Predicting the seed germination response of four rare Grevillea species (Proteaceae) to current and future temperatures
Nathan J. Emery A * , Ruby Paroissien
A * , Ruby Paroissien  A , Stefanie Carusi A , Graeme Errington A , Samuel Padgett
A , Stefanie Carusi A , Graeme Errington A , Samuel Padgett  A , Katherine Thomson
A , Katherine Thomson  A and Laura Watts A
A and Laura Watts A
A
Abstract
Grevillea is one of Australia’s largest plant genera, with 15% of species being federally listed as threatened. Although seed germination studies have largely focused on common species, knowledge of germination in threatened species remains limited. This gap is critical to address effective conservation actions, including translocations.
This study used a bi-directional thermogradient plate (TGP) to examine the germination responses of four threatened Grevillea species from New South Wales to varying temperatures.
Generalised additive models were used to predict monthly final germination proportions and time to 50% germination (t50) across 36 TGP temperature regimes, representing current and two future climate scenarios (SSP2-4.5 and SSP5-8.5).
Three species exhibited high germination across most temperatures, whereas G. iaspicula had a narrow range, preferring cooler conditions (≤15–20°C). For G. masonii and G. rivularis, t50 was under 5 days at all but the most extreme temperatures. G. wilkinsonii and G. iaspicula showed longer germination periods, with t50 exceeding 15 days for most temperatures. All species except G. iaspicula were predicted to maintain consistent germination across current and future climates, whereas G. iaspicula and G. wilkinsonii showed stable t50, and G. masonii and G. rivularis predicted longer t50 in winter.
Modelling seed germination under current and future temperatures highlights potential climate change risks and helps predict impacts on regeneration.
Germination in G. masonii, G. rivularis, and G. wilkinsonii may be resilient to temperature rises, whereas a cooler temperature preference for G. iaspicula may indicate a sensitivity to future temperature changes.
Keywords: Australia, climate change, ex situ conservation, germination niche, germplasm, seed dormancy, thermogradient plate, threatened species.
Introduction
Globally, plant diversity is under significant threat of loss and extinction, with ~45% of the world’s flora being predicted to be threatened (Bachman et al. 2024). Climate change is a key threat for many plant species because it has a significant influence on recruitment, altering seed dormancy responses and germination, and seedling emergence (Walck et al. 2011; Rawal et al. 2015; Chhetri and Rawal 2017; Carta et al. 2022). Seed germination is a critical and sensitive life-stage of plants, and depending on the species, shifts in climate factors may inhibit, delay or increase the seed germination response (Ooi et al. 2009; Cochrane et al. 2011; Cochrane 2016, 2020a; Rajapakshe et al. 2022). Temperature is an important trigger for relaxing seed dormancy, which then influences the number of seeds that germinate, the rate of germination and seasons when germination is likely to occur (Probert 2000; Collette et al. 2022). Changes to the thermal window of conditions conducive for these seed traits (part of the ‘germination niche’, Grubb 1977) can then affect other life-history traits, including the timing of reproduction, ultimately culminating in changes to local ecosystems (Donohue 2002; Walck et al. 2011; Mok et al. 2012). This sensitivity makes the ‘germination niche’ of species a crucial indicator of their overall response to environmental changes.
Rare, long-lived, and obligate seeding (i.e. non-resprouting) species are particularly sensitive to climate change because of their slow reproductive rates, limited dispersal opportunities, and restricted distributions (Hughes et al. 1996; Parmesan and Yohe 2003). This vulnerability is compounded by additional threats such as habitat fragmentation, disease, and invasive species (Schemske et al. 1994; Burgman et al. 2007). Ex situ conservation strategies, including seedbanking and translocation, are critical to mitigating extinction risks. (Commander et al. 2018). Understanding how seeds respond to environmental triggers is essential for developing effective propagation protocols and selecting appropriate reintroduction sites. Whereas some geographically restricted plants have narrow germination niches, others do not (Cochrane et al. 2011, 2022), underscoring the need for further study.
Grevillea is the third-largest genus of Australian flowering plants, with ~360 currently recognised species (Makinson 2000; Mesaglio et al. 2023). In eastern Australia, many Grevillea species are long-lived, obligate seeding shrubs (up to 50 years) that have an increase in recruitment after fire from the soil seed bank (Benson 1985; Auld and Tozer 1995). Several species have seeds that germinate after fire, responding to heat shock, smoke, or a combination of both (Kenny 2000; Morris 2000; Briggs and Morris 2008). A key factor in this process is the water-permeable seed coat, a physiological dormancy mechanism, which must be compromised or removed to allow the embryo to germinate (Morris et al. 2000; Ma et al. 2015; Briggs et al. 2016). Approximately 15% of Grevillea species are listed under the Australian Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act), with 27 species being also listed under the NSW Biodiversity and Conservation Act 2016 (as of June 2024). These species face significant threats such as herbivory, habitat loss, and weed invasion, making translocation and population supplementation essential long-term conservation actions. This study investigated the germination response of four threatened Grevillea species from New South Wales (NSW), with highly restricted distribution to varying temperatures.
