Stability and change in a changing environment: soft-bottom benthic molluscs in the Peel–Harvey Estuary over 42 years
Fred E. Wells A B * , Marthe Monique Gagnon
A B * , Marthe Monique Gagnon  A , Francis Spilsbury
A , Francis Spilsbury  A and Corey Whisson
A and Corey Whisson  C
C
A School of Molecular and Life Sciences, Curtin University, PO Box U1987, Bentley, WA 6845, Australia.
B Negaunee Integrative Research Center, Field Museum of Natural History, Chicago, IL 60605, USA.
C Western Australian Museum, Locked Bag 49, Welshpool DC, WA 6986, Australia.
Marine and Freshwater Research 73(6) 792-802 https://doi.org/10.1071/MF21283
Submitted: 6 October 2021 Accepted: 22 February 2022 Published: 2 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing.
Abstract
Context: Eutrophication caused extensive macroalgal blooms in the Peel–Harvey Estuary, Western Australia, in the 1970s. Nutrient inputs were reduced and an artificial channel was constructed in 1994 to increase marine flushing.
Aims: This study examines benthic mollusc populations in the estuary in 1978, 2000 and 2020, to determine what changes have occurred in the estuaries over time.
Methods: Quantitative samples were made at 10 sites in autumn and spring of each year; physical and chemical parameters were measured in 2000 and 2020.
Key results: Species composition was stable, dominated by Arthritica semen and Hydrococcus brazieri; however, there have been substantial changes in abundance of these and less common species.
Conclusions: The exact cause(s) of density changes could not be determined, but it is likely to be due to a combination of factors.
Implications: Further changes in mollusc assemblages in south-western Australian estuaries are expected as the climate warms and dries and the estuaries are stressed by human population growth.
Keywords: bivalves, climate change, Dawesville Channel, drying climate, eutrophication, gastropods, seasonality, south-western Australia.
Introduction
Although estuaries are productive aquatic environments (Costanza et al. 2007; Cottingham et al. 2018), they are often heavily affected by substantial concentrations of human populations living on their shorelines. Land is cleared for population centres, farming or other purposes, pollution from diffuse sources is common, estuaries are dredged, altered for canal developments and used extensively for recreation and fishing. Climate changes have led to a reduction in rainfall and drainage into many estuaries (Cottingham et al. 2018). These and other anthropogenic activities have led to a degradation of estuaries on a worldwide scale through eutrophication, fish kills, seagrass loss, etc. (Lotze et al. 2006; Cottingham et al. 2018).
South-western Australian estuaries are particularly extreme environments (Cottingham et al. 2018; Hallett et al. 2018). They are located on a relatively flat coastal plain. River flows are sporadic and seasonal, being almost absent in the high temperature summers and greatest during winter rains. Rainfall in the south-west has declined by 20% since the 1970s, resulting in an 80% drop in stream flows, and the drying trends are continuing (Water Corporation 2022). South-western estuaries are small, shallow, with mostly sandy bottoms. The adjacent ocean is microtidal, usually well under 1 m, with a mixture of semidiurnal and diurnal tides (Bureau of Meteorology 2022). Small estuarine entrance channels restrict the already low tidal ranges. For example, prior to the opening of the Dawesville Channel, the average daily tidal range in the Peel–Harvey Estuary was 0.1 m (Eliot and McCormack 2019). Water flushing is primarily from freshwater inflows from rivers; some estuaries are closed for years by sand bars and there may be extended periods of hypersaline conditions (Brearley 2005, 2013; Cottingham et al. 2018; Hallett et al. 2018).
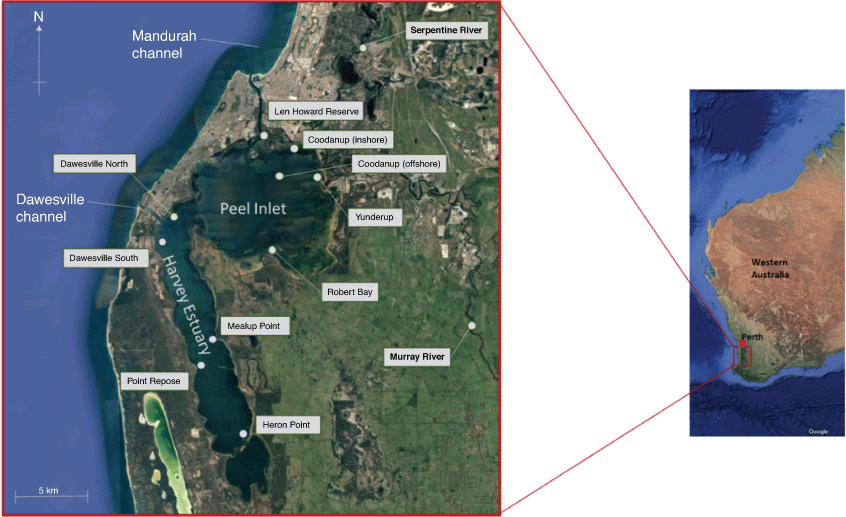
Population centres are located on several of the south-western Australian estuaries. The concentration of people and industries has created significant environmental problems in several of the estuaries, summarised by Brearley (2005). One of the most adversely affected estuaries is the Peel–Harvey Estuary, 80 km south of Perth, at 32°36′S, 115°42′E. It is the largest estuary in south-western Australia, with an area of ~130 km2 composed of Peel Inlet (70 km2) and Harvey Estuary (60 km2; Fig. 1). The Peel–Harvey Estuary has experienced considerable changes in recent decades (Brearley 2005; Potter et al. 2016; Cottingham et al. 2018; Hallett et al. 2018). The population of Mandurah grew from 5938 in 1971 (Australian Bureau of Statistics 2021) to 86 474 in 2020 (City of Mandurah 2021). An estimated 55% of the catchment area has been cleared since 1830 (Thomson 2019). In the 1970s, there was a considerable buildup of algae in the Peel–Harvey Estuary, primarily Willeella brachyclados (previously Cladophora montagneana) in Peel Inlet and Nodularia spumigera in Harvey Inlet (McComb and Lukatelich 1995). The algal blooms died and created malodorous masses of dead vegetation that were removed by bulldozers and transported to a terrestrial landfill site from 1974 until 1983, when a new machine began harvesting the algae in situ (DCE 1985). A major environmental investigation undertaken from 1976 to 1980 determined the primary causes to be excessive use of superphosphate on agricultural land and organic waste from piggery farms. The following three programs were developed to reduce nutrients in the estuary: physical removal of accumulated rotting algae to a terrestrial landfill site; changes in agricultural practices to reduce nutrient runoff; and construction of a new channel from the northern end of Harvey Estuary to the ocean to increase flushing in the estuary (Hodgkin et al. 1980).
