Polyacids as Modulators for the Synthesis of UiO-66
Kyle C. Bentz A , Sergio AyalaA Department of Chemistry and Biochemistry, University of California, San Diego, La Jolla, CA 92093, USA.
B Corresponding author. Email: scohen@ucsd.edu
Australian Journal of Chemistry 72(10) 848-851 https://doi.org/10.1071/CH19271
Submitted: 18 June 2019 Accepted: 6 August 2019 Published: 5 September 2019
Abstract
Poly(acrylic acid) (PAA) and poly(vinylbenzoic acid) (PBA) were synthesized via reversible addition–fragmentation chain transfer (RAFT) polymerization and used as modulators for the synthesis of the metal–organic framework (MOF) UiO-66 (UiO = University of Oslo). Whereas typical syntheses of UiO-66 require large excesses of acid modulators, such as acetic acid or benzoic acid, to achieve controlled particle size and morphology of the resulting MOF particles, the use of polymerized acids allows for narrow particle size distributions at sub-stoichiometric quantities of modulator. We show using scanning electron microscopy and dynamic light scattering techniques that polyacids can act as alternative modulators for the growth of UiO-66.
Introduction
Metal–organic frameworks (MOFs) are porous network solids composed of organic multitopic ligands linked via metal clusters known as secondary building units (SBUs). ZrIV-based MOFs like UiO-66 (composed of Zr6(µ3-O)4(µ3-OH)4 SBUs and 1,4-benzene dicarboxylic acid (H2bdc)) have become a highly studied class of MOFs owing to their high stability and chemical tunability. The solvothermal synthesis of UiO-66 is often accompanied by monotopic ligands known as modulators, typically acetic acid (AA) or benzoic acid (BA).[1] Modulators act to slow the growth of the MOF particles, resulting in narrower particle size distributions and controlled morphologies.[2] However, in order to achieve effective modulation of MOF growth, a large excess of the monofunctional acid is required. Furthermore, modulators are only effective over a narrow range of concentrations, that is, undermodulation can result in uncontrolled particle growth and overmodulation can preclude the formation of the MOF entirely.
Although there are several examples of polymers containing coordinating or structure-directing blocks,[3] and even ligand-containing blocks,[4] the use of polymers explicitly as modulators is largely unreported. An early example of the use of polymers to control the growth of coordination polymers (CPs) was reported by Kitagawa, where the sodium salt of poly(vinylsulfonic acid) was used as an additive to control size in a Cu-based coordination polymer.[5] When the additive was present, the resulting CPs formed much larger crystals than when no additive was present, due to the slowing of nucleation events. Polymers have also been used to template the growth of MOFs into various morphologies or confined geometries. Eddaoudi and coworkers used polyvinylpyrrolidone (PVP) in combination with small-molecule quaternary ammonium compounds to provide excellent control of crystal size morphology in InIII-based square-octahedral MOFs.[6] PVP was also used to selectively control the sizes of the [100] and [111] facets in cubic ZnII-based frameworks.[7] Triblock polymer surfactants have been used to provide both micro- and mesoporous MOFs by directing framework formation around the nanostructures formed from the block polymer assemblies.[8] There are several examples of the use of block polymers containing coordinating blocks to create higher-order assemblies in MOF systems. For example, Cheetham and coworkers used poly(styrene-b-(4-vinyl pyridine)) to create hierarchical superstructures in ZIF-8,[9] and Schmidt and coworkers used poly((ethylene oxide)-b-(methacrylic acid)) to create large hexagonal mesocrystals in both ZnII and CuII-based MOFs.[10] Coordinating block polymers have also been shown to act as modulators for the synthesis of metal–organic polyhedra (MOPs), where polymers containing poly(isophthalic acid) blocks effectively controlled the crystal habit in a variety of Cu24L24 cages.[11]
In addition to the benefit of eliminating large quantities of waste generated by the use of small molecular modulators in stoichiometric excess,[12] herein it is shown that polyacid modulators provide control of MOF growth over a wider range of concentrations when compared with simple molecular acids. In particular, poly(acrylic acid) (PAA) was shown to provide MOF particles at sub-300-nm sizes with very narrow particle size distributions. This is consistent with the work of Lotsch and coworkers, who used commercially available PAA to achieve small particles of HKUST-1 and IRMOF-3;[13] however, a systematic study of the effect of acid concentration was not carried out. Although there have been studies that have elucidated the effect of concentration of small-molecule modulators for UiO-66,[14] no such study with polymer modulators has been performed to date. Herein, we report the use of PAA and poly(vinylbenzoic acid) (PBA) as modulators for the growth of UiO-66 over a very wide range of concentrations, and find that both polymers act as modulators at low concentrations to control both the size and size distribution of UiO-66 nanoparticles.
