Salvaging and replanting 300 mangrove trees and saplings in the arid Arabian Gulf
Paul L. A. Erftemeijer A D , Brae A. Price
A D , Brae A. Price  B , Satoshi Ito C , Hiroshi Yamamoto C , Titus Agastian C and Marion L. Cambridge A
B , Satoshi Ito C , Hiroshi Yamamoto C , Titus Agastian C and Marion L. Cambridge A
A School of Biological Sciences and UWA Oceans Institute, University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
B School of Molecular and Life Sciences, Curtin University, 208 Kent Street, Bentley, WA 6102, Australia.
C Abu Dhabi Oil Co. Ltd (Japan), PO Box 630, Abu Dhabi, United Arab Emirates.
D Corresponding author. Email: paul.erftemeijer@uwa.edu.au
Marine and Freshwater Research 72(11) 1577-1587 https://doi.org/10.1071/MF20381
Submitted: 29 December 2020 Accepted: 17 June 2021 Published: 23 July 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Construction works along a causeway at Mubarraz Island near Abu Dhabi, United Arab Emirates, in the Arabian Gulf necessitated the salvaging and replanting of 300 mangroves (Avicennia marina). Mangroves were excavated manually by shovel (smaller trees and saplings) or mechanically using a backhoe excavator (larger trees), transported with the root ball wrapped in burlap and replanted in a newly created tidal channel. Relocated mangroves were exposed to two different watering regimes and two tidal inundation levels, and were monitored for survival, plant height and leaf health (percentage of green leaves) at 0 and 1 weeks, and then at 3, 7 and 12.5 months. Tree mortality was high in the first week (24%) in mid-summer, with further losses (43%) during the next 3 months. After 12.5 months, 31% of the transplanted trees had survived. There was a significant effect of the duration of tidal inundation on survival and leaf health (higher survival in shallow than deeper plots). There were no significant effects of initial tree height or freshwater treatment on survival or plant performance. These results demonstrate that salvaging of larger mangroves is technically feasible, potentially providing faster ecosystem services (e.g. shoreline protection, source of new propagules) than newly planted seedlings in arid regions where growth is extremely slow.
Keywords: Avicennia marina, mangrove relocation, root damage, tidal inundation, tree salvaging.
Introduction
Marine and coastal habitats and their associated species are under increasing pressure from anthropogenic developments worldwide, including in the Arabian Gulf (Sheppard et al. 2010; Erftemeijer and Shuail 2012; Milani 2018). The ongoing and cumulative effects of coastal construction, extractive resource exploitation and urban and industrial expansion have resulted in the significant loss of coastal habitats, particularly mangroves (Halpern et al. 2008; Polidoro et al. 2010). In response, the past two decades have seen increasing efforts to prevent such impacts through improved selection of construction sites to minimise habitat losses (Gilman 2002), the conservation of critical areas, environmental impact assessments (Naser 2015) and the restoration of damaged areas (Lewis and Streever 2000; Bayraktarov et al. 2016). Salvaging critical and sensitive marine habitats by physical relocation is often seen as a last resort when destruction cannot be avoided (Arlidge et al. 2018). Published cases have highlighted the ability to translocate coastal habitat successfully, but these are few and often small scale (but see Gayle et al. 2005; Rodgers et al. 2017; Park et al. 2018). Reviews of plant translocations suggest that these are relatively high risk, high cost and challenging but, if successful, can reduce the time required for plants to mature and produce viable seeds as a source of second-generation recruitment (Silcock et al. 2019).
Although there are many reports on transplanting nursery-reared mangrove seedlings, there are only three previously published case studies on the relocation of mangrove trees. Pulver (1976; also reviewed in Snedaker and Biber 1996) reported on the successful translocation of 120 mangrove trees (0.5–1.5 m high, comprising three species) in Florida (USA), describing transplanting techniques, the importance of root ball preservation and the beneficial effects of pruning. Pulver (1976) also describes two earlier attempts to excavate (with a crane and backhoe) six Rhizophora mangle trees in Dade County, Florida (five of which were still surviving after 2 years) and the transplanting of ‘some’ Avicennia germinans trees near Marco Island, with 33% survival. Saenger (1996) documented the transplanting of mangrove propagules, seedlings and small shrubs and trees to compensate for the loss of mangroves due to construction works associated with the expansion of Brisbane Airport in subtropical Queensland, Australia. Survival after outplanting was inversely proportional to plant size, with low survival of larger plants >50 cm tall. Abbot and Marohasy (2014) reported on the excavation of ten 7-year old Avicennia marina trees (up to 1.8 m high) in Queensland, Australia, and their subsequent successful cultivation in containers with an automated irrigation system for experimental research. Eight of the ten translocated trees survived under these conditions for over 2 years.
Although these case studies of mangrove trees included valuable information for designing the present study, they were all conducted under ideal humid tropical conditions, so that their findings were not necessarily applicable to salvaging and replanting mangroves in the environmentally extreme Arabian Gulf. Erftemeijer et al. (2020) reported on the long-term restoration of mangroves on Mubarraz Island in the Arabian Gulf, where performance and survival proved to be limited by exceptionally harsh conditions, particularly high salinity, wide temperature variation, extremely low rainfall and nutrient-poor calcareous soils (Al-Habshi et al. 2007). The mangrove restoration program involved planting of ~500 000 A. marina seedlings over 30 years, enabling documentation of methods to improve success, first with wild-collected propagules and then with nursery-reared seedlings, thus providing background on appropriate habitat conditions (site preparation, soil amendments, duration of tidal inundation) for the present study on tree relocation. Seminal work by Lewis (2005) also emphasised the fundamental importance of ensuring appropriate tidal flooding depth above mean sea level (MSL) and duration (inundated <30% of the time) if success were to be achieved at any mangrove restoration site. Recent research demonstrated a beneficial effect of freshwater treatment (spraying of leaves) on the growth of A. marina trees at arid sites, further contributing to treatments with potential for enhancing the survival of relocated mangrove trees in extremely harsh, arid environments (Steppe et al. 2018; Fuenzalida et al. 2019).
Construction works along a causeway at Mubarraz Island, an offshore site in the United Arab Emirates off Abu Dhabi, necessitated the relocation of >300 mangrove trees and saplings (A. marina) that had been planted 5–9 years ago as nursery-reared seedlings (Erftemeijer et al. 2020). The relocation was not a legal requirement, but an example of corporate citizenship on the part of the oil company. Despite their age, these trees had only achieved heights of up to 1.5 m, an indication of the very slow growth rates in such an extreme climate. Here we describe the results of this large-scale mangrove relocation project (undertaken in September 2019), which provided an opportunity to examine the effects of some environmental and biological variables on the success of the relocation.
The objectives of this study were to: (1) assess the feasibility of large-scale mangrove relocation and evaluate the benefits of salvaging larger trees compared with the planting of new seedlings; and (2) determine the effects of environmental conditions at the recipient site on the survival and performance of the salvaged trees and saplings. The hypotheses were that: (1) the survival of relocated mature mangrove trees would be similar to that of transplanted seedlings, but greater benefits would be conferred owing to their larger size; (2) the survival, growth (as an increase in height) and leaf health would be less for relocated trees than control mangroves at the same site; and (3) the survival, growth and leaf health of relocated trees would increase with watering (fresh water addition), decrease with initial tree size and decrease with longer tidal inundation (greater bed depth).
