Do opposites attack? Resource interactions between an alien and native crayfish from the Lake Eyre Basin
Georgia King A * , Stephen Balcombe A , Samantha Capon A and Bernie Cockayne B
A * , Stephen Balcombe A , Samantha Capon A and Bernie Cockayne B
A Australian Rivers Institute, Griffith University, Nathan, Qld 4111, Australia.
B Reef Catchments, Suite 1/85 Gordon Street, Mackay, Qld 4740, Australia.
Marine and Freshwater Research 73(7) 873-883 https://doi.org/10.1071/MF21302
Submitted: 18 October 2021 Accepted: 21 March 2022 Published: 13 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Investigating resource competition between introduced and native species is important to understand the impacts of invasive species, not only on native species, but also with respect to the wider ecosystem. Within the Lake Eyre Basin, there is concern that feral populations of the tropical Cherax quadricarinatus are outcompeting the basins’ native crayfish species, the temperate–subtropical Cherax destructor.
Aims: This study sought to observe the behavioural and inter-specific food competition between juvenile C. quadricarinatus and C. destructor under experimental conditions to inform whether C. quadricarinatus has the potential to outcompete native C. destructor populations.
Methods: Interactions were quantified by establishing a behavioural intensity score, dominance score and recording the total time in possession of the food resource in a range of interspecific and intraspecific experimental pairings at 26°C.
Key results: Cherax quadricarinatus had a significantly higher dominance (z = −2.276, P = 0.023) and behavioural intensity score (t = 4.723, P < 0.001) than did C. destructor, but there was no difference between the two species for time in possession of the food resource (z = −1.334, P = 0.182).
Conclusions and implications: These results have significant ecological implications because the capacity of C. quadricarinatus to displace C. destructor, a keystone species, has the potential to irreversibly alter ecosystem function in invaded habitats.
Keywords: aquarium experiment, behavioural study, Cherax destructor, Cherax quadricarinatus, competition, crayfish behaviour, invasion ecology, species interactions.
Introduction
Human pressures and alterations to the environment create novel pathways that allow species to spread beyond their historical range and invade previously isolated habitats. Such locally alien organisms can pose a significant threat to freshwater ecosystems, which are already extremely vulnerable to biodiversity loss because of many other anthropogenic pressures, including water resource development and climate change (Dudgeon et al. 2006). Although the potential impacts of species invasions are inherently complex, investigating resource interactions affords an opportunity to understand potential trophic interactions and disturbances of invasive species.
Keystone species have a disproportionally high effect on ecosystem function in proportion to abundance (Valiente-Banuet et al. 2015). Within freshwater ecosystems, ecosystem engineers, defined as species that create, destroy or significantly modify a habitat, can hugely affect other species through altering water quality, nutrient content and organic matter (Gallardo et al. 2016). Decapods, such as crayfish, are generalist omnivores that typically act as both ecosystem engineers and keystone species within their natural ecosystem (Strayer 2010). Locally alien crayfish species can therefore cause significant and irreversible alterations to functions and the interactions among the species within an invaded ecosystem (Gherardi et al. 2011; Twardochleb et al. 2013).
The red-claw crayfish (Cherax quadricarinatus), native to northern Australia and Papua New Guinea, is a species with significant invasive potential in Australia. Its attractive features, large maturing size and high flesh to size ratio have made it a sought-after species within the international aquarium and aquaculture trades. The robust and adaptable C. quadricarinatus is a formidable invasive species, with feral populations recorded across the globe originating from escapees of aquarium and aquaculture activities (Twardochleb et al. 2013). Recently, feral populations of C. quadricarinatus have been identified within the Lake Eyre Basin (LEB) of central Australia (Leland et al. 2012; Kingsford et al. 2014). This range expansion is theorised, as in the case of the international examples, to be the combined result of aquaculture escapees, private dam stocking escapees and fishing bait escapees, and there is a growing awareness of the threats posed by this species as an invasive (Leland et al. 2012). The appearance of C. quadricarinatus in the LEB is particularly concerning because of the biologically unique nature of the basin’s catchments. Unlike many other aquatic ecosystems globally and within Australia, rivers and wetlands of the LEB are largely untouched by invasive species, with other recorded invasive species being fish, namely, sleepy cod (Oxyeleotris lineolata), goldfish (Carassius auratus) and mosquito fish (Gambusia affinis; Kingsford et al. 2014; Sternberg and Cockayne 2018).
The LEB is a highly arid environment and the survival of many of its species, both aquatic and terrestrial, rely on periods of high productivity, associated with large flows after unpredictable and uncommon rainfall events (Arthington and Balcombe 2011; Kingsford et al. 2014; Lake Eyre Basin Ministerial Forum 2017). Rarely being the result of local rainfall, these massive flows often originate from monsoonal rainfall in northern Queensland. From there, floodwaters make their way south through the Cooper and Diamantina–Georgina catchments, an event known as a ‘dry flood’ (Kingsford 2017). Characterised by a ‘boom and bust’ ecology, isolated waterholes sustain aquatic biodiversity within the LEB during the dry ‘bust’ period. Here, specialised endemic aquatic species, such as the endangered Cooper Creek catfish (Neosiluroides cooperensis), survive until the next significant flow event fills the river channels, connecting previously isolated waterholes (Arthington and Balcombe 2011; Arthington et al. 2019). During the contrasting ‘boom’ periods, large numbers of wetland birds migrate towards the LEB to feed and reproduce following the significant increase in biomass of aquatic organisms (Arthington and Balcombe 2011; Arthington et al. 2019). This high concentration of endemic aquatic species, and importance to Australia’s wetland birds, contributes to the high conservation value of the LEB (Kingsford et al. 2014; Kingsford 2017). Unlike the neighbouring Murray–Darling Basin, Australia’s other extensive dryland basin system, the LEB is mostly unregulated, resulting in the preservation of the unique biodiversity that relies on ‘boom and bust’ ecology (Costelloe et al. 2003; Kingsford et al. 2014; Kingsford 2017; Lake Eyre Basin Ministerial Forum 2017).
