Inhibitors of lysine biosynthesis enzymes as potential new herbicides
Emily R. R. Mackie A # , Mirrin V. McKay A # , Andrew S. Barrow A and Tatiana P. Soares da Costa A *
A *
A
# These authors contributed equally to this paper
Handling Editor: Rana Munns
Abstract
Lysine is an amino acid that is essential for the growth and development of all organisms owing to its role in a plethora of critical biological functions and reactions. In plants, lysine is synthesised via five sequential enzyme-catalysed reactions collectively known as the diaminopimelate (DAP) pathway, whereas animals are reliant on their plant dietary intake to obtain lysine. Given that lysine is one of the most nutritionally limiting amino acids, several studies have focused on developing strategies to modulate the activity of DAP pathway enzymes to improve the nutritional value of crops. More recently, research has emerged on the potential of inhibiting DAP pathway enzymes for the development of herbicides with a novel mode of action. Over reliance on a small number of modes of action has led to a herbicide resistance crisis, necessitating the development of new modes of action to which no resistance exists. As such, the first herbicidal inhibitors of the DAP pathway have been developed, which target the first three enzymes in lysine biosynthesis. This review explores the structure, function, and inhibition of these enzymes, as well as highlighting promising avenues for the future development of new plant lysine biosynthesis inhibitors.
Keywords: amino acid, catalysis, enzymes, herbicide development, herbicide resistance, inhibitors, lysine, weeds.
Lysine
l-Lysine, hereinafter referred to as lysine, is an essential amino acid that plays a key role in many important biological processes, the most common of which is as a building block for protein synthesis (Luo 2018). Lysine is critical for protein structure and stability, as well as being one of the seven residues most frequently involved in enzymatic catalysis (Holliday et al. 2009; Sokalingam et al. 2012; Luo 2018). Aside from their roles in catalysis, lysine residues facilitate the modulation of protein function as they serve as substrates for enzymes that induce post-translational modifications (Luo, 2018; Wu et al. 2017). The post-translational modifications undergone by the pendant amino group of lysine residues alters protein function, localisation, and interactions with other proteins. This allows for the dynamic modulation of cellular processes such as protein degradation, signal transduction and gene expression, and is therefore imperative to maintaining homeostasis (Wu et al. 2017). Lysine is also a precursor to many important metabolites, including carnitine, which plays a role in oxidative stress protection, and hydroxypipecolic acid, which enhances the immune response to pathogens (Hartmann and Zeier 2018). Given the integral functions of lysine, it is essential for organisms to be able to obtain it either through endogenous synthesis or dietary consumption. Given that the former occurs in plants, the enzymes involved in lysine biosynthesis have recently garnered attention as potential herbicide targets. Conversely, animals must obtain lysine through dietary consumption as they lack the enzymes required for lysine biosynthesis (Yang et al. 2020).
Lysine biosynthesis in plants
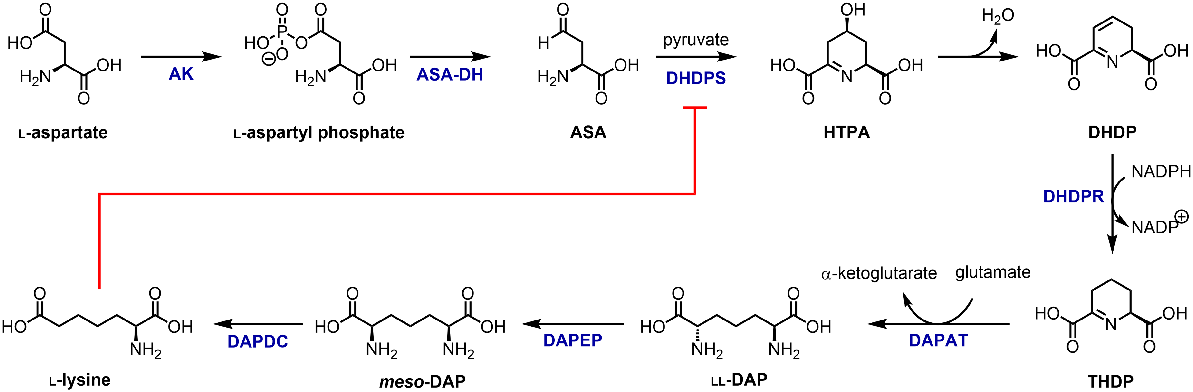
The de novo synthesis of lysine occurs in plants, bacteria, eubacteria, and archaea via the diaminopimelate (DAP) pathway (Fig. 1) (Velasco et al. 2002; Torruella et al. 2009; Fazius et al. 2012). The first substrate in the DAP pathway is produced via the conversion of l-aspartate to 4-phospho-l-aspartate by aspartate kinase (AK, EC 2.7.2.4). The product is subsequently reduced to aspartate semialdehyde (ASA) by ASA dehydrogenase (ASA-DH, EC 1.2.1.11) (Jander and Joshi 2009). From here, ASA serves as a substrate either in the biosynthetic pathways leading to threonine, methionine, and isoleucine production, or in the first committed step towards lysine biosynthesis (Jander and Joshi 2009). This first step of the DAP pathway is catalysed by 4-hydroxy-tetrahydrodipicolinate synthase (EC 4.3.3.7), also known as dihydrodipicolinate synthase (DHDPS), and involves a condensation reaction between ASA and pyruvate to yield 4-hydroxy-2,3,4,5-tetrahydrodipicolinate (HTPA, Fig. 1) (Soares da Costa et al. 2018). This is also the rate limiting step of the pathway, as lysine is an allosteric inhibitor of DHDPS (Hall et al. 2021). HTPA undergoes non-enzymatic dehydration to produce dihydrodipicolinate (DHDP), and the subsequent NAD(P)H-dependent reduction of HTPA by 4-hydroxy-tetrahydrodipicolinate reductase (EC 1.17.1.8), also known as dihydrodipicolinate reductase (DHDPR), yields 2,3,4,5-tetrahydrodipicolinate (THDP, Fig. 1) (Blickling et al. 1997a). To produce the lysine precursor meso-DAP from THDP, there are four variants of the DAP pathway (Velasco et al. 2002). Plants, eubacteria, and archaea utilise the aminotransferase sub-pathway, which involves the conversion of THDP to LL-DAP (hereinafter referred to as DAP) by DAP aminotransferase (DAPAT, EC 2.6.1.83) using glutamate as the amino donor (Fig. 1) (Hudson et al. 2006; Watanabe et al. 2007). DAP epimerase (DAPEP, EC 5.1.1.7) subsequently catalyses the conversion of DAP to meso-DAP (Fig. 1) (Hudson et al. 2006). This product is then decarboxylated by DAP decarboxylase (DAPDC, EC 4.1.1.20) to form lysine in a final step that is common to all four sub-pathways (Fig. 1) (Peverelli et al. 2016). Plants typically possess two isoforms of each DAP pathway enzyme, except for DAPAT where only one isoform has been found.
Modulating lysine levels in plants
Given that lysine is one of the most limiting amino acids in plants, particularly in cereal crops, significant efforts have been made towards the development of high lysine crops (Wang et al. 2017). To achieve this, several approaches have been utilised, including the use of nitrogenous fertilisers and selective breeding of naturally occurring high lysine mutants (Mertz et al. 1964; Nelson et al. 1965; Gauer et al. 1992; Holding et al. 2007; Myers et al. 2011; Wang et al. 2019; Wan et al. 2023). Genetic engineering has also been employed, where overexpression of lysine-rich proteins and deregulation of lysine biosynthesis have been shown to enhance lysine content in crops (Negrutiu et al. 1984; Shaul and Galili 1992; Zhu and Galili 2003; Tamás et al. 2009; Long et al. 2013; Wong et al. 2015; Yang et al. 2021, 2022). Studies investigating the fortification of crops with lysine have been published steadily over the last 60 years and the topic has been reviewed in detail elsewhere (Galili 2002; Wang et al. 2017; Yang et al. 2022). Conversely, research into the depletion of lysine levels in plants as a strategy for the development of novel herbicide modes of action has only recently begun to emerge.
Several commercial herbicides inhibit the production of amino acids, including glyphosate, chlorsulfuron, and glufosinate (Hall et al. 2020). However, no herbicides targeting the enzymes in the lysine biosynthetic pathway have been brought to market. Given the many essential roles of lysine in plants and the fact that animals lack the enzymes required for lysine biosynthesis, curtailing its production may represent a promising strategy for the development of herbicides with a novel mode of action (Hall et al. 2020). Indeed, a historical over reliance on a small number of herbicide modes of action has contributed to an exponential rise in herbicide resistance, making the development of new herbicide modes of action without resistance mechanisms imperative to sustainable agriculture (Duke 2012; Dayan 2019). Recently, the first inhibitors of plant DAP pathway enzymes have been developed, some of which have herbicidal activity (Soares da Costa et al. 2021; Mackie et al. 2022, 2023). These inhibitors target the first three enzymes in the pathway, DHDPS and DHDPR and DAPAT. This review will focus on the structure, function and inhibition of these enzymes. Future opportunities for the development of novel plant lysine biosynthesis inhibitors will also be explored.
Dihydrodipicolinate synthase (DHDPS)
DHDPS-encoding genes
Plants typically possess two DHDPS encoding genes known as DAPA1 and DAPA2. Given that a double gene knockout in Arabidopsis thaliana could not obtained, it has been suggested that plants without DAPA genes are non-viable (Jones-Held et al. 2012). Single gene knockout studies show that despite similar expression levels of both genes, knockouts of DAPA2 resulted in a significant increase in threonine concentrations in all plant organs, whilst DAPA1 knockouts did not affect threonine levels (Craciun et al. 2000; Jones-Held et al. 2012). The viability of the single gene knockout plants indicates that the DAPA1 and DAPA2 genes are functionally redundant. These studies also revealed that DAPA1 contributes towards 30% of the total DHDPS activity, whereas DAPA2 is responsible for 70% (Jones-Held et al. 2012).
