Healthy or unhealthy? Risk factors and biomarkers associated with exposure to infectious agents in wild lowland tapirs (Tapirus terrestris)
Renata Carolina Fernandes-Santos A B C * , Kristin Warren A D , Rebecca Vaughan-Higgins A D E , Emília Patrícia Medici C F G and Mieghan Bruce
A B C * , Kristin Warren A D , Rebecca Vaughan-Higgins A D E , Emília Patrícia Medici C F G and Mieghan Bruce  A B
A B
A
B
C
D
E
F
G
Abstract
Links between tapir health and environmental conditions are well-established, but substantial knowledge gaps on biological and environmental causes of ill-health remain. Furthermore, anthropogenic impacts and climate change effects on disease patterns are escalating issues.
Our study aimed to build on earlier research on wild lowland tapir (Tapirus terrestris) health and investigate risk factors and potential consequences associated with infectious agents.
Between 2008 and 2018, 174 samples from 115 wild lowland tapirs across two contrasting locations in Brazil were screened for four infectious agents (bluetongue virus, porcine parvovirus, Leptospira interrogans serovar Pomona, and Trypanosoma terrestris), along with clinical and haematological findings. Generalised linear models and boosted regression trees were applied to evaluate associations with risk factors, likely disease consequences, and meteorological conditions.
Tapirs in human-modified areas presented higher risk of exposure to livestock pathogens such as bluetongue virus (relative influence (RI) 94.2%) and porcine parvovirus (RI 58.5%), whereas those in pristine habitats exhibited higher risk to Trypanosoma terrestris (RI 92.5%) and Leptospira sp. (RI 39.9%). Bluetongue cases increased from one in Year 2 to 35 in Year 10 (odds ratio 2.90, 95% CI 2.12–3.97, P < 0.001). Significant associations were found between infectious agents and pale mucous membranes (RI 85.5%), high tick burden (RI 78.4%), low red (RI 78.3%) and high white (RI 38.1%) blood cell counts, and presence of wounds (RI 59.1%). Poor body condition was weakly linked to all variables. Elevated alkaline phosphatase, glucose, and total protein levels demonstrated associations with infectious agents, whereas high creatine kinase was linked to capture-related stress. No significant associations with meteorological data were detected.
Our study highlighted the complex influence of biological and environmental conditions on infectious disease dynamics in tapirs. Location emerged as the main risk factor for pathogen occurrence, with biomarkers such as heavy tick burden, pale mucous membranes, presence of wounds, high white blood cell count, and low red blood cell count representing key indicators of tapir health.
Our research has provided robust scientific evidence addressing long-standing hypotheses on tapir health, supporting practical applications and informing wildlife management and disease surveillance research.
Keywords: boosted regression trees, disease, disease risk analysis, epidemiology, health, machine learning, modelling, One Health, Perissodactyla, physiological markers, surveillance, Tapiridae, wildlife.
Introduction
Predicting outbreaks in wildlife is complex and requires understanding a wide range of factors, including species-specific physiology and interactions with other animals, humans, and the environment (Roberts et al. 2021). From both conservation and One Health perspectives, the fundamental step towards predicting and preventing the emergence of infectious diseases requires the ability to accurately assess and monitor health in wild populations (Kophamel et al. 2022). Significant gaps remain in our understanding of wildlife health assessment and the potential impacts of environmental changes, climate change, and close interactions with humans and domestic species on wildlife health. This challenge is particularly relevant for studies targeting species that are widely distributed across diverse landscapes and environmental conditions, where multiple drivers may influence disease transmission.
The lowland tapir (Tapirus terrestris) is an ungulate that inhabits various vegetation physiognomies and ecosystem types, including savannas, wetlands, rainforests, dry forests, mangroves and alpine peaks, across 11 countries in South America (Padilla and Dowler 1994; Flesher and Medici 2022; Medici et al. 2024). The species belongs to the order Perissodactyla, weighs up to 250 kg, requires large home ranges (8 km2), and is currently listed as Vulnerable to extinction (Varela et al. 2019; Medici et al. 2022; IUCN 2025). Few structures act as mechanical barriers to tapir movement, making them highly adaptable to navigating diverse matrix habitats and tolerating disturbed landscapes (Flesher and Medici 2022; Medici et al. 2022). This adaptability creates numerous opportunities for interaction with domestic animals and pathogen exposure within anthropogenic areas.
The direct association between anthropic influence and the health status of wild tapirs was previously reported in lowland tapirs in Brazil and Central American tapirs (T. bairdii) in Mexico. Tapirus terrestris sampled in areas surrounded by sugarcane, soybean and maize crops, exotic tree (Eucalyptus and Pinus) plantations, cattle ranching, and rural communities were considered less healthy than tapirs in well-preserved regions (Fernandes-Santos et al. 2020). Tapirus bairdii individuals were considered healthier in environments located at least 5 km away from human-modified areas and less healthy in the presence of agriculture within a 1-km radius (Pérez-Flores et al. 2020). However, the mechanisms underlying this relationship remain unknown.
Species that thrive in human-altered environments tend to carry more pathogens than those that decline or disappear (Gibb et al. 2020). Several infectious agents have been reported in wild tapir populations, although clinical signs are typically scarce, and most infections are subclinical (Hernandez-Divers et al. 2005; Mangini et al. 2012; Medici et al. 2014; Quse and Fernandes-Santos 2014; Fernandes-Santos et al. 2020; Ordonneau et al. 2024). Comprehensive health assessments of wild tapirs showed poorer body and skin conditions, a higher prevalence of dental problems, and more traumatic lesions in tapirs from a highly threatened area (Cerrado) than in those from a near-pristine habitat (Pantanal) in Brazil. Additionally, several haematological and biochemical parameters significantly differed among populations (Medici et al. 2014; Fernandes-Santos et al. 2020). Nonetheless, these findings could not be directly linked to the serological detection of Orbivirus caerulinguae (bluetongue virus), Protoparvovirus suiformis (porcine parvovirus), and Leptospira interrogans at varying prevalences in different cohorts (Table 1). Additionally, a new species within the Trypanosoma clade named Trypanosoma terrestris, first reported in lowland tapirs in 2013 (Acosta et al. 2013), was isolated in 87.5% of blood samples from Pantanal tapirs (Pérez et al. 2019). The same protozoan was later identified in Cerrado tapirs (Arlei Marcili, unpubl. data); however, neither the underlying drivers of the occurrence of this pathogen nor its impact on tapir health have been investigated.
| Infectious agent | Diagnostic test | Pantanal (n = 36) | Cerrado (n = 35) | |
|---|---|---|---|---|
| Leptospira interrogans (26 serovars) | Microscopic agglutination test | 75 (66.1–83.9A; 63.7–86.3B) | 60 (42.1–76.1) | |
| Bluetongue virus | Agar gel immunodiffusion | 2.8 (0.3–12.6C) | 91 (76.9–98.2) | |
| Porcine parvovirus | Haemagglutination inhibition | 100 (90.3–100C) | 97 (85.1–99.9) |
The long-term risk posed by these established and emerging pathogens to wild tapir population viability and the physiological and environmental variables that might trigger pathological responses and influence disease dynamics remain unclear. Furthermore, health assessments in wild tapirs have not yet established an association between clinical signs and abnormalities in haematological and biochemical parameters with detected infectious agents, which could demonstrate the impact of these pathogens on tapir health.
Using an extensive dataset collected over 10 years (2008–2018) across contrasting environmental conditions, including a near-pristine habitat (Pantanal) and a highly threatened biome (Cerrado) in central-western Brazil, our study aimed to identify risk factors associated with the detection of infectious agents in wild lowland tapirs and potential physiological markers indicative of disease. It builds on earlier work conducted in these tapir populations (Medici et al. 2014; Fernandes-Santos et al. 2020) to uncover likely disease drivers and health impacts.
Materials and methods
Study population
Wild lowland tapirs were sampled across two study areas, named Pantanal and Cerrado (Fig. 1). The Pantanal study site is in a private cattle ranch and eco-tourism facility, Baía das Pedras Ranch, which spans approximately 145 km2 in the Nhecolândia subregion of the Brazilian Pantanal, in Mato Grosso do Sul State (19°20′S, 55°43′W). The Brazilian Pantanal is the largest continuous freshwater wetland on the planet, extending through central-western Brazil, eastern Bolivia, and northeastern Paraguay. During the wet season, up to 80% of the floodplain can be submerged, limiting human activity. Cattle ranching is the main economic practice, mainly involving Nellore cattle (Bos indicus) and horses (Equus ferus caballus). The extensive management approach in this region preserves native vegetation and biodiversity, making it a well-preserved habitat despite being 95% privately owned. Tapir density in the Pantanal study site ranges from 0.41 to 0.69 tapirs/km2, with previous health assessments indicating a healthy population (Medici et al. 2014).
Map illustrating the two study sites, Pantanal and Cerrado, in Mato Grosso do Sul State, Brazil.
