Evaluation and use of a portable field kit for measuring whole-blood lactate in sharks
C. A. Awruch A C , C. Simpfendorfer A and N. W. Pankhurst BA Fishing and Fisheries Research Centre, School of Earth and Environmental Science, James Cook University, Townsville, Qld 4811, Australia.
B Australian Rivers Institute, Griffith University, Gold Coast, Qld 4222, Australia.
C Corresponding author. Email: Cynthia.Awruch@jcu.edu.au
Marine and Freshwater Research 62(6) 694-699 https://doi.org/10.1071/MF10149
Submitted: 18 June 2010 Accepted: 1 April 2011 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Measurement of lactate is becoming a common procedure in assessing the physiological effects of capture stress in sharks, although the necessity to measure the concentrations in the laboratory limits the ability for field assessments. Portable lactate analysers offer an alternative, but await validation against laboratory assays for sharks. The present study assessed the reliability of a portable Lactate Pro analyser for measuring whole-blood lactate in the school shark, Galeorhinus galeus, in the field. Laboratory determination of whole-blood and plasma lactate obtained by spectrophotometry was highly correlated with field determinations. Because shark lactate concentration can exceed the upper detection limit of the portable analysers, which were designed for mammalian use, a method for dealing with values greater than the maximum detection limit was evaluated. Whole-blood diluted by 50% with acidified saline solution, tap water and distilled water gave measured values of 55, 56 and 52%, respectively, of the original values, allowing accurate estimation of concentrations exceeding the upper detection limit of the analyser. These findings indicated that the Lactate Pro can be used to rapidly and reliably measure lactate for sharks in the field.
Additional keywords: elasmobranchs, plasma lactate, portable analyser, stressors.
Introduction
Major declines in population sizes of sharks have been documented in fisheries around the world, as a result of both targeted fishing and fisheries where they are caught as by-catch (Stevens et al. 2000; Dulvy et al. 2008). With more species being protected because of these declines, there will also be increased rates of release from commercial and recreational fisheries. The capture and subsequent release of sharks is a major cause of stress (Skomal 2007; Mandelman and Skomal 2009) and can lead to mortality (Cliff and Thurman 1984; Manire et al. 2001). The ability to identify the fate of released sharks is therefore important in understanding how the release of sharks will benefit conservation efforts.
In recent years, measurement of whole-blood and plasma lactate concentrations in sharks has become a common procedure in research investigating the metabolic response to stressors (Hoffmayer and Parsons 2001; Brill et al. 2008; Frick et al. 2009). Changes in blood chemistry related to capture events provide information about the degree of capture stress in elasmobranchs, with strong evidence that the level of physiological disturbance manifested in the blood of sharks correlates with a wide range of stressors (Cliff and Thurman 1984; Moyes et al. 2006; Mandelman and Skomal 2009).
The analytical technique used to measure both plasma and whole-blood lactate concentration in the laboratory involves the use of enzyme-based spectrophotometry. However, this procedure cannot easily be transferred to field sites and fishing vessels. In recent years, portable gas analysers (e.g. i-STAT, Heska Corporation, Loveland, CO, USA) have also been used to measure lactate and other chemical metabolites (Cooke et al. 2008; Mandelman and Skomal 2009), and hand-held analysers specific for lactate (e.g. Accusport meter, Boehringer Mannheim, East Sussex, UK; and Lactate Pro, Akray Inc., Kyoto, Japan) have become available (Wells and Pankhurst 1999; Brown et al. 2008).
Several studies have shown that portable analysers, designed for human use, offer cheap and reliable measurements of blood lactate for other groups of vertebrates (Venn Beecham et al. 2006; Acierno and Mitchell 2007; Brown et al. 2008). Despite the wider use of hand-held analysers to measure lactate concentrations in teleosts and mammalian blood (Harrenstien et al. 2005; Thorneloe et al. 2007), and although the portable lactate analyser is becoming commonly used in field-based stress investigations on elasmobranchs, no validation of the hand-held analyser against traditional enzymatic assays has been conducted. The relatively unique characteristics of elasmobranch blood – nucleated red blood cells and high levels of nitrogen products (Lai et al. 1997) – make it essential that such testing occurs. In addition, because sharks can have whole-blood and plasma lactate levels >40 mmol L–1 (Hoffmayer and Parsons 2001; Hight et al. 2007; Brill et al. 2008), which is greater than the range of human values, and, hence, the range of the portable analyser, a method for addressing values higher than the upper detection limit is needed.