Both published and unpublished observations have identified C. quadricarinatus populations within the LEB (Kingsford et al. 2014). C. quadricarinatus has the ability to grow to almost twice the weight and twice as fast as the only native crayfish species in the LEB, the blue-claw crayfish (Cherax destructor). Crayfish dominance is highly affected by size, with larger individuals overpowering smaller specimens (Moore 2007). Consequently, there is concern that this obvious size advantage will allow C. quadricarinatus to outcompete and displace the native C. destructor species within the LEB (Masser and Rouse 1997; Moore 2007). This feature may be particularly relevant in terms of food resource competition, because both species are opportunistic omnivores that inhabit the same ecological niche. The replacement of a keystone native species with a larger, more aggressive, faster-growing invasive species has the potential to affect all species within the given ecosystem (Carpenter et al. 1985). There is concern that this interaction may pressure the unique refuge ecosystems of the LEB during the vulnerable ‘bust’ period when resources are limited (Kingsford et al. 2014).
Anecdotal records have recounted some form of interaction between invasive C. quadricarinatus and native C. destructor within the LEB, with a noted reduction in C. destructor numbers when C. quadricarinatus is present (Kingsford et al. 2014; Lake Eyre Basin Ministerial Forum 2017; G. King, unpubl. data). However, there has not previously been any formal research or monitoring effort dedicated to assessing the nature of this interaction. This study investigated behavioural and inter-specific food competition between the locally alien C. quadricarinatus and the native C. destructor under experimental conditions. The primary objective of this study was to examine the dynamics between the two species to better understand their likely behavioural interactions in the field, and to determine whether C. quadricarinatus has the potential to outcompete or exclude C. destructor populations. We hypothesised that C. quadricarinatus would exhibit significantly more aggressive behaviours than would C. destructor and be more dominant in interactions (Hypothesis 1). We also hypothesised that this higher aggression and dominance would translate to C. quadricarinatus being in possession of a food resource for significantly longer than would C. destructor (Hypothesis 2).
Materials and methods
Study species
Cherax destructor is a wide-spread medium-sized Australian crayfish species, with an average maturing occipital carapace length (OCL, measured from the rostrum’s tip to the end of the carapace) of 200 mm. Although native to the temperate—subtropical areas of central and eastern Australia, its natural range spans across all states and territories except for Tasmania and Western Australia (Nguyen et al. 2004). As a result of a continuing decline in native habitat and fragmented native populations, C. destructor was listed as a vulnerable species by the IUCN in 1996 (Crandall 1996). Popular in aquaculture farming and as recreational fishing bait, C. destructor has been introduced to areas outside its natural range and can now be found in all states and territories in Australia and has introduced populations internationally (Lopez et al. 2019). A versatile species, C. destructor, can inhabit a wide range of ecological niches, ranging from semi-arid temporary water systems of the LEB to the cooler, more permanent southern Australia waterbodies (Nguyen et al. 2004).
Cherax quadricarinatus of the tropical northern Australia and Papua New Guinea is the larger of the two species but rarely grows to an OCL longer than 350 mm. C. quadricarinatus has similarly been heavily farmed both nationally and internationally for human food production (Souty-Grosset et al. 2006). Escapees have established feral populations internationally, with populations having been recorded in Costa Rica, Mexico, Africa and Thailand (Bortolini et al. 2007; Azofeifa-Solano et al. 2017; Petersen et al. 2017; Chaichana and Wanjit 2018; Douthwaite et al. 2018). Although being native to northern Australia, C. quadricarinatus has been identified as far south as Lake Ainsworth in New South Wales and within the biologically significant LEB (Leland et al. 2012; Kingsford et al. 2014). This range change is theorised, like the international examples, to be the result of aquaculture escapees from both commercial and private establishments in the area (Leland et al. 2012).
Experimental design
Crayfish collection
Forty individuals of each species were purchased from local and online suppliers, with 24 individuals of each species being included in the experiments. Any crayfish with visible parasites, missing limbs, visible injuries, or that had recently moulted were excluded from the experiment. All crayfish were sexed, because sex influences crayfish behaviour and must be controlled for in behavioural experiments (Moore 2007). Crayfish were sexed on the basis of the presence of genital papillae at the base of the bottom set of legs on males. Females lack this feature and instead have ovary openings at the base of the third set of legs. All crayfish used in the experiments were juveniles, ranging in size from an OCL of 23 to 32 mm. This choice came down to experiment practicality, with juvenile crayfish being easier to source. There is no evidence to suggest that this would affect the behaviour of the crayfish when carapace lengths differ no more than 20% (Moore 2007).
Crayfish housing
Crayfish were individually housed in 15-L opaque aquaria, each containing one PVC pipe for shelter (100-mm length, 45-mm inside diameter), a sand substrate of 2-cm depth and 5 L of rainwater. All aquaria were housed in a controlled laboratory environment, with crayfish being exposed to a natural (uncontrolled) photoperiod of an average 11 h of daylight. Water temperature was maintained at 26°C, with C. quadricarinatus originating from the tropical north, and C. destructor from cooler subtropical–temperate habitats; this is the mid-range of optimal temperature for both species (Masser and Rouse 1997).
During a 1-week acclimation period, crayfish were fed a mixture of bottom-feeder shrimp pellets (API, America), cooked zucchini and raw green prawns 3 times per week. Crayfish were isolated for at least 7 days to allow the natural dominance state of the crayfish to reset after an agonistic interaction (Schneider et al. 1999; Moore 2007). Because the aquaria did not contain any form of filtration, a water change was completed the day after feeding to remove any uneaten food. After the experiment, all crayfish were observed for 1 week, with any crayfish that moulted during this period being excluded from the experiment (Gherardi et al. 2011).