DHDPS structure
The structures of DHDPS from the plant species A. thaliana, Vitis vinifera, and Nicotiana sylvestris have been deposited in the Protein Data Bank (PDB), and all adopt a homotetrameric arrangement comprised of a dimer of tight dimers (Fig. 2) (Blickling et al. 1997b; Atkinson et al. 2012; Griffin et al. 2012; Christensen et al. 2016; Hall et al. 2021). Located at the cleft of each tight dimer interface are two active sites, each in the centre of a (ß/α)8 or TIM barrel formed by the N-termini and two allosteric sites (Fig. 2a) (Soares da Costa et al. 2018). The self-association of the tight dimer interface is essential for DHDPS catalysis as residues from both monomers contribute to active site formation (Griffin et al. 2012; Hall et al. 2021). Tetramerisation occurs via interactions between the C-terminus α-helices and appears to have evolved to maximise catalytic function by reducing overall protein dynamics (Fig. 2b) (Atkinson et al. 2012). This is supported by the finding that residues in the dimer have a higher root mean square fluctuation than that of the equivalent residues in the tetramer, indicating that the enzyme is more stable as a tetramer (Atkinson et al. 2012).
Structure of Arabidopsis thaliana (At) DHDPS. (a) The overlaid crystal structures of A. thaliana apo (teal) (PDB ID: 6VVI) and lysine-bound (magenta) (PDB ID: 6VVH) DHDPS, with the active and allosteric sites shown in the inset within the black and grey rectangles, respectively. RMSD: 0.176 Å. Bound lysine molecules are shown as green sticks. (b) The monomeric structure of AtDHDPS1 highlighting the N-terminal (pink), the C-terminal (cyan) which contains the α-helices (green) involved in tetramerisation.

DHDPS function
The DHDPS-catalysed reaction involves the condensation of pyruvate and ASA to form HTPA (Soares da Costa et al. 2018). Binding studies have shown this reaction occurs via a ping-pong mechanism, whereby pyruvate binds first to the active site lysine (Lys222 in A. thaliana) and forms a Schiff base, which tautomerises to the enamine form (Soares da Costa et al. 2010). This then allows the hydrated form of ASA to bind, which is subsequently dehydrated, reacted with the pre-formed enamine and cyclised to form HTPA (Fig. 3) (Muscroft-Taylor et al. 2010).
In addition to Lys222, other important residues implicated in this reaction mechanism are Thr107, Tyr170, and Tyr194, which comprise the essential catalytic triad (Dobson et al. 2004). During Schiff base formation, Tyr194 donates a proton and accepts one in coordinating the ASA amino group (Dobson et al. 2004). Tyr170 shuttles protons to and from the active site and stabilises the enzyme by spanning the dimer interface, thus interlocking the two monomers (Dobson et al. 2004). Thr107 participates in a hydrogen bonding network, facilitating Schiff base formation followed by cyclisation (Soares da Costa et al. 2018). Another important catalytic residue is Arg199, which binds to the carboxyl group of ASA and stabilises the catalytic triad, thus allowing ASA to condense with pyruvate to form HTPA (Dobson et al. 2005).
DHDPS inhibition
Given that DHDPS catalyses the rate-limiting step in lysine biosynthesis, plant DHDPS enzymes have been characterised from various plant species (Matthews and Widholm 1979; Wallsgrove and Mazelis 1981; Kumpaisal et al. 1987; Frisch et al. 1991; Dereppe et al. 1992; Griffin et al. 2012; Erzeel et al. 2013; Gupta et al. 2018; Hall et al. 2021). In contrast to their bacterial orthologues, plant DHDPS enzymes have been shown to be strongly regulated by lysine through allosteric feedback inhibition (Frisch et al. 1991; Gupta et al. 2018; Hall et al. 2021). Residues implicated in allosteric regulation of DHDPS are conserved and include Asn143 and Tyr169, which interact with the carboxyl group of lysine, whilst the α-amino group of interacts with Gln112, Asn143, and Glu147 and the ε-amino group coordinates with Gly111, Trp116, and His119 (A. thaliana numbering) (Fig. 2a) (Griffin et al. 2012; Soares da Costa et al. 2016; Hall et al. 2021). Indeed, lysine binding significantly enhances the thermostability of V. vinifera DHDPS, indicating structural changes may occur after lysine binding (Atkinson et al. 2013). However, no significant alterations to the DHDPS crystal structures have been observed upon lysine binding (Griffin et al. 2008, 2012; Conly et al. 2014; Hall et al. 2021).
Molecular dynamics studies of V. vinifera DHDPS have suggested significant rotation of several active and allosteric site residues (Atkinson et al. 2013). Specifically, movement of Tyr169 has been proposed to result in disruption of catalytic triad residues and consequently attenuation of proton relay (Atkinson et al. 2013). To corroborate molecular dynamics findings, crystallography studies of DHDPS in the presence and absence of lysine have also noted flipping of Trp116 residue between the ‘open’ and ‘closed’ orientation upon lysine binding (Atkinson et al. 2013; Hall et al. 2021). This is proposed to reduce solvent accessibility and stabilise lysine in the allosteric pocket (Atkinson et al. 2013). Interestingly, inhibition of plant DHDPS has been noted to vary between species as well as isoforms. In vitro characterisation of AtDHDPS1 revealed this isoform was more sensitive to lysine inhibition than AtDHDPS2 (Griffin et al. 2012; Hall et al. 2021). The differential lysine sensitivity was attributed to differences in the positioning of Trp116 (Hall et al. 2021). As such, it was postulated that AtDHDPS1 exists predominantly in the inactive state at intracellular concentrations of lysine (Hall et al. 2021).
Chemical inhibition of plant lysine biosynthesis enzymes has only recently begun to be explored. The first reported plant lysine biosynthesis enzyme inhibitor, (Z)-2-(5-(4-fluorobenzylidene)-2,4-dioxothiazolidin-3-yl) acetic acid, was identified through a high-throughput chemical screen of 87,648 compounds against DHDPS (Soares da Costa et al. 2021). An analogue of this compound, (Z)-2-(5-(4-methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetic acid (MBDTA-2), was subsequently discovered to have improved anti-DHDPS activity in vitro (Table 1) (Soares da Costa et al. 2021). Crystallography studies showed that the mode of action of this class of compounds was through allosteric inhibition at a conserved novel site located at the centre of the homotetramer (Soares da Costa et al. 2021). Despite the distinction between the lysine and MBDTA-2 binding sites, lysine binding residues Trp116 and Glu147 were noted to interact with MBDTA-2. Additionally, as observed for the lysine-bound DHDPS structure, no gross conformational changes ensue following MBDTA-2 binding. However, the same rotation of Trp116 that occurs upon lysine binding was also noted to occur upon MBDTA-2 binding. This is interesting as the orientation of this residue was proposed to result in differential affinity to lysine between DHDPS isoforms (Hall et al. 2021). In planta studies show that MBDTA-2 has pre-emergence herbicidal efficacy against A. thaliana and against the weed species Lolium rigidum, commonly known as annual ryegrass (Hall et al. 2021; Mackie et al. 2022). Importantly, cytotoxicity studies with MBDTA-2 suggest that this inhibitor is plant-specific, as no effects on human or bacterial cell viability were observed at the concentrations tested (Soares da Costa et al. 2021).
| Inhibitor name | Structure | Target | References | |
|---|---|---|---|---|
| (Z)-2-(5-(4-Methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetic acid | 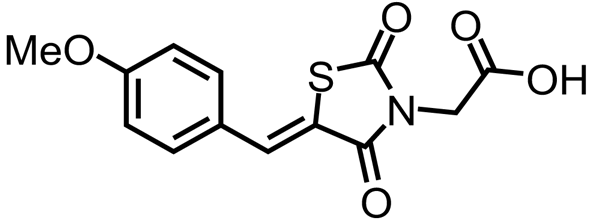 | DHDPS/DHDPR | Soares da Costa et al. (2021), Mackie et al. (2022) | |
| Di(prop-2-yn-1-yl) pyridine-2,6-dicarboxylate | 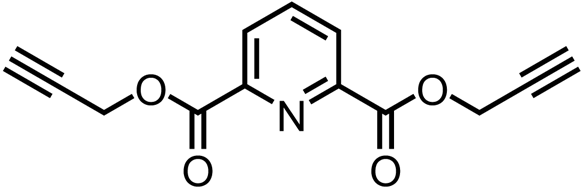 | DHDPR | Mackie et al. (2023) | |
| N-(3-(Hydrazinecarbonyl) naphthalen-2-yl)benzenesulfonamide |  | DAPAT | Fan et al. (2010) | |
| (Z)-5-(5-(2-Chlorobenzyl)-2-hydroxybenzylidene)-3-ethyl-2-thioxothiazolidin-4-one |  | DAPAT | Fan et al. (2010) | |
| (Z)-2-(2-((2,4,6-Trioxo-1-(p-tolyl) tetrahydropyrimidin-5(2H)-ylidene)methyl)-1H-indol-1-yl)acetic acid |  | DAPAT | Fan et al. (2010) | |
| 2-Thioxo-5-((1,2,5-trimethyl-1H-pyrrol-3-yl)methylene)dihydropyrimidine-4,6(1H,5H)-dione | 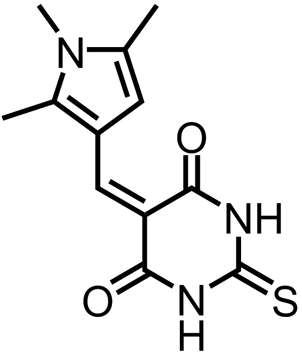 | DAPAT | Fan et al. (2010) |
Dihydrodipicolinate reductase (DHDPR)
DHDPR-encoding genes
The two isoforms of DHDPR, DHDPR1 and DHDPR2, which are present in plants are encoded by the DAPB1 (At2G44040) and DAPB2 (At3G59890) genes, respectively (Mackie et al. 2023). The first study to show the presence of DHDPR activity in plants did so by supplementing bacterial DAPB knockouts with partially purified DHDPR from Zea mays (maize) kernels and observing the synthesis of DAP (Tyagi et al. 1983). Unlike the DAPA genes, there have been no mutagenesis studies of plant DAPB genes to determine their essentiality. Additionally, it remains to be elucidated whether the two DHDPR isoforms are functionally redundant, as is the case for DHDPS (Jones-Held et al. 2012).
