The gibberellic acid responsive GmbHLHm1 transcription factor influences nodule development, nitrogen fixation activity and shoot nitrogen content in soybean (Glycine max)
Die Hu A B , Zhengyu Wen C D , Nijat Imin E , Kanwarpal S. Dhugga C and Brent N. Kaiser A B *
A B *
A
B
C
D
E
Abstract
GmbHLHm1 is a basic Helix-Loop-Helix membrane (bHLHm1) DNA binding transcription factor localized to the symbiosome membrane and nucleus in soybean (Glycine max) nodules. Overexpression of GmbHLHm1 significantly increased nodule number and size, nitrogen fixation activity, and nitrogen delivery to the shoots. This contrasts with reduced nodule numbers per plant, nitrogen fixation activity and poor plant growth when silenced using RNAi. The promoter of GmbHLHm1 was found to be sensitive to exogenous GA supply, decreasing the level of GUS expression in transformed hairy roots in both nodules and roots and reducing native GmbHLHm1 expression in wild-type nodules. In summary, our study suggests that GmbHLHm1 positively regulates soybean nodulation and nitrogen fixation, and that GA can negatively regulate GmbHLHm1 expression in soybean nodules.
Keywords: ammonium, gibberellic acid, Glycine max, GmbHLHm1, nitrogen, nitrogen fixation, nodulation, soybean, symbiosome.
Introduction
Soybean (Glycine max) is an important agricultural crop used in the production of plant-based oils and proteins, animal feeds, food ingredients, and biofuels (Anderson et al. 2019). Soybean is a nitrogen-fixing legume capable of forming a symbiosis with soil-borne rhizobacteria. Compatible rhizobia infect root epidermal cells initiating the formation of specialised organs called root nodules which house the N2-fixing rhizobia called bacteroids (Ferguson et al. 2019; Li et al. 2020). Bacteroids receive carbohydrates from the plant to energise the fixation of atmospheric N2 to NH3 by the bacteroid enzyme, nitrogenase. NH3 is readily assimilated by the plant providing a sustainable Nitrogen (N) resource that can supplement or even replace the need for N fertilisers used in agricultural production systems (Kebede 2021; Barbieri et al. 2023).
GmbHLHm1 (Glyma. 15g061400) encodes a DNA-binding transcription factor (TF) that is expressed in soybean root nodules and non-nodulated root cells (Chiasson et al. 2014). In nodules, its expression is enhanced upon the initiation of symbiotic N2-fixation (Chiasson et al. 2014) with GmbHLHm1 protein found localised on membranes, including the symbiosome, Golgi and plasma. As a TF, GmbHLHm1 is also found localised to the nucleus of infected nodule cells (Kaiser et al. 1998; Chiasson et al. 2014). In roots, GmbHLHm1 is predominantly located in the vascular cells increasing in expression when starved of N. Loss of GmbHLHm1 using RNAi compromises nodule development and symbiotic N2-fixation (Chiasson et al. 2014). Recently, we have identified a role of a GmbHLHm1 orthologue in the symbiotic arbuscular mycorrhizal symbiosis in Medicago truncatula (Ovchinnikova et al. 2023). Loss of MtbHLHm1 expression reduces the mycorrhizal growth response and the mycorrhizal colonisation of infected roots. MtbHLHm1 was found localised to arbuscule containing cells and located across the plasma membrane and nucleus of arbuscule penetrated cells (Ovchinnikova et al. 2023). The MtbHLHm1 TF was also able to bind to the promoter of the ammonium facilitator protein MtAMF1;3 which is also expressed in arbuscule containing infected cells (Ovchinnikova et al. 2023).
Gibberellins (GAs) are plant hormones that regulate cell and tissue growth and influence various developmental processes including plant height (Sasaki et al. 2002) and nodulation (Velandia et al. 2022). Bioactive GAs are recognised by the GID1 (GA INSENSITIVE DWARF1) receptor protein (Ueguchi-Tanaka et al. 2005; Hartweck and Olszewski 2006). GA-activated GID1 proteins bind DELLA proteins, which are transcriptional regulators of GA-induced responses in plants (Murase et al. 2008; Harberd et al. 2009). Studies have reported that the transcripts of gibberellic acid 20-oxidase (GA20ox), which is crucial for bioactive GA synthesis, are upregulated when nodulation occurs on adventitious and lateral roots in a Nod factor-dependent manner in Sesbania rostrata (Lievens et al. 2005). Several GA biosynthesis genes, GmGA20ox1a, GmGA3ox1a, and GmGA2ox1a are also reported to be upregulated during the early nodulation stage in soybean roots (Hayashi et al. 2012; Chu et al. 2022). Unsurprisingly, many rhizobia species such as Bradyrhizobium japonicum produce GA to promote nodulation (Boiero et al. 2007; Nett et al. 2022).
GA-responsive elements, pyrimidine box (P-BOX) and TATC-BOX (TGGGATA), and auxin-responsive TGC elements (AACGAC) have been identified in the promoter region of GmbHLHm1 (Mohammadi Dehcheshmeh 2013). P-BOX motifs were first characterised as a regulator of GA-responsive genes in the barley aleurone (Mena et al. 2002), while sequence analysis of the Brassica napus GA-insensitive dwarf mutant ndf1, identified a mutation in the P-BOX motif of the promoter region of the GA receptor BnGID1 (Li et al. 2011). Similarly, A TATC-BOX (TGGGATA) in the promoter of 4-Coumarate-CoA ligase (4CL) in Pennisetum purpureum has been demonstrated to interact with gibberellic acid (GA). This interaction was confirmed through a combination of deletion analysis, electrophoretic mobility shift assay (EMSA) binding studies involving GA, and experiments using promoter fusions with the β-glucuronidase (GUS) reporter gene (Peng et al. 2016). The GA-responsive elements identified in the GmbHLHm1 promoter suggests a possible regulatory control mechanism that connects endogenous GA to the regulation (positive or negative) of GmbHLHm1.
In this study, we examined the role of GmbHLHm1 in the development and activity of soybean nodules utilising both reverse and forward genetics. Loss of expression compromises nodule growth and function, while overexpression of GmbHLHm1 leads to enhanced nodule growth per plant, nitrogen fixation rates and the % shoot nitrogen. The application of exogenous GA negatively impacts GmbHLHm1 expression levels in soybean nodules and roots.
