Functional traits in arborescent Cactaceae: a guideline for their measurement
Walter F. Paredes Cubas A , Kyle G. Dexter B C , Carlos Reynel Rodríguez D , R. Toby Pennington C E and José Luis Marcelo Peña A *
A , Kyle G. Dexter B C , Carlos Reynel Rodríguez D , R. Toby Pennington C E and José Luis Marcelo Peña A *
A
B
C
D
E
Abstract
The need to understand the impacts of global change on ecosystems has driven interest in studying functional traits, which represent morphological, physiological, or phenological adaptations that determine the ecological performance of organisms. Although standardized methods exist for assessing functional traits in woody and herbaceous plants, protocols for arborescent cacti are still scarce. Cactaceae is a tropical American plant family that reaches high abundance in tropical dry ecosystems and encompasses a great diversity of form and size. Cacti perform fundamental ecosystem functions, are on the list of the most endangered plants globally and represent economically-impactful invasive species outside of the Americas. Here, we propose protocols to measure 12 functional traits in cacti, which are grouped into structural (two traits), morphological (seven traits), hydraulic–mechanical (two traits) and biophysical (one trait) categories, so as to complement ecological studies of plants and improve the understanding of their life cycle and the main environmental challenges faced by cacti.
Keywords: arborescent cacti, biophysical trait, ecological performance, functional traits, hydraulic–mechanical traits, morphological traits, protocol, standardized methods, structural traits, tropical dry ecosystems.
Introduction
The morphological, physiological and phenological characteristics that modulate the survival, growth and reproduction of plants are known as functional traits (Garnier et al. 2016; Nock et al. 2016). Within an ecosystem, functional traits are indicators of the adaptations that plants have to manage and use resources (Leps et al. 2006).
Anthropogenic impacts on ecosystems are evident through changes in the composition and functional diversity of plants, which include the value, type and relative abundance of the functional characteristics of the plant species that make up an ecosystem (Díaz et al. 2007). Research on functional attributes that reflect the ecological capacity of plants is motivated by the growing interest in understanding and predicting the impacts on ecosystems of global change scenarios (Kattge et al. 2011; Matesanz and Valladares 2014; Vela Zevallos 2019). However, the protocols disseminated for the study of plant functional traits have been mainly focused on the study of herbs and woody plants (Garnier et al. 2001; Cornelissen et al. 2003; Pérez-Harguindeguy et al. 2013; Salgado Negret 2015). Despite their ecological importance in many ecosystems, the development of a methodology for the study of cacti has been neglected, which, because of their morphological adaptations, require different protocols.
The Cactaceae family is native to the Americas and comprises approximately 1400 species, including succulents and non-succulents (Oldfield and Hunt 2010; Guerrero et al. 2019). The species in this family are representative of dry environments and are broadly distributed. In the north, they reach the provinces of British Columbia and Alberta in Canada; in the south, Argentine Patagonia; in the west, the Galapagos Islands of Ecuador; and in the east, Fernando de Noronha Island in Brazil (Powell and Weedin 2004; Ostolaza 2014; Prisa 2022). Cactaceae family is over-represented in the list of the most threatened plant species, with 31% of its species in danger of extinction (ranked 5th for plant families; Goettsch et al. 2015). This is due to increasing anthropogenic pressure in arid areas, in addition to limited distribution, characterized by high levels of endemism, and biological factors such as slow growth. Added to this is illegal harvesting for ornamental purposes, driven by the growing interest of succulent plant collectors, which increases its vulnerability.
Species of Cactaceae have important evolutionary modifications that allow them to survive in the limiting conditions of dry environments. The following characteristics stand out: the development of succulent tissue to store water, the anatomical capacity to swell and contract with water without damaging the epidermis, and the replacement of leaves with thorns that control water loss through transpiration (Fleming and Valiente 2002). In most Cactaceae except for the genus Pereskia, photosynthetic activity is performed by the stems, which remain green because of the absence of external bark (Schwertner-Charão et al. 2023).
Succulent cacti perform fundamental ecological functions that benefit other elements of biodiversity in dry, semi-arid and arid biomes. The succulence of their stems offers a source of water for other organisms, allowing those organisms to survive long periods of drought (Orr et al. 2015); the nectar of their flowers is an important food source for hummingbirds, insects and bats that also serve as pollinators (Nayelli Rivera Villanueva and Quirino 2020); and their fruits are a vital resource for organisms. The thorny structure of their stems not only protects the integrity of the plant, but also leads them to be chosen by some birds to establish their nests during the breeding season, because they make access difficult for predators.
Cacti are characterized by a high diversity of shapes and sizes. They may be arborescent, shrubby or creeping; they range from the smallest cacti such as species of the genus Frailea that reach a height of 5 cm to giant species such as Carnegiea gigantea (the ‘Mexican Saguaro’) that reaches up to 16 m in height (Anderson 2001).
In recent years, the need for standardized methods for the assessment of ecologically significant functional traits has increased (Pérez-Harguindeguy et al. 2013; Salgado Negret 2015; Van Cleemput et al. 2019). However, there is a notable lack of studies dedicated to the functional characterization of cacti, especially in seasonally dry biomes. In this context, a specific protocol for the study of functional traits in arborescent cacti is proposed here. Integrating ecosystem monitoring and measuring the functional traits of constituent taxa provides a more complete view of the functional changes that ecosystems experience over time (Esquivel-Muelbert et al. 2019). Given that seasonally dry ecosystems are highly fragile and subject to increasing anthropogenic pressure (Perea 2005; Marcelo-Peña et al. 2016; Linares-Palomino et al. 2022), monitoring their status becomes particularly urgent. A key approach to this end is permanent plot inventories, which often include recording of arborescent cacti (Moonlight et al. 2020). Integrating functional trait assessment into these inventories would allow for a more precise understanding of the response of species and the ecosystem as a whole to short-, medium-, and long-term environmental pressures, in addition to providing relevant information for the ecology, forestry, and conservation of tropical dry forests.
Evaluation of functional traits
Species selection
Species selection will depend on the research objective. If the focus is at the ecosystem level, we recommend prioritizing the most frequent or abundant species in the study area, which represent between 70% and 80% of the individuals or total biomass (Cornelissen et al. 2003; Pérez-Harguindeguy et al. 2013). However, in studies aim at detecting functional relationships or maximizing trait diversity, it is preferable to select species that broaden functional variability, regardless of their abundance, so as to increase the statistical power of the analyses (Garnier et al. 2001; Díaz et al. 2007). In these cases, it is relevant to include both dominant species and species of functional or phylogenetic interest. Likewise, the expected stability or variability of traits in different environments should be considered, because some traits require measurements under multiple conditions to adequately capture their range of variation (Garnier et al. 2016).
Selection of individuals within species and number of samples
Traits should be measured in fully developed, reproductive, and healthy-appearing individuals. Preferably they should be fully exposed to sunlight. Cacti that are attacked by herbivores or infested by pathogens should be excluded from study.
The number of individuals to study varies depending on the trait to be evaluated. For ecosystem-level research, we suggest at least 5–10 replicates. In contrast, if the research focuses on intraspecific variability, more individuals should be included (Cornelissen et al. 2003; Pérez-Harguindeguy et al. 2013).
This number of replicates is based on our experience in the tropical dry forest of northern Peru between 2021 and 2023. After evaluating functional traits in cacti and observing distribution patterns in their vegetative structures, we determined that five replicates are required for homogeneous traits (coefficient of variation <10% for quantitative traits) and 10 for those with greater intraspecific variability (traits such as spine length, stem density, water volume, among others). Furthermore, following the approach proposed by Wright et al. (2005), we recognize that sample size can influence the precision of correlation estimates between functional traits. In particular, small sample sizes tend to increase the variability of observed correlations, whereas larger sizes allow for more robust estimates. Thus, our minimum replication protocol also seeks to optimize the reliability of ecological inferences based on functional traits.
