Novel root clusters in the grasstree Kingia australis (Dasypogonaceae) increase the root surface:volume ratio by 70 times
Byron B. Lamont A § * , Andrew Weinert B and Helen Duff B
A § * , Andrew Weinert B and Helen Duff B
A
B
§ The Editors-in Chief wish to acknowledge Byron Lamont’s long-term contribution to the journal. This is his 28th paper to have been published in the (the first occurring 53 years ago!). Ironically, the first paper was also about specialised roots in the SW Australian flora. Byron’s contribution to the journal has also included serving on the editorial board between 1993 and 1996.
Handling Editor: John Morgan
Abstract
Erosion of a riverbank in south-western Australia exposed previously unrecorded clusters of roots/rootlets produced by the grasstree, Kingia australis (Dasypogonaceae).
Our aim was to prepare an initial report on these roots and consider their possible functions.
Excavation of root clusters, and quantification of the morphological and histological characteristics, and distribution in the soil.
There were 260 clusters per m3 of soil to a depth of 1.6 m, peaking at a depth of 50−70 cm where nutrients and water were accessible all year. Their length × width averaged 8.4 × 5.5 cm, with 520 roots/rootlets per cluster and 5140 rootlets per litre of rhizosphere soil. Clusters comprised a parent lateral (with aerenchyma), hundreds of secondary roots, 50 × 3 mm, and thousands of rootlets, 10 × 1 mm. Clusters are perennial, new roots occasionally emerging from the previous winter-growing-season cluster. We call these novel clusters kingioid roots. Total root length reached 70 m/litre of rhizosphere soil. All roots are covered in root hairs, with parent lateral hairs 250 μm long increasing to 700 μm for rootlets. They possess a ±150-μm thick mucigel layer. Fineness of the rootlets and the dense root-hair cover result in a 70-time increase in the surface:volume ratio compared with the parent roots. No endogenous fungal hyphae or (cyano) bacteria were evident.
Although perennial, these structures link with seasonal root-cluster types (proteoid/dauciform/capillaroid roots) via the abundance of extremely hairy rootlets and mucigel, implying that the structures enhance water and nutrient uptake rather than storage.
As these root clusters are distributionally/morphologically/anatomically distinct they deserve more detailed study.
Keywords: Dasypogonaceae, Kingia, mucigel, nutrient uptake, oligotrophic soils, phosphorus, root clusters, root hairs, root surface area, water uptake.
Introduction
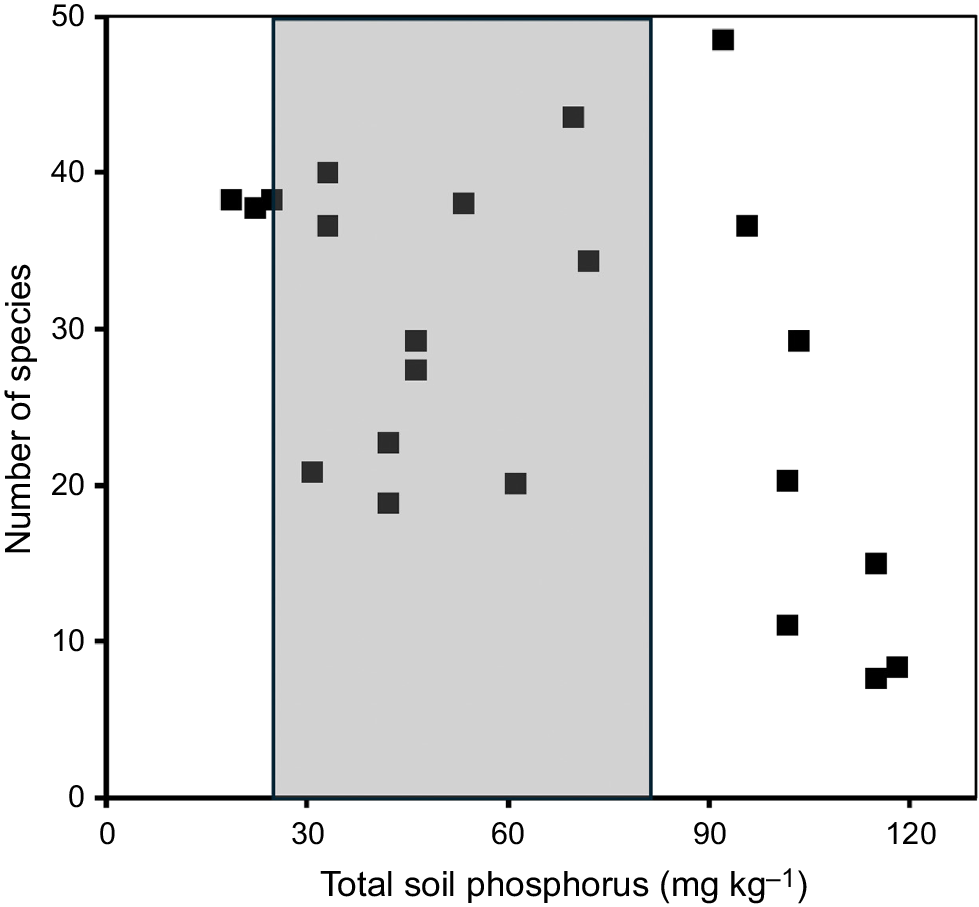
The floras of landscapes with a long history of recurrent fire, nutrient-impoverished soils, prolonged seasonal drought and/or waterlogging, and intense herbivory/granivory are surprisingly species rich. Environmental stresses and disturbances clearly serve to promote species co-occurrence and speciation (Allsopp et al. 2014; Groom and Lamont 2015; Lamont and Keith 2017; He et al. 2019; Brundrett 2021). Species richness increases as the environmental variable becomes less favourable for growth and reproduction although ultimately constrained by the need for the presence of at least a minimal level of that resource (Lamont 2024). This is illustrated in Fig. 1, where the number of species with proteoid root clusters or mycorrhizas quadrupled as total P per unit soil weight halved in the well-documented, nutrient-impoverished, summer-dry soils of sclerophyll shrublands in south-western Australia (Lambers et al. 2013; Lamont and Keith 2017).
Number of species of shrubs with proteoid roots or mycorrhizas per sample site varying in soil-nutrient status (total phosphorus (P) kg−1 soil) in the coastal shrublands 220 km north of Perth, Western Australia (where Kingia australis is abundant in some locations). The grey area corresponds to total P availability (mean ± s.e.) in the A horizon at five sites where the grasstree, Kingia australis, was present, collated from McArthur (1991). Data obtained from Lambers et al. (2013).
There is possibly no better example of this species-environment relationship than for the grasstree, Kingia australis (Dasypogonaceae). The family separated from the palms (Arecaceae) >125 million years ago (Ma) but Kingia originated <34 Ma (Givnish et al. 2018), i.e. as the Earth changed markedly from overall hot, humid climates in the Oligocene, to increasingly (a) cool, dry and seasonal climates, (b) seasonally fire prone, and (c) carbon-dioxide-limited photosynthesis, through the Miocene (Osborne 2008). Kingia became so morphologically distinct that there has even been an attempt to place the genus in a unique family (Kingiaceae Schnizlein). This species (monotypic genus, tribe, subfamily) is endemic to fire prone coastal sclerophyll shrublands (such as those cited in Fig. 1) to open eucalypt forests in south-western Australia (Fig. 2a) (florabase.dbca.wa.gov.au/search/quick?q=kingia+australis, accessed 25 November 2024). The climate is hot to warm mediterranean with 350−1000 mm of rain per annum (prior to recent global warming) mostly falling in winter-spring, with summer drought lasting 4−6 months and temperatures often exceeding 45°C, and frosts are rare. Soils vary from quartzitic sand over a shallow claypan or laterite in seasonally waterlogged lowlands to massive laterite over kaolinitic clay in uplands with extractable P often <10 mg kg−1 surface soil (McArthur 1991).
