Adventures in Translocation: Studies of the Translocator Protein (TSPO) 18 kDa*
Jonathan J. Danon A , Dane F. L. Tregeagle A and Michael Kassiou A B
A B
A School of Chemistry, Faculty of Science, University of Sydney, Sydney, NSW 2006, Australia.
B Corresponding author. Email: michael.kassiou@sydney.edu.au
Australian Journal of Chemistry 74(11) 749-757 https://doi.org/10.1071/CH21176
Submitted: 26 July 2021 Accepted: 13 September 2021 Published: 5 October 2021
Abstract
The 18 kDa translocator protein (TSPO) is an evolutionarily conserved transmembrane protein found embedded in the outer mitochondrial membrane. A secondary target for the benzodiazepine diazepam, TSPO has been a protein of interest for researchers for decades, particularly owing to its well-established links to inflammatory conditions in the central and peripheral nervous systems. It has become a key biomarker for assessing microglial activation using positron emission tomography (PET) imaging in patients with diseases ranging from atherosclerosis to Alzheimer’s disease. This Account describes research published by our group over the past 15 years surrounding the development of TSPO ligands and their use in probing the function of this high-value target.
Keywords: translocator protein, medicinal chemistry, structure–activity relationships, CNS, neuroinflammation, microglia, PET imaging, neurodegeneration, single nucleotide polymorphism.
Introduction
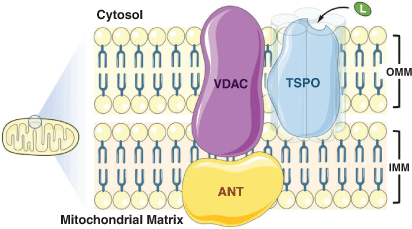
The 18 kDa translocator protein (TSPO, formerly referred to as the peripheral benzodiazepine receptor) is a highly conserved mitochondrial protein predominantly expressed in the outer mitochondrial membrane in steroid-synthesising tissues.[1] It forms part of a larger transmembrane complex (Fig. 1) that facilitates cholesterol translocation across the mitochondrial membranes (the rate-limiting step in steroid synthesis). TSPO is thought to be involved in myriad other cellular processes, although we still lack a precise understanding of the full scope of its functions.[1]
|
TSPO is upregulated in immune cells in response to harmful stimuli, and is therefore a reliable and translatable biomarker for innate immune response to disease and injury. It has long been a target of interest for non-invasive in vivo PET (positron emission tomography) imaging of both microglial activation in the central nervous system (CNS) and macrophage activity in the periphery.[2] Low levels of chronic microglial activation in the CNS have been touted as signposts of the pre-symptomatic stages of neurodegenerative disease. It is critical that researchers have access to techniques that can quantify small changes in expression of neuroinflammatory biomarkers, such as TSPO, in a clinically relevant context. Furthermore, researchers have attempted to target TSPO for therapeutic purposes in several CNS and peripheral indications that comprise inflammatory processes, though no therapeutics that specifically target TSPO are currently available. Understanding how TSPO ligands produce their therapeutic effects remains an important challenge for medicinal chemists and beyond. Our group has been studying the TSPO for nearly two decades, using medicinal chemistry and an array of in vitro and in vivo models to better understand its role in human health and disease. This Account summarises the majority of our work in this area, making reference to significant contributions from other groups where appropriate.
For many years, the isoquinoline carboxamide [11C](R)-PK11195 was the gold standard ligand for use in PET studies for assessing and quantifying expression of TSPO.[1] Over time, however, its limitations as a radiotracer have been better understood. These include poor blood–brain barrier (BBB) permeability, low bioavailability (a consequence of high plasma protein binding), and high lipophilicity and non-specific binding, making it an insensitive probe for detecting subtle changes in TSPO expression in vivo.[3]
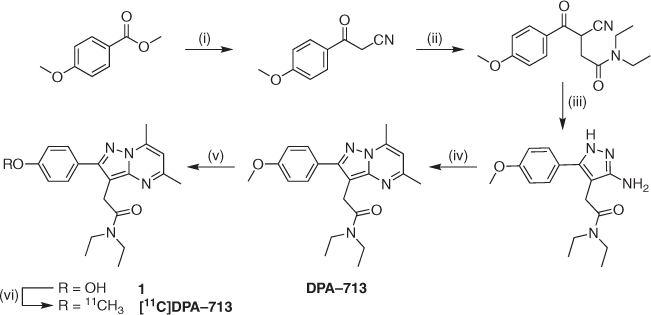
In the mid-2000s, our group was one of several that took on the challenge of designing new and improved TSPO PET tracers. Inspired by a previous study performed by Selleri and co-workers,[4] we reported the synthesis (Scheme 1) and in vivo evaluation (baboon) of a new pyrazolopyrimidine radiotracer: [11C]DPA-713.[5] Maximal brain uptake took 20 min, staying consistent for the duration of the hour-long PET imaging experiment, compared with extremely fast uptake and clearance for [11C](R)-PK11195 (3–5 and 15 min respectively). These improved kinetics and BBB penetration versus [11C](R)-PK11195 were attributed to the new tracer’s lower lipophilicity and plasma protein binding. In vivo selectivity of [11C]DPA-713 for TSPO over the central benzodiazepine receptor (CBR) was confirmed using blocking studies with PK11195 and CBR-selective ligand flumezanil. This initial investigation motivated follow-up validation studies. In 2006, radiolabelling protocols were significantly improved to facilitate automated synthesis.[6]
|
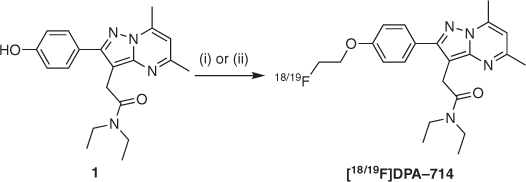
Buoyed by these efforts, our group proceeded to synthesise and assess an 18F-labelled analogue of DPA-713, named [18F]DPA-714[7] (Scheme 2), to take advantage of fluorine-18’s longer radioactive half-life in a clinical setting (109.8 versus 20.4 min for carbon-11).[8] Ex vivo biodistribution and blocking studies in rats with quinolinic acid-induced lesions showed high levels of TSPO-specific uptake into the lesion-containing brain areas. An in vivo biodistribution PET study in a healthy baboon also confirmed specific TSPO binding, as well as rapid and persistent brain uptake.[9] Furthermore, unlabelled DPA-714 acted as an agonist of TSPO, stimulating biosynthesis of pregnenolone – an endogenous precursor to key steroid hormones – at levels 80 % above observed baseline in vitro.[7]
|
The success of these studies laid the foundations for a host of follow-up studies investigating the applicability of [11C]DPA-713 and [18F]DPA-714 in a multitude of animal models of disease, as well as in pre-clinical and clinical settings. Studies such as these have been carried out by dozens of researchers worldwide, many of whom we continue to support by supplying radiotracer precursors and cold standards. We have also carried out traditional medicinal chemistry investigations to probe the structure–binding and structure–activity relationships of TSPO ligands, investigating their potential use as diagnostics and therapeutics in CNS diseases. The following sections discuss a collection of key contributions made in these areas by our and other groups over the past 12 years. Attempts have been made to focus on topics of interest to readers from a chemistry background.