Opening of the Dawesville Channel in 1994 caused considerable changes in the Peel–Harvey Estuary (Brearley 2005; Potter et al. 2016; Cottingham et al. 2018; Hallett et al. 2018; Eliot and McCormack 2019). It was estimated that water exchange with the marine environment would be tripled, phosphorus yearly inputs in the estuary would be reduced by ~100 tonnes (Mg), and nitrogen by 900 Mg (Rogers et al. 2010). The goal of improving water quality was initially met. Tidal flushing increased and mean salinities were 34–37 PSU, essentially marine conditions (Eliot and McCormack 2019; Thomson 2019). Mean chlorophyll a concentrations in both Peel Inlet and Harvey Estuary were highly eutrophic (>50 µg L–1) before the Dawesville Channel, but were dramatically reduced in the years immediately after the channel was opened (Pearce et al. 2000). Algal production declined and seagrasses, particularly Ruppia, became more common (Krumholz 2019).
Molluscs are a dominant benthic invertebrate group in south-western Australian estuaries (Brearley 2005). The present paper compares limited data on the dominant soft-bottom benthic molluscs of the Peel–Harvey Estuary from 1978, before construction of the Dawesville Channel, with more extensive data collected in 2000 and 2020, to assess what, if any, long-term changes in benthic molluscs have occurred as a result of the channel construction. The results are considered in the context of other biological changes in the Peel–Harvey Estuary and other south-western Australian estuaries.
Materials and methods
Both Peel Inlet and Harvey Estuary are shallow, with a mean depth of 0.9 m and a maximum of 2 m in Peel Inlet. They are fringed with extensive shallow banks <0.5 m high. The estuary bottom is sandy with patches of seagrass or macroalgae. The 20 km long Serpentine River and the 24 km long Murray River flow into the eastern side of Peel Inlet. The Harvey River originally flowed into the southern part of Harvey Estuary, but the upper part of the river was diverted in 1935 by the Harvey Diversion Drain to flow directly into the sea (Heritage Council 2022). There is a narrow inlet channel to the ocean at Mandurah, with deltas at both ends. A breakwater has been constructed on the southern side of the inlet where there are a number of artificial hard surfaces (Brearley 2005).
The most abundant species of benthic molluscs were examined as part of the 1970s Peel–Harvey environmental study, with a primary site monitoring the density of molluscs at Coodanup on the northern side of Peel Inlet from March 1977 through February 1979. Surveys were made of these species at 10 sites in the Peel–Harvey Estuary in the summer (16 January) and late winter (30 August) 1978 (Wells et al. 1980). The survey was repeated in early autumn (March) and early spring (September) 2000, to determine whether there were any measurable effects on molluscs attributable to the completion of the Dawesville Channel in 1994; environmental characteristics of the sites were also examined in 2000 (Wells et al. 1980; Whisson et al. 2004).
The 2020, survey was conducted at the same 10 sites as in the 2000 study, and 9 of the 1978 sites (Fig. 1); sites were located in at least 30 cm of water to ensure that they were not exposed at low tide. At each site, a 10-cm-diameter PVC cylinder was driven ~20 cm into the sediment by hand. In March 2020, the submerged end of the tube was capped by hand and the entire sample was sieved through a 500-µm mesh. Material remaining on the sieve was frozen until it could, subsequently, be sorted, identified and counted in a laboratory by using a dissecting microscope. All of the living species collected were small individuals that lived on or near the sediment–water interface. Sorting the lower portion of the sediment was extremely time consuming and no live molluscs were recorded. To reduce the sorting time, the same procedures were followed in September 2020 except that the upper 5 cm of sediment was sieved through a 500-µm mesh and the lower sediment was sieved through a 1-mm mesh; no live molluscs were found in the sediments from depths >5 cm. Identifications were made using Wells (1984), Ponder and Clark (1988), Wells and Bryce (1990) and Wilson (1994).
Sediments and water at the site were quantified in 2020 by using methods detailed in Rose (1994) and Whisson et al. (2004). These included temperature, dissolved oxygen, pH, salinity, redox, sediment grain size and total organic carbon in the sediment. A Handy Polaris 2 Oxyguard was used to measure temperature and dissolved oxygen, whereas a WP-80 TPS was used for pH measurements. Salinity was evaluated with a hand-held refractometer. Temperature, dissolved oxygen, pH and salinity were measured in triplicate, whereas redox, sediment grain size and total organic carbon were measured in duplicate. Redox was estimated using a clear 2.5-cm-diameter cylinder that collected a core of the surface sediment and the top oxidised layer of sediment was measured to the nearest millimetre. Sediment grain size was measured by treating the sediment sample with 6% hydrogen peroxide for 48 h to remove any organic matter, washing overnight, and drying at 60°C for 24 h. The sample was then sieved through a standard set of geological sieves with mesh sizes ranging from 4 mm to 63 μm and weighing each portion to the nearest 0.1 g. For total organic carbon, macrophyte material was removed from a subsample of ~100 g of sediment. The sample was dried for 24 h at 60°C, weighed to the nearest 1 mg, then burned on ignition at 530°C for 16 h and reweighed (Holme and McIntyre 1984).
Multivariate data analyses were conducted using R statistical software (ver. 4.0, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/). Non-metric multidimensional analysis (nMDS) using Bray–Curtis distance to describe similarities in mollusc community assemblages was performed using the Vegan R package (ver. 4.0, J. Oksanen, F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, and H. Wagner, see https://cran.r-project.org/web/packages/vegan/; Dixon 2003). Relationships between environmental factors and the relative abundance of mollusc species was explored using canonical correspondence analysis (CCA), also using the Vegan package. Principal-component analysis (PCA) using the FactoMineR R package (see https://CRAN.R-project.org/package=FactoMineR; Lê et al. 2008) was used to describe differences in sediment and water conditions between estuaries, and between years. The significance of differences among sample sites, seasons or estuaries was established using the Student’s t-test, ANOSIM (Clarke 1993), or ANOVA, as specified in the text, using a significance threshold of P < 0.05. Community diversity was quantified using Shannon’s index (Beck and Schwanghart 2010).
