Valencene derived from essential oils of Psidium guajava L. as multi-target neurodegenerative inhibitor: a computational study
Ram Lal Swagat Shrestha A , Sujan Dhital A , Nirmal Parajuli A , Prabhat Neupane A , Manila Poudel
A , Nirmal Parajuli A , Prabhat Neupane A , Manila Poudel  B , Timila Shrestha A , Samjhana Bharati A , Binita Maharjan A , Bishnu Prasad Marasini
B , Timila Shrestha A , Samjhana Bharati A , Binita Maharjan A , Bishnu Prasad Marasini  C * and Jhashanath Adhikari Subin D *
C * and Jhashanath Adhikari Subin D *
A
B
C
D
Handling Editor: Amir Karton
Abstract
The prevalence of neurodegenerative disorders such as Alzheimer’s, Parkinson’s and Huntington’s has been gradually increasing in recent times. These diseases could respectively be treated through the inhibition of acetylcholinesterase (AChE), monoamine oxidase B (MAO-B) and huntingtin (Htt) proteins. This study aims to identify the compounds present in the oil of Psidium guajava L. using GC-MS analysis and the potent neurodegenerative inhibitors through computational methods. The results revealed the presence of 42 different phytocompounds, and molecular docking calculations demonstrated the firm binding of valencene with the receptor proteins AChE, MAO-B and HTT, with respective binding affinities of −9.246, −9.794 and −9.541 kcal mol–1, better than that of reference drugs. All three complexes, valencene–AChE, valencene–MAO-B and valencene–HTT demonstrated good geometrical stability, showing smooth RMSD curves and ligand RMSD of ~3.5, ~3.0 and ~6.0 Å respectively from 100-ns molecular dynamics simulation. The thermodynamic stability assessed through the MMPBSA method, in terms of binding free energy changes, revealed sustained spontaneity and feasibility of the adduct formations. The pharmacodynamics and pharmacokinetics predicted the drug-like properties of the hit candidate. Therefore, after validating the computational results through in vivo and in vitro experiments, valencene could be a potential remedy for neurological disorders.
Keywords: ADMET, essential oil, free energy changes, molecular docking, molecular dynamics, neurodegenerative disorders, Psidium guajava.
Introduction
Neurodegeneration, characterised by the gradual dysfunction and deterioration of neurons and axons within the central nervous system, stands as the predominant pathological hallmark of both acute and chronic neurodegenerative disorders like Alzheimer’s disease (AD), Parkinson’s disease (PD) and Huntington’s disease (HD).1 The occurrence of neurodegenerative diseases is anticipated to rise with increasing lifespan in most countries.2 Currently, ~50 million individuals are affected by dementia, a number projected to surge to 130 million by 2050.3 AD is the most prevalent form of neurodegenerative disorder resulting in a gradual decline in cognitive function and is becoming one of the most costly, deadliest and burdensome illnesses.4 Similarly, PD stands as the second most prevalent neurodegenerative disorder, affecting over 6 million people worldwide.5 Parkinson’s syndrome is distinguished by the loss of dopaminergic cells and observable irregularities in the spontaneous activity and sensorimotor responses of neurons.6 Likewise, another inherited neurodegenerative disease, HD, is caused by the repetition of CAG sequences (36 repeats or more) in the initial exon of the huntingtin (Htt) protein. This results in an anomalous form of the Htt protein that clumps together to form aggregates,7 characterised by uncontrollable, exaggerated motor movements, alongside emotional and cognitive impairments.8 AD could be treated by inhibiting acetylcholinesterase (AChE), a crucial enzyme responsible for the degradation of acetylcholine, thereby enhancing the effectiveness of brain signaling. AChE inhibitors prevent the breakdown of acetylcholine, consequently fostering enhancements in cholinergic neurotransmission.9 Analogously, inhibition of Monoamine Oxidase B (MAO-B) could lead to effective treatment of PD.10 In patients affected with PD, high levels of MAO-B in the brains and blood platelets have been observed and blocking this enzyme could potentially provide a pathway for curing the condition.11 HD can be addressed by inhibiting the aggregation of mutant Htt by targeting the potential sites that facilitate protein aggregation, thus potentially slowing down the disease’s progression.12
Psidium guajava L. (P. guajava) belongs to the Myrtaceae family and is a commercial crop in numerous subtropical and tropical regions across the globe.13 Owing to the presence of a large number of phytocompounds in the leaves of the guava plant, it has been used for various health benefits such as antidiabetic, antioxidant, anticancer, antimicrobial and hepatoprotection activities since ancient times.14 The leaves have also been used to treat neurodegenerative conditions like AD.15,16 Numerous studies have been performed using various computational approaches, as they are known for their cost effectiveness and efficiency in the drug discovery process.17 The binding affinity and putative binding mode between the receptors and ligands can be identified using computational techniques.18 The aim of this research was to identify the phytocompounds present in the oil of guava leaves using GC-MS analysis and to study their ability to manage neurodegenerative disorders computationally.
