An unusual triterpene fatty acid conjugate from Dodonaea caespitosa
Andrew M. Bloomfield A , Eugene Eades B , Hendra Gunosewoyo
A , Eugene Eades B , Hendra Gunosewoyo  C and Alan D. Payne
C and Alan D. Payne  A *
A *
A
B
C
Abstract
An unusual triterpene fatty acid conjugate was isolated from the leaf resin of Dodonaea caespitosa. Its structure was formulated through spectroscopic analysis of the acetylated natural product and its hydrolysis products (p-hydroxydihydrocinnamic acid, (S)-(+)-8,16-dihydroxypalmitic acid and 30-hydroxybetulin). The stereochemistry of the hydroxyl at C8 of (S)-(+)-8,16-dihydroxypalmitic acid was determined by conversion to the known (S)-(+)-(8)-hydroxypalmitic acid using a Barton–McCombie deoxygenation reaction.
Keywords: 8,16-dihydroxyhexadexanoic acid, cutin monomer, Dodonaea, natural products elucidation, Nowanup, semisynthesis, triterpene, Western Australia.
Triterpene fatty acid conjugates are highly lipophilic natural products, originating from the cuticle layer of plants,1 which play an important role in strengthening the cuticle and preventing water loss at high ambient temperatures.2 Although hundreds of triterpene fatty acid conjugates have been isolated,1 only a single report describes conjugates incorporating a cutin monomer as a linking molecule.3 Inoue et al. showed a set of triterpene glycosides conjugated to 8/9-oxo-16-β-[(D-xylopyranosyl)oxy]hexadecanoic acids through polysaccharides, which likely played a role in the cytotoxicity of the plant.3 To date, non-glycosidic triterpene conjugates employing a cutin monomer are yet to be reported. 30-Hydroxybetulin has limited biological activity,4,5 but its derivatives have, for example, a semisynthetic tyramine–betulin conjugate that has micromolar antiproliferative activity (IC50 = 6 µM) against A-375 human melanoma cells.6 Herein, we report an unusual triterpene-fatty acid–cinnamate conjugate, along with 30-hydroxybetulin from Dodonaea caespitosa, a shrub endemic to Western Australia.7
Air-dried aerial parts of D. caespitosa, collected from Nowanup, WA,8 in 2021, were steeped in diethyl ether for 20 min to afford an oil accounting for 6.0% (w/w) of the dried plant after filtration and concentration. As the products could not be separated by normal phase flash chromatography due to poor solubility, a small portion of the extract was acetylated. The acetylated mixture was purified by normal phase flash chromatography to afford the known 30-hydroxybetulin triacetate 3 in 0.6% and the new lupane fatty acid conjugate 4 in 1% (Fig. 1). Both isolates were acetylated artefacts of the natural products 1 and 2. The triacetate 3 was unambiguously assigned by agreement of experimental NMR and specific rotation data published by Pramanick et al.9
Isolation of 1, 3, 4, 5 and 6 from D. caespitosa, yields of compounds are in weight/weight of dried plant and key HMBC correlations in red.
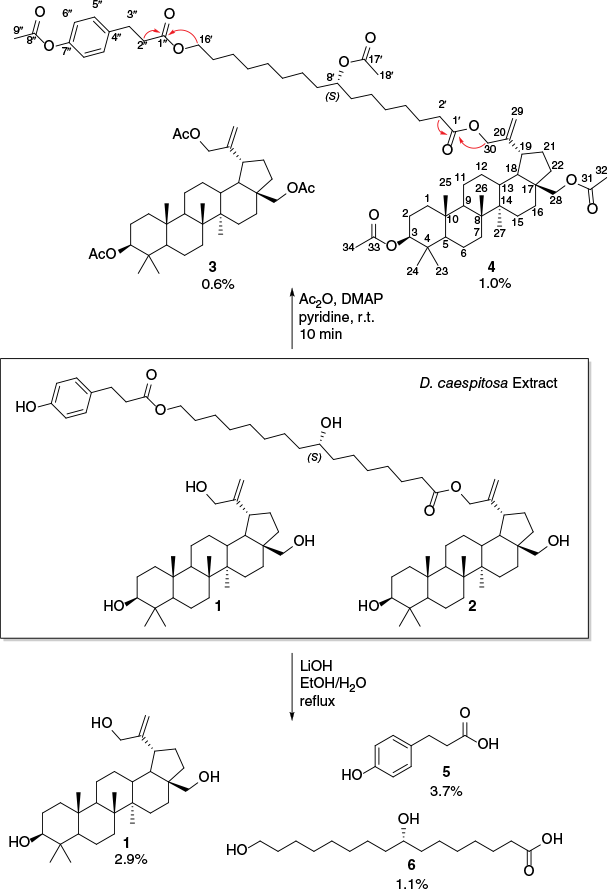
