Ring-expansion reactions of Cyrene and derivatives with ethyl diazoacetate
Johannes Puschnig A , Oscar Lamb A , Christopher J. Sumby B and Ben W. Greatrex A *
A *
A
B
Abstract
The ring expansion and homologation of the biomass derivative Cyrene (6,8-dioxabicyclo[3.2.1]octan-4-one) has been developed by Lewis-acid promoted reactions with ethyl diazoacetate. Insertion into the C3–C4 bond gave a ring-expanded β-ketoester regioisomer as an equilibrating mixture of diastereomers, which was subjected to a one-pot hydrolysis and decarboxylation to give the 7,9-dioxabicyclo[4.2.1]nonan-5-one system (homocyrene). The reactivity of homocyrene was then investigated in a series of transformations known for the parent 6,8-dioxabicyclo[3.2.1]octan-4-one system, including the Baeyer–Villiger oxidation affording S-6-(hydroxymethyl)pyran-2-one, which has been used for the synthesis of jasmine lactone, and another one-carbon ring expansion. The ring-expansion process for Cyrene could be used to prepare chiral C6 and C7 synthons on scale from biomass.
Keywords: carbene, cyrene, diazo compounds, Jasmine lactone, levoglucosenone, organic green chemistry, ring expansion, valerolactone.
Introduction
The biomass derivative levoglucosenone (1) and its reduced analogue Cyrene (2) obtained from acid-catalysed cellulose pyrolysis have been used in a variety of chemical processes to generate pharmaceutical precursors.1–5 The rigid bicyclic structure allows for diastereoselective reactions,6–8 and as the chirality derives from glucose, the products from synthetic processes can be enantiopure. In addition to C6 synthons from ring-opening, a particularly useful transformation for 1, 2 and oxidised derivatives is the Baeyer–Villiger reaction. First reported by Ebata’s group ~30 years ago,9–11 the reaction results in dehomologation giving ribonolactone derivatives and can tolerate a variety of substitution patterns. This allows for the synthesis of branched and oxidised C5 synthons from 1 and 2; however, the preparation of longer linear chains is less well developed and requires a series of functional group interconversions to enable chain-extension.12 The versatility of 1 and 2 would be enhanced if carbon insertion and ring-expansion reactions could be developed. For example, the homologation of 2 to give a 7,9-dioxabicyclo[4.2.1]nonan-5-one ring system followed by Baeyer–Villiger oxidation, might allow the construction of chiral hydroxymethyl δ-valerolactones, which are valuable substrates in organic synthesis and have applications as monomers in material science.13,14 To this end, we have recently developed a synthesis of the 7,9-dioxabicyclo[4.2.1]non-3-en-5-one by dihalocyclopropanation of an enamine derived from 2, followed by dehalogenation.15
An alternative approach for ring enlargement is the Büchner–Curtius–Schlotterbeck reaction, which involves the reaction of diazoalkanes with cycloalkanones.16,17 Reactions of the 6,8-dioxabicyclo[3.2.1]octane ring-system with diazo derivatives has been previously investigated using 2 and derivatives by Kawai et al.18 whereas the reactions of 1 were investigated by Tomilov and coworkers19,20 (Scheme 1). In the reaction of trimethylsilyldiazomethane with 2, epoxidation giving 3 was preferred and a 6% yield of the C4–C5 insertion product 4 was obtained.18 β-Substituted derivatives of 2 gave low yields of ring-expansion products as regioisomeric and diastereomeric mixtures, with the insertion again favoured into the C4–C5 bond. The reaction of methyl diazoacetate with 1 resulted only in cyclopropanation giving 5 or formal C–H insertion resulting in 6 with no ring-expansion products observed, whereas the reaction of diazo cyclopropane and 1 gave only products of 1,3-dipolar cycloaddition.19,20
Previous work18,19 involving reactions of diazo compounds with 1 and 2 and the current synthesis.
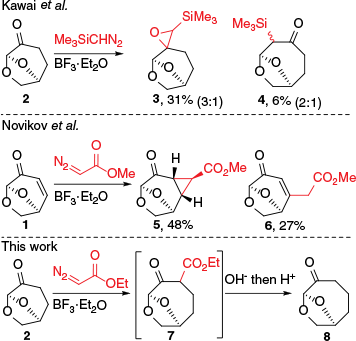
With the current ready availability of 1 and 2 and the potential utility of ring-expanded products, we have examined whether reactions with diazo reagents could be developed into a synthetically useful transformation. The reaction of ethyl diazoacetate (EDA) with 2 has not been reported, and this seemed an appropriate reagent to test due to the many reports of EDA used in ring expansion of cyclic ketones promoted by Lewis acids.21–23 It was presumed that the absence of the olefin in 1, which preferentially reacted in the report by Novikov et al.19 could allow for alternate insertion pathways and ring expansion. EDA could also be used for traceless methylene insertion giving 8, thereby acting as a surrogate for explosive diazomethane or trimethylsilyldiazomethane, as the β-ketoester in 7 could be hydrolysed and decarboxylated.