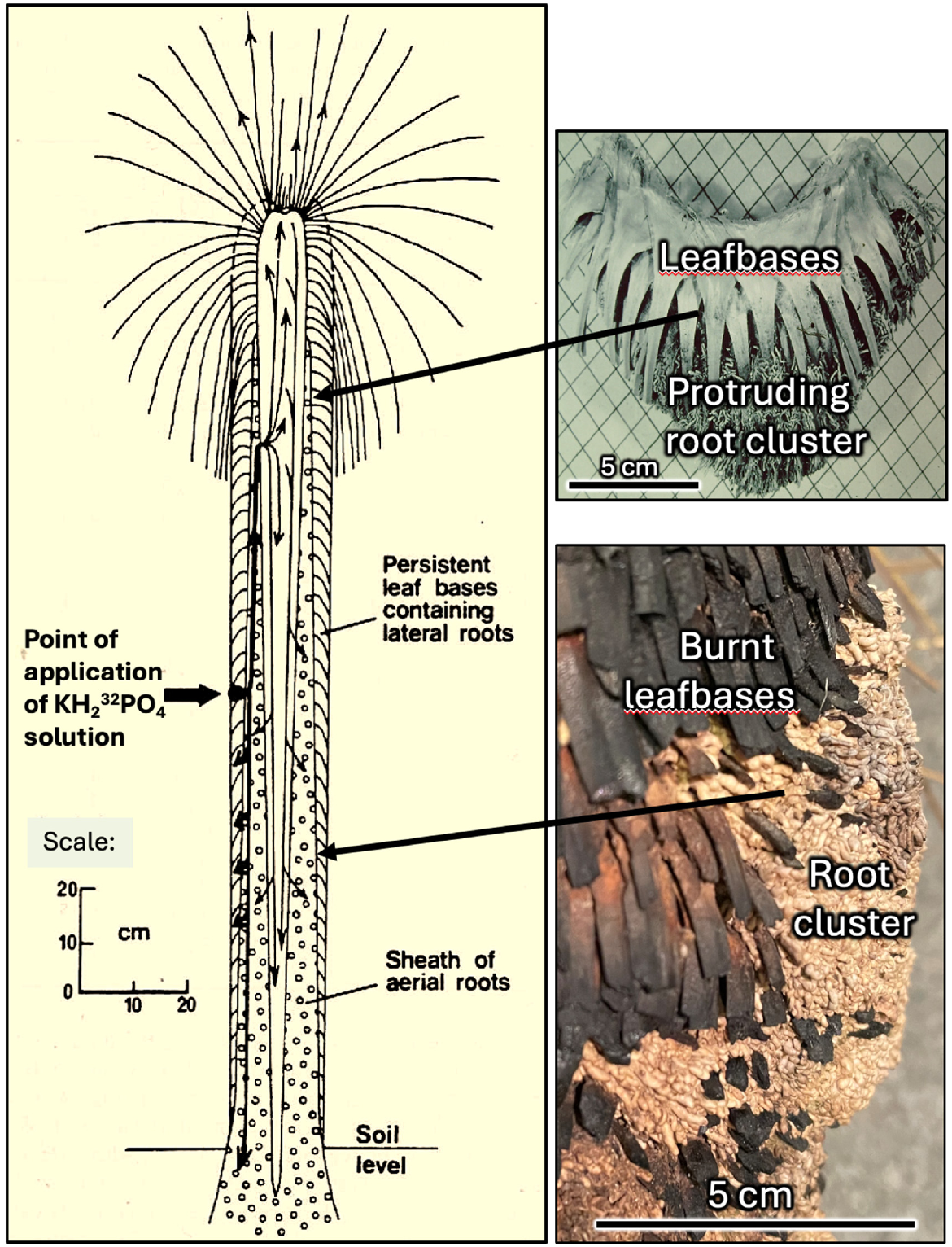
Drawing of section through a 1.67 m tall Kingia australis plant showing the pathway of KH232PO4 injected among a cluster of rootlets present in the persistent leafbases at the horizontal arrow position (images of two typical protruding clusters on the right). This shows rapid uptake of 32P over 6.5 h and translocation throughout the plant. The current work shows that the root clusters among the leafbases are synonymous with those in the soil (from Lamont (1984) based on data in Lamont (1981b) and unpublished work). Images and drawing created by B. B. Lamont.
The long needle-like leaves, rhombic in transection, are highly sclerified with sunken stomata (xeromorphic) and, while strongly herbivore-resistant, are highly flammable. Kingias produce floral primordia every year but prolific flowering requires incineration of the crown by wildfire (Lamont and Downes 1979). Pollination occurs by both insects and birds (e.g. Lichmera indistincta, pers. obs.) that alight on the terminal ‘crown’ of drumstick-shaped inflorescences. Ripe seeds are released explosively from the capsules. The persistent leaves are burnt back to a fire-resistant mantle of leafbases and concealed roots.
Seedlings, or juvenile offshoots from the parent plant, produce one or more tuberous roots that yield liquid when squashed but these are soon replaced by stem-based laterals (Lamont 1981a). Primary aerial roots, with a highly sclerotic stele, arise from near the stem apex and push through the leafbases. Up to 3000 reach the soil of a 6 m tall plant, taking an estimated 35 years to do so (Lamont 1981a, Fig. 2). These form a sheath around the soft primary stem, accounting for 78% of the area of a 20 cm-aboveground cross-section of the caudex of a 190 cm tall plant, with the aerial roots responsible for a 60% greater bulk density than the primary stem (Lamont 1981a, 1981b). Thus, the mantle forms a dense sheath of concealed prop roots, obviating the need for anomalous secondary growth that otherwise characterises palms and other grasstrees, such as Xanthorrhoea spp. (Tomlinson and Zimmermann 1969). Bunches of lateral roots branch out into the leafbases where they are effective in uptake of water and (isotopically labelled) phosphate, KH2PO4 (Lamont 1981b, Fig. 3). The clusters often protrude beyond the leafbases, especially when the crown remains intact when unburnt. This work has shown that, on a volume basis, the leaf bases are a better source of water and nutrients than the sandy surface soil in which the plant resides, possibly due to the accumulation of postfire ash/charcoal, airborne dust and the droppings of pollinators.
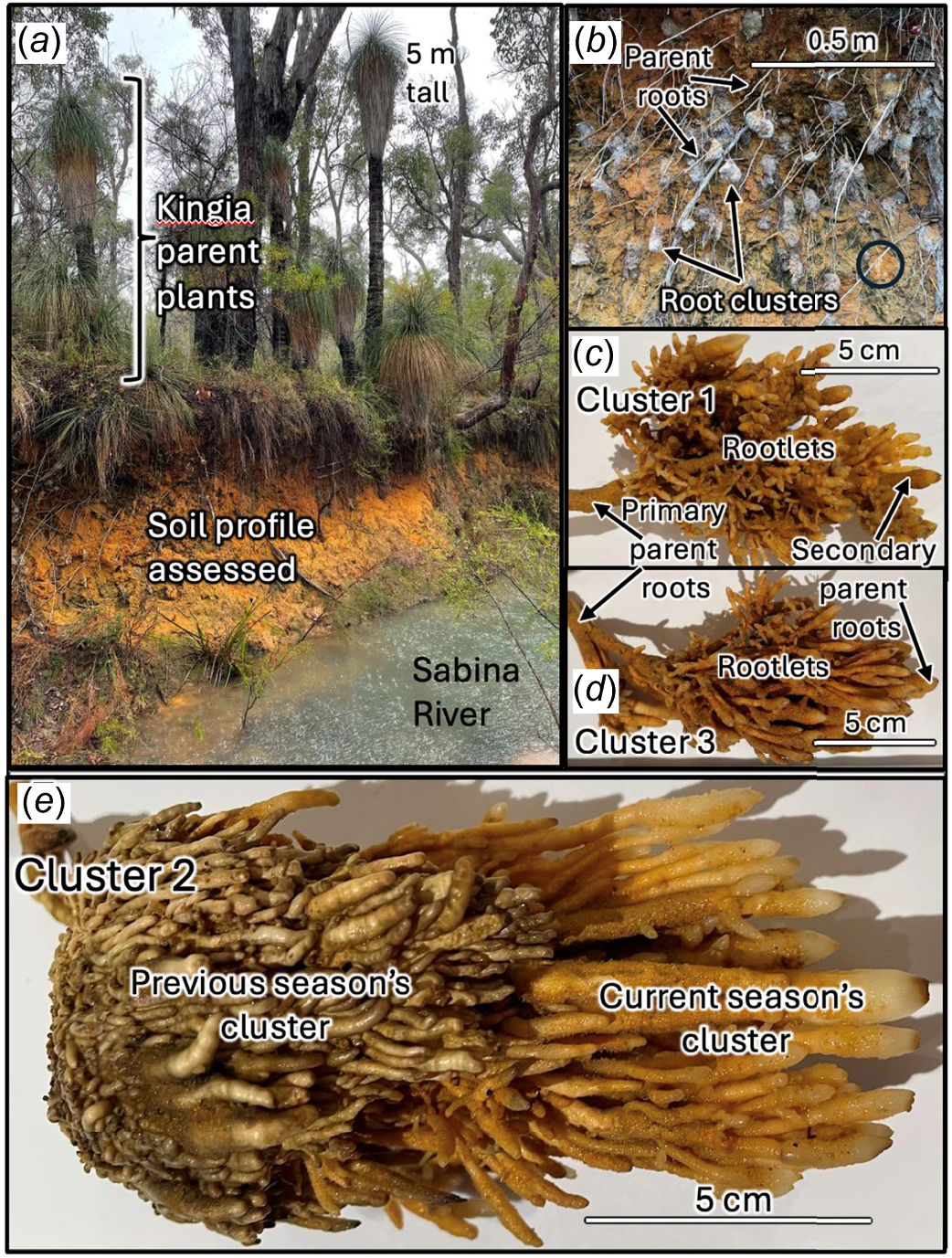
General site, plant and root cluster details. Left: (a) Study site showing location of grasstrees beside Sabina River and soil profile exposed by erosion. (b) ~50 exposed root clusters, averaging 8.4 × 5.5 cm in length × width, in the soil profile attached to grasstree laterals, cluster 1 is circled. (c) Root cluster 1 assessed in detail showing the parent root, secondary roots and rootlets attached to these. (d) Root cluster 3. (e) Root cluster 2 showing perennial nature of clusters with new season’s (mid-winter) cluster emerging from previous wet season’s cluster. Images recorded and annotated by B. B. Lamont.
Kingias often grow in winter/spring-waterlogged soils and the abundant cortical aerenchyma in the primary roots serves to supply oxygen to roots penetrating these soils. The stem pith has remarkable longevity, with a well-defined ‘death front’ separating the living tissues – recorded up to an age of 435 years, from the dead tissues beneath (Lamont 1980). Clearly, this unique combination of traits has enabled kingias to adapt effectively to the special constraints of this highly fire/drought prone, severely nutrient-impoverished and efficient-herbivore-exposed region. Providing only a single contribution to species diversity, kingias make a unique, significant contribution to the region’s world-renowned biodiversity, the conceptual breadth of which covers phylogenetic and functional diversity that is exceptional here (Lamont 1995; Groom and Lamont 2015; Brundrett 2021).