Results and Discussion
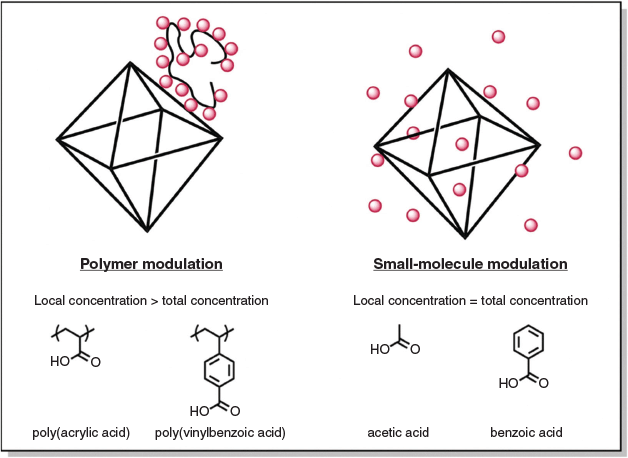
The concept of using polyacids as a modulator for MOF growth stems from the hypothesis that although the total concentration of acid modulator with respect to organic linker may be sub-stoichiometric, the local concentration of acid still remains high (Fig. 1). In contrast, the small-molecule acid modulator always exists in the condition where the local concentration is equal to the total concentration. In other words, although 50 equiv. of small molecule acid is evenly distributed throughout the reaction medium, a polymer with a degree of polymerization (DP) of 50 contains 50 equiv. of acid on a single chain and therefore should behave analogously to a much higher concentration of small-molecule acid. We therefore speculated that the use of polymer modulators would allow controlled MOF particle growth at sub-stoichiometric quantities. Two different polyacids were used in the present study, PAA and PBA, which are structurally analogous to the AA and BA that are commonly used small-molecular modulators for the synthesis of UiO-66. Reversible addition–fragmentation chain transfer (RAFT) polymerization was used to synthesize the PAA and PBA polymers, as RAFT allows polymers of predetermined molecular weights and affords polymers with very narrow molecular weight distributions. 1H NMR confirmed that DPs of 51 and 19 were achieved and gel permeation chromatography revealed narrow dispersities of Đ = 1.04 and 1.10 for PAA and PBA respectively (Figs S1 and S2, Supplementary Material).