The aims of the present study were to (1) determine whether the hand-held analyser was able to reliably measure whole-blood and plasma lactate concentrations in sharks (i.e. to determine the accuracy, precision and bias of the hand-held analyser measurements) and (2) evaluate methods for measuring lactate concentrations greater than the upper detection limit of hand-held analysers (i.e. to test the hypothesis that diluted whole-blood would estimate off-scale lactate levels) and whether the use of different diluents would influence the estimated lactate value.
Materials and methods
Sample collection
For the present study, we used the school shark, Galeorhinus galeus, from the Derwent River (Tasmania, Australia). Forty-one individuals were caught by long lines (soak time, 1 h; depth, 15–20 m). After capture, sharks were rapidly brought to the boat where blood samples (~1.5 mL) were collected by caudal venipuncture with heparinised syringes fitted with 22G needles. After extraction, samples were analysed, without delay, for haematocrit and for whole-blood lactate by using a portable hand-held analyser, Lactate Pro. Packed cell volume (PVC) expressed as haematocrit (%) was calculated by filling microhaematocrit tubes that did not contain heparin with heparinised blood. Tubes were centrifuged on board for 5 min at 4400g with a portable centrifuge (Imbros Pty Ltd, Cambridge, Tasmania, Australia) and haematocrit was determined by measuring the percentage of packed cells relative to the whole-blood volume. The remaining whole-blood samples were stored on dry ice for 6–7 h, until they could be processed. Subsamples of whole-blood were placed on ice for 6–7 h and then centrifuged for 5 min at 1250g. The plasma was collected and immediately processed in the laboratory.
Lactate Pro measurement of whole-blood lactate
A hand-held Lactate Pro analyser was used to measure whole-blood lactate concentration in the field. A small drop of blood (~10 μL) was placed on a test strip impregnated with dried reagent, and lactate concentration was obtained after 60 s (Brown et al. 2008).
Laboratory lactate assay
Whole-blood and plasma lactate concentrations were determined in the laboratory with lactate reagent and a lactate standard set (400 mg L–1) enzymatic kit (Kits 735–10 and 735–11, Trinity Biotech, Bray, Ireland). Samples were vortex-mixed and 10 μL of each sample was placed into an individual cuvette with 1 mL of reagent. Absorbance at 540 nm was measured with a spectrophotometer (Hitachi High-Technologies Corporation, Tokyo, Japan). Because the evaluation of whole-blood (immediately after sampling on the meter) and plasma lactate (for the enzymatic assay) concentrations represents a comparison of two different media, it was necessary to adjust the plasma lactate concentration according to the haematocrit, to allow an approximation of the concentration of the lactate in the whole-blood. This correction was achieved by multiplying the plasma lactate concentration by (1 – haematocrit).
Validation of lactate concentrations outside the range of the Lactate Pro kit
The Lactate Pro reads in the range of 0.8–23.3 mmol L–1, so it was necessary to develop a technique that would estimate concentrations greater than the upper detection limit to quantify the metabolic condition of sharks. For each of the 41 school shark samples, whole-blood lactate was measured twice. The first measurement was as described above (Lactate Pro measurement of the whole-blood lactate), using 10 μL of blood. For the second measurement, whole-blood (60 μL) was diluted by 50% with 60 μL of acidified saline solution (a solution that closely matched the chemical environment of the blood (Wolf 1963)) and 10 μL of this mix was used to obtain a new lactate concentration. The same procedure was repeated, with the acidified saline solution replaced with tap water or distilled water. The three different media were kept on ice during the sampling period, maintaining a constant temperature of 3°C.