Materials and methods
Seeds of soybean (Glycine max L. Merr.) cv. Snowy were sourced from the NSW Department of Primary Industries and surface sterilised by soaking in 1:2 (v/v) water diluted bleach for 2 min and rinsed 3 times with autoclaved water. Surface-sterilised seeds were then transferred to plates with autoclaved Turface (Turface Athletics, USA). Plates were covered with cling wrap to prevent contamination and to maintain humidity and then placed in an incubator with a 14/10 h day/night regime with light intensity at 200 μmol m−2 s−1) photosynthetic flux density at plate level. Seeds were germinated in a day/night temperature cycle of 28–25°C for 7–10 days. Seedlings that had germinated to a length of 3–5 cm were chosen for hairy root transformation. This process was carried out using Agrobacterium rhizogenes strain K599, following the protocol established by Mohammadi-Dehcheshmeh et al. (2014). All hairy roots were inspected for GFP fluorescence, and any non-transformed roots were excised. Plants with transgenic hairy roots were transferred to individual pots (1 L) with a mixed matrix of quartz sand and Turface at 1:1 ratio and grown within covered plastic lids for 2 days at 28°C under light intensity of 400 PAR. After 2 days, the plants were placed into the chamber and inoculated. For nodulation, 50 mL of NoduleN (New Edge Microbiol, Australia) legume inoculant strain CB 1809 (Botha et al. 2004) was mixed in 1 L of distilled water, and 20 mL of the inoculant solution applied to each seedling with a syringe. A nitrogen-free (–N) B&D nutrient solution (Broughton and Dilworth 1971) was applied twice a day using an automated semi-hydroponic system. Where required, 5 mM KNO3 was added to the –N B&D nutrient solution. Plants were grown in a controlled growth chamber with 14/10 h day/night regime, 25/22°C day/night temperature cycle, and 60% humidity for a minimum of 28 days. Seedlings with 28-day-old nodules (days after inoculation) were harvested for further experiments. Root, shoot or nodule samples were also dried at 60°C for 4 days before dry weights (DW) were measured.
Gene silencing and overexpression of GmbHLHm1
For RNAi-mediated gene silencing, a 359-bp porttion of the GmbHLHm1 3′UTR (Glyma. 15g061400) was amplified and inserted into the pK7GWIWG2D(II) vector (Karimi et al. 2002; Chiasson et al. 2014). For overexpression, the full-length GmbHLHm1 CDs (1048 bp) was amplified from reverse transcribed soybean nodule total RNA with primers (forward: GTCCGCGGATGAGGAGTTCTCATATGGAGA) and (reverse: TGGCGCGCCTCACACGAAATATGAAAAAGCT) primers that incorporated a Sac II restriction site on the 5′ end and an Asc I restriction site on 3′ end. The full CDs was inserted into pENTR by double digestion (Sac II and Asc I, NEB) followed by incorporation using T4 ligation (Thermofisher Scientific™). The full-length GmbHLHm1 CDs was then inserted into the pFAST-G02 (Shimada et al. 2010) with the Gateway system and introduced into soybean roots by the A. rhizogenes-mediated hairy root transformation as described by Mohammadi-Dehcheshmeh et al. (2014).
GA treatment of wild-type and nodulating and non-nodulating hairy roots
GA3 was applied to wild-type soybeans or seedlings with transgenic hairy roots 5 days after inoculation with Bradyrhizobium japonicum (CB 1809). GA was applied as 4 ppm (10−5M) GA3 directly to the soil twice a week (Sudadi and Suryono 2015), a similar volume of water was added to the control groups. The surface of the pots were covered with aluminium foil over the treatment period and the plants harvested and analysed at 28 days. GmbHLHm1Pro:GUS transformed plants were supplied GA3 (4 ppm) at 23 days after inoculation with rhizobia, and harvested and analysed at 28 days.
RNA extraction and quantitative PCR (qPCR) analysis
Plants were harvested at 11:00 hours. Nodules were detached from the roots and both tissues immediately frozen in liquid N2 before being transferred to a −80°C freezer. RNA extraction was performed using the PureZOL total RNA extraction reagent (Bio-Rad). First-strand cDNA synthesis was performed using iScript cDNA Synthesis Kits (Bio-Rad). Primers for GmbHLHm1 expression (forward: GCTCGGTGATAACAGCTGGA; reverse: CACGCCATCTCCACCTTAGG) were designed using Geneious software (Geneious). Primer efficiency was tested with SYBR Green Real-Time PCR Master Mix and 1, 1/5, 1/25, 1/125, 1/625 dilution of cDNA synthesised. The primer efficiency was 90–100%. 2 μL of a 1/5 dilution of cDNA was used as the qPCR templates. SYBR Green Real-Time PCR Master Mix was used for all qPCR experiments. Results were normalised against Cons6 as the reference gene and calculated using the 2−ΔΔCt method (Libault et al. 2008).
Measurement of nodule N2-fixation and %N in plant tissues
Intact root systems (transgenic roots and nodules) were collected in the morning (~11:00 hours) and placed individually in 40-mL McCartney vials sealed with a rubber septum. A 5-mL gas seal syringe was used to draw out 4 mL of air from the sealed vial, and 4 mL of instrument grade, dissolved acetylene gas (BOC, Australia) was injected into the vial with another gas-sealed syringe. The starting acetylene-ethylene levels in the vial were measured with GC-2010 Plus gas chromatograph (SHIMADZU, Japan). Vials were then incubated in a 28°C water bath for 1 h and 1 mL extracted and measured for acetylene and ethylene levels with a GC-2010 Plus gas chromatograph (Shimadzu) against a ethylene standard curve prepared from pure (99.99%) Ethylene (Sigma-Aldrich). The samples were removed from the vials, nodules were detached from the roots and placed in a 60°C oven to dry overnight. Dry nodules were weighed, and the nodule dry weight (DW) data were used to calculate the rate of acetylene reduction over time following the methods of Unkovich et al. (2008). To measure the %N in harvested tissues, the top five leaves were sampled and dried at 60°C for 4 days and then ground to a fine powder. A 2-mg sample of powdered leaves was transferred to a tin capsule and the %N content in the plant samples determined using an isotope ratio mass spectrometer (Sercon, Crewe, Cheshire, UK) as described previously by Dechorgnat et al. (2018).
