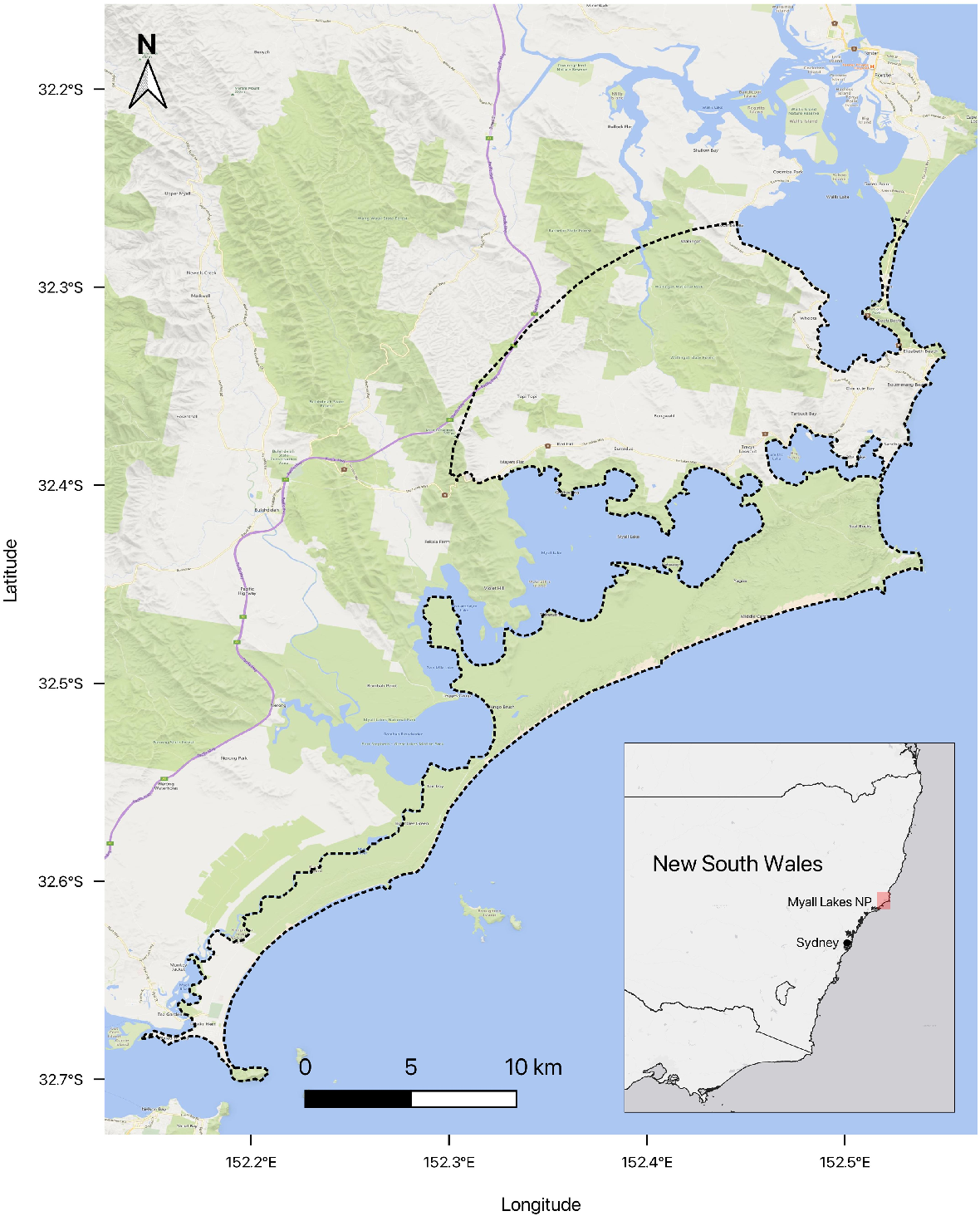
The study area is a temperate coastal lake ecosystem on Worimi Country on the mid-coast of New South Wales, Australia, and comprises Myall Lakes National Park, private land, and small coastal localities of Hawks Nest, Smiths Lake, and Pacific Palms (Fig. 1). The area’s dingo population has approximately six contiguous packs in a 50 km coastal band between Hawks Nest and Seal Rocks, and is fairly geographically isolated due to lakes and rivers to the west and south of the study area (Alting et al. 2024). The dingoes have a mean density of 0.071 dingoes/km2 (95% CI: 0.044–0.115), with the highest density in areas of high human population, such as the town of Hawks Nest (Alting et al. 2024). Dingoes are not routinely trapped or baited in the study site for management, but individual animals may be targeted (in all land tenures) for lethal control if they are identified as engaging in behaviours deemed to pose an unacceptable risk to public safety, as defined under Mid Coast Council’s Dingo Management Procedure (MidCoast Council 2019). Prior to the adoption and implementation of this procedure, and the establishment of substantial research effort facilitating the individual identification of dingoes displaying these behaviours (Alting et al. 2024), indiscriminate culls occurred in and around urban centres, and some private land owners continue to remove multiple dingoes in this way.
Map of the study area (inside dashed line) in the Great Lakes region along the mid-coast of New South Wales, Australia. Populations were modelled within the hatched area (347 km2) for which accurate population estimates (Alting et al. 2024) and dingo population structure data (Myall Lakes Dingo/Dapin Project unpubl. data) were available.
Population viability analysis
To model dingo population viability in the eastern Myall Lakes study site, we conducted a population viability analysis (PVA) through the software package Vortex 10.6.0 (Lacy et al. 2021; Lacy and Pollak 2023). We modelled an unmanaged baseline scenario, with no lethal management, before inputting a series of ‘Harvest’ scenarios where different levels of lethal management were applied. The input parameters for the unmanaged scenario, which was used as a baseline for all subsequent ‘Harvest’ scenarios, are explained below and summarised in Table 1. Table 2 shows the additional ‘Harvest’ inputs which were used to simulate different levels of lethal management. All simulations used 1000 iterations, since Vortex recommends 500–1000 iterations for more rigorous results (Lacy et al. 2021). The timeframe for all simulations was 20 years to focus on relatively short-term population impacts since longer term projections have limited practical use for the population’s management without more complete genetic information. We used life history and demographic data from both mainland and K’gari (formerly Fraser Island) studies.