Materials and methods
Study site
Mubarraz Island is located in the Arabian Gulf, some 100 km north-west of Abu Dhabi, United Arab Emirates. The island is surrounded by a large sand shoal (up to 5 m deep) with extensive seagrass meadows and fringing coral reefs. The Mubarraz shoal features several oil fields (mostly situated in the northern parts of the shoal) that are under concession by the Japanese oil company Abu Dhabi Oil Co. (Japan) Ltd (ADOC). Typically for the arid Arabian Gulf region, environmental conditions are extreme, with water temperatures varying from 17 to 36°C and salinity ranging from 40 to 48. Water levels are governed by a diurnal tide, with a maximum tidal amplitude of 2.3 m. During 1983–85, a channel was dredged across the shoal to facilitate navigational access. The dredged material was used for the construction of a 17-km causeway that protects several oil pipelines and power cables and serves as a road connection between the oil production areas and the main island. Mass planting of ~500 000 nursery-reared mangrove A. marina seedlings along the causeway and island over the past 30 years resulted in the successful establishment of mangrove vegetation along 6.7 km of shoreline, covering an area of 16.5 ha (Erftemeijer et al. 2020).
Site selection
Approximately 300 m of existing mangrove vegetation (planted by ADOC several years prior) had to be removed at two sites along the causeway shoreline to make way for the construction of culvert structures. A new site was created elsewhere along the causeway to translocate the excavated mangrove trees (Fig. 1). The new site consisted of a tidal channel ~200 m long and 6 m wide, excavated parallel to the causeway, as in previous mangrove planting programs at Mubarraz (see Erftemeijer et al. 2020).
Baseline assessment
All mangrove saplings and trees to be removed were counted and their height and number of pneumatophores measured before relocation. Three randomly selected small trees were manually excavated to investigate the depth penetration and horizontal spread of their root systems and to establish a relationship between tree size and pneumatophore abundance.
Site preparations
At the relocation site, bed levels were adjusted (during low tide) with the use of a CAT 966G front loader with a bucket width of 3 m to create the appropriate tidal inundation characteristics conducive to mangrove growth (van Loon et al. 2016). Large limestone rocks were removed from the channel bed before replanting. To allow testing of the relative effect of tidal inundation on the survival and growth of relocated mangroves, two sections were established within the channel that differed in bed depth (shallow, +50 cm above MSL; deep, +30 cm above MSL), corresponding to mean tidal inundations of ~3 and 6 h day–1 respectively. These levels approximated the lower and upper ends of the locally established, site-specific inundation tolerance limits of the mangroves at Mubarraz, based on a detailed hydrological study (DAMCO Consulting 2020; Erftemeijer et al. 2021).
Excavation methods
Two excavation methods were selected for the relocation works (Fig. 1a, b), based on previous literature, an initial investigation of tree sizes to be moved and logistical and environmental constraints, including an assessment of available equipment and staff at Mubarraz, namely manual and mechanical excavation.
Manual excavation, with shovels, was used for the excavation at low tide (Fig. 1a) of smaller saplings up to a height of ~60 cm that had not yet developed extensive cable root systems with pneumatophores. Because of the hardness of the soil (consisting of a mixture of rocks, compacted coral rubble and coarse sand), a pickaxe sometimes had to be used to help loosen the soil around the trees. Given the harsh climatic conditions at the time of the relocation works (with daily air temperatures typically over 40°C), a maximum of 40 saplings could be removed and replanted with this method per day, using a team of 4 people.
Mechanical excavation using an excavator at low tide (backhoe; bucket width 75 cm; Fig. 1b) worked particularly well for the larger trees (from >60 cm up to 2.1 m). The excavator was used to first cut away a square section of root ball, measuring ~70 cm in width. The large excavator was carefully guided by staff standing nearby, who sometimes helped in the excavation with shovels. Care was taken to minimise root damage as much as possible, and secateurs were used to prune exposed and damaged roots where feasible. The root balls of excavated trees, which included the roots and surrounding soil, were wrapped in hessian burlap (Fig. 1c) or plastic. The diameter of the root balls ranged from 25 to 65 cm (mean ± s.e., 38 ± 9 cm; n = 36) depending on the size of the tree.
Special care was taken to try to minimise damage to the root systems of the mangroves during excavation, but this was nearly impossible to avoid, especially for larger trees. The soil in the channels often consisted of predominantly coarse friable material, causing some of the root balls to ‘collapse’ upon excavation, making it difficult to keep the soil and roots together in an intact root ball during excavation.
Relocation and transplanting
Excavated mangroves trees were wrapped in shade cloth to minimise water loss from excessive evapotranspiration, transported by front loader (Fig. 1d) to the new site and then replanted at the new site (at low tide) within 2 h of excavation. Prior to replanting, individual holes were dug by shovel to a depth similar to the size of the root ball and ~200–300 g of peat moss was added to each planting hole. The mangrove trees and saplings were replanted at the same depth as in their original position while making sure that their pneumatophores (if any) were not buried. All plantings were given 6–8 L of fresh water (approximately one bucket per tree) immediately after replanting.
Pruning
To compensate for the loss of root mass, larger-sized trees (>70 cm) were moderately pruned upon transplanting in an effort to avoid excessive water loss through evapotranspiration but taking care not to affect plant height. This reduced the leaf canopy by ~20–30%, following recommendations by Pulver (1976).
Watering
All mangroves were subsequently watered daily (during low tide) with fresh water for the next 7 months. Mangroves in separate experimental plots were given two types of freshwater treatment, either ~2 or ~6 L of fresh water per tree. This differential treatment allowed for testing of the effect of fresh water addition on the survival and growth of the relocated mangroves. Although these volumes (2 and 6 L) were arbitrary (there are no guidelines for the watering of mangroves), they are in the same order of magnitude (m–2) as water volumes commonly applied in the irrigation of agricultural crops and trees (see the Australian irrigation calculator, www.agric.wa.gov.au). The freshwater treatment was applied by spraying the plots with a hose from a water tanker equipped with a metered pump, allowing for the application of approximate measures of watering volumes. Freshwater treatments were continued daily for 7 months after the relocation and then discontinued due to pump breakdown. All plots in the channel (and controls) were also inundated naturally by seawater for several hours during high tides every day.