Recently, we became aware that some of the special features of kingias even existed underground. One of us (A. Weinert) was hiking along the dry bed of the Sabina River located 230 km south of Perth in south-western Australia, when many clumps of roots were observed protruding from the eroded banks (Fig. 3b). The laterals supporting the clumps could be traced back to kingias abutting the banks (Fig. 2a). Inspection of photos by one of us (B. B. Lamont) indicated that these were a type of root cluster possibly new to science (a later revelation showed that these were synonymous with the root clusters formed in the caudex, Fig. 2, that had already been the subject of some study). A project was therefore initiated to examine these structures in more detail with the aims to (a) relate these to what is already known about the root system of kingias, (b) describe their morphological and anatomical characteristics, (c) compare these structures with known root-cluster types that possess abundant rootlets and root hairs with which they seemed most similar, and (d) consider their function in relation to known aspects of the biological characteristics of kingias and soil water and nutrient availability, as background to future experimental research.
Materials and methods
Study site
The study was undertaken at an eroded bank face beside the Sabina River in the Whicher Range National Park in south-western Australia (33.76955° S, 115.45860° E) (Fig. 2). Roots were examined in detail and sampled on 31 July 2024 following substantial rain in July (winter rain, May–July, was 394 mm) and the mean temperature range over that time was 4.3−25.6°C. The river began to flow on 27 July 2024. The soil was lateritic sandy-silt-clay-loam (sand was fine) grading to pisolitic laterite in silt-clay at 1.8 m with massive laterite at 2.0 m (over which the river flows). Soil colour was noted at 10 cm intervals down the profile (Munsell Soil-Color Charts 2009). The pH (slurry with 1:1 rainwater at pH 6.8 with calibrated pH meter) of the kaolinitic clay-loam surrounding four root clusters was 6.0−6.4.
The vegetation was eucalypt woodland, 6−8 m tall (Corymbia calophylla, Eucalyptus marginata), with scattered shrubs, 2−3 m tall (Grevillea manglesioides, Hakea lasianthoides) and grasstrees (Xanthorrhoea preissii, Kingia australis, Dasypogon hookerii), 1−5 m tall and a species-rich, small-shrub−perennial-herb layer (other Proteaceae, other Myrtaceae, Fabaceae, Cyperaceae, Restionceae). Five kingias were located within 1 m of the breakaway face at the study site (Fig. 2a) and the cluster parent laterals were traced to the base of several of these (also see Lamont (1981a) for a comprehensive description of the root system of this species).
Root cluster analyses
Counts were made of the numbers of root clusters evident at 10 cm intervals down the profile for three contiguous 1 × 1.8 m profiles for the most accessible face where clusters were prominent. Assuming that 25 cm of the profile face had been eroded away (by restoring the horizontal position of pendulous clusters as evidence), the data were converted to per soil volume (equivalent to cluster volume). Photographs of the profile were taken, with and without a ruler for scale, and the images used to estimate the length and width of 50 clusters.
Three current season’s clusters embedded in the profile were located, excavated and taken to the ‘laboratory’ (H. Duff’s dining room) in plastic bags for quantitative assessment. Older clusters were also harvested for comparative purposes. Rhizosphere soil was brushed away from each of the three new-season clusters for later determination of Munsell colour and pH. These were repeatedly dunked in rainwater until all remaining loose soil was removed. The three dimensions were subsequently measured with vernier callipers (all values were to 0.1 mm and occasionally to 0.05 mm as required). Clusters were photographed in colour under a frosted, GLS, LED 9-W light source with an iPhone 12 (Apple), double-lens camera with 15.6.1 software. These were separated into primary parent root, secondary parent roots and rootlets, the number counted, and the lengths and widths of 30 representative rootlets per cluster, and the other two root types (up to 30 if more than 30 present) measured with callipers. A few roots were selected for microscopy. The three batches of root types were placed on paper towelling, patted dry and the wet weights taken to 0.01 g. Mathematical formulae were used to determine number of roots per unit volume of soil within the confines of the clusters, total root lengths per unit volume of soil, root volume (LπD3/6), and root surface area (LπD) where L = total length and D = diameter. Root hair values were obtained from Lamont (1981a) as we were not able to acquire sufficiently accurate details here.
Anatomy
Fresh representative roots were placed longitudinally between carrot tuber segments and transections, ~0.3 mm wide, obtained with a safety razor blade. These were placed on glass slides and stained with 1% aqueous brilliant blue, cover slips were added and slides viewed under a dissecting microscope for checking quality. For estimation of root hair length, sections were placed directly on slides with a ruler in mm. Five representative root hairs for each of five roots or different parts of the same root if numbers were insufficient, were measured. Suitable slides were placed under a transmission microscope with camera attachment (Omax, model A3RDF50, China) and images captured with an iPhone 14 Promax. Scales for the images were estimated from known dimensions of the roots. Images were used to count number and width of root hairs, with the assistance of data in Lamont (1981a). To show the presence of aerenchyma confined to the parent roots, a cross-section specimen was critical-point dried and photographed with a scanning electron microscope (Lamont 1983a).
Density was estimated from hairs per unit length of root perimeter × section width. These were converted to root hair volume and area per unit root surface area using the mean root hair length per root type (Table 1), and the above formulae. Total root hair volume and area per L of soil were estimated from mean volumes and surface areas per root type (Table 1). More accurate values for all root hair measurements would require detailed studies of wax-embedded specimens so the estimates of root hair properties must be regarded as approximate, although the trends are clear.
| Attribute | Cluster 1 | Cluster 2 | Cluster 3 | Mean | |
|---|---|---|---|---|---|
| Volume in mL | 76.3 | 122.1 | 121.6 | 106.7 | |
| Fresh weight in g | 18.4 | 20.4 | 21.9 | 20.2 | |
| Fresh weight in g L−1 soil/cluster | |||||
| Parent 1 | 49.5 | 12.9 | 26.8 | 29.7 | |
| Parent 2 | 108.4 | 84.6 | 54.4 | 82.5 | |
| Rootlets | 121.9 | 96.9 | 128.1 | 115.6 | |
| Mean no. roots L−1 soil/cluster | |||||
| Parent 1 | 13 | 8 | 9 | 10 | |
| Parent 2 | 302 | 139 | 198 | 213 | |
| Rootlets | 5270 | 5410 | 4740 | 5140 | |
| Root length range in mm | |||||
| Parent 1 | 82 | 80 | 120 | 94 | |
| Parent 2 | 13−68 | 17−110 | 17−50 | 14−76 | |
| Rootlets | 1−29 | 2−30 | 3−23 | 2−27 | |
| Root length mean in mm | |||||
| Parent 1 | 82.3 | 80.0 | 120 | 94 | |
| Parent 2 | 40.3 | 69.2 | 34.2 | 47.9 | |
| Rootlets | 11.9 | 11.1 | 10.3 | 11.1 | |
| Total root length in m L−1 soil/cluster | |||||
| Parent 1 | 1.1 | 0.6 | 1.0 | 0.9 | |
| Parent 2 | 12.2 | 9.6 | 6.8 | 10.2 | |
| Rootlets | 62.7 | 60.1 | 48.8 | 57.1 | |
| Root diameter range in mm | |||||
| Parent 1 | 7.3 | 4.4−5.5 | 4.6−4.9 | 4.4−7.3 | |
| Parent 2 | 2.3−4.3 | 1.7−4.2 | 2.1−4.0 | 1.7−4.3 | |
| Rootlets | 0.8−1.6 | 0.6−2.3 | 0.7−1.5 | 0.6−2.3 | |
| Root diameter mean in mm | |||||
| Parent 1 | 7.30 | 4.95 | 4.82 | 5.69 | |
| Parent 2 | 3.10 | 2.77 | 2.78 | 2.88 | |
| Rootlets | 1.39 | 1.11 | 1.17 | 1.22 | |
| Root volume as % soil/cluster volume | |||||
| Parent 1 | 4.61 | 1.16 | 1.83 | 2.53 | |
| Parent 2 | 9.23 | 5.79 | 4.13 | 6.38 | |
| Rootlets | 9.52 | 5.82 | 5.25 | 6.86 | |
| Root surface area in cm2 L−1 soil/cluster | |||||
| Parent 1 | 126 | 47 | 76 | 80 | |
| Parent 2 | 594 | 418 | 297 | 462 | |
| Rootlets | 1370 | 1048 | 897 | 1095 | |
| Root hair length range in mm | |||||
| Parent 1 | 0.1−0.6 | 0.1−0.7 | 0.1−0.6 | 0.1−0.7 | |
| Parent 2 | 0.1−0.6 | 0.1−0.7 | 0.2−1.1 | 0.1−1.1 | |
| Rootlets | 0.2−1.0 | 0.4−1.1 | 0.4−1.1 | 0.2−1.1 | |
| Root hair mean length in mm | |||||
| Parent 1 | 0.195 | 0.372 | 0.220 | 0.262 | |
| Parent 2 | 0.375 | 0.380 | 0.620 | 0.458 | |
| Rootlets | 0.564 | 0.804 | 0.743 | 0.704 | |
| Root hair volume as % soil volume | |||||
| Parent 1 | 0.25 | ||||
| Parent 2 | 1.11 | ||||
| Rootlets | 1.92 | ||||
| Root hair surface area in cm2 L−1 soil/cluster | |||||
| Parent 1 | 137 | ||||
| Parent 2 | 1509 | ||||
| Rootlets | 5496 | ||||
Soil/cluster refers to rhizosphere soil located within the confines of the cluster.
