Stable organic radicals and their untapped potential in ionic liquids
Theo A. Ellingsen A , Natasha Hoffmann B , Wesley J. Olivier A , Stuart C. Thickett
A , Natasha Hoffmann B , Wesley J. Olivier A , Stuart C. Thickett  A , Debbie S. Silvester
A , Debbie S. Silvester  B and Rebecca O. Fuller
B and Rebecca O. Fuller  A *
A *
A School of Natural Sciences – Chemistry, University of Tasmania, Hobart, Tas., Australia.
B School of Molecular and Life Sciences, Curtin University, GPO Box U1987, Perth, WA 6845, Australia.
Australian Journal of Chemistry 75(11) 893-898 https://doi.org/10.1071/CH22126
Submitted: 2 June 2022 Accepted: 26 July 2022 Published: 19 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Stable organic radicals have an open shell structure that makes them suitable for use in a diverse set of applications. Specifically, it is the reversible one-electron redox behaviour that makes these species suitable for energy storage and in molecular electronics. Maintaining chemical stability, low redox potential and charge transfer capabilities, are key to the further development of these materials. To date, researchers have largely focused on the the preparation of new molecules with improved redox capabilities for use in traditional solvents. More recently exploration into the use of ionic liquids to stabilise charged species and reduce side reactions has shown promise. Computational and preliminary experimental studies have explored the impact of ionic liquids on radical stabilisation, and notable improvements have been observed for nitroxide-based materials when traditional solvents are replaced by ionic liquids. However, these gains require significant refinement based on the identity of the radical species and the ionic liquid. In this highlight, we focus on the current state of using ionic liquids as solvents to stabilise organic radicals and suggestions on the future direction of the field.
Keywords: Blatter, electrochemistry, ionic liquids, nitroxide, polymer composite, radicals, redox active material, verdazyl.
Introduction
Stable and persistent organic radicals are species that are kinetically and thermodynamically stable despite the molecules possessing an incomplete vacancy. The stabilisation of one or more unpaired electrons in an open shell, results in unique physicochemical (redox, optical and magnetic) properties.[1] Over 100 years have passed since the first reported persistent radical, yet these species continue to be of relevance to contemporary scientific research with a variety of open shell species stabilised through steric protection or the delocalisation of the spin density as well as non-covalent approaches (Fig. 1).[2–4] More than a fundamental curiosity, they are routinely used by biologists to determine the rate of diffusion of molecules through a membrane.[5] Researchers are focused on the production of new radicals,[6] exploring their fundamental structure and bonding,[7] as well as the development of these species into functional materials suitable for use in magnetic, electronic, optoelectronic and biological applications.[8,9] Critical to the advancement of these molecules is the stabilisation of the unpaired electron as well as the fine-tuning of the structure–property relationships towards the intended application.[4]
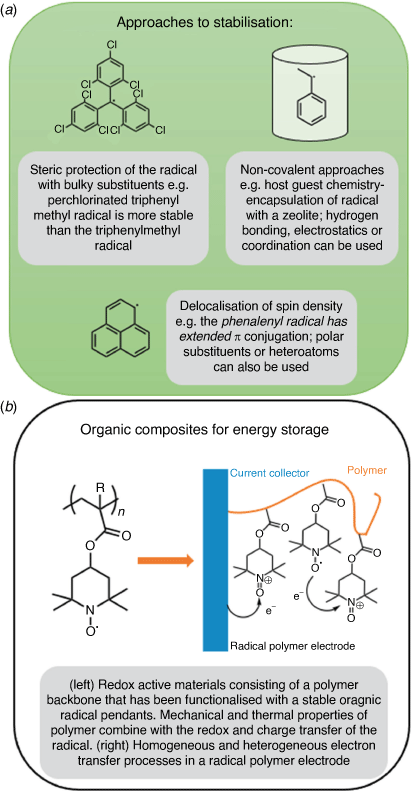
|
Life in the modern world requires access to energy at any time or place, which is made possible through portable power technologies. Lithium-ion batteries (LIBs) are the dominant type of such technologies, due to their high cell voltages (~3.6 V), rechargeability, good cyclability and long lifetimes.[10] Safety and environmental concerns have driven the development of new electrode materials and battery systems. Organic radicals offer a myriad of design choices for energy storage based on their electrochemically active properties.[9,11–14] These molecules can serve as active electrode materials as a consequence of their susceptibility to both oxidation and reduction processes (Fig. 2).[15] Their fast kinetics, reversible redox potentials and high charge and discharge rates make these species well suited to mobile power storage. The preparation of LIB alternatives based on redox-active organic and polymer composite materials is an active area of research and, to date, a variety of materials featuring nitroxides, nitroxyl, phenoxyl and hydrazyls have been prepared.[16] In examples consisting of a non-conducting polymer and radical pendant groups (Fig. 1b), the redox activity and charge transport properties are a result of the open shell moieties. The mechanical and thermal properties of the macromolecule are determined by the polymeric backbone. The TEMPO (2,2,6,6-tetramethylpiperidin-1-yl)oxyl) based system first reported 20 years ago[17] has become one of the most promising electroactive materials based on stable organic radicals.