Results and discussion
GC-MS analysis
The analysis of the GC-MS chromatogram revealed 42 peaks in the oil of P. guajava, as shown in the Supplementary Fig. S1. It confirmed the presence of 42 compounds, with limonene being the most prevalent one, constituting 30.15% of the area percentage. The mass spectra of each phytocompound, along with its structure, are depicted in the Supplementary Fig. S2 and S3. The name, peak number, molecular weight, area percentage, molecular formula and retention time of the obtained compounds are shown in Table 1.
| Peak number | Name of compounds | Molecular formula | Molecular weight (g mol–1) | Retention time (min) | Area (%) | |
|---|---|---|---|---|---|---|
| 1 | α-Pinene | C10H16 | 136.23 | 9.435 | 0.51 | |
| 2 | Myrcene | C10H16 | 136.23 | 11.749 | 0.68 | |
| 3 | 1,4,4-Trimethyl-3,5-dimethylidenecyclopentene | C10H14 | 134.22 | 13.262 | 0.26 | |
| 4 | Limonene | C10H16 | 136.23 | 13.488 | 30.15 | |
| 5 | Eucalyptol | C10H18O | 154.25 | 13.606 | 1.19 | |
| 6 | (E)-β-ocimene | C10H16 | 136.23 | 13.856 | 0.36 | |
| 7 | Lavandulyl 2-methylbutyrate | C15H26O2 | 238.37 | 29.311 | 0.22 | |
| 8 | α-Copaene | C15H24 | 204.35 | 29.935 | 4.54 | |
| 9 | (Z)-caryophyllene | C15H24 | 204.35 | 31.584 | 1.08 | |
| 10 | (E)-Caryophyllene | C15H24 | 204.35 | 31.948 | 16.63 | |
| 11 | Valencene | C15H24 | 204.35 | 33.231 | 0.49 | |
| 12 | α-Humulene | C15H24 | 204.35 | 33.425 | 2.88 | |
| 13 | 9-epi-(E)-caryophyllene | C15H24 | 204.35 | 33.753 | 0.47 | |
| 14 | γ-Muurolene | C15H24 | 204.35 | 34.385 | 0.36 | |
| 15 | Trans-bergamotol | C15H24O | 220.35 | 34.532 | 0.50 | |
| 16 | β-Selinene | C15H24 | 204.35 | 34.857 | 0.29 | |
| 17 | α-amorphene | C15H24 | 204.35 | 35.407 | 0.77 | |
| 18 | β-Bisabolene | C15H24 | 204.35 | 35.678 | 0.39 | |
| 19 | Spathulenol | C15H24O | 220.35 | 36.074 | 5.28 | |
| 20 | Trans-calamenene | C15H22 | 202.33 | 36.381 | 3.59 | |
| 21 | Trans-cadina-1,4-diene | C15H24 | 204.35 | 36.762 | 1.10 | |
| 22 | α-Dehydro-ar-himachalene | C15H20 | 200.32 | 37.061 | 0.22 | |
| 23 | α-Calacorene | C15H20 | 200.32 | 37.227 | 0.51 | |
| 24 | 14-Hydroxy-4,5-dihydro-β-caryophyllene | C15H26O | 222.37 | 37.649 | 0.79 | |
| 25 | Z-Nerolidyl acetate | C17H28O2 | 264.4 | 37.930 | 7.47 | |
| 26 | Caryolan-8-ol | C15H26O | 222.37 | 38.414 | 0.33 | |
| 27 | Aromadendrene | C15H24 | 204.35 | 39.784 | 2.59 | |
| 28 | Copaene | C15H24 | 204.35 | 40.205 | 0.23 | |
| 29 | Cis-calamenene | C15H22 | 202.33 | 40.300 | 0.25 | |
| 30 | 14-Hydroxy-9-epi-(E)-caryophyllene | C15H24O | 220.35 | 40.540 | 0.55 | |
| 31 | α-Cubebene | C15H24 | 204.35 | 40.726 | 3.10 | |
| 32 | Allo-aromadendrene epoxide | C15H24O | 220.35 | 40.840 | 3.32 | |
| 33 | Cis-p-Mentha-1(7),8-dien-2-ol | C10H16O | 152.23 | 40.920 | 0.97 | |
| 34 | Caryophylla-4(12),8(13)-dien-5-ol | C15H24O | 220.35 | 41.076 | 0.47 | |
| 35 | Guaiac acetate | C17H28O2 | 264.4 | 41.200 | 1.05 | |
| 36 | Epicubenol | C15H26O | 222.37 | 41.278 | 1.67 | |
| 37 | α-Muurolol | C15H26O | 222.37 | 41.425 | 1.67 | |
| 38 | δ-Amorphene | C15H24 | 204.35 | 41.755 | 0.94 | |
| 39 | α-Himachalene | C15H24 | 204.35 | 42.340 | 0.43 | |
| 40 | 14-hydroxy-Caryophyllene | C15H24O | 220.35 | 42.416 | 0.90 | |
| 41 | (E)-β-farnesene | C15H26 | 206.37 | 44.176 | 0.56 | |
| 42 | Nootkatene | C15H22 | 202.33 | 50.363 | 0.27 |
Binding affinity from molecular docking calculations
The molecular docking calculation was used to assess the best binding pose of the ligands within the active site of the receptor protein, in terms of binding affinity.19 The results of molecular docking with the MAO-B protein revealed β-bisabolene, nootkatene, allo-aromadendrene epoxide, α-copaene and valencene as the top five best ligands, with binding affinities ranging from −9.993 to −9.794 kcal mol–1, signifying stronger interaction and binding with the receptor. The highest binding affinity was demonstrated by β-bisabolene with −9.993 kcal mol–1. The binding affinity of valencene was −9.794 kcal mol–1, greater than that of the reference drug (−8.817 kcal mol–1).
For the molecular docking calculation with the Htt protein, the best binding affinity was observed with β-bisabolene with a binding affinity of −9.776 kcal mol–1 followed by 9-epi-(E)-caryophyllene and allo-aromadendrene epoxide, with −9.691 and −9.666 kcal mol–1 respectively. Similarly, the binding affinity of −9.541 kcal mol–1 was observed for valencene.