Data analysis
When comparing a new method of measurement (hand-held analyser) with a standard method (spectrophotometry), the reliability (performance) of the new method could be considered as the amount of measurement error that has been considered acceptable for the effective practical use of a new measurement tool (Atkinson and Nevill 1998; Bland and Altman 1999). Reliability can be described in terms of accuracy (how closely a measured value agrees with a true, or accepted, value), precision (how reproducible measurements are) and bias (anything that contributes to a measured value being different from the ‘true’ value or its precision). To test accuracy, a standard linear regression was performed (Quinn and Keough 2002). The slope of the relationship between paired samples (i.e. those obtained with Lactate Pro and by laboratory method) was calculated, and the probability that the slope differed from unity was determined by calculating the 95% confidence intervals (95% CI). Although the regression analysis characterises the degree to which two variables are associated, it does not necessarily indicate the extent to which these values agree or disagree. To overcome this limitation, the approach of quantifying the level of agreement between the two different analysers measuring the same parameter was employed (Atkinson and Nevill 1998). In the Bland and Altman ‘limits of agreement’ analysis (Altman and Bland 1983; Bland and Altman 1995, 1999), the differences between measurements (i.e. with Lactate Pro or by laboratory assays) were plotted against the average of the two measurements. The mean of these differences between measurements was the bias of the method. Standard linear regression analyses were used to examine the relationship between the bias and the average of the two measurements across the entire lactate concentration range. The precision, the 95% limits of the agreement, calculated as 95% range obtained from the standard deviations of a normally distributed population (the mean difference between methods ±1.96 s.d.) provided a reference interval within which 95% of differences between measurements by the two methods were expected to lie. Standard linear regression analyses were performed to measure the association between the lactate concentrations of undiluted samples and those of samples diluted by 50% in the different media. All analyses were undertaken using the SPSS statistical package Base 16.0 (SPSS Inc., an IBM Corporation, Somers, New York, USA).
Results and discussion
Hand-held meter reliability
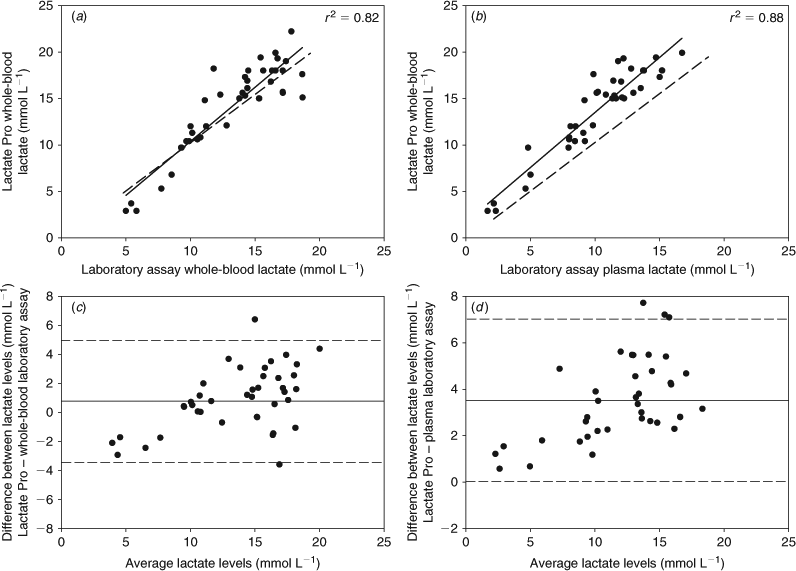
Because one school shark showed a reading above the upper sensitivity limit of 23.3 mmol L–1, this individual was excluded from the analysis of accuracy, bias and precision. Results obtained from the remaining 40 school shark samples showed a strong relationship between the values obtained with the Lactate Pro hand-held meter and those determined by the laboratory reference method for whole-blood lactate across the entire concentration range (r2 = 0.82, P < 0.001) (Fig. 1a). The estimated slope (1.06; 95% CI, 0.98–1.34) was not significantly different from one, demonstrating that hand-held meter readings were accurately agreeable with whole-blood laboratory measurements. Lactate Pro readings were significantly correlated with laboratory plasma measurements (r2 = 0.88, P < 0.001) (Fig. 1b). However, significant differences were found between slopes (1.18; 95% CI, 1.04–1.33), with the hand-held meter readings higher than the laboratory measurements for plasma lactate concentrations (Fig. 1b).