Promoter-GUS fusion construction and GA motif editing
Genomic DNA of soybean cv. Snowy was isolated from nodules using the PureLink Genomic Plant DNA Purification Kit (Thermo Fisher). A 1926-bp section upstream of the GmbHLHm1 (Glyma15g06680) start codon was cloned using primers (forward: AGCATGGCCGTGATTTAACCTAAGAAAACCAATTC; reverse: GACGTAACATTATACTCAAACTACAACATCC). The promoter was cloned into the pCR8 vector and then recombination cloned into the pKGWFS7 vector with the Gateway cloning system (Karimi et al. 2002). The P-Box1, TATA-Box and P-Box2 motifs were edited separately into polyadenylate (aaaaaaa) repeat sequences using PCR primers described in Table 1.
| Primer ID | Primer (5′−3′) | |
|---|---|---|
| p-box1f | aaaaaaaGTAAAAATGAGTTGGGCAAATAACCTTTG | |
| p-box1r | TTCATTATTTTCTAAGTTCCTTTCTTAGATCCT | |
| tatc-boxf | aaaaaaaAGTCTATTATCCTTGGTTGAAAATAGGC | |
| tatc-boxr | TTTTATATTTTAGAGACCCCTTCATGCTG | |
| p-box2f | aaaaaaaAATTACAACAACGAAATATATAATCATCAGCTTC | |
| p-box2r | TATACTCTACCCCACTGTATTACAGCATATAAC |
Constructs were transformed into the A. rhizogenes K599 strain. The Promoter-GUS fusion construct was used for hairy root transformation and cultivation with the method of Mohammadi-Dehcheshmeh et al. (2014). GUS staining was performed on transformed roots according to (Chiasson et al. 2014) with modifications. The harvested transgenic hairy roots with nodules were soaked in 90% (v/v) ice-cold acetone in a 50-mL centrifuge tube. Each sample was rinsed twice in sodium phosphate buffer for 5 min, before being transferred into GUS staining buffer. GUS staining buffer-covered samples were transferred in a vacuum for infiltration for 30 min and then incubated at 37°C for 5 h.
Results
RNAi-mediated gene silencing of Gmbhlhm1 inhibits nodulation and N2-fixation
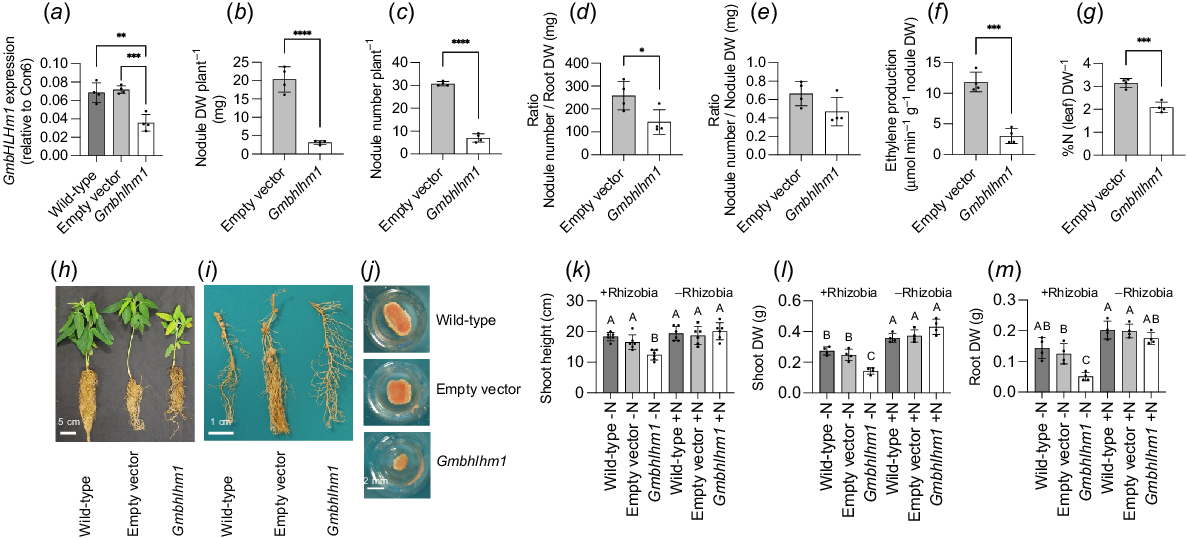
To confirm the function of GmbHLHM1 in root nodulation and N2-fixation, we re-employed RNAi to silence Gmbhlhm1. This post-transcriptional gene silencing (PTGS) approach utilised the binary vector pK7GWIWG2D(II), which contained a Gmbhlhm1 silencing cassette under the control of the constitutive 35S promoter. The construct was introduced into soybean plants through A. rhizogenes-mediated hairy root transformation, allowing for targeted gene silencing in the root system. We verified a reduced expression level in the RNAi line (Gmbhlhm1) of approximately 50% of either the empty vector control and wild-type plants through qPCR on extracted total RNA from harvested nodules (Fig. 1a). Our previous report (Chiasson et al. 2014) characterised the function of GmbHLHm1 in the soybean cv. Djakal. In this study, we observed a consistent phenotype when silencing of GmbHLHm1 in a different soybean cultivar (Snowy), confirming this function of GmbHLHm1 in the rhizobia symbiosis is not cultivar dependent. With reduced GmbHLHm1 expression, total plant nodule weight, nodule number, and the ratio of nodule number to root DW were reduced significantly compared to the controls (Fig. 1b–d). There was no significant change in average nodule size in the Gmbhlhm1 plants (Fig. 1e). After 28 days of growth, most of the initial soybean nodules have begun to fix N2 (Herridge et al. 1990; Imsande 1991; Bergersen et al. 1992). Qualitative images of representative Gmbhlhm1-silenced lines showed yellowed leaves and a root system with fewer nodules (Fig. 1h–j), while empty vector and wild-type plants appeared healthy. The Gmbhlhm1-silenced nodulated hairy roots resulted in reduced shoot height, shoot DW and root DW at 28 DAI (Fig. 1k–m). Each of these growth phenotypes could be restored when grown uninoculated with applied N (5 mM KNO3) (Fig. 1k–m). The silenced nodules showed a reduced effective N2-fixation area (pink area; Fig. 1j) compared to wild-type and empty vector controls. Pink colour in legume root nodules is indicative of the presence of leghaemoglobin, a crucial protein that plays a vital role in N2-fixation in legume root nodules (Ott et al. 2005). Altered nodule development resulted in significant reductions in N2-fixation (measured via the acetylene reduction assay) (4-fold) and a decline in the %N present in harvested leaves (measured by mass spectrometry) (four fold) relative to the empty vector controls (Fig. 1f, g).
Impact of Agrobacterium rhizogenes-mediated post-transcriptional gene silencing of Gmbhlhm1 on composite plants of soybean with and without inoculation with rhizobia. (a) GmbHLHm1 expression in wild-type, empty vector and Gmbhlhm1 RNAi-silenced nodules. Reduction in expression of Gmbhlhm1 in nodulated hairy roots reduced (b) nodule DW per plant, (c) nodule number per plant, (d) ratio of nodule number to root DW per plant, (e) ratio of nodule number to nodule DW, (f) ethylene production from nodulated roots measured using the acetylene reduction assay, and (g) %Nitrogen (N) in leaf DW. Qualitative and representative images of 28 DAI (days after inoculation with Bradyrhizobium japonicum (CB 1809) in (h) wild-type, Empty Vector, and Gmbhlhm1 RNAi whole plants. (i) Nodulated roots and (j) nodule cross sections. (k) Shoot height, (l) shoot DW, and (m) root DW ± rhizobia of inoculated wild-type, empty vector, and Gmbhlhm1 plants supplied without (−N) or with (+N) 5 mM KNO3 for 28 days. The expression of GmbHLHm1 was normalised with Con6 as a reference gene and was calculated using the 2−ΔΔCt method (Libault et al. 2008). Values were means ± s.d. (n = 4–5 individual plants). Significance was determined using either a one-way ANOVA with Sidak post hoc test for multiple comparisons (a, k, l, m) or an unpaired t test (b–g). *P < 0.05; **P < 0.01; ***P < 0.001.