In the case of the AChE protein, the outcome of molecular docking demonstrated the best binding affinity of −9.559 kcal mol–1 for guaiac acetate. The binding affinities of −9.526 and −9.305 kcal mol–1 were obtained for Z-nerolidyl acetate and β-bisabolene respectively. Likewise, the binding affinity of −9.246 kcal mol–1 was shown by valencene, higher than that of the reference drug (−8.883 kcal mol–1).
Among the studied compounds, the molecular docking of β-bisabolene, allo-aromadendrene epoxide, valencene and nootkatene exhibited better binding affinities with all the neurodegenerative receptor proteins; MAO-B, huntingtin and acetylcholinesterase, as shown in Table 2 and Supplementary Table S1.
| Series number | Ligands | PubChem CID | Binding affinity (kcal mol–1) | |||
|---|---|---|---|---|---|---|
| MAO-B | HTT | AChE | ||||
| 1 | β-Bisabolene | 10104370 | −9.993 | −9.776 | −9.305 | |
| 2 | Nootkatene | 25200342 | −9.831 | −9.554 | −9.187 | |
| 3 | Allo-aromadendrene epoxide | 91746712 | −9.803 | −9.666 | −9.247 | |
| 4 | α-Copaene | 70678558 | −9.802 | −9.515 | −9.089 | |
| 5 | Valencene | 9855795 | −9.794 | −9.541 | −9.246 | |
| 6 | E-Caryophyllene | 5281515 | −9.75 | −9.606 | −9.1 | |
| 7 | α-Humulene | 5281520 | −9.745 | −9.548 | −9.114 | |
| 8 | α-Himachalene | 520909 | −9.74 | −9.503 | −9.188 | |
| 9 | Cis-calamenene | 6429077 | −9.719 | −9.384 | −9.028 | |
| 10 | α-Dehydro-ar-himachalene | 15098633 | −9.705 | −9.419 | −9.138 | |
| 11 | Native | – | −10.551 | −9.785 | −11.183 | |
| 12 | Selegiline | 26757 | −8.817 | – | – | |
| 13 | Rivastigmine | 77991 | – | – | −8.883 | |
Valencene is denoted in bold for quick identification and comparison.
Protein–ligand interactions
The three dimensional (3-D) and two dimensional (2-D) representations of the interactions between the ligand and the amino acid residue of the proteins are shown in Fig. 1. It depicts the presence of only hydrophobic interactions, like pi–alkyl, alkyl, pi–sigma and van der Waals between the ligand and receptor proteins, as shown in Table 3. In the interaction of valencene with the MAO-B (4A79) protein, pi–alkyl interactions were observed with the amino acid residues PHE101, TRP117 and TYR324. Likewise, PRO102, LEU162, ILE197 and ILE 314 showed alkyl interaction with the ligand. In the valencene–Htt (7F61) protein complex, the amino acid residues TYR67, TRP83, TYR88, TYR162, PHE166, TYR262, PHE266 and TRP270 exhibited pi–alkyl interactions with the ligand. The alkyl and pi–sigma interaction was observed with amino acid residues LEU84 and PHE266 respectively. For the hydrophobic interaction, pi–alkyl interactions were observed between the valencene and the amino acid residues TRP83, TYR238, PHE329, TYR332 and HIS438 of the AChE (7E3H) protein. Several van der Waals interactions were observed between the ligand (valencene) and different receptor proteins. The interaction between the valencene molecule and the amino acid residues of the proteins depicted a strong hydrophobic interaction, inferring its potential to inhibit the normal functioning of acetylcholinesterase, monoamine oxidase B and huntingtin proteins.
3-D Representation of the docked ligand at the binding site (left) and 2-D interaction profiles (right) of valencene with amino acid residues of (a) MAO-B (4A79), (b) Htt (7F61) and (c) AChE (7E3H) proteins.
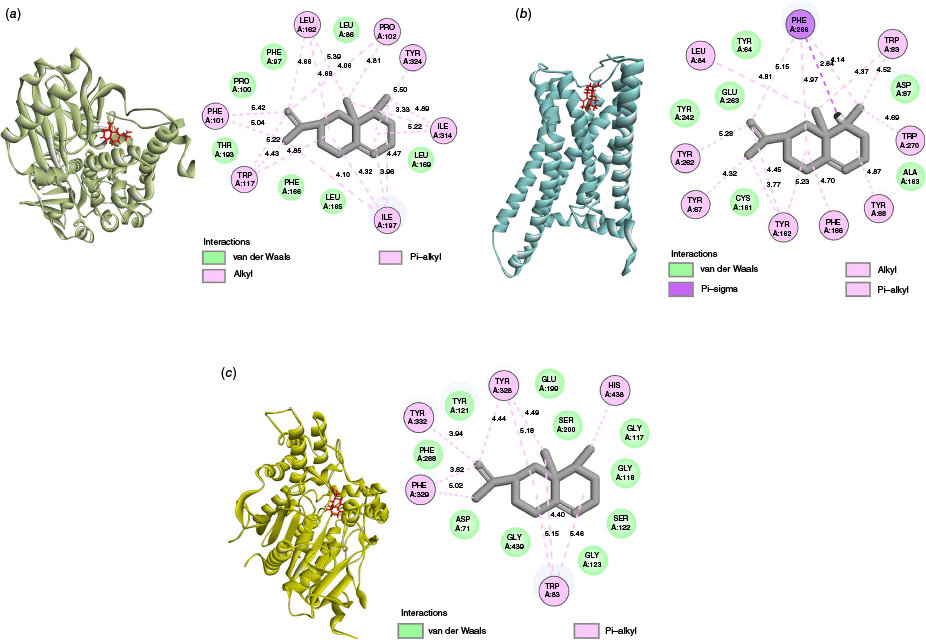
| Proteins | Types of interaction | Active site residues (Å) | |