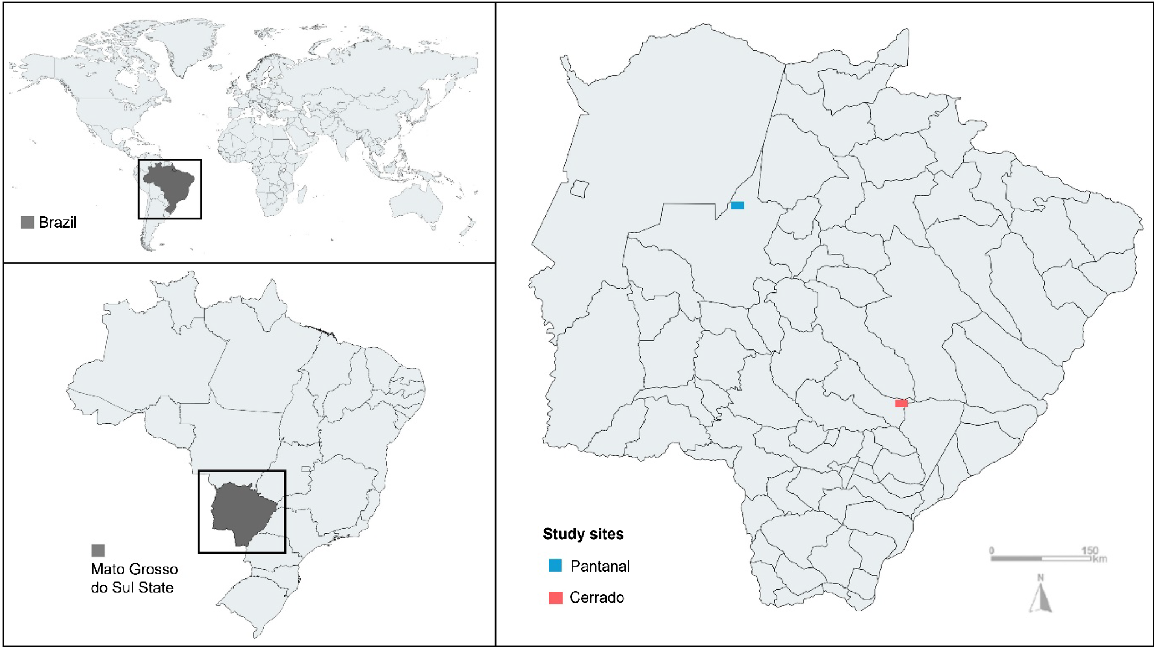
The Cerrado study area, located along the BR-267 highway between Nova Alvorada do Sul and Nova Andradina in Mato Grosso do Sul State (21°60′S, 53°83′W), covers approximately 2200 km2. This region is located at the epicentre of economic development of the country, and wildlife in the Brazilian Cerrado faces numerous threats, including deforestation, roadkill, poaching, bushfires, agricultural expansion, exotic tree plantations, pesticide use, proximity to rural communities, and intensive and high-density cattle ranching. Tapir density in the Cerrado study area ranges from 0.25 to 0.65 tapirs/km2, with the population being previously considered less healthy than in the Pantanal (Fernandes-Santos et al. 2020). The Cerrado site is situated about 350 km from the Pantanal and is 300 m higher in elevation.
Sampling
A longitudinal study conducted over 10 years yielded 174 samples (n) from 115 wild lowland tapirs (N) through capture–recapture methods across two locations in Brazil, Pantanal (n = 135; N = 80) and Cerrado (n = 39; N = 35). Sampling in the Pantanal was conducted from September 2008 to October 2018, whereas sampling in the Cerrado occurred from September 2015 to April 2018, leading to 2.5 years of synchronous sampling. Both study sites are in the central-western Brazilian state of Mato Grosso do Sul. Nonetheless, contrasting environmental conditions were evidenced in these regions, with the Pantanal characterised as a nearly pristine area, whereas the Cerrado is considered a highly disturbed biome (Medici et al. 2022). Capture methods included box traps (n = 132; N = 94), pitfall traps (n = 5; N = 5), and darting from a distance using anaesthetic darts (n = 37; N = 29) (Fig. 2). Box traps were wooden enclosures (3.0 × 1.5 × 2.0 m) placed at salt-baited sites and equipped with a trigger mechanism that closed the door when the animal stepped on the trigger. Pitfall traps (2.3 × 1.8 × 1.8 m) were excavated along frequently used tapir trails, covered with corrugated roofing tiles, and camouflaged with forest debris. Darting from a distance was used through active searching by vehicle or passive observation from a stakeout. In this method, anaesthetic darts equipped with telemetry transmitters were used to facilitate tapir tracking and recovery post-injection (Medici 2010; Quse and Fernandes-Santos 2014). Anaesthetic agents were administered via CO2-powered rifles. Anaesthetic protocols, handling procedures, collection and processing of biological samples of both cohorts have been covered in previous publications (Medici et al. 2014; Pérez et al. 2019; Fernandes-Santos et al. 2020). All animals were monitored until full recovery and released at the capture site.
Selection of infectious agents
In total, 4 of 14 infectious agents previously investigated in those tapir populations were selected, including two viruses (bluetongue virus and porcine parvovirus), one bacterium (Leptospira interrogans serovar Pomona), and one protozoan (Trypanosoma terrestris). The selection of pathogens was based on data availability across populations and sampling periods, along with potential relevance for tapir health. To detect the presence of viruses and bacteria, serologic diagnoses were conducted at Instituto Biológico de São Paulo, São Paulo, Brazil. Diagnostic tests included a microscopic agglutination test (MAT) to investigate Leptospira sp., agar gel immunodiffusion (AGID) to identify bluetongue virus, and haemagglutination inhibition (HI) assay to detect and quantify antibodies against porcine parvovirus (Medici et al. 2014; Fernandes-Santos et al. 2020). To detect trypanosomes, haemocultures were processed at the Brazilian Trypanosomatid Collection and subjected to polymerase chain reaction (PCR) at the Department of Preventive Veterinary Medicine and Animal Health, School of Veterinary Medicine, University of São Paulo, Brazil (Pérez et al. 2019). Complete epidemiological data from samples included in this study are presented as Supplementary material Table S1.
Data processing and modelling
To identify associations between the occurrence of infectious agents and potential drivers and consequences of disease in wild lowland tapirs, a stepwise approach was adopted using the following methods: logistic regression (LR) was applied to predict a binomial response (i.e. positive or negative) for the occurrence of each disease over time, and Poisson regression was utilised as a generalised linear model (GLM) to demonstrate trends in serological titres detected in the study period.
Subsequently, a dataset of relevant biological and ecological categorical explanatory variables was used to investigate likely indicators of disease risk, including location (Pantanal or Cerrado), season (dry or wet), age class (juvenile or adult), and sex (female or male). Data from previous comprehensive health assessments, comprising clinical signs, body condition, tick burden, and abnormalities detected in haematological and biochemical parameters, were used to explore the potential causes and likely consequences of the selected diseases. In our study, tick burden and body condition were assessed by experienced tapir researchers through physical examination of anaesthetised individuals, and standardised to better fit statistical analysis. A semi-quantitative scale from one to three was used, based on tick density and clustering in the inguinal region, with score of one classified as low burden and scores of two or three as high burden (Fig. 3). Body condition was scored on a five-point scale, based on muscle and fat deposition in key anatomical regions (neck, shoulders, ribs, spine, and pelvis) (Clauss et al. 2009; Pérez-Flores et al. 2016). Individuals scoring one or two were considered in poor condition, and those scoring three or four in good body condition (Fig. 4). No individuals were classified as overweight (score 5). For diseases for which serological diagnostic results are given in titres, only individuals presenting recent or currently active infection (potential rising or active post-infection immunity instead of possibly passive maternal protection or past exposure) were assigned as positive. Namely, tapirs that presented titres of ≥1:64 for porcine parvovirus (Lelešius et al. 2006), and >1:800 for Leptospira interrogans serovar Pomona (Chatfield et al. 2013; Pedersen et al. 2018).
Scoring tick burden through physical examination of anaesthetised lowland tapirs (Tapirus terrestris). Credits: Renata Carolina Fernandes-Santos. (a) Inguinal region of a female tapir presenting high tick burden. (b) Inguinal region of a female tapir presenting low tick burden.
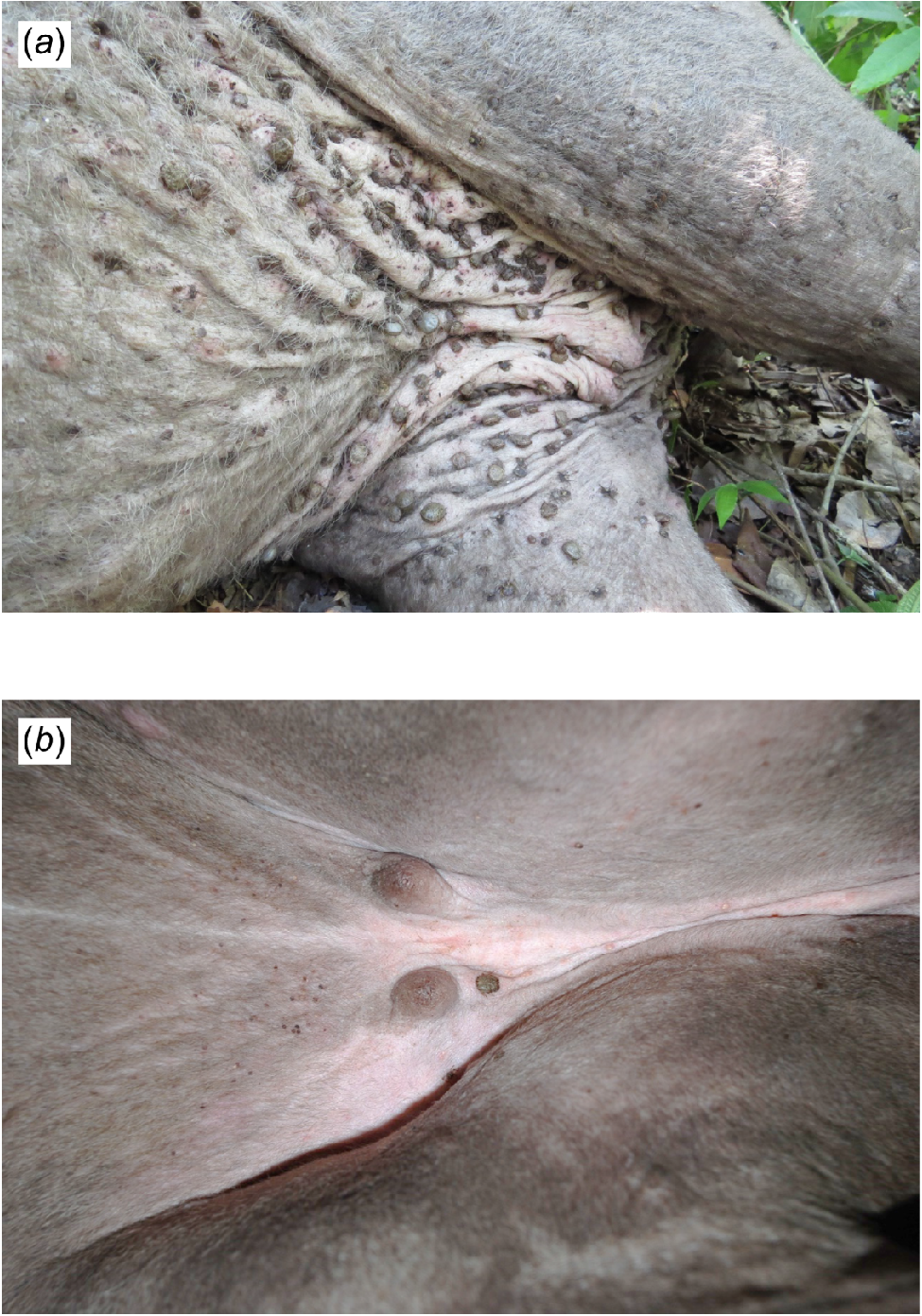
Body condition scoring in lowland tapirs (Tapirus terrestris). Credits: Renata Carolina Fernandes-Santos. (a) Pantanal tapir in good body condition. Note the presence of a wound in the lateral aspect of the left leg of this same individual. (b) Cerrado tapir in poor body condition, with prominent ribs and pelvis visibly indicating muscle and fat depletion.