Results and discussion
The investigation into the ring expansion began by subjecting 2 to solutions of EDA in a variety of solvents and in the presence of a range of Lewis acids. At both room and elevated temperatures, there was no reaction between the ketone and EDA, and only upon the addition of BF3·OEt2 to the solution of 2 and EDA was the evolution of N2 observed. Two dominant products assigned as 7a and 7b were present in crude reaction mixtures as a 1:1 mixture along with small amounts of isomers tentatively assigned as regioisomer 9 and epoxide 10 (Scheme 2). In the 1H NMR spectrum of the mixture, two singlets were observed for the C6 acetal protons at δ 5.22 and 5.18 ppm, consistent with isolated acetal methines, confirming insertion into the C3–C4 bond. There is a strong preference for equatorial substituents at C3 in the parent material 2 which is forced into a 1C4 conformation due to the bicyclic ring-system. The equimolar mixture of 7a/7b indicated that the driving force for pseudo-equatorial group placement of substituents at C4 of the 7,9-dioxabicyclo[4.2.1]nonan-4-one was much lower. Purification of the insertion products by flash column chromatography was challenging due to epimerisation of the β-keto esters 7a/7b and 9 on silica. Practically, it was easier to telescope the entire methylene insertion by hydrolysing the mixture and decarboxylating with acetic acid, affording 7,9-dioxabicyclo[4.2.1]nonan-4-one (8), which could be isolated by flash column chromatography in 42% yield (23% by distillation on 30-g scale). Other Lewis acids, including AlCl3, TiCl4 and SnCl4 gave poor conversion of 2, which remained unreacted once the evolution of N2 had ceased. Et2AlCl resulted in an apparent greater proportion of the regioisomeric 9 in the intermediate mixture and yields of 8 were poor. Changing solvents and the temperature of the reaction also failed to improve the yield (see Table S1 of the Supplementary material).
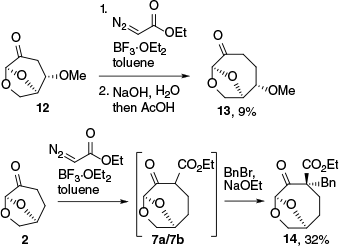
Insertion of ethyl diazoacetate into 2 and the product 8, and subsequent hydrolysis and decarboxylation.
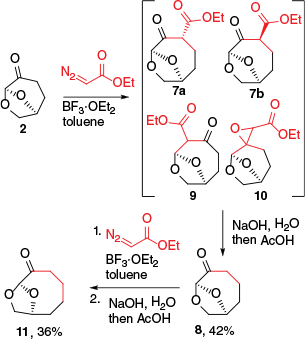
A series of reactions were then performed to examine whether the reaction with EDA could be expanded to derivatives of 2 and to other diazo reagents. Repeating the ring expansion and decarboxylation sequence on 8 gave the doubly homologated material 11 in a low yield (Scheme 2). A set of 3-substituted 6,8-dioxabicyclo[4.2.1]octan-4-one substrates were then examined; however, only 12 with a 2-methoxy substituent resulted in isolable quantities of ring-expansion product 13 following hydrolysis and decarboxylation in yields of up to 9% (Scheme 3 and Table S1). Other substrates gave unreacted ketone and ethyl phenylpropanoate from the reaction of ethyl diazoacetate and the solvent toluene. The reaction of 2 with diazoacetophenone, phenyldiazomethane, diazomethylnitrile or diethyl diazomalonate catalysed by BF3·OEt2 gave no products from insertion and ring expansion. It was envisaged that these targeted products could be prepared if the intermediates from the reaction of 2 with EDA were alkylated prior to decarboxylation. To test this approach, the reaction of the crude insertion mixture (7a, 7b, 9, 10) with base and BnBr led to the isolation of 14 as a single diastereomer; however, the ester was stubbornly unreactive to hydrolysis under forcing conditions due to hindrance around the ester group, and therefore this approach did not produce the decarboxylated material derived from 14. The results of this investigation, along with the previous results reported by Kawai et al.18 suggested that the scope for ring-enlargement was limited, and that the combination of 2 and EDA catalysed by BF3·OEt2 was the most effective way to achieve ring expansion.