Competition experiment
After the acclimation period, all crayfish were starved for 7 days to standardise food motivation. After this starvation period, the OCL of each crayfish was measured. Crayfish pairs were then established, comprising size- and sex-matched C. destructor and C. quadricarinatus, including, in total, 12 inter-specific pairs (comprising six male and six female pairs), 6 C. destructor-only pairs (three male and three female pairs) and 6 C. quadricarinatus-only pairs (three male and three female pairs; see Supplementary material Table S4). Dominance among crayfish individuals is highly dependent on individual carapace size; therefore, OCL size difference was limited to 10% (Hudina et al. 2016; Lopez et al. 2019; see Table S4). Although chelae size also plays a role in dominance among crayfish individuals, it was impossible to control for this variation because of the inherent differences between the species. C. quadricarinatus has skinnier chelae than does C. destructor, an anatomical feature that is particularly evident in adult individuals. Therefore, juvenile crayfish individuals were preferred, allowing a chelae width and length difference of less than 20% (Moore 2007).
Interactions between pairs took place in 60 × 30 × 30-cm aquaria divided into three sections by opaque dividers. The aquaria contained 2 cm of gravel substrate and were held at a constant temperature of 26°C. A food resource (2-cm cube of raw green prawn tissue and 2-cm cube of cooked zucchini) was wrapped in flyscreen and secured to the bottom of the middle compartment, thus allowing the food resource to be interacted with without being manipulated or consumed (Lopez et al. 2019). The crayfish were separately placed in each of the end compartments and allowed 15 min to acclimatise to the new aquaria conditions before interacting with each other and the food resources (Fig. 1).
After the acclimation period, the opaque dividers were removed, and the crayfish pair were allowed to interact with each other and the food resource for 20 min. The whole interaction was video recorded with all persons removed from the laboratory to prevent observer effects. Each experiment occurred at night between 1900 and 2400 hours, illuminated with a red light that is a spectrum that crayfish cannot detect (Dalosto et al. 2015). After the experimental period, the two individuals were returned to their separate aquaria and fed. This experiment was conducted once with each pair.
Behavioural observations
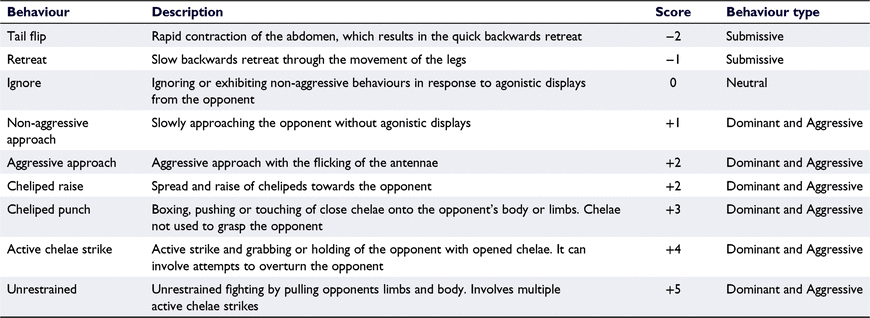
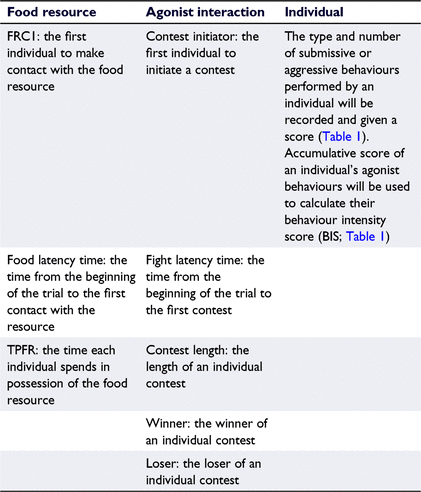
Each recorded experiment was viewed, and the interactions were recorded following the procedures of Dalosto et al. (2015) and Moore (2007). The amount of time an individual spent within 1 cm of the food resource without the competitor was considered ‘time in possession of the food resource’ (TPFR) and was regarded as an individual’s capacity to dominate a food resource. The number of agonistic bouts, in which both individuals were initiated in a fight lasting at least 10 s, was also recorded. The individual that retreated first was identified as the loser, with the crayfish that won the most of these interactions identified as the winner (Dalosto et al. 2015). All interactions were scored through the behavioural ethogram presented in Table 1 (modified from Moore 2007). Additional behaviours that were recorded for further analysis are shown in Table 2. For inter-specific interactions only, the dominance score was calculated for each individual of a pair by the number of contests the individual won divided by the total number of contests between the two contestants. The behavioural intensity score (BIS) for each individual was calculated through the sum total of the associated score for each behaviour observed during the interaction trial, as shown in Table 1.
|
|
Data analysis
For intra-specific interactions, there were not enough trials to ensure statistical robustness. Therefore, no analysis was conducted. Instead, the average of the measured variables (Table 2) was calculated in Microsoft Excel (ver. 16.29.1, Microsoft Corporation, see https://www.microsoft.com). For inter-specific pairs, the differences between individuals in the ‘dominance score’ and ‘time spent in possession of the food resource’ variables were analysed using the non-parametric Wilcoxon signed-ranks test (Quinn and Keough 2002).
Differences between the BIS of the individuals in the inter-specific pairs were analysed using a paired t-test, because all normal distribution assumptions were met. All analyses were conducted in SPSS (ver. 25.0, IBM Corporation) with the significance level set to α = 0.05.