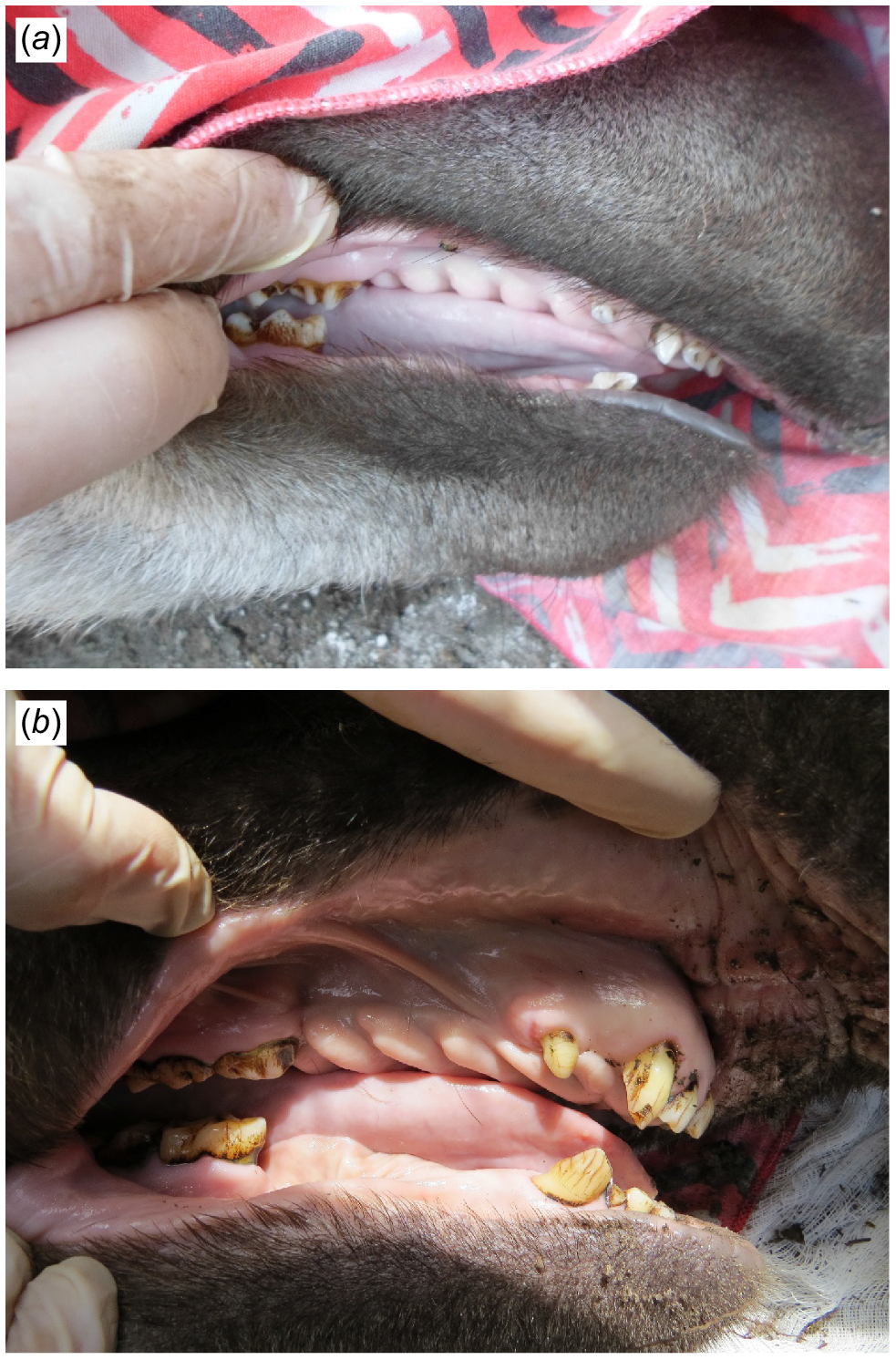
Considering the fundamental nature of wildlife sampling and its inherent limitations concerning unbalanced data, a boosted regression tree (BRT) model was selected to provide a robust response and achieve reduced bias with improved predictive performance (Elith et al. 2008). This modelling approach was successfully applied in similar studies to identify major determinants of disease in different tapir species (Fernandes-Santos et al. 2025) and the most important biological and ecological traits for predicting flavivirus hosts (Pandit et al. 2018). The model was fitted in R (ver. 4.3.1; R Development Core Team 2025), using gbm.auto (ver. 2024.10.01, https://cran.r-project.org/web/packages/gbm.auto; Dedman et al. 2017), dismo (ver. 1.3-16, https://cran.r-project.org/web/packages/dismo), Require (ver. 1.0.1, https://cran.r-project.org/web/packages/Require; McIntire 2023), reproducible (ver. 2.1.2, https://cran.r-project.org/web/packages/reproducible; McIntire and Chubaty 2023), and data.table (ver. 1.17.8, https://CRAN.R-project.org/package=data.table; Dowle and Srinivasan 2019) R packages, as previously described by Fernandes-Santos et al. (2025) and following Elith et al. (2008) working guide. Optimised hyperparameters were set as 15,000 trees with a learning rate of 0.001, bag fraction of 0.95, and maximum tree depth or tree complexity of four. Tapirs were assigned into age classes basied on dentition and tooth wear (Fig. 5), along with long-term camera-trap monitoring. Age categories were defined as calf (up to 11 months old), juvenile (12–17 months), subadult (18–47 months), and adult (over 48 months) (Fernandes-Santos et al. 2020). To better fit the selected model, calves and juveniles were allocated as juveniles, whereas subadults and adults were grouped as adults. Haematological and biochemical parameters were classified as normal and abnormal by using reference values derived from Medici et al. (2014). Values falling below the lower limit of the reference range were considered low, and those exceeding the upper limit were considered high. To allow better-informed clinical interpretation of abnormal results, the percentage of high and low values included in the BRT analysis are presented in Table 2. To avoid automatic hot-encoding, variables were converted to binary responses 1 and −1 before analysis (e.g. positive and negative, present and absent, good and poor, high and low, normal and abnormal). The relative influence of each variable (RI) or overall effect estimate was scaled, with higher numbers indicating a stronger influence on the response. To facilitate interpretation, BRT modelling results are presented in contingency tables.
Dentition of a (a) juvenile and (b) adult lowland tapir (Tapirus terrestris). Credits: Renata Carolina Fernandes-Santos.
| Parameter | Predominantly | Most likely cause | High % (n (N)) | Low % (n (N)) | Abnormal (n (N)) | Total (n (N)) | |
|---|---|---|---|---|---|---|---|
| RBC count | Low | Anaemia | 3.45 (1 (1)) | 96.55 (28 (27)) | 29 (28) | 171 (114) | |
| WBC count | High | Infection, inflammation | 91.30 (63 (58)) | 8.70 (6 (5)) | 69 (63) | 171 (114) | |
| ALT | Low | Not clinically significant | 3 (3 (3)) | 97 (97 (81)) | 100 (84) | 173 (115) | |
| AST | High | Liver dysfunction | 65.79 (25 (23)) | 34.21 (13 (12)) | 38 (35) | 173 (115) | |
| GGT | High | Liver dysfunction | 60.98 (25 (23)) | 39.02 (16 (15)) | 41 (38) | 173 (115) | |
| Alkaline phosphatase | High | Liver dysfunction | 91.30 (21 (18)) | 8.70 (2 (2)) | 23 (20) | 173 (115) | |
| Glucose | High | Inflammatory response, stress, recent feeding | 58.82 (10 (10)) | 41.18 (7 (7)) | 17 (17) | 173 (115) | |
| Total protein | High | Anaemia | 81.40 (35 (30)) | 18.60 (8 (8)) | 43 (38) | 173 (115) | |
| Total bilirubin | Low | Not clinically significant | 30.77 (12 (11)) | 69.23 (27 (25)) | 39 (36) | 173 (115) | |
| Iron | Low | Anaemia | 44.44 (16 (14)) | 55.56 (20 (20)) | 36 (34) | 173 (115) | |
| CK | High | Exertional myopathy | 88.46 (23 (22)) | 11.54 (3 (3)) | 26 (25) | 173 (115) |
n, number of samples; N, number of individuals; RBC, red blood cell; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; and CK, creatine kinase.
Climate change associations
To explore the impact of climate change and the potential associations between meteorological conditions and the occurrence of infectious agents in lowland tapirs, data from two automatic meteorological stations (MS) of the National Institute of Meteorology of Brazil (INMET) were included in the statistical analysis. Studies in the same Brazilian state confirmed the reliability of these stations in representing nearby study sites (Giroux et al. 2022; Giroux et al. 2023). The closest meteorological stations to each study site, named Nhumirim MS for the Pantanal and Rio Brilhante MS for the Cerrado, were selected. Data on local temperature and humidity (daily mean, maximum, and minimum) and daily precipitation were recorded for each day a tapir was sampled. Logistic regression (LR) was used to predict associations between the occurrence of diseases and meteorological conditions on the sampling dates. Despite the challenges of associating meteorological conditions with diseases, which typically require long-term data collected before sampling, this approach aimed to indicate trends over time, considering all tapirs were sampled on different dates over a 10-year period. In addition, the study sites are characterised by stable weather patterns, and daily means are likely to reflect broader conditions during the sampling period.