Experimental design
Relocated mangroves were transplanted in the newly excavated tidal channel according to a randomised block design, with eight plots representing two treatment levels for tidal inundation (bed depth high or low) and two treatment levels for watering regime (daily freshwater treatment 6 or 2 L). This resulted in four treatment combinations (High 6 L, High 2 L, Low 6 L and Low 2 L), each of which was replicated twice within the channel. Excavated trees and saplings of varying sizes were transplanted randomly at a spacing of 1 m across all plots, with a total of 40 mangroves (10 rows of 4) in each plot (except Plots 7 and 8, which only had 32 and 28 seedlings respectively due to insufficient mangroves available for transplanting after relocation). Two control plots along the causeway each consisted of 40 mangroves that were not excavated but had been planted during the same period as those selected for relocation (i.e. 5–9 years earlier). In these control plots, peat moss had also been added with the initial plantings. Controls were monitored for the full duration of the experiment. All mangroves in the experimental and control plots were monitored for survival, plant height and leaf health (% green) at t = 0 (shortly before the relocation) and then at 1 week (i.e. immediately upon completion of the relocation) and 3, 7 and 12.5 months after relocation. Plant height was assessed to the nearest millimetre using a rule measuring from the sediment surface to the top of the tallest branch. The increment in mean height (averaged per plot) between consecutive monitoring events was calculated and taken as proxy for growth. Leaf colour (expressed as a percentage of all leaves on a tree or sapling that were green as opposed to yellow, brown or black) was assessed by visual estimation and used as a metric for leaf health and senescence, in accordance with Benner et al. (1990) and Duke et al. (2005). Trees and saplings that had no leaves (or on which all leaves were black and withered) were presumed to be dead, which served as a metric to determine survival. At the start of the monitoring, we tested for observer bias by repeating monitoring assessments of the same plots by different observers and found a maximum variability of 5% in leaf health estimates (and <2% in plant height measurements) between observers, with no significant difference in the overall assessment results per plot between observers.
Data analysis
Statistical analyses were completed using PRIMER software and the PERMANOVA+ add on to PRIMER (ver. 7, see https://www.primer-e.com/, accessed March 2021; Anderson et al. 2008). A univariate permutational multivariate analysis of variance (PERMANOVA) was completed to test for significant (P < 0.05) differences within plant height, plant survival and leaf health (percentage green) between each treatment by using a three-factor design: (1) Treatment: fixed, five levels (High 6 L, High 2 L, Low 6 L, Low 2 L and Control); (2) Time: fixed, four levels (1 week and 3, 7 and 12.5 months); and (3) Plot: nested within treatment, random, 10 levels (Control Plots 1 and 2 and Experimental Plots 1–8). Additional univariate PERMANOVAs were completed to assess for the effect of plant height, watering regime and tidal inundation on leaf health and plant survival. Plant height was tested using a four-factor design: (1) Treatment: fixed, five levels (High 6 L, High 2 L, Low 6 L, Low 2 L and Control); (2) Plant height: fixed, four levels (0–30, 31–60, 61–90 and >91 cm); (3) Time: fixed, four levels (1 week and 3, 7 and 12.5 months); and (4) Plot: nested within treatment, random, 10 levels (Control Plots 1 and 2 and Experimental Plots 1–8). Bed depth and watering regime were grouped within Treatment in plant height analysis for within and between Plot comparisons.
The effect of watering regime and tidal inundation was tested using four-factor design: (1) Bed depth: fixed, two levels (high and low); (2) Watering regime: fixed, two levels (6 and 2 L); (3) Time: fixed, four levels (1 week and 3, 7 and 12.5 months); and (4) Plot: nested within watering regime and bed depth, random, eight levels (Experimental Plots 1–8). Analyses to determine the effects of tidal inundation (bed depth) and watering regime were treated as individual factors to determine whether each treatment independently affected leaf health or plant survival. All designs including the nested variable plot were used to account for the repeated measurements of the same plants throughout the experiment. PERMANOVA comparisons with 9999 permutations were completed on all plant variables (Anderson et al. 2008) using a Euclidean distance dissimilarity matrix. Pairwise post hoc tests were used to explore significant (P < 0.05) interactions or differences obtained from the PERMANOVA. When there were insufficient permutations (<100) to conduct a rigorous statistical test, Monte Carlo bootstrapping was used to obtain a suitable P-value (PMC; Anderson et al. 2008). We chose to use PERMANOVAs because they are less susceptible to deviations from the assumptions that underlie parametric approaches in repeated-measures analyses of variance (ANOVAs). Our data had considerable variation between and within treatments due to mortality from transplanting that violated sphericity assumptions of a repeated-measures ANOVA. However, PERMANOVAs are largely unaffected by the correlation structure that is associated with sphericity (Anderson and Walsh 2013). The strength of the correlation between the number of pneumatophores per tree and their maximum distance from the stem was explored against mangrove height using regression analyses in RStudio (ver. 4.0.0, see https://rstudio.com/, accessed March 2021). All figures were produced using RStudio. Full statistical results are presented in the Supplementary material (Tables S1–S9).
Results
Baseline assessment of mangrove trees before relocation
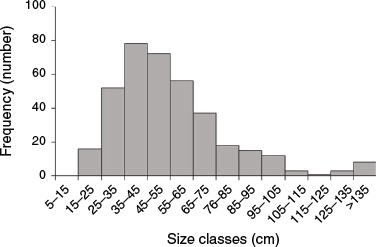
In all, 300 mangroves were selected for relocation from the two construction sites (Fig. 1f), consisting of small trees and saplings, with a mean (±s.e.) height of 54.3 ± 15.0 cm (maximum 210 cm). Only 17 trees were taller than 1 m, and most (n = 204) were smaller than 55 cm (Fig. 2). The small size of these mangroves, which had been planted during multiple planting campaigns over the past 9 years, is typical of both natural and planted mangrove stands in the arid Arabian Gulf region (Al-Khayat and Balakrishnan 2014; Erftemeijer et al. 2020). Trees and saplings for relocation were similar in size to those in the two control plots, with comparable size frequency distribution at the start of the relocation program (Fig. 2). Mean tree height differed between the two control plots (69 v. 39 cm), but was within the range of mean tree heights (42–76 cm) recorded in the treatment plots immediately after transplanting (t = 0). Mean pneumatophore abundance ranged from 0.1 to 15.6 per tree at the control plots and from 0.2 to 6.6 per tree (after transplanting) at the relocation plots.
|
Root systems
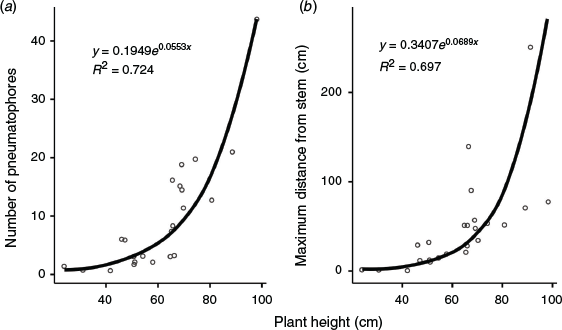
The root systems of the trees and saplings rarely extended beyond ~1 m from the stem and did not generally penetrate much deeper than 30 cm. There was a strong exponential relationship between lateral extension (R2 = 0.697) and number of pneumatophores (R2 = 0.724) with the size of the tree (Fig. 3). Mangroves smaller than 60 cm generally had few or no pneumatophores, but the number of pneumatophores increased markedly with tree size for mangroves taller than 60 cm. The maximum distance of pneumatophores from the stem was generally not more than tree height in the trees selected for relocation. Pneumatophore height ranged from a mean (±s.e.) of 12.5 ± 7.4 cm for smaller trees (n = 14) to 23.8 ± 9.8 cm (n = 17; overall mean 18.9 ± 10.4 cm).