DPA-713 and DPA-714 in Animal Models of Disease
In 2007, the utility of [11C]DPA-713 for imaging TSPO in AMPA-induced (AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate) rat models of neuroinflammation was shown through a series of in vivo PET and ex vivo autoradiography experiments.[10] [11C]DPA-713 displayed improved signal-to-noise ratio versus [11C]PK11195 (attributed to lower non-specific binding). A follow-up head-to-head study published in 2009 compared these two tracers with [18F]DPA-714 in the same rat models.[11] It concluded that [18F]DPA-714 performed best in terms of binding potential and bioavailability. A separate study that compared the same three tracers in a less invasive rat model of herpes encephalitis was published around the same time.[12] It confirmed that specific binding and utility of [11C]DPA-713 were far superior to that of [11C]PK11195 for assessing small changes in TSPO levels. Lower specific binding of [18F]DPA-714 than expected was attributed to its agonistic activity and potential heterogenous affinity states of TSPO depending on the activated microglial phenotype. This pointed to the possibility of using different PET tracers to image different aspects of neuroinflammation. Our group has since published several further studies using [11C]DPA-713 and [18F]DPA-714 to successfully image neuroinflammation in rat models of focal cerebral ischemia,[13–15] arthritis,[16] and LPS-induced (LPS = lipopolysaccharide) neuroinflammation.[17] It must be mentioned that other groups have also performed proof-of-principle animal studies using these tracers.[18–20]
DPA-713 and DPA-714 in Human Studies
The road from pre-clinical to first in-human studies is typically less convoluted and onerous for radiotracers than for therapeutics. Unlike a therapeutic drug, the dose of a radiotracer required for PET imaging is very low (typically in the nano- to picogram range), minimising the likelihood of encountering toxicity issues.[8] We reported the first dosimetry, biodistribution, and imaging studies in healthy humans for [11C]DPA-713 and [18F]DPA-714 between 2009 and 2012.[21–23] Sufficient in vivo stability, brain uptake, and TSPO selectivity were observed and, despite accumulation in other organs (e.g. lungs, spleen, kidneys), these investigations concluded that these tracers were safe and could potentially be useful tools for assessing neuroinflammation in human patients pending further studies.
Our group has engaged with several collaborators over the past decade who have continued to validate the use of these tracers in humans in a diverse range of conditions. For [11C]DPA-713, these have included individuals with HIV,[24] systemic lupus erythematosus,[25] and post-treatment Lyme disease syndrome.[26] A complementary pair of studies used [11C]DPA-713 to investigate the link between microglial activation (inferred from TSPO uptake) and brain injury and repair in control groups versus active and recently retired NFL players.[27,28] [18F]DPA-714 has been used to study post-stroke neuroinflammation,[29] as well as patients with amyotrophic lateral sclerosis (ALS)[30] and progressive multiple sclerosis.[31] An additional study pitted [11C]DPA-713, [18F]DPA-714, and [11C](R)-PK11195 against each other in a proof-of-concept study imaging the wrists and hands of rheumatoid arthritis patients. Background uptake of both DPA tracers was lower than for [11C](R)-PK11195, with [11C]DPA-713 yielding the highest-quality imaging results.