Results
Intra-specific interactions
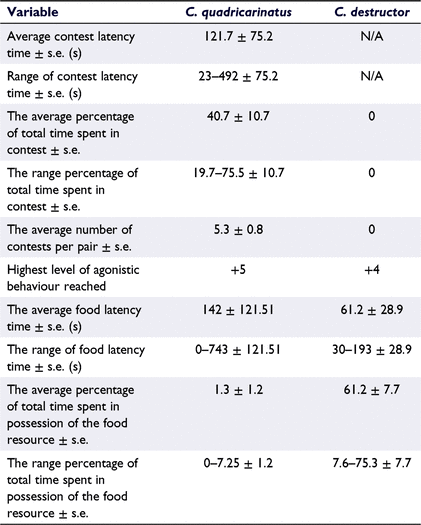
Cherax quadricarinatus in intra-specific pairs spent an average of 40% of the time in contest, averaging 5.3 contests per pair (Table 3). The maximum intensity reached was unrestrained fighting (+5), with an average contest latency time of 121.7 s (Table 3). C. quadricarinatus pairs had an average combined time in possession of the resource of 1.3%, with a latency time of 142 s. By contrast, none of the six C. destructor pairs engaged in contests, with the maximum level of agonistic behaviour reached of active chelae strike (+4; Table 3). On average, however, the C. destructor pairs reached the food resource twice as fast as the C. quadricarinatus at 61.2 s and spent an average of 61.2% of the total trial time in possession of the food resource (Table 3).
|
Inter-specific interactions
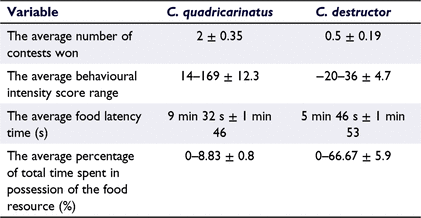
In the inter-specific pairs, C. quadricarinatus specimens were ∼3.5 times more dominant than were the C. destructor specimens, with the Wilcoxon signed-rank test identifying this as a significant difference (z = −2.276, P = 0.023; Fig. 2, see Supplementary material Table S1). With an average of 2.5 contests per pair, C. quadricarinatus won 4 times as many contests as did C. destructor (Table 4). The maximum number of fights conducted by a pair was nine, with a minimum of one fight per pair. C. quadricarinatus individuals were significantly more aggressive, with a BIS ∼7 times higher than that of their C. destructor opponents (t = 4.723, P < 0.001; Fig. 3, Table 4). On average, C. destructor possessed the food resource 6 times longer than did C. quadricarinatus (Fig. 4, Table 4); although this was not a significant difference (z = −1.334, P = 0.182). On occasions when C. destructor was able to take possession of the food resource, it did so almost twice as fast as did C. quadricarinatus (Table 4).
|
|
|
|
|
Cherax destructor displayed significantly more submissive and neutral behaviours, identified as tail flip (z = −2.527, P = 0.012), retreat (z = −2.083, P = 0.037) and ignore (z = −2.032, P = 0.042), than did their C. quadricarinatus opponents (Fig. 5). Comparatively, C. quadricarinatus performed significantly more aggressive behaviours, identified as active chelae strike (z = −2.955, P = 0.003), cheliped punch (z = −2.680, P = 0.007) and aggressive approach (z = P = 0.012) (Fig. 5). The frequency of the behaviours of unrestrained combat and non-aggressive approach was not significantly different between the species (Fig. 5).
|
|
Discussion
Invasion of an alien crayfish into a large and highly intact river basin poses significant threats to global pursuits to protect freshwater ecosystems and their assemblages from invasive species. The translocation of C. quadricarinatus to the LEB has become a significant concern to the scientific and general community over the recent decade, particularly in relation to its interactions with the native C. destructor (Kingsford et al. 2014; Firn et al. 2015; Schmarr et al. 2016). Unpublished data by King, as well as the Lake Eyre Basin Ministerial Forum (2017), has located established C. quadricarinatus populations within the three major catchments of the Queensland segment of the LEB, the Diamantina, Georgina and the Cooper catchments. Sites with C. quadricarinatus detected had an observed reduction in C. destructor captured compared with sites where C. quadricarinatus was not detected, with the Lake Eyre Basin Ministerial Forum (2017) classifying C. quadricarinatus as a high-risk invasive. The results of our study suggested that where C. quadricarinatus has established populations within the LEB, it may pose a risk to the native C. destructor through its aggressive and dominant behaviours, although the mechanisms enabling C. quadricarinatus to dominate C. destructor may be more complex than originally thought.
In accordance with Hypothesis 1 that C. quadricarinatus will show significantly more aggressive behaviours than does C. destructor and will also be more dominant, the invasive C. quadricarinatus displayed more aggressive behaviours leading to a higher dominance than for the native C. destructor in experimental interactions, supporting this hypothesis. However, this clear dominance did not result in a higher resource-holding capacity, with C. destructor possessing the food resource for a longer, albeit statistically non-significant, time. This result does not support the predictions of Hypothesis 2 that C. quadricarinatus will possess a food resource for significantly longer than does the C. destructor during laboratory-based pairwise trials.
Cherax quadricarinatus and C. destructor interaction dynamics
Dominance
Dominance, defined here as the ability to win more agonistic contests, is one of the many factors that contribute to the displacement of endemics by invasive species (Moore 2007). For crayfish, in particular, dominance plays an essential role in the success of an invasive species within a habitat that already contains a native crayfish species (Thomas Lorenz et al. 2019). This dominance can translate into the possession of food resource, shelter and territory, all of which enhance an individual’s chance of survival (Moore 2007). The results of this study indicated that the trend for invasive crayfish species to be more dominant than their native counterparts, as was also indicated in a meta-analysis by Twardochleb et al. (2013), extends to the interactions between the invasive C. quadricarinatus and the native C. destructor in the LEB. The results of this study indicated that C. destructor is likely to be a submissive, almost placid, contestant. The placid behaviour of C. destructor was highlighted in the intra-specific trials, where same-species contestants behaved in a communal nature, choosing to share the resource rather than fight for it. This starkly contrasts to the behaviour of C. quadricarinatus in the intra-specific trials, which displayed a high motivation to establish dominance through aggression. The intra-specific trials further pronounced the behaviours observed in the inter-specific trials, particularly that of C. destructor, which had no recorded fights.