DHDPR structure
DHDPR structures from two plant species, A. thaliana and Selaginellamoellendorffii, have been deposited recently in the PDB (Watkin et al. 2018; Mackie et al. 2023). Plant DHDPR enzymes exist in a dimeric arrangement, which is in contrast to the bacterial orthologues that form homotetramers (Griffin et al. 2012; Watkin et al. 2018). Each subunit of the dimer is comprised of two domains, one of which is the N-terminal domain that contains a Rossman fold where the nucleotide cofactor NAD(P)H binds, and the C-terminal domain contains the active site where the substrate binds (Fig. 4). DHDPR can adopt an open or a closed conformation, the latter of which arises following rotation of the N-terminal domain relative to the C-terminal domain, facilitated by two hinge regions (Fig. 4) (Watkin et al. 2018). In the closed conformation, the nucleotide cofactor and substrate are brought within range of each other to allow for hydride transfer. Some evidence exists to suggest that the domain movement necessary for the enzyme to adopt the closed conformation may be induced by cofactor and substrate binding (Scapin et al. 1997; Janowski et al. 2010; Lee et al. 2018). However, multiple DHDPR crystal structures exist where the enzyme lacks any bound ligand, yet adopts a closed conformation (Girish et al. 2011; Watkin et al. 2018; Mackie et al. 2023). It is therefore likely that the domain movement either occurs spontaneously, or that the binding-dependency is species-specific (Girish et al. 2011; Watkin et al. 2018). The substrate binding loop of plant DHDPR enzymes appears to be highly flexible. This may be responsible for the entry of a new substrate molecule into the active site before the oxidised cofactor has been released, which is the proposed mechanism of substrate inhibition (Griffin et al. 2012; Watkin et al. 2018).
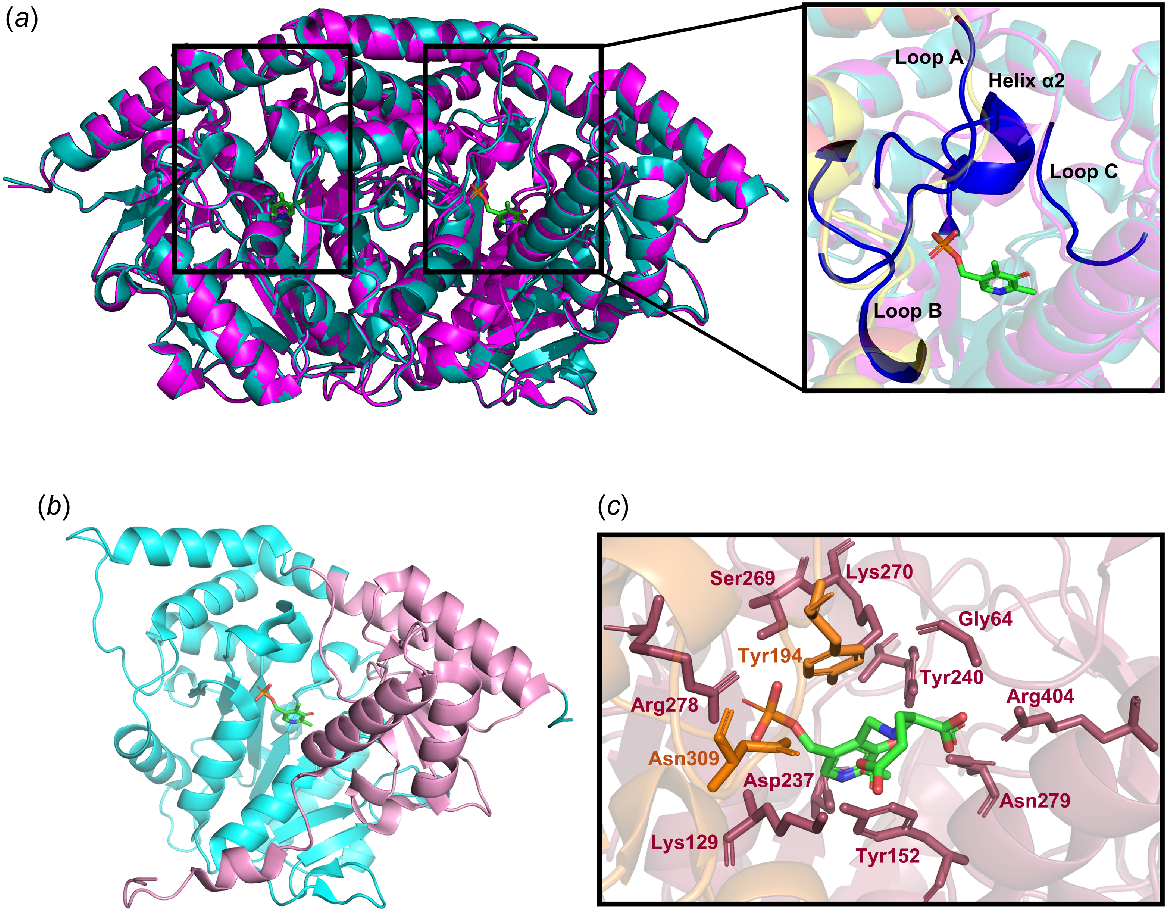
Structure of Arabidopsis thaliana (At) DHDPR. (a) The overlaid dimeric crystal structures of apo A. thaliana (teal) (PDB ID: 5UA0) and NADH-bound Selaginella moellendorffii (magenta) (PDB ID: 5U5N) DHDPR, with the nucleotide binding site shown in the inset. RMSD: 1.105 Å. The two nucleotide binding sites and active sites present in the dimer are indicated by rectangles and circles, respectively. (b) The monomeric structure of AtDHDPR highlighting the N-terminal domain (pink), the C-terminal domain (cyan) that contains the substrate binding loop (green) and hinge regions one (blue) and two (orange), which facilitate conformational changes.
DHDPR function
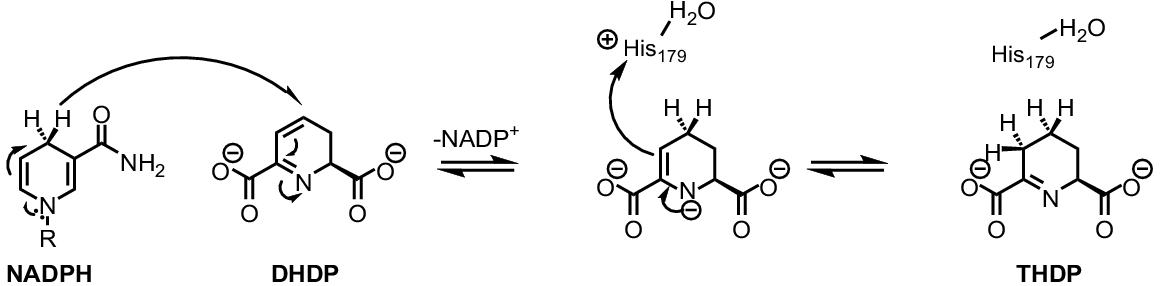
DHDPR catalysis follows a compulsory-order ternary complex mechanism where the binding of a nucleotide cofactor precedes the binding of substrate (Dommaraju et al. 2011). Unlike many oxidoreductase enzymes, DHDPR displays promiscuous nucleotide specificity as either the phosphorylated (NADPH) or unphosphorylated (NADH) form of the cofactor can be utilised (Watkin et al. 2018). Interestingly, cofactor preference has been shown to vary greatly between species, with plant DHDPR orthologues exhibiting a higher catalytic rate when using NADPH as the cofactor in vitro (Dommaraju et al. 2011; Lee et al. 2018; Pote et al. 2018). NADPH is most likely the biologically relevant cofactor in plants given that DHDPR is localised to the chloroplast, where NADPH is generated during light-dependent reactions (Griffin et al. 2012). In the DHDPR catalysed reaction, a hydride ion is transferred from NADPH to DHDP (Fig. 5) (Scapin et al. 1997). Subsequent tautomerisation and protonation of the intermediate, facilitated by the interaction of His179 (A. thaliana numbering) with a water molecule, yields the product THDP (Fig. 5) (Scapin et al. 1997). Plant DHDPR is prone to substrate inhibition, more so than the bacterial orthologues, however the substrate is unlikely to accumulate to a high enough level for inhibition to occur under normal biological conditions (Watkin et al. 2018; Mackie et al. 2023).