The Bland–Altman analysis of differences in lactate concentrations determined with Lactate Pro and those obtained from whole-blood and plasma laboratory assays showed that the mean bias between the two methods was 0.89 mmol L–1 (±2.14 s.d.) for whole-blood and 3.50 mmol L–1 (±1.78 s.d.) for plasma samples (Fig. 1c, d). Concentrations of lactate determined with hand-held Lactate Pro are expected to lie between 3.32 mmol L–1 below and 5.10 mmol L–1 above (±0.33 s.e.) those obtained from whole-blood laboratory assays, and between 0.03 mmol L–1 and 7.01 mmol L–1 (±0.28 s.e.) above those obtained from plasma laboratory assays, which corresponds to the 95% limits of agreement between methods (hand-held meter and whole-blood and plasma laboratory assays) (Fig. 1c, d). A weak but significant relationship in the degree of disparity (bias) between the two methods measuring whole-blood lactate (Lactate Pro – laboratory assay) across the entire lactate concentration range was observed (r2 = 0.26, y = 0.2638x – 2.6971, P < 0.001) (Fig. 1c). However, because only five values fell below 8 mmol L–1 (which could cause misinterpretations of the results), the regression analysis was recalculated excluding these values, finding a non-significant relationship (r2 = 0.03, y = 0.1291x – 0.585, P = 0.72). For plasma lactate measurements, no significant relationship in the bias between methods (Lactate Pro – laboratory assay) was found, either including or excluding values <8 mmol L–1 (r2 = 0.31, y = 0.2399x + 0.652, P = 0.88).
Our results support the use of the Lactate Pro as a reliable analyser for whole-blood lactate for sharks caught in the wild. On average, a low bias (<0.9 mmol L–1) was obtained, and the data from the Lactate Pro appeared to be homoscedastic, because the differences between analysers did not depend on the magnitude of lactate concentration. The degree of heteroscedasticity found for values <8 mmol L–1 appears to be a result of a low representative sample size and did not have clinical significance. However, further studies on whole-blood samples with very low lactate concentration are required. A high level of precision was concluded, because the limits of agreement <5.1 mmol L–1 through a physiological range of 0.8 ±23.3 mmol L–1 are not likely to have an impact on management and prognosis. These observations are consistent with those from other taxa for which this type of device has been tested (Acierno and Mitchell 2007; Brown et al. 2008), and have confirmed that the characteristics of shark blood do not affect the performance of the device. The Lactate Pro hand-held analyser is therefore a tool that field researchers can use for rapid determination of whole-blood lactate concentrations in a field setting.
The results for plasma lactate showed that the hand-held analyser overestimated the concentrations, although in a predictable way. For lactate levels from 2 mmol L–1 to 17 mmol L–1, the hand-held meter overestimated whole-blood lactate levels compared with plasma lactate concentrations determined by laboratory assays, by 18% (±7%). Therefore, we recommend that users either calculate their own bias-adjustment curves or correct for the bias by using the following equation: y = 1.65 + 1.18x (Fig. 1b). Similar results have been reported for other groups of vertebrate (Wells and Pankhurst 1999; Venn Beecham et al. 2006). This greater difference between the methods could have been further confounded by the 6–7 h that heparinised whole-blood was temporarily stored before centrifugation, potentially affecting the concentration of plasma lactate when left in contact with red blood cells (Venn Beecham et al. 2006). Storage of shark whole-blood before analysis can lead to measurement biases because the erythrocytes are nucleated and possess high metabolic activity, leading to potentially significant changes in lactate concentrations during transport and storage (Baldwin and Wells 1990; Lai et al. 1997). Even so, the hand-held analyser was able to effectively detect metabolic changes, providing a useful tool when relative, rather than absolute, changes in lactate concentrations are used to evaluate and compare responses to stressors.