A recent search for ‘DPA-714’ on ClinicalTrials.gov (accessed 19 July 2021) showed 24 results comprising 2 completed, 14 recruiting, 3 not yet recruiting, 1 withdrawn, and 4 whose status is undefined (start dates ranged from October 2009 to June 2022). These trials indicate use of [18F]DPA-714 to assess inflammation in disorders including but not limited to multiple sclerosis, Alzheimer’s disease, Parkinson disease, post-traumatic stress disorder (PTSD), ALS, myocardial infarction, and triple-negative breast cancer. This a clear additional indication of the extensive uptake of [18F]DPA-714 by the medical community, although it must be noted that other radiotracers targeting TSPO are also being used for in human studies of diseases with neuroinflammatory components, including [11C]PBR-28,[32] [11C]-ER176,[33] [18F]FEPPA,[34] and [18F]-GE-180.[35] For more detailed discussion of contributions to TSPO PET tracer development from other research groups, we refer readers to an excellent review that was recently published by Zhang et al.[1]
Limitations of [11C]DPA-713 and [18F]DPA-714 as Radiotracers
A critical finding from early PET imaging studies targeting TSPO was an inherent binding variability across human patients when using second-generation radiotracers, compromising imaging sensitivity and clinical usefulness of the data acquired.[36] It was eventually deduced that this was the result of a co-dominantly expressed single nucleotide polymorphism (rs6971) in the human TSPO gene that results in a non-conservative alanine to threonine substitution at residue 147.[37] The consequence of this substitution is the presence of three distinct binding statuses in humans for second-generation TSPO ligands: Ala/Ala (wild type, high-affinity binding), Ala/Thr (mixed-affinity binding), and Thr/Thr (low-affinity binding). It is therefore necessary to genotype patients before commencing imaging studies, accounting for sensitivity differences in high- and medium-affinity binders when analysing data and excluding low-affinity binders from participating entirely.[1] This discovery has hastened the search for third-generation TSPO radiotracers that bind to both isoforms of the protein with excellent affinity and target specificity, while also maintaining desirable pharmacokinetic properties for PET imaging. It has also been shown that this polymorphism has functional consequences, affecting steroidogenic pathways in rats and humans[38] and potentially being causally associated with bipolar disorder.[39]
Investigating the Structure–Binding and Structure–Activity Relationships of TSPO Ligands
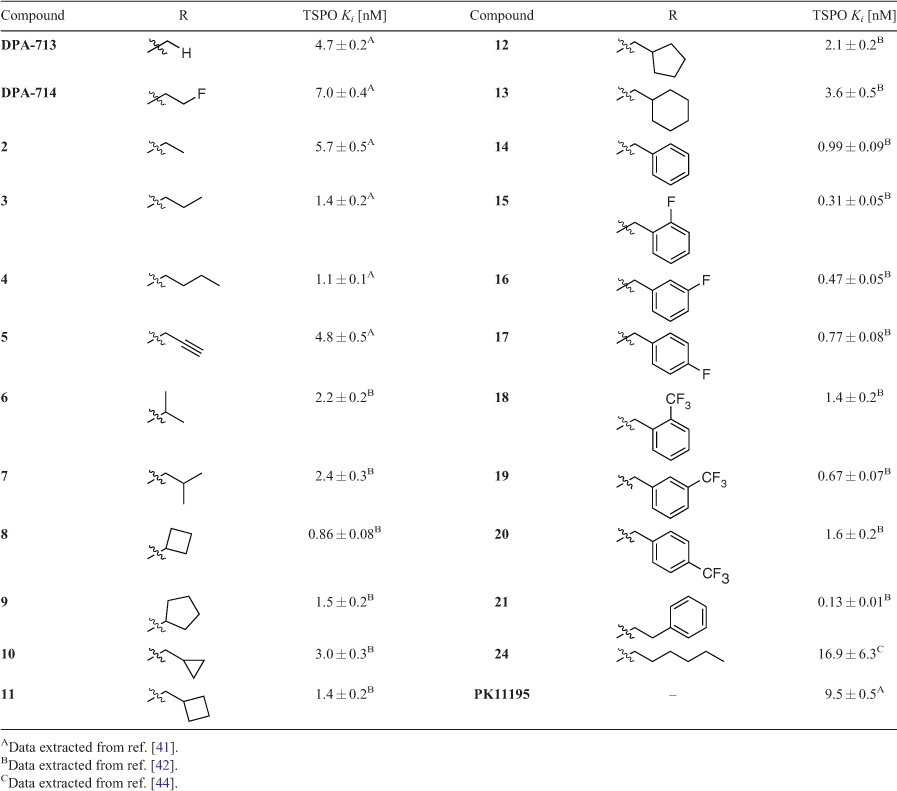
In 2012, we published a new, scalable synthetic route to DPA-714 that allowed access to multigram quantities of key intermediate phenol 1.[40] This efficient, chromatography-free route not only underpinned our ability to generate plentiful supplies of PET tracer precursors for radiolabelling, but also facilitated our early efforts to probe how DPA-like ligands interact with the TSPO through traditional structure–binding and structure–activity relationships studies. We first chose to explore the effects of different alkyl substitutions on TSPO binding and function. Williamson ether reaction conditions were employed using a variety of alkyl halides to generate several small libraries of DPA analogues (Table 1). Our first study compared alkyl and propargyl derivatives 2–5 with DPA-713, DPA-714, and PK11195.[41] Binding affinities for TSPO consistently ranged between 1 and 10 nM, while DPA-714, 3, 4, and 5 were all shown to increase pregnenolone biosynthesis in rat C6 glioma cells between 80 and 175 % above baseline levels. All compounds tested also showed complete selectivity over the CBR. A follow-up study used a similar approach to synthesise branched alkyl (6–7), cycloalkyl (8–13), benzyl (14–20), and phenethyl (21) derivatives, which all possessed higher affinity for TSPO than DPA-713 and DPA-714 and increased pregnenolone biosynthesis by up to 331 %.[42] With this glut of new data available, propargyl DPA (5), DPA-713, and DPA-714 were used as tool compounds to probe the effect of TSPO ligands on levels of microglial activation in a quinolinic acid rat model of excitotoxic neurodegeneration.[43] All three pyrazolopyrimidine ligands outperformed PK11195, inhibiting microglial activation and promoting survival of neurons (though DMSO, the drug vehicle, also exhibited some neuroprotective effects via a different signalling pathway).
A selection of DPA-based compounds (4–7, 9, 14, and 24) was later screened for binding and anti-proliferative/pro-apoptotic activity against human T98G glioblastoma and foetal astroglial SVG p12 cell lines.[44] While binding affinities remained similar (although lower than when measured against rat TSPO in previous studies), a diverse array of functional behaviour was observed across this series. Compounds 4, 7, and 9 modestly decreased proliferation in both cell lines. However, the most potent compounds were 14 (IC50 71.5 ± 0.5 μM) and 24 (IC50 64.6 ± 1.6 μM), which decreased proliferation only in T98G cells. All compounds except 5 induced significant levels of apoptosis in T98G cells, with evidence pointing to a role in mitochondrial membrane potential dissipation. At the time, we proposed that residence time, rather than binding affinity, could be the driving factor for this range of activity.[45,46] Further experiments are required to investigate this hypothesis more thoroughly.