Materials and methods
Study species
Grevillea iaspicula McGill, G. masonii Olde & Marriott, G. rivularis L.A.S.Johnson & MacGill., and G. wilkinsonii Makinson belong to two higher-order groups proposed by Makinson (2000), namely, Pteridifolia and Floribunda. These species are listed as either Endangered (G. iaspicula and G. masonii) or Critically Endangered (G. rivularis and G. wilkinsonii) under the EPBC Act (as of July 2025). All are threatened because of their highly restricted distributions in New South Wales, with recorded Extent of Occurrence and Area of Occupancy ranging between 4 and 270 km2 and 4 and 56 km2 respectively. Each species has fewer than 1000 known extant individuals, occurring across no more than eight populations. Regeneration can occur only from seed for all species except G. masonii, which has a lignotuber. Seed germination research, propagation and translocation have been recommended as future conservation management actions for each species. For these reasons, the species were selected for this study.
Seeds of Grevillea iaspicula, G. masonii, G. rivularis and G. wilkinsonii were collected from wild populations across the eastern coast of Australia, between Lawrence (29.49°S, 153.10°E) and Tumut (35.31°S, 148.23°E), NSW. This area experiences mean annual minimum and maximum temperatures between 8.3°C and 25.9°C respectively, and mean annual rainfall between 624 and 997 mm. The species were found in open, dry woodlands dominated by eucalypt species or in open shrubland. Mature seeds were collected post-dispersal by placing fine mesh bags over immature fruiting branches of representative plants from one population of each species, with collections made 4–6 weeks later. Seeds were processed and cleaned in a laboratory and placed in an environmentally controlled drying room (15°C and 15% RH) for at least 2 weeks. Sufficient Grevillea rivularis seeds could not be collected in 2024 because of the limited availability of seed from the only extant population. As a result, a historical seed collection from 2010 was used for this study. These seeds were stored at −20°C in vacuum-sealed foil packets and retrieved from storage for use. Prior to experimentation, seeds were equilibrated at ambient temperature and humidity for 24 h. A germination test was conducted on the stored seeds to assess potential viability loss after 14 years in cold storage; 100% viability was recorded (data not shown). Seeds of the remaining three species were collected in January 2024. For each collection, seeds were pooled representatively of seed production from multiple maternal plants from a single population, so as to give the required number of seeds for the experiment.
Experimental design
Experiments were conducted in May and June 2024. Because we were interested in the germination response to temperature, the seed coat was removed from all seeds with a scalpel and fine forceps to overcome physiological dormancy imposed by the seed coat. The bi-directional thermogradient plate (TGP) was set-up in a 36-cell half grid (eight cells along the x- and y-axis) so that only cells that received 12 h of light during the warm part of the diurnal cycle were used. Two species were run concurrently by using sealed 90 mm sterile polycarbonate bi-plates (Livingstone International, Australia), which have a built-in barrier to separate seeds of each species, and the plates were rotated a random amount every 2–3 days to overcome within-cell temperature differences. Seeds were sown onto a water agar medium (7 g L−1, depth ~5 mm) and each cell contained 10 seeds of each species, resulting in a total of 360 seeds for each species. Germination was checked every 2–3 days for 5 weeks and was scored when the radicle emerged >2 mm from the seed. Post-experiment seed viability was not determined because visibly damaged or otherwise non-viable seeds were discarded when the seed coat was removed, and extreme temperatures on the TGP can affect seed viability differently from mild temperatures. TGP cell temperatures were recorded using an infra-red thermometer (ZyTemp TN408LC, Bacto Laboratories, Mount Pritchard, NSW, Australia) on the surface of the plate, during light and dark periods on the days that germination was checked. Final cell temperatures were calculated as an average of temperature readings over the experimental period.