Lactate concentrations outside the meter range
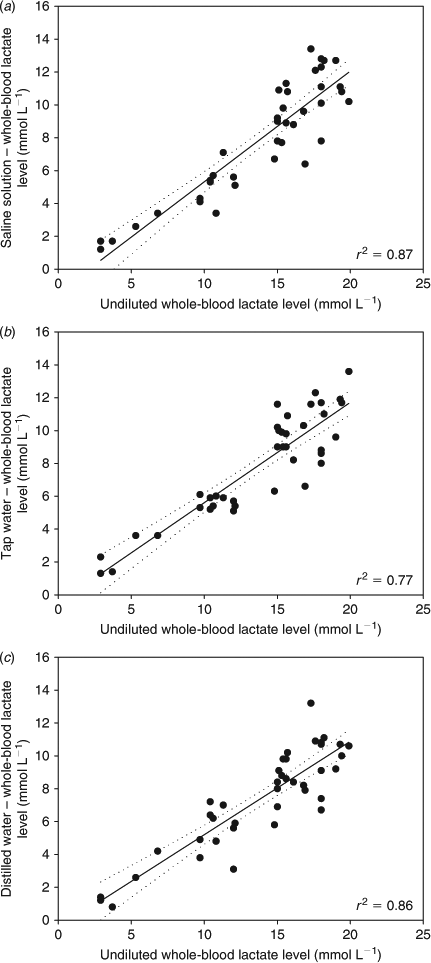
Comparisons of lactate concentrations between undiluted samples and samples diluted by 50% in the different media showed that the lactate concentrations in diluted samples were very similar, independent of the dilution medium applied (Fig. 2). The mean lactate concentration of non-diluted whole-blood obtained by Lactate Pro was 13.84 mmol L–1 (±0.74 s.e., n = 40), whereas the mean lactate concentration obtained by diluting the whole-blood in acidified saline was 7.91 mmol L–1 (±0.55 s.e.), in tap water 7.79 mmol L–1 (±0.51 s.e.) and in distilled water 7.39 mmol L–1 (±0.48 s.e.). The ratio between diluted concentrations and original non-diluted concentrations was 0.55 for acidified saline solution, 0.56 for tap water and 0.52 for distilled water.
|
During the dilution trial, one school shark showed a value higher than the Lactate Pro upper limit. The whole-blood was diluted with the three different media, giving new readings of 13.1, 13.4 and 13.9 mmol L–1 for acidified saline solution, tap water and distilled water, respectively. By using the mean correction factor (0.55, 0.56 and 0.52, respectively, from ratios obtained above), an undiluted concentrations of 23.81 mmol L–1 (acidified saline solution), 23.92 mmol L–1 (tap water) and 26.73 mmol L–1 (distilled water) were assigned. The corresponding whole-blood lactate concentration measured by spectrophotometry in the laboratory was 25 mmol L–1.
In the present study, only a few samples showed concentrations below the detection limit of the Lactate Pro analyser, and all occurred immediately after capture. Whole-blood lactate concentrations obtained by using enzymatic assays have been reported to be <0.8 mmol L–1 in shark species such as Squalus acanthias (Mandelman and Farrington 2007) and Heterodontus portusjacksoni (Cooper and Morris 1998). Thus, although some readings from sharks may be below the detectable concentration (0.8 mmol L–1), they indicated a very low level of metabolic disturbance and provided information that would be of value in assessing the metabolic status of the animal.
Previous studies have reported both whole-blood and plasma lactate concentrations >23.3 mmol L–1 (the upper limit for the Lactate Pro) in several species of sharks, including Isurus oxyrinchus and Alopias vulpinus (Hight et al. 2007), Prionace glauca (Moyes et al. 2006), Rhizoprionodon terraenovae (Hoffmayer and Parsons 2001), Carcharhinus obscurus (Cliff and Thurman 1984; Mandelman and Skomal 2009) and C. plumbeus (Brill et al. 2008). In the present study, diluting the whole-blood by 50%, whether using acidified saline, tap water or distilled water, provided a simple and reliable method for measurement of potentially off-scale lactate concentrations. The use of acidified saline solution as a diluent showed no measurable advantage over using distilled water or tap water, indicating that simple dilution in water was an effective technique. However, because the quality of the tap water might vary, the use of distilled water would be a more reliable and better diluent to employ. The temperature of the diluent may also be important. In the current study, chilled diluents were used and these produced reliable lactate concentrations; however, in a separate study, use of diluents at temperatures >20°C produced highly variable results (C. A. Awruch, unpubl. data).