|---|---|---|---|
| MAO-B (4A79) | Alkyl | PRO102 (4.06, 4.81), LEU162 (4.66, 4.68, 5.39), ILE197 (3.96, 4.10, 4.32, 4.47), ILE314 (3.33, 4.89, 5.22) | |
| Pi–alkyl | PHE101 (5.04, 5.42), TRP117 (4.43, 4.85, 5.22), TYR324 (5.50) | ||
| Van der Waals | LEU86, PHE97, PRO100, LEU165, PHE166, LEU169, THR193 | ||
| HTT (7F61) | Alkyl | LEU84 (4.81) | |
| Pi–alkyl | TYR67 (4.32), TRP83 (4.37, 4.52), TYR88 (4.87), TYR162 (3.77, 4.45, 5.23), PHE166 (4.70), TYR262 (5.28), PHE266 (4.14, 4.97, 5.15), TRP270 (4.69) | ||
| Pi–sigma | PHE266 (2.64) | ||
| Van der Waals | TYR64, ASP87, CYS161, ALA163, TYR242, GLU263 | ||
| AChE (7E3H) | Pi–alkyl | TRP83 (4.40, 5.15, 5.46), TYR328 (4.44, 4.49, 5.18), PHE329 (3.82, 5.02), TYR332 (3.94), HIS438 (4.51) | |
| Van der Waals | ASP71, GLY117, GLY118, TYR121, SER122, GLY123, GLU199, SER200, PHE288, GLY439 |
Molecular Dynamics Simulation (MDS) analysis
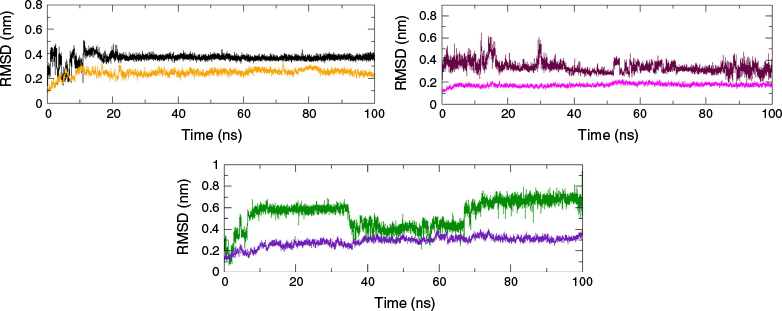
The geometrical stability of ligands in their adducts can be assessed through the root mean square deviation (RMSD) profiling obtained from the MDS trajectory.20 The RMSD of the protein backbone and ligand, with respect to the protein backbone, depicts the overall stability of the formed adducts as shown in Fig. 2.21 The molecular dynamics simulation of top-docked ligands showed the good stability of valencene with proteins (PDB ID: 4A79, 7F61 and 7E3H), with RMSD below 6.5 Å. The fairly smooth RMSD curve of the ligand demonstrated the stability of the adduct during the production run. An exceptional stability was shown by valencene with the MAO-B protein, with a consistently smoother RMSD trajectory of ~3.5 Å despite some initial spikes. The ligand RMSD of ~3.0 Å, with slight fluctuation and spikes, was depicted by valencene with the AChE protein, inferring that the ligand remained docked at the same location throughout the production run. However, valencene exhibited moderate stability with the Htt protein, with a RMSD of ~6.0 Å, with some fluctuations. Despite major fluctuations and spikes in the trajectory, the system attained equilibrium after 70 ns. A smooth curve with a RMSD of ~2.0 Å of the protein backbone inferred the stability of the protein structure upon ligand binding. No significant structural changes were observed.
RMSD of valencene with respect to three different protein backbones in three adducts with MAO-B (black), AChE (maroon) and Htt (green); RMSD of protein backbones with respect to protein backbone in three adducts (orange, MAO-B; magenta, AChE; and indigo, Htt).
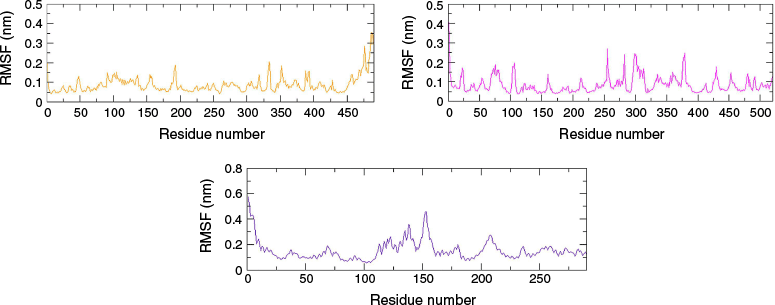
The root mean square fluctuation (RMSF) depicts the extent of fluctuation and flexibility of the amino acid residues of a protein over the 100-ns production run. Higher RMSF indicates greater flexibility during simulation, whereas lower RMSF indicates minimal changes, implying a tightly bound protein–ligand complex and enhanced system stability.22 The RMSF plot (Fig. 3) depicted that the fluctuation of α-carbon atoms of the MAO-B protein was less than 2.0 Å for almost all amino acid residues except for the terminal part. Similarly, the RMSF of the amino acid residue of the AChE and Htt proteins were less than 3.0 Å, with exceptions at ~140 and 155 amino acid residue numbers of the 7F61 protein.
RMSF plots of protein backbone of 4A79–valencene (orange), 7E3H–valencene (magenta) and 7F61–valencene (indigo) complexes.