The behaviour of C. destructor contrasts with the findings of Lynas et al. (2007), where C. destructor won significantly more interactions during contests with Cherax cainii. Lynas et al. (2007) linked this result to the larger chelae of C. destructor, than that of C. cainii, a physiological characteristic that is closely linked to crayfish dominance (Moore 2007). Although precise chelae size was not used in the size matching of individuals within this study, all crayfish pairs had a chelae-size difference of less than 20%, a degree which Moore (2007) reported would not significantly affect dominance in crayfish. This decision was made as C. quadricarinatus generally has thinner chelae than does C. destructor; therefore, it was not possible to match individuals on the basis of both chelae and carapace size. A carapace size match was prioritised over chelae, because this was determined to play a larger role in determining dominance (Moore 2007; see Table S4).
The results of this study, and that of Lynas et al. (2007), suggested that crayfish dominance varies across species. Although a species may be submissive in one situation with one opponent species, it may be dominant in another interaction with a different species. This pattern of behaviour appears to be the case with C. destructor, which has a varied level of dominance depending on the opponent species. This pattern further unfolds when comparing the results of Lynas et al. (2007) and Lopez et al. (2019). Lopez et al. (2019) similarly undertook behavioural interactions between carapace size-matched C. destructor (the invader) and a native crayfish species. Contrasting to the results of Lynas et al. (2007), the native crayfish species, Euastacus dharawalus, was much more aggressive, with C. destructor taking on a submissive role. Many factors could contribute to this variation in behaviour, including differences in the species physiological makeup, including body and chela ratio and differences in neurochemistry and motivation (Moore 2007). A notable factor that may also influence such behavioural variations is the invaded species’ historic interactions with the invader. Lopez et al. (2019) collected both invaded and invader from the same creek system. Invaded specimens that are already familiar with the invader may exhibit more aggressive behaviours than one that is naïve to the invasive species (Moore 2007). A meta-analysis by Twardochleb et al. (2013) showed that, overall, crayfish that are invasive are more likely to display aggressive and dominant behaviours; this was linked to the enhanced competitive ability of many invasive crayfish species, although deviations from this trend are present, as can be observed with the behaviour of C. destructor.
Agonistic behaviours
Similar to patterns in dominance, invasive crayfish species generally exhibit more agonistic behaviours than do their native contestants (Dalosto et al. 2015). As was the case with dominance, this trend extends to the interactions between C. quadricarinatus and C. destructor observed in this study, with C. quadricarinatus exhibiting predominately agonistic behaviours and C. destructor exhibiting predominately submissive behaviours. Because dominance and the performance of agonistic behaviours are closely linked, this was not unexpected.
On average, C. destructor performed tail flips 6 times more than did C. quadricarinatus during the inter-specific trials. Tail flips have been identified as a significant indication of a crayfish’s submission during agonistic interactions (Moore 2007). Although tail flips are common in laboratory studies and do not always indicate the termination of further contests, fights in wild conditions rarely end in a tail flip, with the fight breaking up and the submissive individual retreating before the fight intensity warrants a hasty escape (Bergman and Moore 2003). The tendency for invasive crayfish to display more aggressive behaviours than does native crayfish during inter-specific interactions was highlighted in the meta-analysis of the impact of invasive crayfish by Twardochleb et al. (2013), although it was also noted that the link between more aggressive behaviours and a decline in native crayfish populations was highly variable within the literature.
Resource-holding capacity
Although many studies have concluded that invasive crayfish species are more aggressive and dominant, directly increasing their resource-holding capacity (such as Procambarus clarkii in studies by Dalosto et al. 2015), this was not observed in the inter-specific interactions performed in our study (Gherardi et al. 2011; Twardochleb et al. 2013). In particular, the high aggression of C. quadricarinatus did not translate to a high resource-holding capacity. This surprising result is not the consequence of an inadequate food source that one species prefers, because preliminary trials testing appropriate food resources found that both species were attracted to, and consumed, the chosen food resource of a raw green prawn and cooked zucchini cube. Similar results were found in a study by Lopez et al. (2019), in which C. destructor had a significantly higher resource-holding capacity than did the more aggressive E. dharawalus. In this case, the role of C. destructor was reversed from that of the current study, with it being the invasive species within native habitat of E. dharawalus.
In the intra-specific trials conducted here, the readiness of C. quadricarinatus to fight with its own species was surprising because multiple studies have labelled it as gregarious, and it is often farmed happily in high densities (Masser and Rouse 1997; Bortolini et al. 2007). We suspect that this social nature does not extend to periods when food availability is low and, after the initial contest to determine social hierarchy, C. quadricarinatus might be a more social species. The disconnect between crayfish aggression and resource-holding capacity observed here would suggest that, rather than aggression being the main indicator of the ability to acquire an essential resource, some other factors may be at play. Factors involving the establishment of an invasive crayfish species may be much more complex than variables such as aggression and resource-holding capacity. In both the inter-specific and intra-specific trials, C. destructor spent more time in possession of the food resource. This suggests that C. destructor may have a higher food motivation, prioritising food possession over the assertion of dominance, than does C. quadricarinatus, which instead has a high fight drive, prioritising the assertion of dominance over resource possession (Lopez et al. 2019). This would explain the polar opposite reactions of the two species within the intra-specific trails in relation to food and agonistic interactions.
Additional factors
Environmental factors such as temperature may affect species behaviour, with temperature effects driving metabolic responses (Díaz et al. 2004; Tattersall et al. 2012). This response to temperature is likely to vary among species depending on climatic preferences. In this instance, the study observed two species hailing from different climates and temperature preferences (Nguyen et al. 2004; Souty-Grosset et al. 2006). Recorded optimal temperature range varies in the literature for both species, although generally, C. destructor is described as having an optimal survival range of 15–32°C, with a preferred temperature of 25°C, whereas C. quadricarinatus had an optimal range of 22–31°C with a preferred temperature of 28°C (King 1994; Verhoef et al. 1998; Verhoef and Austin 1999; Meade et al. 2002). Owing to time constraints, it was not possible to conduct this experiment under a variety of temperatures. Instead, 26°C was chosen as within the optimal thermal range of both species and between their preferred temperatures (King 1994; Verhoef et al. 1998; Verhoef and Austin 1999; Meade et al. 2002).