GmbHLHm1 expression responds to GA treatments
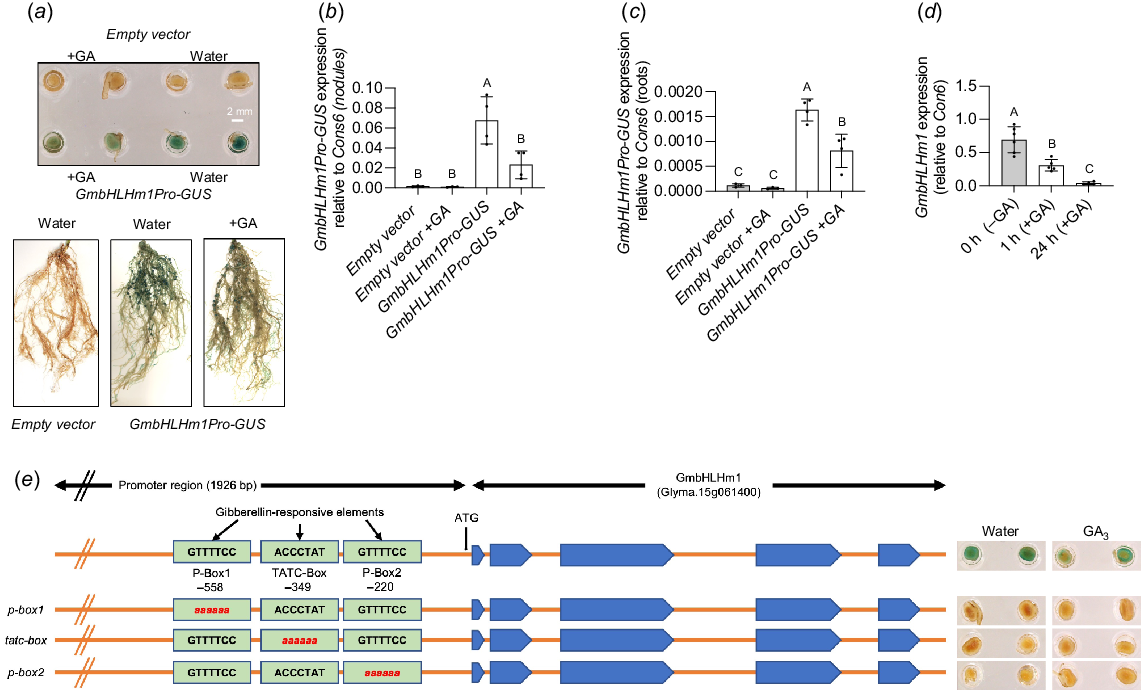
Three putative GA responsive elements (P-Box1, TATA-Box, P-Box2) were identified in the promoter region of GmbHLHm1 (Fig. 2a) using the Plant-CARE database (Lescot et al. 2002). To test their potential role in GA regulation of GmbHLHm1, a full-length GmbHLHm1 promoter (1926 bp upstream of the start codon) was cloned from genomic DNA and inserted upstream of the reporter GUS in the pKGWFS7 (Promoter-GUS reporter) vector to generate transgenic hairy roots and nodules after inoculated with B. japonicum (CB 1809). After 23 days, nodulated roots were treated daily with 4 ppm GA3 or with water (control) for 5 days. At 28 days, nodules attached to roots were harvested to measure GUS expression. As shown in Fig. 2a, the GA treatment reduced the level of GUS staining in both nodules (Fig. 2a, e) and roots (Fig. 2a), compared to the water treated controls. Quantification of gene expression (qPCR) revealed that GA significantly reduced transcript levels of the GmbHLHm1 promoter-GUS transgene in both roots (P < 0.0017) and nodules (P < 0.0085) (Fig. 2b, c). Application of GA3 to empty vector controls, reduced endogenous GmbHLHm1 expression after 1 (P < 0.0014) and 24 h (P < 0.0001) exposure (D). Editing of any of the three proposed GA elements in the GmbHLHm1 promoter completely disrupted promoter activity, eliminating GUS expression in hairy-root derived nodules (Fig. 2e). Collectively, the qPCR and GUS staining experiments indicates a negative relationship between long-term GA exposure and GmbHLHm1 promoter activity (reduced GUS expression) and gene expression. Promoter editing indicates that all three GA-motifs are required for promoter functionality but does not show a direct relationship between GA and the proposed GmbHLHm1 GA promoter elements.
GA recognised elements in the promoter of GmbHLHm1 (Glyma. 15g061400). (a) GA repression of GmbHLHm1Pro-GUS in GUS stained GmbHLHm1Pro-GUS hairy root nodules and roots with the application of GA (4 ppm GA3) or water twice per week. Corresponding GUS expression in GmbHLHm1Pro-GUS and empty vector control (b) nodule and (c) root tissues. (d) Changes in empty vector control GmbHlHm1 expression (18 days nodules) in response to short-term (1 and 24 h) of 4 ppm GA3 treatment. (e) Diagram highlighting three GA-responsive DNA elements (P-Box1, TATC-Box, and P-Box2) in the upstream region of the GmbHLHm1 promoter. Loss of GA element function in nodules through selected nucleotide substitutions across p-box1, tatc-box and p-box2. The expression of GUS was normalised with Con6 as a reference gene and was calculated using the 2−ΔΔCt method (Libault et al. 2008). Values were means ± s.d. (n = 3–6 individual plant events). Significance was determined using one-way ANOVA (b, c, e) with a multiple comparison test (Sidak). Different letters above bars indicate a level of significance (P < 0.05).