(+)-HRESIMS analysis of the conjugate 4 revealed multiple [M + H] ions, which made it unclear which formula was the molecular ion. Analysis of the 1H and 13C NMR spectra of the conjugate 4 indicated that the key resonances assigned to the triacetate 3 were also present in the spectra of the conjugate 4; however, the conjugate was significantly more complex in structure, showing 61 distinct resonances in the 13C NMR spectrum. Further NMR analysis indicated the appearance of three ester carbonyl groups (δC 173.6), (δC 173.0) and (δC 169.7); an oxygenated sp2 carbon (δC 149.2); two sp2 methines (δH 7.20 dd, J = 10.8, 8.4 Hz; δC 129.4, C-5′) and (δH 6.99 d, J = 8.4 Hz; δC 129.4, C-6′); a quaternary sp2 carbon (δC 138.3); an oxymethine (δH 4.85 pent., J = 6.2 Hz; δC 74.4); an oxymethylene (δH 4.05 t, J = 6.7 Hz; δC 74.4); three deshielded methylenes (δH 2.94 t, J = 7.8 Hz; δC 30.5, C-3′), (δH 2.61 t, J = 7.8 Hz; δC 36.0, C-2′) and (δH 2.34 t, J = 7.5 Hz; δC 34.5); and an acetate methyl group (δH 2.28 s), as well as 14 aliphatic methylene groups.
As a high number of overlapping resonances in the 1H NMR spectrum were observed, 2-D-NMR data could not be used to elucidate the structure of the conjugate 4. Thus, a small portion of the unmodified crude extract was subjected to hydrolysis and separated by standard acid–base partitioning to afford the three known natural products 30-hydroxybetulin 1, para-hydroxydihydrocinnamic acid 5 and 8,16-dihydroxypalmitic acid 6 as the only products. 30-hydroxybetulin 1 and para-hydroxydihydrocinnamic acid 5 were unambiguously assigned based on agreement with literature reported spectroscopic data,5,10,11 whereas 8,16-dihydroxypalmitic acid 6 was assigned by preparation of the corresponding methyl ester 7, which agreed with Siddiqui et al.’s reported NMR data.12 Although NMR data for the palmitate methyl ester 7 have been previously reported,12 the stereochemistry of the secondary alcohol (C-8) had never been assigned and required evaluation for the assignment of the betulin conjugate 4.
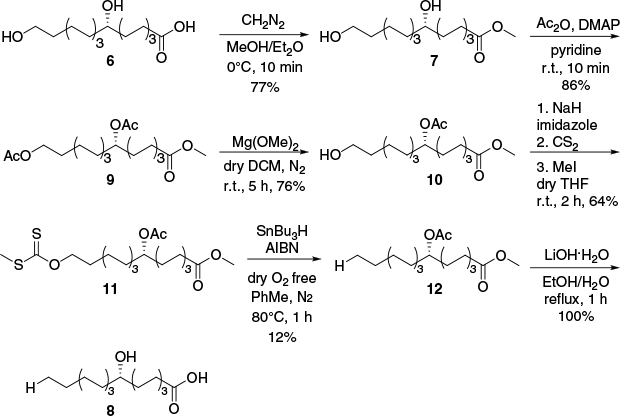
Owing to the high symmetry of 8,16-dihydroxypalmitic acid 6 and the lack of chromophores near the C-8 secondary alcohol, standard methods of stereochemical evaluation, such as Mosher’s esters and circular dichroism, were unsuitable. The stereochemistry at C-8 was therefore determined by conversion of the isolated 8,16-dihydroxypalmitic acid 6 to the known (S)-(+)-8-hydroxypalmitic acid 8 by a six-step synthesis employing a Barton–McCombie deoxygenation. The 1H and 13C NMR spectra of the final reaction product were in agreement with literature reported data for (S)-(+)-8-hydroxypalmitic acid 8 confirming the connectivity of the reaction product.13,14 The reported specific rotation values for (S)-(+)-8-hydroxypalmitic acid 8 varied from +0.15° to +1.23°14–17; however, they agree with the observed sign and magnitude of the isolated product (+1.00° to +2.45°). As (S)-(+)-(8)-hydroxyplamitic acid 8 was obtained by semisynthesis from 8,16-dihydroxypalmitic acid 6, the C-8 stereochemistry of the starting material was also confirmed as (S)-configured (Fig. 2).
Semisynthesis of (S)-(+)-(8)-hydroxypalmitic acid 8 from (S)-(+)-8,16-dihydroxypalmitic acid 6 employing a Barton–McCombie deoxygenation.