Animal ethics
All protocols for capture, physical and chemical restraint, handling, collection and analyses of biological samples included in this study have been reviewed and approved by the Veterinary Advisors of the Association of Zoos and Aquariums (AZA) Tapir Taxon Advisory Group (TAG), and the Veterinary Committee of the International Union for Conservation of Nature (IUCN) Species Survival Commission (SSC) Tapir Specialist Group (TSG). These procedures were performed in the presence of experienced veterinarians and required research permits were renewed annually by the Brazilian Federal Agency for Biodiversity Conservation (ICMBio), Federal Environmental Ministry, under Certificate numbers 38332 and 14603.
Results
Longitudinal detection of infectious agents
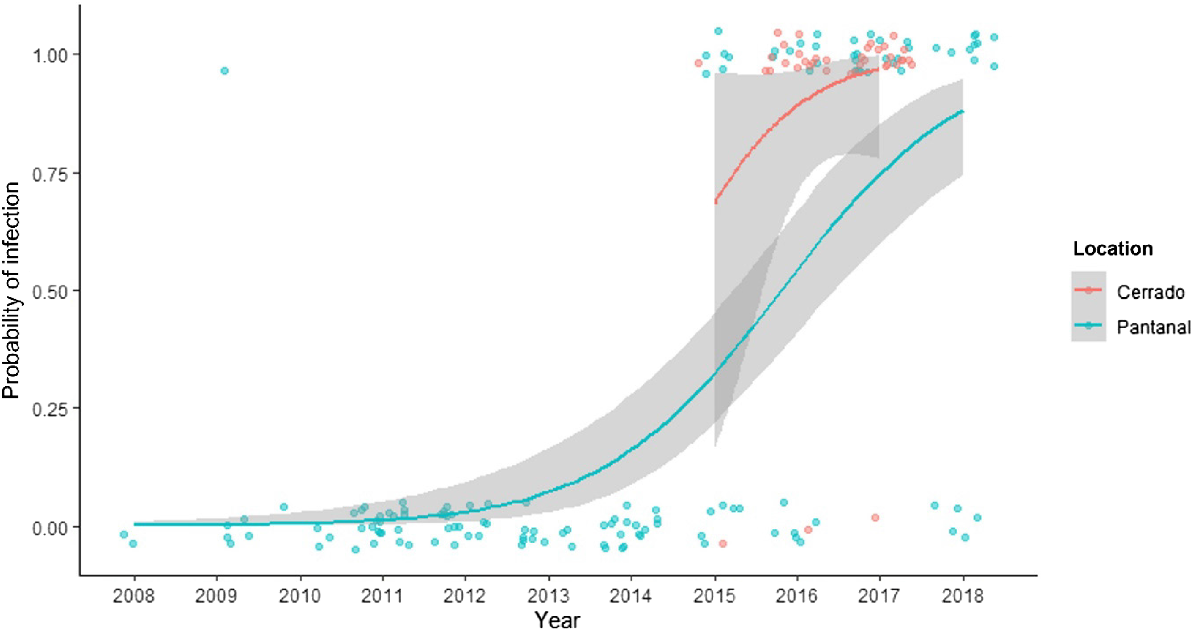
Logistic regression demonstrated a highly significant variation in the occurrence of bluetongue virus, with the odds of seropositivity increased by 2.90 (95% CI 2.12–3.97, P < 0.001) over time, and the highest odds in the last year of data collection in each cohort (Fig. 6). Only one case was detected before 2015. In contrast, cases of Trypanosoma terrestris infection gradually decreased, with an odds ratio (OR) of 0.60 (95% CI 0.43–0.84, P = 0.003). No significant variation was detected for the overall occurrence of Leptospira sp. (P = 0.141) and porcine parvovirus (P = 0.757) between 2008 and 2018. Nevertheless, generalised linear models showed a significant association between the sampling year and the level of antibody response against Leptospira sp. (OR 0.937, 95% CI 0.936–0.939, P < 0.001) and porcine parvovirus (OR 1.04, 95% CI 1.03–1.05, P < 0.001), with the former steadily dropping titres while the latter slightly increased titres over time. Meteorological conditions such as environmental temperature and humidity (daily mean, maximum, and minimum) and daily precipitation were not significantly associated with the occurrence of selected infectious agents within the studied period, with P-values ranging between 0.077 and 0.829.
Factors associated with the detection of infectious agents
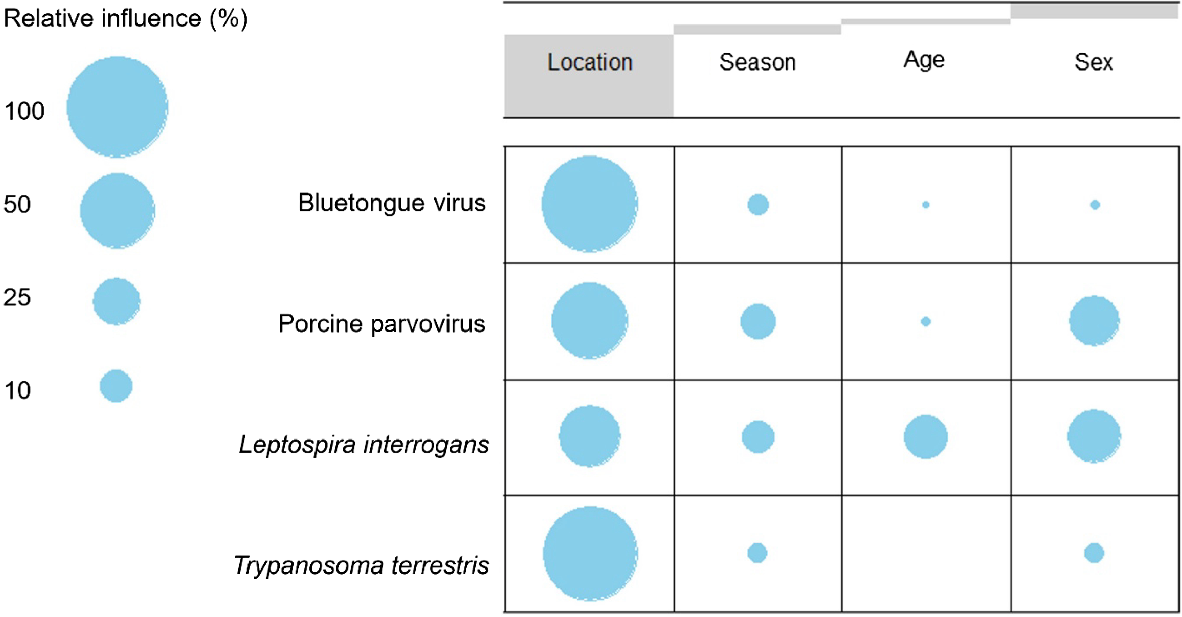
Boosted regression tree models demonstrated a strong influence of the location in the occurrence of all selected infectious agents, with tapirs living in the Cerrado study site being at higher risk of exposure to bluetongue virus (RI 94.2%) and porcine parvovirus (RI 58.5%), whereas tapirs from the Pantanal are at higher risk of exposure to Trypanosoma terrestris (RI 92.5%) and Leptospira sp. serovar Pomona (RI 39.9%). Sex represented the second-most relevant variable for the occurrence of high serological response for Leptospira sp. (RI 29%) and porcine parvovirus (RI 27.3%), with females being at greater risk of exposure to the bacteria, whereas the virus represented a higher risk for males. For bluetongue virus and Trypanosoma terrestris, the season was the second-most important variable, with the dry season representing an increased risk of exposure to the virus (RI 4.1%) and the wet season to the protozoan (RI 3.9%). Higher risk in the dry season was also observed for porcine parvovirus (RI 12.7%) and Leptospira sp. (RI 10.9%). Age was the least relevant variable for the occurrence of porcine parvovirus (RI 1.5%), bluetongue (RI 0.4%), and Trypanosoma terrestris (RI 0.1%). For Leptospira sp. serovar Pomona, age (RI 20.2%) demonstrated a stronger influence than did season, with adult tapirs being at greater risk of presenting an active serological response to bacterial infection than juveniles (Fig. 7).
Contingency table demonstrating the relative influence of risk factors in the occurrence of selected infectious agents in wild lowland tapirs (Tapirus terrestris). Stripes through the top row represent proportional marginal distributions (or overall percentages) for each risk factor within all variables investigated.
Association between the detection of infectious agents and health parameters
Clinical signs and abnormalities in haematological and biochemical parameters demonstrated both positive and negative associations with infectious agents (Fig. 8). Overall, findings that demonstrated a higher positive association with the detection of selected infectious agents comprised the presence of pale mucous membranes (RI 85.5%), high infestation by ticks (RI 78.4%), abnormal (predominantly low) red blood cell (RBC) count (RI 78.3%), presence of wounds (RI 59.1%), and abnormal (predominantly high) white blood cell (WBC) count (RI 38.1%). The presence of reactive lymphocytes (RI 79%) and poor teeth conditions (RI 50.2%) were strongly influenced by the location rather than any other variable, with tapirs living in the Cerrado at higher risk of presenting these abnormalities. Poor body condition was weakly linked with all variables, with the most relevant correlation being with tapirs being reactive to bluetongue virus (RI 11.4%).
Contingency table demonstrating positive (blue) and negative (grey) associations between host health parameters and potential drivers, including the occurrence of selected infectious agents in wild lowland tapirs (Tapirus terrestris). Stripes through the top row represent proportional marginal distributions (or overall percentages) for each driver within all variables investigated.