Functional traits to evaluate in arborescent cacti
We recommend evaluating as many traits as possible in a single individual because several functional traits exhibit allometric relationships and measuring them in the same organism maintains biological coherence among them (Portelli et al. 2023). Likewise, in research that is conducted in permanent plots and that considers the measurement of characters by collecting samples (e.g. cutting branches), individuals should be selected outside the plots to avoid possible alterations of natural growth.
This protocol proposes 12 characters, representing structural, morphological, hydraulic/mechanical, and biophysical variation (Table 1). Together, these characterize the life cycle of arborescent cacti and the challenges involved in acquiring resources, regeneration and responses to changes in the environment.
| Trait type | Traits | Unit | Obtaining the trait | Function | |
|---|---|---|---|---|---|
| Structural | Maximum vegetative height (MVH) | m | Field | Ability to compete for light, hydraulic limitation and longevity of the species. This can also be expressed relative to maximum diameter. | |
| Diameter of the largest stem (DLS) | cm | Field | Stability and support of the aerial part. Also, related to the transport or storage capacity of water and nutrients. | ||
| Morphological | Number of areoles (NA) | Number per cm2 | Field | Main survival mechanism, they group the spines strategically. | |
| Distance between areoles (DA) | cm | Field | |||
| Number of spines per areole (NSA) | Number | Field | Related to the protection of the stem against herbivory. | ||
| Spine length (SL) and basal diameter (SBD) | cm | Field/laboratory | Limit the damage of the sun rays and capture moisture from the environment. | ||
| Number of ribs (NR) | Number | Field | They swell with water in times of rain, preventing the epidermis from breaking. | ||
| Number of branches (NB) | Number | Field | They increase the photosynthetic surface and the capacity to store water. | ||
| Height of the first branch (HFB) | m | Field | Responds to environmental conditions and stress as a result of human damage or herbivory. | ||
| Hydraulic–mechanical | Stem specific density (SSD) | g per cm3 | Laboratory | Associated with the resistance of the trunk to natural enemies, mechanical damage and drought. | |
| Water volume (WV) | mL per cm3 | Laboratory | Ability to survive periods with high temperatures. | ||
| Biophysics | Surface area to volume ratio (S:V) | per cm | Field/laboratory | Influences transpiration, photosynthesis and the volume of water stored in cacti. |
Structural features
This measure corresponds to the vertical distance from the point of emergence of the main stem from the ground to the vegetative apex, excluding reproductive structures (Wenk et al. 2024), of the tallest recorded individual of a species in its adult or mature state. Maximum height is related to the plant’s position in the vertical light gradient, competitive vigor, seed dispersal distance, longevity, and survival capacity in the face of disturbance events (Falster and Westoby 2003).
The height of arborescent cacti can be estimated using different methods, depending on field conditions and the size of the individuals. Optical tools (such as the Suunto clinometer and Leiss hypsometer), instruments based on geometric principles (such as the Merritt and Christen hypsometers), electronic devices (laser distance meters), and manual methods such as the telescopic pole and the similar triangles technique are used (Table 2).
| Method | Accuracy | Advantages | Limitations | Applicability in cacti | |
|---|---|---|---|---|---|
| Telescopic pole | High (<5 m) | Simple, inexpensive | Limited to shorter individuals | High for juveniles | |
| Optical clinometer (Suunto) | Medium | Portable, fast | Affected by angular errors | High | |
| Geometric hypsometer | Medium | Low cost | Requires training | Moderate | |
| Electronic distance meter (EDM) | High | Precise, fast | Expensive, error under intense light | High in open field | |
| Similar triangles method | Low | Very inexpensive | Lower precision, human error | Only for shorter individuals |
Each method has advantages and limitations depending on the morphological characteristics of the cacti; the presence of scattered branches and irregular columnar stems can affect visual precision when using clinometers or manual rulers. Electronic meters offer high accuracy but their effectiveness decreases with dense surfaces or under intense light conditions. Although less precise, the similar triangles method is useful in areas with difficult access or for individuals of smaller height (<5 m).
Vertical growth is related to the diameter of the stem in plants, so as to provide security to their structure and to avoid the phenomenon of elastic buckling, the most likely anomaly that the stem can suffer (Niklas 1994). Stems are extraordinary mechanical constructions and give trees the largest stature among the plants that inhabit our planet (Vargas-Silva 2019). In the case of cacti, the stems have specialized succulent tissues to store water that can be used in times of water scarcity; therefore, the diameter and height condition their structure to resist the load of the stored water and avoid buckling.
The diameter of the stem is measured in most forest inventories and serves to quantify other variables such as volume, basal area and biomass. (Malleux 1982). In the study of functional traits, it is important to identify the thickest stem diameter that a cactus individual can achieve.
Stem diameter is measured at a height of 1.30 m above ground level (Fig. 1). This measurement can be taken from the same individuals whose height has been recorded, as well as from other individuals observed in the field that present thicker stems.
Diagram showing the structures of the cactus and the point where the DLS is measured. In Figs 2, 3, the DLS corresponds to the stem identified with the number, because it is the thickest among the measured stems (≥5 cm at 1.30 m height), rather than those labeled with letters.
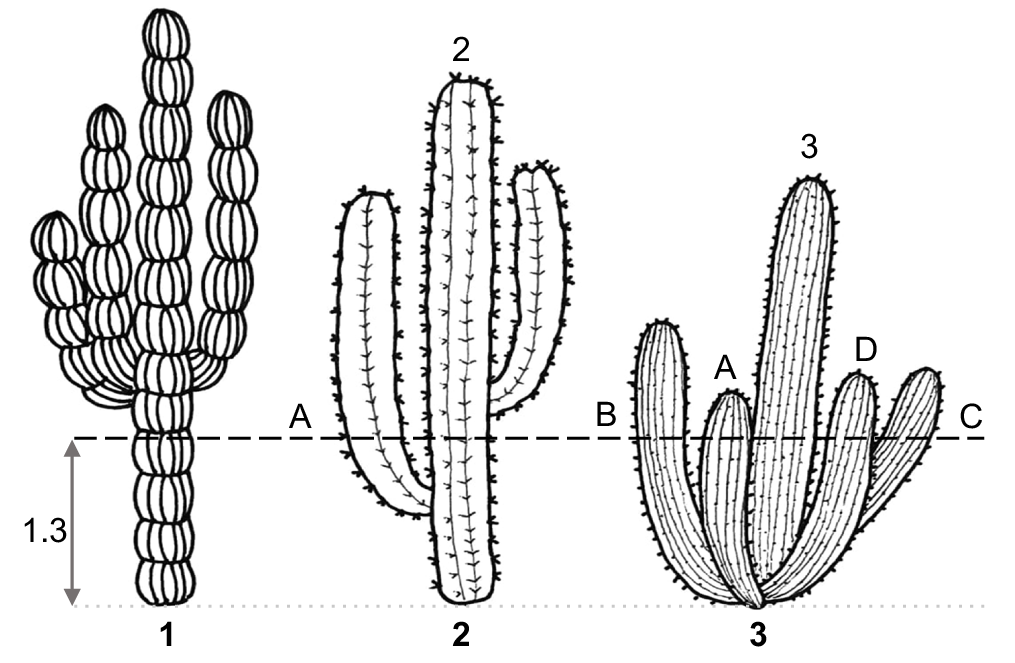
Because arborescent cacti commonly exhibit multiple branches or main stems, making it difficult to identify a single dominant axis, the diameter of all stems with a cross-sectional width of 5 cm or greater at 1.30 m height should be measured. Among these, the thickest stem is identified, and its diameter recorded as the DLS for that individual. This approach consistently captures the most structurally relevant value without causing confusion with terms such as ‘maximum DBH’ at the population or species level.
The following options are available to measure the diameter in cacti:
Use a diameter tape.