Results
2020 survey
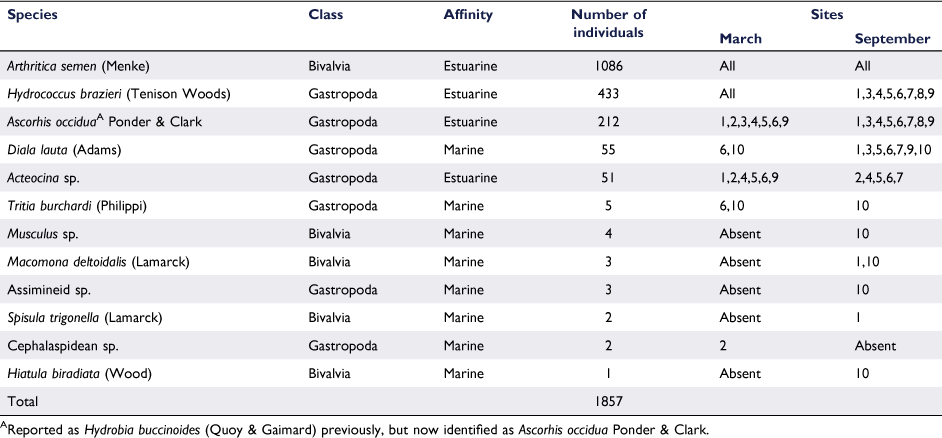
In total, 1857 individuals of 12 species of molluscs were collected during the 2020 surveys (Table 1). Four estuarine species dominated, including 1086 Arthritica semen, 433 Hydrococcuus brazieri, 212 Ascorhis occidua, and 51 Acteocina spp. Together, the four estuarine species represented 95.9% of all molluscs collected. All of these species are small, <4 mm long. In total, 8 of the 12 species collected were marine, but the total marine component was 4.1% of all molluscs collected. The most common marine species was Diala lauta with 55 individuals, which reaches up to 8 mm (Wells 1984). There was a total of 20 individuals of the remaining 7 marine species (Table 2).
|
|
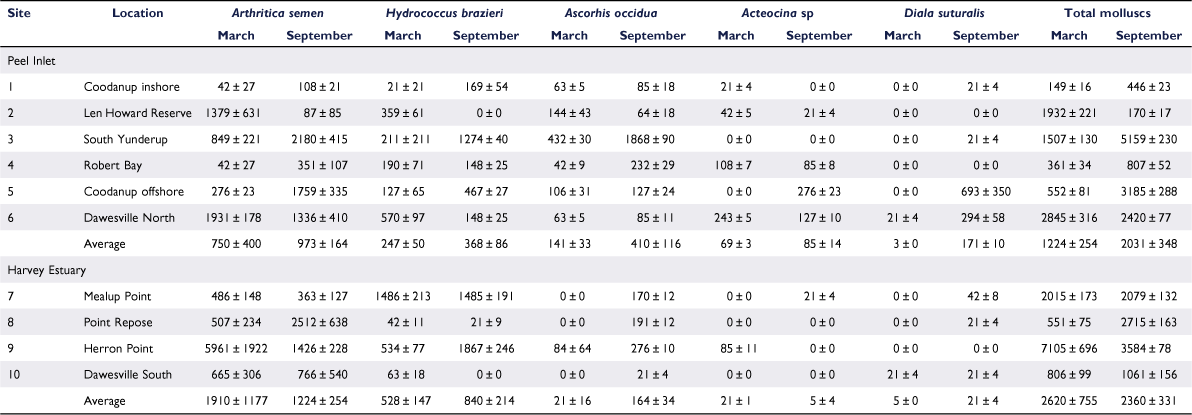
Mean molluscan density in the estuary increased from 1778 ± 346 (s.e.) m−2 in March 2020 to 2157 ± 246 m−2 in September and in Peel Inlet from 1224 ± 254 to 2031 ± 348 m−2, but decreased in Harvey Estuary from 2620 ± 755 to 2360 ± 330 m−2. None of these variations was statistically significant (t-test, P > 0.05).
The bivalve Arthritica semen was the dominant mollusc in the estuary, with 58.5% of total individuals. The distribution of A. semen was patchy, ranging from 42 ± 27 m−2 at Coodanup inshore and Robert Bay in March to 5961 ± 1922 m−2 at Herron Point in September (Table 2). Density at Coodanup offshore (3185 ± 288 m−2) in September was much greater than at Coodanup inshore (108 ± 21 m−2), a few hundred metres away (t = 4.82, P < 0.05). Densities at all stations increased in September except for Dawesville North, which decreased insignificantly from 1931 ± 178 to 1336 ± 410 m−2 (t = 0.42, P > 0.05). Mean A. semen density in Harvey Estuary was greater than in Peel Inlet in both seasons.
The distribution of Hydrococcus brazieri was also patchy (Table 2), but as the species was less common than A. semen, the density differences were not as great. Densities ranged from 0 ± 0 m−2 at Len Howard Reserve and Dawesville South to 1867 ± 246 m−2 at Herron Point in September. Like A. semen, H. brazieri was more abundant in Harvey Estuary than Peel Inlet in both seasons.
The third-most common species, Ascorhis occidua, also had a very patchy distribution (Table 2). Low densities were recorded in March 2020 in all stations in Peel Inlet, with the highest at Yunderup (432 ± 30 m−2), but it was detected only at Herron Point (85 ± 11 m−2) in the Harvey Estuary. Densities at all stations increased in September, except for Len Howard Reserve, where there was an insignificant decline from 144 ± 43 to 64 ± 18 m−2 (t = 0.003, P > 0.05). In contrast to March, A. occidua was present in low densities (276 ± 10 m−2 or less) at all stations in the Harvey Estuary.