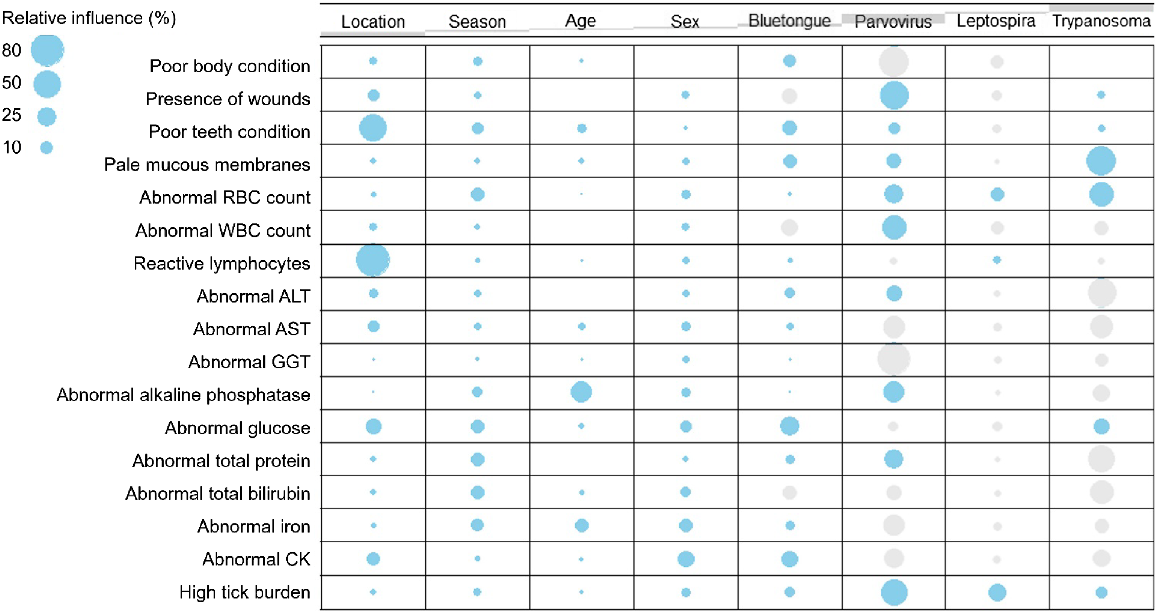
Parameters that indicated a positive correlation with high serological response for Leptospira sp. serovar Pomona included high infestation by ticks (RI 21.4%), low red blood cell (RBC) count (RI 12.5%), and presence of reactive lymphocytes (RI 4.3%). Trypanosoma terrestris infection in tapirs was mostly associated with the presence of pale mucous membranes (RI 59.1%) and low red blood cell (RBC) count (RI 40.3%). A strong association among several physiological parameters was observed for tapirs actively infected by porcine parvovirus, including the presence of wounds (RI 54.1%), high infestation by ticks (RI 48.3%), and high white blood cell (WBC) count (RI 38.1%). Similarly, bluetongue virus infection showed positive associations with various parameters, with the strongest observed being for predominantly high glucose concentrations (RI 24.6%) (Fig. 8).
Discussion
This study investigated the risk factors associated with exposure to four selected infectious agents and their potential impacts on the health of wild lowland tapirs across contrasting environmental conditions. We identified a strong association between the occurrence of livestock pathogens, such as bluetongue virus and porcine parvovirus, in tapirs inhabiting human-modified areas. Conversely, tapirs from pristine habitats exhibited a higher risk of exposure to Trypanosoma terrestris and Leptospira interrogans serovar Pomona. We also observed a distinct longitudinal variation in the occurrence of bluetongue virus throughout the study period in both cohorts. Moreover, we detected significant associations between infectious agents and clinical signs and abnormalities in physiological parameters, which lays the groundwork for future research on potential biomarkers for assessing tapir health.
Risk factors associated with exposure to infectious agents in tapirs
Building on recent research on tapir disease dynamics (Fernandes-Santos et al. 2025), our results corroborated that geographical location significantly affects the prevalence of the four infectious agents studied in tapir populations. Our findings indicated that Cerrado tapirs face a higher risk of exposure to bluetongue virus and porcine parvovirus, both being considered primarily livestock diseases. Domestic animals frequently outnumber wildlife hosts of shared pathogens, facilitating the risk of spill-over or cross-species transmission in interconnected ecosystems (Daszak et al. 2001; Karmacharya et al. 2024). The higher livestock biomass in the Cerrado may account for the increased exposure risk for tapirs in this region. This dynamic was recently demonstrated by a study that identified cattle density as the most significant predictor for the distribution and concentration of bluetongue vectors, among several environmental and climatic variables (De Klerk et al. 2024). Additionally, previous research suggested that feral pigs (or wild boars) may serve as important maintenance hosts and play a key role in the transmission of porcine parvovirus to wild tapirs (Medici et al. 2014). Infection with this virus is common among domestic pigs (Sus scrofa) in Brazil (Streck et al. 2011). Having become feral over 150 years ago, these pigs are now widespread across the country (Herrera et al. 2005; Ruiz-Fons et al. 2007) and are found in high densities at both study sites. In contrast, tapirs formerly sampled in a protected area of the Brazilian Atlantic Forest, where there are no feral or domestic pigs, showed no serological response to porcine parvovirus (Medici et al. 2014).
As a vector-borne disease, bluetongue prevalence may also be linked to environmental conditions that favour insect survival, such as high rainfall, temperature, and humidity (Tomich et al. 2009; Araujo et al. 2010). However, weather conditions in Brazil are conducive to year-round bluetongue transmission (El Moustaid et al. 2021), which may explain the minimal seasonal impact on bluetongue occurrence in tapirs. Despite the relatively small seasonal variation, our longitudinal study showed a significant temporal influence in bluetongue detection in tapirs over a 10-year period. The first case was identified in 2009, with no cases being reported between 2010 and 2014, followed by an exponential increase in cases in both tapir cohorts after 2015. Considering the diagnostic test sensitivity and specificity, it is possible that the 2009 case was a false positive. This suggests that the disease may have been introduced into tapirs only in 2015. Although this variation may also reflect fluctuations in mosquito and cattle populations or climate conditions, necessitating further investigation and highlighting the dynamic nature of disease risk assessments (Knox et al. 2024).
Leptospirosis is another infectious disease for which weather and environmental conditions play a pivotal role in its epidemiological cycle. The incidence of this widespread bacterium is strongly associated with heavy rains, standing water, and hot climates (Uhart et al. 2010). The Pantanal, a seasonally inundated floodplain, provides an ideal environment for pathogens reliant on water (Medici et al. 2014). The increased risk of exposure to Leptospira sp. in Pantanal tapirs is likely to be due to the flooding regime, water availability, and concentration during the dry season, rather than anthropogenic impact. Drier weather conditions can lead to increased interactions among individuals at limited watering points. This higher usage of shared water sources may facilitate the spread of Leptospira among tapirs. Previous research indicated that cattle access to flooded areas and rodent presence are significant risk factors, with swampy areas enabling Leptospira survival for up to 180 days in the environment (Da Silva et al. 2022). Furthermore, leptospirosis has a complex aetiology and epidemiology, with over 250 antigenically distinct serovars and several natural hosts (Hagedoorn et al. 2024). Our study focused on serovar Pomona, the most prevalent in tapirs in the studied regions (Medici et al. 2014; Fernandes-Santos et al. 2020). This serovar infects a wide range of hosts, including wild mammals, cattle, pigs, and humans, with pigs being considered potential maintenance hosts (Arent et al. 2017). We detected a higher risk of infection by Leptospira sp. serovar Pomona for female tapirs. Consistent with our findings, a recent study demonstrated that female wild boars had an increased risk of infection by the same serovar (Tadić et al. 2025).
In line with that, sex emerged as a significant determinant for infectious disease risk in our study, surpassing age in relative influence for all four investigated pathogens. Different sexes were linked to higher risks for different pathogens. Age was considered more relevant than sex in previous research on tapir diseases (Fernandes-Santos et al. 2025), and our conservative approach grouping individuals into two age classes (i.e. juvenile and adult) owing to unbalanced data might explain this discrepancy. Increasing the number of samples and segregating the dataset into more age categories might yield different results, warranting further investigation.
Overall, our findings emphasise the complex influence of both biological and environmental conditions on infectious agent dynamics in tapir populations, even for well-researched pathogens. Novel pathogens present additional challenges. Trypanosoma terrestris infection was primarily influenced by location, with minor influences from sex and season. This host-specific protozoan has been detected exclusively in lowland tapirs, both wild and captive (Acosta et al. 2013; Pérez et al. 2019), with its epidemiological cycle and transmission pathways yet to be elucidated. Another Trypanosoma species (T. evansi) was reported to cause acute gastroenteric illness in a captive Malayan tapir (T. indicus), resulting in mortality (Soetisno 1933; Desquesnes et al. 2013).
Potential biomarkers to assess health in wild lowland tapirs
Our study identified a strong association between the overall detection of infectious agents and increased tick burden in tapirs. Tapirs are continuously infested by various tick species in their natural environment, predominantly in the inguinal and axillary regions, abdomen, and around the ears, with Amblyomma sculpum being the most abundant on tapirs in Pantanal and Cerrado (Labruna et al. 2021). Several factors can influence tick distribution and abundance, including weather conditions, host density, immunity, vegetation coverage, and human activities (Szabo et al. 2003; Fyumagwa et al. 2007; Anderson et al. 2013; Dantas-Torres 2015; Ellingwood et al. 2020). Our model demonstrated that heavy tick infestation in tapirs might indicate underlying health conditions, although it remains unclear whether infectious agents may cause immunosuppression and, consequently, increase susceptibility to ticks or vice versa. Previous studies linked high ectoparasite abundance to poor host immunity in free-ranging African buffalo (Syncercus caffer) (Fyumagwa et al. 2007; Anderson et al. 2013). In equines, ectoparasite infestation is known to cause anaemia, supporting further examination in tapirs (Hernandez-Divers et al. 2005). Large tick burdens and associated pathogens pose a substantial health risk to large mammals, as evidenced by rhino mortality linked to vector-borne diseases (Nijhof et al. 2003). We recommend developing and applying more accurate methods for scoring and monitoring tick burden in tapir health research (Szabo et al. 2003; Quse and Fernandes-Santos 2014). This could support tailoring management and intervention strategies for disease prevention and control at both individual and population levels, which requires balancing effectiveness with potential environmental consequences. For instance, acaricide-treated cattle in African savannas have successfully reduced tick abundance in wildlife (Keesing et al. 2013). However, widespread use may promote resistance in tick populations and lead to environmental contamination and unintended impacts on non-target species (Obaid et al. 2022).