Data analysis
All data analyses were performed in the R statistical platform (R Core Team 2021) and repeated for each species to determine how germination changes with future temperature predictions. Germination data were analysed following Collette et al. (2022), by using the open-access ThermoGradient R-notebook workflow (ver. 1.2) (Collette et al. 2024). The individual seed was the unit of replication for each TGP temperature cell. We interpolated and predicted final germination proportion and time to 50% germination (t50) for all 36 temperatures by using generalised additive model (GAM) selection with a binomial (final proportion germination) or gaussian (t50) distribution. The raw data and best model outputs were visualised and checked using quilt plots. Next, the decimal latitude and longitude coordinates of the local seed collection site for each species were utilised to obtain minimum and maximum monthly temperature data (tmin and tmax) under current and future climate scenarios. Current temperature predictions were modelled using WorldClim 2.1 and obtained from the WorldClim online database (https://www.worldclim.org/data/index.html). Future climate predictions for tmin and tmax were derived from the Coupled Model Intercomparison Project Phase 6 (CMIP6) models, also available from WorldClim (https://worldclim.org/data/cmip6/cmip6_clim2.5 m.html). We used the ‘middle of the road’ scenario (SSP2-4.5) and the ‘fossil-fuel development’ scenario (SSP5-8.5) to project germination data, representing both moderate and worst-case scenarios. Eight CMIP6 model variations for SSP2-4.5 and SSP5-8.5 were included for the years 2081–2100, with a spatial resolution of 2.5 min (approximately 4.5 km2). The monthly temperature data were averaged to generate the required datasets. Final germination proportions and t50 were predicted for both current and future temperature scenarios by using the selected GAM.
Results
Observed data
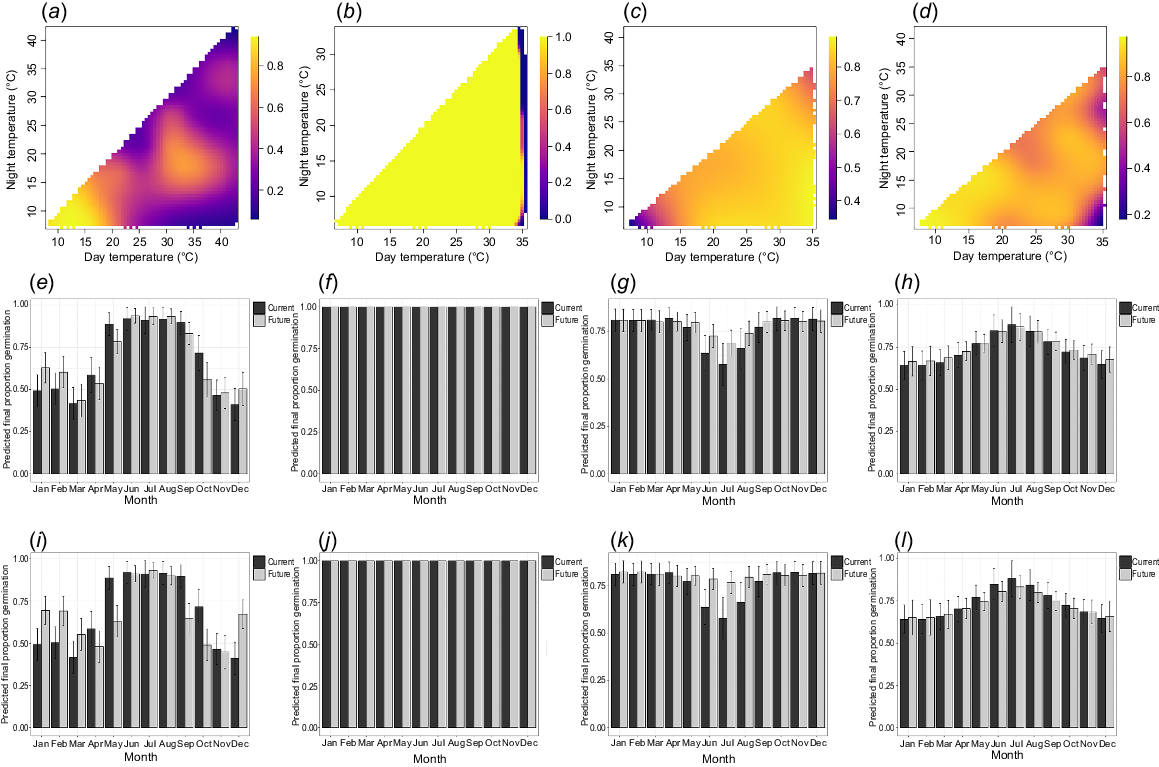
Complete germination (1.0) was achieved across a range of temperatures for all four species (Fig. 1). Nearly all G. masonii seeds (0.96) germinated within 30 days. Seeds of G. wilkinsonii and G. rivularis exhibited slightly higher final germination proportions at milder temperatures (10–15°C). G. wilkinsonii preferred constant and low-amplitude temperature regimes, whereas G. rivularis showed a slight preference for high-amplitude temperatures (Fig. 1). In contrast, G. iaspicula displayed a narrow germination response, favouring cooler temperatures (≤15–20°C) and, to a lesser extent, warmer temperatures including 33/17°C and 30/22°C.