Acteocina sp. was present in low densities (243 ± 5 m−2 or less) in most of the stations in Peel Inlet in March, but was absent at three of the four stations in Harvey Estuary. In contrast to the other species, there was no general change in the density of Acteocina sp. in the estuary in September.
Three larger-sized species of marine bivalves were recorded in very low numbers, including Macomona deltoidalis at Coodanup inshore and Dawesville south, Spisula trigonella at Coodanup inshore, and Hiatula biradiata at Dawesville south (Table 1). All were juveniles <1 cm long. Numerous dead shells of S. trigonella were observed at Point Repose, but no live individuals were collected.
Temporal changes
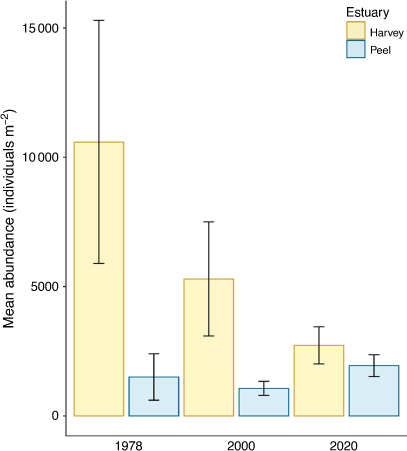
Compared with data from surveys in previous years, changes in the mollusc assemblage at each site were evident in 2020, although the same species still dominated molluscan composition assemblages. In 1978, before the dredging of the Dawesville Channel, the abundance of molluscs in the Harvey Estuary was much higher than in the more sparsely populated Peel Inlet (Fig. 2). Overall abundance of molluscs in the Harvey Estuary declined in successive decades, with mean concentrations of 10 600 ± 4700 m−2 in 1978 falling to 5300 ± 2200 m−2 and then 2700 ± 700 m−2 in 2000 and 2020 respectively. Conversely, the mean Peel Inlet mollusc abundance increased slightly from 1500 ± 900 in 1978 to 1900 ± 400 m−2 in 2020. By 2020, there was no significant difference in mean mollusc abundance at sites in the Peel Inlet and Harvey Estuary (t-test, P = 0.18).
|
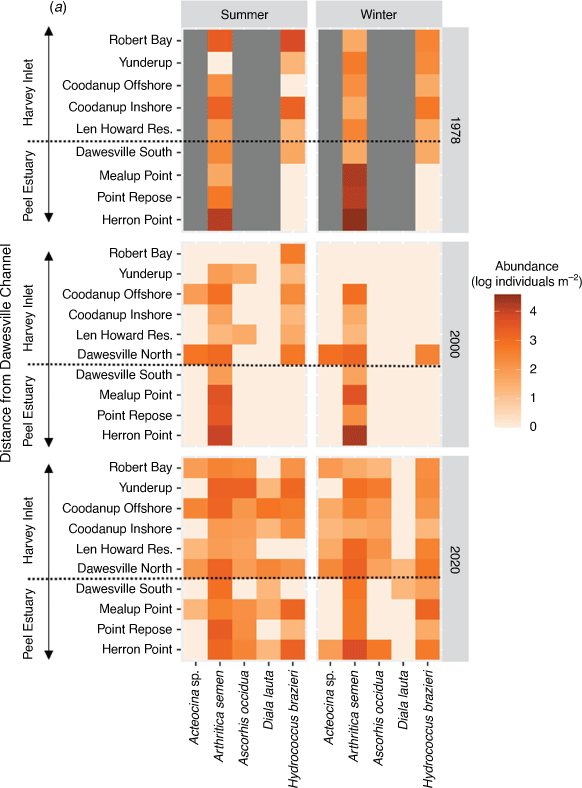
Among sites in each estuary, the relative abundances of the five most prolific species, which, combined, represent greater than 95% of all molluscs (Arthritica semen, Hydrococcuus brazieri, Ascorhis occidua, Acteocina sp. and Diala lauta) in any of the surveys, increased in 2020 compared with 2000 (Fig. 3a, b). The mean Shannon’s index of community diversity for the whole Peel–Harvey Estuary increased from 0.57 ± 0.12 in 2000 to 0.86 ± 0.07 in 2020, but the increase was not significant (t-test, P = 0.065; Supplementary Fig. S1).
|
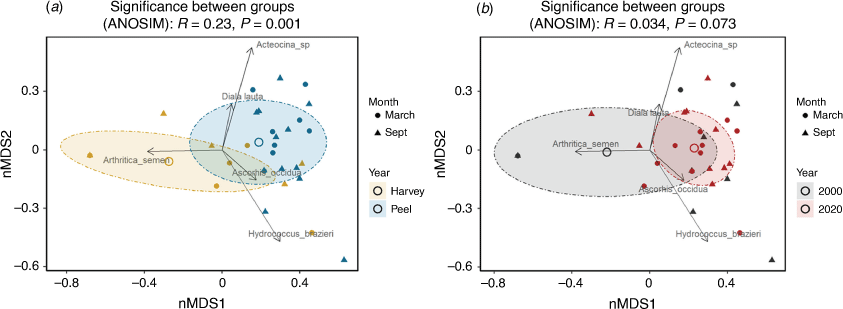
The nMDS analysis shows that the 2020 mollusc assemblages in Peel Inlet and Harvey Estuary (Fig. 4a) were significantly different (ANOSIM, R = 0.23, P = 0.001). Assemblages in the overall Peel–Harvey Estuary in 2000 were not significantly different from those in 2020 (ANOSIM, R = 0.034, P = 0.073; Fig. 4b).