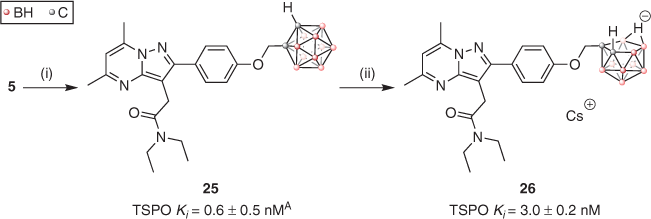
In a proof-of-concept collaboration with the Rendina group, we showed that the DPA scaffold could be appropriated for a new purpose – development of boron-rich compounds for use in boron neutron capture therapy (BNCT).[47] Two new carborane-containing ligands were synthesised starting from propargyl compound 5, as depicted in Scheme 3. The 1,2-closo-DPA (25) and 7,8-nido-DPA (Cs·26) analogues bound TSPO with high affinity, though the latter displayed behaviour indicative of a two-site binder. Good uptake and low cytotoxicity were observed in T98G cells, making these compounds promising tumour-targeting agents for BNCT. A follow-up study reported two carborane-containing indole carboxamide TSPO ligands with even better uptake and toxicity profiles; plans for in vivo studies are currently in the pipeline.[48]
|
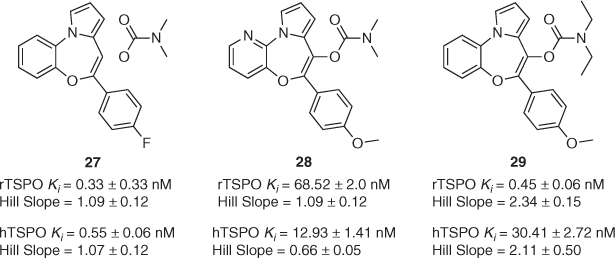
Over the years, we have also reported on the synthesis of TSPO ligands not based on the pyrazolopyrimidine scaffold. Taking inspiration from a 1996 study by Campiani and co-workers,[49] we synthesised a library of 10 pyrrolobenzoxazepines to better understand their binding and functional properties at TSPO through systematic in vitro investigations (structures of three of these compounds that are shown in Fig. 2).[50] Scarf and co-workers succeeded in exposing some of the many complexities associated with scrutinising this high-value target, in particular highlighting the differences in ligand behaviour at human and rat TSPO. This is exemplified by compounds 27, 28, and 29 (Fig. 2). Small changes to the composition of the western aromatic ring (C to N), carbamate N-substitution (Me to Et) or para-position of the southern phenyl ring (F to OMe) result in significant changes to binding affinities, even reversing preference entirely. Furthermore, the associated Hill slopes for compounds 28 and 29 (among others not shown here) suggested complex binding profiles at human and/or rat TSPO. As with our previous studies, functional assay results did not correlate with these binding affinities. For example, while 27 and 29 had no effect on pregnenolone release in rat C6 glioma cells, 28 increased it by 100 % versus baseline production. This is despite the fact that 28 possesses the lowest binding affinity of the three for rat TSPO. Finally, all three compounds displayed similar anti-proliferative properties in MCF-7 human breast cancer cells, inhibiting proliferation by 65–80 %. We concluded that accounting for ligand binding cooperativity and obtaining a better understanding of which binding site residues are most important for binding in different species were key considerations for future rational design of TSPO-based therapeutics.
|
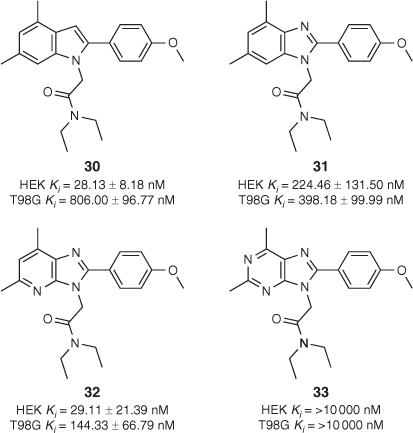
We also conducted a separate study wherein the number and arrangement of nitrogen atoms on DPA-713 were altered to further probe complex binding events at and function of TSPO in HEK293 and T98G cells.[51] The four compounds that were synthesised are shown in Fig. 3. Key differences in both binding affinity and binding mode (inferred from Hill slopes) were observed. Purine derivative 33 did not bind to TSPO in either cell line, while imidazopyridine 32 bound preferentially to TSPO in HEK293 cells in a one-site manner. Indole-based compound 30 bound to TSPO nearly 30 times more weakly in the human glioblastoma (T98G) cells than in HEK293 cells. Moreover, with a Hill slope more negative than –1, it appeared that 30 was displaying positive ‘allosteric-type’ modulatory behaviour in the presence of tritiated PK11195 used in radioligand binding assays. Benzimidazole analogue 31 bound to TSPO with similar affinity in both cell lines but curiously had a Hill slope more shallow than –1 in T98G cells. This indicated either negative modulation of PK11195 (a hypothesis seemingly refuted by 31’s antiproliferative properties) or the presence of a heterogenous receptor with multiple binding sites or protein–protein interfaces to which ligand could bind in glioma-like conditions. It was proposed that this could be congruent with the ligand interacting heterogeneously with monomeric, dimeric, and higher-order TSPO oligomers that form dynamically in response to the surrounding cellular environment.[52]
|
Solving the Single Nucleotide Polymorphism (SNP) Problem
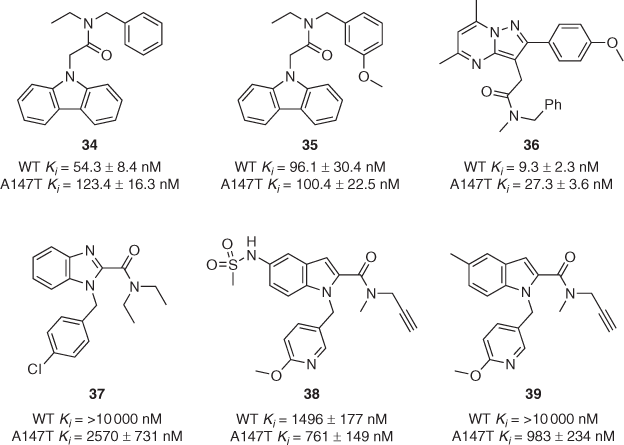
In order to address the issue facing second-generation TSPO ligands such as DPA-713 and DPA-714, our group established isogenic cell lines (HEK-293T) that stably overexpress either wild-type (WT) or A147T TSPO as a basis for a series of structure–binding relationship studies.[53] As part of the same study, a series of N-acetamide-substituted carbazole ligands was synthesised and evaluated using membranes from this new cell line. Compound 34 displayed 2.3-fold binding discrimination between the two isoforms of TSPO (Fig. 4), representing the best result from this series. In 2019, we reported the synthesis and in vitro binding assessment of a more diverse series of carbazole ligands containing dialkyl and alkyl/benzyl N-acetamide-substitutions.[54] For the first time, we were pleased to observe that one of our compounds, 35, bound with high affinity (Ki ≈ 100 nM) to both WT and A147T TSPO. Notably, few of the compounds from these two studies induced increased pregnenolone biosynthesis or showed significant antiproliferative effects in functional assays.