Overexpression of GmbHLHm1 increased nodule size and nodule N2-fixation
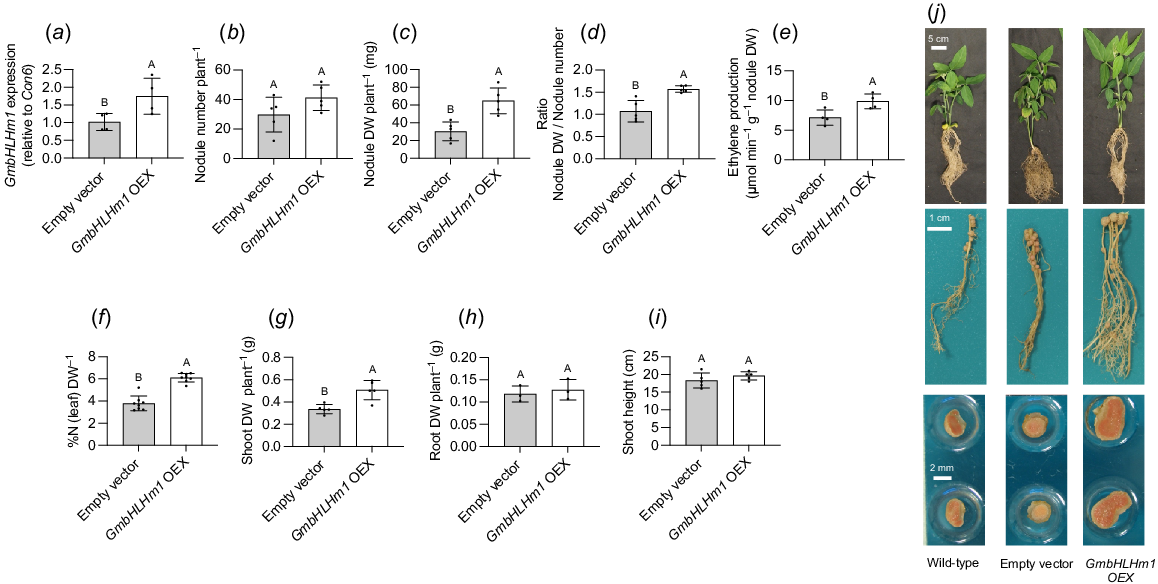
GmbHLHm1 was overexpressed (GmbHLHm1 OEX) in hairy roots (P < 0.05 relative to the empty vector control) using a 35S promoter (Fig. 3a). At 28 days after rhizobia inoculation, changes in nodule development were evident, including significant increases (P < 0.05) in total nodule DW per plant (Fig. 3c), nodule size (Fig. 3d, j), N2 -fixation rates (ARA activity) (Fig. 3e), %N in aerial leaf tissues (Fig. 3f). and shoot DW (Fig. 3g). There were no changes in either nodule number per root system, root DW or shoot height in GmbHLHm1 OEX plants (Fig. 3b, h, i, respectively).
Overexpression of GmbHLHm1 in soybean hairy roots. (a) Overexpression of GmbHLHm1 using the 35S promoter. Changes to nitrogen fixation activities and plant growth in GmbHLHm1 OEX and empty vector hairy root soybean plants: (b) nodule number per plant root; (c) nodule DW per plant root; (d) ratio of nodule DW (mg) to nodule number; (e) nodule acetylene reduction to ethylene per plant root nodule DW; (f) %N (DW) in aerial leaf tissues; (g) shoot DW per plant; (h) root DW per plant; and (i) shoot height at harvest. (j) Qualitative and representative images of whole plants, nodulated roots and extracted nodules from wild-type, empty vector, and GmbHLHm1 OEX lines. The expression of GmbHLHm1 was normalised with Con6 as a reference gene and was calculated using the 2−ΔΔCt method (Libault et al. 2008). Values were means ± s.d. (n = 3–8 individual plants). Significance was determined using un-paired t-tests. Different letters above bars indicate the level of significance (P < 0.05).
Discussion
Legume root systems have two roles in plant N acquisition strategies: (1) the uptake and assimilation on exogenous N (inorganic and organic) from the soil solution; and (2) the establishment of a symbiotic N2-fixation symbiosis with soil-borne rhizobia bacteria. Both activities result in the transport of reduced N (NH4+, NO3−, and amino acids) to developing shoot and root tissues. GmbHLHm1 expression is linked to nodule development and activity (Chiasson et al. 2014). For plants grown without N or when grown uninoculated, GmbHLHm1 expression was shown to be strongly upregulated, while repressed when inoculated with N2-fixing rhizobia (Chiasson et al. 2014). We employed a post-transcriptional gene silencing (PTGS) approach to reduce the expression of GmbHLHm1 in soybean roots. As first observed by Chiasson et al. (2014), the reduction of GmbHLHm1 expression in hairy roots led to poorly developed nodules, and poor growth of the plant when grown in the absence of N fertilisers. Our study further detailed this response, showing a reduction in nodule DW, nodule number per plant and a significant decrease in the rate of nitrogenase activity and a corresponding reduction in the %N content in the shoots (Fig. 1). As a result, Gmbhlhm1-RNAi plants grown solely on nodule-derived N showed symptoms of N deficiency such as a yellowing of the leaves and reduced shoot growth (Fig. 1h) relative to the empty vector controls and wild-type plants. Interestingly, we show for the first time that shoot height, shoot and root growth (DW) in the Gmbhlhm1-RNAi lines can be recovered with the supply of exogenous nitrogen fertiliser (5 mM KNO3−), which suggests the growth deficiencies are linked to Gmbhlhm1-RNAi compromised nodule activities and not changes in root N assimilation or root N redistribution (Fig. 1k–m).
When GmbHLHm1 was overexpressed with the constitutive 35S promoter in a hairy-root transformation system, nodule weight (DW) and nodule size increased relative to the empty vector controls (Fig. 3c, d) with larger older nodules evident on the root system (Fig. 3j). This translated into higher rates of N2-fixation, the %N observed in aerial leaves and an increase in the shoot DW (Fig. 3d–f). These positive changes to nodule development and function would suggest GmbHLHm1 expression and activity are negatively regulated (repressed) in the context of long-term symbiotic N2-fixation. A prior time course analysis of GmbHLHm1 expression revealed a strong upregulation of expression at 20 days after rhizobia inoculation (Chiasson et al. 2014), which coincides with the development of a matured nodule and the onset of measurable N2-fixation activity in soybean (Herridge et al. 1990; Imsande 1991; Bergersen et al. 1992). This enhanced expression in nodules then decreases as the plant transitions to a reproductive growth phase (Chiasson et al. 2014). In this experiment, nodule expression of GmbHLHm1 was evident in the OEX lines at 28 days after rhizobia inoculation, suggesting that constitutive over-expression of GmbHLHm1 (OEX) supported an extended N2-fixation capacity, possibly through enhanced nodule development and activities. We are cognisant of the native diel expression pattern previously recorded for GmbHLHm1 (Chiasson et al. 2014) and that our measurement of GmbHLHm1 expression (taken at 11:00 hours) may actually be an underestimation of its potential level of expression at night.