| Parameter | Value | |
|---|---|---|
| Scenario settings | ||
| Number of iterations | 1000 | |
| Number of years | 20 | |
| Duration of each ‘year’ in days | 365 | |
| Extinction definition | Only 1 sex remains | |
| Number of populations | 1 | |
| Species description | ||
| Inbreeding depression | Yes | |
| Lethal equivalents | 6.29 | |
| Percent due to recessive lethals | 50 | |
| Environmental Variation (EV) correlation between reproduction and survival | 0.5 | |
| Reproductive system | ||
| Mating system | Long-term monogamy | |
| Age of first offspring for females/males | 2/2 | |
| Max. age of reproduction for females/males | 10/10 | |
| Max. lifespan | 13 | |
| Maximum number of broods per year | 1 | |
| Maximum number of progeny per brood | 10 | |
| Sex ratio at birth – in % males | 51 | |
| Reproductive rates | ||
| % adult females breeding (probability of reproduction) | 47, s.d. 10 | |
| The distribution of no. of offspring per female per brood | 4.5, s.d. 1.8 | |
| Mortality rates (annual means) | ||
| Age 0–1 year (juveniles) | 58, s.d. 16 | |
| Age 1–2 years (subadults) | 28.57, s.d. 23 | |
| Age 2+ years (adults) | 9.6, 4.29 s.d. | |
| Mate monopolisation | ||
| % males successfully siring offspring | 80 | |
| Initial population size | ||
| Population size | 27, stable age distribution | |
| Carrying capacity (K) | 43 | |
| Intervention | Scenario (number/frequency of harvest) | |
|---|---|---|
| Intervention 1: targeting individual problem animals | One subadult male/year | |
| Two subadult males/year | ||
| Three subadult males/year | ||
| Four subadult males/year | ||
| Five subadult males/year | ||
| Six subadult males/year | ||
| Seven subadult males/year | ||
| Eight subadult males/year | ||
| Nine subadult males/year | ||
| Ten subadult males/year | ||
| One subadult male/2 years | ||
| Three subadult males/2 years | ||
| Six subadult males/2 years | ||
| Six subadult males/3 years | ||
| Intervention 2: whole pack removal | Pack/year | |
| Pack/2 years | ||
| Pack/3 years | ||
| Intervention 3: removal of different demographics | One subadult female/year | |
| One adult male/year | ||
| One adult female/year |
Note subadults are age 1–2 years, adults are age 2+ years, and a pack is modelled as two subadult males, two subadult females, one adult male, and one adult female.
Unmanaged scenario parameters
Reproduction inputs were possible to determine with reasonable accuracy using the available literature, with some uncertainty in the mating system and percentage of females breeding. Dingoes have an annual generation cycle, with litters born in winter each year (June–August), so the duration of each ‘year’ was 365 days in the model (Purcell 2010). We used the Vortex defaults for inbreeding depression, where fitness is reduced due to lowered genetic diversity through inbreeding, following Appleby et al. (2025) and Merli et al. (2023). The default values for lethal equivalents are calculated from a study by O’Grady et al. (2006), but because the potential for inbreeding depression within the population is uncertain, we also modelled a scenario without inbreeding depression, and a scenario with more severe inbreeding effects at 12 lethal equivalents (Supplementary Table S1). We modelled long-term monogamy for the mating system, assuming pairs stay stable season-to-season. This aligns with observed long-term monogamy on K’gari (Appleby et al. 2025), and unpublished observations of the study population (Myall Lakes Dingo/Dapin Project unpublished data). However, Tatler et al. (2021) provide genetic evidence from dingoes in central Australia for both long-term monogamy and extreme promiscuity in mating strategies. Because of this, we also modelled a polygynous system (Table S1).
The median age of first breeding (defined in Vortex as when first offspring are born) is 2 years for males and females, and Allen et al. (2015) records most dingoes reach sexual maturity by their second year. Studies on K’gari by Behrendorff and Allen (2016) and Allen et al. (2015) provided longevity and reproduction parameters. We modelled a 51% male sex ratio at birth, which has been observed by Catling et al. (1992) in central Australia. Allen et al. (2015) records litters as having approximately equal males/females. We modelled 47% of adult females as breeding capable, since observational data from Myall Lakes show that in 2023–2024, 7/15 (47%) adult females successfully produced offspring. This number aligns with Appleby et al. (2025) middle estimate of 50% adult female breeding success. Allen et al. (2015) records approximately one successful litter per pack annually, although two litters in a pack are not unusual, and this is supported by data from the population study. With six known core packs in the study area, approximately six to seven successful litters each year seems a reasonable estimate. The distribution of number of offspring per female per brood is a mean of 4.5 pups (s.d. 1.8) in dingoes, following Allen et al. (2015) observations of 54 litters a few months after pups were born on K’gari. Mills et al. (2008) study on juvenile Eastern wolves in Canada found similar litter sizes, recording mean litter size as 4.6 ± 0.6.
Unmanaged mortality rates were difficult to determine, with little data available in the literature from dingo populations that are not lethally managed in some way. Mortality rates in the Myall Lakes population are unknown and we used a combination of other studies and observational data to inform the model’s stage-specific mortality rates. Juvenile (age 0–1) mortality rates in other social canids vary but can be up to 50% (Mills et al. 2008; Alting et al. 2024), so we used a high juvenile mortality estimate to give conservative results of the population’s likely persistence. We modelled juveniles (age 0–1) as 58% annual mortality (s.d. was calculated using =SQRT((0.58 × (1 − 0.58))/9, assuming a population of nine juveniles as indicated by site observations). We calculated subadult (age 1–2) mortality from Allen et al. (2015) study which found that only ~30% of pups survive to reach age two. Adult (age 2+) mortality at Myall Lakes was difficult to determine, but falls between an upper limit of 39.39% (s.d. 8.57), estimated from discrepancies between individual dingo sightings each year, and a lower limit of 5.42% (s.d. 5.05), estimated from the annual known deaths in the population. The upper limit does not account for dispersal or unseen individuals, while the lower limit may not account for some unknown mortality. Because of the wide window of uncertainty, we chose to follow O’Neill et al. (2016) published figure of a mean 9.6% (4.29 s.d.) annual ‘natural’ mortality (i.e. excluding culls) from 2010 to 2015 on K’gari. Since adult mortality was particularly difficult to determine, we also modelled a scenario where the adult mortality was doubled to 19.2%, given in the supplementary materials (Table S1).
Population size and carrying capacity estimates are likely reasonably accurate, due to a recent population study in the area (Alting et al. 2024). Spatial Capture–Recapture (SCR) models estimated a mean density of 0.071 dingoes/km2 (95% CI, range 0.044–0.115) in the study area, resulting in a population size estimate of 27 dingoes in the study area (extrapolating the density estimate, range 17–43, 95% CI) (Alting et al. 2024). This density estimate is similar to Namadgi National Park, at 0.06/km2 (Forsyth et al. 2019). We modelled carrying capacity (K) as 43, which is the upper limit of the estimated population range of 17–43 (95% CI), and extrapolated from density estimates given by (Alting et al. 2024). Assuming the population is currently near carrying capacity, K was set to the upper limit of the population range.