DHDPR inhibition
The first plant DHDPR inhibitor was identified in a follow-up study on the mechanism of action of the plant DHDPS inhibitor MBDTA-2 (Mackie et al. 2022). The authors noted that the similarity in MBDTA-2 potency against the DHDPS enzymes in vitro and against whole plants plant was unusual, given that the amount of inhibitor reaching the target site within a plant is expected to be less than the amount applied (Mackie et al. 2022). This prompted the investigation of MBDTA-2 as a potential DHDPR inhibitor, to address the hypothesis that the compound may have an additional mode of action beyond DHDPS inhibition. MBDTA-2 was found to represent the first plant DHDPR inhibitor and the first dual-target lysine biosynthesis inhibitor. Herbicides with multiple sites of action have the potential to circumvent the emergence of resistance, given that weeds harbouring a single resistance-conferring target site mutation should not survive due to inhibition of another target. Considering that no commercially available herbicides are multi-targeted, the discovery of a herbicidal compound that has a novel mode of action at two target enzymes could have significant implications for herbicide resistance mitigation. In contrast to the allosteric mode of DHDPS inhibition by MDBTA-2, docking studies and substrate competition assays revealed it to be an active site inhibitor of DHDPR.
The recent publication of structural information about plant DHDPR enzymes has facilitated the identification of other inhibitors of plant DHDPR. Defining the similarity of DHDPR orthologues between bacteria and plants gave rise to the hypothesis that an inhibitor of Escherichia coli DHDPR may also bind to the plant orthologues (Reddy et al. 1995; Scapin et al. 1997; Mackie et al. 2023). This inhibitor, 2,6-pyridinedicarboxylic acid (2,6-PDC), was first identified in a study focussed on the inhibition of bacterial lysine biosynthesis as an antibiotic development strategy (Reddy et al. 1995). However, 2,6-PDC was not reported to have any efficacy against intact bacteria and thus did not progress through the antibiotic development pipeline (Paiva et al. 2001). Whilst 2,6-PDC did inhibit plant DHDPR in vitro, it did not exert herbicidal effects against soil grown A. thaliana at the concentration tested. Nevertheless, a structure-activity relationship investigation yielded several 2,6-PDC analogues with pre-emergence herbicidal efficacy against A. thaliana. The lead compound was found to also exhibit pre-emergence herbicidal activity against both L. rigidum and Raphanus raphanistrum (wild radish) (Mackie et al. 2023). This work exemplifies the potential of re-examining inhibitors identified against bacterial enzymes that are closely related to essential plant enzymes. The resultant bypassing of laborious and costly steps involved in herbicide lead identification could offer an efficient way to identify much-needed herbicides with new modes of action.
Diaminopimelate aminotransferase (DAPAT)
DAPAT-encoding genes
Plants possess a single DAPAT enzyme that is encoded by the DAPL gene (At4G33680), which was previously annotated as ABBERANT GROWTH AND DEATH 2 (Song et al. 2004; Dobson et al. 2011). The first study to examine the essentiality of DAPL isolated a mutant with a T-DNA insertion in the first exon of the gene (Song et al. 2004). The heterozygous mutant had an aborted embryo phenotype and no homozygous mutants were present in 64 of the progeny, suggesting that homozygous embryos were not viable (Song et al. 2004). Reciprocal crosses revealed that no transmission defects were present in the pollen or ovules, demonstrating that a functional DAPL gene is essential for post-fertilisation plant development (Song et al. 2004). This was further supported by the finding that complementation of the heterozygous mutant with the wild-type DAPL gene allowed for the isolation of homozygous mutants (Song et al. 2004). A subsequent study used embryo lethality screening to validate that the phenotype was due to the essentiality of DAPL rather than the presence of multiple T-DNA insertions (Dobson et al. 2011). It was found that a heterozygous T-DNA insertion in the promoter region of the DAPL gene resulted in the same aborted embryo phenotype seen previously (Dobson et al. 2011). Furthermore, homozygous mutant plants could not be isolated, corroborating the finding that a functional DAPL gene is essential in plants (Dobson et al. 2011).
DAPAT structure
The crystal structure of DAPAT from A. thaliana has been deposited in the PDB, in addition to those from two bacterial and one algal species (Dobson et al. 2011; Watanabe et al. 2007, 2011). DAPAT exists as a homodimer, which is the case for many aminotransferase enzymes given that residues from both subunits comprise the active site (Fig. 6) (Schneider et al. 2000; Watanabe et al. 2007; Dobson et al. 2011). Additionally, in silico studies have suggested that dimerisation of DAPAT is important for stability (Adams et al. 2020). Each monomeric unit consists of a large and small domain, which both have an α/β fold (Fig. 6) (Watanabe et al. 2007). The monomers come together to form a dimer with two active sites where the interface is stabilised primarily by hydrophobic interactions (Watanabe et al. 2007). The N-termini also makes extensive hydrogen bonding interactions and salt bridges with the neighbouring monomer (Watanabe et al. 2007).
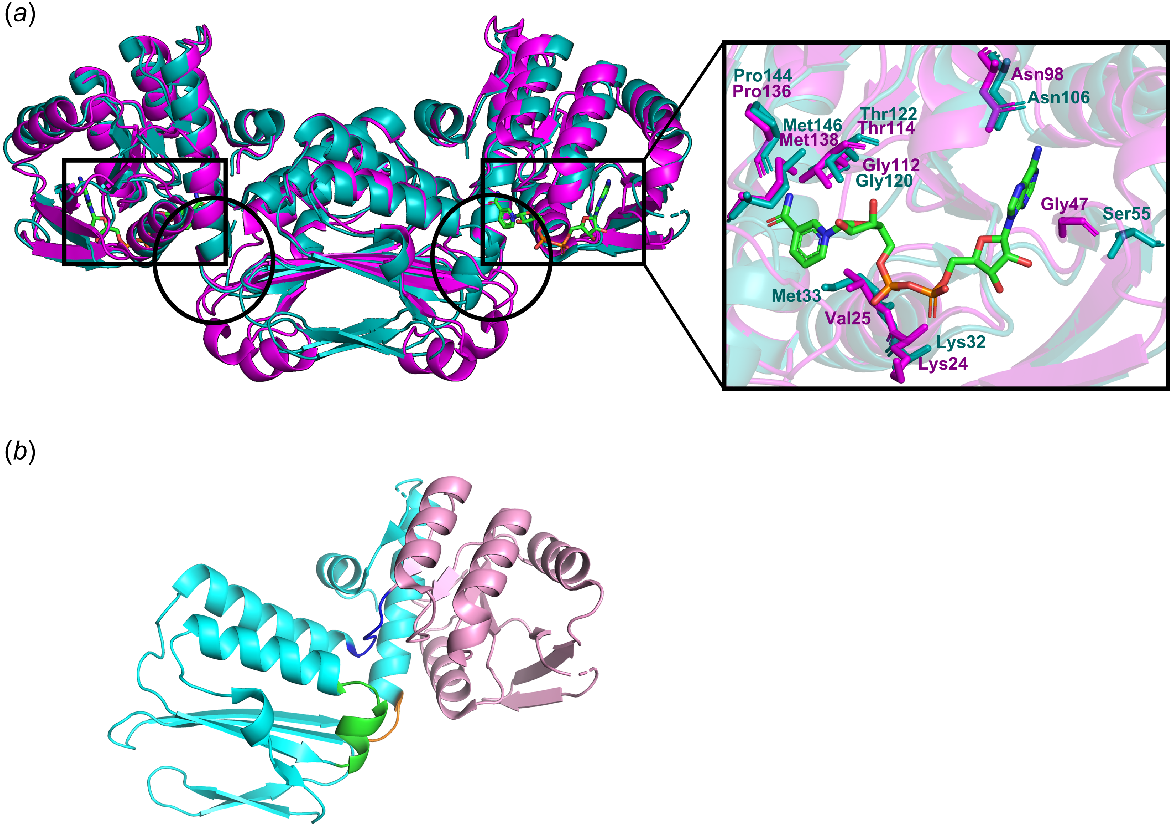
Structure of AtDAPAT. (a) The overlaid dimeric crystal structures of non-covalently cofactor-bound (magenta) (PDB ID: 2Z20) and apo (teal) (PDB ID: 3EI7) AtDAPAT, with shifts in loops A-C and helix α2 that result in ordering of the active site upon cofactor binding shown in the inset. RMSD: 0.215. The two active sites present in the dimer are indicated by rectangles. (b) The monomeric structure of cofactor-bound AtDAPAT highlighting the small (pink) and large (cyan) domains. (c) The AtDAPAT active site. Residues from both chain A (mauve) and chain B (orange) contribute to the stabilisation of the cofactor and substrate in the active site.
DAPAT function
Until recently, it was not known how the conversion of THDP to DAP took place in plants, as the activity of enzymes used by other species for this conversion could not be detected in plant extracts (Hudson et al. 2005, 2006). DAPAT was identified as the enzyme that bridges this metabolic gap by determining that it was capable of converting THDP to DAP in vitro (Hudson et al. 2006). Its presence in plants was confirmed with the finding that functional complementation of E. coli auxotrophs lacking the succinylase pathway enzymes bridging THDP and DAP could be achieved with A. thaliana DAPAT (Hudson et al. 2006).