|
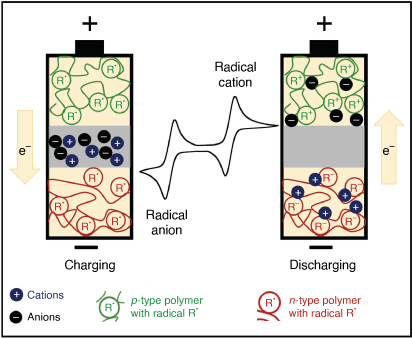
The simplest organic battery involves replacing the LIB cathode with a radical macromolecule (cf. LIB uses LiCoO2) bound to glassy carbon (Fig. 1b).[11] A poly(2,2,6,6-tetramethylpiperidinyloxy methacrylate) cathode was found to be stable, highly reversible, capable of multiple charge/discharge cycles and have a potential of ~3.6 V (vs Li/Li+).[18] All-organic batteries represent an alternative type of cell in which electrochemically active polymers are employed for both the anode and cathode materials. These cells have potential for application in devices powered by thin-film batteries (Fig. 3). Organic cells have demonstrable performance in terms of their charging speed and cycling stability, although the voltage generated is relatively low (~1 V).[19,20] However, the possibility of lower cost and higher flexibility justify a small sacrifice in performance. Increased sustainability can be offered via radical pendant polypeptide examples.[21]
Organic redox flow batteries are an alternative cell type, suited to grid based power storage. The organic radical materials have a similar design to the traditional vanadium flow systems with the anode and cathode materials dissolved into the electrolyte solution.[22,23] Redox-active monomers have been used directly, with organic radicals being inexpensive, exhibiting redox stability and the capacity to be tuned through functionalisation when compared to vanadium. The most promising candidates are bipolar, whereby a single radical species can be used as both the anolyte and the catholyte.[24]
|
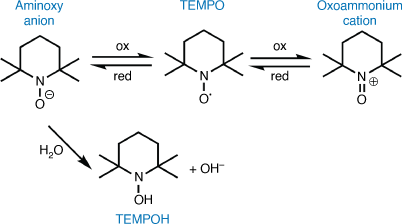
Regardless of the organic electrode material used, the reactivity of the radical with the electrolyte or other species represents a challenge to the development of these materials in batteries. The irreversibility of the redox reaction and poor stability of the radical or its electrogenerated product is also a significant problem, and is often caused by side-reactions of the radical species and dissolution in the solvent.[16] It is well known that the reduced form of TEMPO (aminoxy anion) degrades via fast environmental proton transfer (Fig. 2)[25] and nucleophilic attack[26] with the decomposition leading to a loss in the reversibility. The inherent instability can be overcome by adding stabilising functional groups, such as trifluoromethyl (–CF3), to the nitroxide structure.[27,28] This tailored approach is not necessarily useful in preventing side-reactions (e.g. self degradation and degradation via reaction with the neutral nitroxide radical)[29] or reducing problems associated with dissolution of the active polymer into standard electrolytes that results in loss of battery charge.[20] In recent times, ionic liquid (IL) electrolytes have been studied as a more promising generalised approach to resolving these limitations (Fig. 4c). [30,31]
|
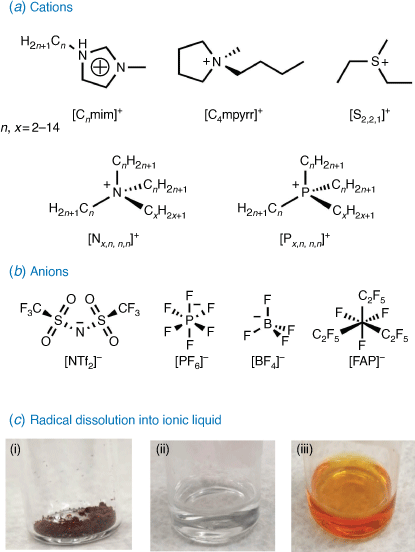
ILs (Fig. 4) are a unique class of solvent well suited to applications in energy storage.[32,33] They differ from molecular solvents as they consist entirely of ionic species (cations and anions) with a melting point of < 100°C.[30] ILs are attractive solvents for electrochemistry as the charged ions allow an IL to act as both the solvent and electrolyte in an electrochemical system.[34] ILs also possess favourable properties such as low volatility, high polarity, high thermodynamic and chemical stability, wide electrochemical windows and structural designability.[34–36] In the context of organic radical-based batteries, ILs may offer a solution to the poor stability and electrochemical irreversibility of redox species observed in conventional organic solvents.[30,31,37] ILs may reduce or prevent the undesired dissolution of radical macromolecules at times observed with conventional solvents, as the ionic nature means that they differ substanitally in polarity compared to organic molecules.[37] Being electrochemically and thermodynamically stable over a wide range of redox potentials makes ILs a suitable medium for reversible reactions.[38] ILs remain liquid over a wide-range of temperatures[38] and are stable to decomposition over a large electrochemical window, which minimises side-reactions.[39] These properties can be optimised by tailoring the combination of IL cation and anion to allow for better reversibility of the dissolved redox system.[38]
The use of ILs to stabilise organic radicals[40,41] in spin probes is a well-developed field,[42] however their application in electrochemically active energy storage materials has received much less attention. Computational chemists have led recent interest into understanding the value of ILs for improving the stability and redox potential in radical composites for organic batteries. We aim to highlight the current state of play in this exciting field and the future directions of this work.