To explore the influence of parameters on model predictions, we conducted sensitivity analyses on the baseline unmanaged scenario using the ‘Single-Factor’ Sensitivity Testing setup in Vortex10, with values varied ±20% and tested in 5% increments (i.e. −20%, −15%, −10%, −5%, +5%, +10%, +15%, +20%). We tested the parameters of juvenile (age 0–1) mortality, subadult (age 1–2) mortality, adult (age 2+) mortality, percentage of adult females breeding, initial population size, carrying capacity, and lethal equivalents (Table S2).
Lethal management scenario parameters
When modelling lethal control of individuals (or ‘harvests’ in Vortex), different number and frequencies of lethal control events were added to the baseline unmanaged scenario. Our lethal control scenarios replicated three hypothetical interventions:
Intervention 1 involved scenarios in which subadult males were targeted. This replicated the current program of targeted destruction of high-risk animals in the study population (MidCoast Council 2019). Incidents determined to require lethal control interventions are rare, but when they do occur, they usually involve subadult males. In the period from 2019 to November 2024, there were 313 dingo reports from the public in the study area. Of these, three were ‘Class 4’, meaning an animal engaged in behaviour deemed high-risk to humans and was targeted for destruction. Two of these were subadult males, and were euthanised, and one involved a dominant male that evaded substantial targeted control efforts and subsequently de-escalated its behaviour. K’gari has a lethal management program (Appleby et al. 2018), and 110 dingoes were destroyed from 2001 to 2013, with approximately 66% being <18 months old and 65% being male (Allen et al. 2015). A subordinate adult female dingo was only destroyed once in this period, and in any given year, no more than four female dingoes were destroyed during mating/whelping seasons (Allen et al. 2015). In K’gari serious incident reports from 2001 to 2015, subadult males were involved in 47% of dingo–human conflicts compared with subadult females being involved in only 3% of incidents (out of 90 incidents where sex and age-class were recorded) (Appleby et al. 2018). Fourteen scenarios were modelled for Intervention 1, involving different numbers (1–10) and frequencies (yearly to every 3 years) of lethal control.
Intervention 2 involved scenarios which replicated an entire pack being removed from the population. This scenario has occurred in the study area, and can occur when private landowners manage animals on their land outside of the framework, targeting individual problem animals. Pack removal would be a likely culling scenario if targeted lethal management was not employed, where packs are targeted in areas near human habitation or campsites, and when one pack is destroyed, new animals will likely eventually take their place. This intervention also gave an indication of the likely population impact of more indiscriminate culls, with varied age-sex classes. A pack was modelled as two subadult males, two subadult females, one adult male, and one adult female, which replicated common pack structures in the study site, and aligned with a small pack size without pups (Corbett 1988). Three scenarios were modelled for Intervention 2, involving different frequencies (yearly to every 3 years) of pack removal.
Intervention 3 gave a comparison of removing different demographics of dingoes from the population. This provided comparison to targeting subadult males, and demonstrated the different levels of impact that removing different age-sex classes may have on population viability. We modelled removing subadult males (age 1–2), subadult females (age 1–2), adult males (age 2+), and adult females (age 2+) to investigate whether age and sex had an impact on population viability. Subadults have not yet reached sexual maturity, so dingo reproduction could be affected by removing adults compared to subadults. Three scenarios were modelled for Intervention 3, involving different age-sex classes, all with the same number (one) and frequency (yearly) of lethal control.
Results
Modelled lethal management decreased the probability of population survival in all scenarios tested, particularly when individuals above the age of 2 years were targeted, and when the frequency of lethal control was modelled to increase (Table 3). Our models indicated that lethal management targeted towards subadult males increased the probability of population survival compared with indiscriminate culls in the form of whole pack removal.
| Intervention | Scenario | P[survive] (%) | Mean final population (±s.e.) | Mean growth rate (±s.e.) | |
|---|---|---|---|---|---|
| No intervention | Unmanaged | 98.2 | 33.77 ± 0.34 | 0.0793 ± 0.0017 | |
| Intervention 1: targeting problem animals | One subadult male/year | 95.7 | 30.08 ± 0.40 | 0.0585 ± 0.0017 | |
| Two subadult males/year | 84.8 | 24.49 ± 0.47 | 0.0311 ± 0.0017 | ||
| Three subadult males/year | 65.4 | 17.54 ± 0.46 | 0.0026 ± 0.0017 | ||
| Four subadult males/year | 44.6 | 10.52 ± 0.36 | −0.0251 ± 0.0017 | ||
| Five subadult males/year | 24.8 | 6.63 ± 0.29 | −0.0402 ± 0.0017 | ||
| Six subadult males/year | 12.9 | 4.13 ± 0.19 | −0.0505 ± 0.0017 | ||
| Seven subadult males/year | 5.5 | 2.76 ± 0.13 | −0.0550 ± 0.0017 | ||
| Eight subadult males/year | 2.7 | 2.07 ± 0.10 | −0.0589 ± 0.0017 | ||
| Nine subadult males/year | 1.1 | 1.66 ± 0.08 | −0.0602 ± 0.0017 | ||
| Ten subadult males/year | 0.2 | 1.39 ± 0.06 | −0.0608 ± 0.0017 | ||
| One subadult male/2 years | 97.7 | 32.69 ± 0.36 | 0.0710 ± 0.0017 | ||
| Three subadult males/2 years | 96.2 | 30.42 ± 0.40 | 0.0526 ± 0.0017 | ||
| Six subadult males/2 years | 95.6 | 29.26 ± 0.41 | 0.0421 ± 0.0017 | ||
| Six subadult males/3 years | 96.2 | 29.86 ± 0.40 | 0.0499 ± 0.0017 | ||
| Intervention 2: pack removal | Pack/year | 8.4 | 1.46 ± 0.18 | −0.1430 ± 0.0028 | |
| Pack/2 years | 57.7 | 14.52 ± 0.48 | −0.0215 ± 0.0022 | ||
| Pack/3 years | 76.7 | 20.91 ± 0.50 | 0.0086 ± 0.0020 | ||
| Intervention 3: comparison of demographics | One subadult female/year | 91.5 | 25.91 ± 0.43 | 0.0365 ± 0.0017 | |
| One adult male/year | 79.2 | 25.94 ± 0.48 | 0.0476 ± 0.0018 | ||
| One adult female/year | 64.7 | 19.83 ± 0.49 | 0.0207 ± 0.0018 |
No intervention
Our models projected that the population mostly persists with no management intervention. An unmanaged (or baseline) scenario with no lethal management projected a 98.2% probability of survival over the next 20 years. The projected mean final population was 33.77 ± 0.34 (17.32 male and 16.45 female). Throughout the simulation, the mean population size remained fairly stable, with the mean population climbing from 27.00 to 37.11 individuals in the first 7 years before remaining at a mean of 35.69 ± 0.31. Of the 18/1000 populations which went extinct in this scenario, the mean time to first extinction was 15.72 ± 0.75 years. Across all years, prior to carrying capacity truncation, the mean growth rate (r) of the population/year was 0.0793 ± 0.0017. When a polygynous mating system was modelled rather than long-term monogamy, there was a 99% probability of survival with a projected mean final population of 34.15 ± 0.32. When adult (age 2+) mortality was doubled, there was an 86.3% probability of survival with a projected mean final population of 19.56 ± 0.43. Without inbreeding depression, there was a 99.3% probability of survival with a projected mean final population of 36.61 ± 0.28 (Table S1).