After identifying all the hydrolysis products of the conjugate 4 as (S)-(+)-8,16-dihydroxypalmitic acid 6, para-hydroxydihydrocinnamic acid 5, and 30-hydroxybetulin 1, focus was returned to finalising the connectivity of the conjugate 4. Upon revisiting the 1H NMR spectrum of the conjugate 4, the new oxymethine (δH 4.85 pent., J = 6.2 Hz; δC 74.4), oxymethylene (δH 4.05 t, J = 6.7 Hz; δC 74.4) and deshielded methylene group resonances (δH 2.34 t, J = 7.5 Hz; δC 34.5) were associated with (S)-(+)-8,16-dihydroxypalmitic acid 6. Moreover, the two new sp2 methines (δH 7.20 dd, J = 10.8, 8.4 Hz; δC 129.4, C-5′), (δH 6.99 d, J = 8.4 Hz; δC 129.4, C-6′) and coupled deshielded methylenes (δH 2.94 t, J = 7.8 Hz; δC 30.5, C-3′), (δH 2.61 t, J = 7.8 Hz; δC 36.0, C-2′), were associated with p-hydroxydihydrocinnamic acid 5.
The 1H–13C HMBC spectrum of the conjugate 4, when compared to the spectrum of the triacetate 3, confirmed that the acetates connected to the oxymethine (C-3) and oxymethylene (C-28) in the triacetate 3 were also present in the conjugate 4. Further analysis indicated that the allylic alcohol (C-30) no longer showed correlation to an acetate carbonyl in the conjugate 4 and instead correlated to the carbonyl group (δC 173.6) of the (S)-(+)-8,16-dihydroxypalmitic acid 6 portion. Further analysis of the HMBC spectrum indicated a correlation between the oxymethylene of (S)-(+)-8,16-dihydroxypalmitic acid 6 (δH 4.05 t, J = 6.7 Hz) and the carbonyl group (δC 173.0) of p-hydroxydihydrocinnamic 5. The connectivity of the conjugate 4 was therefore finalised as 30-(16-para-acetoxydihydrocinnimate-8-(S)-acetoxypalmitate)betulin diacetate 4. As the absolute configuration of the hydrolysed (S)-(+)-8,16-dihydroxypalmitic acid 6 and 30-hydroxybetulin 1 were assigned by specific rotation measurements, the absolute configuration of the conjugate 4 was determined as 3-(S), 5-(R), 8-(R), 9-(R), 10-(R), 13-(R), 14-(R), 17-(S), 18-(S), 19-(R), 8′-(S). Based on the high overlap of both proton and carbon resonances, most resonances associated with the (S)-(+)-8,16-dihydroxypalmitic acid portion could not be unambiguously assigned. In further proof of the assignment, returning to the (+)-HRESIMS spectrum after NMR analysis revealed an [M + H – AcOH] ion at m/z 985.6763, consistent with the molecular formula C63H96O12. As the 1H NMR spectrum of the initial extract lacked resonances characteristic of acetate functionalities, it was clear that the betulin conjugate 2 was the natural product present within the leaf resin and that the conjugate 4 was an acetylated artefact.
In summary, purification of the acetylated diethyl ether extract afforded the triacetate 3 and the novel betulin conjugate 4 in 0.6 and 1% respectively. The natural products present in the leaf resin were therefore 30-hydroxybetulin 1 and the betulin conjugate 2. As (S)-(+)-8,16-dihydroxypalmitic acid 6 is a known monomer of cutin,18 it is highly likely that (S)-(+)-8,16-dihydroxypalmitic acid 6 incorporated into the betulin conjugate 4 originated from the same source. The conjugate 4 marks the first isolation of a cutin monomer as a linking molecule directly conjugated to a triterpene. Although not completed within this study, exploration of the biological activity should be investigated to evaluate if conjugate 4 provides an ecological purpose independent of cuticle strengthening and plant moisture retention. Finally, a simple extraction, hydrolysis and partitioning procedure afforded 30-hydroxybetulin 3 in 2.9% (w/w) without chromatography. This method provides rapid access to a medicinally relevant molecule which could be used for semisyntheses, such as in the preparation of anti-cancer agents.
Acknowledgements
We acknowledge that the plant was collected from Goreng Nyungar land and extend our appreciation to the Nowanup community for their ongoing support.