Juvenile specimens were exclusively used in this study, owing to both availability and financial constraints. Whereas this does allow a foundation to be laid in behavioural dynamics between C. destructor and C. quadricarinatus, more robust studies in the future should include a variety of OCL sizes, with an emphasis on mature specimens. This, particularly if paired with multiple experiment temperatures, will allow for results that more closely mimic that of the variable nature of the LEB.
Conclusions
This study has demonstrated that where C. quadricarinatus is present within the LEB, it is likely to pose a significant threat to C. destructor populations because of it higher dominance and readiness to perform agonistic behaviours. C. quadricarinatus’ aggressive behaviour resulted in its dominance over the more submissive native C. destructor; however, this did not translate to dominance over a food resource at 26°C. The results suggested that C. quadricarinatus may well be affecting the C. destructor population within the Lake Eyre Basin, but that this interaction is not food-based competition. Instead, C. quadricarinatus may be more driven by territory establishment than food resources, which would explain why the presence of C. quadricarinatus within the Lake Eyre Basin has resulted in an observed reduction of C. destructor numbers (G. King, unpubl. data). The ability of C. quadricarinatus to grow substantially bigger than its native competitor only increases its ability to exclude C. destructor. This is of particular concern during the ‘bust’ periods within the Lake Eyre Basin when habitat is reduced to small remnant pools, reducing the amount of available territory and leaving the C. destructor with no escape from its aggressive invaders. Further research is needed to confirm which factors are contributing to the exclusion of C. destructor by C. quadricarinatus.
Supplementary material
Supplementary material is available online.
Data availability
Data are available from the authors upon reasonable request.
Conflicts of interest
Samantha Capon is an Associate Editor of Marine and Freshwater Research but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practise when handling manuscripts submitted by an editor to this journal. Marine and Freshwater Research encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
The authors thank the Australian Government, through the Department of Agriculture, Water and the Environment, for providing financial assistance to conduct this study in the form of the ‘Lake Eyre Basin – Justin Costelloe PhD Scholarship’ (2019). The authors also thank the Lamington Natural History Association for providing further financial support through the ‘Lamington Natural History Bursary’ (2019).
Acknowledgements
The authors thank and acknowledge the pastoralists and Traditional Owners of the land in which this study was conducted.
References
Arthington, AH, and Balcombe, SR (2011). Extreme flow variability and the ‘boom and bust’ ecology of fish in arid-zone floodplain rivers: a case history with implications for environmental flows, conservation and management. Ecohydrology 4, 708–720.| Extreme flow variability and the ‘boom and bust’ ecology of fish in arid-zone floodplain rivers: a case history with implications for environmental flows, conservation and management.Crossref | GoogleScholarGoogle Scholar |
Arthington AH, Sternberg D, Cockayne B, Schmarr DW (2019) Cooper creek catfish Neosiluroides cooperensis. In ‘The IUCN Red List of Threatened Species 2019’. e.T122900149A123382031. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/122900149/123382031
Azofeifa-Solano, JC, Naranjo-Elizondo, B, Rojas-Carranza, AH, and Cedeno-Fonseca, M (2017). Presence of the Australian redclaw crayfish Cherax quadricarinatus (von Martens, 1868) (Parastacidae, Astacoidea) in a freshwater system in the Caribbean drainage of Costa Rica. BioInvasions Records 6, 351–355.
| Presence of the Australian redclaw crayfish Cherax quadricarinatus (von Martens, 1868) (Parastacidae, Astacoidea) in a freshwater system in the Caribbean drainage of Costa Rica.Crossref | GoogleScholarGoogle Scholar |
Bergman, DA, and Moore, PA (2003). Field observations of intraspecific agonistic behavior of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. The Biological Bulletin 205, 26–35.
| Field observations of intraspecific agonistic behavior of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats.Crossref | GoogleScholarGoogle Scholar | 12917219PubMed |
Bortolini, JL, Alvarez, F, and Rodríguez-Almaraz, G (2007). On the presence of the Australian redclaw crayfish, Cherax quadricarinatus, in Mexico. Biological Invasions 9, 615–620.
| On the presence of the Australian redclaw crayfish, Cherax quadricarinatus, in Mexico.Crossref | GoogleScholarGoogle Scholar |
Carpenter, SR, Kitchell, JF, and Hodgson, JR (1985). Cascading trophic interactions and lake productivity. Bioscience 35, 634–639.
| Cascading trophic interactions and lake productivity.Crossref | GoogleScholarGoogle Scholar |
Chaichana, R, and Wanjit, C (2018). Impacts, control and perception of introduced crayfish in Thailand. Aquatic Ecosystem Health & Management 21, 60–69.
| Impacts, control and perception of introduced crayfish in Thailand.Crossref | GoogleScholarGoogle Scholar |
Costelloe, JF, Puckridge, JT, Reid, JRW, Pritchard, J, Hudson, P, Bailey, V, and Good, M (2003). Environmental flow requirements in arid zone rivers – a case study from the Lake Eyre Basin, central Australia. Water Science and Technology 48, 65–72.
| Environmental flow requirements in arid zone rivers – a case study from the Lake Eyre Basin, central Australia.Crossref | GoogleScholarGoogle Scholar | 14653635PubMed |
Crandall K (1996). Cherax destructor. In ‘The IUCN Red List of Threatened Species’. e.T4622A11042150. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/4622/11042150
Dalosto, MM, Palaoro, AV, Souty-Grosset, C, Bueno, SLDS, Loureiro, TG, Almerão, MP, De Araujo, PB, and Santos, S (2015). One step ahead of the enemy: investigating aggressive interactions between invasive and native crayfish before the contact in nature. Biological Invasions 17, 3503–3515.