Use a vernier or caliper. If using these, we recommend taking the measurement at multiple points, at the same height, moving the calipers around the stem, and to then calculate the average.
We recommend using leather gloves or other material that is resistant to being punctured by spines.
The DLS is influenced by the hydration status of succulent tissue. For comparisons among species, sites, or campaigns, it is suggested that measurements should be taken during the rainy season or immediately after rainfall events, when stems are at their most hydrated, thus reducing variability induced by environmental factors.
For studies of intraspecific variation or functional plasticity in the face of water stress, sampling can also be conducted during the dry season, allowing for analysis of how this trait varies depending on water availability.
Morphological features
The areoles are cushion-shaped structures that are located on the ribs or the cladodes and have two meristems, one that is vegetative and produces new stems, spines, glochidia, bristles, hair, wool or felt and another that is reproductive and produces flowers (McAuliffe and Hendricks 1988; Ceroni and Castro 2014).
The development of spiny areoles was an important evolutionary change during the transition from woody and leafy ancestral species to plants with succulent stems (Altesor and Ezcurra 2003). Regarding the protection of the stem, they are responsible for organizing the development of the spines (Arroyo-Cosultchi et al. 2010); groups of spines are formed in the areole and offer a more effective defence than solitary spines (Gibson and Nobel 1986).
The areoles have a uniform distribution pattern around the stem, known as phyllotactic arrangement, which is strongly linked to the internal distribution of the vascular bundles (Gibson and Nobel 1986; Mauseth 2017) and therefore reflects how the tissues of the stem are arranged, which in turn support the cactus (Altesor and Ezcurra 2003). When the plant emits new development stimuli, these are received by the areole and the vascular bundles, the traces of the buds are strengthened with new cells that, in addition to improving their capacity to transport water and nutrients, also provide support to maintain a flower or a new branch (Schwager et al. 2015).
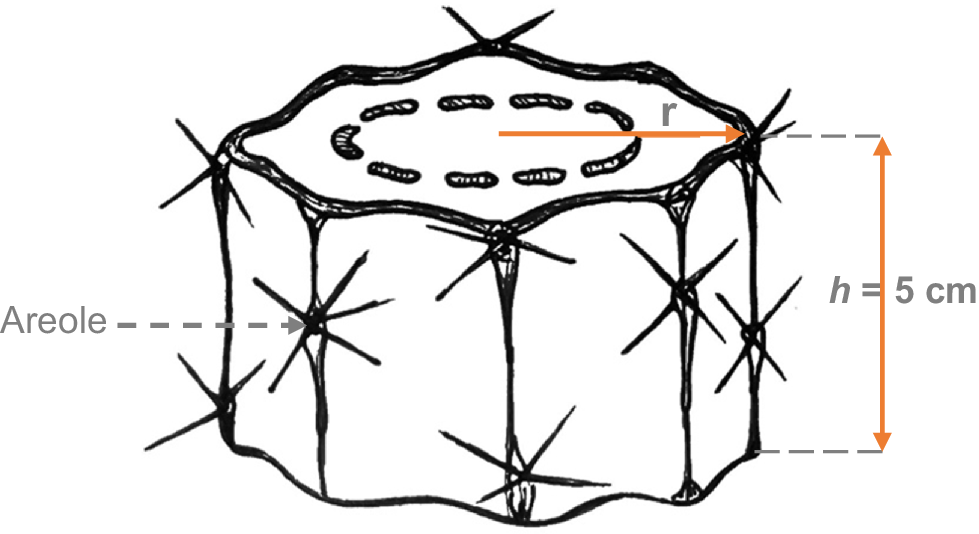
The number of areoles in the lateral area of a 5 cm high cylinder should be counted (Fig. 2) in at least five samples for each species. For this, the samples collected in the study of the hydraulic–mechanical features can be used.
Because the area of the curved surface = 2πrh, the units for NAR is number per cm2.
The number of areoles (NAR), expressed as density (number per cm2), is calculated by counting the areoles present on the lateral surface of a 5 cm tall cylindrical stem segment. The estimate is based on the surface area of the cylinder (A = 2πrh), so any variation in the stem radius will directly influence the area used to calculate this density. Because cactus stems have the ability to expand or contract in response to water status (particularly after rainfall or during periods of drought), the stem radius can fluctuate throughout the year, thus altering the calculated density without resulting in an actual change in the number of areoles. Therefore, we recommended recording the stem radius at the time of measurement or to take measurements under similar degree of hydration for each individual and species. Alternatively, this variable can be complemented with the absolute number of areolas per unit length (e.g. 5 cm) as an additional indicator of the trait, regardless of stem geometry.
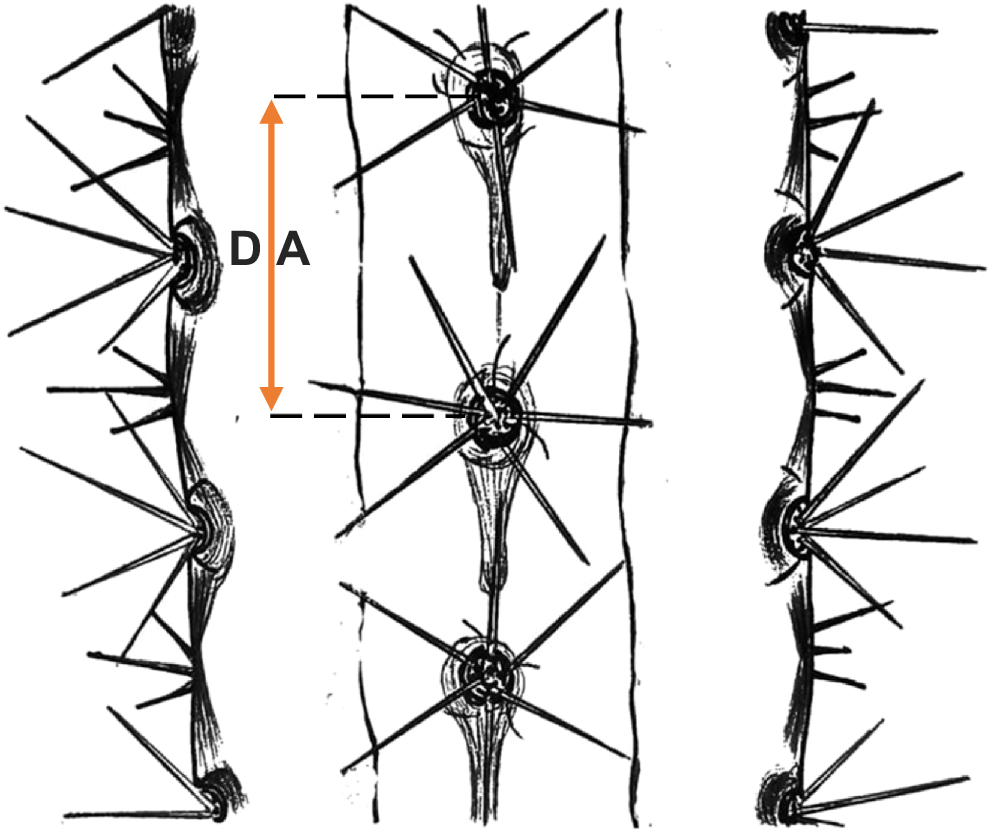
Distance between areoles can be related to the length, number and arrangement of spines. Closely packed areoles predetermined by phyllotaxis result in a dense network of spines that uniformly surrounds the plant (Altesor and Ezcurra 2003). In contrast, a greater distance between the areoles could reflect greater length of the spines or less investment in defence or other benefits derived from areoles.
The distance between areoles can strengthen the functions attributed to the spines, because a greater density of areoles makes the regulation of extreme temperatures more effective, provides greater protection against herbivores and greater uptake of atmospheric water.