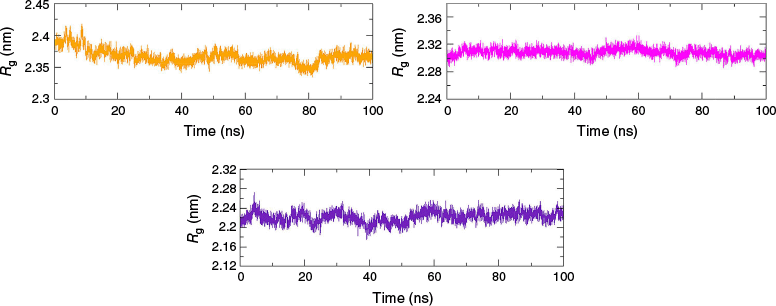
The radius of gyration (Rg) obtained through MDS reflects the compactness of protein–ligand complexes, with smaller Rg suggesting a more compact structure.23 From the calculation of Rg, a steady and smooth trajectory at ~23 ± 0.7 Å indicated no significant expansion or shrinkage of the proteins, implying the stability of all three proteins (4A79, 7E3H and 7F61) upon the binding of the ligand, as evident from Fig. 4.
Rg of protein in MAO-B–valencene (orange), AChE–valencene (magenta) and Htt–valencene (indigo) complexes.
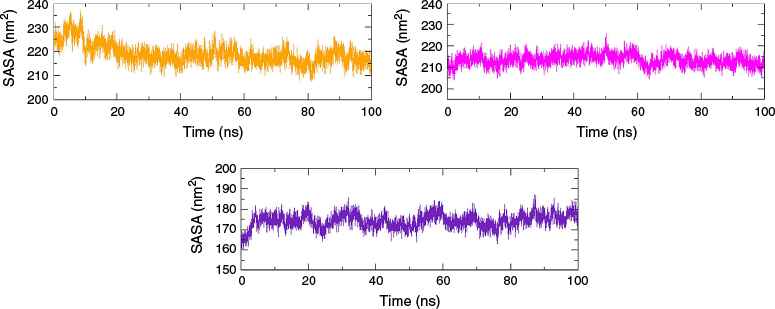
The solvent-accessible surface areas (SASA) refer to the area of a protein’s surface that interacts with its solvent molecules.24 A reasonably smooth trajectory with SASA of 216 ± 4 nm2 was observed with MAO-B–valencene and AChE–valencene complexes respectively. Likewise, a smooth SASA curve with a value of ~175 nm2 was obtained for the Htt–valencene complex (Fig. 5). The SASA remained almost stable throughout the production run without any significant bumps and spikes, indicating no change in the wettable area of the protein upon ligand binding. The combined analysis of SASA, RMSF and Rg demonstrated the stability of the protein structure in the holo form.
Binding free energy changes (ΔGBFE) or thermodynamic stability
The binding free energy changes (ΔGBFE) was used to assess the feasibility and spontaneity of the complex formation: the lower the negative value of ΔGBFE, the greater the stability of the protein–ligand system.25,26 The free energy changes of the adducts calculated for an equilibrated part of the trajectory (20 ns) are shown in Table 4. The best ΔGBFE was observed for the MAO-B–valencene complex with −25.18 ± 2.30 kcal mol–1. Similarly, negative binding free energy changes of −15.17 ± 3.44 and −18.98 ± 2.38 kcal mol–1 were observed for the AChE–valencene and Htt–valencene complexes respectively. The negative ΔGBFE values for all three complexes suggested the spontaneous nature of complex formation.
| Complexes | ΔEVDWAALS | ΔEEL | ΔEPB | ΔENPOLAR | ΔGGAS | ΔGSOLV | ΔGBFE | |
|---|---|---|---|---|---|---|---|---|
| 4A79–valencene | −37.81 ± 1.65 | −1.14 ± 0.45 | 16.78 ± 1.58 | −3.01 ± 0.05 | −38.95 ± 1.71 | 13.77 ± 1.58 | −25.18 ± 2.30 | |
| 7E3H–valencene | −28.94 ± 1.88 | −0.36 ± 1.12 | 17.31 ± 3.47 | −3.17 ± 0.09 | −29.31 ± 2.23 | 14.14 ± 3.44 | −15.17 ± 3.44 | |
| 7F61–valencene | −29.87 ± 2.19 | −0.11 ± 0.86 | 13.87 ± 1.78 | −3.08 ± 0.09 | −29.76 ± 2.17 | 10.79 ± 1.76 | −18.98 ± 2.38 |
Drug-likeness and toxicity
The pharmacokinetic and pharmacodynamic characteristics of valencene were predicted by in silico tools to evaluate its drug-likeness and suitability for human consumption, as depicted in Table 5. From the prediction of absorption, metabolism, excretion, distribution and toxicity (ADMET), it was observed that the valencene molecule belongs to toxicity class 5 and obeys the Lipinski’s rule of 5 (RO5), suggesting a probability for drug-likeness. The compound did not show any sign of immunotoxicity, carcinogenicity, mutagenicity, cytotoxicity, or hepatotoxicity but exhibited skin sensitisation and neurotoxicity, analogous to the reference drugs, selegiline and rivastigmine. The blood–brain barrier (BBB) plays a very crucial role in the treatment of neurodegenerative disorders, as a majority of molecules encounter difficulties in crossing this barrier.27 The hit candidate and both reference drugs demonstrated the potential to cross the BBB, indicating their capability to function as brain-targeting drugs. The metabolic activity of valencene was similar to that of the drugs and exhibited no hERG-blocking properties, implying that the compound does not induce fatal ventricular arrhythmia. The compound demonstrated high intestinal absorption, like the reference drugs, representing effective passage of the compound through the intestinal cell membranes suitable as an oral formulation.28 The total clearance of the compound was almost similar to the clearance of the reference drug, suggesting its potential to be used as a drug.