Modelled data of final proportion germinated for (a, e, i) Grevillea iaspicula, (b, f, j) G. masonii, (c, g, k) G. rivularis and (d, h, l) G. wilkinsonii under multiple temperature regimes. Data were generated using a TGP, and modelled with generalised additive modelling. The first row (a–d) shows rasterised quilt plots of the modelled data, with day temperature (°C) on the x-axis, night temperature (°C) on the y-axis and final germination proportion on the z-axis. These plots show the best fitting models from Table 1. The middle row (e–h) shows the final germination proportion predicted into real world current and future predicted temperature for each month at the location of the source population for each species under the IPPC shared socio-economic pathway SSP2-4.5, for the years 2081–2100. The final row (i–l) shows the same data but for the SSP5-8.5 climate scenario for the years 2081–2100.
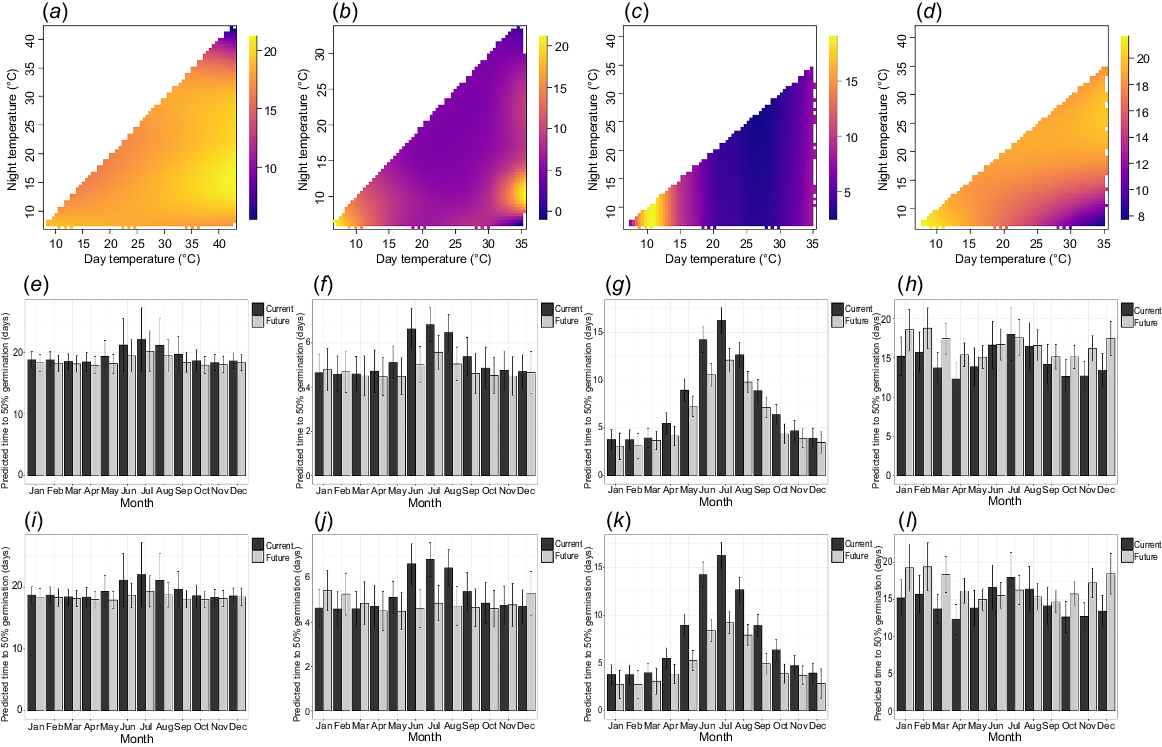
The t50 was less than 5 days for temperatures above 15°C for G. masonii and G. rivularis (Fig. 2). G. wilkinsonii and G. iaspicula germinated over a longer period, with most temperatures resulting in a t50 greater than 15 days (Fig. 2).
Modelled data of time to 50% germination (t50) for (a, e, i) Grevillea iaspicula, (b, f, j) G. masonii, (c, g, k) G. rivularis and (d, h, l) G. wilkinsonii under multiple temperature regimes. Data were generated using a TGP, and modelled with generalised additive modelling. The first row (a–d) shows rasterised quilt plots of the modelled data, with day temperature (°C) on the x-axis, night temperature (°C) on the y-axis and time to 50% germination (t50) on the z-axis. These plots show the best fitting models from Table 1. The middle row (e–h) shows the t50 predicted into real world current and future predicted temperature for each month at the location of the source population for each species under the IPPC shared socio-economic pathway SSP2-4.5, for the years 2081–2100. The final row (i–l) shows the same data but for the SSP5-8.5 climate scenario for the years 2081–2100.