The distance between areoles is measured directly in the field with a metal ruler. The measurement should capture the distance separating an areole from its immediate predecessor in the vertical sequence (Fig. 3). Measurements should be made on mature individuals, at the diameter at breast height (DBH), so as to ensure comparability among specimens and to minimize variability related to apical or juvenile growth. Measurements should be taken from five individuals per species, obtaining five records per individual. Considering the relatively homogeneous distribution of areoles along the stem, the values obtained can be averaged for each individual and, subsequently, for each species, so as to generate a representative estimator of this functional trait.
The DA is determined by linear measurement between an areole and its immediate predecessor along the longitudinal axis of the stem, by using a metal ruler directly in the field. This trait represents a static morphological attribute, related to phyllotaxis, and with defined developmental patterns during primary growth. Because stem swelling or contraction in cacti occurs mainly in the radial direction (diameter), associated with variations in the water content of the aquifer parenchyma, the longitudinal dimension of the stem is not affected by this phenomenon. Consequently, the interareola distance is considered a stable morphological trait against seasonal fluctuations in water status and does not require adjustment for diameter variation. However, to ensure comparability among individuals and minimize variability associated with apical development or tissue maturity, measurements on mature individuals and at DBH are recommended.
Spines develop on the areoles and function as a protective shield on the surface of the stems (Gibson and Nobel 1986).
Cactus spines represent an evolutionary innovation; they are an adaptation of the leaves to conserve accumulated water and protect plants from herbivory. In this sense, spines are capable of acting as a protective barrier against attack by a wide variety of herbivores, from mammals to insects to mollusks (Aliscioni et al. 2021).
In addition, the spines also protect the succulent stems from damage that could be caused by the sun rays. Nobel (1994) determined that a dense cover of spines is capable of reducing the temperature of the chlorenchyma by up to 10°C, thereby increasing its chances of survival in hot environments.
Although spines are a natural defence for cactus stems, they become an adverse factor during fires (Thomas 1991). Mature spines, composed of dead tissue, act as fuel, intensifying the flames and decreasing the probability of survival.
Because of the presence of multiple spines, it can be difficult to quantify the number present on the main stem up to 1.30 m. Therefore, the number of spines contained in the areole should be counted, which should be selected on the main stem below 1.3 m height, with five in total being assayed for each individual of the sampled species.
Some species in their mature stage can lose their spines, leaving the areoles exposed and keep the spines only on the youngest branches, for example, Armatocereus rauhii. In this situation, spines will be recorded only when they are observed on the main stem below 1.30 m.
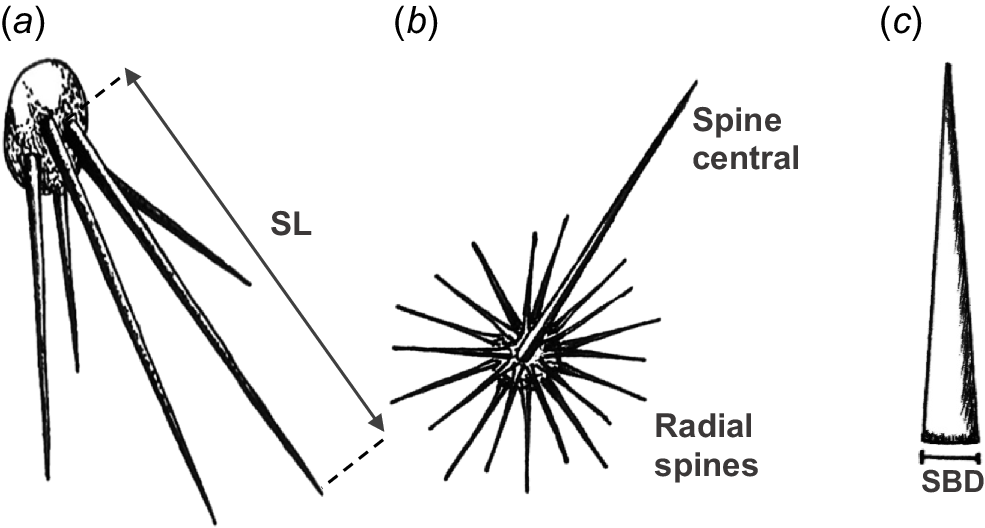
Spine length (SL) and spine basal diameter (SBD) measurements can be performed directly in the field or in the laboratory, and both traits are linked to important ecological functions. Cactus spines have been associated with the following three main functions: defence against herbivores, thermal regulation of the stem, and atmospheric water capture. Long spines cast shade over the stem surface, reducing its temperature and protecting it from direct solar radiation (Gibson and Nobel 1986). Cacti in warmer environments have been observed to develop longer spines (Drezner 2017), and these structures are also involved in water collection by fog condensation (Aliscioni et al. 2021).
In contrast, the SBD provides key information about their structural rigidity, which directly influences their effectiveness as a defence against herbivores. Spines with a larger basal diameter tend to be harder and more piercing, functioning as effective physical barriers (Pérez-Harguindeguy et al. 2013; Medina-Villar et al. 2022). In contrast, spines with small basal diameters are more flexible and are associated with functions such as solar radiation reflection or water capture. The inclusion of both parameters allows for a more comprehensive characterization of the functional role of spines in arborescent cacti.
The length and basal diameter of spines should be measured in five individuals per species. From each individual, at least five mature spines should be selected, preferably fully developed and representative of the plant. For length, a metal ruler or caliper should be used, measuring from the base to the tip of the spine. For basal diameter, the width of the spine at the point of insertion into the areola should be measured with a graduated ruler or caliper with millimeter precision.
When a marked difference is observed between central and radial spines, both should be measured and recorded separately. The data obtained can be averaged by individual and then by species to obtain representative estimates of both functional traits. (Fig. 4).
The ribs are edges that protrude from the stems and function similar to an accordion. In the rainy season, they have the ability to absorb a large amount of water without damaging the epidermis; likewise, in times of drought, the accumulated water is used and causes the contraction of the stems without affecting the epidermis (Ostolaza 2014; Pizarro 2014; Kuru et al. 2020).
The columnar shape of the stems faces the problem of vertical growth, associated with the loss of mechanical resistance caused by succulence. As a solution to this problem, natural selection gave rise to vascular bundles organized into long ribs. Any number of ribs proportional to the total height of the plant will fulfill the adaptive purpose of providing mechanical support (Altesor and Ezcurra 2003).
The dermal tissues of new shoots are considerably extensible, unlike mature tissues, so the total surface area in a region of the mature stem remains constant (Mauseth 2000). According to the above, in the case of cacti that tend to branch, the ribs of the stem can be related to mechanical support and the ribs of the branches to the volume of succulence. Selection for water storage favors a greater number of ribs (Cody 1984).
According to their branching patterns, cacti have different growth forms; if there is an absence of branching, it is a simple pillar; if the trunk is branched above the base, they are arborescent; and if the branching is at the base or they lack a main trunk, and the growth form is like that of a shrub or bush (Hernández-Hernández et al. 2011).
Branches in cacti allow them to increase their photosynthetic surface and their capacity to store water (Drezner 2014). Likewise, they are an evolutionary adaptation associated with competitive vigor; growth in height and surface area allows them to eclipse other plants (Cody 1984).
The abundance of branches allows for a greater number of flowers and fruits, increasing seed production and the survival of the species. In addition, these branches offer shelter to birds, reptiles and insects that find a safe place to nest, hide and feed (Drezner 2014).
Branching height is related to the dynamics of photosynthesis and water storage, as well as to the surface:volume ratio, which decreases as the plant grows (Delgado 2017).
The height at which the first branching occurs can be determined by different climates. In warm tropical environments, branching usually begins in the upper section of the plant, but in the warmer desert regions of mid-latitudes, branching tends to originate in the lower part of the stem (Cornejo and Simpson 1997).
The branching pattern can be modified by external factors such as wind, frost, human damage or herbivory (Zavala-Hurtado and Díaz-Solís 1995). These events can divert or break branches, forcing a plant to resprout from different points and to adapt its growth pattern.