| ADMET parameters | Compounds | |||
|---|---|---|---|---|
| Valencene | Selegiline | Rivastigmine | ||
| Toxicity class | 5 | 4 | 4 | |
| Lipinski’s rule of 5 (RO5) | Yes | Yes | Yes | |
| Immunotoxicity | No | No | Yes | |
| Hepatotoxicity | No | No | No | |
| Carcinogenicity | No | No | No | |
| Mutagenicity | No | No | No | |
| Neurotoxicity | Yes | Yes | Yes | |
| Cytotoxicity | No | No | No | |
| Skin sensitisation | Yes | Yes | No | |
| hERG I inhibitor | No | No | No | |
| hERG II inhibitor | No | No | No | |
| CYP2D6 substrate | No | No | No | |
| CYP3A4 substrate | Yes | No | Yes | |
| CYP1A2 inhibitor | No | Yes | No | |
| CYP2C19 inhibitor | No | No | No | |
| CYP2C9 inhibitor | Yes | Yes | No | |
| CYP2D6 inhibitor | No | No | No | |
| CYP3A4 inhibitor | No | No | No | |
| BBB permeability | Yes | Yes | Yes | |
| Skin permeability | Low | Low | High | |
| Caco-2 permeability | High | High | High | |
| Intestinal absorption | High | High | High | |
| Total clearance log (mL min–1 kg–1) | 1.205 | 1.007 | 0.557 | |
The comparative evaluation of toxicity and pharmacokinetics of valencene with reference drugs revealed its less toxic nature along with its potential to treat neurodegenerative disorders. The ADMET results suggested further exploration of the hit compound through experimental trials, in vivo and in vitro, to assess its suitability for human consumption.
Conclusion
Among the 42 compounds identified from the GC-MS analysis of the essential oil of Psidium guajava L., the in silico approach revealed valencene as a potent inhibitor of acetylcholinesterase, monoamine oxidase B and huntingtin proteins. The compound exhibited strong thermodynamic and geometric stability with all the proteins as adducts and could possibly inhibit the normal functioning possibly leading to the treatment of neurodegenerative diseases. ADMET analysis suggested that valencene could be a drug-like candidate with safe toxicity levels. However, further in vivo and in vitro experimentation is necessary to validate the in silico findings. Hence, plant-derived compounds could be employed in addressing neurodegenerative disorders.
Experimental
Collection of plant samples and extraction of essential oil
The leaves of Psidium guajava L. were collected from Kathmandu, Nepal, and verified by the National Herbarium and Plant Laboratories, Lalitpur, Nepal. The essential oil from the leaves was extracted through hydrodistillation using a Clevenger-type apparatus.29
GC-MS analysis
Gas chromatography–mass spectrometry (GCMS) experiments were performed using a GCMS-QP 2010 instrument. Helium was used as the carrier gas, passing through an RTX-5MS column of dimension 60 m × 0.32 mm × 0.25 μm. The temperature profile included a gradual increase from 80 to 300°C, with respective hold times of 2.0 and 5.0 min. Throughout the analysis, the ion source and interface were consistently maintained at 200 and 250°C respectively, and the compound identification relied on MS comparison using the Flavor and Fragrance Natural and Synthetic Compounds (FFNSC, ver. 4.0) library.30
In silico approach
A database composed of 42 ligands, obtained from the GC-MS analysis of the oil of P. guajava was prepared. The 3-D structure of the ligand was downloaded from the PubChem database in SDF format31 (see https://pubchem.ncbi.nlm.nih.gov/) and the Avogadro software (ver. 1.2.0, see https://avogadro.cc/) was used to check their bond order and molecular structure.32 The 3-D crystalline structure of the proteins with PDB ID: 4A79 (X-ray resolution = 1.89 Å, expression system: Komagataella pastoris), 7E3H (X-ray resolution = 2.45 Å, expression system: Homo sapiens) and 7F61 (X-ray resolution = 2.60 Å, expression system: Spodoptera frugiperda) were obtained in pdb format from the RCSB protein data bank server33 (see https://www.rcsb.org/). The protein was then cleaned using the PyMOL program (ver. 2.5.2, see https://www.pymol.org/34) and saved as an apo structure.
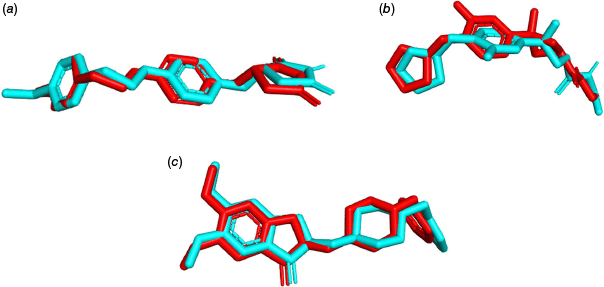
The molecular docking calculation was carried out using the DockThor server (see https://dockthor.lncc.br/) to determine the best binding pose of the ligand within the receptor protein.35 The docking program was selected because of its capability to perform numerous docking calculations quickly,being freely available and for its ability to reproduce the results. Different control parameters were used for the docking of ligands with different proteins. For the protein 4A79, box coordinates (51, 157, 31), box size (15, 15, 15), discretisation (0.16), population size (750), number of evaluations (1,000,000) and number of runs (24) were selected. The box size (16, 16, 16), box coordinates (−43, 36, −32), discretisation (0.17), population size (750), number of runs (24) and evaluations (1,000,000) were selected for protein 7E3H. Similarly, box coordinates (−19, 50, −1), box size (15, 15, 15), discretisation (0.16), population size (750), number of runs (24) and evaluations (1,000,000) were used for protein 7F61. The validation of the docking protocol was done by obtaining the RMSD less than 2 Å through the superimposition of native ligands in the crystalline complex with re-docked ligands in the docked complex as shown in Fig. 6.