Sensitivity testing showed that varying the parameters of least certainty – mortality, % adult females breeding, and the potential for inbreeding depression – had only minor effects on model outcomes, with the probability of survival remaining above 90% (Table S2). Changes to juvenile mortality (age 0–1) had the greatest effect on model outcomes, with probability of survival being 91.6% at +20% and 99.4% at −20%. All other parameters’ probability of survival remained above 96% at every ±20% increment.
Lethal management interventions
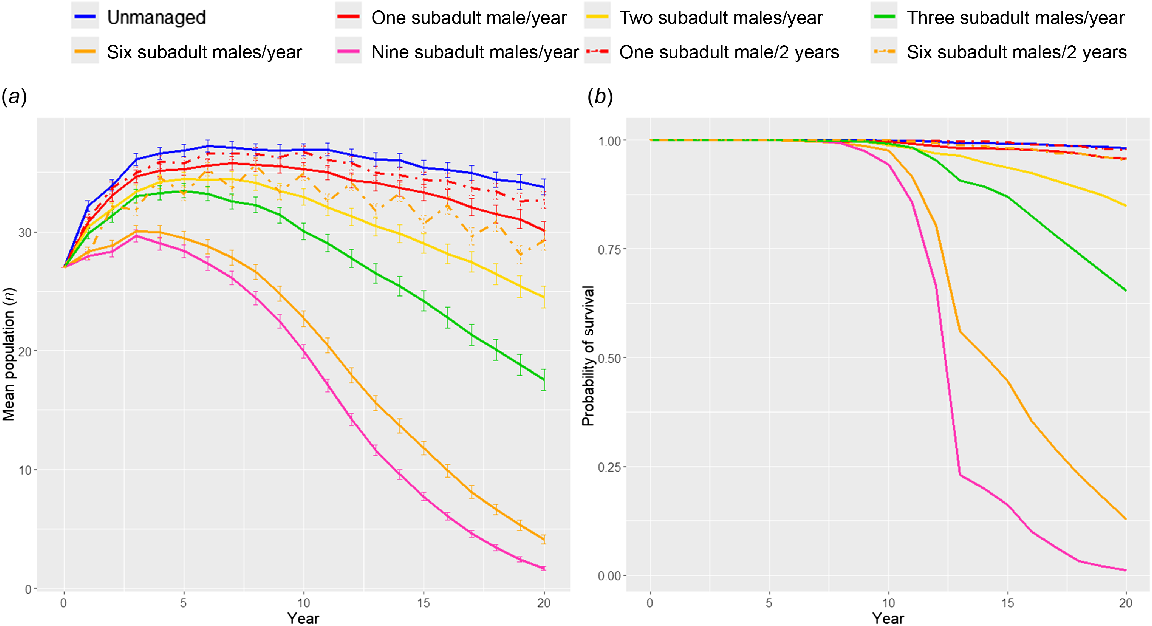
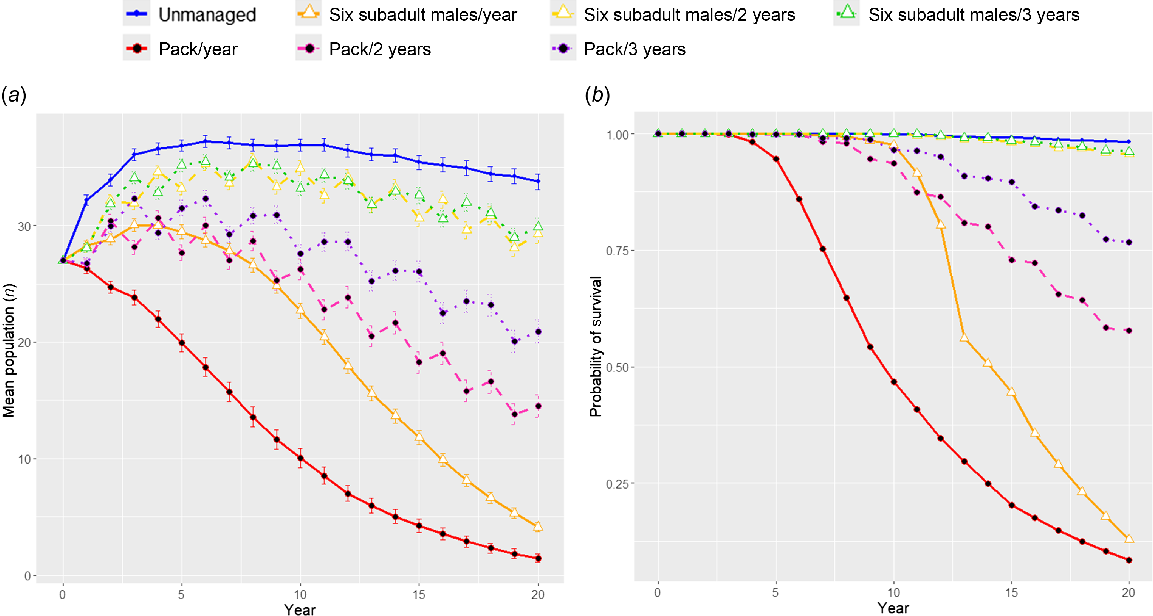
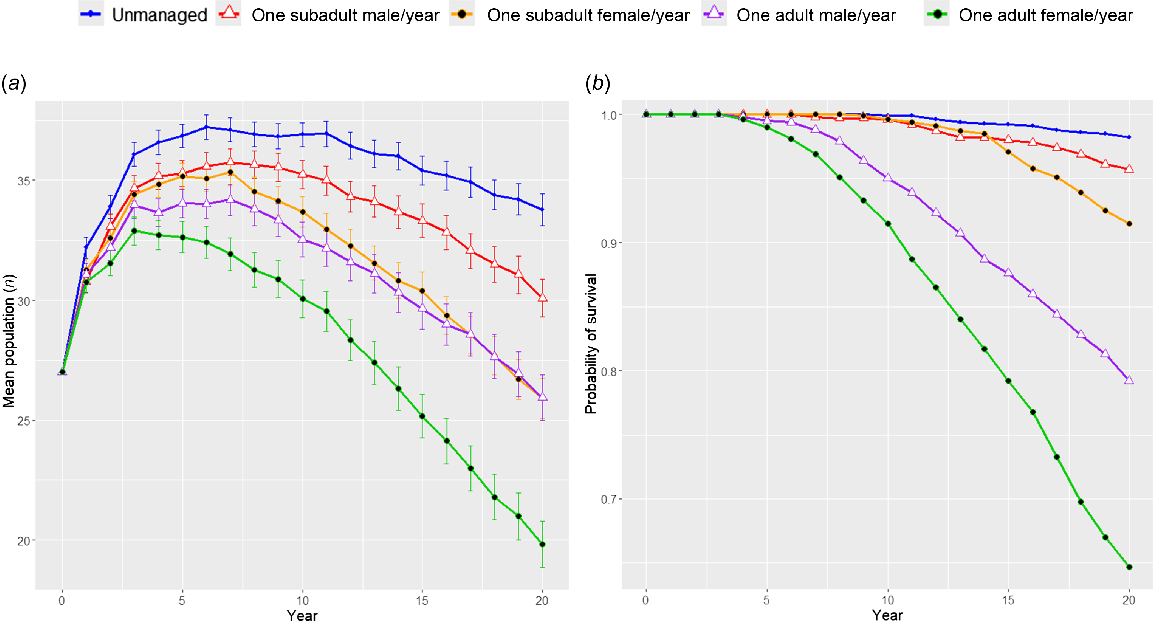
Intervention 1 simulated targeting subadult males with lethal management (Fig. 2). Highly frequent (annual) destruction of multiple subadult males had the largest impact on the probability of population survival within this category, with less frequent (every 2 years) destruction allowing the population to replenish and increasing the probability of population survival. Intervention 2 simulated the removal of a pack, i.e. removing mixed demographics similar to a pack. Here, the probability of survival was much lower than when removing the same number of subadult males (Fig. 3). When pack removal was less frequent, probability of survival increased. Intervention 3 compared the demographics being lethally controlled (Fig. 4). In all scenarios, any level of lethal management negatively affected the probability of population persistence, and the age-sex characteristics of the individuals being killed determined the severity of the effect. Lethal management of subadult males (age 1–2) affected the population the least, and adult females (age 2+) affected the population the most.
Model prediction outputs from Vortex 10.6.0 (Lacy and Pollak 2023) comparing population trajectories at various lethal control levels of subadult (age 1–2) males (Intervention 1). Solid lines indicate control occurs annually, and dashed lines indicate control occurs every 2 years. Graph (a) plots the mean population size with error bars showing 95% CI of mean, graph (b) plots the mean probability of survival.
Model prediction outputs from Vortex 10.6.0 (Lacy and Pollak 2023) comparing population trajectories where lethal control of subadult males or pack removal occurs (Intervention 2). Note ‘pack removal’ involved removing 2 subadult (age 1–2) males, 2 subadult (age 1–2) females, 1 adult (age 2+) male, and 1 adult (age 2+) female. White triangles indicate the population with subadult males removed and black circles indicate pack removals. Solid lines indicate control occurs annually, dashed lines indicate control occurs every 2 years, and dotted lines indicate control occurs every 3 years. Graph (a) plots the mean population size with error bars showing 95% CI of mean, graph (b) plots the mean probability of survival.
Model prediction outputs from Vortex 10.6.0 (Lacy and Pollak 2023) comparing population trajectories where lethal control of subadult (age 1–2) males, subadult (age 1–2) females, adult (age 2+) males, and adult (age 2+) females occurs (Intervention 3). White triangles indicate males and black circles indicate females. Graph (a) plots the mean population size with error bars showing 95% CI of mean, graph (b) plots the mean probability of survival.
Discussion