|
Tree survival
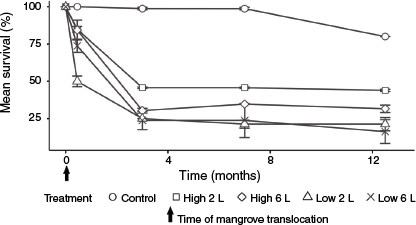
The survival of mangrove trees and saplings was significantly less in experimental than control plots (P < 0.05), indicating relocation mortality (Table S1). The survival of mangroves showed a relatively consistent response to relocation across all plots, with an initial mean loss of 24% following excavation and transplantation (Week 1) and a further loss of 43% during the first 3 months after transplantation (Fig. 4). Mean survival stabilised at ~33% at 3 months after transplantation, and remained stable over the subsequent months, with 31% of the trees surviving after 12.5 months. After 7 months, only low levels of ‘new’ mortality were recorded in the treatment plots over time until the end of the experiment, similar to the levels of mortality observed in the control plots (Table S2). Survival of mangroves in shallow tidal plots (41%) was significantly (P < 0.05) greater than in deep plots (19%) (Tables S3, S4). There was no significant effect of initial plant height or watering regime on the survival of the relocated trees.
|
Leaf health
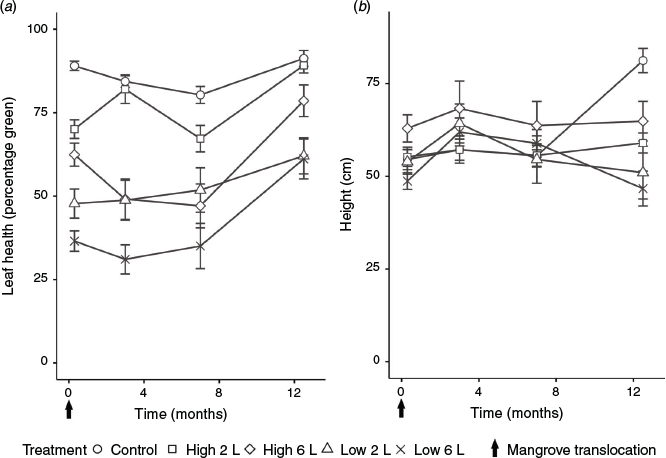
A significant interaction between treatment and time was found in the leaf health of mangroves (Table S5). Mangroves in all experimental plots (except the High 6-L treatment) showed a significant decrease in the percentage of green leaves (P < 0.05) compared with the control plots immediately after transplanting, indicating stress associated with relocation (Fig. 5a; Table S6). The percentage of green leaves (indicative of the health of the surviving trees and saplings) increased during the last 5 months in all plots (Fig. 5a; Table S6). Initial plant height did not have a significant effect on the leaf health of the relocated trees (Table S5). However, there was significant variation in the leaf health of mangroves between and within different size classes and treatments (Tables S7, S8). Most notably, mangrove leaf health was significantly lower in Low 6-L treatment plots for every size class (0–30, 31–60, 61–90 and ≥91 cm), and in the High 2-L and High 6-L treatment plots for trees >91 cm compared with control plots (Tables S8, S9). There was a significant (P < 0.05) effect of duration of tidal inundation on leaf health, with the leaf health of mangroves in shallow tidal plots (34%), being significantly greater than that of mangroves in deep plots (12%; Tables S3, S4).
|
Tree height and growth
At the start of the monitoring period immediately after transplanting (and in some cases pruning), mean (±s.e.) tree height in the experimental plots was comparable to that of the control plots (55.8 ± 0.7 v. 54.5 ± 2.7 cm respectively). Trees in the control plots showed a mean height increase of ~20 cm year–1. Growth in the control plots showed marked seasonality (Fig. 6), with substantial growth during summer months but none during the cold winter months. Trees in the treatment plots did not show any significant mean height increase throughout the experiment. The height increase observed in trees in the treatment plots was variable, whereas the mean height of trees in five of the treatment plots decreased by up to 19 cm, possibly as a result of the death of some of the larger trees in these plots (Fig. 5b).
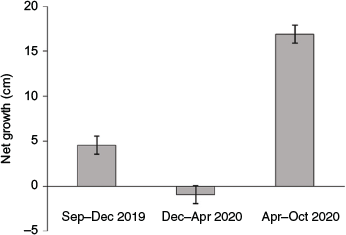
|
The overall height distribution of relocated mangroves (regardless of treatment) was similar to that of trees in the control plots during the first 7 months (Fig. 7). At the end of the 12.5-month monitoring period, the mean height of mangroves in the control plots had increased substantially due to summer growth, whereas there was no increase in the height of mangroves in the treatment plots (Fig. 7). Variations in the initial plant height had no significant effect on the growth of relocated trees.
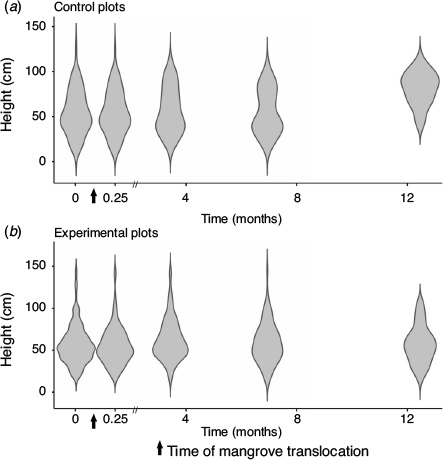
|
The abundance of pneumatophores in Control Plot 1 increased from a mean (±s.e.) of 15.6 ± 3.7 per tree at the start of the monitoring (t = 0) to 30.2 ± 7.1 per tree after 12.5 months. In all other plots, pneumatophore numbers were low (generally less than six per tree, and many with none, depending on size) and remained low until the end of the 12.5 months with no apparent effect of any of the treatments. No substantial flowering or fruiting was observed in any of the plots over the entire monitoring period, with only a few trees bearing fruits (up to a maximum of 3 of 40 trees (8%) in Control Plot 1 at the onset of monitoring).
Discussion
This study investigated the feasibility of excavating and relocating mature mangrove trees and saplings during a salvage operation at an offshore island site in the Arabian Gulf. By comparing the results of the present study with those of a previously published evaluation of three decades of mangrove planting efforts using seedlings at the same site (Erftemeijer et al. 2020), we were able to consider the relative success, costs and benefits of salvaging large trees compared with the planting of new seedlings. Our results showed that when relocating mature mangrove trees, minimisation of root damage during excavation and ensuring appropriate tidal inundation at the recipient site were the most important factors determining survival, whereas the effects of initial tree size and freshwater treatment were not significant.
The outcome of this study demonstrated that large-scale relocation of mangrove trees is practically feasible, and that salvaging and replanting larger trees can be advantageous over the planting of new seedlings. Although the overall costs of the tree relocation were not dissimilar to those of the conventional planting of nursery-reared seedlings, the replanting of salvaged trees conferred greater benefits in terms of enhanced survival and faster provision of ecosystem services owing to their larger size.