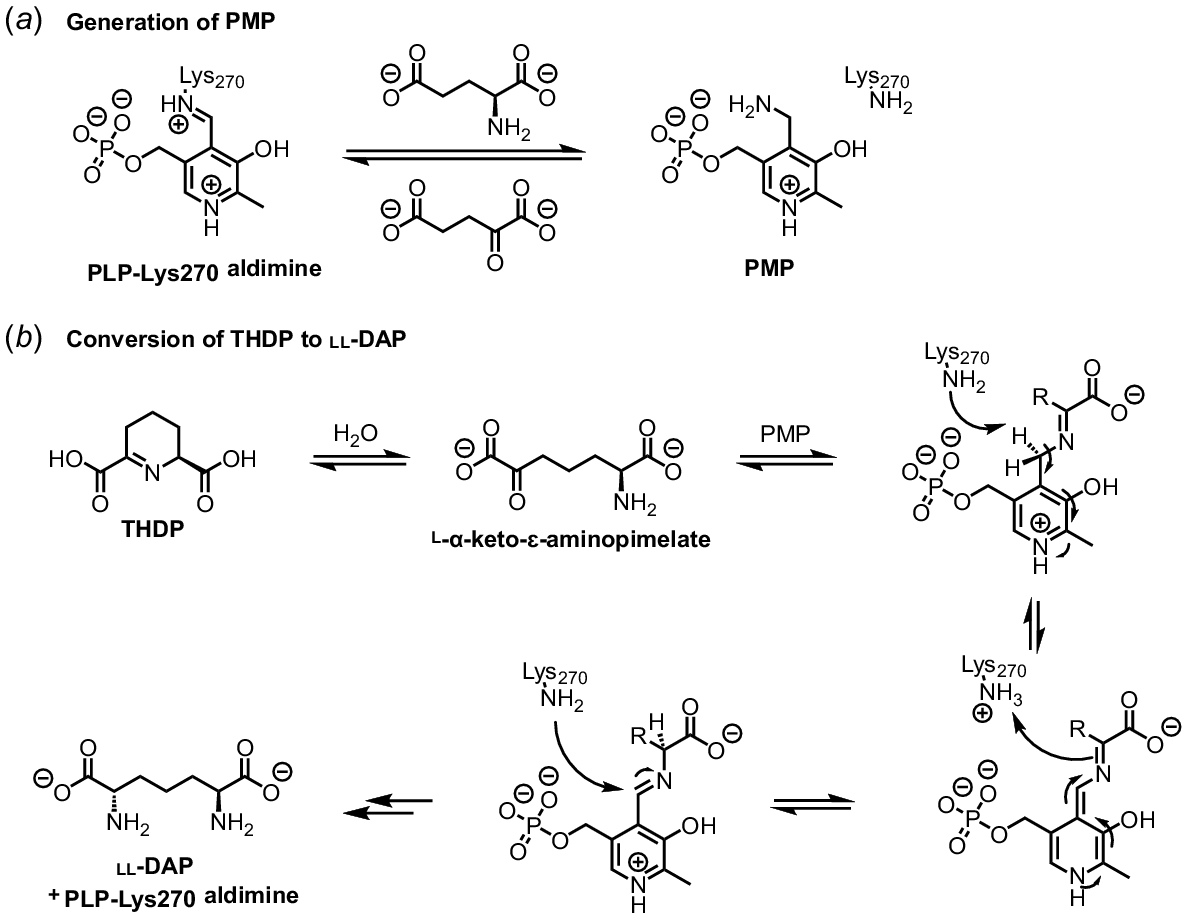
Catalysis by the DAPAT enzyme follows a ping-pong mechanism (Watanabe et al. 2007). First, a Schiff base is formed via the covalent imine linkage of the cofactor pyridoxal 5-phosphate (PLP) to Lys270 (A. thaliana numbering). PLP binding induces conformational changes in loops A, B and C and helix α2 of the enzyme to form an ordered active site (Watanabe et al. 2008). PLP is stabilised by an extensive network of interactions including hydrogen bonds with Ty240, Asn209, Lys129, Ser269, Tyr94, Asn309, and Asp237 and a salt bridge with Arg278. The subsequent binding of glutamate via a salt bridge with Arg404 and hydrogen bonds with Gly64, Glu97, Lys129, Tyr152 and Asn209 results in conformational changes in the side chains of active site residues (Watanabe et al. 2008). Notably, Tyr152 moves away from the active site cavity, bringing the aromatic ring of Tyr152 within stacking proximity of the PLP pyridine ring, further stabilising PLP (Watanabe et al. 2008). Next, glutamate acts as an amino donor to produce pyridoxamine phosphate (PMP) from the PLP-Lys270 aldimine, yielding α-ketoglutarate, which leaves the active site (Watanabe et al. 2007, 2008) (Fig. 7a). The DAPAT substrate THDP exists in equilibrium with its hydrolysis product l-α-keto-ɛ-aminopimelate in aqueous solution, which is most likely to be the form accepted by the enzyme (Caplan et al. 2001; Watanabe et al. 2007, 2008). This species is stabilised in the active site by contacts with Asn309, allowing attack by PMP to form a ketimine intermediate (Watanabe et al. 2008). Tautomerisation mediated by Lys270 generates the key aldimine intermediate, which undergoes a transamination reaction with Lys270 to generate ll-DAP and regenerate the PLP-Lys270 aldimine (Watanabe et al. 2007) (Fig. 7b).
DAPAT inhibition
The first DAPAT inhibitors were identified in a chemical screen of 29,201 drug-like compounds against the A. thaliana enzyme (Fan et al. 2010). A high percentage of the lead compounds from this screen contained barbiturate or thiobarbiturate rings (Table 1) (Fan et al. 2010). Additionally, rhodanine-based and hydrazide inhibitors were amongst the top 46 lead compounds (Table 1). The most potent compound belonged to the latter group, and had an IC50 of 5.0 μM against the recombinant enzyme (Table 1) (Fan et al. 2010). Analogues of this compound were synthesised; however, all had decreased potency relative to the parent compound (Fan et al. 2010). In a later study, the lead inhibitors from each of the barbiturate, thiobarbiturate, rhodanine and hydrazide classes were tested against DAPAT orthologues from the bacterial species Leptospira interrogans (Li) and Verrucomicrobium spinosum (Vs), as well as the algae Chlamydomonas reinhardtii, relative to AtDAPAT (McKinnie et al. 2014). The inhibitors exhibited differential inhibition against the orthologues despite complete conservation of the sequence and arrangement of the substrate recognition and binding residues (McKinnie et al. 2014). However, the crystal structure of CrDAPAT and the homology models of LiDAPAT and VsDAPAT do not have PLP bound in the active site (McKinnie et al. 2014). Given that PLP binding leads to rearrangement of the active site, it is possible that the differential inhibition may be due to differences in the PLP-bound enzyme (McKinnie et al. 2014). Alternatively, it is possible that these inhibitors bind in a pocket distal from the substrate binding site. The mode of binding for these compounds, and thus the grounds for the differential response, are yet to be determined (McKinnie et al. 2014). Moreover, whether inhibitors of DAPAT have in planta activity remains to be elucidated.
Towards the discovery of novel plant lysine biosynthesis inhibitors
Inhibitors of the last two enzymes in the plant lysine biosynthesis pathway, DAPEP and DAPDC, are yet to be developed. Mutagenesis studies to characterise the essentiality of DAPEP and DAPDC in plants may aid in validating them as potential targets for herbicide development. Alternatively, the identification of herbicidal inhibitors of these enzymes may represent anotherroute to target validation, as exemplified by studies with DHDPR (Mackie et al. 2023). However, without confirmation that the enzymes are essential to plant survival, it may be difficult to delineate if poor herbicidal activity of in vitro inhibitors is due to the plant tolerating inhibition of the enzyme or if the physiochemical properties of the inhibitor are limiting uptake and translocation promoting degradation.
Crystal structures of A. thaliana DAPEP and DAPDC enzymes have been deposited in the PDB, and studies to characterise their function and catalytic mechanism have been undertaken (Pillai et al. 2009; Crowther et al. 2019). This information could be used to examine the degree of homology between inhibitor binding sites of the bacterial enzymes with those of the plant enzymes, to determine the likelihood of inhibitors also being able to bind the plant orthologues. This could facilitate the repurposing of compounds that have failed to progress through the antibiotic development pipeline as scaffolds for herbicide development, as was accomplished for the development of herbicidal inhibitors of DHDPR. Indeed, many examples of inhibitors of bacterial lysine biosynthesis enzymes exist, which have been reviewed elsewhere, and could be explored as candidates for repurposing (Muduli et al. 2023).
Whilst inhibitors of plant lysine biosynthesis have been shown to exert herbicidal effects against the weed species L. rigidum and R. raphanistrum, their potency against the enzyme orthologues from these species remains unknown. Indeed, all plant lysine biosynthesis inhibitors developed to date have only been tested for in vitro inhibition of the A. thaliana enzymes. This is due to the availability of the A. thaliana genome allowing the production of recombinant proteins from the model species. Whilst lysine biosynthesis enzymes are highly conserved across diverse species, subtle changes in amino acid sequence and protein structure can result in differential inhibition (Hall et al. 2021). However, a recent sharp increase in the number of high-quality genome assemblies available for weed species, owing to advances in sequencing technology, will enable herbicidal compounds to be increasingly designed and tested against the biologically relevant enzyme orthologues (Montgomery et al. 2024). Furthermore, continuous improvements in ab initio protein structure prediction software such as AlphaFold will allow in silico docking of potential inhibitors with the weed targets, reducing the time and cost associated with crystallisation and structure determination for rational design or high-throughput screening for inhibitor discovery (Abramson et al. 2024).