Dingo population viability analysis indicates that targeting individuals for lethal management is more sustainable for the population than indiscriminate culls. When lethal management occurs in the context of ensuring human safety, evidence from Myall Lakes and K’gari indicate that it is often subadult males which are deemed high-risk and are targeted (Allen et al. 2015; Appleby et al. 2018). Our results indicate that removing subadult (age 1–2) males affects the population less than other age-sex classes, so targeted lethal management poses less risk to population persistence than indiscriminate culls, since culling is more likely to remove older animals and females. Increases in frequency and severity of lethal control impact population viability, and decrease the population’s likelihood of survival. Under current conditions, with no lethal management intervention, the dingo population in eastern Myall Lakes is likely to persist in the long term. Targeted lethal management of subadult males impacts the population’s survival likelihood, but not as clearly as if subadult females, adult males, or adult females are killed instead. Modelling suggests that indiscriminate culls or removal of entire packs may have serious consequences for the population’s persistence.
Our results indicate that targeted lethal management has some impact on dingo population viability, and the severity of this population impact changes depending on the age-sex class and reproductive status of the individual removed. Non-targeted culls have a more tangible impact, and can remove breeder individuals which can alter carnivore behaviour and intensify conflict with humans (Lorand et al. 2022). Targeted removal of individual ‘problem’ coyotes in urban Colorado, USA, was found to have little effect on the population’s viability, and the removal of those individuals usually reduced subsequent human–coyote conflicts for several years afterwards (Breck et al. 2017). However, when certain individuals such as breeders are targeted, even a small number of removals can have negative impacts on social and reproductive structures. In wolves, removing breeders can disrupt the social stability of packs, and Brainerd et al. (2008) recommends that managers consider the possible social impacts of lethal control, only removing solitary individuals or territorial pairs where possible (rather than breeding animals). The role individuals play in group dynamics should be considered, with some keystone individuals being important for maintaining social structures (Swan et al. 2017). Similarly, we found that the age-sex class of the individuals removed from a population makes a considerable difference to the probability of population survival. Our modelled scenarios indicate that removing subadult males from the population has the least negative impact, followed by subadult females, then adult males, then adult females. In dingoes, management programs which target the destruction of specific ‘problem’ individuals, such as the current programs in the study area in Myall Lakes and on K’gari, suggest that most individuals removed are subadult males (Allen et al. 2015; Appleby et al. 2018). Studies on Baltic grey seals and introduced cats suggest males are overrepresented in the removal of problem individuals (Kauhala et al. 2015; Moseby et al. 2015; Swan et al. 2017). These results indicate that when non-breeding subadult male individuals are targeted for lethal management, the population has a substantially higher probability of survival than when other demographics or a mixed demographic are removed.