|
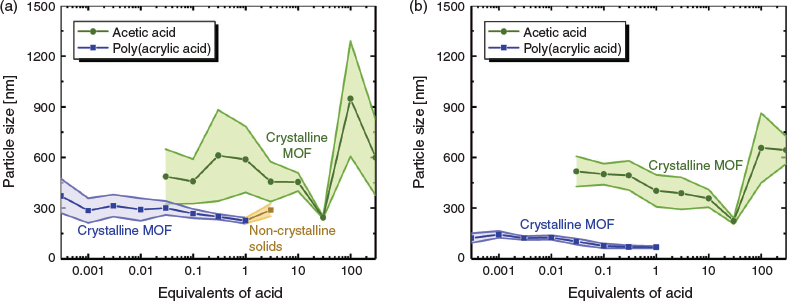
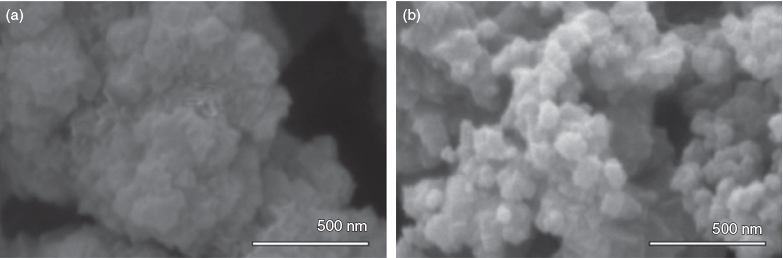
For a variety of applications in which MOFs are envisioned, control over particle size and size distribution is critical. Therefore, monotopic acid modulators are often introduced during synthesis to control particle nucleation and slow growth, leading to particles with narrow size distributions. In this study, particle sizes were characterized by both dynamic light scattering (DLS) and scanning electron microscopy (SEM). Although SEM can provide useful information regarding particle morphology, accurate determination of particle size can be challenging, especially for samples with large size distributions. However, the use of DLS provides an unambiguously, bias-free quantification of particle sizes and distributions. Particle sizes and distributions were calculated using a cumulants analysis of the second-order autocorrelation function G2(τ) (for further details see Supplementary Material).
When AA was used as the modulator for UiO-66 synthesis, only a very narrow range of acid concentrations provided effective modulation of the MOF particles. Indeed, only when 30 equiv. of AA was used was the average particle size <300 nm as measured by both DLS and SEM (Fig. 2). When >30 equiv. of AA was used, the resulting MOF particles grew markedly in size and the size distribution widened considerably. Addition of >300 equiv. of AA in the UiO-66 synthesis resulted in overmodulation and no isolable solids were obtained. In contrast, the use of PAA allowed a narrow distribution of particles sizes, as measured by both DLS and SEM, over a very wide range of concentrations (Fig. 2). The use of PAA as the modulator for UiO-66 synthesis gave particles with diameters <400 nm as measured by DLS over the entire concentration range, spanning greater than four orders of magnitude. Measurements of particle size using SEM images corroborate these results, indicating a very tight distribution of particle sizes when PAA is used as the modulator, giving highly controlled particles over the entire range of number of equivalents tested. SEM shows a tighter distribution of particle sizes for AA-modulated UiO-66 only in a very narrow range of modulator concentration (Fig. 2). In addition to controlling the growth of UiO-66, the use of PAA also endows the resulting MOF particles with superior colloidal stability. Particles synthesized using AA sediment from solution quite rapidly (<1 day), but particles made under identical conditions with PAA modulator remain exceptionally well dispersed after standing for 1 week (Fig. S5, Supplementary Material). We hypothesize that this additional colloidal stability is due to coordinated polymer that remains exposed on the surface, which provides stabilization and prevents aggregation events.