Another relevant clinical sign detected in our study was the presence of wounds. In tapirs, wounds are typically linked to social behaviour and result from agonistic intraspecific (territoriality, mating disputes) or interspecific (predation, competition) interactions rather than diseases (Medici et al. 2014; Reyna-Hurtado et al. 2025). We found a strong association between wounds and active infection by porcine parvovirus, with male tapirs being at greater risk. This virus, primarily transmitted through direct contact, may spread during agonistic interactions among tapirs. Although unreported, similar interactions with feral pigs in overlapping habitats may also contribute to the observed wound patterns and viral transmission. Wounds were also associated with Trypanosoma infection, for which transmission pathways remain unclear. Transmission of other Trypanosoma species occurs mainly through vectors such as biting flies, and ticks may also be implicated. Both porcine parvovirus and Trypanosoma infections have been linked to skin lesions in previous research. Trypanosoma infection was associated with multifocal inflammatory processes in the dermis and subcutaneous tissue in mice (Capewell et al. 2016), whereas porcine parvovirus was linked to cutaneous lesions in piglets (Lager and Mengeling 1994). However, we hypothesise that wounds in tapirs are more likely to be due to agonistic interactions, which increase the risk of direct contact and pathogen transmission, rather than being a direct result of disease. Developing a standardised approach to wound assessment and monitoring, comprising characteristics such as tissue type (e.g. necrotic, slough, granulation), affected body area, size and depth, and signs of infection or inflammation (e.g. erythema, exudate, swelling), would benefit further research in this area.
Furthermore, pale mucous membranes in tapirs were substantially linked to bluetongue, parvovirus and Trypanosoma infections. There are several possible reasons for this finding in tapirs, from anaesthesia-related issues (e.g. respiratory depression and hypoxia) to anaemia and infectious diseases (Quse and Fernandes-Santos 2014), and additional research is required to support this association.
The weak association between poor body condition and the occurrence of infectious agents in our study suggests that this parameter may not be a reliable indicator for disease status in tapirs. Body condition loss might be better explained by factors not addressed in our research, such as nutritional constraints, seasonal variation in food availability, and environmental stressors. For instance, proximity to agricultural areas has been linked to poor body scores in Central American (Pérez-Flores et al. 2016, 2020) and lowland tapirs (Fernandes-Santos et al. 2020), which is likely to reflect environmental pressures rather than disease. We caution that tapirs are commonly asymptomatic, and integrative health assessments that combine clinical, pathological, and ecological data are essential for accurately evaluating their health (Fernandes-Santos et al. 2025). In addition, our model indicated several associations among explanatory variables, suggesting potential confounded factors (Knox et al. 2024). Therefore, clinical manifestations that demonstrated stronger links, such as heavy tick burden, wounds, or pale mucous membranes, should be particularly noted when accompanied by additional evidence, such as abnormal haematological and biochemical parameters, and positive results in disease screening tests. This underscores the need to identify the most relevant biomarkers while ensuring comprehensive health assessments in tapir health research.
High white blood cell (WBC) count and low red blood cell (RBC) count are relevant haematological parameters in any health assessment and are commonly linked to infectious diseases, which was supported by our model. Abnormal WBC count (predominantly high) was particularly relevant for active porcine parvovirus infection in tapirs, whereas abnormal RBC (predominantly low) count was positively associated with the four infectious agents investigated. Complete haematological evaluations, including leukocyte differential count, are cost-effective and highly recommended to support the detection of specific health conditions in tapirs.
The biochemical parameters most strongly associated with the detection of infectious agents were alkaline phosphatase (ALP), glucose, and total protein, all of which were predominantly elevated. High ALP, although a marker for liver dysfunction in adult horses, is not organ-specific in other large animals and can be elevated in various disorders. Our results demonstrated that high ALP was equally linked to active porcine parvovirus infection and age, with juveniles particularly affected. This enzyme is also released by metabolically active bones, which could account for the higher serum ALP levels observed in growing individuals than in adults (Stämpfli and Oliver-Espinosa 2020). This suggests that age-related physiological processes may confound ALP interpretation as a disease marker, and elevated results may reflect normal skeletal development rather than pathology in juvenile tapirs. Clinical interpretations should be supported by concomitant alterations in more organ-specific enzymes such as gamma-glutamyl transferase (GGT) (Stämpfli and Oliver-Espinosa 2020).
Both glucose and total protein showed varied associations with numerous variables. Hyperglycaemia is a well-documented physiological response to acute stress in wildlife, often triggered by capture, restraint, or handling. Similarly, total protein levels may fluctuate as a result of dehydration, inflammation, anaemia, or nutritional status, making isolated interpretation challenging without supporting clinical and environmental data.
Elevated creatine kinase (CK) was mildly linked with sex and bluetongue infection, although smaller associations were found with other parameters. High CK is widely recognised as a specific and sensitive marker of muscle damage across species, potentially indicating exertional myopathy in wildlife, especially when combined with elevated aspartate aminotransferase (AST) (Hartup et al. 1999). In this study, we hypothesise that the observed CK elevations in tapirs are primarily due to capture-related stress rather than disease. This biochemical alteration was more likely to be detected in juvenile and subadult males, suggesting a greater susceptibility to capture stress within these demographic groups. Although all capture, restraint, and handling protocols were reviewed and approved by the appropriate agencies and conducted by experienced researchers, wild animals remain highly susceptible to stress, and many cases of exertional myopathy may be clinically inapparent. Our findings highlight the importance of refining welfare and capture protocols and implementing targeted interventions to mitigate the effects of exertional myopathy, such as appropriate fluid therapy and supportive care, particularly for high-risk individuals. Long-term monitoring of wildlife health also provides valuable data on the effectiveness of these strategies and supports continuous improvements.
The presence of reactive lymphocytes and poor teeth condition in tapirs were strongly linked to their location, with Cerrado tapirs being at higher risk. Cerrado tapirs exhibited dental issues such as tooth loss, fractures, periodontitis, and gingival retraction (Fernandes-Santos et al. 2020). The causes of these dental abnormalities are unknown but may be related to differences in diet or resource availability, compared with the Pantanal. The presence of reactive lymphocytes suggests an active antigenic response to infectious or non-infectious disorders. It is hypothesised that tapirs in the Cerrado may be exposed to diseases or other unexplored hazards not included in our analysis. Previous reports have indicated an impact on tapir health because of exposure to environmental contaminants such as pesticides and metals, which are widely used in monoculture plantations in the Cerrado (Fernandes-Santos et al. 2020; Medici et al. 2021). The observation of reactive lymphocytes in these tapirs may potentially be a response to these toxic substances. Additional research is recommended to support the development of mitigation strategies for enhancing environmental health.
Given that some pathogens are critically dependent on climatic conditions (Pandit et al. 2018; Cohen et al. 2020), our study included key meteorological variables from the sampling days to explore potential associations with the occurrence of infectious diseases in tapirs. However, no significant associations were detected, and our methodological approach did not show any indicators. Although this method may not adequately reflect the temporal complexity of pathogen cycles, particularly for chronic or latent infections, it is possible that other anthropogenic factors may overshadow more subtle environmental changes. Climate change appears to be altering the patterns and intensity of infectious diseases, with different pathogens potentially responding variably to these changes. For instance, recent research has indicated that viral infections exhibit less obvious relationships with climate change than do fungal, bacterial, and endoparasitic diseases (Cohen et al. 2020). Additionally, a recent study demonstrated that the Cerrado landscape is undergoing significant changes over time, becoming less resilient to severe climate changes (De Miranda Santos et al. 2024). Therefore, future studies should continue to explore the occurrence of infectious agents in tapirs over time in the context of both land-use and climatic changes, particularly when associated with extreme weather events. Incorporating long-term climate datasets (spanning 30 years or more) that capture seasonal and interannual variability, along with time-lagged modelling approaches that assess climate conditions weeks or months prior to sampling, may more effectively identify environmental drivers of disease, particularly for chronic or vector-borne infections (Chen and Moraga 2025). These associations also depend on pathogen-specific epidemiological data, such as incubation periods and host–pathogen dynamics, which should be further investigated in future studies.
Ungulates, primates, and bats are major zoonotic reservoirs in wildlife trade (Shivaprakash et al. 2021), making it crucial to develop appropriate surveillance and monitoring programs for emerging diseases in these wildlife groups. As the interface among wildlife, domestic animals, and humans narrows, an integrative approach is increasingly important (Balseiro et al. 2020; Karmacharya et al. 2024). Our results emphasise that each infectious agent has unique epidemiological cycles and a broad range of potential drivers and consequences, which may contrast across different locations and individuals. This complexity highlights the need for both comprehensive and targeted investigation in wildlife health surveillance. We addressed significant knowledge gaps, particularly regarding the main risk factors and the selection of specific haematological, biochemical, and physiological markers to assess tapir health. Our research has provided robust scientific evidence addressing long-standing questions and hypotheses regarding tapir health assessments and underscored important implications for wildlife management, supporting practical applications in current and future tapir research.
Data availability
All data supporting the findings of this study can be made available upon request to the corresponding author, subject to approval from the Lowland Tapir Conservation Initiative (LTCI) – Instituto de Pesquisas Ecológicas (IPÊ) in Brazil.
Declaration of funding
This paper used data gathered by tapir health research conducted by the Lowland Tapir Conservation Initiative (LTCI) – Instituto de Pesquisas Ecológicas (IPÊ) in Brazil which has been funded by several national and international agencies, including over 50 zoological institutions, 20 NGOs and foundations, corporations, as well as private individuals. The LTCI Pantanal Program is supported by Baía das Pedras Ranch. The main supporters of the LTCI Cerrado Program have been Foundation Segré, Houston Zoo, and the Whitley Fund for Nature. Specific grants received for tapir health studies included Chicago Zoological Society, Cleveland Metroparks Zoo, Conservation, Food and Health Foundation, Idea Wild, and Roger Williams Park Zoo. Supporting sources were not involved in the preparation of the data or paper or the decision to submit for publication. The final stages of this research were part of RCFS doctoral studies supported by a Murdoch International Postgraduate Scholarship (MIPS), Murdoch University, Australia, under the Australian Government Research Training Program.
Author contributions
Renata Carolina Fernandes-Santos: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, software, validation, visualisation, funding acquisition, writing – original draft, writing – review and editing. Kristin Warren: conceptualisation, supervision, writing – review and editing. Rebecca Vaughan-Higgins: conceptualisation, supervision, writing – review and editing. Emília Patrícia Medici: conceptualisation, data curation, investigation, methodology, project administration, funding acquisition, writing – review and editing. Mieghan Bruce: conceptualisation, methodology, supervision, validation, writing – review and editing.