The height of the first branch (HFB) is measured as the vertical distance from the base of the stem in contact with the ground to the point where the first branch attaches to the main axis of the plant. This measurement should be taken only on individuals with clearly defined branching.
For cacti whose first branch is accessible from the ground, use a tape measure, ensuring it is placed perpendicularly from the base of the stem to the base of the first branch. If the first branch is out of reach, use a forest hypsometer or equivalent device, pointing at the point where the branch joins the stem to accurately record the height.
The measurement should be expressed in centimeters (cm) and is recommended to be taken under adequate visibility to ensure accurate readings.
Hydraulic–mechanical traits
Stem specific density (SSD)
Stem specific density (SSD) is a trait that is measured in the laboratory and consists of dividing the dry weight of the sample by the fresh volume (g per cm3). According to Pérez-Harguindeguy et al. (2013), SSD is an essential functional character because of its relationship with important properties for the plant and the ecosystem. It contributes to structural stability, stem defence, hydraulic characteristics, carbon absorption and the potential growth of plants (Cornelissen et al. 2003).
In the context of functional ecology, stem density represents an integrated measure that reflects distinct adaptive strategies of plants. Low density is often associated with less costly tissues to construct, greater storage capacity, and hydraulic efficiency, whereas high density indicates greater investment in mechanical strength and structural tolerance to physical or biotic stresses (Pérez-Harguindeguy et al. 2013). These trade-offs are not exclusive to woody species but are also relevant in arborescent cacti, where succulent stems fulfill multiple functional roles. Additionally, Chave et al. (2009) highlighted wood density as a key functional trait within the economic spectrum of wood, which integrates structural, hydraulic, and storage properties. Although their focus is on woody species, their concepts are applicable to arborescent cacti, whose succulent stems simultaneously perform support, storage, and transport functions. Stem density in these systems therefore reflects a balance between hydraulic efficiency, biomechanical stability, and water storage capacity, and is a comprehensive indicator of plant functional performance under different ecological contexts.
In the particular case of cacti, the stems must accumulate large amounts of water and nutrients, and the mechanical support for these large masses falls on a limited number of vascular bundles arranged in a specific biomechanical distribution (Altesor et al. 1994); however, this evolutionary adaptation has generated physical costs; approximately 80% of mortality in adult cacti occurs because of falls driven by their own weight (Díaz-Maeda 1991).
In addition, stem density in combination with other quantitative variables such as height and diameter at breast height (DBH), serves to estimate carbon capture and storage (Nogueira et al. 2005; Lin et al. 2012).
Collections must be made from individuals that meet the conditions described in ‘Selection of individuals within species and number of samples’. It is advisable to work with cacti selected for morphological traits, five in total for each species and between two to three samples per individual. To minimize impact on the plant, accessible secondary branches or mature shoots emerging from the base should be cut. From each of these, a cylindrical section of stem approximately 5 cm long should be removed, suitable for volume and dry weight measurements required in the laboratory.
Immediately after sectioning the sample, it should be wrapped in moistened newspaper and placed inside clean or preferably new polyethylene bags, to then be sealed and properly labeled. It is important to transfer the samples to the laboratory as soon as possible, ideally on the same day as collection, to take the following measurements.
The laboratory work begins by determining the volume of the sample and Pérez-Harguindeguy et al. (2013) proposed two easy-to-execute procedures, which can be adapted to work with cacti.
Water displacement method. This procedure consists of using a beaker with an adequate amount of water in such a way that it allows the sample to be completely submerged without spilling. Place the glass of water on the scale and tare; a punch is inserted into the stem log and with the help of a universal support the sample is completely submerged in the water, avoiding touching the sides or bottom of the glass. Once the sample is submerged, the water level rises and the weight increases (weight of the displaced water). This value is equivalent to the volume of the sample (cm3) since the water has a density of 1 g per cm3. It is recommended to quickly read the weight recorded on the scale.
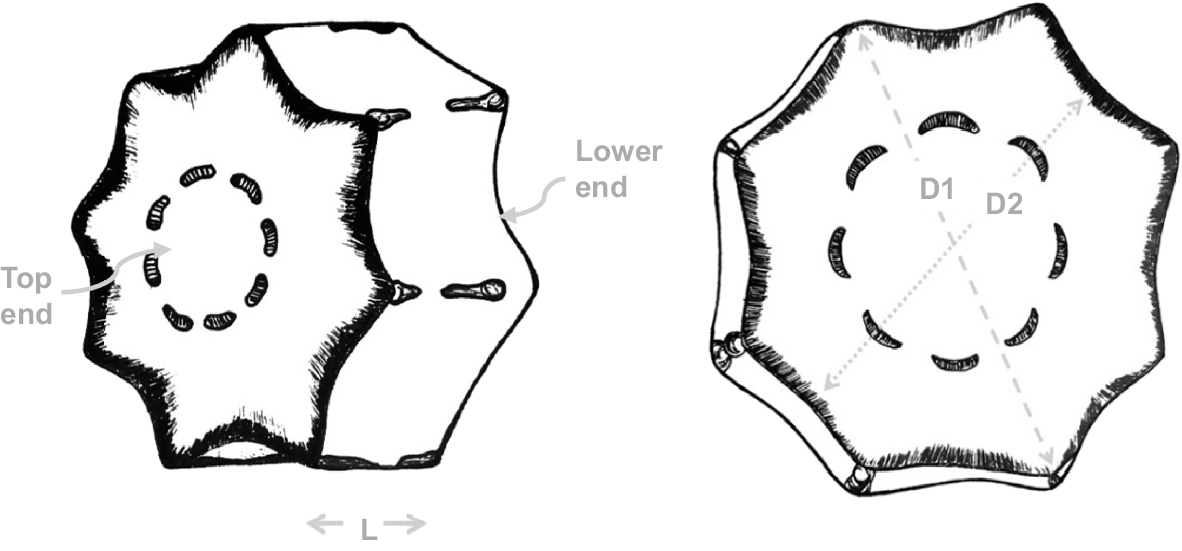
Dimensional method. The procedure consists of measuring the diameter (D) and length (L) of the sample with a vernier caliper. Because of the presence of ribs, four measurements must be made for the diameter, two measurements at the upper end of the sample, i.e. one measurement between the ends of the ribs (largest diameter, D1), the other between the slits (smallest diameter, D2); and two measurements at the lower end (Fig. 5). Four measurements must also be made for the length.
The recorded values will be averaged for D and L respectively, then the volume (V) can be calculated using the following formula:
Once the volume has been determined, the samples should be placed in the oven at 70°C until a constant weight is achieved. Starting on the third day, the first weight should be recorded, then they will be returned to the oven and weighed again every 12 h. The constant weight is usually achieved after 4–5 days of drying; at the time of weighing, the sample should be taken immediately to the scale, to prevent ambient humidity from influencing it.
In the case of evaluating the traits of species within permanent plots, sampling for density assessment should be performed on individuals located near but not within the plot, under the same health and lighting conditions established previously, with the purpose of not altering the natural conditions that long-term vegetation monitoring investigations require.
In the specific case of species with monopodial architecture, that is, those with a single main stem without evident lateral branches, cuts should not be made in the main axis of living individuals, because this contains the determining apical meristem, the removal of which would irreversibly compromise the organism’s viability. For these species, it is best to obtain samples exclusively from individuals that have died of natural causes, or, failing that, to work in populations outside the monitoring area, thus ensuring the integrity of the populations under study and the continuity of the ecological processes being evaluated.
The SSD, calculated as the ratio of dry weight to fresh volume (g per cm3), is directly influenced by the tissue water status. In cacti, stem shrinkage and swelling can modify its volume without altering its dry mass, thus affecting the SSD estimate. Therefore, this trait reflects both structural properties and physiological conditions at the time of sampling.