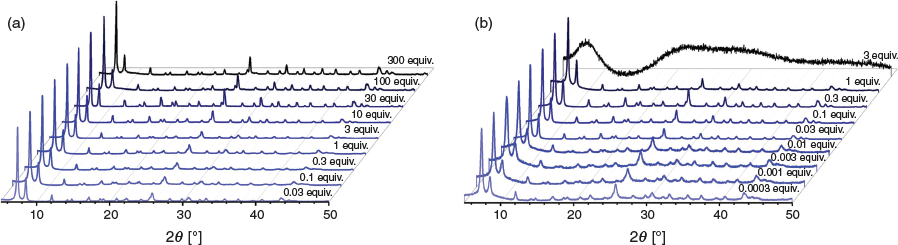
Across the entire range of acid equivalents tested with AA, the crystallinity of the particles was very high, as determined by powder X-ray diffraction patterns (PXRD, Fig. 3). Although low concentrations of small molecular modulator gave materials with uncontrolled particle size and broad size distributions, crystallinity remained high throughout the concentration range. However, when PAA was used as the modulator for UiO-66 growth, crystalline particles were not produced until the concentration was reduced to stoichiometric equivalence with respect to H2bdc ligand concentration. This observation supports the hypothesis that polymer acids have very high effective local concentration. In contrast to small molecule AA, which required >300 equiv. to achieve overmodulation and preclude particle growth, only 3 equiv. of PAA resulted in overmodulation, highlighting the efficiency of the polymer acid. Interesting to note as well is that although overmodulation with AA causes no solids to form during synthesis, when UiO-66 growth is overmodulated by PAA, non-crystalline solids are formed, likely composed of metal-coordinated crosslinked polymers. These metal-coordinated polymers may have interesting potential as amorphous MOFs or metallogels under the proper synthetic conditions.[15]
|
Crystal morphology is largely unaffected by the use of PAA modulator at low concentrations (Fig. 4). Although particle sizes were smaller and size distributions were narrower when PAA was used as the modulator for UiO-66, highly faceted or regular geometries were not obtained. It has been previously shown that the use of doubly hydrophilic block polymers provides morphology control in ZnII- and CuII-based MOFs.[10] In their system, poly(methacrylic acid) acted as the coordinating block to slow the crystal growth, while a poly(ethylene glycol) block worked to stabilize the growing MOFs and provide assembly of mesocrystals into large, micrometre-sized hexagonal crystals. Perhaps the use of block polymers for modulation and morphology control in the growth of UiO-66 will result in similar particle morphology control, but that is outside the scope of the present study.
Additionally, BA and PBA were used to modulate UiO-66 (Figs S3 and S4, Supplementary Material). When BA was used as a modulator, 10 equiv. provided particles with both the smallest diameters and the narrowest size distributions. Crystalline particles with narrow size distributions were produced when 30 equiv. of BA were used, but at numbers of equivalents >30, no particles were produced as a consequence of overmodulation. At <3 equiv., modulation by BA became increasingly less controlled, with very large particle sizes and size distributions. Modulation by PBA provided improved levels of control compared with BA, with very narrow size distributions afforded by equivalents of acid as low as 0.03. Below these levels, particle size and size distributions increased markedly. Interestingly, overmodulation with PBA occurred at >0.3 equiv., demonstrating the remarkable modulation efficiency of this polymer. As in the case of PAA, overmodulation by PBA resulted in non-crystalline solids.
Lastly, Brunauer–Emmett–Teller (BET) surface area measurements were performed on selected samples to assess how modulator type and concentration affect the porosity of the resulting UiO-66 particles (Figs S6–S13 and Table S1, Supplementary Material). Particles modulated with PAA showed a lower surface area compared with particles synthesized with 30 equiv. of AA, but were comparable with particles modulated with 1 equiv. of AA. In contrast, particles synthesized with PBA retained high surface areas, comparable with materials prepared with BA. When BA was used at 10 and 0.3 equiv., surface areas of 1046 ± 194 and 1018 ± 86 m2 g−1 were achieved respectively. Similarly, when PBA was used at 0.3 and 0.03 equiv., surface areas of 946 ± 13 and 1010 ± 25 m2 g−1 were achieved respectively, indicating that PBA is an effective modulator for UiO-66 even at sub-stoichiometric concentrations.
Conclusions
In conclusion, we have shown that polymer acids are useful modulators for the synthesis of UiO-66, providing narrow particle size distributions over a wide range of concentrations, and at equivalencies orders of magnitude lower than their small-molecule analogues. Average particle sizes and size distributions were determined by both SEM and DLS techniques. In addition to providing efficient levels of modulation, synthesis of UiO-66 using PAA as a modulator resulted in particles with high colloidal stability, that remained well suspended in solution after more than 1 week sitting undisturbed.