The overall survival of relocated A. marina mangroves (31% after 1 year) was higher than the long-term survival (26%) of nursery-reared mangrove seedlings in earlier plantings at this same location (Erftemeijer et al. 2020) but relatively low compared with survival rates (<50–90%) reported from other geographic areas (Pulver 1976; Saenger 1996; Abbot and Marohasy 2014). The low survival observed in the present study is attributed to the environmentally extreme conditions in the Arabian Gulf region, further exacerbated by the hot summer conditions at the time of relocation (September 2019), with daily air temperatures up to ~48°C, which is likely to have further increased the water stress experienced by the trees and saplings during relocation.
The overall success of any mangrove relocation will be a function of multiple factors, related to both the method of excavation and replanting, as well as site conditions at the new transplanting site and other environmental and climatological constraints. Our results suggest that the following factors are particularly critical to ensure success: minimising root damage upon excavation, avoiding the extreme temperatures of summer (particularly in extreme climates such as the Gulf region) and ensuring optimal site suitability at the destination site (tidal hydrology, soil conditions).
Tidal hydrology, in particular the mean duration of inundation during high tide, is known to be an important variable determining site suitability for mangroves (Choy and Booth 1994; van Loon et al. 2016; Alsumaiti and Shahid 2019). Although the response of A. marina and other mangrove species to waterlogging is generally well understood (Kozlowski 1997; van Loon et al. 2007, 2016), their specific tolerance thresholds to the duration of tidal inundation may reflect site-specific factors (Friess et al. 2012). The effect of tidal inundation on the health and survival of the transplants in the present study is comparable to observations by Gorman and Turra (2016), who found differences in survival for mangrove seedlings established at sheltered v. exposed locations that reflected differences in tidal height and sediment grain size. Soil conditions are also known to be important, with particle size distribution (in particular the proportion of the coarse sand fraction), nutrient availability, the occurrence of an anaerobic layer in the profile, and surface layer salinity influencing both the establishment and growth of mangrove plants (Duarte et al. 1998; Bhat and Suleiman 2004).
Because field measurements of root biomass in mangroves are both labour intensive and difficult, there is considerable uncertainty regarding estimates of below-ground biomass in mangroves (Njana et al. 2015; Adame et al. 2017). Published data further suggest that there is substantial variation in root biomass in relation to environmental conditions (Adame et al. 2017). It is therefore difficult to give a reliable estimate of the extent of root loss as a result of the excavation of the mangrove trees and saplings for the present relocation. However, a considerable proportion (~34%) of the total below-ground biomass in A. marina is stored in the root crown (Njana et al. 2015). Given that the root crown of the excavated trees was mostly intact and large quantities of cable roots with pneumatophores were excavated successfully in most cases, the damage and loss of root mass in our study is estimated to have been <60% for most trees (depending on their size), and much less for saplings.
Our results did not reveal a significant effect of initial height of the transplanted mangroves on their survival. However, Saenger (1996) reported the survival rates of relocated mangroves in Queensland to be inversely proportional to tree size, finding that for plants >50 cm in height, survival rates fell below 50% within 1 month. This is believed to be primarily related to the fact that it takes longer for larger transplanted trees to re-establish a root : shoot ratio comparable to non-transplanted trees (Watson 2005). Rapid regeneration of the root system is essential for successful re-establishment of any transplanted trees (Watson and Himelick 1982). Transplanting stress is a temporary condition of distress resulting from root injuries and depletion due to impaired function (a reduction in the acquisition and assimilation of water and essential minerals and expenditure of stored carbohydrates to regenerate new roots). Similarly, ‘transplant shock’ is largely due to stresses resulting from the removal of a substantial portion of the transplanted tree root systems, which creates a root : shoot imbalance (Watson 2005). Moderate canopy pruning upon transplanting may alleviate this imbalance and the associated transplant stress, even in mangrove trees (Pulver 1976). Regardless of pruning, in most trees it takes ~1 year for the root system to regenerate to an extent where the shoot : root ratios and pretransplant growth rates are restored (Watson 2005).
Freshwater treatment in the present study did not have a significant effect on the health and survival of relocated mangroves. In fact, some of the data even seem to suggest that a high dosage of (daily) fresh water may have had an adverse effect on plant survival and health or growth, although this difference was not significant due to high variability in the data. Our findings contrast with physiological studies of A. marina by Steppe et al. (2018) and Fuenzalida et al. (2019), who found that leaf wetting events allowing direct uptake of fresh water by the canopy during episodic rainfall can be important for A. marina, especially in arid areas where rehydration from the soil is limited. The growth of A. marina seedlings is known to be adversely affected by high salinity, showing higher growth at reduced salinities, with optimum growth and biomass observed around a salinity of ~15 (Patel et al. 2010; Nguyen et al. 2015; Santini et al. 2015). The mean height increase of trees (~20 cm year–1) measured in control plots in the present study are well within the range of growth rates reported for A. marina from other locations within the arid Arabian Gulf region (Bhat et al. 2004; Loughland et al. 2020). The slow growth (or even reduction in height) recorded in the experimental plots in the present study is attributed to the stress of excavation and transplantation, and the priority for the trees to first restore their severed below-ground root biomass following the relocation (sensu Watson and Himelick 1982).
Although the results of this study and some previous literature may suggest that relocation of mangrove trees and shrubs is technically feasible with reasonable survival rates, it is always preferable to avoid relocation in the first place. In this context, a common principle that is widely applied in environmental considerations of development projects is ‘mitigation hierarchy’ (avoid-minimise-remediate-offset) to guide development activities towards limiting negative effects on biodiversity (Arlidge et al. 2018). It would be wrong to think that mangrove trees can be easily moved and to use that as an excuse to approve developments that would result in widespread losses of mangroves rather than explore non-destructive alternatives. Salvaging should only be considered as a last resort, such as in situations where damaging effects on mangroves or other valuable habitats cannot be avoided, or the loss of threatened species is imminent (Silcock et al. 2019).
This also poses the question whether it would make more sense to plant new mangroves from nursery-raised seedlings (e.g. Loughland et al. 2020) rather than relocate the older trees and saplings. Does size matter? In laboratory studies in Queensland, under ideal tropical conditions mangrove propagules planted in laboratory pots outgrew well-developed saplings that had been translocated from the field into similar laboratory pots within 2 years (N. Duke, pers. comm.). However, in arid regions (including the Arabian Gulf), where soils are nutrient poor and mangroves are typically dwarfed and very slow growing (Naidoo 2009), it may take well over 10 years for the seedlings to reach heights comparable to natural mangrove stands (AboEl-Nil 2001; Ochieng and Erftemeijer 2002; Bhat et al. 2004; Almahasheer et al. 2016, Erftemeijer et al. 2018, 2020).