To ensure comparability across species, sites, or campaigns, it is recommended to measure under conditions of maximum hydration, such as during the rainy season. However, if the objective is to evaluate the response to water stress, sampling can also be undertaken during the dry season. In all cases, it is essential to record the phenological and climatic state, or estimate the tissue moisture content, to correctly interpret the results.
Water volume (WV)
Cacti are turgid, fleshy or juicy plants because of the water they accumulate. This property is known as succulence and is an evolutionary adaptation mechanism to resist prolonged periods of drought and survive in xeric environments. Responding to this need, they have modeled a shape similar to spheres or cylinders, geometric structures that cover a greater volume in a smaller surface area (Ostolaza 2014; Lee et al. 2019).
Succulence is achieved through the presence of an unspecialized parenchyma that accumulates water and other resources in the form of mucilage (Pizarro 2014). These resources, acquired under favorable conditions, ensure photosynthesis, growth, and reproduction during hot and dry periods; however, storage capacity varies according to the arborescent cactus species (Huber et al. 2018). Large species with solitary stems tend to develop greater aboveground biomass per individual, which translates into a significantly higher water storage capacity, up to five times greater, than in multi-stemmed species (Hultine et al. 2016).
Water volume should be calculated in the samples used to evaluate stem specific density. First the fresh weight of the sample should be obtained and then the constant dry weight should be subtracted from it (procedure explained in the density feature). On the basis of the property for the density of water (1 kg = 1 L), volume can be calculated from mass; the trait will be expressed in milliliters per square centimeter. It is very important to indicate the weather conditions or the season of the year in which the measure is obtained.
The volume of water present in succulent tissues varies significantly depending on the plant’s water status, which is directly influenced by the season and recent rainfall events. In arborescent cacti, the ability of the stem to swell allows for significant water storage during the wet season, whereas in the dry season, tissue volume gradually shrinks without compromising the integrity of the epidermis or tissue functionality. Therefore, estimating water volume should consider the phenological and climatic context at the time of collection, accurately recording whether the sample was obtained during the dry or wet season, or immediately after a rainfall event. Ideally, it is recommended to standardize sampling timing across sites, collecting samples during the peak rainy or dry season, depending on the study objectives, so as to maximize data comparability across species or locations.
Biophysical traits
Surface area to volume ratio (S:V)
In the functional characterization of plants, a widely used variable is specific leaf area (SLA), which is part of the leaf economic spectrum (LES), along with other traits such as water content, leaf thickness, and density (Wright et al. 2004). These traits are closely associated with physiological strategies for resource acquisition, photosynthetic efficiency, and tissue longevity. However, in arborescent cacti, characterized by the absence of true leaves, these functions are shifted to succulent stems, which act as photosynthetic, storage, and defence organs. Because of this specialization, the surface area:volume (S:V) ratio is proposed as an analogous metric to SLA, because it determines the efficiency of gas exchange, heat dissipation, and water retention capacity in photosynthetic stems (Williams et al. 2014).
In plants adapted to withstand drought seasons, the S:V ratio directly affects transpiration, photosynthetic activity and the ability to store water. A high S:V ratio can be advantageous for those species that develop in mostly humid habitats and with short periods without precipitation; on the contrary, those species that will endure extended periods of drought need high amounts of succulent tissue and a low S:V ratio (Mauseth 2000).
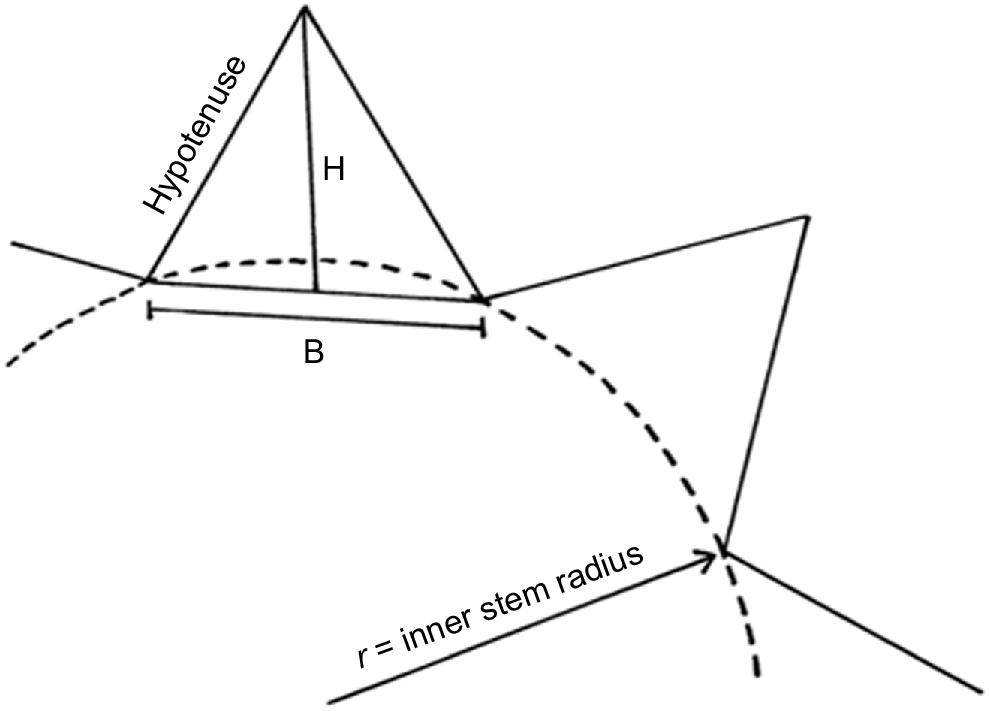
This should be measured from the cacti selected to measure stem specific density. The calculation should be performed by using the following formula established by Mauseth (2000):
where N = number of ribs, H = height of the right triangle that will be formed in a rib (Fig. 6), and r = radius of the inner circumference of the stem
Top view of a cross-section of the stem. H, height of rib; B, base of rib; r, inner radius of stem (adapted from Mauseth 2000).
Conclusions
This protocol was specifically designed for the evaluation of functional traits in arborescent cacti, based on studies conducted in permanent climate change monitoring plots in dry forests of northern Peru, where this growth habit is highly representative ecologically and structurally. However, it is recognized that the Cactaceae family exhibits a wide diversity of growth forms, including columnar, globose, and creeping, among others, that also fulfill key ecological functions in arid and semi-arid ecosystems. This morpho-functional diversity raises the need to adapt and complement the methods proposed here to achieve a more comprehensive characterization.
Whereas several of the traits included in this protocol can be applied non-destructively to small growth forms (such as the number and arrangement of areoles, spines, and ribs), other traits that involve tissue extraction require alternative methods or the development of strategies that avoid affecting the apical meristem, whose removal would compromise the viability of the individual.
The development of a protocol for the evaluation of functional traits in arborescent cacti represents a fundamental step toward a better understanding of the adaptive strategies of these species in arid and semi-arid environments. However, it is important to recognize that this protocol focuses on a specific set of structural, morphological, hydromechanical, and biophysical traits, ignoring other functional attributes that are also relevant in the comprehensive ecological study of cacti.
Among these, traits related to reproduction stand out, particularly those associated with seeds (such as size, mass, and viability) and seedlings (establishment rates, initial growth, and survival), which are essential for understanding natural regeneration processes and population dynamics. Similarly, aspects related to post-disturbance regrowth, vegetative growth capacity, and structural resilience to mechanical damage or loss of aboveground biomass are essential for evaluating the functional response of species to environmental or anthropogenic pressures.
Furthermore, non-destructive tools such as photosynthetic stem reflectance spectroscopy open up new methodological possibilities. The application of spectrometry to cacti would allow indirect inferences of biochemical and physiological traits related to photosynthetic efficiency, such as chlorophyll content, carboxylative capacity (Vc,max), or relative water content, from specific spectral signatures. These approaches, already tested in broadleaf species and diverse forests, could be adapted to the cactus context, considering their anatomical and functional particularities.