Possible functions
Locations bearing stands of Kingia australis were sought in McArthur (1991). Five sites were identified and records for mineral-nutrient concentrations through the profile were tabulated. Values given as cmol/kg were converted to mg/kg and all mass/soil mass values changed to mass/soil volume. Where some horizons were missed in the original data, the mean was obtained by interpolation of the adjacent values. Using the data on cluster morphology and distribution of soil nutrients, estimates were made of the potential for storing soil water and accessing and absorbing nutrients (next paragraph). Limitations of the data were that our values for mean number of clusters per unit soil volume referred to a single large grasstree (Lamont 1981a) and that nutrient values for the soil profiles did not extend beyond 95 cm, somewhat shorter than the limit of occurrence of root clusters.
The possible role of kingioid root clusters in storing water was calculated as follows: number of clusters per unit soil volume (260 m−3, Table 1) was multiplied by volume of a typical cluster (0.107 L, Table 1), and percentage of soil occupied by clusters (19.1%, Table 1) as a fraction of the total soil volume occupied by the plant (2.57 m−3, Lamont 1981a) to give volume of root clusters (x). Estimates of the volume of primary roots below ground were made (78.8% of total, y) as well as volume of an entire 3.7 m tall plant aboveground (164 L, z) from Lamont (1981a). Assuming that the mean concentration of water in tissues above and below ground was the same (‘available’ water constitutes 35% of leaf volume of some sclerophylls (Lamont and Lamont 2000) but, on the assumption of equality, any value could have been used as the focus was on relative availablity) and this resulted in the fraction of total water stored by root clusters belowground = x/(x + y + z). The quantity of water stored within the rhizosphere soil of clusters (w) was estimated as follows: the fraction of cluster volume occupied by soil was calculated (100 − 19.1 = 80.9%) and multiplied by saturated water availability on a volume basis for silt-loam (15%, Ratliff et al. 1983) and this resulted in w = (80.9/19.1)x × 0.15. Available nutrient content in the total cluster rhizosphere soil per kingia (n) was determined as follows: the mean total/extractable/exchangeable concentrations by weight (e) of N, P, K, Ca and Mg in the B horizon as given in McArthur (1991) for five sites occupied by kingias was multiplied by the bulk density of silt-loam (1.5 kg L−1, Zeri et al. 2018) and resulted in n = (80.9/19.1)x × e × 1.5 (soil weights and volumes cancel out to leave absolute mass of nutrient).
Soil profile distribution
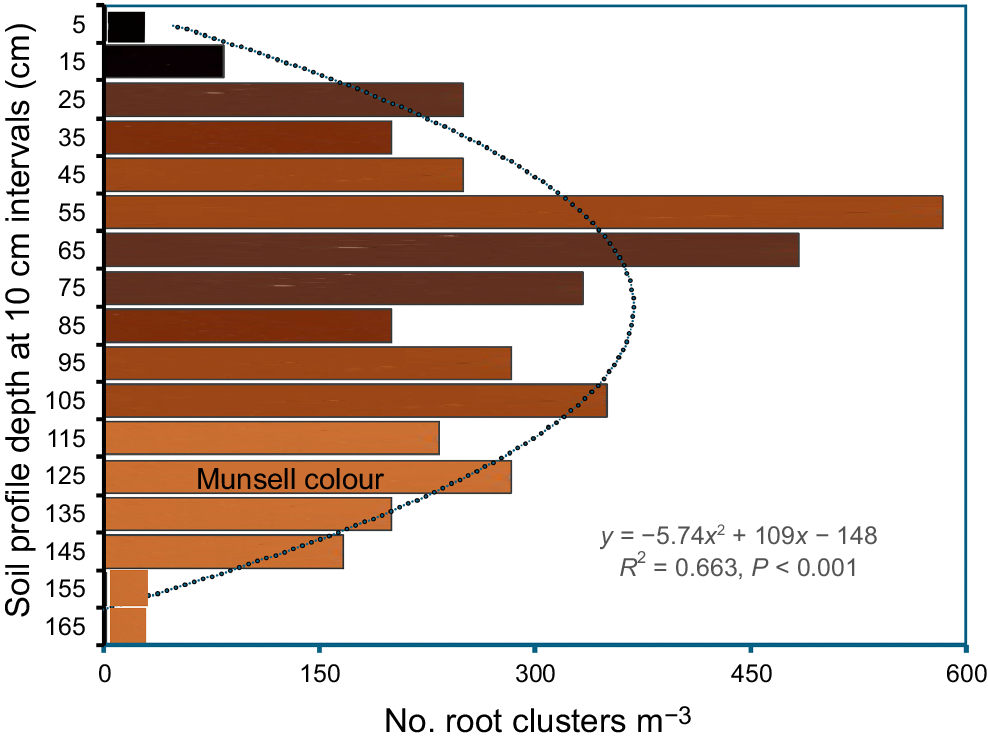
Soil colour was in the range of yellow–red with black humus the dominant colour in the uppermost 20 cm (A1 horizon), becoming brown (Munsell 7.5YR 4/4–5/6) at a depth of 60−80 cm and orange from 110 cm (6/6, Fig. 3, also see Fig. 2a). Fifty root clusters varied in length from 8 to 28 cm and width from 4 to 15 cm, with a mean ± 95% confidence interval of 15.2 ± 2.0 × 10.0 ± 2.0 cm. Clusters were rare in the A horizon, peaking in the B horizon at 45−75 cm, averaging almost 500 clusters per m3 of soil in that section and declining (as with the supporting laterals) to zero by 170 cm, the distribution thereby forming a highly significant quadratic curve (Fig. 4). This yields a mean of 260 clusters per m3 in the uppermost 150 cm of soil. Elsewhere, we noted that where the soil was deeper, some clusters occurred at a maximum depth of 200 cm.
Distribution of root clusters (number per unit volume) through the soil profile at Sabina River. The colour of each 10 cm section of the profile has been added from the Munsell Soil-Color Charts (Munsell Color 2009). A highly significant quadratic curve (dotted line) has been added to the data.
Cluster morphology
There was minor variation in cluster volume and wet weight among the three root clusters analysed in detail, averaging 107 mL and 20 g (Table 1). Wet weight averaged 230 g per L of cluster. Sometimes clusters were aggregated into 3–4 clumps, possibly representing different seasons, making identification of individual clusters difficult. The finer the root type the more this contributed to mass, with rootlets averaging 50% total weight and secondary parent roots 37%. The primary parent root varied greatly in its (small) contribution to mass as care was not taken to ensure that these were always within the confines of the cluster. Secondary root abundance was 20 × that of the primary, and rootlets were 24 × more abundant than the secondary roots, with rootlets accounting for 96% of the total number on average, with 520 roots/rootlets per cluster and 5350 roots/rootlets per L of soil within the confines of the cluster (Table 1).
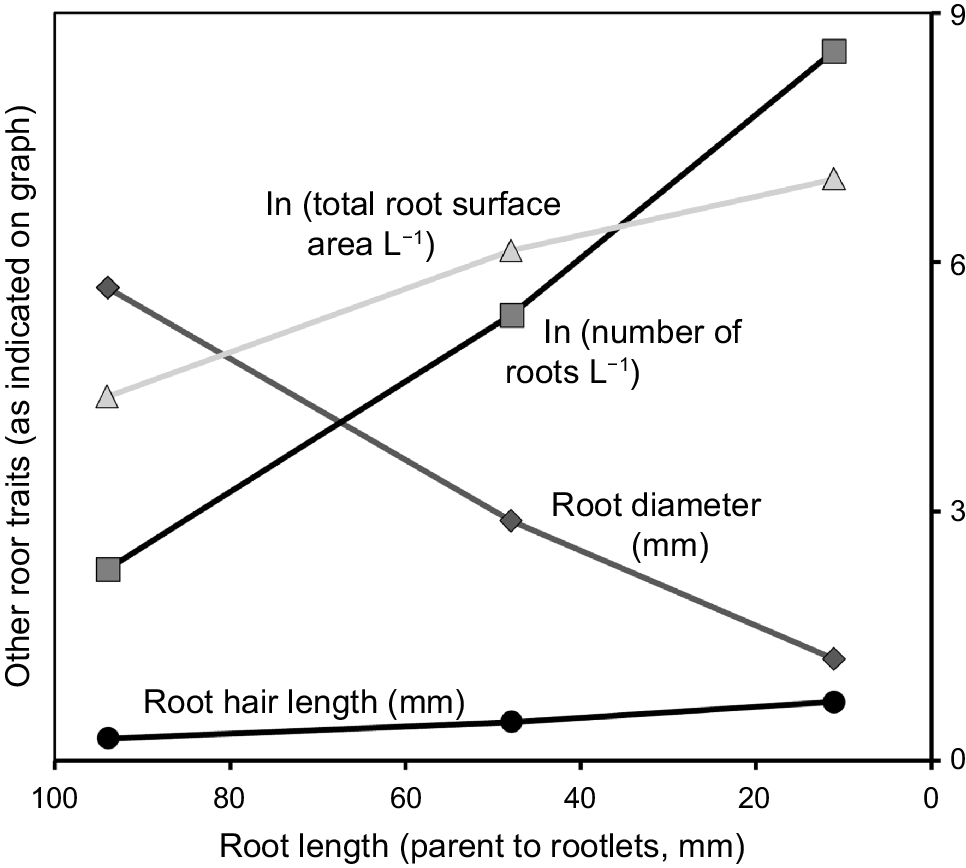
Root length within the secondary and rootlet categories varied greatly as these were actively growing and producing new roots at the collection time (Table 1). Secondary roots (47 mm) averaged four times the length of rootlets (11 mm). Rootlets accounted for 84% of total root length with a mean of 57 m per L of soil/cluster. Root diameter was less variable within each category, with the primary root averaging twice that of the secondary (2.9 mm), which in turn was twice that of the rootlets (1.2 mm). Fig. 5 shows the trends graphically, with root diameter decreasing and root hair length increasing progressively from parent root to secondary root to rootlets. In contrast, number of roots/rootlets and total surface area increased exponentially. Combining these values showed that rootlets accounted for 43.5% of root volume that occupied 16% of the soil volume within the confines of the cluster, but 67% of total root surface area, equivalent to an area of 40.5 × 40.5 cm2 per L of cluster/rhizosphere soil.