Model predictions
The models with the best fit are outlined in Table 1. Model fit for final germination proportion was good, with mean error and RMSE values ranging from 0.06 to 0.18 and from 0.01 to 0.14 respectively, and correlations between the observed and predicted data ranging from 0.76 to 0.99. G. rivularis had the poorest fit because of its highly variable but broad germination response across all temperatures.
| Species | Model | Mean error | Lower 95% | Upper 95% | RMSE | Correlation | |
|---|---|---|---|---|---|---|---|
| G. iaspicula | prop_germ ~ s(day_temp, night_temp, bs = ‘tp’) | 0.173 | 0.061 | 0.29 | 0.096 | 0.947 | |
| t50_Farooq ~ te(day_temp, night_temp, bs = ‘tp’) | 5.636 | 2.686 | 10.067 | 4.737 | 0.552 | ||
| G. masonii | prop_germ ~ te(day_temp, night_temp, bs = ‘cr’) | 0.059 | 0 | 0.35 | 0.001 | 0.999 | |
| t50_Farooq ~ te(day_temp, night_temp, bs = ‘cr’) | 2.81 | 0.324 | 9.786 | 1.029 | 0.975 | ||
| G. rivularis | prop_germ ~ s(day_temp, night_temp, bs = ‘tp’) | 0.152 | 0.053 | 0.31 | 0.137 | 0.756 | |
| t50_Farooq ~ s(day_temp, bs = ‘tp’) + s(night_temp, bs = ‘tp’) | 2.743 | 0.716 | 8.19 | 1.969 | 0.908 | ||
| G. wilkinsonii | prop_germ ~ s(day_temp, night_temp, bs = ‘tp’) | 0.177 | 0.072 | 0.348 | 0.097 | 0.912 | |
| t50_Farooq ~ te(day_temp, night_temp, bs = ‘tp’) | 4.590 | 1.857 | 8.078 | 4.161 | 0.584 |
Mean error was estimated using hold-out samples through Monte Carlo resampling. In each of 100 iterations, 90% of the data were used for modelling, and predictions were made for the remaining 10%. The 95% confidence intervals were determined using the mean error, based on the 95th percentile of the error distribution. Root mean squared error (RMSE) was used as a standard measure of model error. Model performance was further assessed using Pearson’s correlation, which quantified the relationship between the modelled and observed data.
When the data were modelled under current temperatures at the local site, final germination was predicted to be consistent year-round for G. masonii, whereas G. iaspicula showed higher germination predicted from late autumn to early spring (May to September; 0.89 ± 0.07 to 0.92 ± 0.07). G. rivularis was predicted to have its highest germination between spring and autumn (October to April; 0.82 ± 0.05 to 0.83 ± 0.05), and G. wilkinsonii showed a slight preference for winter temperatures.
Under future climate scenarios SSP2-4.5 and SSP5-8.5, germination was predicted to remain consistent with current climate predictions, except for increases in predicted germination at summer temperatures for G. iaspicula (December to February, SSP2-4.5: 0.50 ± 0.10 to 0.63 ± 0.09; SSP5-8.5: 0.45 ± 0.10 to 0.69 ± 0.09), and winter temperatures for G. rivularis (June to August, SSP2-4.5: 0.74 ± 0.06 to 0.78 ± 0.06; SSP5-8.5: 0.79 ± 0.05 to 0.81 ± 0.05).
Model fit for t50 was good for G. rivularis and G. masonii, with mean error and RMSE values of 2.74–2.81 and 1.03–1.97 respectively, and correlations of 0.91–0.98. By contrast, poor model fit for t50 was observed for G. wilkinsonii and G. iaspicula, with mean error and RMSE values of 4.59–5.64 and 4.16–4.74 respectively, and correlations of 0.58 and 0.55.
Under current climate, t50 was predicted to be lowest during summer months and highest during winter months for all species. The t50 predictions for G. masonii ranged across the year from 4.60 ± 0.81 to 6.83 ± 0.76 days under the current climate, and were faster by an average of 0.54 ± 0.5 and 0.43 ± 0.08 days under the SSP2-4.5 and SSP5-8.5 future scenarios respectively.
Similarly for G. iaspicula and G. wilkinsonii, t50 was consistent throughout the year (18.29 ± 1.31 to 22.03 ± 5.13 days and 15.79 ± 1.41 to 21.69 ± 4.13 days, respectively) and was predicted to have a negligible change under both future climate scenarios. For G. rivularis, t50 was >10 days for winter months and <10 days for every other month. Under the SSP2-4.5 and SSP5-8.5 future climate scenarios, t50 was predicted to be faster every month by an average of 1.70 ± 0.11 d and 2.87 ± 0.18 days, respectively.