Molecular dynamics simulations of the best ligand–protein complexes were carried out using the GROMACS program (vers. 2021.2, see http://www.gromacs.org/)36 and the SwissParam server (see https://www.swissparam.ch/).37 An adduct in a triclinic box system of 10-Å spacing was solvated using the TIP3P water model.38 An isotonic solution of NaCl (0.15 M) was used and Na+ or Cl− ions were added to neutralise the system. It was equilibrated at the temperature of 310 K in four stages i.e. two NVT equilibria and two final NPT equilibria (500 ps each). Other parameters were adopted from recent literature39 and the final production run was conducted for 100 ns with a step size of 2 fs.
The binding free energy change of the protein–ligand complex is given by the mathematical equation40:
where ΔGcomplex is the free energy of the protein–ligand complex, ΔGprotein is the free energy of the protein, and ΔGligand is the free energy of the ligand.
The absorption, metabolism, excretion, distribution and toxicity of the compound was assessed through the servers ProTox (ver. 3.0, see https://tox.charite.de/protox3/)41, pkCSM (see https://biosig.lab.uq.edu.au/pkcsm/)42 and SwissADME (see http://www.swissadme.ch/).43
Supplementary material
The chromatogram, mass spectra, chemical structure of compounds identified from GC-MS analysis (Supplementary Fig. S1–S3) and binding affinity table (Supplementary Table S1) are available as supplementary material. Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online Supplementary material.
Acknowledgements
The authors acknowledge Department of Plant Resources, Kathmandu, Nepal, for the GC-MS experiments.
References
1 Amor S, Puentes F, Baker D, Van Der Valk P. Inflammation in neurodegenerative diseases. Immunology 2010; 129(2): 154-169.
| Crossref | Google Scholar | PubMed |
2 Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med 2004; 10(10): 1055-1063.
| Crossref | Google Scholar |
3 Prince M, Wimo A, Guerchet M, Gemma-Claire A, Wu YT, Prina M. World Alzheimer Report 2015: The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International; 2015. Available at https://www.alzint.org/u/WorldAlzheimerReport2015.pdf
4 Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet 2021; 397(10284): 1577-1590.
| Crossref | Google Scholar | PubMed |
5 GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18(1): 88-106.
| Crossref | Google Scholar | PubMed |
6 Lang A, Lozano A. Parkinson’s disease. N Engl J Med 1998; 339(16): 1130-1143.
| Crossref | Google Scholar |
7 Smalley JL, Breda C, Mason RP, Kooner G, Luthi-Carter R, Gant TW, et al. Connectivity mapping uncovers small molecules that modulate neurodegeneration in Huntington’s disease models. J Mol Med 2016; 94(2): 235-45.
| Crossref | Google Scholar | PubMed |
8 Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis 2010; 5: 40.
| Crossref | Google Scholar | PubMed |
9 Talesa VN. Acetylcholinesterase in Alzheimer’s disease. Mech Ageing Dev 2001; 122(16): 1961-1969.
| Crossref | Google Scholar | PubMed |
10 Riederer P, Danielczyk W, Grünblatt E. Monoamine oxidase-B inhibition in Alzheimer’s disease. Neurotoxicology 2004; 25(1–2): 271-277.
| Crossref | Google Scholar | PubMed |
11 Zhou G, Miura Y, Shoji H, Yamada S, Matsuishi T. Platelet monoamine oxidase B and plasma β-phenylethylamine in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2001; 70: 229-231.
| Crossref | Google Scholar | PubMed |
12 Kohli H, Kumar P, Ambasta RK. In silico designing of putative peptides for targeting pathological protein HTT in Huntington’s disease. Heliyon 2021; 7(2): e06088.
| Crossref | Google Scholar | PubMed |
13 Singh SP. Guava (Psidium guajava L.) In: Yahia EM, editor. Postharvest Biology and Technology of Tropical and Subtropical Fruits. Woodhead Publishing Limited; 2011; pp. 213–246. 10.1533/9780857092885.213.
14 Kumar M, Tomar M, Amarowicz R, Saurabh V, Nair MS, Maheshwari C, et al. Guava (Psidium guajava L.) leaves: nutritional composition, phytochemical profile, and health-promoting bioactivities. Foods 2021; 10(752): 1-20.
| Crossref | Google Scholar | PubMed |
15 Jeong CH, Jeong HR, Choi GN, Kwak JH, Kim JH, Park SJ, et al. Neuronal cell protective effects of hot water extracts from guava (Psidium guajava L.) fruit and leaf. Korean J Food Preserv 2011; 18(1): 124-129.
| Crossref | Google Scholar |
16 Aly SH, Eldahshan OA, Al-Rashood ST, Binjubair FA, El Hassab MA, Eldehna WM, et al. Chemical constituents, antioxidant, and enzyme inhibitory activities supported by in silico study of n-hexane extract and essential oil of guava leaves. Molecules 2022; 27(24): 8979.
| Crossref | Google Scholar | PubMed |
17 Neupane P, Dhital S, Parajuli N, Shrestha T, Bharati S, Maharjan B, et al. Exploration of anti-diabetic potential of rubus ellipticus smith through molecular docking, molecular dynamics simulation, and MMPBSA calculation. J Nepal Phys Soc 2023; 9(2): 95-105.
| Crossref | Google Scholar |
18 Shrestha RLS, Panta R, Maharjan B, Shrestha T, Bharati S, Dhital S, et al. Molecular docking and ADMET prediction of compounds from Piper longum L. detected by GC-MS analysis in diabetes management. Mor J Chem 2024; 12(2): 776-798.