Recent developments with radicals in ionic liquids
Nitroxide radical systems
The importance of understanding stabilisation differences between the neutral and charged radical species is becoming prevalent for decreasing the decomposition and improving the cyclability of nitroxide-based batteries.[43] Recent theoretical studies have highlighted that the propensity of the aminoxy anion to undergo side reactions can be decreased through replacement of the aqueous media with an appropriate IL. [44–48] Tailoring the IL, rather than substitution of the nitroxide, can decrease reactivity by increasing diffusion rates,[44] increasing the redox potential of the nitroxide, 4.7–5.5 eV (cf. 2.2 eV in aqueous media),[45] and improving reversibility in flow systems.[25] The choice of IL is crucial because not all ILs are capable of preventing environmental proton transfer. Optimisation of the cation–anion pair can reduce proton transfer to the aminoxy anion. ILs with positive Gibbs free energy values are found to preferentially stabilise the anionic radical compared to hydroxy-TEMPO (TEMPOH).[25] Specifically, interactions between TEMPO and imidazolium ILs are predicted to be dominated through electrostatics.[46] The functionalisation of TEMPO influences both solubility and oxidation potential.[48] However a general trend towards specific ILs that will improve performance across the family has not yet been reported.[29] In addition to significant computational studies, Wylie and coworkers undertook an experimental investigation into the electrochemistry of three TEMPO analogues in ILs.[29] Specific focus was on examining the degradation mechanisms known to impact on the aminoxy anion, with the 4-cyano-substituted TEMPO found to exhibit the greatest stability in the reduced form. It should be noted that the constitution of the IL requires optimisation depending on the exact TEMPO analogue.
Related nitroxide alternatives have also been studied, including aqueous-based catholytes such as a nitroxide radical functionalised with an imidazolium cation for aqueous redox flow batteries.[49] Cathodes based on polymeric ILs with redox-active pendants have also shown promise.[50] Half cells based on the polyvinylimidazolium backbone bearing TEMPO groups exhibited improved performance in terms of charge capacity and current density.[51] Heterogenous systems in which the nitroxide radical is dissolved into ILs have been shown to influence localisation of the radical molecule compared to traditional solvents.[52]
Alternative radical systems
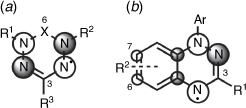
The nature of the radical species is an often overlooked but essential component in the development of radical composite materials. The majority of contemporary examples feature nitroxide-based radicals, which is in part due to the ease with which these species can be prepared from commercial materials.[6] However, stable nitroxide radicals possess notable deficiencies. Specifically, TEMPO has a strong localisation of charge, which can limit charge transfer and reduce electronic conductivity in the radical polymer system.[53] Alternative open shell species (Fig. 5) with a high degree of delocalisation could overcome the prevalence of mismatched intermolecular conformations and their use in a conducting polymer system may alleviate and improve this deficiency.[53]
|
Verdazyl radicals (Fig. 5a) are one example in which the unpaired electron is delocalised in a π symmetry orbital that spans the four nitrogen atoms of the tetrazine ring. They are generally capable of undergoing reversible one electron oxidation and reduction processes. Their redox potentials tend to vary based on the verdazyl type with the 6-oxo analogues generating larger cell voltages (~1.5 V) than the methylene bridge Kuhn verdazyls (~1 V).[7] The singly occupied molecular orbital (SOMO) is further influenced by functionalisation at N1,5. For example, an inductively withdrawing but resonance donating p-methoxy-phenyl group lowered the oxidation potential when compared to an unsubstituted phenyl analogue.[7] Spectroelectrochemical methods have been used to investigate the impact of an extended π system at the N1,5 on the redox behaviour.[54] A preliminary investigation of the cyclic voltammetric behaviour of 1,5-dimethyl-3-phenyl-6-oxoverdazyl in several ILs found that the IL structure influences the reduction process.[34] Polymer systems with verdazyl pendants have a high degree of redox tunability and have been synthesised via oxidation of a polymethacrylate bearing tetrazine[55] and post-polymerisation via copper-assisted azide–alkyne cycloaddition (CuAAC) reactions.[56] In Blatter (benzo[e][1,2,4]triazinyl) radicals, the spin distribution of the SOMO largely resides across the triazine ring, with some smaller elements found in the neighbouring aryl group (Fig. 5b).[57] Functionalisation of the phenyl group at N1 has been shown to impact the redox potential of the species, as well as functionalisation at C3 or C6 and C7. An electroactive polymer based on Blatter’s radicals has been made via CuAAC.[58] Only basic electrochemical studies of Blatter radicals and polymers have so far been undertaken.[59] This delocalised system has been shown to undergo reversible one electron oxidation (10π cation) and reduction (12π anion) processes with a narrow cell potential.[60,61] Detailed structural relation studies have yet to be undertaken for this radical species.