Supplementary Material
Synthetic procedures, powder X-ray diffraction patterns, scanning electron microscopy images, gel permeation chromatograms, dynamic light scattering data, and BET isotherms are available on the Journal’s website.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
We acknowledge financial support from the Department of Energy, Office of Basic Energy Sciences, Division of Materials Science and Engineering under Award no. DE-FG02–08ER46519 (S.M.C.). S.A. was supported, in part, by a fellowship from the Department of Education Graduate Assistance in Areas of National Need (GAANN) Training Grant and the Inamori Foundation. M.K. is supported by the Department of Defence (DoD) through the National Defence Science and Engineering Graduate (NDSEG) Fellowship Program. SEM imaging was performed at the San Diego Nanotechnology Infrastructure (SDNI) of UC San Diego, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (grant ECCS-1542148).
References
[1] G. C. Shearer, S. Chavan, S. Bordiga, S. Svelle, U. Olsbye, K. P. Lillerud, Chem. Mater. 2016, 28, 3749.| Crossref | GoogleScholarGoogle Scholar |
[2] W. Morris, S. Wang, D. Cho, E. Auyeung, P. Li, O. K. Farha, C. A. Mirkin, ACS Appl. Mater. Interfaces 2017, 9, 33413.
| Crossref | GoogleScholarGoogle Scholar | 28509530PubMed |
[3] D. Bradshaw, E.-H. Samir, L. Lupica-Spagnolo, Chem. Soc. Rev. 2014, 43, 5431.
| Crossref | GoogleScholarGoogle Scholar | 24811778PubMed |
[4] S. Ayala, K. C. Bentz, S. M. Cohen, Chem. Sci. 2019, 10, 1746.
| Crossref | GoogleScholarGoogle Scholar | 30842840PubMed |
[5] T. Uemura, Y. Hoshino, S. Kitagawa, K. Yoshida, S. Isoda, Chem. Mater. 2006, 18, 992.
| Crossref | GoogleScholarGoogle Scholar |
[6] M. Pang, A. J. Cairns, Y. Liu, Y. Belmabkhout, H. C. Zeng, M. Eddaoudi, J. Am. Chem. Soc. 2012, 134, 13176.
| Crossref | GoogleScholarGoogle Scholar | 22812681PubMed |
[7] S. Wang, Y. Lv, Y. Yao, H. Yu, G. Lu, Inorg. Chem. Commun. 2018, 93, 56.
| Crossref | GoogleScholarGoogle Scholar |
[8] M.-H. Pham, G.-T. Vuong, F.-G. Fontaine, T.-O. Do, Cryst. Growth Des. 2012, 12, 1008.
| Crossref | GoogleScholarGoogle Scholar |
[9] S. Cao, G. Gody, W. Zhao, S. Perrier, X. Peng, C. Ducati, D. Zhao, A. K. Cheetham, Chem. Sci. 2013, 4, 3573.
| Crossref | GoogleScholarGoogle Scholar |
[10] J. Hwang, T. Heil, M. Antonietti, B. V. K. J. Schmidt, J. Am. Chem. Soc. 2018, 140, 2947.
| Crossref | GoogleScholarGoogle Scholar | 29390606PubMed |
[11] T.-H. Chen, L. Wang, J. V. Trueblood, V. H. Grassian, S. M. Cohen, J. Am. Chem. Soc. 2016, 138, 9646.
| Crossref | GoogleScholarGoogle Scholar | 27400759PubMed |
[12] M. Rubio-Martinez, C. Avci-Camur, A. W. Thornton, I. Imaz, D. Maspoch, M. R. Hill, Chem. Soc. Rev. 2017, 46, 3453.
| Crossref | GoogleScholarGoogle Scholar | 28530737PubMed |
[13] A. Ranft, S. B. Betzler, F. Haase, B. V. Lotsch, CrystEngComm 2013, 15, 9296.
| Crossref | GoogleScholarGoogle Scholar |
[14] G. Lu, C. Cui, W. Zhang, Y. Liu, F. Huo, Chem. Asian J. 2013, 8, 69.
| Crossref | GoogleScholarGoogle Scholar | 23065843PubMed |
[15] K. C. Bentz, S. M. Cohen, Angew. Chem. Int. Ed. 2018, 57, 14992.
| Crossref | GoogleScholarGoogle Scholar |