Discussion
Our findings showed that seed germination occurred across a broad range of temperatures in three of the four Grevillea species examined. Our models indicated that germination of G. masonii, G. rivularis and G. wilkinsonii is predicted to remain stable year-round under current temperatures, with only minor variations in final germination proportion and t50 under future temperature scenarios. This suggests potential resilience for seeds of these three species to the higher temperatures associated with climate change. In contrast, G. iaspicula exhibited a restricted germination response, favouring cooler temperatures (<20°C). The predicted data suggested a higher probability of germination from late autumn to early spring, with decreased germination in early autumn and late spring, and increased germination in summer under future climate scenarios. These results imply that G. iaspicula may require additional dormancy breaking conditions and seasonal success of germination may be altered by rising temperatures.
The germination response of seeds to different temperatures has not been previously studied in Grevillea. Previous experiments of Grevillea species under laboratory conditions have incubated seeds only at mild temperatures between 15°C and 23°C (Morris 2000; Pickup et al. 2003; Barrett and Cochrane 2007). In all cases, scarification or removal of the seed coat, a physiological dormancy mechanism, was a necessary pre-treatment for germination to occur. That geographically restricted and threatened species do not have corresponding narrow germination niches has been previously reported in other species (Cochrane et al. 2011, 2020b; Venn et al. 2021). Environmental factors (e.g. soil type, moisture, dispersal mechanisms, biotic interactions) or threats (e.g. habitat fragmentation, isolation, competition from invasive species) may also contribute to the restricted locations of these species, including those tested in the current study.
For G. masonii, G. rivularis and G. wilkinsonii, the thermal germination niche may not be a significant barrier to recruitment, but other climate factors, such as precipitation, may have a more pronounced effect on germination success. For example, decreasing water potential was shown to reduce the germination of Grevillea juniperina by ~50% at −0.6 MPa (Briggs et al. 2016). Moisture stress also interacts with temperature and may do so unpredictably among related taxa (Cochrane et al. 2014; Duncan et al. 2019; Emery and Collette 2021). Furthermore, fire-related cues such as smoke may increase the growth potential of the embryo at lower water potentials (Briggs et al. 2016); but their effect relies on these cues overcoming the restriction of the seed coat.
In the regions where our study species occur, climate change models predict a reduction in rainfall during winter and spring as well as a decrease in soil moisture levels in all seasons by 2090 (Dowdy et al. 2015; Grose et al. 2015; Timbal et al. 2015). This suggests that while future temperatures may remain suitable for germination, the timing of germination may be restricted to seasons with a higher likelyhood of precipitation to support adequate available moisture (e.g. summer and autumn). With its preference for cooler winter temperatures, there is a higher risk of the germination niche of G. iaspicula being negatively affected under these future projections. This highlights the need to examine how Grevillea seeds respond to moisture availability under laboratory and field conditions.
Microhabitat variability within different habitat types can significantly influence germination outcomes for threatened Grevillea species. Although the vegetation types in which the study species occur, namely open shrubland and dry woodland, are broadly characterised by high light availability and low canopy cover, fine-scale differences in soil moisture, temperature, litter cover, and shading can affect seed dormancy break and seedling emergence (Ooi et al. 2012). For example, Grevillea iaspicula and G. rivularis, which occur in dry sclerophyll shrublands, may experience heterogeneous litter layers and patchy shading from surrounding shrubs, altering temperature cues that are critical for germination. However, this may be less influential for G. rivularis if its seeds can germinate across a broad range of temperatures, as identified in this study. G. iaspicula occurs on limestone-derived skeletal soils, which are characterised by high temperature fluctuations and low water retention. The preference for cooler temperatures observed in this study may reflect microhabitat preferences for establishment in rock crevices or at the base of cliffs, where moisture is retained for longer periods. Incorporating microhabitat conditions into future germination models may improve the ecological realism of projections under climate change, particularly for rare species occurring in narrow habitats and limited dispersal capacity.
Understanding the seed germination response to a range of environmental conditions is a key conservation issue for threatened plants and will help predict the extent to which regeneration may be affected by changing climate. Understanding the relationship between seed germination and temperature is the first step to identifying species that may be at greater risk of future regeneration failure. However, it is important to consider that our germination trials were conducted under controlled conditions that do not fully capture natural seed coat weathering or decay processes. In the field, higher temperatures may accelerate seed coat degradation, potentially leading to rapid germination rates if adequate moisture is also available. This interaction between temperature and seed coat decay could significantly influence natural recruitment patterns, especially under warming climate scenarios. Therefore, future research should examine the germination response to moisture stress and the combined effects of temperature and seed coat ageing to better define the germination niche and seed bank dynamics of these threatened species.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This work was supported through the seed collection project which is funded through the New South Wales Department of Climate Change, Energy, the Environment and Water ‘Saving Our Species’ program.