Consequently, the protocol proposed here could be expanded by progressively incorporating reproductive, regeneration, and photosynthetic efficiency traits, using both conventional methodologies and emerging technologies. This integrative effort will contribute to establishing a solid foundation for the functional study of cacti in diverse ecosystems, improving their representation within global trait ecology frameworks.
Data availability
The data supporting the findings of this study are available upon reasonable request from the corresponding author. These include trait measurements, methodological details, and any additional analyses generated during the study. Although no new datasets were formally archived in a public repository, the authors are committed to facilitating data sharing for academic and non-commercial purposes.
Author contributions
Walter Fachini Paredes Cubas, José Luis Marcelo Peña, and Kyle Dexter designed the methodology. All authors conceived ideas for the paper, and outlined and drafted early versions of the text. Walter Fachini Paredes Cubas performed data collection and figure preparation.
Acknowledgements
We extend our sincere thanks to members of the Seedbed of Research in Ecology and Restoration (SIERE) and the Biodiversity Research and Monitoring Group (GIMBIO), for support during the field data collection phase for the development of the protocol. Your dedication, enthusiasm, and professionalism have been the fundamental pillars for the success of this research. To project 147-2020-FONDECY, we express our deepest gratitude for the opportunity they gave us to participate in field expeditions to the Marañón Valley. These experiences allowed us to gain in-depth knowledge of the ecosystems studied and collect valuable data that served as a fundamental basis for the preparation of this manual.
References
Aliscioni NL, Delbón N, Gurvich DE (2021) Spine function in Cactaceae, a review. Journal of the Professional Association for Cactus Development 23, 1-11.
| Crossref | Google Scholar |
Altesor A, Ezcurra E (2003) Functional morphology and evolution of stem succulence in cacti. Journal of Arid Environments 53(4), 557-567.
| Crossref | Google Scholar |
Altesor A, Silva C, Ezcurra E (1994) Allometric neoteny and the evolution of succulence in cacti. Botanical Journal of the Linnean Society 114(3), 283-292.
| Crossref | Google Scholar |
Arroyo-Cosultchi G, Terrazas T, Arias S, López-Mata L (2010) Delimitación de Neobuxbaumia mezcalaensis y N. multiareolata (Cactaceae) con base en análisis multivariados. Boletín de la Sociedad Botánica de México 86, 53-64 [In Spanish].
| Google Scholar |
Ceroni AH, Castro V (2014) Manual de cactus: identificación y origen. Available at https://www.minam.gob.pe/diversidadbiologica/wp-content/uploads/sites/21/2014/02/manual+de+cactus.compressed.pdf [In Spanish]
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecology Letters 12, 351-366.
| Crossref | Google Scholar | PubMed |
Cody ML (1984) Branching patterns in columnar cacti. In ‘Being alive on land. Tasks for vegetation science’. (Eds NS Margaris, M Arianoustou-Faraggitaki, WC Oechel) vol. 13, pp. 201–236. (Springer: Dordrecht, Netherlands) 10.1007/978-94-009-6578-2_23
Cornejo DO, Simpson BB (1997) Analysis of form and function in North American columnar cacti (tribe Pachycereeae). American Journal of Botany 84(11), 1482-1501.
| Crossref | Google Scholar |
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51(4), 335-380.
| Crossref | Google Scholar |
Delgado M (2017) Edad, tasas de crecimiento y alometría del cardón (Pachycereus pringlei) en la península de Baja California. Tesis de doctorado, Centro de Investigaciones Biológicas del Noroeste, S.C. Available at http://cibnor.repositorioinstitucional.mx/jspui/handle/1001/864 [In Spanish]
Díaz S, Lavorel S, Chapin FS, Tecco PA, Gurvich DE, Grigulis K (2007) Functional Diversity at the crossroads between ecosystem functioning and environmental filters. In ‘Terrestrial ecosystems in a changing world. global change – The IGBP Series’. (Eds JG Canadell, DE Pataki, LF Pitelka) pp. 81–91 (Springer: Berlin, Heidelberg, Germany) 10.1007/978-3-540-32730-1_7
Díaz-Maeda PG (1991) Efectos dependientes de la densidad en una cactácea columnar (Neobuxbeumie tetetzo (Coulter) Backeberg) del valle de Zapotitlán de las Salinas, Puebla. Doctoral dissertation, Tesis licenciatura, Facultad De Ciencias, UNAM, México, DF. Available at https://tesiunamdocumentos.dgb.unam.mx/ptd2013/anteriores/0163733/0163733.pdf [In Spanish]
Drezner TD (2014) Regional branching relationships in Carnegiea gigantea, a keystone cactus. Western North American Naturalist 74(2), 155-161.
| Crossref | Google Scholar |
Drezner TD (2017) Shade, reproductive effort and growth of the endangered native cactus, Opuntia humifusa Raf. in Point Pelee National Park, Canada. The Journal of the Torrey Botanical Society 144(2), 179-190.
| Crossref | Google Scholar |
Esquivel-Muelbert A, Baker TR, Dexter KG, Lewis SL, Brienen RJ, Feldpausch TR, Lloyd J, Monteagudo-Mendoza A, Arroyo L, Álvarez-Dávila E, Higuchi N, Marimon B, Marimon-Junior B, Silveira M, et al. (2019) Compositional response of Amazon forests to climate change. Global Change Biology 25(1), 39-56.
| Crossref | Google Scholar | PubMed |
Falster DS, Westoby M (2003) Plant height and evolutionary games. Trends in Ecology & Evolution 18(7), 337-343.
| Crossref | Google Scholar |
Garnier E, Shipley B, Roumet C, Laurent G (2001) A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology 15(5), 688-695.
| Crossref | Google Scholar |
Goettsch B, Hilton-Taylor C, Cruz-Piñón G, et al. (2015) High proportion of cactus species threatened with extinction. Nature Plants 1(10), 15142.
| Crossref | Google Scholar |
Guerrero PC, Majure LC, Cornejo-Romero A, Hernández-Hernández T (2019) Phylogenetic relationships and evolutionary trends in the cactus family. Journal of Heredity 110(1), 4-21.
| Crossref | Google Scholar | PubMed |
Hernández-Hernández T, Hernández H, De-Nova A, Puente R, Eguiarte L, Magallón S (2011) Phylogenetic relationships and evolution of growth form in Cactaceae (Caryophyllales, Eudicotyledoneae). American Journal of Botany 98(1), 44-61.
| Crossref | Google Scholar |
Huber J, Dettman DL, Williams DG, Hultine KR (2018) Gas exchange characteristics of giant cacti species varying in stem morphology and life history strategy. American Journal of Botany 105(10), 1688-1702.
| Crossref | Google Scholar | PubMed |
Hultine KR, Williams DG, Dettman DL, Butterfield BJ, Puente-Martinez R (2016) Stable isotope physiology of stem succulents across a broad range of volume-to-surface area ratio. Oecologia 182, 679-690.
| Crossref | Google Scholar | PubMed |
Kattge J, Diaz S, Lavorel S, et al. (2011) TRY – a global database of plant traits. Global Change Biology 17(9), 2905-2935.
| Crossref | Google Scholar |
Kuru A, Oldfield P, Bonser S, Fiorito F (2020) A framework to achieve multifunctionality in biomimetic adaptive building skins. Buildings 10(7), 114.
| Crossref | Google Scholar |
Lee SJ, Ha N, Kim H (2019) Superhydrophilic–superhydrophobic water harvester inspired by wetting property of cactus stem. ACS Sustainable Chemistry & Engineering 7(12), 10561-10569.
| Crossref | Google Scholar |
Leps J, de Bello F, Lavorel S, Berman S (2006) Quantifying and interpreting functional diversity of natural communities: practical considerations matter. Preslia 78(4), 481-501.