Conclusions
The development of stable organic radicals for energy storage continues to remain a highly topical field. Increasing the energy density and preventing side reactions that result in decomposition are key challenges. Using ILs to overcome these deficiencies is an established concept, with substantial work on nitroxide-based radicals already reported. Renewed contemporary interest in this area has been driven by computational research. Overcoming irreversibility/quenching of the redox process is essential and, promisingly, ILs are predicted to significantly stabilise the reduction process compared to traditional solvents. However, stabilisation of each nitroxide analogue with an appropriate IL appears to require considerable refinement. Significant work is required for the advancement of this field and to mitigate common failings. The real possibility in this field arguably lies in the employment of materials based on under-utilised stable organic radicals that possess delocalised spin density. Although significant steps have been taken to understand the fundamentals of how ILs can be used to help mitigate some of the common failings, there is still much work needed to understand which combinations of IL cations and anions can best stabilise the radicals, their electrogenerated products, and maximise the physical properties for use in batteries.
Data availability
Data sharing is not applicable as no new data was generated. Analysis has been performed on published materials which can be accessed by permission/license.
Conflicts of interest
There are no conflicts to declare.
Declaration of funding
ROF was supported by an Australian Research Council (ARC) Discovery Early Career Researcher Award (DE180100112).
Acknowledgements
DSS thanks the ARC for a Future Fellowship (FT170100315).
References
[1] D Griller, KU Ingold, Persistent carbon-centered radicals. Acc Chem Res 1976, 9, 13.| Persistent carbon-centered radicals.Crossref | GoogleScholarGoogle Scholar |
[2] M Gomberg, An instance of trivalent carbon: triphenylmethyl. J Am Chem Soc 1900, 22, 757.
| An instance of trivalent carbon: triphenylmethyl.Crossref | GoogleScholarGoogle Scholar |
[3] RG Hicks, What’s new in stable radical chemistry? Org Biomol Chem 2007, 5, 1321.
| What’s new in stable radical chemistry?Crossref | GoogleScholarGoogle Scholar |
[4] B Tang, J Zhao, J-F Xu, X Zhang, Tuning the stability of organic radicals: from covalent approaches to non-covalent approaches. Chem Sci 2020, 11, 1192.
| Tuning the stability of organic radicals: from covalent approaches to non-covalent approaches.Crossref | GoogleScholarGoogle Scholar |
[5] Z Yang, M Bridges, MT Lerch, C Altenbach, WL Hubbell, Saturation recovery EPR and nitroxide spin labeling for exploring structure and dynamics in proteins. Methods Enzymol 2015, 564, 3.
| Saturation recovery EPR and nitroxide spin labeling for exploring structure and dynamics in proteins.Crossref | GoogleScholarGoogle Scholar |
[6] RO Fuller, MR Taylor, M Duggin, AC Bissember, AJ Canty, MM Judd, N Cox, SA Moggach, GF Turner, Enhanced synthesis of oxo-verdazyl radicals bearing sterically-and electronically-diverse C3-substituents. Org Biomol Chem 2021, 19, 10120.
| Enhanced synthesis of oxo-verdazyl radicals bearing sterically-and electronically-diverse C3-substituents.Crossref | GoogleScholarGoogle Scholar |
[7] JB Gilroy, SDJ McKinnon, BD Koivisto, RG Hicks, Electrochemical studies of verdazyl radicals. Org Lett 2007, 9, 4837.