| One step ahead of the enemy: investigating aggressive interactions between invasive and native crayfish before the contact in nature.Crossref | GoogleScholarGoogle Scholar |
Díaz, F, Re, AD, Sierra, E, and Amador, G (2004). Behavioural thermoregulation and critical limits applied to the culture of red claw crayfish Cherax quadricarinatus (Von Martens). Freshwater Crayfish 14, 90–98.
| Behavioural thermoregulation and critical limits applied to the culture of red claw crayfish Cherax quadricarinatus (Von Martens).Crossref | GoogleScholarGoogle Scholar |
Douthwaite, RJ, Jones, EW, Tyser, AB, and Vrdoljak, SM (2018). The introduction, spread and ecology of redclaw crayfish Cherax quadricarinatus in the Zambezi catchment. African Journal of Aquatic Science 43, 353–366.
| The introduction, spread and ecology of redclaw crayfish Cherax quadricarinatus in the Zambezi catchment.Crossref | GoogleScholarGoogle Scholar |
Dudgeon, D, Arthington, AH, Gessner, MO, Kawabata, ZI, Knowler, DJ, Lévêque, C, Naiman, RJ, Prieur-Richard, AH, Soto, D, Stiassny, MLJ, and Sullivan, CA (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews of the Cambridge Philosophical Society 81, 163–182.
| Freshwater biodiversity: importance, threats, status and conservation challenges.Crossref | GoogleScholarGoogle Scholar | 16336747PubMed |
Firn, J, Maggini, R, Chadès, I, Nicol, S, Walters, B, Reeson, A, Martin, TG, Possingham, HP, Pichancourt, JB, Ponce-Reyes, R, and Carwardine, J (2015). Priority threat management of invasive animals to protect biodiversity under climate change. Global Change Biology 21, 3917–3930.
| Priority threat management of invasive animals to protect biodiversity under climate change.Crossref | GoogleScholarGoogle Scholar | 26179346PubMed |
Gallardo, B, Clavero, M, Sánchez, MI, and Vilà, M (2016). Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biology 22, 151–163.
| Global ecological impacts of invasive species in aquatic ecosystems.Crossref | GoogleScholarGoogle Scholar | 26212892PubMed |
Gherardi, F, Aquiloni, L, Diéguez-Uribeondo, J, and Tricarico, E (2011). Managing invasive crayfish: is there a hope? Aquatic Sciences 73, 185–200.
| Managing invasive crayfish: is there a hope?Crossref | GoogleScholarGoogle Scholar |
Hudina, S, Hock, K, Radović, A, Klobučar, G, Petković, J, Jelić, M, and Maguire, I (2016). Species-specific differences in dynamics of agonistic interactions may contribute to the competitive advantage of the invasive signal crayfish (Pacifastacus leniusculus) over the native narrow-clawed crayfish (Astacus leptodactylus). Marine and Freshwater Behaviour and Physiology 49, 147–157.
| Species-specific differences in dynamics of agonistic interactions may contribute to the competitive advantage of the invasive signal crayfish (Pacifastacus leniusculus) over the native narrow-clawed crayfish (Astacus leptodactylus).Crossref | GoogleScholarGoogle Scholar |
King, CR (1994). Growth and survival of redclaw crayfish hatchlings (Cherax quadricarinatus von Martens) in relation to temperature, with comments on the relative suitabitity of Cherax quadricarinatus and Cherax destructor for culture in Queensland. Aquaculture 122, 75–80.
| Growth and survival of redclaw crayfish hatchlings (Cherax quadricarinatus von Martens) in relation to temperature, with comments on the relative suitabitity of Cherax quadricarinatus and Cherax destructor for culture in Queensland.Crossref | GoogleScholarGoogle Scholar |
Kingsford RT (2017) ‘Lake Eyre Basin rivers: environmental, social and economic importance.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Kingsford RT, Costelloe JF, Sheldon F (2014) Lake Eyre Basin – challenges for managing the world’s most variable river system. In ‘River basin management in the twenty-first century: understanding people and place’. (Eds VR Squires, HM Milner, KA Daniell) pp. 346–367. (CRC Press: Boca Raton, FL, USA)
Lake Eyre Basin Ministerial Forum (2017) Lake Eyre Basin: State of the Basin Condition Assessment 2016 Report. Department of Agriculture and Water Resources, Canberra, ACT, Australia.
Leland, JC, Coughran, J, and Furse, JM (2012). Further translocation of the redclaw, Cherax quadricarinatus (Decapoda: Parastacidae), to Lake Ainsworth in northeastern New South Wales, Australia. Crustacean Research Special2012, 1–4.
| Further translocation of the redclaw, Cherax quadricarinatus (Decapoda: Parastacidae), to Lake Ainsworth in northeastern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Lopez, LK, Hendry, K, Wong, MYL, and Davis, A (2019). Insight into invasion: interactions between a critically endangered and invasive crayfish. Austral Ecology 44, 78–85.
| Insight into invasion: interactions between a critically endangered and invasive crayfish.Crossref | GoogleScholarGoogle Scholar |
Lynas, J, Storey, AW, and Knott, B (2007). Aggressive interactions between three species of freshwater crayfish of the genus Cherax (Decapoda: Parastacidae). Marine and Freshwater Behaviour and Physiology 40, 105–116.
| Aggressive interactions between three species of freshwater crayfish of the genus Cherax (Decapoda: Parastacidae).Crossref | GoogleScholarGoogle Scholar |
Masser MP, Rouse DB (1997) Australian red claw crayfish. Publication number 244. Southern Regional Aquaculture Center, Stoneville, MS, USA.