Some advantages of larger trees and saplings over seedlings may include that they are less likely to be uprooted and washed away, they offer a promise of greater and faster shoreline protection and they are more likely to rapidly serve as a source of propagules for natural recruitment and further replanting. All these should be given due consideration when evaluating the cost-effectiveness of relocation (older trees and saplings) v. new plantations (nursery-reared seedlings). Apart from some expert technical guidance (10 days) and the involvement of a backhoe excavator (5 days), the costs of relocating trees in this project were not really different from the costs of planting new seedlings because the same channel preparation, man-power and time investment were involved. It should be noted that access to heavy equipment is rarely a constraint within the context of larger construction projects, and the costs for relocation may constitute only a fraction of the overall project construction costs.
In conclusion, this study of relocating 300 mangroves in extreme environmental conditions highlighted two factors that were paramount to success: minimisation of root damage during excavation and ensuring site suitability at the replanting site, particularly the appropriate tidal hydrological conditions. Our findings further demonstrate that the salvaging of adult mangrove trees is worthwhile, especially because relocated larger trees may provide greater and faster ecosystem services, adding another viable option to the usual approaches of planting propagules and nursery-reared seedlings (Vanderklift et al. 2020) that are currently used across the globe to achieve marine coastal restoration (www.decadeonrestoration.org).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was funded by the Abu Dhabi Oil Co. (Japan) Ltd.
Acknowledgements
Many Abu Dhabi Oil Co. (Japan) Ltd (ADOC) staff in Abu Dhabi and at Mubarraz Island offered valuable support during the work. The authors extend their sincere thanks to Koji Ueno (current General Manager) and Hiroyuki Yamamoto (previous General Manager) of ADOC for their approval and support of this work. In addition, the authors thank the Mubarraz Marine Team and the Safety and Environment Team – Mubarraz Island for providing invaluable assistance and indispensable support during the relocation and monitoring work at Mubarraz Island. Permission for this publication was granted by the Abu Dhabi National Oil Co.
References
Abbot, J., and Marohasy, J. (2014). The excavation and cultivation in containers of mature grey mangroves, Avicennia marina. Wetlands Ecology and Management 22, 641–646.| The excavation and cultivation in containers of mature grey mangroves, Avicennia marina.Crossref | GoogleScholarGoogle Scholar |
AboEl-Nil, M. M. (2001). Growth and establishment of mangrove (Avicennia marina) on the coastlines of Kuwait. Wetlands Ecology and Management 9, 421–428.
| Growth and establishment of mangrove (Avicennia marina) on the coastlines of Kuwait.Crossref | GoogleScholarGoogle Scholar |
Adame, M. F., Sherian, S., Reef, R., and Stewart-Koster, B. (2017). Mangrove root biomass and the uncertainty of belowground carbon estimations. Forest Ecology and Management 403, 52–60.
| Mangrove root biomass and the uncertainty of belowground carbon estimations.Crossref | GoogleScholarGoogle Scholar |
Al-Habshi, A., Youssef, T., Aizpuru, M., and Blasco, F. (2007). New mangrove ecosystem data along the UAE coast using remote sensing. Aquatic Ecosystem Health & Management 10, 309–319.
| New mangrove ecosystem data along the UAE coast using remote sensing.Crossref | GoogleScholarGoogle Scholar |
Al-Khayat, J. A., and Balakrishnan, P. (2014). Avicennia marina around Qatar: tree, seedling and pneumatophore densities in natural and planted mangroves using remote sensing. International Journal of Sciences 3, 18–27.
Almahasheer, H., Duarte, C. M., and Irigoien, X. (2016). Phenology and growth dynamics of Avicennia marina in the Central Red Sea. Scientific Reports 6, 37785.
| Phenology and growth dynamics of Avicennia marina in the Central Red Sea.Crossref | GoogleScholarGoogle Scholar | 27892956PubMed |
Alsumaiti, T. S., and Shahid, S. A. (2019). Mangroves among most carbon-rich ecosystem living in hostile saline rich environment and mitigating climate change – a case of Abu Dhabi. Journal of Agricultural and Crop Research 7, 1–8.
| Mangroves among most carbon-rich ecosystem living in hostile saline rich environment and mitigating climate change – a case of Abu Dhabi.Crossref | GoogleScholarGoogle Scholar |
Anderson, M. J., and Walsh, D. C. I. (2013). PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecological Monographs 83, 557–574.
| PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing?Crossref | GoogleScholarGoogle Scholar |
Anderson, M., Gorley, R. N., and Clarke, R. K. (2008). ‘Permanova+ for Primer: Guide to Software and Statistical Methods.’ (PRIMER-e: Plymouth, UK.)
Arlidge, W. N. S., Bull, J. W., Addison, P. F. E., Burgass, M. J., Gianuca, D., Gorham, T. M., Jacob, C., Shumway, N., Sinclair, S. P., Watson, J. E. M., Wilcox, C., and Milner-Gulland, E. J. (2018). A Global Mitigation Hierarchy for Nature Conservation. Bioscience 68, 336–347.
| A Global Mitigation Hierarchy for Nature Conservation.Crossref | GoogleScholarGoogle Scholar |
Bayraktarov, E., Saunders, M. I., Abdullah, S., Mills, M., Beher, J., Possingham, H. P., Mumby, P. J., and Lovelock, C. E. (2016). The cost and feasibility of marine coastal restoration. Ecological Applications 26, 1055–1074.
| The cost and feasibility of marine coastal restoration.Crossref | GoogleScholarGoogle Scholar | 27509748PubMed |
Benner, R., Weliky, K., and Hedges, J. I. (1990). Early diagenesis of mangrove leaves in a tropical estuary: Molecular-level analyses of neutral sugars and lignin-derived phenols. Geochimica et Cosmochimica Acta 54, 1991–2001.
| Early diagenesis of mangrove leaves in a tropical estuary: Molecular-level analyses of neutral sugars and lignin-derived phenols.Crossref | GoogleScholarGoogle Scholar |
Bhat, N. R., and Suleiman, M. K. (2004). Classification of soils supporting mangrove plantation in Kuwait. Archives of Agronomy and Soil Science 50, 535–551.
| Classification of soils supporting mangrove plantation in Kuwait.Crossref | GoogleScholarGoogle Scholar |
Bhat, N. R., Suleiman, M. K., and Shahid, S. A. (2004). Mangrove, Avicennia marina, establishment and growth under the arid climate of Kuwait. Arid Land Research and Management 18, 127–139.
| Mangrove, Avicennia marina, establishment and growth under the arid climate of Kuwait.Crossref | GoogleScholarGoogle Scholar |
Choy, S. C., and Booth, W. E. (1994). Prolonged inundation and ecological changes in an Avicennia mangrove: implications for conservation and management. Hydrobiologia 285, 237–247.
| Prolonged inundation and ecological changes in an Avicennia mangrove: implications for conservation and management.Crossref | GoogleScholarGoogle Scholar |
DAMCO Consulting (2020). Mubarraz – mangrove and seagrass studies. Technical Report for Abu Dhabi Oil Co. (Japan) Ltd, February 2020, DAMCO Consulting, Perth, WA, Australia.