We modelled real or realistic management scenarios, but there were also several model limitations. Brook et al. (2000) write that generic PVA packages such as Vortex are useful in species management because they are widely used, and therefore widely scrutinised and frequently updated. PVA is particularly useful in comparing alternative potential management plans for a particular population (Reed et al. 2002). Limitations of PVA include the amount of detailed life history data needed for accurate modelling, and studies advise caution to prevent misuse of PVA results (Coulson et al. 2001; Reed et al. 2002; Chaudhary and Oli 2020). A limitation for our models was that sometimes a harvest could not be completed in a given year of a lethal management iteration, since not enough individuals of that age-sex class were present to be harvested. This could potentially give an iteration with a large harvest and small population size an extra year of survival before the next scheduled harvest. Additionally, Vortex does not provide spatially explicit models, which is a limitation since demographic parameters are necessarily location-specific in the real world (Pollock et al. 2012). In the current study, we would expect targeted lethal control to occur in specific areas (urban and peri-urban areas and campgrounds) and so modelling the removal of individuals or packs in and around these specific locations would be particularly informative. Despite limitations surrounding the accuracy of predicting future population sizes and extinction probability, Coulson et al. (2001) finds that PVA is useful for a comparative study of different management strategies, and for determining how different model assumptions or parameters might affect a population. PVA was useful in this way for our study: primarily as a method of comparison for different lethal management scenarios. In future studies, it will be important to consider the effects of lethal control beyond population persistence, including impacts on social structure and intraspecific conflict, which is beyond the scope of the Vortex software package, but could be included in a PVA if behavioural responses could be mathematically described. A study by O’Neill et al. (2016) on the K’gari lethal control program suggests that even when lethal management does not affect population viability, it may perpetuate conspecific conflict and human–dingo conflict. Because of this, it is important that future research considers the social ecology of lethally managed dingoes.
Information is lacking on the genetic diversity of dingo populations (Conroy et al. 2021). Recent studies indicate the presence of domestic dog ancestry is less common than previously thought (Cairns et al. 2023), with most wild canids in southeastern Australia having majority dingo ancestry, and only 2% being offspring of a dingo × dog or offspring of two dingo × dog hybrids (F1 or F2 hybrids) (Cairns et al. 2022). Inbreeding depression is a major concern, since if the influence of inbreeding depression on extinction risk is disregarded, models can overestimate the survival probability of wild vertebrate populations (O’Grady et al. 2006). Vortex 10.6.0 models inbreeding depression as a reduction in first-year survival among inbred individuals (Lacy et al. 2021). The default value of 6.29 lethal equivalents, used by prior studies (Merli et al. 2023; Appleby et al. 2025), is calculated from the mean effect of inbreeding on fecundity and first year survival in a study by O’Grady et al. (2006). Effects of inbreeding on species fitness may be best modelled even higher, possibly at 12 (Lacy et al. 2021), which we tested and it did not impact the probability of survival or mean population size after 20 years, which may be due to the short timeframe of our analysis (Table S1). To assess the uncertainty in inbreeding depression, we conducted sensitivity tests on lethal equivalents, and found that variations of up to ±20% did not bring the probability of survival below 97% (Table S2). Removing inbreeding depression from the unmanaged scenario made the population 1.1% more likely to survive after 20 years (See Tables 3 and S1), but while this is a small difference within the model’s time frame, true levels of inbreeding depression remain uncertain.
Our results show several areas in which dingo life history information is lacking in the literature. Dingo mortality rates are difficult to determine due to limited studies on dingo populations which are not baited or culled. We found it particularly difficult to separate dispersal of individuals beyond the study site from mortality within the study area. Sensitivity testing showed that data deficiency in mortality and % adult females breeding only minorly affected the baseline model, with juvenile mortality having the greatest effect. Our results indicate a polygynous mating system raises the likelihood of survival by 0.8% (Table S1), so the prevalence of polygyny is worth further investigation following Tatler et al. (2021) results. Along with other studies, we recommend further genetic and demographic monitoring to better understand dingo ecology and increase the likelihood of persistence for small carnivore populations (Conroy et al. 2021; Appleby et al. 2025). Further attention could be paid to how individual variations in carnivores such as differences in resource selection may influence how they interact with humans (Swan et al. 2017). Dingo populations that are not culled or baited are understudied, and it would be valuable to better understand them for future management of the species.
Targeted lethal control is a relatively new management strategy for dingoes in urban and peri-urban areas where non-lethal management options are not available or must be supplemented, and where managers aim to maintain a sustainable dingo population while remaining sensitive to Aboriginal cultural values and broader community sentiment. By comparing the likely population impacts of different lethal control scenarios on an unbaited coastal dingo population, we found targeted control of specific ‘problem’ animals (subadult males) was more sustainable than other lethal management approaches. Where it is possible to identify individual ‘problem’ animals, and where population persistence is a desired management outcome, targeted control of specific problem animals should be employed.
Data availability
The data that support this study are available in the article and accompanying online supplementary material, or from cited literature.
Declaration of funding
The Myall Lakes Dingo/Dapin Project has received funding and support from The Hermon Slade Foundation, Oatley Flora and Fauna Conservation Society, Australian Mammal Society, Taronga Conservation Society Australia, Paddy Palin Foundation, NSW National Parks and Wildlife Service, MidCoast Council, Australia and Pacific Science Foundation, the Marine Estate Management Strategy (MEMS), and the University of New South Wales.
Acknowledgements
Data used in this paper were primarily collected on Worimi Country, and we acknowledge the Worimi people, past and present, and their historical and ongoing connection to dingoes and Country. The Myall Lakes Dingo/Dapin Project receives funding and support from The Hermon Slade Foundation, Oatley Flora and Fauna Conservation Society, Australian Mammal Society, Taronga Conservation Society Australia, NSW National Parks and Wildlife Service, MidCoast Council, Australia and Pacific Science Foundation, the Marine Estate Management Strategy (MEMS), and the University of New South Wales. Thanks to Amelia Jeffery for her work on Fig. 1. Demographic information on the dingo population underpinning this work was collected by a range of researchers, practitioners, and volunteers with additional information gratefully received from the broader community. In particular, we would like to thank Brendan Alting, Dushmantha Gamage, Michelle Campbell, Alex Dibnah, Amelia Jeffery, Deanne Phillips, Fiona Miller, Tamara Campbell, Mathew Bell, Nicholas Colman, Robbie Paulson, Katrina Blow, and Jacob Hagne.