| Electrochemical studies of verdazyl radicals.Crossref | GoogleScholarGoogle Scholar |
[8] ZX Chen, Y Li, F Huang, Persistent and stable organic radicals: design, synthesis, and applications. Chem 2021, 7, 288.
| Persistent and stable organic radicals: design, synthesis, and applications.Crossref | GoogleScholarGoogle Scholar |
[9] I Ratera, J Veciana, Playing with organic radicals as building blocks for functional molecular materials. Chem Soc Rev 2012, 41, 303.
| Playing with organic radicals as building blocks for functional molecular materials.Crossref | GoogleScholarGoogle Scholar |
[10] M Winter, B Barnett, K Xu, Before Li ion batteries. Chem Rev 2018, 118, 11433.
| Before Li ion batteries.Crossref | GoogleScholarGoogle Scholar |
[11] K-A Hansen, JP Blinco, Nitroxide radical polymers – a versatile material class for high-tech applications. Polym Chem 2018, 9, 1479.
| Nitroxide radical polymers – a versatile material class for high-tech applications.Crossref | GoogleScholarGoogle Scholar |
[12] MB Casu, Nanoscale studies of organic radicals: surface, interface, and spinterface. Acc Chem Res 2018, 51, 753.
| Nanoscale studies of organic radicals: surface, interface, and spinterface.Crossref | GoogleScholarGoogle Scholar |
[13] T Janoschka, N Martin, U Martin, C Friebe, S Morgenstern, H Hiller, MD Hager, US Schubert, An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 2015, 527, 78.
| An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials.Crossref | GoogleScholarGoogle Scholar |
[14] S Muench, A Wild, C Friebe, B Häupler, T Janoschka, US Schubert, Polymer-based organic batteries. Chem Rev 2016, 116, 9438.
| Polymer-based organic batteries.Crossref | GoogleScholarGoogle Scholar |
[15] C Karlsson, T Suga, H Nishide, Quantifying TEMPO redox polymer charge transport toward the organic radical battery. ACS Appl Mater Interfaces 2017, 9, 10692.
| Quantifying TEMPO redox polymer charge transport toward the organic radical battery.Crossref | GoogleScholarGoogle Scholar |
[16] Y Chen, C Wang, Designing high performance organic batteries. Acc Chem Res 2020, 53, 2636.
| Designing high performance organic batteries.Crossref | GoogleScholarGoogle Scholar |
[17] K Nakahara, S Iwasa, M Satoh, Y Morioka, J Iriyama, M Suguro, E Hasegawa, Rechargeable batteries with organic radical cathodes. Chem Phys Lett 2002, 359, 351.
| Rechargeable batteries with organic radical cathodes.Crossref | GoogleScholarGoogle Scholar |
[18] L Bugnon, CJH Morton, P Novak, J Vetter, P Nesvadba, Synthesis of poly(4-methacryloyloxy-TEMPO) via group-transfer polymerization and its evaluation in organic radical battery. Chem Mater 2007, 19, 2910.
| Synthesis of poly(4-methacryloyloxy-TEMPO) via group-transfer polymerization and its evaluation in organic radical battery.Crossref | GoogleScholarGoogle Scholar |
[19] A Wild, M Strumpf, B Häupler, MD Hager, US Schubert, All-organic battery composed of thianthrene- and TCAQ-based polymers. Adv Energy Mater 2017, 7, 1601415.
| All-organic battery composed of thianthrene- and TCAQ-based polymers.Crossref | GoogleScholarGoogle Scholar |
[20] T Janoschka, MD Hager, US Schubert, Powering up the future: radical polymers for battery applications. Adv Mater 2012, 24, 6397.
| Powering up the future: radical polymers for battery applications.Crossref | GoogleScholarGoogle Scholar |
[21] TP Nguyen, AD Easley, N Kang, S Khan, S-M Lim, YH Rezenom, S Wang, DK Tran, J Fan, RA Letteri, X He, L Su, C-H Yu, JL Lutkenhaus, KL Wooley, Polypeptide organic radical batteries. Nature 2021, 593, 61.
| Polypeptide organic radical batteries.Crossref | GoogleScholarGoogle Scholar |
[22] GL Soloveichik, Flow batteries: current status and trends. Chem Rev 2015, 115, 11533.
| Flow batteries: current status and trends.Crossref | GoogleScholarGoogle Scholar |
[23] J Cao, J Tian, J Xu, Y Wang, Organic flow batteries: recent progress and perspectives. Energy Fuels 2020, 34, 13384.
| Organic flow batteries: recent progress and perspectives.Crossref | GoogleScholarGoogle Scholar |
[24] M Li, J Case, SD Minteer, Bipolar redox-active molecules in non-aqueous organic redox flow batteries: status and challenges. ChemElectroChem 2021, 8, 1215.