Conclusion
The persistence of altered states of lactate concentration following metabolic acidosis indicates that lactate is useful for measuring stress responses in sharks in the field (Mandelman and Farrington 2007; Skomal 2007; Mandelman and Skomal 2009). This, combined with the strong association between lactate acidosis and subsequent mortality in sharks (Mandelman and Skomal 2009), suggests that field measurements with meters will improve understanding of species ability to survive human impacts and also help improve handling practices. An important advantage of the portable lactate analyser is the ability to measure lactate concentrations at the point of sampling to assist in the rapid assessment of shark stress levels. Use in this type of situation will allow improved understanding of the release condition of sharks and assist in making decisions about, for example, which animals may be suitable for tagging, surgery or transport for research or husbandry purposes.
Acknowledgements
We are very grateful to Jonah Yick (University of Tasmania, Hobart, Tasmania) and Amos Mapleston (James Cook University, Townsville, Queensland) for their help during the field work. Dr John Mandelman provided very valuable comments on the manuscript. We are also thankful to the Guest Editor, Dr Richard Brill, and one anonymous referee for very constructive revisions. This research was supported by Save Our Seas Foundation (Grant No. SOSF A07/69). This research operated under James Cook University Animal Care and Ethics Committee research approval A1337. Use of specific brand-name equipment does not imply endorsement by the investigators or the funding organisation.
References
Acierno, M. J., and Mitchell, M. A. (2007). Evaluation of four point-of-care meters for rapid determination of blood lactate concentrations in dogs. Journal of the American Veterinary Medical Association 230, 1315–1318.| Evaluation of four point-of-care meters for rapid determination of blood lactate concentrations in dogs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotVCmsbk%3D&md5=6ec1d15d7bd10b797c5b89af11791045CAS | 17472555PubMed |
Altman, D. G., and Bland, J. M. (1983). Measurement in medicine – the analysis of method comparison studies. The Statistician 32, 307–317.
| Measurement in medicine – the analysis of method comparison studies.Crossref | GoogleScholarGoogle Scholar |
Atkinson, G., and Nevill, A. M. (1998). Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Medicine 26, 217–238.
| Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M%2FjvVelsQ%3D%3D&md5=eeed0f7c66a5759a48adb31535dd4a1fCAS | 9820922PubMed |
Baldwin, J., and Wells, R. M. G. (1990). Oxygen-transport potential in tropical elasmobranchs from the Great Barrier Reef – relationship between hematology and blood-viscosity. Journal of Experimental Marine Biology and Ecology 144, 145–155.
| Oxygen-transport potential in tropical elasmobranchs from the Great Barrier Reef – relationship between hematology and blood-viscosity.Crossref | GoogleScholarGoogle Scholar |
Bland, J. M., and Altman, D. G. (1995). Comparing methods of measurement – why plotting difference against standard method is misleading. Lancet 346, 1085–1087.
| Comparing methods of measurement – why plotting difference against standard method is misleading.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28%2FisF2gsw%3D%3D&md5=9d9307b3c2281996eddb2de96ecae52fCAS | 7564793PubMed |
Bland, J. M., and Altman, D. G. (1999). Measuring agreement in method comparison studies. Statistical Methods in Medical Research 8, 135–160.
| Measuring agreement in method comparison studies.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MvivFOmug%3D%3D&md5=1c73797c369a8a367e99bd8eaec127e1CAS | 10501650PubMed |
Brill, R., Bushnell, P., Schroff, S., Seifert, R., and Galvin, M. (2008). Effects of anaerobic exercise accompanying catch-and-release fishing on blood-oxygen affinity of the sandbar shark (Carcharhinus plumbeus, Nardo). Journal of Experimental Marine Biology and Ecology 354, 132–143.