|
In 2020, we published another systematic structure–binding relationship study wherein four different heterocyclic scaffolds (pyrazolopyrimidines, carbazoles, pyrazolobenzodiazepines, and dibenzodiazepines) were decorated with four different pendant acetamide groups (Me/Me, Et/Et, Me/Bn, Et/Bn).[55] Unsurprisingly, the DPA-like pyrazolopyrimidine series proved the highest-affinity binders, with Me/Bn derivative 36 displaying 2.9-fold discrimination with both Kis below 30 nM (Fig. 4), approaching the binding affinity required for effective imaging of TSPO using PET. In contrast, the Et/Bn derivative displayed 13-fold discrepancy between isoforms (WT Ki = 6.2 ± 1.0 nM; A147T Ki = 80.6 ± 4.3 nM). Me/Bn acetamide substitution across all four scaffolds tended to display lower-discrepancy profiles, while Me/Me substitution conferred relatively poor binding across the entire series. In the same year, a thorough investigation of three more scaffolds (benzimidazole-2-carboxamides, indole-2-carboxamides, and acetanilides) with various pendant moieties yielded several compounds that bound preferentially to A147T rather than WT TSPO.[56] Three examples of these (37–39) are shown in Fig. 4, with up to 10-fold selectivity for A14T7 reported for compound 39.
It should be mentioned that there are a small number of third-generation, non-discriminating TSPO ligands being used in preliminary human PET studies. Notable examples are [11C]-ER176[57] and [18F]GE-180,[35,58] though the latter reportedly suffers from poor brain uptake and high activity in blood vessels.[59] In summary, the race is still on for our group and others to develop a truly useful one-size-fits-all TSPO ligand. Improved understanding of structure–binding and structure–activity relationships will allow the development of new imaging agents and therapeutic drug candidates going forward.
Summary and Future Directions
The TSPO continues to be a target of great interest for drug discovery researchers from both fundamental and application-based perspectives. Since 2005, our group has published and patented work surrounding the synthesis and validation of two widely used TSPO PET tracers in [11C]DPA-713 and [18F]DPA-714. Despite the discovery of countless new TSPO ligands in recent years, these tracers remain widely used for assessing neuroinflammation. We have also reported several structure–binding and structure–activity relationship studies that have probed how ligands bind to and affect the function of TSPO. However, the consensus remains that even after decades of research, we are still unravelling the complexities of its role as a biomarker of neuroinflammation and its functional role in healthy and unhealthy humans alike. For example, recent publications have challenged longstanding assumptions by proposing that an increase in TSPO PET signal under pro-inflammatory conditions correlates to increased microglial density rather than the upregulated expression of the protein in humans.[60,61] Moreover, the longstanding assertion that increased TSPO expression is correlated only with microglial activity has been repeatedly challenged, with several studies also detecting its presence in astrocytes and endothelial cells.[62] Furthermore, a key gap in our knowledge is the lack of an atomic structure of human TSPO, with many researchers relying on structures of mouse[63] or bacterial[64] TSPO to construct homology models to guide their work. Improved insights into the key structural features of TSPO that influence ligand binding and its function under pathological conditions will also guide medicinal chemists in their attempts to rationally design drugs that have potent and predictable neuroprotective effects.[65] For those of us focusing our efforts on targeting TSPO, there are undoubtedly more adventures ahead.
Data Availability Statement
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Declaration of Funding
The work presented herein was supported in part by NH&MRC and the European Union’s Seventh Framework Programme (FP7/2007–2013) INMiND (Grant Agreement No. HEALTH-F2–2011–278850).
References
[1] L. Zhang, K. Hu, T. Shao, L. Hou, S. Zhang, W. Ye, L. Josephson, J. H. Meyer, M.-R. Zhang, N. Vasdev, et al. Acta Pharm. Sin. B 2021, 11, 373.| Crossref | GoogleScholarGoogle Scholar | 33643818PubMed |
[2] F. Dolle, C. Luus, A. Reynolds, M. Kassiou, Curr. Med. Chem. 2009, 16, 2899.
| Crossref | GoogleScholarGoogle Scholar | 19689272PubMed |
[3] L. Best, C. Ghadery, N. Pavese, Y. F. Tai, A. P. Strafella, Curr. Neurol. Neurosci. Rep. 2019, 19, 24.
| Crossref | GoogleScholarGoogle Scholar | 30941587PubMed |
[4] S. Selleri, F. Bruni, C. Costagli, A. Costanzo, G. Guerrini, G. Ciciani, B. Costa, C. Martini, Bioorg. Med. Chem. 2001, 9, 2661.
| Crossref | GoogleScholarGoogle Scholar | 11557354PubMed |
[5] M. L. James, R. R. Fulton, D. J. Henderson, S. Eberl, S. R. Meikle, S. Thomson, R. D. Allan, F. Dolle, M. J. Fulham, M. Kassiou, Bioorg. Med. Chem. 2005, 13, 6188.
| Crossref | GoogleScholarGoogle Scholar | 16039131PubMed |
[6] C. Thominiaux, F. Dollé, M. L. James, Y. Bramoullé, H. Boutin, L. Besret, M. C. Grégoire, H. Valette, M. Bottlaender, B. Tavitian, et al. Appl. Radiat. Isot. 2006, 64, 570.
| Crossref | GoogleScholarGoogle Scholar | 16427784PubMed |
[7] M. L. James, R. R. Fulton, J. Vercoullie, D. J. Henderson, L. Garreau, S. Chalon, F. Dolle, S. Selleri, D. Guilloteau, M. Kassiou, J. Nucl. Med. 2008, 49, 814.
| Crossref | GoogleScholarGoogle Scholar | 18413395PubMed |
[8] S. M. Ametamey, M. Honer, PET Chemistry 2007 (Springer: Berlin).