| Bipolar redox-active molecules in non-aqueous organic redox flow batteries: status and challenges.Crossref | GoogleScholarGoogle Scholar |
[25] L Wylie, T Blesch, R Freeman, K Hatakeyama-Sato, K Oyaizu, M Yoshizawa-Fujita, EI Izgorodina, Reversible reduction of the TEMPO radical: one step closer to an all-organic redox flow battery. ACS Sustain Chem Eng 2020, 8, 17988.
| Reversible reduction of the TEMPO radical: one step closer to an all-organic redox flow battery.Crossref | GoogleScholarGoogle Scholar |
[26] JP Blinco, JL Hodgson, BJ Morrow, JR Walker, GD Will, ML Coote, SE Bottle, Experimental and theoretical studies of the redox potentials of cyclic nitroxides. J Org Chem 2008, 73, 6763.
| Experimental and theoretical studies of the redox potentials of cyclic nitroxides.Crossref | GoogleScholarGoogle Scholar |
[27] N Dotti, E Heintze, M Slota, R Hübner, F Wang, J Nuss, M Dressel, L Bogani, Conduction mechanism of nitronyl–nitroxide molecular magnetic compounds. Phys Rev B 2016, 93, 165201.
| Conduction mechanism of nitronyl–nitroxide molecular magnetic compounds.Crossref | GoogleScholarGoogle Scholar |
[28] T Suga, Y-J Pu, S Kasatori, H Nishide, Cathode- and anode-active poly(nitroxylstyrene)s for rechargeable batteries: p- and n-type redox switching via substituent effects. Macromolecules 2007, 40, 3167.
| Cathode- and anode-active poly(nitroxylstyrene)s for rechargeable batteries: p- and n-type redox switching via substituent effects.Crossref | GoogleScholarGoogle Scholar |
[29] L Wylie, K Hakatayama-Sato, C Go, K Oyaizu, EI Izgorodina, Electrochemical characterization and thermodynamic analysis of TEMPO derivatives in ionic liquids. Phys Chem Chem Phys 2021, 23, 10205.
| Electrochemical characterization and thermodynamic analysis of TEMPO derivatives in ionic liquids.Crossref | GoogleScholarGoogle Scholar |
[30] P Wasserscheid, W Keim, Ionic liquids – new “solutions” for transition metal catalysis. Angew Chem Int Ed 2000, 39, 3772.
| Ionic liquids – new “solutions” for transition metal catalysis.Crossref | GoogleScholarGoogle Scholar |
[31] M Armand, F Endres, DR MacFarlane, H Ohno, B Scrosati, Ionic-liquid materials for the electrochemical challenges of the future. Nat Mater 2009, 8, 621.
| Ionic-liquid materials for the electrochemical challenges of the future.Crossref | GoogleScholarGoogle Scholar |
[32] M Watanabe, ML Thomas, S Zhang, K Ueno, T Yasuda, K Dokko, Application of ionic liquids to energy storage and conversion materials and devices. Chem Rev 2017, 117, 7190.
| Application of ionic liquids to energy storage and conversion materials and devices.Crossref | GoogleScholarGoogle Scholar |
[33] J Zhang, B Sun, Y Zhao, A Tkacheva, Z Liu, K Yan, X Guo, AM McDonagh, D Shanmukaraj, C Wang, T Rojo, M Armand, Z Peng, G Wang, A versatile functionalized ionic liquid to boost the solution-mediated performances of lithium-oxygen batteries. Nat Commun 2019, 10, 602.
| A versatile functionalized ionic liquid to boost the solution-mediated performances of lithium-oxygen batteries.Crossref | GoogleScholarGoogle Scholar |
[34] J Lee, C Caporale, AJ McKinley, RO Fuller, DS Silvester, Electrochemical properties of a verdazyl radical in room temperature ionic liquids. Aust J Chem 2020, 73, 1001.
| Electrochemical properties of a verdazyl radical in room temperature ionic liquids.Crossref | GoogleScholarGoogle Scholar |
[35] VM Ortiz-Martínez, L Gómez-Coma, G Pérez, A Ortiz, I Ortiz, The roles of ionic liquids as new electrolytes in redox flow batteries. Sep Purif Technol 2020, 252, 117436.
| The roles of ionic liquids as new electrolytes in redox flow batteries.Crossref | GoogleScholarGoogle Scholar |
[36] F Endres, S Zein El Abedin, Air and water stable ionic liquids in physical chemistry. Phys Chem Chem Phys 2006, 8, 2101.
| Air and water stable ionic liquids in physical chemistry.Crossref | GoogleScholarGoogle Scholar |
[37] P Gerlach, A Balducci, A critical analysis about the underestimated role of the electrolyte in batteries based on organic materials. ChemElectroChem 2020, 7, 2364.