| Effects of anaerobic exercise accompanying catch-and-release fishing on blood-oxygen affinity of the sandbar shark (Carcharhinus plumbeus, Nardo).Crossref | GoogleScholarGoogle Scholar |
Brown, J. A., Watson, J., Bourhill, A., and Wall, T. (2008). Evaluation and use of the Lactate Pro, a portable lactate meter, in monitoring the physiological well-being of farmed Atlantic cod (Gadus morhua). Aquaculture 285, 135–140.
| Evaluation and use of the Lactate Pro, a portable lactate meter, in monitoring the physiological well-being of farmed Atlantic cod (Gadus morhua).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtl2qtb3I&md5=8c648ed584db3f7a879ef374ed9eca3fCAS |
Cliff, G., and Thurman, G. D. (1984). Pathological and physiological effects of stress during capture and transport in the juvenile dusky shark, Carcharhinus obscurus. Comparative Biochemistry and Physiology 78A, 167–173.
| 1:CAS:528:DyaL2cXksFaiu7s%3D&md5=986bfee1bb865b77a6aa8ee90c963facCAS |
Cooke, S. J., Suski, C. D., Danylchuk, S. E., Danylchuk, A. J., Donaldson, M. R., et al. (2008). Effects of different capture techniques on the physiological condition of bonefish Albula vulpes evaluated using field diagnostic tools. Journal of Fish Biology 73, 1351–1375.
| Effects of different capture techniques on the physiological condition of bonefish Albula vulpes evaluated using field diagnostic tools.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlyju7bF&md5=40f1cae2e1ed27afa103fffe6e9a6689CAS |
Cooper, A. R., and Morris, S. (1998). The blood respiratory, haematological, acid-base and ionic status of the Port Jackson shark, Heterodontus portusjacksoni, during recovery from anaesthesia and surgery: a comparison with sampling by direct caudal puncture. Comparative Biochemistry and Physiology Part A 119, 895–903.
| The blood respiratory, haematological, acid-base and ionic status of the Port Jackson shark, Heterodontus portusjacksoni, during recovery from anaesthesia and surgery: a comparison with sampling by direct caudal puncture.Crossref | GoogleScholarGoogle Scholar |
Dulvy, N. K., Baum, J. K., Clarke, S., Compagno, L. J. V., Cortés, E., et al. (2008). You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquatic Conservation: Marine and Freshwater Ecosystems 18, 459–482.
| You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays.Crossref | GoogleScholarGoogle Scholar |
Frick, L. H., Reina, R. D., and Walker, T. I. (2009). The physiological response of Port Jackson sharks and Australian swellsharks to sedation, gill-net capture, and repeated sampling in captivity. North American Journal of Fisheries Management 29, 127–139.
| The physiological response of Port Jackson sharks and Australian swellsharks to sedation, gill-net capture, and repeated sampling in captivity.Crossref | GoogleScholarGoogle Scholar |
Harrenstien, L. A., Tornquist, S. J., Miller-Morgan, T. J., Fodness, B. G., and Clifford, K. E. (2005). Evaluation of a point-of-care blood analyzer and determination of reference ranges for blood parameters in rockfish. Journal of the American Veterinary Medical Association 226, 255–265.
| Evaluation of a point-of-care blood analyzer and determination of reference ranges for blood parameters in rockfish.Crossref | GoogleScholarGoogle Scholar | 15706978PubMed |
Hight, B. V., Holts, D., Graham, J. B., Kennedy, B. P., Taylor, V., et al. (2007). Plasma catecholamine levels as indicators of the post-release survivorship of juvenile pelagic sharks caught on experimental drift longlines in the Southern California Bight. Marine and Freshwater Research 58, 145–151.
| Plasma catecholamine levels as indicators of the post-release survivorship of juvenile pelagic sharks caught on experimental drift longlines in the Southern California Bight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVeju7k%3D&md5=3ef26af70dee5633d6bffcdc82180e3aCAS |
Hoffmayer, E. R., and Parsons, G. R. (2001). The physiological response to capture and handling stress in the Atlantic sharpnose shark, Rhizoprionodon terraenovae. Fish Physiology and Biochemistry 25, 277–285.