|
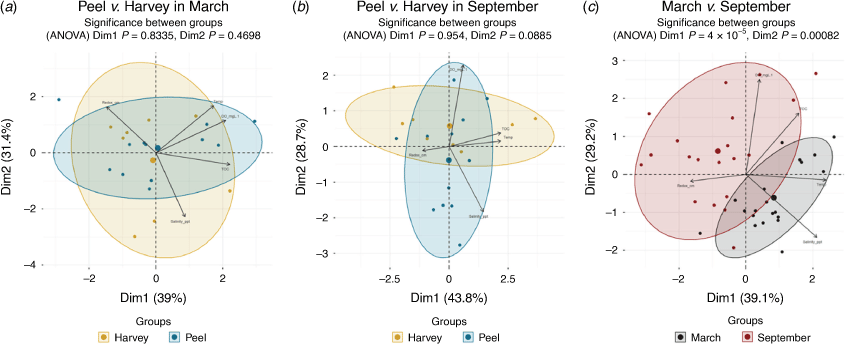
A PCA of five environmental parameters (salinity, pH, water temperature, total organic carbon (TOC) and sediment redox depth) comparing the 2020 data measured in Peel Inlet and Harvey Estuary showed that there was no statistically significant difference in the environmental conditions between the two estuaries in March (Fig. 5a; ANOVA, P > 0.764) or September (Fig. 5b; ANOVA, P > 0.088). However, between the seasons, there was a highly significant difference in environmental conditions in the Peel–Harvey Estuary (Fig. 5c; ANOVA, P < 0.0001), driven by large increases in salinity in March (44.9 ± 1.2 PSU) compared with September (22.1 ± 2.2 PSU). This is consistent with seasonal changes in rainfall for the region, as is the small seasonal decrease in water temperature from 21.0 ± 0.8 to 17.7 ± 0.9°C in March and September respectively.
Measured environmental conditions between 2000 and 2020 were generally similar, although the mean TOC increased significantly (t-test, P = 0.0004) from 0.77 ± 0.3% in 2000 to 1.85 ± 0.5% in 2020, and the sediment redox depth decreased from 2.00 ± 0.9 to 1.61 ± 0.4 cm in 2000 and 2020 respectively, although this was not statistically significant (t-test, P = 0.22). Same-season PCA analysis of the environmental conditions showed that overall environmental conditions changed slightly, but not statistically significantly, between 2000 and 2020 (March ANOVA, Dim1: P = 0.055, Dim2: P = 0.055; September ANOVA: Dim1: P = 0.006, Dim2: P = 0.109; Supplementary Fig. S2). The CCA showed that only 35.6% of the alterations in the mollusc communities were attributed to changes in the five environmental factors, and none of them significantly (ANOVA, P > 0.214).
Discussion
South-western Australian has a Mediterranean climate, characterised by cool wet winters and dry hot summers. Such environments are predicted to become hotter and drier with climate change (Hallett et al. 2018). Estuaries in south-western Australia are particularly susceptible to climate change because they are small, shallow, and have narrow entrances that restrict inflows from a microtidal marine environment (Cottingham et al. 2018; Hallett et al. 2018). Increasing population centres on many of the estuaries are creating more stresses (e.g. Brearley 2005; Rogers et al. 2010). In recent decades, a 20% decrease in rainfall has led to an 80% decline in runoff (Water Corporation 2022). The Peel–Harvey Estuary is the largest estuary in the south-west. It was heavily eutrophic in the 1970s, leading to the creation of the Dawesville Channel to increase marine inflows (McComb and Lukatelich 1995; Brearley 2005; Potter et al. 2016; Cottingham et al. 2018; Hallett et al. 2018; Eliot and McCormack 2019). Extensive environmental studies in the system allow the Peel–Harvey Estuary to be used as a model to develop an understanding of the effects of climate and other changes on these vulnerable estuaries.
A number of studies have documented biotic changes since the Dawesville Channel was constructed. For example, Wildsmith et al. (2011) compared macroinvertebrates in the Peel–Harvey Estuary before (1986–1987) and after (2003–2004) construction of the Dawesville Channel. As expected, macroinvertebrate density declined, but taxonomic distinctiveness also declined and species composition became more variable. There were differences in major groups; reductions in density and diversity occurred in sensitive crustaceans and molluscs, but the reverse occurred in polychaetes, which are more robust. Young and Potter (2003) compared the fish biota of the Peel–Harvey Estuary in 1980–1981 with that of 1996–1997, just after completion of the Dawesville Channel. Weed-associated species were significantly less abundant, whereas marine species had increased. Rogers et al. (2010) attributed the changes to reductions in habitat diversity owing to the reduction in macroalgae. Concurrently the movement patterns of blue swimmer crabs (Portunus pelagicus) and prawns were altered, reducing their catchability (Rogers et al. 2010).
It is now more than a quarter of a century since the Dawesville Channel was completed. Despite the initial success of the channel, the Peel–Harvey Estuary has returned to a partially degraded state, partly owing to causes independent of estuary management such as climate change, but also because of changes in the estuary itself. Cottingham et al. (2018) found that the flow reduction increased eutrophication of the mouths of the three rivers in the estuary. The major contributor to the recent degradation of the Peel–Harvey Estuary is probably due to the growth of the human population in Mandurah (Rogers et al. 2010).
Key species of molluscs were surveyed in the Peel–Harvey Estuary in 1978 as part of the initial environmental studies (Wells and Threlfall 1981). The survey was repeated in 2000, 6 years after the Dawesville Channel was completed and broadened to include all benthic molluscs and selected environmental factors (Whisson et al. 2004). The 2000 survey was repeated in 2020 to assess any further changes.
Four small (<4 mm) soft-bottom estuarine species of molluscs were present in all three surveys, namely, A. semen, H. brazieri, A. occidua and Acteocina sp. Although population densities of these species were variable, A. semen and H. brazieri dominated the molluscan fauna in all 3 years, comprising 82% in 2020 and 89% in both 1978 and 2000. The life cycles and population densities of these two molluscs were studied in detail by Wells et al. (1980) and Wells and Threlfall (1982a, 1982b, 1982c). Wells et al. (1980) monitored densities of H. brazieri and A. semen at Coodanup on Peel Inlet from March 1977 to February 1979: densities of H. brazieri ranged from 700 to 19 959 m−2 and A. semen varied from 751 to 45 491 m−2. A. semen matures in ~6 months and reaches its maximum size in 9 months. H. brazieri matures in ~4 months and reaches maximum size in 7–8 months. Although the lifespans have not been determined, it is probably 1 year or less (Wells et al. 1980). European Hydrobia ventrosa and H. neglecta in Denmark are of similar size and ecology. They are spawned one summer, overwinter and may survive through the following summer (Siegismund 1982). Hydrococcus brazieri and A. semen have only moderate fecundity, but they have adaptations that ensure high survivorship of the young. Fertilised eggs of A. semen are retained in a brood pouch until they are released as crawling juveniles ~0.5 mm long. Female H. brazieri individuals deposit eggs in capsules in which a single young snail (rarely two) develops to 0.3 mm; capsules are attached to any hard surface. The lack of a planktonic larval stage ensures that young are not washed from the estuary by tidal currents. The brood pouches and capsules also help buffer sensitive young from rapid environmental changes in temperature and salinity. The temperature and salinity tolerances of both species are wide, allowing them to thrive in changing estuarine conditions (Wells and Threlfall 1982a, 1982c). Continuous reproduction facilitates rapidly increasing populations when conditions are favourable. Their small size makes them easily distributed in the estuary entrapped in algae, floating debris or on bird feet; this could explain the recent distribution of H. brazieri into Harvey estuary. These characteristics have allowed A. semen and H. brazieri to survive and thrive in the highly variable environment of the Peel–Harvey Estuary and other estuaries of the south-west.