Root traits plotted against decreasing mean root length per cluster (parent root to rootlets). Note that total surface area in cm2 per L soil/cluster and number of roots per L are given as natural logs (ln). Individual cluster means and ranges are provided in Table 1.
Microscopy
The three root categories recognised here have a typical monocotyledonous anatomy with a well-defined stele and cortex with a dense cover of medium-sized root hairs (Fig. 6b, d). Root hair length varied from 0.1 to 1.1 mm (Table 1). The hairs of rootlets averaged 54% longer (0.7 mm) than for the secondary roots (0.5 mm) and 270% longer (0.26 mm) than for the primary roots. Widths varied from a mean of 12.5 μm for primary laterals to 13.6 μm for secondary laterals and rootlets (from Lamont 1981a). Density averaged 167 root hairs per mm2 of root surface. Combining these data yields rootlets contributing 60% of root hair volume while adding only 3.3% to the volume of the cluster plus associated soil (Table 1). Root hairs add 7150 cm2 per L−1 soil/cluster, increasing the total area exposed to the soil by 4.36 times, with rootlets accounting for 77% of this increase. Overall, the ultimate rootlets increased the surface:volume ratio of the parent roots by 69 times.
Root and rootlet cross sections. (a) Transection showing highly lignified stele with rings of 19 phloem strands (stained black) and spiraliform vessels, and 8 central vessels, parenchymatous cortex and mucigel layer wrapped around rootlet. (b) Dense cover of simple root hairs emerging from mucigel layer showing individual colloid particles. (c) Sector showing root hairs emerging from dense mucigel layer adhering to rootlet surface. Note absence of any hyphae or other microorganisms. (d) Scanning electron micrograph of parent root showing aerenchymatous parenchyma and sparse root hair cover. (a−c) Stained with brilliant blue. Prepared or imaged by A. Weinert, H. Duff and B. B. Lamont, edited by W. Lamont.
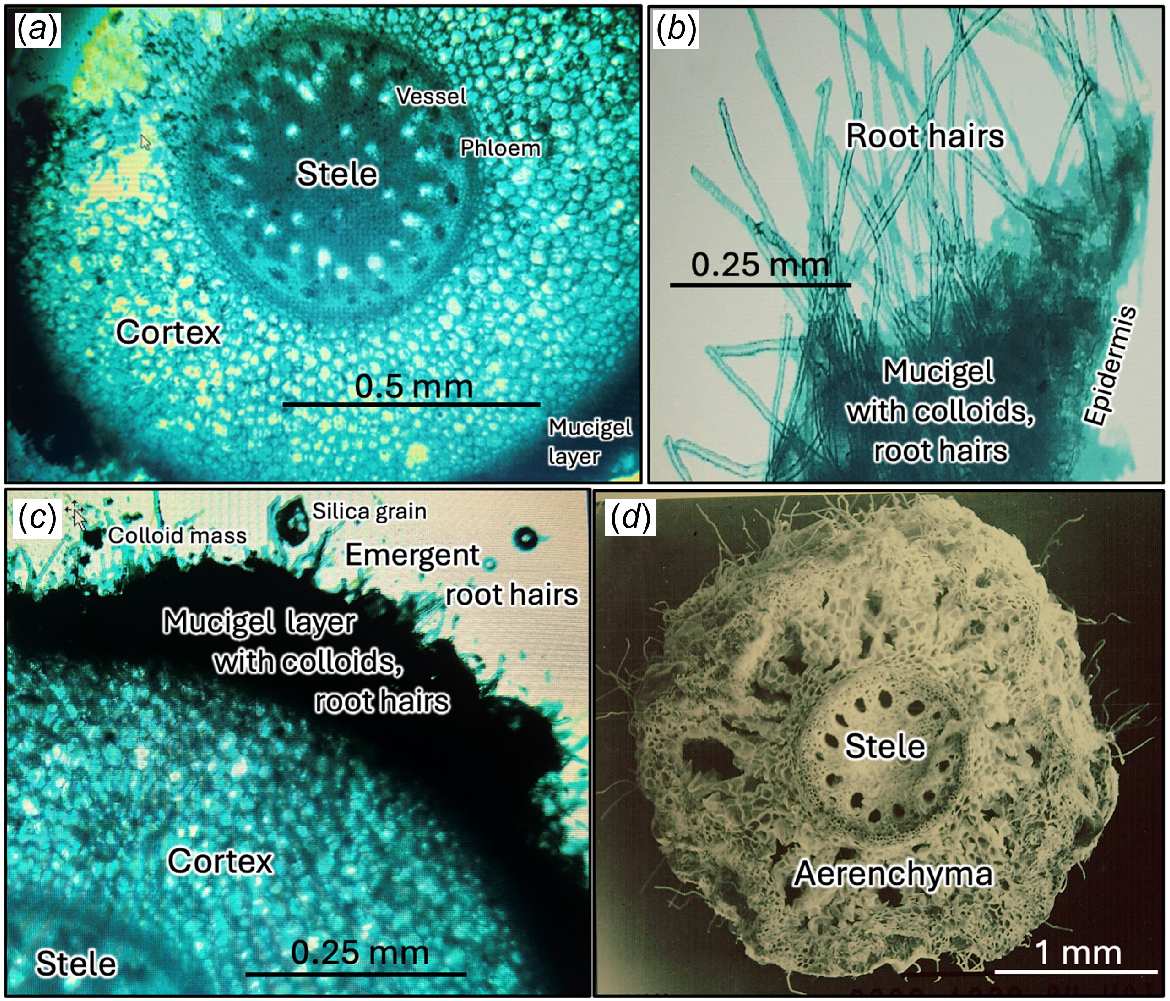
The parent laterals possessed large, radially aligned, schizogynous airspaces in the cortex (aerenchyma) that make the root spongy to the touch (Fig. 6d). The root hairs were sometimes crowded onto micromounds of 30 or more hairs that produce pincushion-like structures resulting in hairs from adjacent mounds interweaving with slight depressions in between. This adds to the root sponginess and appears to facilitate water retention. The daughter roots lacked this interwoven structure of the hairs as there were no mounds. These also lacked aerenchyma, consisting entirely of standard parenchyma cells (Fig. 6a). Cells were not swollen, nor was the parenchyma extensive compared with the size of the vascular system, and no starch grains were detected as would be expected of tuberous roots. The sections were not washed and relatively thick, therefore any hyphae or (cyano)bacteria should have been visible in the cortex but none was evident. A prominent mucigel layer, 100−200 μm thick, surrounded the secondary roots and rootlets (Fig. 6). This layer stained darkly with brilliant blue. Root hairs need to push through this mucigel layer to reach the soil. Colloids, apparently iron-oxide impregnated and associated with the lateritic soil that occasionally formed microlumps, were present in the mucigel that appeared to cause the orange appearance of the roots (Fig. 3). Humic and clay particles, and tiny silica grains were also embedded in the mucigel (Fig. 6c). Daughter roots/rootlets arose randomly from the pericycle of the parent root.
Possible functions
The volume of available water stored by the root clusters per grasstree, using the method described above, was 4.77 L. Available water stored underground by primary roots was 17.68 L and that aboveground for an entire grasstree was 57.40 L. Clusters were therefore estimated to account for 6.0% of total water stored in a 3.7 m tall plant. Available water stored within the rhizosphere of clusters at saturation was estimated at 8.66 L.