Duarte, C. M., Geertz-Hansen, O., Thampanya, U., Terrados, J., Fortes, M. D., Kamp-Nielsen, L., Borum, J., and Boromthanarath, S. (1998). Relationship between sediment conditions and mangrove Rhizophora apiculata seedling growth and nutrient status. Marine Ecology Progress Series 175, 277–283.
| Relationship between sediment conditions and mangrove Rhizophora apiculata seedling growth and nutrient status.Crossref | GoogleScholarGoogle Scholar |
Duke, N., Bell, M., Pederson, D. K., Roelfsema, C. M., and Nash, S. B. (2005). Herbicides implicated as the cause of severe mangrove dieback in the Mackay region, NE Australia – serious implications for marine plant habitats of the GBR World Heritage Area. Marine Pollution Bulletin 51, 308–324.
| Herbicides implicated as the cause of severe mangrove dieback in the Mackay region, NE Australia – serious implications for marine plant habitats of the GBR World Heritage Area.Crossref | GoogleScholarGoogle Scholar | 15757730PubMed |
Erftemeijer, P. L. A., and Shuail, D. A. (2012). Seagrass habitats in the Arabian Gulf: distribution, tolerance thresholds and threats. Aquatic Ecosystem Health & Management 15, 73–83.
| Seagrass habitats in the Arabian Gulf: distribution, tolerance thresholds and threats.Crossref | GoogleScholarGoogle Scholar |
Erftemeijer, P. L. A., Wylie, N., and Hooper, G. J. (2018). Successful mangrove establishment along an artificially created tidal creek at Port Hedland, Western Australia. Marine and Freshwater Research 69, 134–143.
| Successful mangrove establishment along an artificially created tidal creek at Port Hedland, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Erftemeijer, P. L. A., Agastian, T., Yamamoto, H., Cambridge, M. L., Hoekstra, R., Toms, G., and Ito, S. (2020). Mangrove planting on dredged material: three decades of nature-based coastal defence along a causeway in the Arabian Gulf. Marine and Freshwater Research 71, 1062–1072.
| Mangrove planting on dredged material: three decades of nature-based coastal defence along a causeway in the Arabian Gulf.Crossref | GoogleScholarGoogle Scholar |
Erftemeijer, P., Ito, S., and Yamamoto, H. (2021). Creating mangrove habitat for shoreline protection: Working with Nature in the Arabian Gulf. Terra et Aqua 162, 16–27.
Friess, D. A., Krauss, K. W., Horstman, E. M., Balke, T., Bouma, T. J., Galli, D., and Webb, E. L. (2012). Are all intertidal wetlands naturally created equal? Bottlenecks, thresholds and knowledge gaps to mangrove and saltmarsh ecosystems. Biological Reviews of the Cambridge Philosophical Society 87, 346–366.
| Are all intertidal wetlands naturally created equal? Bottlenecks, thresholds and knowledge gaps to mangrove and saltmarsh ecosystems.Crossref | GoogleScholarGoogle Scholar | 21923637PubMed |
Fuenzalida, T. I., Bryant, C. J., Ovington, L. I., Yoon, H. J., Oliveira, R. S., Sack, L., and Ball, M. C. (2019). Shoot surface water uptake enables leaf hydraulic recovery in Avicennia marina. New Phytologist 224, 1504–1511.
| Shoot surface water uptake enables leaf hydraulic recovery in Avicennia marina.Crossref | GoogleScholarGoogle Scholar |
Gayle, P. M. H., Wilson-Kelly, P., and Green, S. (2005). Transplantation of benthic species to mitigate impacts of coastal development in Jamaica. Revista de Biología Tropical 53, 105–115.
Gilman, E. (2002). Guidelines for coastal and marine site-planning and examples of planning and management intervention tools. Ocean and Coastal Management 45, 377–404.
| Guidelines for coastal and marine site-planning and examples of planning and management intervention tools.Crossref | GoogleScholarGoogle Scholar |
Gorman, D., and Turra, A. (2016). The role of mangrove revegetation as a means of restoring macrofaunal community structure along subtropical coasts. The Science of the Total Environment 566–567, 223–229.
| The role of mangrove revegetation as a means of restoring macrofaunal community structure along subtropical coasts.Crossref | GoogleScholarGoogle Scholar | 27220099PubMed |
Halpern, B. S., Walbridge, S., Selkoe, K. A., Kappel, C. V., Micheli, F., D’Agrosa, C., Bruno, J. F., Casey, K. S., Ebert, C., Fox, H. E., Fujita, R., Heinemann, D., Lenihan, H. S., Madin, E. M. P., Perry, M. T., Selig, E. R., Spalding, M., Steneck, R., and Watson, R. (2008). A global map of human impact on marine ecosystems. Science 319, 948–952.
| A global map of human impact on marine ecosystems.Crossref | GoogleScholarGoogle Scholar | 18276889PubMed |
Kozlowski, T. T. (1997). Responses of woody plants to flooding and salinity. Tree Physiology Monograph number 1 (Heron Publishing: Victoria, BC, Canada.)
Lewis, R. R. (2005). Ecological engineering for successful management and restoration of mangrove forests. Ecological Engineering 24, 403–418.
| Ecological engineering for successful management and restoration of mangrove forests.Crossref | GoogleScholarGoogle Scholar |
Lewis, R. R., and Streever, B. (2000). Restoration of mangrove habitat. WRP Technical Notes Collection, ERDC TN-WRP-VN-RS-3.2. (US Army Engineer Research and Development Center: Vicksburg, MS, USA.) Available at http://www.fao.org/forestry/10559-0f0e6548b08e46a08a3d5723c354ead69.pdf
Loughland, R., Butt, S. J., and Nithyanandan, M. (2020). Establishment of mangrove ecosystems on man-made islands in Kuwait: Sustainable outcomes in a challenging and changing environment. Aquatic Botany 167, 103273.
| Establishment of mangrove ecosystems on man-made islands in Kuwait: Sustainable outcomes in a challenging and changing environment.Crossref | GoogleScholarGoogle Scholar |
Milani, A. S. (2018). Mangrove forests of the Persian Gulf and the Gulf of Oman. In ‘Threats to Mangrove Forests’. (Eds C. Makowski and C. W. Finkl.) Coastal Research Library 25, pp. 53–75. (Springer International Publishing AG.)