Wells (1984) surveyed molluscs in 16 south-western Australian estuaries. Both A. semen and H. brazieri were widespread, recorded in 9 and 10 estuaries respectively, where they are often dominant species of molluscs (Brearley 2005). Wells and Threlfall (1980) compared mollusc assemblages at Coodanup on Peel Inlet with the much more marine conditions near the entrance to Oyster Harbour. Molluscs were sorted from sieves with 1- and 2-mm meshes. In contrast to Peel Inlet, where a single bivalve was collected on the 2-mm mesh, there were 18 species with a total density of 202.5 m−2 on the 2-mm mesh in Oyster Harbour. However, H. brazieri (4674 m−2) dominated on the 1-mm mesh in Oyster Harbour and there was a low density of A. semen (137 m−2). Semeniuk and Wurm (2000) examined molluscs in Leschenault Inlet, south of the Peel–Harvey Estuary, over 5 years from 1982 to 1987. As in the Peel–Harvey Estuary, A. semen (densities typically in the hundreds to thousands per square metre) and H. brazieri (tens to hundreds per square metre) were abundant estuarine species; Acteocina sp. was also common. Densities of all three species fluctuated erratically; for a single species, there could be substantial increases between samples at one site, but correspondingly large decreases at another site. A. semen had generally high densities in the first year of the study, declined for 1–2 years, then increased near the end of the study. At one site, H. brazieri was abundant, then was absent for over a year from February 1983 to May 1984, before reappearing. The substantial density variations in A. semen and H. brazieri recorded by Semeniuk and Wurm (2000) are similar to those recorded in the Peel–Harvey Estuary from March 1977 to February 1979 (Wells et al. 1980).
Semeniuk and Wurm (2000) measured a number of physical parameters, and found no apparent relationship between any of the measured parameters and population fluctuations. They did suggest that the absence of H. brazieri from one site from February 1983 until May 1984 may have been caused by a combination of high summer salinities (45 PSU) and temperatures at the site that caused the population to crash. Similarly, there is no clear cause of the short-term population variations reported here for the Peel–Harvey Estuary. Rogers et al. (2010) suggested that habitat diversity decreased due to the loss of macroalgae after the Dawesville Channel opened, causing changes in fish populations. Wildsmith et al. (2011) also found decreases in the molluscs and crustaceans between 1986–1987 and 2003–2004 but increases in polychaetes. Although they could not determine a specific cause, they noted that there was markedly increased urbanisation, recreational boating and fishing and canal development during the period between their samples, resulting in a cumulative deterioration of the benthic marine environment. Another major environmental change in the local environment has been a recent series of marine heatwaves along the entire Western Australian west coast, between North West Cape and Cape Leeuwin. The most intense of these began to develop off North West Cape in November 2010, progressively moving south over the following months and dissipating by May 2011. Inshore sea-surface temperatures were elevated by 3°C for 3 months; shorter-term peaks of up to 5°C were experienced in some areas in late February and early March (Pearce and Feng 2013; Oliver et al. 2018). Extensive long-term changes in the marine biota of the region have been recorded (Ruthrof et al. 2018; Caputi et al. 2019), but the sandy bottom molluscs of the Peel–Harvey Estuary were unaffected by the marine heatwaves.
In summary, this study has provided several comparisons of the soft-bottom molluscs in the Peel–Harvey Estuary between 1978 and 2020:
The dominant species of soft-bottom estuarine molluscs were the same small species in 2020 as in 1978 and 2000, Arthritica semen and Hydrococcus brazieri. These species have rapid fluctuations in population density (Wells et al. 1980; Wells and Threlfall 1982c), the causes of which are unknown.
H. brazieri was recorded in Harvey Estuary in 2020, but not 1978 or 2000. The species is easily transported attached to drifting algae, on logs or bird feet, so the presence in Harvey Estuary is not surprising.
Mollusc numbers remained relatively constant in Peel Inlet between 1978 and 2000, but decreased by 75% in 2020, possibly owing to decreasing habitat diversity as a reduction in macroalgae biomass.
Despite the increased salinity of the estuary caused by the opening of the Dawesville Channel in 1994, populations of marine affinity species remained low in 2020, with 4.1% of total mollusc density.
The diversity of these molluscan communities also remained stable through sampling years, suggesting that potential recovery to high abundances as measured in 1978 is still possible if the Peel–Harvey Estuary returns to favourable conditions.
The marine environment outside the Peel–Harvey Estuary was changed considerably by a marine heatwave in 2011, but there was no discernible effect on benthic molluscs in the Peel–Harvey Estuary.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Supplementary material
Supplementary material is available online.
Acknowledgements
This work was undertaken under a permit approved by the Western Australian Department of Primary Industry and Regional Development (DPIRD). We thank Dr Tom Rose for help in locating sites and collection of the March 2020 samples. We very much appreciate the constructive comments of two anonymous referees, which considerably improved the paper.