The mechanisms by which the GmbHLHm1 transcription factor increases nodule growth and activity remains unknown. A range of different genetic markers have been aligned to nodule growth and development. A QTL was identified on Chromosome 11(B1) linked to nodule size and nodule weight (Hwang et al. 2014). This was eventually fine mapped to reveal the presence of a cell wall localised β-expansin, GmINS1 (INCREASING NODULE SIZE1) (Li et al. 2018). β-expansins belong to a superfamily of Expansin proteins that are involved in pH dependent cell-wall extensions by disrupting hydrogen bonds between cellulose microfibrils and cross-linking glycans (Li et al. 2003). Overexpression of GmINSI resulted in increased nodule size, nodule weight, and nodule number per plant relative to the empty vector controls. Another cell wall β-expansin, GmEXPB2, was identified through soybean Pi starvation assay (Li et al. 2015). Like GmINSI, overexpression of the nodule localised GmEXPB2, increased nodule size and number while suppression of expression reduced nodule development and activity. A third expansin, GmEXPA11 (Glyma. 04g222100) has recently been linked to nodule enlargement, increased N2-fixation and N content when overexpressed (Xing et al. 2025). Interestingly, GmEXPA11 is positively regulated through the overexpression of the soybean bHLH transcription factor GmPFT1 (Yang et al. 2021; Zhang et al. 2024). We have no direct evidence linking expansin activity to GmbHLHm1 in soybean nodules, but we have previously seen the reduction in expression of an EXPANSIN A7 (Glyma. 11g027600) in soybean roots when GmbHLHm1 is silenced (Mohammadi Dehcheshmeh 2013), suggesting a possible link in activities. Future research will explore the transcriptional targets of GmbHLHm1 through targeted RNA sequence experiments and DIP-SEQ assays.
The presence of GA responsive elements on the GmbHLHm1 promoter suggested a putative regulatory pathway linking GmbHLHm1, plant GA and nodule activity. Application of GA3 to transformed hairy roots expressing GmbHLHm1Pro:GUS significantly reduced GUS expression and the intensity of visualised GUS signal in both nodules and roots. However, base pair changes to any of the three GA responsive elements individually disrupted GmbHLHm1Pro:GUS activity. At this stage it remains unclear what regulatory role the GA elements in the GmbHLHm1 promoter have on GmbHLHm1 expression. Nevertheless, the reduction of GmbHLHm1 expression by short-term GA treatment suggests a nodule-linked responsiveness to GA. For example, exogenous GA3 application before rhizobia inoculation inhibits nodulation in Lotus japonicus through the disruption of infection thread formation (Maekawa et al. 2009). Treatment with various GA biosynthesis inhibitors, including chlormequat chloride and uniconazole-P, reduces lateral root-based nodulation in S. rostrata in a similar fashion that high levels of applied bioactive GA does (Lievens et al. 2005). GA biosynthetic deficient mutants of pea (Pisum sativum), show decreased nodulation, while the application of 10−6 M GA3 could restore nodulation to wild-type levels (Ferguson et al. 2005). Moreover, low (10−9−10−6 M) GA3 applications can increase nodule numbers in wild-type plants, while increased GA3 levels (up to 10−3 M) decrease nodule numbers in both wild-type and GA deficient mutant peas (Ferguson et al. 2005). Akamatsu et al. (2021) reported that GA biosynthesis is activated during nodule formation inside the vascular bundles. Chu et al. (2022) reported that spatio-temporal changes of GA biosynthesis genes and their distribution in the nodulated root system play an important role during nodulation. A mutation study revealed that both high and low GA concentrations influence GA signalling in GA biosynthesis deficiency mutants of pea (na, ls, lh) and a constitutive GA signalling mutant, results in suppression of nodule formation (Ferguson et al. 2005; Ferguson et al. 2011). The links between GA and transcription factors was documented through a GA-responsive cis-acting region discovered on the NIN promoter, indicating endogenous GA may play a central role in coordinating nodule development through NIN activity (Akamatsu et al. 2021; Shen and Feng 2024). McAdam et al. (2018) suggested that biosynthesised GA promotes nodule organogenesis into N2-fixing nodules via the activity of the DELLA protein. In M. truncatula, GA signalling mediated by DELLA1 decreased bioactive cytokinin (CK) in roots and negatively regulated the Cytokinin Response1 (CRE1)-dependent NF activation, including CK-signalling genes as well as the CK-regulated early nodulation genes (NODULATION SIGNALLING PATHWAY 2 (NSP2) and Ethylene Response Factor Required for Nodulation1) (Fonouni-Farde et al. 2016). High levels of GA would have an impact on DELLA protein stability and could influence several GA-responsive transcription factors including GmbHLHm1. Recent evidence has revealed that GA helps regulate root endodermal cells promoting lateral root and nodule organogenesis, as well as help repress rhizobial infection through the induction of secondary signals (Velandia et al. 2024). This regulation is most likely linked to cellular GA pools. Using a GA biosensor (GIBERELLIN PERCEPTION SENSOR 2), GA accumulation is identified at the site of nodule primordia in M. truncatula, increasing in concentration in nodule cortical cells and finally in the nodule meristem as it matures (Drapek et al. 2024).
The GmbHLHm1 TF has a significant impact on nodulation and nodule development in soybean. Reduced activity curtails nodule growth, N2-fixation and the delivery of reduced N to aerial tissues, while overexpression increases nodule activity (growth and N2-fixation) while delivering increased N to aerial tissues. This study also suggests a relationship between GA and GmbHLHm1 expression, possibly acting as a negative regulator. We observed that extended GA treatments had a negative impact on promoter activity. However, we did not observe a direct link to either of the three identified GA-motifs located on the GmbHLHm1 promoter, where disruption of either motif eliminated promoter activities independent of GA treatment. This suggests that each of these elements may be required to function as a promoter per se, while possibly having a yet defined role in a GA signalling cascade. It will be important to further understand if a direct relationship to endogenous GA exists with GmbHLHm1 to help encourage or negate nodule development and function.
Data availability
All background data (raw data) and DNA constructs will be made available upon request.
Conflicts of interest
The authors declare that they have no competing interests, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Declaration of funding
This research was funded through an ARC Discovery Grant (DP210100956) awarded to BN Kaiser, KS Dhugga and N Imin.
Acknowledgements
D. H. planned and executed all experiments, analysed data and co-wrote the manuscript and prepared figures. Z. W. planned experiments and reviewed the main manuscript and figures. N. I. reviewed the manuscript and figures, helped analyse the data and helped develop the research topic. K. S. D. planned experiments and reviewed the manuscript and figures. B. N. K. planned, co-wrote and reviewed the main manuscript and figures. This paper forms part of the PhD thesis of Dr Die Hu (2023).