Naidoo, G. (2009). Differential effects of nitrogen and phosphorus enrichment on growth of dwarf Avicennia marina mangroves. Aquatic Botany 90, 184–190.
| Differential effects of nitrogen and phosphorus enrichment on growth of dwarf Avicennia marina mangroves.Crossref | GoogleScholarGoogle Scholar |
Naser, H. A. (2015). The role of environmental impact assessment in protecting coastal and marine environments in rapidly developing islands: the case of Bahrain, Arabian Gulf. Ocean and Coastal Management 104, 159–169.
| The role of environmental impact assessment in protecting coastal and marine environments in rapidly developing islands: the case of Bahrain, Arabian Gulf.Crossref | GoogleScholarGoogle Scholar |
Nguyen, H. T., Stanton, D. E., Schmitz, N., Farquhar, G. D., and Ball, M. C. (2015). Growth responses of the mangrove Avicennia marina to salinity: development and function of shoot hydraulic systems require saline conditions. Annals of Botany 115, 397–407.
| Growth responses of the mangrove Avicennia marina to salinity: development and function of shoot hydraulic systems require saline conditions.Crossref | GoogleScholarGoogle Scholar | 25600273PubMed |
Njana, M. A., Eid, T., Zahabu, E., and Malimbwi, R. (2015). Procedures for quantification of belowground biomass of three mangrove tree species. Wetlands Ecology and Management 23, 749–764.
| Procedures for quantification of belowground biomass of three mangrove tree species.Crossref | GoogleScholarGoogle Scholar |
Ochieng, C. A., and Erftemeijer, P. L. A. (2002). Phenology, litterfall and nutrient resorption of Avicennia marina (Forssk.) Vierh in Gazi Bay, Kenya. Trees 16, 167–171.
| Phenology, litterfall and nutrient resorption of Avicennia marina (Forssk.) Vierh in Gazi Bay, Kenya.Crossref | GoogleScholarGoogle Scholar |
Park, A., Lemieux, G., Emmett, B., McMillan, D., Troffe, P., Davis, S., Bodman, M., Waters, M., and Robinson, C. (2018). Techniques for understory kelp salvage and recolonization of disturbed sites to mitigate temporal habitat loss. In ‘Proceedings of the 2018 Salish Sea Ecosystem Conference’, 4–6 April 2018. Seattle, WA, USA. p. 543. (bepress.) Available at https://cedar.wwu.edu/ssec/2018ssec/allsessions/543
Patel, N. T., Gupta, A., and Pandey, A. N. (2010). Salinity tolerance of Avicennia marina (Forssk.) Vierh. from Gujarat coasts of India. Aquatic Botany 93, 9–16.
| Salinity tolerance of Avicennia marina (Forssk.) Vierh. from Gujarat coasts of India.Crossref | GoogleScholarGoogle Scholar |
Polidoro, B. A., Carpenter, K. E., Collins, L., Duke, N. C., Ellison, A. M., Ellison, J. C., Farnsworth, E. J., Fernando, E. S., Kathiresan, K., Koedam, N. E., Livingstone, S. R., Miyagi, T., Moore, G. E., Nam, V. N., Ong, J. E., Primavera, J. H., Salmo, S. G., Sanciangco, J. C., Sukardjo, S., Wang, Y., and Yong, J. W. H. (2010). The loss of species: mangrove extinction risk and geographic areas of global concern. PLoS One 5, e10095.
| The loss of species: mangrove extinction risk and geographic areas of global concern.Crossref | GoogleScholarGoogle Scholar | 20386710PubMed |
Pulver, T. R. (1976). Transplant techniques for sapling mangrove trees, Rhizophora mangle, Laguncularia racemosa, and Avicennia germinans, in Florida. Florida Marine Research Publications number 22, Florida Department of Natural Resources, Marine Research Laboratory, Saint Petersburg, FL, USA.
Rodgers, K. S., Lorance, K., Richards Donà, A., Stender, Y., Lager, C., and Jokiel, P. L. (2017). Effectiveness of coral relocation as a mitigation strategy in Kane‘ohe Bay, Hawai’i. PeerJ 5, e3346.
| Effectiveness of coral relocation as a mitigation strategy in Kane‘ohe Bay, Hawai’i.Crossref | GoogleScholarGoogle Scholar | 28584703PubMed |
Saenger, P. (1996). Mangrove restoration in Australia: A case study of Brisbane International Airport. In ‘Restoration of Mangrove Ecosystems’. (Ed C. Field.) pp. 45–46. (ITTO / ISME: Okinawa, Japan.)
Santini, N. S., Reef, R., Lockington, D. A., and Lovelock, C. E. (2015). The use of fresh and saline water sources by the mangrove Avicennia marina. Hydrobiologia 745, 59–68.
| The use of fresh and saline water sources by the mangrove Avicennia marina.Crossref | GoogleScholarGoogle Scholar |
Sheppard, C., Al-Husiani, M., Al-Jamali, F., Al-Yamani, F., Baldwin, R., Bishop, J., Benzoni, F., Dutrieux, E., Dulvy, N., Durvasula, S., Jones, D., Loughland, R., Medio, D., Nithyanandan, M., Pilling, G., Polikarpov, I., Price, A., Purkis, S., Riegl, B., Saburova, M., Namin, K., Taylor, O., Wilson, S., and Zainal, K. (2010). The Gulf: a young sea in decline. Marine Pollution Bulletin 60, 13–38.
| The Gulf: a young sea in decline.Crossref | GoogleScholarGoogle Scholar | 20005533PubMed |
Silcock, J. L., Simmons, C. L., Monks, L., Dillon, R., Reiter, N., Jusaitis, M., Vesk, P. A., Byrne, M., and Coates, D. J. (2019). Threatened plant translocation in Australia: A review. Biological Conservation 236, 211–222.
| Threatened plant translocation in Australia: A review.Crossref | GoogleScholarGoogle Scholar |
Snedaker, S. C., and Biber, P. D. (1996). Restoration of mangroves in the United States of America. In ‘Restoration of Mangrove Ecosystems’. (Ed C. Field.) pp. 170–188. (ITTO / ISME: Okinawa, Japan).
Steppe, K., Vandegehuchte, M. W., Van de Wal, B. A. E., Hoste, P., Guyot, A., Lovelock, C. E., and Lockington, D. A. (2018). Direct uptake of canopy rainwater causes turgor-driven growth spurts in the mangrove Avicennia marina. Tree Physiology 38, 979–991.
| Direct uptake of canopy rainwater causes turgor-driven growth spurts in the mangrove Avicennia marina.Crossref | GoogleScholarGoogle Scholar | 29562244PubMed |
van Loon, A. F., Dijksma, D., and van Mensvoort, M. E. F. (2007). Hydrological classification in mangrove areas: a case study in Can Gio, Vietnam. Aquatic Botany 87, 80–82.
| Hydrological classification in mangrove areas: a case study in Can Gio, Vietnam.Crossref | GoogleScholarGoogle Scholar |
van Loon, A. F., Te Brake, B., van Huijgevoort, M. H. J., and Dijksma, R. (2016). Hydrological classification, a practical tool for mangrove restoration. PLoS One 11, e0150302.
| Hydrological classification, a practical tool for mangrove restoration.Crossref | GoogleScholarGoogle Scholar | 27008277PubMed |
Vanderklift, M. A., Doropoulos, C., Gorman, D., Leal, I., Minne, A. J. P., Statton, J., Steven, A. D. L., and Wernberg, T. (2020). Using propagules to restore coastal marine ecosystems. Frontiers in Marine Science 7, 724.
| Using propagules to restore coastal marine ecosystems.Crossref | GoogleScholarGoogle Scholar |
Watson, W. T. (2005). Influence of tree size on transplant establishment and growth. HortTechnology 15, 118–122.
| Influence of tree size on transplant establishment and growth.Crossref | GoogleScholarGoogle Scholar |
Watson, G. W., and Himelick, E. B. (1982). Root distribution of nursery trees and its relationship to transplanting success. Journal of Arboriculture 8, 225–229.