References
Allen BL, Goullet M, Allen LR, Lisle A, Leung LK-P (2013a) Dingoes at the doorstep: preliminary data on the ecology of dingoes in urban areas. Landscape and Urban Planning 119, 131-135.
| Crossref | Google Scholar |
Allen BL, Fleming PJS, Allen LR, Engeman RM, Ballard G, Leung LK-P (2013b) As clear as mud: a critical review of evidence for the ecological roles of Australian dingoes. Biological Conservation 159, 158-174.
| Crossref | Google Scholar |
Allen BL, Higginbottom K, Bracks JH, Davies N, Baxter GS (2015) Balancing dingo conservation with human safety on Fraser Island: the numerical and demographic effects of humane destruction of dingoes. Australasian Journal of Environmental Management 22(2), 197-215.
| Crossref | Google Scholar |
Alting BF, Pitcher BJ, Rees MW, Ferrer-Paris JR, Jordan NR (2024) Population density and ranging behaviour of a generalist carnivore varies with human population. Ecology and Evolution 14(5), e11404.
| Crossref | Google Scholar |
Appleby R, Smith B, Bernede L, Jones D (2017) Utilising aversive conditioning to manage the behaviour of K’gari (Fraser Island) dingoes (Canis dingo). Pacific Conservation Biology 23(4), 335-358.
| Crossref | Google Scholar |
Appleby R, Mackie J, Smith B, Bernede L, Jones D (2018) Human–dingo interactions on Fraser Island: an analysis of serious incident reports. Australian Mammalogy 40(2), 146-156.
| Crossref | Google Scholar |
Appleby R, Smith BP, Jones D, Conroy G, Behrendorff L (2025) A population viability analysis of K’gari (Fraser Island) wongari (dingoes). Australian Mammalogy 47(1), AM23009.
| Crossref | Google Scholar |
Behrendorff L, Allen BL (2016) From den to dust: longevity of three dingoes (Canis lupus dingo) on Fraser Island (K’gari). Australian Mammalogy 38(2), 256-260.
| Crossref | Google Scholar |
Brainerd SM, Andrén H, Bangs EE, Bradley EH, Fontaine JA, Hall W, Iliopoulos Y, Jimenez MD, Jozwiak EA, Liberg O, Mack CM, Meier TJ, Niemeyer CC, Pedersen HC, Sand H, Schultz RN, Smith DW, Wabakken P, Wydeven AP (2008) The effects of breeder loss on wolves. The Journal of Wildlife Management 72(1), 89-98.
| Crossref | Google Scholar |
Breck SW, Poessel SA, Bonnell MA (2017) Evaluating lethal and nonlethal management options for urban coyotes. Human-Wildlife Interactions 11(2), 133-145.
| Crossref | Google Scholar |
Brook BW, O’Grady JJ, Chapman AP, Burgman MA, Akçakaya HR, Frankham R (2000) Predictive accuracy of population viability analysis in conservation biology. Nature 404(6776), 385-387.
| Crossref | Google Scholar | PubMed |
Cairns KM, Crowther MS, Nesbitt B, Letnic M (2022) The myth of wild dogs in Australia: are there any out there? Australian Mammalogy 44(1), 67-75.
| Crossref | Google Scholar |
Cairns KM, Crowther MS, Parker HG, Ostrander EA, Letnic M (2023) Genome-wide variant analyses reveal new patterns of admixture and population structure in Australian dingoes. Molecular Ecology 32, 4133-4150.
| Crossref | Google Scholar | PubMed |
Catling PC, Corbett LK, Newsome AE (1992) Reproduction in captive and wild dingoes (Canis familiaris dingo) in temperate and arid environments of Australia. Wildlife Research 19(2), 195-209.
| Crossref | Google Scholar |
Chaudhary V, Oli MK (2020) A critical appraisal of population viability analysis. Conservation Biology 34(1), 26-40.
| Crossref | Google Scholar | PubMed |
Conroy GC, Lamont RW, Bridges L, Stephens D, Wardell-Johnson A, Ogbourne SM (2021) Conservation concerns associated with low genetic diversity for K’gari–Fraser Island dingoes. Scientific Reports 11(1), 9503.
| Crossref | Google Scholar |
Corbett LK (1988) Social dynamics of a captive dingo pack: population regulation by dominant female infanticide. Ethology 78(3), 177-198.
| Crossref | Google Scholar |
Coulson T, Mace GM, Hudson E, Possingham H (2001) The use and abuse of population viability analysis. Trends in Ecology & Evolution 16(5), 219-221.
| Crossref | Google Scholar | PubMed |
Dickman AJ (2010) Complexities of conflict: the importance of considering social factors for effectively resolving human–wildlife conflict. Animal Conservation 13(5), 458-466.
| Crossref | Google Scholar |
Dickman CR, Newsome TM, van Eeden LM (2021) The dingo dilemma: a brief history of debate. Australian Zoologist 41(3), 298-321.
| Crossref | Google Scholar |
Forsyth DM, Ramsey DSL, Woodford LP (2019) Estimating abundances, densities, and interspecific associations in a carnivore community. The Journal of Wildlife Management 83(5), 1090-1102.
| Crossref | Google Scholar |
Glen AS, Dickman CR, Soulé ME, Mackey BG (2007) Evaluating the role of the dingo as a trophic regulator in Australian ecosystems. Austral Ecology 32(5), 492-501.
| Crossref | Google Scholar |
Grente O, Bauduin S, Santostasi NL, Chamaillé-Jammes S, Duchamp C, Drouet-Hoguet N, Gimenez O (2024) Evaluating the effects of wolf culling on livestock predation when considering wolf population dynamics in an individual-based model. Wildlife Biology 2024(6), e01227.
| Crossref | Google Scholar |
Heinsohn R, Lacy R, Elphinstone A, Ingwersen D, Pitcher BJ, Roderick M, Schmelitschek E, Van Sluys M, Stojanovic D, Tripovich J, Crates R (2022) Population viability in data deficient nomadic species: What it will take to save regent honeyeaters from extinction. Biological Conservation 266, 109430.