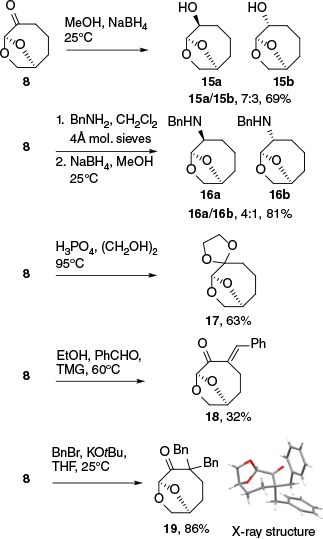
Cyrene and levoglucosenone have been used to prepare multiple chiral building blocks and pharmaceutical intermediates,3 and it was of interest to determine whether the homologue 8 could participate in reactions known for 2. Thus, the reduction of 8 with NaBH4 gave a 7:3 ratio of epimers 15a and 15b, compared to 9:1 when the reduction was performed on 2 (Scheme 4).24 Both steric and Cieplak effects support the approach from the exo face in reactions of 2, which may be reduced in the larger ring-system.25 When 8 was subject to reductive amination by forming the imine from benzylamine and reducing with NaBH4, a 4:1 ratio of diastereomers 16a/16b resulted, which mirrors the results observed when 2 is subjected to reductive amination using NaBH(OAc)3 (ratio of 3:1 to 5:1 endo/exo).24 The carbonyl could also be protected under standard conditions giving rise to the dioxolane 17, which is a homologated analogue of the green solvent candidate ‘Cygnet’.26
Reactions at the C3 position adjacent to the ketone in 2 can be used as a starting point to generate a variety of useful materials such as substituted lactones following Baeyer–Villiger reactions.27,28 The aldol reaction with benzaldehyde afforded benzylidene 18 in 32% yield, and alkylation of 8 with 3 equivalents of benzyl bromide resulted in dibenzylated ketone 19, a homologue of the ketone used to prepare an 8-phenylmenthol like chiral auxiliary (Scheme 4).29 Ketone 19 was crystalline and this allowed for confirmation of the structure and conformation by X-ray crystallography, verifying that the ring expansion had taken place and that the stereochemistry of the parent structure was retained (absolute structure determined by anomalous dispersion).
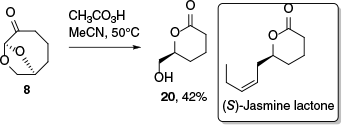
The Baeyer–Villiger reaction on 8 was investigated using peracetic acid and pleasingly afforded the δ-lactone 20 after an acid-catalysed lactonisation and then formate hydrolysis (Scheme 5). Lactone 20 was especially prone to polymerisation, which agrees with findings that δ-valerolactones more readily undergo polymerisation than the γ-butyrolactones obtained from 2 without similar issues in handling.30 When crude reaction mixtures of 20 were concentrated under acidic conditions, polymerisation was spontaneous and purification by column chromatography afforded only moderate yields of 20. Preparation of lactone 20 provides a formal synthesis of (S)-jasmine lactone, a key flavour component in jasmine tea and highly valuable material in the perfume industry.31,32
Conclusion
The ring expansion of the biomass derived compound 2 has been developed using EDA as the carbene source, with hydrolysis and decarboxylation giving traceless methylene insertion. The process has been used to generate 7,9-dioxabicyclo[4.2.1]nonan-5-one (8), a compound which we term homocyrene. The addition of other diazo reagents and modified substrates was unsuccessful, suggesting that the ring expansion of 2 is more limited than other cyclic ketones, although the ring expansion of 8 allowed the construction of 8,10-dioxabicyclo[5.2.1]decan-6-one (11). We envisage that the ready accessibility of 8 and the corresponding Baeyer–Villiger product 20 will enable new applications of 1 and 2 in enantioselective synthesis.
Supplementary material
Copies of 1H and 13C NMR spectra, table of optimisation conditions, and procedures for new compounds. Details of the structure determination of compound 19 by X-ray crystallography. Supplementary material is available online.
Data availability
The data that support this study are available in Zenodo.33 Details include 1H and 13C NMR FIDs and HRMS spectra for all new compounds. Full details of the structure determination have been deposited with the Cambridge Crystallographic Data Centre as CSD 2395098.
Conflicts of interest
Circa Group AS, a manufacturer of 1 and 2, fund a PhD studentship in the same research group where this work was conducted. The authors declare that they have no other conflicts of interest.
Declaration of funding
J. Puschnig thanks the Australian Government for a Research Training (RTP) Scholarship. C. J. Sumby acknowledges the Australian Research Council for Linkage Infrastructure, Equipment and Facilities funding (LE210100163).