Acknowledgements
The study of tapir health has been an important component of the long-term activities of the Lowland Tapir Conservation Initiative (LTCI) – Instituto de Pesquisas Ecológicas (IPÊ) in Brazil. The LTCI has institutional support from the International Union for Conservation of Nature Species Survival Commission Tapir Specialist Group, the Association of Zoos and Aquariums Tapir Taxon Advisory Group, and the European Association of Zoos and Aquariums Tapir Taxon Advisory Group. The LTCI–IPÊ partners with several diagnostic laboratories in Brazil, including Instituto Biológico de São Paulo and the Department of Preventive Veterinary Medicine and Animal Health, School of Veterinary Medicine, University of São Paulo. The Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis and Instituto Chico Mendes de Conservação da Biodiversidade provided annual permits for tapir capture, immobilisation and sample collection. The authors thank Arlei Marcili and the Brazilian Trypanosomatid Collection for processing all Trypanosoma samples, and Tatiane Micheletti for her valuable guidance in selecting statistical methods and discussing the best modelling approach for this study. Last, but definitely not least, the authors thank José Maria de Aragão, Caroline Testa José, Ariel da Costa Canena and Vinícius Gasparotto for their invaluable support during data and sample collection in the field.
References
Acosta IdC, Da Costa AP, Nunes PH, Gondim MFN, Gatti A, Rossi JL, Jr, Gennari SM, Marcili A (2013) Morphological and molecular characterization and phylogenetic relationships of a new species of trypanosome in Tapirus terrestris (lowland tapir), Trypanosoma terrestris sp. nov., from Atlantic Rainforest of southeastern Brazil. Parasites & Vectors 6(1), 349.
| Crossref | Google Scholar |
Anderson K, Ezenwa VO, Jolles AE (2013) Tick infestation patterns in free ranging African buffalo (Syncercus caffer): effects of host innate immunity and niche segregation among tick species. International Journal for Parasitology: Parasites and Wildlife 2, 1-9.
| Crossref | Google Scholar | PubMed |
Araujo JP Jr, Nogueira MF, Cruz TF, Haigh JC (2010) Viral diseases. In ‘Neotropical cervidology: biology and medicine of Latin American deer’. (Eds JMB Duarte, S Gonzalez) pp. 330–341. (International Union for Conservation of Nature, Gland, Switzerland, and Fundacao de Apoio a Pesquisa, Ensino e Extensao: Jaboticabal, Sao Paulo, Brazil)
Arent ZJ, Gilmore C, San-Miguel Ayanz JM, Neyra LQ, García-Peña FJ (2017) Molecular epidemiology of Leptospira serogroup pomona infections among wild and domestic animals in Spain. EcoHealth 14(1), 48-57 Scopus.
| Crossref | Google Scholar | PubMed |
Balseiro A, Thomas J, Gortázar C, Risalde MA (2020) Development and challenges in animal tuberculosis vaccination. Pathogens 9(6), 472.
| Crossref | Google Scholar |
Capewell P, Cren-Travaillé C, Marchesi F, Johnston P, Clucas C, Benson RA, Gorman T-A, Calvo-Alvarez E, Crouzols A, Jouvion G, Jamonneau V, Weir W, Stevenson ML, O’Neill K, Cooper A, Swar N-RK, Bucheton B, Ngoyi DM, Garside P, Rotureau B, MacLeod A (2016) The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. eLife 5, e17716.
| Crossref | Google Scholar | PubMed |
Chatfield J, Milleson M, Stoddard R, Bui DM, Galloway R (2013) Serosurvey of Leptospirosis in Feral Hogs (Sus scrofa) in Florida. Journal of Zoo and Wildlife Medicine 44(2), 404-407.
| Crossref | Google Scholar | PubMed |
Chen X, Moraga P (2025) Assessing dengue forecasting methods: a comparative study of statistical models and machine learning techniques in Rio de Janeiro, Brazil. Tropical Medicine and Health 53(1), 52.
| Crossref | Google Scholar |
Clauss M, Wilkins T, Hartley A, Hatt J-M (2009) Diet composition, food intake, body condition, and fecal consistency in captive tapirs (Tapirus spp.) in UK collections. Zoo Biology 28(4), 279-291.
| Crossref | Google Scholar | PubMed |
Cohen JM, Sauer EL, Santiago O, Spencer S, Rohr JR (2020) Divergent impacts of warming weather on wildlife disease risk across climates. Science 370(6519), eabb1702.
| Crossref | Google Scholar |
Da Silva JF, Alba DAH, Jorge S, Gindri P, Bialves TS, De Souza GN, Bruhn FRP, Pegoraro LMC, Dellagostin OA (2022) Leptospirosis in dairy cattle from Southern Brazil – risk factors. Acta Scientiae Veterinariae 50, 1857.
| Crossref | Google Scholar |
Dantas-Torres F (2015) Climate change, biodiversity, ticks and tick-borne diseases: the butterfly effect. International Journal for Parasitology: Parasites and Wildlife 4(3), 452-461.
| Crossref | Google Scholar | PubMed |
Daszak P, Cunningham AA, Hyatt AD (2001) Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Tropica 78(2), 103-116.
| Crossref | Google Scholar | PubMed |
De Klerk J, Tildesley M, Labuschagne K, Gorsich E (2024) Modelling bluetongue and African horse sickness vector (Culicoides spp.) distribution in the Western Cape in South Africa using random forest machine learning. Parasites and Vectors 17(1), 354.
| Crossref | Google Scholar |
De Miranda Santos FF, Durigan G, Boschi RS, Ivanauskas N, Rodrigues RR (2024) Tree community dynamics in the cerradão (2002–2016): a case of biome shift. Forest Ecology and Management 555, 121698.
| Crossref | Google Scholar |
Dedman S, Officer R, Clarke M, Reid DG, Brophy D (2017) Gbm.auto: a software tool to simplify spatial modelling and marine protected area planning. PloS ONE 12(12), e0188955.
| Crossref | Google Scholar |
Desquesnes M, Holzmuller P, Lai D-H, Dargantes A, Lun Z-R, Jittaplapong S (2013) Trypanosoma evansi and surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. BioMed Research International 2013, 194176.
| Crossref | Google Scholar | PubMed |
Dowle M, Srinivasan A (2019) Data.table: extension of ‘data.frame’. R package version. Available at https://CRAN.R-project.org/package=data.table
El Moustaid F, Thornton Z, Slamani H, Ryan SJ, Johnson LR (2021) Predicting temperature-dependent transmission suitability of bluetongue virus in livestock. Parasites & Vectors 14(1), 382.
| Crossref | Google Scholar | PubMed |
Elith J, Leathwick JR, Hastie T (2008) A working guide to boosted regression trees. Journal of Animal Ecology 77(4), 802-813.
| Crossref | Google Scholar | PubMed |
Ellingwood DD, Pekins PJ, Jones H, Musante AR (2020) Evaluating moose Alces alces population response to infestation level of winter ticks Dermacentor albipictus. Wildlife Biology 2020(2), wlb.00619.
| Crossref | Google Scholar |
Fernandes-Santos RC, Medici EP, Testa-José C, Micheletti T (2020) Health assessment of wild lowland tapirs (Tapirus terrestris) in the highly threatened Cerrado biome, Brazil. Journal of Wildlife Diseases 56(1), 34-46.
| Crossref | Google Scholar | PubMed |
Fernandes-Santos RC, Warren K, Vaughan-Higgins R, Micheletti T, Bruce M (2025) Disease dynamics and mortality risk in tapirs (Perissodactyla: Tapiridae) through a systematic literature review: implications for preventive medicine and conservation. Preventive Veterinary Medicine 239, 106470.
| Crossref | Google Scholar | PubMed |
Flesher KM, Medici EP (2022) The distribution and conservation status of Tapirus terrestris in the South American Atlantic Forest. Neotropical Biology and Conservation 17(1), 1-19.
| Crossref | Google Scholar |
Fyumagwa RD, Runyoro V, Horak IG, Hoare R (2007) Ecology and control of ticks as disease vectors in wildlife of the Ngorongoro Crater, Tanzania. South African Journal of Wildlife Research 37(1), 79-90.
| Crossref | Google Scholar |
Gibb R, Redding DW, Chin KQ, Donnelly CA, Blackburn TM, Newbold T, Jones KE (2020) Zoonotic host diversity increases in human-dominated ecosystems. Nature 584, 398-402.
| Crossref | Google Scholar | PubMed |
Giroux A, Ortega Z, Bertassoni A, Desbiez ALJ, Kluyber D, Massocato GF, De Miranda G, Mourão G, Surita L, Attias N, Bianchi RdC, Gasparotto VPdO, Oliveira-Santos LGR (2022) The role of environmental temperature on movement patterns of giant anteaters. Integrative Zoology 17(2), 285-296.
| Crossref | Google Scholar | PubMed |
Giroux A, Ortega Z, Attias N, Desbiez ALJ, Valle D, Börger L, Rodrigues Oliveira-Santos LG (2023) Activity modulation and selection for forests help giant anteaters to cope with temperature changes. Animal Behaviour 201, 191-209.
| Crossref | Google Scholar |
Hagedoorn NN, Maze MJ, Carugati M, Cash-Goldwasser S, Allan KJ, Chen K, Cossic B, Demeter E, Gallagher S, German R, Galloway RL, Habuš J, Rubach MP, Shiokawa K, Sulikhan N, Crump JA (2024) Global distribution of Leptospira serovar isolations and detections from animal host species: a systematic review and online database. Tropical Medicine & International Health 29(3), 161-172.
| Crossref | Google Scholar |
Hartup BK, Kollias GV, Jacobsen MC, Valentine BA, Kimber KR (1999) Exertional myopathy in translocated river otters from New York. Journal of Wildlife Diseases 35(3), 542-547.
| Crossref | Google Scholar | PubMed |
Hernandez-Divers SM, Aguilar R, Leandro-Loria D, Foerster CR (2005) Health evaluation of a radiocollared population of free-ranging baird’s tapirs (Tapirus Bairdii) in costa rica. Journal of Zoo and Wildlife Medicine 36(2), 176-187.
| Crossref | Google Scholar | PubMed |
Herrera HM, Norek A, Freitas TPT, Rademaker V, Fernandez O, Jansen AM (2005) Domestic and wild mammals infection by Trypanosoma evansi in a pristine area of the Brazilian Pantanal region. Parasitology Research 96, 121-126.
| Crossref | Google Scholar | PubMed |
IUCN (2025) IUCN Red List of Threatened Species. Available at https://www.iucnredlist.org/en [retrieved 20 August 2025]
Karmacharya D, Herrero-García G, Luitel B, Rajbhandari R, Balseiro A (2024) Shared infections at the wildlife–livestock interface and their impact on public health, economy, and biodiversity. Animal Frontiers 14(1), 20-29.