Concluding remarks
Weeds reduce the profitability of agricultural industries and threaten their ability to provide a secure supply of food. Herbicides remain the most effective and economical weed control method. However, the rapid emergence and spread of weeds that are resistant to current herbicides is imperilling their utility. It is evident that new herbicide modes of action without existing resistance mechanisms are urgently needed. Until recently, the potential of lysine biosynthesis as a novel herbicide mode of action remained unexplored. The detailed in vitro characterisation of plant DHDPS, DHDPR and DAPAT has allowed for the identification of the first inhibitors of these enzymes, some of which have herbicidal efficacy. Target validation and inhibitor discovery are essential stages in the herbicide development pipeline. However, developing enzyme inhibitors into commercial products is a complex and costly undertaking, which is in part due to the increasingly stringent safety assessments required for herbicide registration (Flynn 2008; Swanton et al. 2011; Sparks and Lorsbach 2017). Additionally, given the high degree of sequence conservation of lysine biosynthesis enzymes across plant species, specificity against the enzyme is unlikely to be achieved (Hall et al. 2021). However, herbicide susceptibility may differ between plant species due to differences in the absorption or translocation of the compound as well as the morphological and physiological differences between the plant species (Blanco et al. 2015). As such, testing against panels of weed and crop species will be pertinent to inform application strategies. Alternatively, herbicide-resistant crops can be developed to allow for broad-spectrum herbicides to be used at various plant life cycle stages without damaging crops and so the development of crops resistant to lysine biosynthesis inhibitors should be investigated (Green and Owen 2011). Furthermore, the dosage of the herbicidal compound is another important consideration. Administration of sublethal doses has been shown to result in accumulation of resistant phenotypes over several generations (Norsworthy et al. 2021). However, excessive application of herbicides can extend their persistence in the environment, which is not desirable (Hanke et al. 2010; Kanissery et al. 2019). As such, investigations into the potency, soil persistence and degradation fate of the compounds needs to be studied as part of the development. Nevertheless, the studies on the structure, function and inhibition of plant lysine biosynthesis discussed here have contributed vital groundwork towards the development of lysine biosynthesis inhibition as a much-needed novel herbicide mode of action.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Declaration of funding
We acknowledge the Australian Research Council (DP250102939, FT230100203, IC220100050, DP220101901, DE190100806) for contributing to funding the primary research described in this review.
References
Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, Ronneberger O, Willmore L, Ballard AJ, Bambrick J, Bodenstein SW, Evans DA, Hung C-C, O’Neill M, Reiman D, Tunyasuvunakool K, Wu Z, Žemgulytė A, Arvaniti E, Beattie C, Bertolli O, Bridgland A, Cherepanov A, Congreve M, Cowen-Rivers AI, Cowie A, Figurnov M, Fuchs FB, Gladman H, Jain R, Khan YA, Low CMR, Perlin K, Potapenko A, Savy P, Singh S, Stecula A, Thillaisundaram A, Tong C, Yakneen S, Zhong ED, Zielinski M, Žídek A, Bapst V, Kohli P, Jaderberg M, Hassabis D, Jumper JM (2024) Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493-500.
| Crossref | Google Scholar | PubMed |
Adams LE, Rynkiewicz P, Babbitt GA, Mortensen JS, North RA, Dobson RCJ, Hudson AO (2020) Comparative molecular dynamics simulations provide insight into antibiotic interactions: a case study using the enzyme L,L-diaminopimelate aminotransferase (DapL). Frontiers in Molecular Biosciences 7, 46.
| Crossref | Google Scholar |
Atkinson SC, Dogovski C, Downton MT, Pearce FG, Reboul CF, Buckle AM, Gerrard JA, Dobson RCJ, Wagner J, Perugini MA (2012) Crystal, solution and in silico structural studies of dihydrodipicolinate synthase from the common grapevine. PLoS ONE 7, e38318.
| Crossref | Google Scholar | PubMed |
Atkinson SC, Dogovski C, Downton MT, Czabotar PE, Dobson RCJ, Gerrard JA, Wagner J, Perugini MA (2013) Structural, kinetic and computational investigation of Vitis vinifera DHDPS reveals new insight into the mechanism of lysine-mediated allosteric inhibition. Plant Molecular Biology 81, 431-446.
| Crossref | Google Scholar | PubMed |
Blanco FMG, Ramos YG, Scarso MF, Jorge LAC (2015) Determining the selectivity of herbicides and assessing their effect on plant roots – A case study with indaziflam and glyphosate herbicides. In ‘Herbicides, physiology of action, and safety’. (Eds AD Price, JED Kelton, L Sarunaite). (IntechOpen). 10.5772/61721
Blickling S, Renner C, Laber B, Pohlenz H-D, Holak TA, Huber R (1997a) Reaction mechanism of Escherichia coli dihydrodipicolinate synthase investigated by X-ray crystallography and NMR spectroscopy. Biochemistry 36, 24-33.
| Crossref | Google Scholar | PubMed |
Blickling S, Beisel HG, Bozic D, Knäblein J, Laber B, Huber R (1997b) Structure of dihydrodipicolinate synthase of Nicotiana sylvestris reveals novel quaternary structure. Journal of Molecular Biology 274, 608-621.
| Crossref | Google Scholar | PubMed |
Caplan JF, Sutherland A, Vederas JC (2001) The first stereospecific synthesis of L-tetrahydrodipicolinic acid; a key intermediate of diaminopimelate metabolism. Journal of the Chemical Society, Perkin Transactions 1 [18] 2217-2220.
| Google Scholar |
Christensen JB, Soares da Costa TP, Faou P, Pearce FG, Panjikar S, Perugini MA (2016) Structure and function of cyanobacterial DHDPS and DHDPR. Scientific Reports 6, 37111.
| Crossref | Google Scholar | PubMed |
Conly CJT, Skovpen YV, Li S, Palmer DRJ, Sanders DAR (2014) Tyrosine 110 plays a critical role in regulating the allosteric inhibition of Campylobacter jejuni dihydrodipicolinate synthase by lysine. Biochemistry 53, 7396-7406.
| Crossref | Google Scholar | PubMed |
Craciun A, Jacobs M, Vauterin M (2000) Arabidopsis loss-of-function mutant in the lysine pathway points out complex regulation mechanisms. FEBS Letters 487, 234-238.
| Crossref | Google Scholar | PubMed |
Crowther JM, Cross PJ, Oliver MR, Leeman MM, Bartl AJ, Weatherhead AW, North RA, Donovan KA, Griffin MDW, Suzuki H, Hudson AO, Kasanmascheff M, Dobson RCJ (2019) Structure-function analyses of two plant meso-diaminopimelate decarboxylase isoforms reveal that active-site gating provides stereochemical control. Journal of Biological Chemistry 294, 8505-8515.
| Crossref | Google Scholar | PubMed |
Dayan FE (2019) Current status and future prospects in herbicide discovery. Plants 8, 341.
| Crossref | Google Scholar | PubMed |
Dereppe C, Bold G, Ghisalba O, Ebert E, Schär H-P (1992) Purification and characterization of dihydrodipicolinate synthase from pea. Plant Physiology 98, 813-821.
| Crossref | Google Scholar | PubMed |
Dobson RCJ, Valegård K, Gerrard JA (2004) The crystal structure of three site-directed mutants of Escherichia coli dihydrodipicolinate synthase: further evidence for a catalytic triad. Journal of Molecular Biology 338, 329-339.
| Crossref | Google Scholar | PubMed |
Dobson RCJ, Devenish SRA, Turner LA, Clifford VR, Pearce FG, Jameson GB, Gerrard JA (2005) Role of arginine 138 in the catalysis and regulation of Escherichia coli dihydrodipicolinate synthase. Biochemistry 44, 13007-13013.
| Crossref | Google Scholar | PubMed |
Dobson RCJ, Girón I, Hudson AO (2011) L,L-diaminopimelate aminotransferase from Chlamydomonas reinhardtii: a target for algaecide development. PLoS ONE 6, e20439.
| Crossref | Google Scholar | PubMed |
Dommaraju SR, Dogovski C, Czabotar PE, Hor L, Smith BJ, Perugini MA (2011) Catalytic mechanism and cofactor preference of dihydrodipicolinate reductase from methicillin-resistant Staphylococcus aureus. Archives of Biochemistry and Biophysics 512, 167-174.
| Crossref | Google Scholar | PubMed |
Duke SO (2012) Why have no new herbicide modes of action appeared in recent years? Pest Management Science 68, 505-512.
| Crossref | Google Scholar | PubMed |
Erzeel E, Van Bochaute P, Thu TT, Angenon G (2013) Medicago truncatula dihydrodipicolinate synthase (DHDPS) enzymes display novel regulatory properties. Plant Molecular Biology 81, 401-415.
| Crossref | Google Scholar | PubMed |
Fan C, Clay MD, Deyholos MK, Vederas JC (2010) Exploration of inhibitors for diaminopimelate aminotransferase. Bioorganic & Medicinal Chemistry 18, 2141-2151.
| Crossref | Google Scholar | PubMed |
Fazius F, Shelest E, Gebhardt P, Brock M (2012) The fungal α-aminoadipate pathway for lysine biosynthesis requires two enzymes of the aconitase family for the isomerization of homocitrate to homoisocitrate. Molecular Microbiology 86, 1508-1530.
| Crossref | Google Scholar | PubMed |
Frisch DA, Gengenbach BG, Tommey AM, Sellner JM, Somers DA, Myers DE (1991) Isolation and characterization of dihydrodipicolinate synthase from maize. Plant Physiology 96, 444-452.
| Crossref | Google Scholar | PubMed |
Galili G (2002) New insights into the regulation and functional significance of lysine metabolism in plants. Annual Review of Plant Biology 53, 27-43.
| Crossref | Google Scholar | PubMed |
Gauer LE, Grant CA, Bailey LD, Gehl DT (1992) Effects of nitrogen fertilization on grain protein content, nitrogen uptake, and nitrogen use efficiency of six spring wheat (Triticum aestivum L.) cultivars, in relation to estimated moisture supply. Canadian Journal of Plant Science 72, 235-241.