References
1 Ebata T, Matsushita H. Levoglucosenone: old but new optically active starting material. J Syn Org Chem Jpn 1994; 52: 1074-1082.
| Crossref | Google Scholar |
2 Camp JE. Bio‐available solvent cyrene: synthesis, derivatization, and applications. ChemSusChem 2018; 11: 3048-3055.
| Crossref | Google Scholar | PubMed |
3 Camp JE, Greatrex BW. Levoglucosenone: bio-based platform for drug discovery. Front Chem 2022; 10: 902239.
| Crossref | Google Scholar | PubMed |
4 Comba MB, Tsai Yh, Sarotti AM, Mangione MI, Suárez AG, Spanevello RA. Levoglucosenone and its new applications: valorization of cellulose residues. Eur J Org Chem 2018; 2018: 590-604.
| Crossref | Google Scholar |
5 Pacheco CM, Lima W, Lima FA, Gomez M, da Silva IG, Miranda L, Esteves PM, Itabaiana I, Jr, Wojcieszak R, Leão R, de Souza R. Levoglucosenone as a starting material for cascade continuous-flow synthesis of (R)-γ-carboxy-γ-butyrolactone. RSC Adv 2024; 14: 34611-34619.
| Crossref | Google Scholar | PubMed |
6 Sarotti AM, Spanevello RA, Suárez AG, Echeverría GA, Piro OE. 1,3-Dipolar cycloaddition reactions of azomethine ylides with a cellulose-derived chiral enone. A novel route for organocatalysts development. Org Lett 2012; 14: 2556-2559.
| Crossref | Google Scholar | PubMed |
7 Martínez S, Carrau G, Gonzalez D, Veiga N. Diels–Alder reaction of levoglucosenone with a protected cis-cyclohexadienediol: structural and electronic basis behind the unexpected stereoselectivity. ChemistrySelect 2017; 2: 11223-11230.
| Crossref | Google Scholar |
8 Nishikawa T, Asai M, Ohyabu N, Yamamoto N, Fukuda Y, Isobe M. Synthesis of a common key intermediate for (−)-tetrodotoxin and its analogs. Tetrahedron 2001; 57: 3875-3883.
| Crossref | Google Scholar |
9 Okano K, Ebata T, Koseki K, Kawakami H, Matsumoto K, Matsushita H. Formal synthesis of (+)-grandisol from levoglucosenone. Chem Pharm Bull 1993; 41: 861-865.
| Crossref | Google Scholar |
11 Koseki K, Ebata T, Kawakami H, Matsushita H, Naoi Y, Itoh K. A method for easy preparation of optically pure (S)‐5‐hydroxy‐2‐penten‐4‐olide and (S)‐5‐hydroxypentan‐4‐olide. Heterocycles 1990; 31: 423-426.
| Crossref | Google Scholar |
12 Peru AAM, Flourat AL, Gunawan C, Raverty W, Jevric M, Greatrex BW, Alais F. Chemo-enzymatic synthesis of chiral epoxides ethyl and methyl (S)-3-(oxiran-2-yl)propanoates from renewable levoglucosenone: an access to enantiopure (S)-dairy lactone. Molecules 2016; 21: 988.
| Crossref | Google Scholar | PubMed |
13 Corey EJ, Pyne SG, Su W-g. Total synthesis of leukotriene B5. Tetrahedron Lett 1983; 24: 4883-4886.
| Crossref | Google Scholar |
14 Bińczak J, Dziuba K, Chrobok A. Recent developments in lactone monomers and polymer synthesis and application. Materials 2021; 14: 2881.
| Crossref | Google Scholar | PubMed |
15 Puschnig J, Greatrex BW. Ring‐expansion reactions of the biomass derivative cyrene via enamine dihalocyclopropanation. Eur J Org Chem 2024; 27: e202400031.
| Crossref | Google Scholar |
16 Shershnev I, Dar’in D, Chuprun S, Kantin G, Bakulina O, Krasavin M. The use of ∝-diazo-γ-butyrolactone in the Büchner–Curtius–Schlotterbeck reaction of cyclic ketones: a facile entry into spirocyclic scaffolds. Tetrahedron Lett 2019; 60: 1800-1802.
| Crossref | Google Scholar |
17 Gogula SS, Prasanna DV, Sridhar B, Lincoln CA, Reddy PM, Reddy BS. Sc(OTf) 3‐Catalyzed synthesis of 3‐substituted lawsones through a Buchner–Curtius–Schlotterbeck reaction. Eur J Org Chem 2024; 27: e202400362.