References
Australian Bureau of Statistics (2021) Census of Population and Housing (1971). (ABS) Available at https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/2105.01971?OpenDocument [Verified 12 March 2021]Beck, J, and Schwanghart, W (2010). Comparing measures of species diversity from incomplete inventories: an update. Methods in Ecology and Evolution 1, 38–44.
| Comparing measures of species diversity from incomplete inventories: an update.Crossref | GoogleScholarGoogle Scholar |
Brearley A (2005) ‘Ernest Hodgkin’s Swanland: Estuaries and Coastal Lagoons of South-Western Australia.’ (UWA Publishing: Perth, WA, Australia)
Brearley A (2013) ‘Revisiting the Blackwood River and the Hardy Inlet. 40 Years of Change. An Environmental Review of the Blackwood Estuary, Western Australia 1974–2010.’ (Ernest Hodgkin Trust for Estuary Education and Research: Perth, WA, Australia)
Bureau of Meteorology (2022) Tide tables for Western Australia. (BOM: Canberra, ACT, Australia) Available at http://www.bom.gov.au/oceanography/projects/ntc/wa_tide_tables.shtml [Verified 9 January 2022]
Caputi, N, Kangas, M, Chandrapavan, A, Hart, A, Feng, M, Marin, M, and de Lestang, S (2019). Factors affecting the recovery of invertebrate stocks from the 2011 Western Australian extreme marine heatwave. Frontiers in Marine Science 6, 484.
| Factors affecting the recovery of invertebrate stocks from the 2011 Western Australian extreme marine heatwave.Crossref | GoogleScholarGoogle Scholar |
City of Mandurah (2021) Facts and figures. Available at https://www.mandurah.wa.gov.au/learn/about-mandurah/fact-and-figures [Verified 12 March 2021]
Costanza, R, Fisher, B, Mulder, K, Liu, S, and Christopher, T (2007). Biodiversity and ecosystem services: a multi-scale empirical study of the relationship between species richness and net primary production. Ecological Economics 61, 478–491.
| Biodiversity and ecosystem services: a multi-scale empirical study of the relationship between species richness and net primary production.Crossref | GoogleScholarGoogle Scholar |
Clarke, KR (1993). Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18, 117–143.
Cottingham, A, Huang, P, Hipsey, MR, Hall, NG, Ashworth, E, Williams, J, and Potter, IC (2018). Growth, condition and maturity schedules of an estuarine fish species change in estuaries following increased hypoxia due to climate change. Ecology and Evolution 8, 7111–7130.
| Growth, condition and maturity schedules of an estuarine fish species change in estuaries following increased hypoxia due to climate change.Crossref | GoogleScholarGoogle Scholar | 30073071PubMed |
(1985). Peel–Harvey Estuary progress. Managing the algal problem: how will the estuary change. Western Australian Department of Conservation and Environment Bulletin 146, 1–6.
Dixon, P (2003). VEGAN, a package of R functions for community ecology. Journal of Vegetation Science 14, 927–930.
| VEGAN, a package of R functions for community ecology.Crossref | GoogleScholarGoogle Scholar |
Eliot M, McCormack G (2019) Observed water level responses to opening a large channel to Peel–Harvey Estuary. In ‘Australasian Coasts & Ports 2019 Conference’, 10–13 September 2019. (Engineers Australia: Hobart, Tas., Australia). Available at https://search.informit.org/doi/epdf/10.3316/informit.797049682891688 [Verified 25 February 2022]
Hallett, CS, Hobday, AJ, Tweedley, JR, Thompson, PA, McMahon, K, and Valesini, FJ (2018). Observed and predicted impacts of climate change on the estuaries of south-western Australia, a Mediterranean climate region. Regional Environmental Change 18, 1357–1373.
| Observed and predicted impacts of climate change on the estuaries of south-western Australia, a Mediterranean climate region.Crossref | GoogleScholarGoogle Scholar |
Heritage Council (2022) Harvey Diversion Drain. (Heritage Council, Government of Western Australia). Available at http://inherit.stateheritage.wa.gov.au/Public/Inventory/Details/10543e1a-a9aa-46e8-b31a-3590f3986c9e [Verified 7 January 2022]
Hodgkin EP, Birch PB, Black RE, Humphries RB (1980) The Peel–Harvey Estuarine System Study (1976-1980). A report to the Estuarine & Marine Advisory Committee December 1980. Report 9, Western Australian Department of Conservation and Environment, Perth, WA, Australia.
Holme, NA, and McIntyre, AD (1984). ‘Methods for the Study of Marine Benthos.’ (Blackwell Scientific Publications: London, UK)
Krumholz O (2019) Macrophyte communities in the Peel–Harvey Estuary: historical trends and current patterns in biomass and distribution. BSc(Hons) Thesis, Murdoch University, Perth, WA, Australia.
Lê, S, Josse, J, and Husson, F (2008). FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25, 1–18.
| FactoMineR: an R package for multivariate analysis.Crossref | GoogleScholarGoogle Scholar |
Lotze, HK, Lenihan, HS, Bourque, BJ, Bradbury, RH, Cooke, RG, Kay, MC, Kidwell, SM, Kirby, MX, Peterson, CH, and Jackson, JBC (2006). Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809.
| Depletion, degradation, and recovery potential of estuaries and coastal seas.Crossref | GoogleScholarGoogle Scholar | 16794081PubMed |
McComb AJ, Lukatelich RJ (1995) The Peel–Harvey Estuarine System, Western Australia. In ‘Eutrophic Shallow Estuaries and Lagoons’. (Ed. AJ McComb) pp. 5–17. (CRC Press: Boca Raton, FL, USA)
Oliver, ECJ, Donat, MG, Burrows, MT, et al. (2018). Longer and more frequent marine heatwaves over the past century. Nature Communications 9, 1324.
| Longer and more frequent marine heatwaves over the past century.Crossref | GoogleScholarGoogle Scholar | 29636482PubMed |
Pearce, AF, and Feng, M (2013). The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/2011. Journal of Marine Systems 111–112, 139–156.
| The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/2011.Crossref | GoogleScholarGoogle Scholar |
Pearce A, Helleren S, Marinelli M (2000) Review of productivity levels of Western Australian coastal and estuarine waters for mariculture planning purposes. Fisheries Research Report 123, pp. 1–67, Fisheries Western Australia.