| Crossref | Google Scholar |
Kauhala K, Kurkilahti M, Ahola MP, Herrero A, Karlsson O, Kunnasranta M, Tiilikainen R, Vetemaa M (2015) Age, sex and body condition of Baltic grey seals: are problem seals a random sample of the population? Annales Zoologici Fennici 52(1–2), 103-114.
| Crossref | Google Scholar |
König HJ, Kiffner C, Kramer-Schadt S, Fürst C, Keuling O, Ford AT (2020) Human–wildlife coexistence in a changing world. Conservation Biology 34(4), 786-794.
| Crossref | Google Scholar | PubMed |
Lacy RC, Pollak JP (2023) Vortex: a stochastic simulation of the extinction process (version 10.6.0). Available at https://scti.tools/vortex/ [accessed 11 February 2025]
Lacy RC, Miller PS, Traylor-Holzer K (2021) Vortex 10 user’s manual (30 March 2021 update). Available at https://scti.tools/downloads/#SoftwareAndManuals [accessed 11 February 2025]
Lennox RJ, Gallagher AJ, Ritchie EG, Cooke SJ (2018) Evaluating the efficacy of predator removal in a conflict-prone world. Biological Conservation 224, 277-289.
| Crossref | Google Scholar |
Letnic M, Ritchie EG, Dickman CR (2012) Top predators as biodiversity regulators: the dingo (Canis lupus dingo) as a case study. Biological Reviews 87(2), 390-413.
| Crossref | Google Scholar | PubMed |
Lorand C, Robert A, Gastineau A, Mihoub J-B, Bessa-Gomes C (2022) Effectiveness of interventions for managing human-large carnivore conflicts worldwide: scare them off, don’t remove them. Science of The Total Environment 838, 156195.
| Crossref | Google Scholar |
Merli E, Mattioli L, Bassi E, Bongi P, Berzi D, Ciuti F, Luccarini S, Morimando F, Viviani V, Caniglia R, Galaverni M, Fabbri E, Scandura M, Apollonio M (2023) Estimating wolf population size and dynamics by field monitoring and demographic models: implications for management and conservation. Animals 13(11), 1735.
| Crossref | Google Scholar |
MidCoast Council (2019) Procedure on the management of dingoes in residential areas and council managed spaces in Hawks Nest. Available at https://www.midcoast.nsw.gov.au/files/assets/public/v/1/document-resources/council/projects-documents/dingo-management/hawks-nest-dingo_wild-dog-management-procedure.pdf [accessed 11 February 2025]
Mills KJ, Patterson BR, Murray DL (2008) Direct estimation of early survival and movements in eastern wolf pups. The Journal of Wildlife Management 72(4), 949-954.
| Crossref | Google Scholar |
Morehouse AT, Boyce MS (2017) Troublemaking carnivores: conflicts with humans in a diverse assemblage of large carnivores. Ecology and Society 22(3), 4.
| Crossref | Google Scholar |
Moseby KE, Peacock DE, Read JL (2015) Catastrophic cat predation: a call for predator profiling in wildlife protection programs. Biological Conservation 191, 331-340.
| Crossref | Google Scholar |
Nyhus PJ (2016) Human-wildlife conflict and coexistence. Annual Review of Environment and Resources 41, 143-171.
| Crossref | Google Scholar |
O’Grady JJ, Brook BW, Reed DH, Ballou JD, Tonkyn DW, Frankham R (2006) Realistic levels of inbreeding depression strongly affect extinction risk in wild populations. Biological Conservation 133(1), 42-51.
| Crossref | Google Scholar |
O’Neill A, Cairns K, Kaplan G, Healy E (2016) Managing dingoes on Fraser Island: culling, conflict, and an alternative. Pacific Conservation Biology 23, 4-14.
| Crossref | Google Scholar |
Possingham HP, Lindenmayer DB, Norton TW (1994) A framework for the improved management of threatened species based on Population Viability Analysis (PVA). Pacific Conservation Biology 1(1), 39-45.
| Crossref | Google Scholar |
Reed JM, Mills LS, Dunning JB, Jr., Menges ES, McKelvey KS, Frye R, Beissinger SR, Anstett M-C, Miller P (2002) Emerging issues in population viability analysis. Conservation Biology 16(1), 7-19.
| Crossref | Google Scholar | PubMed |
Sergio F, Newton I, Marchesi L, Pedrini P (2006) Ecologically justified charisma: preservation of top predators delivers biodiversity conservation. Journal of Applied Ecology 43(6), 1049-1055.
| Crossref | Google Scholar |
Slotta-Bachmayr L, Boegel R, Kaczensky P, Stauffer C, Walzer C (2004) Use of population viability analysis to identify management priorities and success in reintroducing Przewalski’s horses to southwestern Mongolia. The Journal of Wildlife Management 68(4), 790-798.
| Crossref | Google Scholar |
Smith BP, Litchfield CA (2009) A review of the relationship between Indigenous Australians, dingoes (Canis dingo) and domestic dogs (Canis familiaris). Anthrozoös 22(2), 111-128.
| Crossref | Google Scholar |
Smith BP, Appleby RG, Jordan NR (2021a) Co-existing with dingoes: challenges and solutions to implementing non-lethal management. Australian Zoologist 41(3), 491-510.
| Crossref | Google Scholar |
Smith BP, Jaques NB, Appleby RG, Morris S, Jordan NR (2021b) Automated shepherds: responses of captive dingoes to sound and an inflatable, moving effigy. Pacific Conservation Biology 27(2), 195-201.
| Crossref | Google Scholar |
Swan GJF, Redpath SM, Bearhop S, McDonald RA (2017) Ecology of problem individuals and the efficacy of selective wildlife management. Trends in Ecology & Evolution 32(7), 518-530.
| Crossref | Google Scholar | PubMed |
Tatler J, Prowse TAA, Roshier DA, Cairns KM, Cassey P (2021) Phenotypic variation and promiscuity in a wild population of pure dingoes (Canis dingo). Journal of Zoological Systematics and Evolutionary Research 59(1), 311-322.
| Crossref | Google Scholar |
van Eeden LM, Crowther MS, Dickman CR, Macdonald DW, Ripple WJ, Ritchie EG, Newsome TM (2018) Managing conflict between large carnivores and livestock. Conservation Biology 32(1), 26-34.
| Crossref | Google Scholar | PubMed |
van Eeden LM, Crowther MS, Dickman CR, Newsome TM (2021) Wicked ‘wild dogs’: Australian public awareness of and attitudes towards dingoes and dingo management. Australian Zoologist 41(3), 467-479.
| Crossref | Google Scholar |