| Crossref | Google Scholar | PubMed |
Keesing F, Allan BF, Young TP, Ostfeld RS (2013) Effects of wildlife and cattle on tick abundance in central Kenya. Ecological Applications 23(6), 1410-1418.
| Crossref | Google Scholar | PubMed |
Knox F, Jelocnik M, Stephens N, Sims C, Jackson B, Cowen S, Rayner K, Garretson S, Yeap L, Warren K, Vaughan-Higgins R (2024) Chlamydia in wild Australian rodents: a cross-sectional study to inform disease risks for a conservation translocation. Wildlife Research 51(1), WR23060.
| Crossref | Google Scholar |
Kophamel S, Illing B, Ariel E, Difalco M, Skerratt LF, Hamann M, Ward LC, Méndez D, Munns SL (2022) Importance of health assessments for conservation in noncaptive wildlife. Conservation Biology 36(1), e13724.
| Crossref | Google Scholar | PubMed |
Labruna MB, Martins TF, Acosta ICL, Serpa MCA, Soares HS, Teixeira RHF, Fernandes-Santos RC, Medici EP (2021) Ticks and rickettsial exposure in lowland tapirs (Tapirus terrestris) of three Brazilian biomes. Ticks and Tick-Borne Diseases 12(3), 101648.
| Crossref | Google Scholar | PubMed |
Lager KM, Mengeling WL (1994) Porcine parvovirus associated with cutaneous lesions in piglets. Journal of Veterinary Diagnostic Investigation 6(3), 357-359.
| Crossref | Google Scholar | PubMed |
Lelešius R, Sereika V, Zienius D, Michalskien I (2006) Serosurvey of wild boar population for porcine parvovirus and other selected infectious diseases in lithuania. Bulletin of the Veterinary Institute in Pulawy 50(2), 143-147 Available at https://hdl.handle.net/20.500.12512/87584.
| Google Scholar |
Mangini PR, Medici EP, Fernandes-Santos RC (2012) Tapir health and conservation medicine. Integrative Zoology 7(4), 331-345.
| Crossref | Google Scholar | PubMed |
McIntire EJB (2023) Require: installing and loading R packages for reproducible workflows. Available at https://CRAN.R-project.org/package=Require
McIntire EJB, Chubaty AM (2023) Reproducible: enhance reproduciblity of R code. Available at https://reproducible.predictiveecology.org
Medici EP, Mangini PR, Fernandes-Santos RC (2014) Health assessment of wild lowland tapir (Tapirus Terrestris) populations in the Atlantic Forest and pantanal biomes, Brazil (1996–2012). Journal of Wildlife Diseases 50(4), 817-828.
| Crossref | Google Scholar | PubMed |
Medici EP, Fernandes-Santos RC, Testa-José C, Godinho AF, Brand A-F (2021) Lowland tapir exposure to pesticides and metals in the Brazilian Cerrado. Wildlife Research 48(5), 393-403.
| Crossref | Google Scholar |
Medici EP, Mezzini S, Fleming CH, Calabrese JM, Noonan MJ (2022) Movement ecology of vulnerable lowland tapirs between areas of varying human disturbance. Movement Ecology 10(1), 14.
| Crossref | Google Scholar | PubMed |
Medici P, Velez J, Silva AR (2024) Chapter 3: lowland tapir Tapirus terrestris (Linnaeus, 1758). In ‘Tapirs of the world: ecology, conservation and management’. (Eds M Melletti, R Reyna-Hurtado, P Medici) pp. 63–78. (Springer Nature: Switzerland) doi:10.1007/978-3-031-65311-7
Nijhof AM, Penzhorn BL, Lynen G, Mollel JO, Morkel P, Bekker CPJ, Jongejan F (2003) Babesia bicornis sp. nov. and Theileria bicornis sp. nov.: tick-borne parasites associated with mortality in the black rhinoceros (Diceros bicornis). Journal of Clinical Microbiology 41(5), 2249-2254.
| Crossref | Google Scholar |
Obaid MK, Islam N, Alouffi A, Khan AZ, Da Silva Vaz I, Jr, Tanaka T, Ali A (2022) Acaricides resistance in ticks: selection, diagnosis, mechanisms, and mitigation. Frontiers in Cellular and Infection Microbiology 12, 941831.
| Crossref | Google Scholar | PubMed |
Ordonneau D, Fernandes-Santos RC, Zimmerman D, Pukazhenthi B, Rojas-Jimenez J, Pérez-Flores J, Navas-Suarez PE (2024) Chapter 11: tapir health. In ‘Tapirs of the world: ecology, conservation and management’. (Eds M Melletti, R Reyna-Hurtado, P Medici) pp. 167–205. (Springer Nature: Switzerland) doi:10.1007/978-3-031-65311-7
Padilla M, Dowler RC (1994) Tapirus terrestris. Mammalian Species 481(481), 1-8.
| Crossref | Google Scholar |
Pandit PS, Doyle MM, Smart KM, Young CCW, Drape GW, Johnson CK (2018) Predicting wildlife reservoirs and global vulnerability to zoonotic Flaviviruses. Nature Communications 9(1), 5425.
| Crossref | Google Scholar | PubMed |
Pedersen K, Anderson TD, Maison RM, Wiscomb GW, Pipas MJ, Sinnett DR, Baroch JA, Gidlewski T (2018) Leptospira antibodies detected in wildlife in the USA and the US Virgin Islands. Journal of Wildlife Diseases 54(3), 450-459.
| Crossref | Google Scholar | PubMed |
Pérez SD, Grummer JA, Fernandes-Santos RC, José CT, Medici EP, Marcili A (2019) Phylogenetics, patterns of genetic variation and population dynamics of Trypanosoma terrestris support both coevolution and ecological host-fitting as processes driving trypanosome evolution. Parasites & Vectors 12(1), 473.
| Crossref | Google Scholar | PubMed |
Pérez-Flores J, Calmé S, Reyna-Hurtado R (2016) Scoring body condition in wild baird’s tapir (Tapirus bairdii) using camera traps and opportunistic photographic material. Tropical Conservation Science 9(4), 194008291667612.
| Crossref | Google Scholar |
Pérez-Flores J, Weissenberger H, López-Cen A, Calmé S (2020) Environmental factors influencing the occurrence of unhealthy tapirs in the southern yucatan peninsula. EcoHealth 17(3), 359-369.
| Crossref | Google Scholar | PubMed |
R Development Core Team (2025) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Reyna-Hurtado R, Huerta-Rodríguez JO, Rojas-Flores E (2025) Extreme fighting and vocalisations in Tapirus bairdii: observations from aguadas of Calakmul, social arenas for the species. Neotropical Biology and Conservation 20(1), 67-78.
| Crossref | Google Scholar |
Roberts M, Dobson A, Restif O, Wells K (2021) Challenges in modelling the dynamics of infectious diseases at the wildlife–human interface. Epidemics 37, 100523.
| Crossref | Google Scholar | PubMed |
Ruiz-Fons F, Vidal D, Höfle U, Vicente J, Gortázar C (2007) Aujeszky’s disease virus infection patterns in European wild boar. Veterinary Microbiology 120(3–4), 241-250.
| Crossref | Google Scholar | PubMed |
Shivaprakash KN, Sen S, Paul S, Kiesecker JM, Bawa KS (2021) Mammals, wildlife trade, and the next global pandemic. Current Biology 31(16), 3671-3677.e3.
| Crossref | Google Scholar |
Soetisno M (1933) Surra bij een tapir (Tapirus indicus). [Surra in a Tapir (Tapirus indicus).]. Nederlandsch-Indische Bladen Voor Diergeneeskunde 45, 126-128.
| Google Scholar |
Stämpfli H, Oliver-Espinosa O (2020) Chapter 22: clinical chemistry tests. In ‘Large animal internal medicine’. 6th edn. (Eds BP Smith, DC Van Metre, N Pusterla) pp. 395–420.e2. (Elsevier Mosby) doi:10.1016/B978-0-323-55445-9.00022-7
Streck A, Bonatto SL, Homeier T, Souza CK, Gonalves KR, Gava D, Canal CW, Truyen U (2011) High rate of viral evolution in the capsid protein of porcine parvovirus. The Journal of General Virology 92(11), 2628-2636.
| Crossref | Google Scholar | PubMed |
Szabo MPJ, Labruna MB, Pereira MC, Duarte JMB (2003) Ticks (Acari: Ixodidae) on wild marsh-deer (Blastocerus dichotomus) from southeast Brazil: infestations before and after habitat loss. Journal of Medical Entomology 40(3), 268-274.
| Crossref | Google Scholar |
Tadić M, Konjević D, Perko VM, Štritof Z, Zečević I, Benvin I, Milas Z, Turk N, Bujanić M, Hađina S, Habuš J (2025) The occurrence of Leptospira spp. Serogroup Pomona infections in wild boars. Veterinarska Stanica 56(2), 225-233.
| Crossref | Google Scholar |
Tomich RGP, Nogueira MF, Lacerda ACR, Campos FS, Tomas WM, Herrera HM, Lima-Borges PA, Pellegrin AO, Lobato ZIP, Silva RAMS, Pellegrin LA, Barbosa-Stancioli EF (2009) Sorologia para o vírus da língua azul em bovinos de corte, ovinos e veados campeiros no Pantanal sul-mato-grossense. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 61(5), 1222-1226.
| Crossref | Google Scholar |
Uhart MM, Mangini PR, Galvez CES, Corti P, Milano FA, Jorge MC, Girio RJS, Mathias LA, Schettino AM, Catena MC, Terragno R, Aprile G (2010) Bacterial diseases. In ‘Neotropical cervidology, biology and medicine of Latin American deer’. (Eds JMB Duarte, S Gonzalez) pp. 342–362. (International Union for Conservation of Nature, Gland, Switzerland, and Fundacao de Apoio a Pesquisa, Ensino e Extensao: Jaboticabal, Sao Paulo, Brazil)
Varela D, Flesher K, Cartes JL, De Bustos S, Chalukian S, Ayala G, Richard-Hansen C (2019) Tapirus terrestris, Lowland Tapir. The IUCN red list of threatened species. Available at https://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T21474A45174127.en