[9] A. Damont, F. Hinnen, B. Kuhnast, M. A. Schöllhorn-Peyronneau, M. James, C. Luus, B. Tavitian, M. Kassiou, F. Dollé, J. Labelled Comp. Radiopharm. 2008, 51, 286.
| Crossref | GoogleScholarGoogle Scholar |
[10] H. Boutin, F. Chauveau, C. Thominiaux, M. C. Grégoire, M. L. James, R. Trebossen, P. Hantraye, F. Dollé, B. Tavitian, M. Kassiou, J. Nucl. Med. 2007, 48, 573.
| Crossref | GoogleScholarGoogle Scholar | 17401094PubMed |
[11] F. Chauveau, N. Van Camp, F. Dollé, B. Kuhnast, F. Hinnen, A. Damont, H. Boutin, M. James, M. Kassiou, B. Tavitian, J. Nucl. Med. 2009, 50, 468.
| Crossref | GoogleScholarGoogle Scholar | 19223401PubMed |
[12] J. Doorduin, H. C. Klein, R. A. Dierckx, M. James, M. Kassiou, E. F. J. de Vries, Mol. Imaging Biol. 2009, 11, 386.
| Crossref | GoogleScholarGoogle Scholar | 19330384PubMed |
[13] A. Martín, R. Boisgard, B. Thézé, N. Van Camp, B. Kuhnast, A. Damont, M. Kassiou, F. Dollé, B. Tavitian, J. Cereb. Blood Flow Metab. 2010, 30, 230.
| Crossref | GoogleScholarGoogle Scholar | 19794397PubMed |
[14] A. Martín, R. Boisgard, M. Kassiou, F. Dollé, B. Tavitian, Mol. Imaging Biol. 2011, 13, 10.
| Crossref | GoogleScholarGoogle Scholar | 20383592PubMed |
[15] H. Boutin, C. Prenant, R. Maroy, J. Galea, A. D. Greenhalgh, A. Smigova, C. Cawthorne, P. Julyan, S. M. Wilkinson, S. D. Banister, et al. PLoS One 2013, 8, e56441.
| Crossref | GoogleScholarGoogle Scholar | 23418569PubMed |
[16] Y. Y. Gent, K. Weijers, C. F. Molthoff, A. D. Windhorst, M. C. Huisman, M. Kassiou, G. Jansen, A. A. Lammertsma, C. J. van der Laken, Arthritis Res. Ther. 2014, 16, R70.
| Crossref | GoogleScholarGoogle Scholar | 24625077PubMed |
[17] S. Sridharan, F. X. Lepelletier, W. Trigg, S. Banister, T. Reekie, M. Kassiou, A. Gerhard, R. Hinz, H. Boutin, Mol. Imaging Biol. 2017, 19, 77.
| Crossref | GoogleScholarGoogle Scholar | 27481358PubMed |
[18] S. Lavisse, K. Inoue, C. Jan, M. A. Peyronneau, F. Petit, S. Goutal, J. Dauguet, M. Guillermier, F. Dollé, L. Rbah-Vidal, et al. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 478.
| Crossref | GoogleScholarGoogle Scholar | 25488184PubMed |
[19] A. M. Chaney, E. M. Johnson, H. C. Cropper, M. L. James, J. Vis. Exp. 2018, 2018, 1.
| Crossref | GoogleScholarGoogle Scholar |
[20] T. Rodríguez-Chinchilla, A. Quiroga-Varela, F. Molinet-Dronda, A. Belloso-Iguerategui, L. Merino-Galan, H. Jimenez-Urbieta, B. Gago, M. C. Rodriguez-Oroz, Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2602.
| Crossref | GoogleScholarGoogle Scholar | 32206840PubMed |
[21] C. J. Endres, M. G. Pomper, M. James, O. Uzuner, D. A. Hammoud, C. C. Watkins, A. Reynolds, J. Hilton, R. F. Dannals, M. Kassiou, J. Nucl. Med. 2009, 50, 1276.
| Crossref | GoogleScholarGoogle Scholar | 19617321PubMed |
[22] C. J. Endres, J. M. Coughlin, K. L. Gage, C. C. Watkins, M. Kassiou, M. G. Pomper, J. Nucl. Med. 2012, 53, 330.
| Crossref | GoogleScholarGoogle Scholar | 22241913PubMed |
[23] N. Arlicot, J. Vercouillie, M. J. Ribeiro, C. Tauber, Y. Venel, J. L. Baulieu, S. Maia, P. Corcia, M. G. Stabin, A. Reynolds, et al. Nucl. Med. Biol. 2012, 39, 570.
| Crossref | GoogleScholarGoogle Scholar | 22172392PubMed |
[24] J. M. Coughlin, Y. Wang, S. Ma, C. Yue, P. K. Kim, A. V. Adams, H. V. Roosa, K. L. Gage, M. Stathis, R. Rais, et al. J. Neurovirol. 2014, 20, 219.
| Crossref | GoogleScholarGoogle Scholar | 24567030PubMed |
[25] Y. Wang, J. M. Coughlin, S. Ma, C. J. Endres, M. Kassiou, A. Sawa, R. F. Dannals, M. Petri, M. G. Pomper, Lupus 2017, 26, 170.
| Crossref | GoogleScholarGoogle Scholar | 27387599PubMed |
[26] J. M. Coughlin, T. Yang, A. W. Rebman, K. T. Bechtold, Y. Du, W. B. Mathews, W. G. Lesniak, E. A. Mihm, S. M. Frey, E. S. Marshall, et al. J. Neuroinflammation 2018, 15, 346.