Acknowledgements
Seeds were collected under a scientific license (SL100569) issued by the New South Wales Department of Climate Change, Energy, the Environment and Water. We thank Asho and Leonie Ashley for granting permission for seeds to be collected from their property.
References
Auld TD, Tozer M (1995) Patterns in emergence of Acacia and Grevillea seedlings after fire. Proceedings of the Linnean Society of NSW 15, 5-15.
| Google Scholar |
Bachman SP, Brown MJM, Leão TCC, Nic Lughadha E, Walker BE (2024) Extinction risk predictions for the world’s flowering plants to support their conservation. New Phytologist 242(2), 797-808.
| Crossref | Google Scholar | PubMed |
Barrett S, Cochrane A (2007) Population demography and seed bank dynamics of the threatened obligate seeding shrub Grevillea maxwellii McGill (Proteaceae). Journal of the Royal Society of Western Australia 90, 165-174.
| Google Scholar |
Benson DH (1985) Maturation periods for fire-sensitive shrub species in Hawkesbury sandstone vegetation. Cunninghamia 1(3), 339-349.
| Google Scholar |
Briggs CL, Morris EC (2008) Seed-coat dormancy in Grevillea linearifolia: little change in permeability to an apoplastic tracer after treatment with smoke and heat. Annals of Botany 101(5), 623-632.
| Crossref | Google Scholar | PubMed |
Briggs CL, Morris EC, Stone G (2016) Micropylar seed coat restraint and embryonic response to heat shock and smoke control seed dormancy in Grevillea juniperina. Seed Science Research 26(2), 111-123.
| Crossref | Google Scholar |
Burgman M, Keith D, Hopper SD, Widyatmoko D, Drill C (2007) Threat syndromes and conservation of the Australian flora. Biological Conservation 134(1), 73-82.
| Crossref | Google Scholar |
Carta A, Fernández-Pascual E, Gioria M, Müller JV, Rivière S, Rosbakh S, Saatkamp A, Vandelook F, Mattana E (2022) Climate shapes the seed germination niche of temperate flowering plants: a meta-analysis of European seed conservation data. Annals of Botany 129(7), 775-786.
| Crossref | Google Scholar | PubMed |
Chhetri SB, Rawal DS (2017) Germination phenological response identifies flora risk to climate change. Climate 5(3), 73.
| Crossref | Google Scholar |
Cochrane A (2016) Can sensitivity to temperature during germination help predict global warming vulnerability? Seed Science Research 26(1), 14-29.
| Crossref | Google Scholar |
Cochrane JA (2020a) Thermal requirements underpinning germination allude to risk of species decline from climate warming. Plants 9(6), 796.
| Crossref | Google Scholar |
Cochrane A (2020b) Temperature thresholds for germination in 20 short-range endemic plant species from a Greenstone Belt in southern Western Australia. Plant Biology 22(S1), 103-112.
| Crossref | Google Scholar |
Cochrane A, Daws MI, Hay FR (2011) Seed-based approach for identifying flora at risk from climate warming. Austral Ecology 36(8), 923-935.
| Crossref | Google Scholar |
Cochrane JA, Hoyle GL, Yates CJ, Wood J, Nicotra AB (2014) Evidence of population variation in drought tolerance during seed germination in four Banksia (Proteaceae) species from Western Australia. Australian Journal of Botany 62(6), 481-489.
| Crossref | Google Scholar |
Collette JC, Sommerville KD, Lyons MB, Offord CA, Errington G, Newby Z-J, Von Richter L, Emery NJ (2022) Stepping up to the thermogradient plate: a data framework for predicting seed germination under climate change. Annals of Botany 129(7), 787-794.
| Crossref | Google Scholar | PubMed |
Collette J, Emery N, Paroissien R (2024) JustinCollette/ThermoGradient: ThermoGradient (1.2). Zenodo. Available at https://doi.org/10.5281/zenodo.13835870
Donohue K (2002) Germination timing influences natural selection on life-history characters in Arabidopsis thaliana. Ecology 83(4), 1006-1016.
| Crossref | Google Scholar |
Dowdy A, Abbs D, Bhend J, Chiew F, Church J, Ekström M, et al. (2015) East coast cluster report, climate change in Australia projections for Australia’s natural resource management regions: cluster reports. (Eds M Ekström, P Whetton, C Gerbing, M Grose, L Webb, J Risbey) (CSIRO and Bureau of Meteorology: Australia)
Duncan C, Schultz NL, Good MK, Lewandrowski W, Cook S (2019) The risk-takers and -avoiders: germination sensitivity to water stress in an arid zone with unpredictable rainfall. AoB Plants 11(6), plz066.