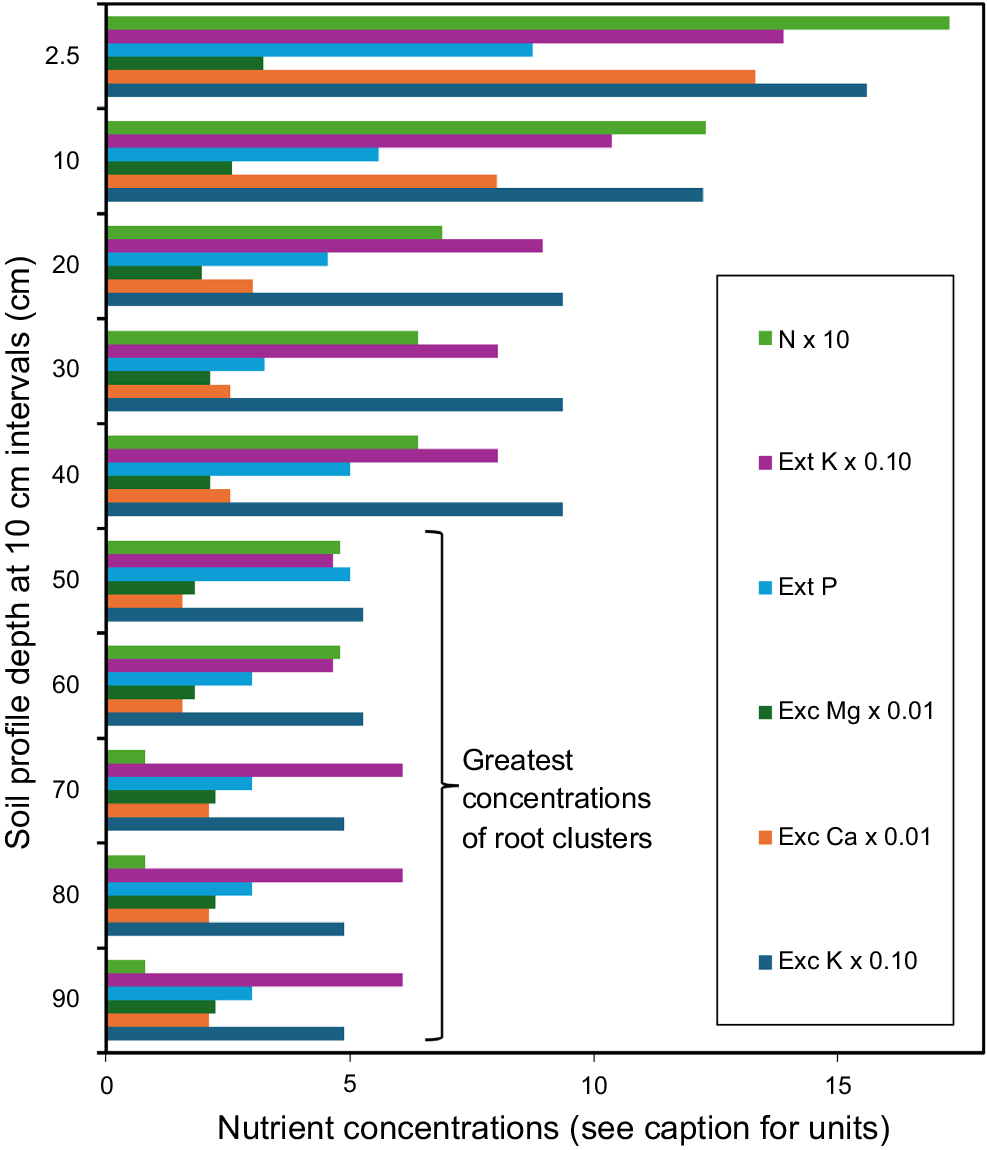
The concentration of mineral nutrients is highest in the uppermost 15 cm of soil (A1/12 horizons) (Fig. 7) and intermediate at 15–45 cm (B1/12). Calcium (Ca) and nitrogen (N) levels continue to decline beneath 45 cm where most root clusters are located (B2/21/22) but remain at moderate levels for phosphorus (P), magnesium (Mg) and potassium (K). Direct comparisons are provided in Table 2. Soils were not sampled at depths exceeding 95 cm, therefore the potential for nutrient uptake by the presence of kingioid roots at this depth is unknown.
Distribution of six mineral nutrients through the soil profile for five locations in SW Australia where Kingia australis plants were recorded, collated from McArthur (1991). Units for N: mass per unit soil volume (g kL−1 × 10 gives the correct concentration), extractable (Ext) K (g kL−1 × 0.10), extractable P (g kL−1), exchangeable (Exc) Mg (g kL−1 × 0.01), exchangeable Ca (g kL−1 × 0.01), exchangeable K (g kL−1 × 0.10).
| Nutrients | B horizon concentration (g kL−1) A | Estimated quantity in cluster soil (g) | B horizon concentration (% of A horizon) | |
|---|---|---|---|---|
| Extractable P | 3.64 | 0.21 | 50.8 | |
| Total N | 529.6 | 30.58 | 29.1 | |
| Extractable K | 62.3 | 3.60 | 51.3 | |
| Exchangeable K | 62.7 | 3.62 | 50.6 | |
| Exchangeable Ca | 208.1 | 12.02 | 19.5 | |
| Exchangeable Mg | 209.5 | 12.10 | 72.0 |
Discussion
The discovery of abundant clusters of roots through the soil profile in a grove of Kingia australis shows that the clumps of lateral roots previously reported protruding from the caudexes of this species (Fig. 2, Lamont 1981a) are not only synomymous with the clusters now described but they are far more abundant in the soil. These are now shown to be discrete root clusters, an order of magnitude larger than the other three root-cluster types currently recognised, other than compound proteoid roots (Table 3). The root clusters terminate lateral roots ultimately arising from the primary prop roots embedded in the persistent leafbases of the grasstree (Fig. 2). In deference to the apparent restriction of these structures to Kingia, we refer to these as kingioid roots. The precedent is proteoid roots, although these were shown not to be confined to the Proteaceae (Lamont 1972a) 12 years after the original description (Purnell 1960) and this is also possible here. The absence of longitudinal rows of rootlets distinguishes these root clusters from proteoid roots. There is no basal swelling of the rootlets as in dauciform roots and the root clusters do not form a soil-surface mat as in capillaroid roots. At an average width of 2.9 mm (secondary parent) and 1.2 mm (rootlets), the roots are coarser than other root-cluster types. The occurrence of two sizes of root division is also unique, although sometimes daughter proteoid roots may arise within the confines of a parent proteoid root (Lamont 1983b).
| Root cluster type | Family | Subfamily | Genus | Region and vegetation types | Reference | |
|---|---|---|---|---|---|---|
| Kingioid | Dasypogonaceae | None | Kingia | Southwestern Australia, shrubby wetlands to forests | This paper | |
| Proteoid | ||||||
| Simple | Proteaceae | Proteoideae | Protea | Africa, shrublands and savannas | Lamont (1983b) | |
| Simple | Proteaceae | Proteoideae | Beauprea | New Caledonia, rainforest | (Lamont, unpubl. data) | |
| Simple | Proteaceae | Grevilleoideae | Hakea | Australia, shrublands to forests | Lamont (1972b) | |
| Simple | Proteaceae | Grevilleoideae | Embothrium | Northern South America, forests | Zúñiga-Feest et al. (2010) | |
| Compound | Proteaceae | Proteoideae | Leucospermum | Southern Africa, shrublands | Lamont (1983b) | |
| Compound | Proteaceae | Grevilleoideae | Banksia | Australia, shrublands to forests | Purnell (1960) | |
| Simple | Fabaceae | Faboideae | Viminaria | Southern Australia, shrubby wetlands | Lamont (1972a) | |
| Simple | Fabaceae | Faboideae | Aspalathus | Southern Africa shrublands | Allsopp and Stock (1993) | |
| Simple | Fabaceae | Faboideae | Lupinus | Europe, shrublands to woodlands | Crocker and Schwintzer (1993) | |
| Simple | Fabaceae | Faboideae | Kennedia | Australia, shrublands to forests | Trinick (1977) | |
| Simple | Fabaceae | Mimosoideae | Acacia | Australia, shrublands to forests | Sward (1978) | |
| Simple | Myricaceae | Myricoideae | Myrica | Eurasia/North America, shrubby wetlands | Louis et al. (1990) | |
| Simple | Casuarinaceae | None | Casuarina | Australia, woodlands to forests | Reddell et al. (1997) | |
| Simple | Cucurbitaceae | Cucurbitoideae | Cucurbita | Mexico/Florida, coastal wetlands | Waters and Blevins (2000) | |
| Simple | Elaeagnaceae | None | Hippophae | Eurasia, coastal to mountain woodlands | Skene (1998) | |
| Simple | Betulaceae | Betuloideae | Alnus | Eurasia/North America, riverine or shrublands | Hurd and Schwintzer (1996) | |
| Simple | Moraceae | Ficoideae | Ficus | Myanmar to North Australia, rain/monsoon forest | Rosenfield et al. (1991) | |
| Simple | Pittosporaceae | Pittosporoideae | Marianthus | Australia, woodlands to forests | (Lamont, unpubl. data) | |
| Dauciform | ||||||
| Cyperaceae | Cyperoideae | Cyathochaeta | Southwestern Australia, wetlands | Lamont (1974) | ||
| Cyperaceae | Cyperoideae | Cladium | Cosmopolitan, wetlands, peat swamps | Lamont (1982) | ||
| Cyperaceae | Cyperoideae | Carex | Europe, moist lowland to upland heaths | Davies et al. (1973) | ||
| Cyperaceae | Schoenoideae | Schoenus | South Africa/New Zealand, wetlands | Lamont (1982) | ||
| Juncaceae | None | Juncus | New Zealand, wetlands | Powell (1973) | ||
| Capillaroid | ||||||
| Restionaceae | Restionoideae | Elegia | South Africa, wetlands/shrublands | Lamont (1982) | ||
| Restionaceae | Leptocarpoideae | Hypolaena | New Zealand/Southeastern Australia, wetlands | Campbell (1981) |
Additional information is provided in Lamont (1982), Skene (1998) and Lambers et al. (2006).