Meade, ME, Doeller, JE, Kraus, D, and Watts, SA (2002). Effects of temperature and salinity on weight gain, oxygen consumption rate, and growth efficiency in juvenile red-claw crayfish Cherax quadricarinatus. Journal of the World Aquaculture Society 33, 188–198.
| Effects of temperature and salinity on weight gain, oxygen consumption rate, and growth efficiency in juvenile red-claw crayfish Cherax quadricarinatus.Crossref | GoogleScholarGoogle Scholar |
Moore PA (2007). Agonistic behavior in freshwater crayfish: the influence of intrinsic and extrinsic factors on aggressive behavior and dominance. In ‘Evolutionary ecology of social and sexual systems: Crustacea as model organisms’. (Eds JE Duffy, M Thiel) pp. 90–114. (Oxford University Press: Oxford, UK)
Nguyen, TTT, Austin, CM, Meewan, MM, Schultz, MB, and Jerry, DR (2004). Phylogeography of the freshwater crayfish Cherax destructor Clark (Parastacidae) in inland Australia: historical fragmentation and recent range expansion. Biological Journal of the Linnean Society 83, 539–550.
| Phylogeography of the freshwater crayfish Cherax destructor Clark (Parastacidae) in inland Australia: historical fragmentation and recent range expansion.Crossref | GoogleScholarGoogle Scholar |
Petersen, RM, Hoffman, AC, Kotze, P, and Marr, SM (2017). First record of the invasive Australian redclaw crayfish Cherax quadricarinatus (Von Martens, 1868) in the Crocodile river, Kruger National Park, South Africa. Koedoe 59, 1–3.
| First record of the invasive Australian redclaw crayfish Cherax quadricarinatus (Von Martens, 1868) in the Crocodile river, Kruger National Park, South Africa.Crossref | GoogleScholarGoogle Scholar |
Quinn GP, Keough MJ (2002) ‘Experimental design and data analysis for biologists.’ (Cambridge University Press: New York, NY, USA)
Schmarr D, Duguid A, Cheshire D, Mathwin R, Sternberg D, Cockayne B (2016). Lake Eyre Basin Rivers Assessment (LEBRA) 2015/16 monitoring report. Department of Agriculture and Water Resources, Canberra, ACT, Australia.
Schneider, RAZ, Schneider, RWS, and Moore, PA (1999). Recognition of dominance status by chemoreception in the red swamp crayfish, Procambarus clarkii. Journal of Chemical Ecology 25, 781–794.
| Recognition of dominance status by chemoreception in the red swamp crayfish, Procambarus clarkii.Crossref | GoogleScholarGoogle Scholar |
Souty-Grosset C, Holdich D, Noël P, Reynolds JD, Haffner P (2006) ‘Atlas of crayfish in Europe.’ (Muséum national d’Histoire naturelle: Paris, France)
Sternberg, D, and Cockayne, B (2018). The ongoing invasion of translocated sleepy cod (Oxyeleotris lineolata) in the Lake Eyre Basin, central Australia. Wildlife Research 45, 164–175.
| The ongoing invasion of translocated sleepy cod (Oxyeleotris lineolata) in the Lake Eyre Basin, central Australia.Crossref | GoogleScholarGoogle Scholar |
Strayer, DL (2010). Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology 55, 152–174.
| Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future.Crossref | GoogleScholarGoogle Scholar |
Tattersall, GJ, Luebbert, JP, LePine, OK, Ormerod, KG, and Mercier, AJ (2012). Thermal games in crayfish depend on establishment of social hierarchies. Journal of Experimental Biology 215, 1892–1904.
| Thermal games in crayfish depend on establishment of social hierarchies.Crossref | GoogleScholarGoogle Scholar | 22573768PubMed |
Thomas Lorenz, O, Craddock, E, Baxter, C, and Palacio, A (2019). Agonistic behavior of an invasive crayfish (Orconectes palmeri) toward the Muckalee crayfish (Procambarus gibbus): effects of residence, size, and cover. Journal of Freshwater Ecology 34, 19–26.
| Agonistic behavior of an invasive crayfish (Orconectes palmeri) toward the Muckalee crayfish (Procambarus gibbus): effects of residence, size, and cover.Crossref | GoogleScholarGoogle Scholar |
Twardochleb, LA, Olden, JD, and Larson, ER (2013). A global meta-analysis of the ecological impacts of nonnative crayfish. Freshwater Science 32, 1367–1382.
| A global meta-analysis of the ecological impacts of nonnative crayfish.Crossref | GoogleScholarGoogle Scholar |
Valiente-Banuet, A, Aizen, MA, Alcántara, JM, Arroyo, J, Cocucci, A, Galetti, M, García, MB, García, D, Gómez, JM, Jordano, P, Medel, R, Navarro, L, Obeso, JR, Oviedo, R, Ramírez, N, Rey, PJ, Traveset, A, Verdú, M, and Zamora, R (2015). Beyond species loss: the extinction of ecological interactions in a changing world. Functional Ecology 29, 299–307.
| Beyond species loss: the extinction of ecological interactions in a changing world.Crossref | GoogleScholarGoogle Scholar |
Verhoef, GD, and Austin, CM (1999). Combined effects of temperature and density on the growth and survival of juveniles of the Australian freshwater crayfish, Cherax destructor Clark: Part 1. Aquaculture 170, 37–47.
| Combined effects of temperature and density on the growth and survival of juveniles of the Australian freshwater crayfish, Cherax destructor Clark: Part 1.Crossref | GoogleScholarGoogle Scholar |
Verhoef, GD, Austin, CM, Jones, PL, and Stagnitti, F (1998). Effect of temperature on molt increment and intermolt period of a juvenile Australian fresh-water crayfish, Cherax destructor. Journal of Crustacean Biology 18, 673–679.
| Effect of temperature on molt increment and intermolt period of a juvenile Australian fresh-water crayfish, Cherax destructor.Crossref | GoogleScholarGoogle Scholar |