References
Akamatsu A, Nagae M, Nishimura Y, Romero Montero D, Ninomiya S, Kojima M, Takebayashi Y, Sakakibara H, Kawaguchi M, Takeda N (2021) Endogenous gibberellins affect root nodule symbiosis via transcriptional regulation of NODULE INCEPTION in Lotus japonicus. The Plant Journal 105(6), 1507-1520.
| Crossref | Google Scholar | PubMed |
Anderson EJ, Ali ML, Beavis WD, Chen P, Clemente TE, Diers BW, Graef GL, Grassini P, Hyten DL, McHale LK, Nelson RL, Parrott WA, Patil GB, Stupar RM, Tilmon KJ (2019) Soybean [Glycine max (L.) Merr.] breeding: history, improvement, production and future opportunities. In ‘Advances in plant breeding strategies: legumes. Vol. 7’. (Eds J Al-Khayri, S Jain, D Johnson) pp. 431–516. (Springer)
Barbieri P, Starck T, Voisin A-S, Nesme T (2023) Biological nitrogen fixation of legumes crops under organic farming as driven by cropping management: a review. Agricultural Systems 205, 103579.
| Crossref | Google Scholar |
Bergersen FJ, Turner GL, Peoples MB, Gault RR, Morthorpe LJ, Brockwell J (1992) Nitrogen fixation during vegetative and reproductive growth of irrigated soybeans in the field: application of d15N methods. Australian Journal of Agricultural Research 43, 145-153.
| Crossref | Google Scholar |
Boiero L, Perrig D, Masciarelli O, Penna C, Cassán F, Luna V (2007) Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Applied Microbiology and Biotechnology 74, 874-880.
| Crossref | Google Scholar | PubMed |
Botha WJ, Jaftha JB, Bloem JF, Habig JH, Law IJ (2004) Effect of soil bradyrhizobia on the success of soybean inoculant strain CB 1809. Microbiological Research 159(3), 219-231.
| Crossref | Google Scholar | PubMed |
Broughton WJ, Dilworth MJ (1971) Control of leghaemoglobin synthesis in snake beans. Biochemical Journal 125(4), 1075-1080.
| Crossref | Google Scholar |
Chiasson DM, Loughlin PC, Mazurkiewicz D, Mohammadidehcheshmeh M, Fedorova EE, Okamoto M, McLean E, Glass ADM, Smith SE, Bisseling T, Tyerman SD, Day DA, Kaiser BN (2014) Soybean SAT1 (Symbiotic Ammonium Transporter 1) encodes a bHLH transcription factor involved in nodule growth and NH4+ transport. Proceedings of the National Academy of Sciences 111(13), 4814-4819.
| Crossref | Google Scholar |
Chu X, Su H, Hayashi S, Gresshoff PM, Ferguson BJ (2022) Spatiotemporal changes in gibberellin content are required for soybean nodulation. New Phytologist 234(2), 479-493.
| Crossref | Google Scholar |
Dechorgnat J, Francis KL, Dhugga KS, Rafalski JA, Tyerman SD, Kaiser BN (2018) Root ideotype influences nitrogen transport and assimilation in maize. Frontiers in Plant Science 9, 531.
| Crossref | Google Scholar |
Drapek C, Rizza A, Mohd-Radzman NA, Schiessl K, Dos Santos Barbosa F, Wen J, Oldroyd GED, Jones AM (2024) Gibberellin dynamics governing nodulation revealed using GIBBERELLIN PERCEPTION SENSOR 2 in Medicago truncatula lateral organs. The Plant Cell 36(10), 4442-4456.
| Crossref | Google Scholar |
Ferguson BJ, Ross JJ, Reid JB (2005) Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiology 138(4), 2396-2405.
| Crossref | Google Scholar | PubMed |
Ferguson BJ, Foo E, Ross JJ, Reid JB (2011) Relationship between gibberellin, ethylene and nodulation in Pisum sativum. New Phytologist 189(3), 829-842.
| Crossref | Google Scholar | PubMed |
Ferguson BJ, Mens C, Hastwell AH, Zhang M, Su H, Jones CH, Chu X, Gresshoff PM (2019) Legume nodulation: the host controls the party. Plant, Cell & Environment 42(1), 41-51.
| Crossref | Google Scholar | PubMed |
Fonouni-Farde C, Tan S, Baudin M, Brault M, Wen J, Mysore KS, Niebel A, Frugier F, Diet A (2016) DELLA-mediated gibberellin signalling regulates Nod factor signalling and rhizobial infection. Nature Communications 7(1), 12636.
| Crossref | Google Scholar |
Harberd NP, Belfield E, Yasumura Y (2009) The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. The Plant Cell 21(5), 1328-1339.
| Crossref | Google Scholar |
Hartweck LM, Olszewski NE (2006) Rice GIBBERELLIN INSENSITIVE DWARF1 is a gibberellin receptor that illuminates and raises questions about GA signaling. The Plant Cell 18(2), 278-282.
| Crossref | Google Scholar |
Hayashi S, Reid DE, Lorenc MT, Stiller J, Edwards D, Gresshoff PM, Ferguson BJ (2012) Transient Nod factor-dependent gene expression in the nodulation-competent zone of soybean (Glycine max [L.] Merr.) roots. Plant Biotechnology Journal 10(8), 995-1010.
| Crossref | Google Scholar | PubMed |
Herridge DF, Bergersen FJ, Peoples MB (1990) Measurement of nitrogen fixation by soybean in the field using the ureide and natural 15N abundance methods. Plant Physiology 93(2), 708-716.
| Crossref | Google Scholar |
Hwang S, Ray JD, Cregan PB, King CA, Davies MK, Purcell LC (2014) Genetics and mapping of quantitative traits for nodule number, weight, and size in soybean (Glycine max L.[Merr.]). Euphytica 195(3), 419-434.
| Crossref | Google Scholar |
Imsande J (1991) Regulation of nodule efficiency by the undisturbed soybean plant1. Journal of Experimental Botany 42(5), 687-691.
| Crossref | Google Scholar |
Kaiser BN, Finnegan PM, Tyerman SD, Whitehead LF, Bergersen FJ, Day DA, Udvardi MK (1998) Characterization of an ammonium transport protein from the peribacteroid membrane of soybean nodules. Science 281(5380), 1202-1206.
| Crossref | Google Scholar |
Karimi M, Inzé D, Depicker A (2002) GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science 7(5), 193-195.
| Crossref | Google Scholar | PubMed |
Kebede E (2021) Contribution, utilization, and improvement of legumes-driven biological nitrogen fixation in agricultural systems. Frontiers in Sustainable Food Systems 5, 767998.
| Crossref | Google Scholar |
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research 30(1), 325-327.
| Crossref | Google Scholar | PubMed |
Li Y, Jones L, McQueen-Mason S (2003) Expansins and cell growth. Current Opinion in Plant Biology 6(6), 603-610.