| A critical analysis about the underestimated role of the electrolyte in batteries based on organic materials.Crossref | GoogleScholarGoogle Scholar |
[38] LE Barrosse-Antle, AM Bond, RG Compton, AM O’Mahony, EI Rogers, DS Silvester, Voltammetry in room temperature ionic liquids: comparisons and contrasts with conventional electrochemical solvents. Chem Asian J 2010, 5, 202.
| Voltammetry in room temperature ionic liquids: comparisons and contrasts with conventional electrochemical solvents.Crossref | GoogleScholarGoogle Scholar |
[39] W Kunz, K Häckl, The hype with ionic liquids as solvents. Chem Phys Lett 2016, 661, 6.
| The hype with ionic liquids as solvents.Crossref | GoogleScholarGoogle Scholar |
[40] RG Evans, AJ Wain, C Hardacre, RG Compton, An electrochemical and ESR spectroscopic study on the molecular dynamics of TEMPO in room temperature ionic liquid solvents. ChemPhysChem 2005, 6, 1035.
| An electrochemical and ESR spectroscopic study on the molecular dynamics of TEMPO in room temperature ionic liquid solvents.Crossref | GoogleScholarGoogle Scholar |
[41] Y Maruyama, K Nagamine, A Nomura, S Iwasa, S Tokito, Electrochemical characterization of TEMPO radical in ionic liquids. Electrochemistry 2020, 88, 34.
| Electrochemical characterization of TEMPO radical in ionic liquids.Crossref | GoogleScholarGoogle Scholar |
[42] V Strehmel, Radicals in ionic liquids. ChemPhysChem 2012, 13, 1649.
| Radicals in ionic liquids.Crossref | GoogleScholarGoogle Scholar |
[43] G Gryn’ova, ML Coote, Origin and scope of long-range stabilizing interactions and associated SOMO–HOMO conversion in distonic radical anions. J Am Chem Soc 2013, 135, 15392.
| Origin and scope of long-range stabilizing interactions and associated SOMO–HOMO conversion in distonic radical anions.Crossref | GoogleScholarGoogle Scholar |
[44] L Wylie, ZL Seeger, AN Hancock, EI Izgorodina, Increased stability of nitroxide radicals in ionic liquids: more than a viscosity effect. Phys Chem Chem Phys 2019, 21, 2882.
| Increased stability of nitroxide radicals in ionic liquids: more than a viscosity effect.Crossref | GoogleScholarGoogle Scholar |
[45] L Wylie, K Oyaizu, A Karton, M Yoshizawa-Fujita, EI Izgorodina, Toward improved performance of all-organic nitroxide radical batteries with ionic liquids: a theoretical perspective. ACS Sustain Chem Eng 2019, 7, 5367.
| Toward improved performance of all-organic nitroxide radical batteries with ionic liquids: a theoretical perspective.Crossref | GoogleScholarGoogle Scholar |
[46] S Zhang, G Wang, Y Lu, W Zhu, C Peng, H Liu, The interactions between imidazolium-based ionic liquids and stable nitroxide radical species: a theoretical study. J Phys Chem A 2016, 120, 6089.
| The interactions between imidazolium-based ionic liquids and stable nitroxide radical species: a theoretical study.Crossref | GoogleScholarGoogle Scholar |
[47] B Gizatullin, C Mattea, S Stapf, Molecular dynamics in ionic liquid/radical systems. J Phys Chem B 2021, 125, 4850.
| Molecular dynamics in ionic liquid/radical systems.Crossref | GoogleScholarGoogle Scholar |
[48] TM Ismail, N Mohan, PK Sajith, Theoretical study of hydrogen bonding interactions in substituted nitroxide radicals. New J Chem 2021, 45, 3866.
| Theoretical study of hydrogen bonding interactions in substituted nitroxide radicals.Crossref | GoogleScholarGoogle Scholar |
[49] Z Chang, D Henkensmeier, R Chen, Shifting redox potential of nitroxyl radical by introducing an imidazolium substituent and its use in aqueous flow batteries. J Power Sources 2019, 418, 11.
| Shifting redox potential of nitroxyl radical by introducing an imidazolium substituent and its use in aqueous flow batteries.Crossref | GoogleScholarGoogle Scholar |
[50] T Hagemann, J Winsberg, M Grube, I Nischang, T Janoschka, N Martin, MD Hager, US Schubert, An aqueous all-organic redox-flow battery employing a (2,2,6,6-tetramethylpiperidin-1-yl)oxyl-containing polymer as catholyte and dimethyl viologen dichloride as anolyte. J Power Sources 2018, 378, 546.