| Crossref | Google Scholar |
Girish TS, Navratna V, Gopal B (2011) Structure and nucleotide specificity of Staphylococcus aureus dihydrodipicolinate reductase (DapB). FEBS Letters 585, 2561-2567.
| Crossref | Google Scholar | PubMed |
Green JM, Owen MDK (2011) Herbicide-resistant crops: utilities and limitations for herbicide-resistant weed management. Journal of Agricultural and Food Chemistry 59, 5819-5829.
| Crossref | Google Scholar | PubMed |
Griffin MDW, Dobson RCJ, Pearce FG, Antonio L, Whitten AE, Liew CK, Mackay JP, Trewhella J, Jameson GB, Perugini MA, Gerrard JA (2008) Evolution of quaternary structure in a homotetrameric enzyme. Journal of Molecular Biology 380, 691-703.
| Crossref | Google Scholar | PubMed |
Griffin MDW, Billakanti JM, Wason A, Keller S, Mertens HDT, Atkinson SC, Dobson RCJ, Perugini MA, Gerrard JA, Pearce FG (2012) Characterisation of the first enzymes committed to lysine biosynthesis in Arabidopsis thaliana. PLoS ONE 7, e40318.
| Crossref | Google Scholar |
Gupta R, Hogan CJ, Perugini MA, Soares da Costa TP (2018) Characterization of recombinant dihydrodipicolinate synthase from the bread wheat Triticum aestivum. Planta 248, 381-391.
| Crossref | Google Scholar | PubMed |
Hall CJ, Mackie ER, Gendall AR, Perugini MA, Soares da Costa TP (2020) Review: amino acid biosynthesis as a target for herbicide development. Pest Management Science 76, 3896-3904.
| Crossref | Google Scholar | PubMed |
Hall CJ, Lee M, Boarder MP, Mangion AM, Gendall AR, Panjikar S, Perugini MA, Soares da Costa TP (2021) Differential lysine-mediated allosteric regulation of plant dihydrodipicolinate synthase isoforms. FEBS Journal 288, 4973-4986.
| Crossref | Google Scholar | PubMed |
Hanke I, Wittmer I, Bischofberger S, Stamm C, Singer H (2010) Relevance of urban glyphosate use for surface water quality. Chemosphere 81, 422-429.
| Crossref | Google Scholar | PubMed |
Hartmann M, Zeier J (2018) l-lysine metabolism to N-hydroxypipecolic acid: an integral immune-activating pathway in plants. Plant Journal 96, 5-21.
| Crossref | Google Scholar |
Holding DR, Otegui MS, Li B, Meeley RB, Dam T, Hunter BG, Jung R, Larkins BA (2007) The maize floury1 gene encodes a novel endoplasmic reticulum protein involved in zein protein body formation. The Plant Cell 19, 2569-2582.
| Crossref | Google Scholar | PubMed |
Holliday GL, Mitchell JBO, Thornton JM (2009) Understanding the functional roles of amino acid residues in enzyme catalysis. Journal of Molecular Biology 390, 560-577.
| Crossref | Google Scholar | PubMed |
Hudson AO, Bless C, MacEdo P, Chatterjee SP, Singh BK, Gilvarg C, Leustek T (2005) Biosynthesis of lysine in plants: evidence for a variant of the known bacterial pathways. Biochimica et Biophysica Acta (BBA) - General Subjects 1721, 27-36.
| Crossref | Google Scholar | PubMed |
Hudson AO, Singh BK, Leustek T, Gilvarg C (2006) An LL-diaminopimelate aminotransferase defines a novel variant of the lysine biosynthesis pathway in plants. Plant Physiology 140, 292-301.
| Crossref | Google Scholar | PubMed |
Jander G, Joshi V (2009) Aspartate-derived amino acid biosynthesis in Arabidopsis thaliana. The Arabidopsis Book 7, e0121.
| Crossref | Google Scholar | PubMed |
Janowski R, Kefala G, Weiss MS (2010) The structure of dihydrodipicolinate reductase (DapB) from Mycobacterium tuberculosis in three crystal forms. Acta Crystallographica Section D: Biological Crystallography 66, 61-72.
| Crossref | Google Scholar | PubMed |
Jones-Held SA, Ambrozevicius LP, Campbell M, Drumheller B, Harrington E, Leustek T (2012) Two Arabidopsis thaliana dihydrodipicolinate synthases, DHDPS1 and DHDPS2, are unequally redundant. Functional Plant Biology 39, 1058-1067.
| Crossref | Google Scholar | PubMed |
Kanissery R, Gairhe B, Kadyampakeni D, Batuman O, Alferez F (2019) Glyphosate: its environmental persistence and impact on crop health and nutrition. Plants 8, 499.
| Crossref | Google Scholar | PubMed |
Kumpaisal R, Hashimoto T, Yamada Y (1987) Purification and characterization of dihydrodipicolinate synthase from wheat suspension cultures. Plant Physiology 85, 145-151.
| Crossref | Google Scholar | PubMed |
Lee CW, Park SH, Lee SG, Park HH, Kim HJ, Park H, Park H, Lee JH (2018) Crystal structure of dihydrodipicolinate reductase (PaDHDPR) from Paenisporosarcina sp. TG-14: structural basis for NADPH preference as a cofactor. Scientific Reports 8, 7936.
| Crossref | Google Scholar |
Long X, Liu Q, Chan M, Wang Q, Sun SSM (2013) Metabolic engineering and profiling of rice with increased lysine. Plant Biotechnology Journal 11, 490-501.
| Crossref | Google Scholar | PubMed |
Luo M (2018) Chemical and biochemical perspectives of protein lysine methylation. Chemical Reviews 118, 6656-6705.
| Crossref | Google Scholar | PubMed |
Mackie ERR, Barrow AS, Christoff RM, Abbott BM, Gendall AR, Soares da Costa TP (2022) A dual-target herbicidal inhibitor of lysine biosynthesis. eLife 11, e78235.
| Crossref | Google Scholar |
Mackie ERR, Barrow AS, Giel M-C, Hulett MD, Gendall AR, Panjikar S, Soares da Costa TP (2023) Repurposed inhibitor of bacterial dihydrodipicolinate reductase exhibits effective herbicidal activity. Communications Biology 6, 550.
| Crossref | Google Scholar |
Matthews BF, Widholm JM (1979) Expression of aspartokinase, dihydrodipicolinic acid synthase and homoserine dehydrogenase during growth of carrot cell suspension cultures on lysine- and threonine-supplemented media. Zeitschrift für Naturforschung C 34, 1177-1185.
| Crossref | Google Scholar |
McKinnie SMK, Rodriguez-Lopez EM, Vederas JC, Crowther JM, Suzuki H, Dobson RCJ, Leustek T, Triassi AJ, Wheatley MS, Hudson AO (2014) Differential response of orthologous l,l-diaminopimelate aminotransferases (DapL) to enzyme inhibitory antibiotic lead compounds. Bioorganic and Medicinal Chemistry 22, 523-530.
| Crossref | Google Scholar | PubMed |
Mertz ET, Bates LS, Nelson OE (1964) Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science 145, 279-280.
| Crossref | Google Scholar | PubMed |
Montgomery J, Morran S, MacGregor DR, McElroy JS, Neve P, Neto C, Vila-Aiub MM, Sandoval MV, Menéndez AI, Kreiner JM, Fan L, Caicedo AL, Maughan PJ, Martins BAB, Mika J, Collavo A, Merotto A, Jr., Subramanian NK, Bagavathiannan MV, Cutti L, Mazharul Islam M, Gill BS, Cicchillo R, Gast R, Soni N, Wright TR, Zastrow-Hayes G, May G, Malone JM, Sehgal D, Kaundun SS, Dale RP, Vorster BJ, Peters B, Lerchl J, Tranel PJ, Beffa R, Fournier-Level A, Jugulam M, Fengler K, Llaca V, Patterson EL, Gaines TA (2024) Current status of community resources and priorities for weed genomics research. Genome Biology 25, 139.
| Crossref | Google Scholar | PubMed |
Muduli S, Karmakar S, Mishra S (2023) The coordinated action of the enzymes in the L-lysine biosynthetic pathway and how to inhibit it for antibiotic targets. Biochimica et Biophysica Acta (BBA) - General Subjects 1867, 130320.
| Crossref | Google Scholar | PubMed |
Muscroft-Taylor AC, Soares da Costa TP, Gerrard JA (2010) New insights into the mechanism of dihydrodipicolinate synthase using isothermal titration calorimetry. Biochimie 92, 254-262.
| Crossref | Google Scholar | PubMed |
Myers AM, James MG, Lin Q, Yi G, Stinard PS, Hennen-Bierwagen TA, Becraft PW (2011) Maize opaque5 encodes monogalactosyldiacylglycerol synthase and specifically affects galactolipids necessary for amyloplast and chloroplast function. The Plant Cell 23(6), 2331-2347.
| Crossref | Google Scholar |
Negrutiu I, Cattoir-Reynearts A, Verbruggen I, Jacobs M (1984) Lysine overproducer mutants with an altered dihydrodipicolinate synthase from protoplast culture of Nicotiana sylvestris (Spegazzini and Comes). Theoretical and Applied Genetics 68, 11-20.
| Crossref | Google Scholar | PubMed |
Nelson OE, Mertz ET, Bates LS (1965) Second mutant gene affecting the amino acid pattern of maize endosperm proteins. Science 150, 1469-1470.
| Crossref | Google Scholar | PubMed |
Norsworthy JK, Varanasi VK, Bagavathiannan M, Brabham C (2021) Recurrent selection with sub-lethal doses of mesotrione reduces sensitivity in Amaranthus palmeri. Plants 10, 1293.