These special root structures share the following traits: (a) morphologically characterised by clusters of crowded rootlets, (b) occurrence at discrete locations along otherwise bare parent roots, (c) presence of a dense cover of root hairs, and (c) lack of endogenous microbial symbionts. At least some proteoid roots are also covered in mucigel (Dell et al. 1980; Lamont 2003), although these do not seem to be a feature of dauciform and capillaroid roots. Attempts by Brundrett and Abbott (1991) to inoculate kingia roots with arbuscular mycorrhizas failed and this confirms our observations of a lack of microbial symbionts within these roots. The references cited to accompany Table 3 show that similar, non-symbiotic clusters of roots densely covered in root hairs are widespread throughout the world’s nutrient-impoverished soils, especially if these are also seasonally waterlogged as is the case with some habitats of kingias.
Kingioid root clusters support the emerging rule that, as roots become thinner, these become hairier, individually shorter, the length per unit of total root mass increases (by definition) and the surface area increases exponentially with an almost negligible increase in root volume or mass (Table 1, Fig. 5). Area of contact with the rhizosphere soil increases markedly, especially in the presence of copious mucigel, and can be expected to enhance root function on three fronts: (a) exudation of organic and phenolic acids, sugar derivatives and enzymes, (b) enhanced activity of beneficial microbes, and (c) absorption of water and nutrients mobilised by these processes (Lamont 2003; Lambers et al. 2006). Despite this, the rootlets of kingioid roots are not as fine as the structures listed in Table 3 and these are therefore doubtfully as metabolically efficient. Nevertheless, their unique features and location in the soil profile may provide these roots with adaptive advantages yet to be explored. The abundance of root clusters in wetlands (sedges, restios, Viminaria, Hakea sulcata) needs further consideration, and may reflect the unsuitable conditions for symbiotic microbes in waterlogged soils and/or ease with which root hairs can form at the soil surface. Lamont (1976) showed how proteoid root production of hakeas is enhanced in waterlogged soils (low redox potential) despite severe reduction in growth of the remainder of the root system. The ancestry of kingias involving waterlogging is indicated by the abundance of aerenchyma in the primary prop roots (Fig. 6d), even among upland plants, only ceasing on reaching the root clusters.
Intriguingly, kingioid roots are not preferentially located in the high-humus part of the soil profile as with other root-cluster types (Lamont 2003) but peak at a depth of 45–75 cm (Fig. 3). (A reviewer pointed out that the deepening of colour (Munsell 7.5YR) from reddish-yellow (6/6) to brown (4/4) in this zone may well represent the effect of the additional humus created by decaying roots in this zone). This pattern is consistent with the lateral spread of parent roots through the profile of this species (Lamont 1981a). Therefore, the prior question may be why the parent roots concentrate in this region? The primary roots have a direct downward path when emerging from the caudex from which these later spread and subsequently stop production when approaching the water table or bedrock (Lamont 1981a), as here. The parent roots may not need a microbial stimulus to promote initiation, unlike proteoid roots (Lamont et al. 2015) or root nodules or mycorrhizas that obliges these roots to form in the microbially-rich humus layers.
The B horizon may still be a suitable source of exchangeable nutrients, such as P or K. Pate et al. (2001) showed how organic acids exuded by proteoid roots chelate Fe and Al phosphates in the A horizon where these are carried to, and subsequently deposited in, the B horizon. This is the reason why these lateritic soils are so orange in the B horizon, as are the embedded roots (Fig. 2). This horizon is therefore possibly a source of insoluble nutrients that can be accessed by the well-established solubilising powers of root clusters via organic acids and phosphatases (Lambers et al. 2006). The rhizosphere soil of current season roots averaged a pH of 6.2 compared with 6.7 around old roots, i.e. the rhizosphere of active roots is three times more acidic than moribund roots, supporting this possibility. This is confirmed by earlier work that showed that these clusters (undefined at the time) are highly efficient at absorbing (isotopically labelled) phosphate, as KH2PO4, injected between the leaf bases where this compound is translocated throughout the plant (Fig. 2, Lamont 1981b).
To further examine this possibility, nutrient data for soil profiles at five sites where kingias occur were collated from McArthur (1991). This showed that the availability of P and K in the B horizon (B1/12/22) is at least 50% that in the A horizon (A1/12) (Table 2). The B horizon is a relatively poor source of N (29%) and Ca (19%). Interestingly, Lamont (1981b) showed that available N in the leafbases was twice that in an equal volume of A1/12 soil beneath the plant, 4.2 times for exchangeable P and 7 times for available K. Perhaps these various sources of nutrients help to balance out the metabolic needs of the plant? Soil within the confines of the clusters is effective in the uptake of P (Shane et al. 2004). Rhizosphere soil is also a poor source of K, such that mass flow and diffusion processes would be required to overcome potential deficits. Cluster soil is a three times greater source of exchangeable Ca and Mg, and a surprisingly high repository of N altogether (31 g), according to our (preliminary) estimates.
Unlike proteoid and dauciform roots but as in root nodules, kingioid roots appear to be not only terminal to the parent lateral but also perennial. Clearly, a new cluster may develop from within the previous season’s cluster (Fig. 3e). Multiple clumps of rootlets cohering into a massive structure were sometimes observed. To what extent old rootlets remain metabolically active is less clear. The root hairs whither and the mucigel-colloid mix turns a powdery grey (Fig. 3b). However, these roots have been exposed to the air so clusters not so exposed may remain functional. At this depth in the profile, the soil is more likely to remain moist throughout the year and adds to possible reasons for their preferential formation in the B horizon, and explains their perenniality. Certainly, the abundant mucigel will serve to enhance water and nutrient uptake by maintaining contact between the rootlets and rhizosphere soil, especially as the soils dry out in summer.
The upper reaches of the soil in which grasstrees occur are ‘bone-dry’ over summer-autumn, yet leaf initiation continues unabated (Lamont 1981a; Korczynskyj and Lamont 2005). This implies that the soil water potential must be below the wilting point for active roots through summer-autumn at some critical depth in the B horizon. As the leafbases of kingias remain moist during summer, lateral and primary roots are also initiated at this time (Lamont 1981a). This is also possible in the soil as most clusters are located in the B horizon, at least part of which must remain sufficiently moist for leaf growth, and possibly even allow ‘hydraulic lift’ into the root sheath to explain how this region remains sufficiently moist for root growth during the drought season. At one kingia site, the water potential was 0.02 MPa at a depth of 50 cm (the deepest tested and where clusters may be abundant according to Fig. 4) in early summer (Lamont 1981b) that supports the proposition described above. Assuming a soil-water availability of 15%, the rhizosphere soil within the total sum of clusters of a 3.7 m tall plant is able to hold 8.66 L of water according to our estimates (Table 2). This seems to be a substantial reservoir, even without replenishment from the surrounding soil as uptake occurs. Even if only part of this is available over summer-autumn, these root clusters appear to be key to sustained growth during the dry season.
The clusters are perennial, and the rootlets are notably rather thicker than proteoid roots; therefore, a colleague suggested that these roots may play a key role in water storage. However, their morphological characterisitcs are the antithesis of root tubers that young kingias do produce (Lamont 1981a). These clearly serve to store water (and nutrients) during the growth stage during which the plants do not have access to soil water over summer–autumn. These tubers are lost as the plant matures, indicating that a water-storage function is no longer required. Similar calculations to those conducted for the nutrients show that the total sum of clusters is able to store 4.77 L of available (utilisable) water. This contributes only 6.0% of the volume of water in the entire plant; therefore water storage can be dismissed as a key function of these clusters.
The calculated values are preliminary and only refer to a particular set of circumstances so lack any range or probability dimensions. However, while these appear to be in the correct order of magnitude, detailed studies focusing on each of the possible functions are required. Notably, 22 years passed before the functions of proteoid roots began to be elucidated (Gardner et al. 1982). We would welcome interest from other researchers in ‘taking up the reins’ to further examine the anatomy, morphogenesis, causal initiation, and microbiological, ecological and physiological characteristics of these fascinating structures, the further pursuit of which is beyond our expertise and capabilities.
Data availability
All data used for analysis are provided here (Table 1) or can be provided by B. B. Lamont (Figs 2, 3, 6). An early version of this manuscript was published as a preprint entitled ‘Abundance of root clusters in Kingia australis (Dasypogonaceae) highlights widespread non-symbiotic adaptations to nutrient-impoverished soils’ that is available through the link: https://www.researchsquare.com/article/rs-5090992/v1.
Author contributions
A. Weinert initiated this project, all authors participated in field and laboratory work, and B. B. Lamont undertook all literature reviews, mathematical analyses, figure and table preparation, and wrote the first draft of the manuscript. All authors approved the submitted manuscript.
Acknowledgements
We thank the Department of Biodiversity, Conservation and Attractions in Western Australia for permission to collect specimens from the Whicher Range National Park, Richard Clark for field assistance, anonymous reviewers for useful comments, Wesley Lamont for technical assistance, and Hans Lambers and Mark Brundrett (University of Western Australia) for ‘devil’s advocate’ comments needed at this early stage in the scientific exploration of these fascinating roots.