| An aqueous all-organic redox-flow battery employing a (2,2,6,6-tetramethylpiperidin-1-yl)oxyl-containing polymer as catholyte and dimethyl viologen dichloride as anolyte.Crossref | GoogleScholarGoogle Scholar |
[51] M Aqil, F Ouhib, A Aqil, A El Idrissi, C Detrembleur, C Jérôme, Polymer ionic liquid bearing radicals as an active material for organic batteries with ultrafast charge-discharge rate. Eur Polym J 2018, 106, 242.
| Polymer ionic liquid bearing radicals as an active material for organic batteries with ultrafast charge-discharge rate.Crossref | GoogleScholarGoogle Scholar |
[52] MY Ivanov, OA Krumkacheva, SA Dzuba, MV Fedin, Microscopic rigidity and heterogeneity of ionic liquids probed by stochastic molecular librations of the dissolved nitroxides. Phys Chem Chem Phys 2017, 19, 26158.
| Microscopic rigidity and heterogeneity of ionic liquids probed by stochastic molecular librations of the dissolved nitroxides.Crossref | GoogleScholarGoogle Scholar |
[53] Y Tan, NC Casetti, BW Boudouris, BM Savoie, Molecular design features for charge transport in nonconjugated radical polymers. J Am Chem Soc 2021, 143, 11994.
| Molecular design features for charge transport in nonconjugated radical polymers.Crossref | GoogleScholarGoogle Scholar |
[54] VJ Kumar, J-Z Wu, M Judd, E Rousset, M Korb, SA Moggach, N Cox, PJ Low, The syntheses, structures and spectroelectrochemical properties of 6-oxo-verdazyl derivatives bearing surface anchoring groups. J Mater Chem C 2022, 10, 1896.
| The syntheses, structures and spectroelectrochemical properties of 6-oxo-verdazyl derivatives bearing surface anchoring groups.Crossref | GoogleScholarGoogle Scholar |
[55] JT Price, JA Paquette, CS Harrison, R Bauld, G Fanchini, JB Gilroy, 6-Oxoverdazyl radical polymers with tunable electrochemical properties. Polym Chem 2014, 5, 5223.
| 6-Oxoverdazyl radical polymers with tunable electrochemical properties.Crossref | GoogleScholarGoogle Scholar |
[56] F Magnan, JS Dhindsa, M Anghel, P Bazylewski, G Fanchini, JB Gilroy, A divergent strategy for the synthesis of redox-active verdazyl radical polymers. Polym Chem 2021, 12, 2786.
| A divergent strategy for the synthesis of redox-active verdazyl radical polymers.Crossref | GoogleScholarGoogle Scholar |
[57] JA Grant, Z Lu, DE Tucker, BM Hockin, DS Yufit, MA Fox, R Kataky, V Chechik, AC O’Donoghue, New Blatter-type radicals from a bench-stable carbene. Nat Commun 2017, 8, 15088.
| New Blatter-type radicals from a bench-stable carbene.Crossref | GoogleScholarGoogle Scholar |
[58] A Saal, C Friebe, US Schubert, Polymeric Blatter's radical via CuAAC and ROMP. Macromol Chem Phys 2021, 222, 2100194.
| Polymeric Blatter's radical via CuAAC and ROMP.Crossref | GoogleScholarGoogle Scholar |
[59] FJM Rogers, PL Norcott, ML Coote, Recent advances in the chemistry of benzo[e][1,2,4]triazinyl radicals. Org Biomol Chem 2020, 18, 8255.
| Recent advances in the chemistry of benzo[e][1,2,4]triazinyl radicals.Crossref | GoogleScholarGoogle Scholar |
[60] AC Savva, SI Mirallai, GA Zissimou, AA Berezin, M Demetriades, A Kourtellaris, CP Constantinides, C Nicolaides, T Trypiniotis, PA Koutentis, Preparation of Blatter radicals via Aza-Wittig chemistry: the reaction of N-aryliminophosphoranes with 1-(Het)aroyl-2-aryldiazenes. J Org Chem 2017, 82, 7564.
| Preparation of Blatter radicals via Aza-Wittig chemistry: the reaction of N-aryliminophosphoranes with 1-(Het)aroyl-2-aryldiazenes.Crossref | GoogleScholarGoogle Scholar |
[61] AA Berezin, G Zissimou, CP Constantinides, Y Beldjoudi, JM Rawson, PA Koutentis, Route to benzo- and pyrido-fused 1,2,4-triazinyl radicals via N′-(Het)aryl-N′-[2-nitro(het)aryl]hydrazides. J Org Chem 2014, 79, 314.
| Route to benzo- and pyrido-fused 1,2,4-triazinyl radicals via N′-(Het)aryl-N′-[2-nitro(het)aryl]hydrazides.Crossref | GoogleScholarGoogle Scholar |