Ponder, WF, and Clark, GA (1988). A morphological and electrophoretic examination of ‘Hydrobia buccinoides’, a variable brackish-water gastropod from temperate Australia (Mollusca: Hydrobiidae). Australian Journal of Zoology 36, 661–689.
| A morphological and electrophoretic examination of ‘Hydrobia buccinoides’, a variable brackish-water gastropod from temperate Australia (Mollusca: Hydrobiidae).Crossref | GoogleScholarGoogle Scholar |
Potter, IC, Veale, L, Tweedley, J, and Clarke, KR (2016). Decadal changes in the ichthyofauna of a eutrophic estuary following a remedial engineering modification and subsequent environmental shifts. Estuarine, Coastal and Shelf Science 181, 345–363.
| Decadal changes in the ichthyofauna of a eutrophic estuary following a remedial engineering modification and subsequent environmental shifts.Crossref | GoogleScholarGoogle Scholar |
Rogers P, Hall N, Valesini F (2010) Science strategy for the Peel Harvey Estuary. Prepared for the Peel–Harvey Catchment Council. (Centre for Fish and Fisheries Research, Murdoch University: Perth, WA, Australia) Available at https://peel-harvey.org.au/wp-content/uploads/2011/11/2010_Science_Strategy.pdf [Verified 7 January 2022]
Rose TH (1994) Comparisons of the benthic and zooplankton communities in the eutrophic Peel–Harvey and nearby Swan Estuaries in south-western Australia. PhD Thesis, Murdoch University, Perth, WA, Australia.
Ruthrof, KX, Breshears, DD, Fontaine, JB, Froend, RH, Matusick, G, Kala, J, Miller, BP, Mitchell, PJ, Wilson, SK, van Keulen, M, Enright, NJ, Law, DJ, Wernberg, T, and Hardy, GESJ (2018). Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses. Nature Scientific Reports 8, 13094.
| Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses.Crossref | GoogleScholarGoogle Scholar |
Semeniuk, V, and Wurm, PAS (2000). Molluscs of the Leschenault Inlet estuary: their diversity, distribution, and population dynamics. Journal of the Royal Society of Western Australia 83, 377–418.
Siegismund, HR (1982). Life Cycle and Production of Hydrobia ventrosa and H. neglecta (Mollusca: Prosobranchia). Marine Ecology - Progress Series 7, 75–82.
| Life Cycle and Production of Hydrobia ventrosa and H. neglecta (Mollusca: Prosobranchia).Crossref | GoogleScholarGoogle Scholar |
Thomson CE (2019) Regional estuaries initiative, estuary condition report: Peel–Harvey 2016/17. (Department of Water and Environmental Regulation: Perth, WA, Australia) Available at https://www.watercorporation.com.au/Our-water/Climate-change-and-WA/Climate-and-Southern-WA [Verified 10 January 2022]
Water Corporation (2022) Climate & Southern WA. Available at https://www.watercorporation.com.au/Our-water/Climate-change-and-WA/Climate-and-Southern-WA [Verified 9 January 2022]
Wells FE (1984) ‘A Guide to the Common Molluscs of South-Western Australian Estuaries.’ (Western Australian Museum: Perth, WA, Australia)
Wells FE, Bryce CW (1990) ‘Seashells of Western Australia’, revised edn. (Western Australian Museum: Perth, WA, Australia)
Wells, FE, and Threlfall, TJ (1980). A comparison of molluscan communities on intertidal sand flats in Oyster Harbour and Peel Inlet, Western Australia. Journal of Molluscan Studies 46, 300–311.
Wells, FE, and Threlfall, TJ (1981). Molluscs of the Peel–Harvey Estuarine System, with comparison to other south-western Australian estuaries. Journal of the Malacological Society of Australia 5, 101–111.
| Molluscs of the Peel–Harvey Estuarine System, with comparison to other south-western Australian estuaries.Crossref | GoogleScholarGoogle Scholar |
Wells, FE, and Threlfall, TJ (1982a). Salinity and temperature tolerance of Hydrococcus brazieri (T. Woods, 1876) and Arthritica semen (Menke, 1843) from the Peel–Harvey Estuarine System, Western Australia. Journal of the Malacological Society of Australia 5, 151–156.
Wells, FE, and Threlfall, TJ (1982b). Reproductive strategies of Hydrococcus brazieri and Arthritica semen in Peel Inlet, Western Australia. Journal of the Malacological Society of Australia 5, 157–166.
| Reproductive strategies of Hydrococcus brazieri and Arthritica semen in Peel Inlet, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Wells, FE, and Threlfall, TJ (1982c). Density fluctuations, growth and production of Hydrococcus brazieri and Arthritica semen in the Peel–Harvey Estuarine System, Western Australia. Journal of Molluscan Studies 48, 310–320.
| Density fluctuations, growth and production of Hydrococcus brazieri and Arthritica semen in the Peel–Harvey Estuarine System, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Wells FE, Threlfall TJ, Wilson BR (1980) The Peel–Harvey Estuarine System Study 1976–1980, Technical Report, Biology of Molluscs. Bulletin 97, Department of Conservation and Environment, Perth, WA, Australia.
Whisson, CS, Wells, FE, and Rose, T (2004). The benthic invertebrate fauna of the Peel–Harvey Estuary of south-western Australia after completion of the Dawesville Channel. Records of the Western Australian Museum 22, 81–90.
| The benthic invertebrate fauna of the Peel–Harvey Estuary of south-western Australia after completion of the Dawesville Channel.Crossref | GoogleScholarGoogle Scholar |
Wildsmith, MD, Rose, TH, Potter, IC, Warwick, RM, and Clarke, KR (2011). Benthic macroinvertebrates as indicators of environmental deterioration in a large microtidal estuary. Marine Pollution Bulletin 62, 525–538.
| Benthic macroinvertebrates as indicators of environmental deterioration in a large microtidal estuary.Crossref | GoogleScholarGoogle Scholar | 21195437PubMed |
Wilson BR (1994) ‘Australian Marine Shells Prosobranch Gastropods.’ (Odyssey Printing: Perth, WA, Australia)
Young, GC, and Potter, IC (2003). Influence of an artificial entrance channel on the ichthyofauna of a large estuary. Marine Biology 142, 1181–1194.
| Influence of an artificial entrance channel on the ichthyofauna of a large estuary.Crossref | GoogleScholarGoogle Scholar |