| Crossref | GoogleScholarGoogle Scholar | 30567544PubMed |
[27] J. M. Coughlin, Y. Wang, C. A. Munro, S. Ma, C. Yue, S. Chen, R. Airan, P. K. Kim, A. V. Adams, C. Garcia, et al. Neurobiol. Dis. 2015, 74, 58.
| Crossref | GoogleScholarGoogle Scholar | 25447235PubMed |
[28] J. M. Coughlin, Y. Yuchuanwang, I. Minn, N. Bienko, E. B. Ambinder, X. Xu, M. E. Peters, J. W. Dougherty, M. Vranesic, S. M. Koo, et al. J. Am. Med. Assoc. Neurol. 2017, 74, 67.
| Crossref | GoogleScholarGoogle Scholar |
[29] M.-J. Ribeiro, J. Vercouillie, S. Debiais, J.-P. Cottier, I. Bonnaud, V. Camus, S. Banister, M. Kassiou, N. Arlicot, D. Guilloteau, EJNMMI Res. 2014, 4, 28.
| Crossref | GoogleScholarGoogle Scholar | 25006546PubMed |
[30] P. Corcia, C. Tauber, J. Vercoullie, N. Arlicot, C. Prunier, J. Praline, G. Nicolas, Y. Venel, C. Hommet, J. L. Baulieu, et al. PLoS One 2012, 7, e52941.
| Crossref | GoogleScholarGoogle Scholar | 23300829PubMed |
[31] M. H. J. Hagens, S. V. Golla, M. T. Wijburg, M. Yaqub, D. Heijtel, M. D. Steenwijk, P. Schober, J. J. P. Brevé, R. C. Schuit, T. A. Reekie, et al. J. Neuroinflammation 2018, 15, 314.
| Crossref | GoogleScholarGoogle Scholar |
[32] M. Fujita, M. Imaizumi, S. S. Zoghbi, Y. Fujimura, A. G. Farris, T. Suhara, J. Hong, V. W. Pike, R. B. Innis, Neuroimage 2008, 40, 43.
| Crossref | GoogleScholarGoogle Scholar | 18093844PubMed |
[33] M. Ikawa, T. G. Lohith, S. Shrestha, S. Telu, S. S. Zoghbi, S. Castellano, S. Taliani, F. Da Settimo, M. Fujita, V. W. Pike, et al. J. Nucl. Med. 2017, 58, 320.
| Crossref | GoogleScholarGoogle Scholar | 27856631PubMed |
[34] P. M. Rusjan, A. A. Wilson, P. M. Bloomfield, I. Vitcu, J. H. Meyer, S. Houle, R. Mizrahi, J. Cereb. Blood Flow Metab. 2011, 31, 1807.
| Crossref | GoogleScholarGoogle Scholar | 21522163PubMed |
[35] Z. Fan, V. Calsolaro, R. A. Atkinson, G. D. Femminella, A. Waldman, C. Buckley, W. Trigg, D. J. Brooks, R. Hinz, P. Edison, J. Nucl. Med. 2016, 57, 1753.
| Crossref | GoogleScholarGoogle Scholar | 27261523PubMed |
[36] D. R. J. Owen, R. N. Gunn, E. A. Rabiner, I. Bennacef, M. Fujita, W. C. Kreisl, R. B. Innis, V. W. Pike, R. Reynolds, P. M. Matthews, et al. J. Nucl. Med. 2011, 52, 24.
| Crossref | GoogleScholarGoogle Scholar |
[37] D. R. Owen, A. J. Yeo, R. N. Gunn, K. Song, G. Wadsworth, A. Lewis, C. Rhodes, D. J. Pulford, I. Bennacef, C. A. Parker, et al. J. Cereb. Blood Flow Metab. 2012, 32, 1.
| Crossref | GoogleScholarGoogle Scholar | 22008728PubMed |
[38] D. R. Owen, J. Fan, E. Campioli, S. Venugopal, A. Midzak, E. Daly, A. Harlay, L. Issop, V. Libri, D. Kalogiannopoulou, et al. Biochem. J. 2017, 474, 3985.
| Crossref | GoogleScholarGoogle Scholar | 29074640PubMed |
[39] A. Colasanti, D. R. Owen, D. Grozeva, E. A. Rabiner, P. M. Matthews, N. Craddock, A. H. Young, Psychoneuroendocrinology 2013, 38, 2826.
| Crossref | GoogleScholarGoogle Scholar | 23942012PubMed |
[40] S. D. Banister, S. M. Wilkinson, R. Hanani, A. J. Reynolds, D. E. Hibbs, M. Kassiou, Tetrahedron Lett. 2012, 53, 3780.
| Crossref | GoogleScholarGoogle Scholar |
[41] A. Reynolds, R. Hanani, D. Hibbs, A. Damont, E. Da Pozzo, S. Selleri, F. Dollé, C. Martini, M. Kassiou, Bioorg. Med. Chem. Lett. 2010, 20, 5799.
| Crossref | GoogleScholarGoogle Scholar | 20727749PubMed |
[42] S. D. Banister, C. Beinat, S. M. Wilkinson, B. Shen, C. Bartoli, S. Selleri, E. Da Pozzo, C. Martini, F. T. Chin, M. Kassiou, Eur. J. Med. Chem. 2015, 93, 392.
| Crossref | GoogleScholarGoogle Scholar | 25725375PubMed |
[43] K. R. Leaver, A. Reynolds, S. Bodard, D. Guilloteau, S. Chalon, M. Kassiou, ACS Chem. Neurosci. 2012, 3, 114.
| Crossref | GoogleScholarGoogle Scholar | 22860181PubMed |
[44] E. L. Werry, V. A. King, M. L. Barron, S. D. Banister, R. Sokias, M. Kassiou, Eur. J. Pharm. Sci. 2017, 96, 186.
| Crossref | GoogleScholarGoogle Scholar | 27658888PubMed |
[45] B. Costa, E. Da Pozzo, C. Giacomelli, S. Taliani, S. Bendinelli, E. Barresi, F. Da Settimo, C. Martini, Apoptosis 2015, 20, 383.