References
Allsopp N, Stock WD (1993) Mycorrhizal status of plants growing in the Cape Floristic Region, South Africa. Bothalia 23(1), 91-104.
| Crossref | Google Scholar |
Brundrett MC (2021) One biodiversity hotspot to rule them all: Southwestern Australia – an extraordinary evolutionary centre for plant functional and taxonomic diversity. Journal of the Royal Society of Western Australia 104, 91-122.
| Crossref | Google Scholar |
Brundrett MC, Abbott LK (1991) Roots of jarrah forest plants. I. Mycorrhizal associations of shrubs and herbaceous plants. Australian Journal of Botany 39(5), 445-457.
| Crossref | Google Scholar |
Crocker LJ, Schwintzer CR (1993) Factors affecting formation of cluster roots in Myrica gale seedlings in water culture. Plant and Soil 152, 287-298.
| Crossref | Google Scholar |
Davies J, Briarty LG, Rieley JO (1973) Observations on the swollen lateral roots of the Cyperaceae. New Phytologist 72, 167-174.
| Google Scholar |
Dell B, Kuo J, Thomson GJ (1980) Development of proteoid roots in Hakea obliqua R.Br. (Proteaceae) grown in water culture. Australian Journal of Botany 28(1), 27-37.
| Crossref | Google Scholar |
Gardner WK, Parbery DG, Barber DA (1982) The acquisition of phosphorus by Lupinus albus L. I. Some characteristics of the soil/root interface. Plant and Soil 68, 19-32.
| Crossref | Google Scholar |
Givnish TJ, Zuluaga A, Spalink D, Soto Gomez M, Lam VKY, Saarela JM, Sass C, Iles WJD, De Sousa DL, Leebens-Mack J, Chris Pires J, Zomlefer WB, Gandolfo MA, Davis JI, Stevenson DW, De Pamphilis C, Specht CD, Graham SW, Barrett CF, Ané C (2018) Monocot plastid phylogenomics, timeline, net rates of species diversification, the power of multi-gene analyses, and a functional model for the origin of monocots. American Journal of Botany 105(11), 1888-1910.
| Crossref | Google Scholar | PubMed |
He T, Lamont BB, Pausas JG (2019) Fire as a key driver of Earth’s biodiversity. Biological Reviews 94, 983-2010.
| Crossref | Google Scholar |
Hurd TM, Schwintzer CR (1996) Formation of cluster roots in Alnus incana ssp. rugosa and other Alnus species. Canadian Journal of Botany 74(11), 1684-1686.
| Crossref | Google Scholar |
Korczynskyj D, Lamont BB (2005) Grasstree (Xanthorrhoea preissii) leaf growth in relation to season and water availability. Austral Ecology 30(7), 765-774.
| Crossref | Google Scholar |
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98(4), 693-713.
| Crossref | Google Scholar | PubMed |
Lambers H, Ahmedi I, Berkowitz O, Dunne C, Finnegan PM, Hardy GESJ, Jost R, Laliberté E, Pearse SJ, Teste FP (2013) Phosphorus nutrition of phosphorus-sensitive Australian native plants: threats to plant communities in a global biodiversity hotspot. Conservation Physiology 1(1), cot010.
| Crossref | Google Scholar |
Lamont B (1972a) ‘Proteoid’ roots in the legume Viminaria juncea. Search 3, 90-91.
| Google Scholar |
Lamont B (1972b) The morphology and anatomy of proteoid roots in the genus Hakea. Australian Journal of Botany 20(2), 155-174.
| Crossref | Google Scholar |
Lamont B (1974) The biology of dauciform roots in the sedge Cyathochaete avenacea. New Phytologist 73(5), 985-996.
| Crossref | Google Scholar |
Lamont B (1976) The effects of seasonality and waterlogging on the root systems of a number of Hakea species. Australian Journal of Botany 24(6), 691-702.
| Crossref | Google Scholar |
Lamont BB (1980) Tissue longevity of the arborescent monocotyledon, Kingia australis (Xanthorrhoeaceae). American Journal of Botany 67(8), 1262-1264.
| Crossref | Google Scholar |
Lamont B (1981a) Morphometrics of the aerial roots of Kingia australis (Liliales). Australian Journal of Botany 29(1), 81-25.
| Crossref | Google Scholar |
Lamont B (1981b) Availability of water and inorganic nutrients in the persistent leaf bases of the grasstree Kingia australis, and uptake and translocation of labelled phosphate by the embedded aerial roots. Physiologia Plantarum 52, 181-186.
| Crossref | Google Scholar |
Lamont B (1982) Mechanisms for enhancing nutrient uptake in plants, with particular reference to mediterranean South Africa and Western Australia. The Botanical Review 48, 597-689.
| Crossref | Google Scholar |
Lamont B (1983a) Root hair dimensions and surface/volume/weight ratios of roots with the aid of scanning electron microscopy. Plant and Soil 74, 149-152.
| Crossref | Google Scholar |
Lamont B (1983b) Proteoid roots in the South African Proteaceae. South African Journal of Botany 49(2), 103-123.
| Crossref | Google Scholar |
Lamont BB (1995) Testing the effect of ecosystem composition/structure on its functioning. Oikos 74(2), 283-295.
| Crossref | Google Scholar |
Lamont BB (2003) Structure, ecology and physiology of root clusters – a review. Plant and Soil 248, 1-19.
| Google Scholar |
Lamont BB (2024) The species richness–resource availability relationship is hump-shaped. Perspectives in Plant Ecology, Evolution and Systematics 65, 125824.
| Crossref | Google Scholar |
Lamont BB, Downes S (1979) The Longevity, flowering and fire history of the grasstrees Xanthorrhoea preissii and Kingia australis. The Journal of Applied Ecology 16(3), 893-899.
| Crossref | Google Scholar |
Lamont BB, Lamont HC (2000) Utilizable water in leaves of eight arid species as derived from pressure-volume curves and chlorophyll fluorescence. Physiologia Plantarum 110, 64-71.
| Google Scholar |
Lamont BB, Pérez-Fernández M, Rodríguez-Sánchez J (2015) Soil bacteria hold the key to root cluster formation. New Phytologist 206(3), 1156-1162.
| Crossref | Google Scholar | PubMed |
Louis I, Racette S, Torrey JG (1990) Occurrence of cluster roots on Myrica cerifera L. (Myricaceae) in water culture in relation to phosphorus nutrition. New Phytologist 115(2), 311-317.
| Crossref | Google Scholar | PubMed |
Osborne CP (2008) Atmosphere, ecology and evolution: what drove the Miocene expansion of C4 grasslands? Journal of Ecology 96(1), 35-45.
| Crossref | Google Scholar | PubMed |
Pate JS, Verboom WH, Galloway PD (2001) Co-occurrence of Proteaceae, laterite and related oligotrophic soils: coincidental associations or causative inter-relationships? Australian Journal of Botany 49(5), 529-560.
| Crossref | Google Scholar |
Purnell HM (1960) Studies of the family Proteaceae. I. Anatomy and morphology of the roots of some Victorian species. Australian Journal of Botany 8(1), 38-50.
| Crossref | Google Scholar |
Ratliff LF, Ritchie JT, Cassel DK (1983) Field-measured limits of soil water availability as related to laboratory-measured properties. Soil Science Society of America Journal 47(4), 770-775.
| Crossref | Google Scholar |
Reddell P, Yun Y, Shipton WA (1997) Cluster roots and mycorrhizae in Casuarina cunninghamiana: their occurrence and formation in relation to phosphorus supply. Australian Journal of Botany 45(1), 41-51.
| Crossref | Google Scholar |
Rosenfield C-L, Reed DW, Kent MW (1991) Dependency of iron reduction on development of a unique root morphology in Ficus benjamina L. Plant Physiology 95(4), 1120-1124.
| Crossref | Google Scholar | PubMed |
Shane MW, Cramer MD, Funayama-Noguchi S, Cawthray GR, Millar AH, Day DA, Lambers H (2004) Developmental physiology of cluster-root carboxylate synthesis and exudation in harsh hakea. Expression of phosphoenolpyruvate carboxylase and the alternative oxidase. Plant Physiology 135(1), 549-560.
| Google Scholar |
Skene KR (1998) Cluster roots: some ecological considerations. Journal of Ecology 86(6), 1060-1064.
| Crossref | Google Scholar |
Tomlinson PB, Zimmermann MH (1969) Vascular anatomy of monocotyledons with secondary growth – an introduction. Journal of the Arnold Arboretum 50(2), 159-179.
| Crossref | Google Scholar |
Trinick MJ (1977) Vesicular-arbuscular infection and soil phosphorus utilization in Lupinus spp. New Phytologist 78(2), 297-304.
| Crossref | Google Scholar |
Waters BM, Blevins DG (2000) Ethylene production, cluster root formation, and localization of iron(III) reducing capacity in Fe deficient squash roots. Plant and Soil 225, 21-31.
| Crossref | Google Scholar |
Zeri M, Alvalá RCS, Carneiro R, Cunha-Zeri G, Costa JM, Rossato Spatafora L, Urbano D, Vall-Llossera M, Marengo J (2018) Tools for communicating agricultural drought over the Brazilian Semiarid using the soil moisture index. Water 10(10), 1421.
| Crossref | Google Scholar |
Zúñiga-Feest A, Delgado M, Alberdi M (2010) The effect of phosphorus on growth and cluster-root formation in the Chilean Proteaceae: Embothrium coccineum (R. et J. Forst.). Plant and Soil 334, 113-121.
| Crossref | Google Scholar |