| Crossref | Google Scholar | PubMed |
Paiva AM, Vanderwall DE, Blanchard JS, Kozarich JW, Williamson JM, Kelly TM (2001) Inhibitors of dihydrodipicolinate reductase, a key enzyme of the diaminopimelate pathway of Mycobacterium tuberculosis. Biochimica et Biophysica Acta - Protein Structure and Molecular Enzymology 1545, 67-77.
| Crossref | Google Scholar |
Peverelli MG, Soares da Costa TP, Kirby N, Perugini MA (2016) Dimerization of bacterial diaminopimelate decarboxylase is essential for catalysis. The Journal of Biological Chemistry 291, 9785-9795.
| Crossref | Google Scholar | PubMed |
Pillai B, Moorthie VA, van Belkum MJ, Marcus SL, Cherney MM, Diaper CM, Vederas JC, James MNG (2009) Crystal structure of diaminopimelate epimerase from Arabidopsis thaliana, an amino acid racemase critical for l-lysine biosynthesis. Journal of Molecular Biology 385, 580-594.
| Crossref | Google Scholar | PubMed |
Pote S, Pye SE, Sheahan TE, Gawlicka-Chruszcz A, Majorek KA, Chruszcz M (2018) 4-hydroxy-tetrahydrodipicolinate reductase from Neisseria gonorrhoeae - structure and interactions with coenzymes and substrate analog. Biochemical and Biophysical Research Communications 503, 1993-1999.
| Crossref | Google Scholar | PubMed |
Reddy SG, Sacchettini JC, Blanchard JS (1995) Expression, purification, and characterization of Escherichia coli dihydrodipicolinate reductase. Biochemistry 34, 3492-3501.
| Crossref | Google Scholar | PubMed |
Scapin G, Reddy SG, Zheng R, Blanchard JS (1997) Three-dimensional structure of Escherichia coli dihydrodipicolinate reductase in complex with NADH and the inhibitor 2,6-pyridinedicarboxylate. Biochemistry 36, 15081-15088.
| Crossref | Google Scholar | PubMed |
Schneider G, Käck H, Lindqvist Y (2000) The manifold of vitamin B6 dependent enzymes. Structure 8, R1-R6.
| Crossref | Google Scholar | PubMed |
Shaul O, Galili G (1992) Increased lysine synthesis in tobacco plants that express high levels of bacterial dihydrodipicolinate synthase in their chloroplasts. Plant Journal 2, 203-209.
| Crossref | Google Scholar |
Soares da Costa TP, Muscroft-Taylor AC, Dobson RCJ, Devenish SRA, Jameson GB, Gerrard JA (2010) How essential is the ‘essential’ active-site lysine in dihydrodipicolinate synthase? Biochimie 92, 837-845.
| Crossref | Google Scholar | PubMed |
Soares da Costa TP, Desbois S, Dogovski C, Gorman MA, Ketaren NE, Paxman JJ, Siddiqui T, Zammit LM, Abbott BM, Robins-Browne RM, Parker MW, Jameson GB, Hall NE, Panjikar S, Perugini MA (2016) Structural determinants defining the allosteric inhibition of an essential antibiotic target. Structure 24, 1282-1291.
| Crossref | Google Scholar | PubMed |
Soares da Costa TP, Abbott BM, Gendall AR, Panjikar S, Perugini MA (2018) Molecular evolution of an oligomeric biocatalyst functioning in lysine biosynthesis. Biophysical Reviews 10, 153-162.
| Crossref | Google Scholar | PubMed |
Soares da Costa TP, Hall CJ, Panjikar S, Wyllie JA, Christoff RM, Bayat S, Hulett MD, Abbott BM, Gendall AR, Perugini MA (2021) Towards novel herbicide modes of action by inhibiting lysine biosynthesis in plants. eLife 10, e69444.
| Crossref | Google Scholar | PubMed |
Sokalingam S, Raghunathan G, Soundrarajan N, Lee SG (2012) A study on the effect of surface lysine to arginine mutagenesis on protein stability and structure using green fluorescent protein. PLoS ONE 7, e40410.
| Crossref | Google Scholar | PubMed |
Song JT, Lu H, Greenberg JT (2004) Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and death2 and AGD2-LIKE DEFENSE RESPONSE PROTEIN1, encoding novel aminotransferases. Plant Cell 16, 353-366.
| Crossref | Google Scholar | PubMed |
Sparks TC, Lorsbach BA (2017) Perspectives on the agrochemical industry and agrochemical discovery. Pest Management Science 73, 672-677.
| Crossref | Google Scholar | PubMed |
Swanton CJ, Mashhadi HR, Solomon KR, Afifi MM, Duke SO (2011) Similarities between the discovery and regulation of pharmaceuticals and pesticides: in support of a better understanding of the risks and benefits of each. Pest Management Science 67, 790-797.
| Crossref | Google Scholar | PubMed |
Tamás C, Kisgyörgy BN, Rakszegi M, Wilkinson MD, Yang M-S, Láng L, Tamás L, Bedő Z (2009) Transgenic approach to improve wheat (Triticum aestivum L.) nutritional quality. Plant Cell Reports 28, 1085-1094.
| Crossref | Google Scholar | PubMed |
Torruella G, Suga H, Riutort M, Peretó J, Ruiz-Trillo I (2009) The evolutionary history of lysine biosynthesis pathways within eukaryotes. Journal of Molecular Evolution 69, 240-248.
| Crossref | Google Scholar | PubMed |
Tyagi VV, Henke RR, Farkas WR (1983) Partial purification and characterization of dihydrodipicolinic acid reductase from maize. Plant Physiology 73, 687-691.
| Crossref | Google Scholar | PubMed |
Velasco AM, Leguina JI, Lazcano A (2002) Molecular evolution of the lysine biosynthetic pathways. Journal of Molecular Evolution 55, 445-449.
| Crossref | Google Scholar | PubMed |
Wallsgrove RM, Mazelis M (1981) Spinach leaf dihydrodipicolinate synthase: partial purification and characterization. Phytochemistry 20, 2651-2655.
| Crossref | Google Scholar |
Wan C, Gao L, Wang J, Lei X, Tao J, Feng B, Gao J (2023) Effects of nitrogen fertilizer on protein synthesis, accumulation, and physicochemical properties in common buckwheat. The Crop Journal 11, 941-950.
| Crossref | Google Scholar |
Wang G, Xu M, Wang W, Galili G (2017) Fortifying horticultural crops with essential amino acids: a review. International Journal of Molecular Sciences 18, 1306.
| Crossref | Google Scholar | PubMed |
Wang W, Niu S, Dai Y, Wang M, Li Y, Yang W, Zhao D (2019) The Zea mays mutants opaque2 and opaque16 disclose lysine change in waxy maize as revealed by RNA-Seq. Scientific Reports 9, 12265.
| Crossref | Google Scholar | PubMed |
Watanabe N, Cherney MM, van Belkum MJ, Marcus SL, Flegel MD, Clay MD, Deyholos MK, Vederas JC, James MNG (2007) Crystal structure of LL-diaminopimelate aminotransferase from Arabidopsis thaliana: a recently discovered enzyme in the biosynthesis of L-lysine by plants and Chlamydia. Journal of Molecular Biology 371, 685-702.
| Crossref | Google Scholar | PubMed |
Watanabe N, Clay MD, van Belkum MJ, Cherney MM, Vederas JC, James MNG (2008) Mechanism of substrate recognition and PLP-induced conformational changes in LL-diaminopimelate aminotransferase from Arabidopsis thaliana. Journal of Molecular Biology 384, 1314-1329.
| Crossref | Google Scholar | PubMed |
Watanabe N, Clay MD, van Belkum MJ, Fan C, Vederas JC, James MNG (2011) The structure of ll-diaminopimelate aminotransferase from Chlamydia trachomatis: implications for its broad substrate specificity. Journal of Molecular Biology 411, 649-660.
| Crossref | Google Scholar | PubMed |
Watkin SAJ, Keown JR, Richards E, Goldstone DC, Devenish SRA, Grant Pearce F (2018) Plant DHDPR forms a dimer with unique secondary structure features that preclude higher-order assembly. Biochemical Journal 475, 137-150.
| Crossref | Google Scholar | PubMed |
Wong HW, Liu Q, Sun SSM (2015) Biofortification of rice with lysine using endogenous histones. Plant Molecular Biology 87, 235-248.
| Crossref | Google Scholar | PubMed |
Wu Z, Connolly J, Biggar KK (2017) Beyond histones – the expanding roles of protein lysine methylation. FEBS Journal 284, 2732-2744.
| Crossref | Google Scholar | PubMed |
Yang Q, Zhao D, Liu Q (2020) Connections between amino acid metabolisms in plants: lysine as an example. Frontiers in Plant Science 11, 928.
| Crossref | Google Scholar |
Yang QQ, Yu WH, Wu HY, Zhang CQ, Sun SSM, Liu QQ (2021) Lysine biofortification in rice by modulating feedback inhibition of aspartate kinase and dihydrodipicolinate synthase. Plant Biotechnology Journal 19, 490-501.
| Crossref | Google Scholar | PubMed |
Yang Q, Zhao D, Zhang C, Sreenivasulu N, Sun SS-M, Liu Q (2022) Lysine biofortification of crops to promote sustained human health in the 21st century. Journal of Experimental Botany 73, 1258-1267.
| Crossref | Google Scholar | PubMed |
Zhu X, Galili G (2003) Increased lysine synthesis coupled with a knockout of its catabolism synergistically boosts lysine content and also transregulates the metabolism of other amino acids in Arabidopsis seeds. Plant Cell 15, 845-853.
| Crossref | Google Scholar | PubMed |