| Crossref | Google Scholar |
Emery NJ, Collette JC (2021) Drought stress affects the germination of four co-occurring Eucalyptus species from north-west New South Wales. Australian Journal of Botany 69(3), 143-151.
| Crossref | Google Scholar |
Grose M, Abbs D, Bhend J, Chiew F, Church J, Ekström M, et al. (2015) Southern slopes cluster report, climate change in Australia projections for Australia’s natural resource management regions: cluster reports. (Eds M Ekström, P Whetton, C Gerbing, M Grose, L Webb, J Risbey) (CSIRO and Bureau of Meteorology: Australia)
Grubb PJ (1977) The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews 52(1), 107-145.
| Crossref | Google Scholar |
Hughes L, Cawsey EM, Westoby M (1996) Climatic range sizes of Eucalyptus species in relation to future climate change. Global Ecology and Biogeography Letters 5(1), 23-29.
| Crossref | Google Scholar |
Kenny BJ (2000) Influence of multiple fire-related germination cues on three Sydney Grevillea (Proteaceae) species. Austral Ecology 25(6), 664-669.
| Google Scholar |
Ma X, Guo J, Han X, Yan G (2015) Grevillea (Proteaceae) seed coats contain inhibitors for seed germination. Australian Journal of Botany 63(7), 566-571.
| Crossref | Google Scholar |
Mesaglio T, Sauquet H, Coleman D, Wenk E, Cornwell WK (2023) Photographs as an essential biodiversity resource: drivers of gaps in the vascular plant photographic record. New Phytologist 238(4), 1685-1694.
| Crossref | Google Scholar | PubMed |
Mok H-F, Arndt SK, Nitschke CR (2012) Modelling the potential impact of climate variability and change on species regeneration potential in the temperate forests of south-eastern Australia. Global Change Biology 18(3), 1053-1072.
| Crossref | Google Scholar |
Morris EC (2000) Germination response of seven east Australian Grevillea species (Proteaceae) to smoke, heat exposure and scarification. Australian Journal of Botany 48(2), 179-189.
| Crossref | Google Scholar |
Morris EC, Tieu A, Dixon K (2000) Seed coat dormancy in two species of Grevillea (Proteaceae). Annals of Botany 86(4), 771-775.
| Crossref | Google Scholar |
Ooi MKJ, Auld TD, Denham AJ (2009) Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence. Global Change Biology 15(10), 2375-2386.
| Crossref | Google Scholar |
Ooi MKJ, Auld TD, Denham AJ (2012) Projected soil temperature increase and seed dormancy response along an altitudinal gradient: implications for regeneration and range shifts in a warming climate. Ecology and Evolution 2(3), 540-552.
| Google Scholar |
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37-42.
| Crossref | Google Scholar | PubMed |
Pickup M, McDougall KL, Whelan RJ (2003) Fire and flood: soil-stored seed bank and germination ecology in the endangered Carrington Falls Grevillea (Grevillea rivularis, Proteaceae). Austral Ecology 28(2), 128-136.
| Crossref | Google Scholar |
R Core Team (2021) ‘R: A language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Rajapakshe RPVGSW, Cross AT, Turner SR, Tomlinson S (2022) Understanding the interplay of temperature and moisture on the germination niche to improve management of threatened species impacted by mining. Restoration Ecology 30(S1), e13708.
| Crossref | Google Scholar |
Rawal DS, Kasel S, Keatley MR, Nitschke CR (2015) Environmental effects on germination phenology of co-occurring eucalypts: implications for regeneration under climate change. International Journal of Biometeorology 59, 1237-1252.
| Crossref | Google Scholar | PubMed |
Schemske DW, Husband BC, Ruckelshaus MH, Goodwillie C, Parker IM, Bishop JG (1994) Evaluating approaches to the conservation of rare and endangered plants. Ecology 75(3), 584-606.
| Crossref | Google Scholar |
Timbal B, Abbs D, Bhend J, Chiew F, Church J, Ekström M, et al. (2015) Murray basin cluster report, change in Australia projections for Australia’s natural resource management regions: cluster reports. (Eds M Ekström, P Whetton, C Gerbing, M Grose, L Webb, J Risbey) (CSIRO and Bureau of Meteorology: Australia)
Venn SE, Gallagher RV, Nicotra AB (2021) Germination at extreme temperatures: implications for alpine shrub encroachment. Plants 10, 327.
| Crossref | Google Scholar |
Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P (2011) Climate change and plant regeneration from seed. Global Change Biology 17(6), 2145-2161.
| Crossref | Google Scholar |