| The physiological response to capture and handling stress in the Atlantic sharpnose shark, Rhizoprionodon terraenovae.Crossref | GoogleScholarGoogle Scholar |
Lai, N. C., Korsmeyer, K. E., Katz, S., Holts, D. B., Laughlin, L. M., et al. (1997). Hemodynamics and blood properties of the shortfin Mako shark (Isurus oxyrinchus). Copeia 1997, 424–428.
| Hemodynamics and blood properties of the shortfin Mako shark (Isurus oxyrinchus).Crossref | GoogleScholarGoogle Scholar |
Mandelman, J. W., and Farrington, M. A. (2007). The physiological status and mortality associated with otter-trawl capture, transport, and captivity of an exploited elasmobranch, Squalus acanthias. ICES Journal of Marine Science 64, 122–130.
| The physiological status and mortality associated with otter-trawl capture, transport, and captivity of an exploited elasmobranch, Squalus acanthias.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmtl2itL8%3D&md5=5528dfef66acd3b24afc9e2adc5a33f6CAS |
Mandelman, J. W., and Skomal, G. (2009). Differential sensitivity to capture stress assessed by blood acid–base status in five carcharhinid sharks. Journal of Comparative Physiology Part B 179, 267–277.
| Differential sensitivity to capture stress assessed by blood acid–base status in five carcharhinid sharks.Crossref | GoogleScholarGoogle Scholar |
Manire, C. A., Hueter, R. E., Hull, E., and Spieler, R. (2001). Serological changes associated with gill-net capture and restraint in three species of sharks. Transactions of the American Fisheries Society 130, 1038–1048.
| Serological changes associated with gill-net capture and restraint in three species of sharks.Crossref | GoogleScholarGoogle Scholar |
Moyes, C. D., Fragoso, N., Musyl, M. K., and Brill, R. W. (2006). Predicting postrelease survival in large pelagic fish. Transactions of the American Fisheries Society 135, 1389–1397.
| Predicting postrelease survival in large pelagic fish.Crossref | GoogleScholarGoogle Scholar |
Quinn, G. P., and Keough, M. J. (2002). ‘Experimental Design and Data Analysis for Biologists.’ (Cambridge University Press: Cambridge, UK.)
Skomal, G. B. (2007). Evaluating the physiological and physical consequences of capture on post-release survivorship in large pelagic fishes. Fisheries Management and Ecology 14, 81–89.
| Evaluating the physiological and physical consequences of capture on post-release survivorship in large pelagic fishes.Crossref | GoogleScholarGoogle Scholar |
Stevens, J. D., Bonfil, R., Dulvy, N. K., and Walker, P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystem. Journal of Marine Science 57, 476–494.
Thorneloe, C., Bedard, C., and Boysen, S. (2007). Evaluation of a hand-held lactate analyzer in dogs. The Canadian Veterinary Journal. La Revue Veterinaire Canadienne 48, 283–288.
| 1:CAS:528:DC%2BD2sXks1Gmtbs%3D&md5=96c80f3916e4c2a6fc600cffa64028c1CAS | 17436905PubMed |
Venn Beecham, R., Small, B. C., and Minchew, C. D. (2006). Using portable lactate and glucose meters for catfish research: acceptable alternatives to established laboratory methods? North American Journal of Aquaculture 68, 291–295.
| Using portable lactate and glucose meters for catfish research: acceptable alternatives to established laboratory methods?Crossref | GoogleScholarGoogle Scholar |
Wells, R. M. G., and Pankhurst, N. W. (1999). Evaluation of simple instruments for the measurement of blood glucose and lactate, and plasma protein as stress indicators in fish. Journal of the World Aquaculture Society 30, 276–284.
| Evaluation of simple instruments for the measurement of blood glucose and lactate, and plasma protein as stress indicators in fish.Crossref | GoogleScholarGoogle Scholar |
Wolf, K. (1963). Physiological salines for fresh-water teleosts. Progressive Fish-Culturist 25, 135–140.
| Physiological salines for fresh-water teleosts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2MXmvVCksg%3D%3D&md5=2dd2aa98af19aabc5137b9c2e4958abcCAS |