| Crossref | Google Scholar |
18 Kawai T, Isobe M, Peters SC. Factors affecting reaction of 1,6-anhydrohexos-2-ulose derivatives. Aust J Chem 1995; 48: 115-131.
| Crossref | Google Scholar |
19 Novikov RA, Rafikov RR, Shulishov EV, Konyushkin LD, Semenov VV, Tomilov YV. Reactions of levoglucosenone and its derivatives with diazo compounds. Russ Chem Bull 2009; 58: 327-334.
| Crossref | Google Scholar |
20 Rafikov RR, Novikov RA, Shulishov EV, Konyushkin LD, Semenov VV, Tomilov YV. Reaction of levoglucosenone with diazocyclopropane. Russ Chem Bull 2009; 58: 1927-1933.
| Crossref | Google Scholar |
21 Liu HJ, Majumdar SP. On the regioselectivity of boron trifluoride catalyzed ring expansion of cycloalkanones with ethyl diazoacetate. Synth Commun 1975; 5: 125-130.
| Crossref | Google Scholar |
22 Paterna R, André V, Duarte MT, Veiros LF, Candeias NR, Gois PM. Ring‐expansion reaction of isatins with ethyl diazoacetate catalyzed by dirhodium(II)/DBU metal–organic system: en route to viridicatin alkaloids. Eur J Org Chem 2013; 2013: 6280-6290.
| Crossref | Google Scholar |
23 Hashimoto T, Naganawa Y, Maruoka K. Desymmetrizing asymmetric ring expansion of cyclohexanones with α-diazoacetates catalyzed by chiral aluminum Lewis acid. J Am Chem Soc 2011; 133: 8834-8837.
| Crossref | Google Scholar | PubMed |
24 Dekker T, Harteveld JW, Wágner G, de Vries MCM, Custers H, van de Stolpe AC, de Esch IJP, Wijtmans M. Green drug discovery: novel fragment space from the biomass-derived molecule dihydrolevoglucosenone (Cyrene). Molecules 2023; 28: 1777.
| Crossref | Google Scholar | PubMed |
25 Cieplak AS. Stereochemistry of nucleophilic addition to cyclohexanone. The importance of two-electron stabilizing interactions. J Am Chem Soc 1981; 103: 4540-4552.
| Crossref | Google Scholar |
26 Milescu RA, Zhenova A, Vastano M, Gammons R, Lin S, Lau CH, Clark JH, McElroy CR, Pellis A. Polymer chemistry applications of Cyrene and its derivative Cygnet 0.0 as safer replacements for polar aprotic solvents. ChemSusChem 2021; 14: 3367-3381.
| Crossref | Google Scholar | PubMed |
27 Podversnik H, Camp JE, Greatrex BW. Practical and scalable enantioselective synthesis of (+)-majoranolide from Cyrene. Org Biomol Chem 2024; 22: 950-953.
| Crossref | Google Scholar | PubMed |
28 Ledingham ET, Stockton KP, Greatrex BW. Efficient synthesis of an indinavir precursor from biomass-derived (–)-levoglucosenone. Aust J Chem 2017; 70: 1146-1150.
| Crossref | Google Scholar |
29 Klepp J, Sumby CJ, Greatrex BW. Synthesis of a chiral auxiliary family from levoglucosenone and evaluation in the Diels–Alder reaction. Synlett 2018; 29: 1441-1446.
| Crossref | Google Scholar |
30 Houk KN, Jabbari A, Hall HK, Jr, Aleman C. Why δ-valerolactone polymerizes and γ-butyrolactone does not. J Org Chem 2008; 73: 2674-2678.
| Crossref | Google Scholar | PubMed |
31 Blaser F, Deschenaux P-F, Kallimopoulos T, Jacot-Guillarmod A. Synthèse des (−)-(6R)-et(+)-(6S)-tétrahydro-6-[(Z)-pent-2-ényl]-2H-pyran-2-one, lactones de Jasminum grandiflorum L. et de Polianthes tuberosa L. Helv Chim Acta 1991; 74: 787-790 [In French].
| Crossref | Google Scholar |
32 Mase N, Inoue A, Nishio M, Takabe K. Organocatalytic α-hydroxymethylation of cyclopentanone with aqueous formaldehyde: easy access to chiral δ-lactones. Bioorg Med Chem Lett 2009; 19: 3955-3958.
| Crossref | Google Scholar | PubMed |
33 Puschnig J, Lamb O, Greatrex B. Primary data for manuscript provisionally titled, ‘Ring-expansion reactions of Cyrene and derivatives with ethyl diazoacetate’. Zenodo 2024; 2024: version v1 [Dataset, published 7 November 2024].
| Crossref | Google Scholar |