| Google Scholar |
Lin D, Lai J, Muller-Landau HC, Mi X, Ma K (2012) Topographic variation in aboveground biomass in a subtropical evergreen broad-leaved forest in China. PLoS ONE 7(10), e48244.
| Crossref | Google Scholar | PubMed |
Linares-Palomino R, Huamantupa-Chuquimaco I, Padrón E, La Torre-Cuadros MDLÁ, Roncal-Rabanal M, Choquecota Castillo NM, Collazos Huamán JL, Elejalde Romero RE, Vergara Camarena N, Marcelo-Peña JL (2022) Los bosques estacionalmente secos del Perú: un re-análisis de sus patrones de diversidad y relaciones florísticas. Revista Peruana de Biología 29(4), e21613.
| Crossref | Google Scholar |
Marcelo-Peña JL, Huamantupa-Chuquimaco I, Särkinen T, Tomazello M (2016) Identifying conservation priority areas in the Marañón valley (Peru) based on floristic inventories. Edinburgh Journal of Botany 1(1), 1-29.
| Crossref | Google Scholar |
Matesanz S, Valladares F (2014) Ecological and evolutionary responses of Mediterranean plants to global change. Environmental and Experimental Botany 103, 53-67.
| Crossref | Google Scholar |
Mauseth JD (2000) Theoretical aspects of surface-to-volume ratios and water-storage capacities of succulent shoots. American Journal of Botany 87(8), 1107-1115.
| Crossref | Google Scholar | PubMed |
Mauseth JD (2017) An introduction to Cactus Areoles. Part I. Cactus and Succulent Journal 89(3), 128-134.
| Crossref | Google Scholar |
McAuliffe JR, Hendricks P (1988) Determinants of the vertical distributions of woodpecker nest cavities in the Sahuaro Cactus. The Condor 90(4), 791-801.
| Crossref | Google Scholar |
Medina-Villar S, Vázquez de Aldana BR, Herrero A, et al. (2022) The green thorns of Ulex europaeus play both defensive and photosynthetic roles: consequences for predictions of the enemy release hypothesis. Biol Invasions 24, 385-398.
| Crossref | Google Scholar |
Moonlight P, Banda K, Phillips OL, et al. (2020) ‘Manual DryFlor: protocolo para el establecimiento y monitoreo de parcelas de bosque seco.’ 1st edn. ForestPlots.net. Available at https://doi.org/10.5521/forestplots.net/2020_4b [In Spanish]
Nayelli Rivera Villanueva A, Quirino R (2020) Síndrome de quiropterofilia en cactus columnares. Desde El Herbario CICY 12, 149-153 Available at http://cicy.repositorioinstitucional.mx/jspui/handle/1003/2596 [In Spanish].
| Google Scholar |
Niklas KJ (1994) Interspecific allometries of critical buckling height and actual plant height. American Journal of Botany 81(10), 1275-1279.
| Crossref | Google Scholar |
Nock CA, Vogt RJ, Beisner BE (2016) Functional traits. In ‘Encyclopedia of Life Sciences (eLS)’. pp. 1–8. (John Wiley & Sons: Hoboken, NJ) 10.1002/9780470015902.a0026282
Nogueira EM, Nelson BW, Fearnside PM (2005) Wood density in dense forest in central Amazonia, Brazil. Forest Ecology and Management 208(1–3), 261-286.
| Crossref | Google Scholar |
Oldfield S, Hunt D (2010) The conservation of cacti and succulents in botanic gardens. BGjournal 7(1), 15-17 Available at https://www.jstor.org/stable/24811073.
| Google Scholar |
Orr TJ, Newsome SD, Wolf BO (2015) Cacti supply limited nutrients to a desert rodent community. Oecologia 178, 1045-1062.
| Crossref | Google Scholar | PubMed |
Ostolaza C (2014) ‘Todos los cactus del Perú.’ (Franco EIRL). Available at https://www.minam.gob.pe/diversidadbiologica/wpcontent/uploads/sites/21/2014/02/document.pdf[In Spanish]
Perea M (2005) Aspectos funcionales y biogeográficos en desiertos cálidos de América: Desierto Sonorense (México) y Desierto del Monte (Argentina). Tesis doctoral, Centro de Investigaciones Biológicas del Noreste, S.C. Available at https://cibnor.repositorioinstitucional.mx/jspui/bitstream/1001/1743/1/perea_m.pdf [In Spanish]
Pérez-Harguindeguy N, Díaz S, Garnier E, et al. (2013) New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61, 167-234.
| Crossref | Google Scholar |
Portelli AM, Windecker SM, Pollock LJ, Neal WC, Morris WK, Khot R, Vesk PA (2023) From mallees to mountain ash, specific leaf area is coordinated with eucalypt tree stature, resprouting, stem construction, and fruit size. Australian Journal of Botany 71, 506-522.
| Crossref | Google Scholar |
Prisa D (2022) Botany and uses of cacti. GSC Biological and Pharmaceutical Sciences 21(1), 287-297.
| Crossref | Google Scholar |
Salgado Negret B (2015) ‘La ecología funcional como aproximación al estudio, manejo y conservación de la biodiversidad: protocolos y aplicaciones.’ 1st edn. (Alexander Von Humboldt). Available at http://biblioteca.humboldt.org.co/es/boletines-y-comunicados/item/839-eco-funcional [In Spanish]
Schwager H, Neinhuis C, Mauseth JD (2015) Secondary growth of the leaf and bud traces in Hylocereus undatus (Cactaceae) during the formation of branches or flowers. International Journal of Plant Sciences 176, 762-769.
| Crossref | Google Scholar |
Schwertner-Charão L, Treviño-Carreón J, Delgado-Martínez R (2023) Las fascinantes adaptaciones de las cactáceas y su historia evolutiva. CIENCIA ergo-sum 30(2),.
| Crossref | Google Scholar |
Thomas PA (1991) Response of succulents to fire: a review. International Journal of Wildland Fire 1(1), 11-22.
| Crossref | Google Scholar |
Van Cleemput E, Roberts DA, Honnay O, Somers B (2019) A novel procedure for measuring functional traits of herbaceous species through field spectroscopy. Methods in Ecology and Evolution 10(8), 1332-1338.
| Crossref | Google Scholar |
Vargas-Silva G (2019) Biomecánica de los árboles: crecimiento, anatomía y morfología. Madera y Bosques 25(3), 1-18.
| Crossref | Google Scholar |
Vela Zevallos AW (2019) Rasgos funcionales asociados al servicio ecosistémico de mitigación del cambio climático en árboles de colinas altas del bosque reservado de la UNAS − Tingo María. Tesis de posgrado, Universidad Nacional Agraria de la Selva. Available at https://hdl.handle.net/20.500.14292/1722[In Spanish]
Wenk EH, Sauquet H, Gallagher RV, et al. (2024) The AusTraits plant dictionary. Scientific Data 11, 537.
| Crossref | Google Scholar | PubMed |
Williams DG, Hultine KR, Dettman DL (2014) Functional trade-offs in succulent stems predict responses to climate change in columnar cacti. Journal of Experimental Botany 65(13), 3405-3413.
| Crossref | Google Scholar | PubMed |
Wright I, Reich P, Westoby M, et al. (2004) The worldwide leaf economics spectrum. Nature 428, 821-827.
| Crossref | Google Scholar | PubMed |
Wright IJ, Reich PB, Cornelissen JHC, et al. (2005) Assessing the generality of global leaf trait relationships. New Phytologist 166(2), 485-496.
| Crossref | Google Scholar | PubMed |
Zavala-Hurtado JA, Díaz-Solís A (1995) Repair, growth, age and reproduction in the gigant columnar cactus Cephalocereus columna-trajani (Karwinski ex. Pfeiffer) Schumann (Cactaceae). Journal of Arid Environments 31, 21-31.
| Crossref | Google Scholar |