| Crossref | GoogleScholarGoogle Scholar | 25413799PubMed |
[46] B. Costa, E. Da Pozzo, C. Giacomelli, E. Barresi, S. Taliani, F. Da Settimo, C. Martini, Sci. Rep. 2016, 6, 18164.
| Crossref | GoogleScholarGoogle Scholar | 26750656PubMed |
[47] E. L. Crossley, F. Issa, A. M. Scarf, M. Kassiou, L. M. Rendina, Chem. Commun. 2011, 12179.
| Crossref | GoogleScholarGoogle Scholar |
[48] R. Narlawar, C. J. D. Austin, J. Kahlert, S. Selleri, E. Da Pozzo, C. Martini, E. L. Werry, L. M. Rendina, M. Kassiou, Chem. Asian J. 2018, 13, 3321.
| Crossref | GoogleScholarGoogle Scholar | 30369074PubMed |
[49] G. Campiani, V. Nacci, I. Fiorini, M. P. De Filippis, A. Garofalo, S. M. Ciani, G. Greco, E. Novellino, D. C. Williams, D. M. Zisterer, et al. J. Med. Chem. 1996, 39, 3435.
| Crossref | GoogleScholarGoogle Scholar | 8784441PubMed |
[50] A. M. Scarf, C. Luus, E. Da Pozzo, S. Selleri, C. Guarino, C. Martini, L. M. Ittner, M. Kassiou, Curr. Mol. Med. 2012, 12, 488.
| Crossref | GoogleScholarGoogle Scholar | 22348617PubMed |
[51] R. Narlawar, E. L. Werry, A. M. Scarf, R. Hanani, S. W. Chua, V. A. King, M. L. Barron, R. N. Martins, L. M. Ittner, L. M. Rendina, et al. J. Med. Chem. 2015, 58, 8743.
| Crossref | GoogleScholarGoogle Scholar | 26461041PubMed |
[52] F. Delavoie, H. Li, M. Hardwick, J.-C. Robert, C. Giatzakis, G. Péranzi, Z.-X. Yao, J. Maccario, J.-J. Lacapère, V. Papadopoulos, Biochemistry 2003, 42, 4506.
| Crossref | GoogleScholarGoogle Scholar | 12693947PubMed |
[53] R. Sokias, E. L. Werry, S. W. Chua, T. A. Reekie, L. Munoz, E. C. N. Wong, L. M. Ittner, M. Kassiou, MedChemComm 2017, 8, 202.
| Crossref | GoogleScholarGoogle Scholar | 30108706PubMed |
[54] H. W. A. Cheng, R. Sokias, E. L. Werry, L. M. Ittner, T. A. Reekie, J. Du, Q. Gao, D. E. Hibbs, M. Kassiou, J. Med. Chem. 2019, 62, 8235.
| Crossref | GoogleScholarGoogle Scholar |
[55] R. Sokias, E. L. Werry, H. W. Alison Cheng, J. H. Lloyd, G. Sohler, J. J. Danon, A. P. Montgomery, J. J. Du, Q. Gao, D. E. Hibbs, et al. Eur. J. Med. Chem. 2020, 207, 112725.
| Crossref | GoogleScholarGoogle Scholar | 32920427PubMed |
[56] S. V. Vo, S. D. Banister, I. Freelander, E. L. Werry, T. A. Reekie, L. M. Ittner, M. Kassiou, RSC Med. Chem. 2020, 11, 511.
| Crossref | GoogleScholarGoogle Scholar | 33479652PubMed |
[57] M. Ikawa, T. G. Lohith, S. Shrestha, S. Telu, S. S. Zoghbi, S. Castellano, S. Taliani, F. Da Settimo, M. Fujita, V. W. Pike, et al. J. Nucl. Med. 2017, 58, 320.
| Crossref | GoogleScholarGoogle Scholar | 27856631PubMed |
[58] A. Nack, M. Brendel, J. Nedelcu, M. Daerr, S. Nyamoya, C. Beyer, C. Focke, M. Deussing, C. Hoornaert, P. Ponsaerts, et al. Cells 2019, 8, 94.
| Crossref | GoogleScholarGoogle Scholar |
[59] P. Zanotti-Fregonara, M. Veronese, B. Pascual, R. C. Rostomily, F. Turkheimer, J. C. Masdeu, Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1205.
| Crossref | GoogleScholarGoogle Scholar | 30656358PubMed |
[60] D. R. Owen, N. Narayan, L. Wells, L. Healy, E. Smyth, E. A. Rabiner, D. Galloway, J. B. Williams, J. Lehr, H. Mandhair, et al. J. Cereb. Blood Flow Metab. 2017, 37, 2679.
| Crossref | GoogleScholarGoogle Scholar | 28530125PubMed |
[61] E. Nutma, E. Gebro, M. C. Marzin, P. Valk, P. M. Matthews, D. R. Owen, S. Amor, Glia 2021, 69, 2447.
| Crossref | GoogleScholarGoogle Scholar | 34145928PubMed |
[62] E. Nutma, K. Ceyzériat, S. Amor, S. Tsartsalis, P. Millet, D. R. Owen, V. Papadopoulos, B. B. Tournier, Eur. J. Nucl. Med. Mol. Imaging 2021,
| Crossref | GoogleScholarGoogle Scholar | 33433698PubMed |
[63] Ł. Jaremko, M. Jaremko, K. Giller, S. Becker, M. Zweckstetter, Science 2014, 343, 1363.
| Crossref | GoogleScholarGoogle Scholar | 24653034PubMed |
[64] F. Li, J. Liu, Y. Zheng, R. M. Garavito, S. Ferguson-Miller, Science 2015, 347, 555.
| Crossref | GoogleScholarGoogle Scholar | 25635101PubMed |
[65] J.-J. Lacapere, L. Duma, S. Finet, M. Kassiou, V. Papadopoulos, Trends Pharmacol. Sci. 2020, 41, 110.
| Crossref | GoogleScholarGoogle Scholar | 31864680PubMed |
* Michael Kassiou is the recipient of the 2020 RACI Applied Research Award.