| Crossref | Google Scholar | PubMed |
Li H, Wang Y, Li X, Gao Y, Wang Z, Zhao Y, Wang M (2011) A GA-insensitive dwarf mutant of Brassica napus L. correlated with mutation in pyrimidine box in the promoter of GID1. Molecular Biology Reports 38(1), 191-197.
| Crossref | Google Scholar | PubMed |
Li X, Zhao J, Tan Z, Zeng R, Liao H (2015) GmEXPB2, a cell wall β-Expansin, affects soybean nodulation through modifying root architecture and promoting nodule formation and development. Plant Physiology 169(4), 2640-2653.
| Crossref | Google Scholar | PubMed |
Li X, Zheng J, Yang Y, Liao H (2018) INCREASING NODULE SIZE1 expression is required for normal rhizobial symbiosis and nodule development. Plant Physiology 178(3), 1233-1248.
| Crossref | Google Scholar | PubMed |
Li R, Chen H, Yang Z, Yuan S, Zhou X (2020) Research status of soybean symbiosis nitrogen fixation. Oil Crop Science 5(1), 6-10.
| Crossref | Google Scholar |
Libault M, Thibivilliers S, Bilgin DD, Radwan O, Benitez M, Clough SJ, Stacey G (2008) Identification of four soybean reference genes for gene expression normalization. The Plant Genome 1(1), 44-54.
| Crossref | Google Scholar |
Lievens S, Goormachtig S, Den Herder J, Capoen W, Mathis Ŕ, Hedden P, Holsters M (2005) Gibberellins are involved in nodulation of Sesbania rostrata. Plant Physiology 139(3), 1366-1379.
| Crossref | Google Scholar | PubMed |
Maekawa T, Maekawa-Yoshikawa M, Takeda N, Imaizumi-Anraku H, Murooka Y, Hayashi M (2009) Gibberellin controls the nodulation signaling pathway in Lotus japonicus. The Plant Journal 58(2), 183-194.
| Crossref | Google Scholar | PubMed |
McAdam EL, Reid JB, Foo E (2018) Gibberellins promote nodule organogenesis but inhibit the infection stages of nodulation. Journal of Experimental Botany 69(8), 2117-2130.
| Crossref | Google Scholar | PubMed |
Mena M, Cejudo FJ, Isabel-Lamoneda I, Carbonero P (2002) A role for the DOF transcription factor BPBF in the regulation of gibberellin-responsive genes in barley aleurone. Plant Physiology 130(1), 111-119.
| Crossref | Google Scholar | PubMed |
Mohammadi-Dehcheshmeh M, Ebrahimie E, Tyerman SD, Kaiser BN (2014) A novel method based on combination of semi-in vitro and in vivo conditions in Agrobacterium rhizogenes-mediated hairy root transformation of Glycine species. In Vitro Cellular & Developmental Biology - Plant 50(2), 282-291.
| Crossref | Google Scholar |
Murase K, Hirano Y, Sun T-P, Hakoshima T (2008) Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 456(7221), 459-463.
| Crossref | Google Scholar |
Nett RS, Bender KS, Peters RJ (2022) Production of the plant hormone gibberellin by rhizobia increases host legume nodule size. The ISME Journal 16(7), 1809-1817.
| Crossref | Google Scholar | PubMed |
Ott T, van Dongen JT, Gunther C, Krusell L, Desbrosses G, Vigeolas H, Bock V, Czechowski T, Geigenberger P, Udvardi MK (2005) Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Current Biology 15, 531-535.
| Crossref | Google Scholar |
Ovchinnikova E, Chiasson D, Wen Z, Wu Y, Tahaei H, Smith PMC, Perrine-Walker F, Kaiser BN (2023) Arbuscular-mycorrhizal symbiosis in medicago regulated by the transcription factor MtbHLHm1;1 and the ammonium facilitator protein MtAMF1;3. International Journal of Molecular Sciences 24(18), 14263.
| Crossref | Google Scholar |
Peng X-Q, Ke S-W, Liu J-Q, Chen S, Zhong T-X, Xie X-M (2016) Deletion and hormone induction analyses of the 4-coumarate: CoA ligase gene promoter from Pennisetum purpureum in transgenic tobacco plants. Plant Cell, Tissue and Organ Culture (PCTOC) 126(3), 439-448.
| Crossref | Google Scholar |
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M (2002) A mutant gibberellin-synthesis gene in rice. Nature 416(6882), 701-702.
| Crossref | Google Scholar |
Shen L, Feng J (2024) NIN—at the heart of NItrogen-fixing Nodule symbiosis. Frontiers in Plant Science 14, 1284720.
| Crossref | Google Scholar |
Shimada TL, Shimada T, Hara-Nishimura I (2010) A rapid and non-destructive screenable marker, FAST, for identifying transformed seeds of Arabidopsis thaliana. The Plant Journal 61(3), 519-528.
| Crossref | Google Scholar | PubMed |
Sudadi S, Suryono S (2015) Exogenous application of tryptophan and indole acetic acid (IAA) to induce root nodule formation and increase soybean yield in acid, neutral and alkaline soil. AGRIVITA Journal of Agricultural Science 37(1), 37-44.
| Crossref | Google Scholar |
Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow T-y, Hsing Y-iC, Kitano H, Yamaguchi I, Matsuoka M (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437(7059), 693-698.
| Crossref | Google Scholar |
Velandia K, Reid JB, Foo E (2022) Right time, right place: the dynamic role of hormones in rhizobial infection and nodulation of legumes. Plant Communications 3(5), 100327.
| Crossref | Google Scholar |
Velandia K, Correa-Lozano A, McGuiness PM, Reid JB, Foo E (2024) Cell-layer specific roles for gibberellins in nodulation and root development. New Phytologist 242(2), 626-640.
| Crossref | Google Scholar | PubMed |
Xing X, Du H, Yang Z, Zhang H, Li N, Shao Z, Li W, Kong Y, Li X, Zhang C (2025) GmEXPA11 facilitates nodule enlargement and nitrogen fixation via interaction with GmNOD20 under regulation of GmPTF1 in soybean. Plant Science 355, 112469.
| Crossref | Google Scholar |
Yang Z, Gao Z, Zhou H, He Y, Liu Y, Lai Y, Zheng J, Li X, Liao H (2021) GmPTF1 modifies root architecture responses to phosphate starvation primarily through regulating GmEXPB2 expression in soybean. The Plant Journal 107(2), 525-543.
| Crossref | Google Scholar | PubMed |
Zhang X, Chen J-X, Lian W-T, Zhou H-W, He Y, Li X-X, Liao H (2024) Molecular module GmPTF1a/b-GmNPLa regulates rhizobia infection and nodule formation in soybean. New Phytologist 241(4), 1813-1828.
| Crossref | Google Scholar | PubMed |