| Crossref | Google Scholar |
19 Shrestha RLS, Neupane P, Dhital S, Parajuli N, Maharjan B, Shrestha T, et al. Selected phytochemicals as potent acetylcholinesterase inhibitors: an in silico prediction. J Serbian Chem Soc 2025; 90: 187-200.
| Crossref | Google Scholar |
20 Shrestha RLS, Parajuli N, Neupane P, Dhital S, Maharjan B, Shrestha T, et al. A computational approach of anti-diabetic potential evaluation of flower and seed of Nyctanthes arbor tristis Linn. Turk Comp Theor Chem 2025; 9(1): 1-18.
| Crossref | Google Scholar |
21 Salo-Ahen OMH, Alanko I, Bhadane R, Alexandre AM, Honorato RV, Hossain S, et al. Molecular dynamics simulations in drug discovery and pharmaceutical development. Processes 2021; 9: 71.
| Crossref | Google Scholar |
22 Abdizadeh R, Hadizadeh F, Abdizadeh T. In silico analysis and identification of antiviral coumarin derivatives against 3-chymotrypsin-like main protease of the novel coronavirus SARS-CoV-2. Mol Divers 2022; 26(2): 1053-1076.
| Crossref | Google Scholar | PubMed |
23 Lobanov MY, Bogatyreva NS, Galzitskaya O V. Radius of gyration as an indicator of protein structure compactness. Mol Biol 2008; 42(4): 623-628.
| Crossref | Google Scholar |
24 da Fonseca AM, Caluaco BJ, Madureira JMC, Cabongo SQ, Gaieta EM, Djata F, et al. Screening of potential inhibitors targeting the main protease structure of SARS-CoV-2 via molecular docking, and approach with molecular dynamics, RMSD, RMSF, H-Bond, SASA and MMGBSA. Mol Biotechnol 2024; 66: 1919-1933.
| Crossref | Google Scholar | PubMed |
25 Cournia Z, Allen B, Sherman W. Relative binding free energy calculations in drug discovery: recent advances and practical considerations. J Chem Inf Mod 2017; 57: 2911-2937.
| Crossref | Google Scholar | PubMed |
26 Dhital S, Parajuli N, Poudel M, Shrestha T, Bharati S, Maharjan B, et al. Spatial and energetic stability assessment of the adducts of phytocompounds of Piper longum L. with α-amylase by computational approach. Biointerface Res Appl Chem 2024; 14(7): 126.
| Crossref | Google Scholar |
27 Pardridge WM. Treatment of Parkinson’s disease with biologics that penetrate the blood–brain barrier via receptor-mediated transport. Front Aging Neurosci 2023; 15: 1276376.
| Crossref | Google Scholar | PubMed |
28 Shen J, Cheng F, Xu Y, Li W, Tang Y. Estimation of ADME properties with substructure pattern recognition. J Chem Inf Model 2010; 50(6): 1034-1041.
| Crossref | Google Scholar | PubMed |
29 Elyemni M, Louaste B, Nechad I, Elkamli T, Bouia A, Taleb M, et al. Extraction of essential oils of Rosmarinus officinalis L. by two different methods: hydrodistillation and microwave assisted hydrodistillation. Sci World J 2019; 2019: 3659432.
| Crossref | Google Scholar | PubMed |
30 Pradhan S, Paudel HR, Maharjan R, Sharma K. Essential oils from six aromatic plants of Langtang National Park: insights on their chemical constituents via GC-MS analysis. Separations 2023; 10(1): 52.
| Crossref | Google Scholar |
31 Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2023 update. Nucleic Acids Res 2023; 51(D1): D1373-D1380.
| Crossref | Google Scholar | PubMed |
32 Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 2012; 4(8): 17.
| Crossref | Google Scholar | PubMed |
33 Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic Acids Res 2000; 28(1): 235-242.
| Crossref | Google Scholar | PubMed |
34 Yuan S, Chan HCS, Hu Z. Using PyMOL as a platform for computational drug design. Vol. 7, Wiley Interdisciplinary Reviews: Computational Molecular Science. Blackwell Publishing Inc.; 2017. 10.1002/wcms.1298
35 Santos KB, Guedes IA, Karl ALM, Dardenne LE. Highly flexible ligand docking: benchmarking of the DockThor program on the LEADS-PEP protein–peptide data set. J Chem Inf Model 2020; 60(2): 667-683.
| Crossref | Google Scholar | PubMed |
36 Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015; 1–2: 19-25.
| Crossref | Google Scholar |
37 Zoete V, Cuendet MA, Grosdidier A, Michielin O. SwissParam: a fast force field generation tool for small organic molecules. J Comput Chem 2011; 32(11): 2359-2368.
| Crossref | Google Scholar | PubMed |
38 Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys 1983; 79(2): 926-935.
| Crossref | Google Scholar |
39 Neupane P, Adhikari Subin J, Adhikari R. Assessment of iridoids and their similar structures as antineoplastic drugs by in silico approach. J Biomol Struct Dyn 2024; 1-16.
| Crossref | Google Scholar | PubMed |
40 Valdés-Tresanco MS, Valdés-Tresanco ME, Valiente PA, Moreno E. Gmx_MMPBSA: a new tool to perform end-state free energy calculations with GROMACS. J Chem Theory Comput 2021; 17(10): 6281-6291.
| Crossref | Google Scholar | PubMed |
41 Banerjee P, Kemmler E, Dunkel M, Preissner R. ProTox 3.0: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res 2024; 52(W1): W513-W520.
| Crossref | Google Scholar | PubMed |
42 Pires DEV, Blundell TL, Ascher DB. pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem 2015; 58(9): 4066-4072.
| Crossref | Google Scholar | PubMed |
43 Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports 2017; 7(1): 42717.
| Crossref | Google Scholar |