References
1 Liu J, Yin X, Kou C, Thimmappa R, Hua X, Xue Z. Classification, biosynthesis, and biological functions of triterpene esters in plants. Plant Commun 2024; 5(4): 100845.
| Crossref | Google Scholar | PubMed |
2 Busta L, Schmitz E, Kosma DK, Schnable JC, Cahoon EB. A co-opted steroid synthesis gene, maintained in sorghum but not maize, is associated with a divergence in leaf wax chemistry. Proc Natl Acad Sci USA 2021; 118(12): e2022982118.
| Crossref | Google Scholar | PubMed |
3 Inoue M, Ohtani K, Kasai R, Okukubo M, Andriantsiferana M, Yamasaki K, Koike T. Cytotoxic 16-beta-[(D-xylopyranosyl)oxy]oxohexadecanyl triterpene glycosides from a Malagasy plant, Physena sessiliflora. Phytochemistry 2009; 70(9): 1195-1202.
| Crossref | Google Scholar | PubMed |
4 Sun IC, Wang H-K, Kashiwada Y, Shen J-K, Cosentino LM, Chen C-H, Yang L-M, Lee K-H. Anti-AIDS agents. 34. Synthesis and structure−activity relationships of betulin derivatives as anti-HIV agents. J Med Chem 1998; 41(23): 4648-4657.
| Crossref | Google Scholar | PubMed |
5 Macías FA, Simonet AM, Esteban MD. Potential allelopathic lupane triterpenes from bioactive fractions of melilotus messanensis. Phytochemistry 1994; 36(6): 1369-1379.
| Crossref | Google Scholar |
6 Mar AA, Szotek EL, Koohang A, Flavin WP, Eiznhamer DA, Flavin MT, Xu Z-Q. Synthesis, proapoptotic screening, and structure–activity relationships of novel aza-lupane triterpenoids. Bioorg Med Chem Lett 2010; 20(18): 5389-5393.
| Crossref | Google Scholar | PubMed |
7 Paczkowska G. Dodonaea caespitosa Diels. In: Florabase – the Western Australian Flora. Perth, WA, Australia: Western Australian Herbarium; 2025. Available at https://florabase.dbca.wa.gov.au/browse/profile/4756 [Verified July 2025]
9 Pramanick S, Mandal S, Mukhopadhyay S, Jha S. Allylic hydroxylation through acid catalysed epoxy ring opening of betulinic acid derivatives. Synth Commun 2005; 35(16): 2143-2148.
| Crossref | Google Scholar |
10 Yamashita H, Matsuzaki M, Kurokawa Y, Nakane T, Goto M, Lee K-H, Shibata T, Bando H, Wada K. Four new triterpenoids from the bark of Euonymus alatus forma ciliato-dentatus. Phytochem Lett 2019; 31: 140-146.
| Crossref | Google Scholar | PubMed |
11 Chitalia VCPD, Prabakaran, D, Bharti A, Jayatilake GS, Fu X. Compositions and methods of use. World Intellectual Property Organization; 2010. International Publication Number WO2010/083311 A3. Available at https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2010083311
12 Siddiqui S, Begum S, Hafeez F, Siddiqui BS. Isolation and structure of neriumol and nerifol from the leaves of Nerium odorum. Planta Med 1987; 53(01): 47-49.
| Crossref | Google Scholar | PubMed |
13 Chitalia VCPD, Bharti A, Jayatilake GS, Fu X. Compositions and methods of use for cancer. World Intellectual Property Organization; 2021. International Publication Number WO2021/086610 A1. Available at https://patentimages.storage.googleapis.com/1a/28/ad/fd45fd5b897fb5/WO2022221194A1.pdf
14 Shimojo M, Matsumoto K, Hatanaka M. Enzyme-mediated preparation of optically active 1,2-diols bearing a long chain: enantioselective hydrolysis of cyclic carbonates. Tetrahedron 2000; 56(47): 9281-9288.
| Crossref | Google Scholar |
15 Ishihara K, Hanaki N, Yamamoto H. Highly diastereoselective acetal cleavages using novel reagents prepared from organoaluminum and pentafluorophenol. J Am Chem Soc 1993; 115(23): 10695-10704.
| Crossref | Google Scholar |
16 Masaoka Y, Sakakibara M, Mori K. Synthesis and absolute configuration of (+)-8-hydroxyhexadecanoic acid, an endogenous inhibitor for spore germination in Lygodium japonicum. Agric Biol Chem 1982; 46(9): 2319-2324.
| Crossref | Google Scholar |
17 Tulloch AP. The oxygenated fatty acids from the oil of spores of Lycopdium species. Can J Chem 1965; 43(2): 415-420.
| Crossref | Google Scholar |
18 Schweizer P, Felix G, Buchala A, Müller C, Métraux J. Perception of free cutin monomers by plant cells. Plant J 1996; 10(2): 331-341.
| Crossref | Google Scholar |


