Evaluation of a novel research trap for surveys of blue swimmer crab populations
Roshan Hanamseth A B * , Daniel D. Johnson B , Hayden T. Schilling
A B * , Daniel D. Johnson B , Hayden T. Schilling  A C , Iain M. Suthers
A C , Iain M. Suthers  A C and Matthew D. Taylor
A C and Matthew D. Taylor  A B
A B
A School of Biological, Earth and Environmental Science, University of New South Wales, NSW 2052, Australia.
B Port Stephens Fisheries Institute, New South Wales Department of Primary Industries, Locked Bag 1, Nelson Bay, NSW 2315, Australia.
C Sydney Institute of Marine Science, Mosman, NSW 2088, Australia.
Marine and Freshwater Research 73(6) 812-822 https://doi.org/10.1071/MF21005
Submitted: 7 January 2021 Accepted: 9 March 2022 Published: 3 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Surveying free-ranging crab populations is important for monitoring the health of exploited stocks and predicting future productivity. Here, we present a novel research trap design for use in fisheries-independent surveys of blue swimmer crab (Portunus armatus) populations, and evaluate the trap against some existing approaches within an independent survey framework. Specifically, the trap design aimed to improve efficiency for capturing smaller crabs, without adversely affecting catches of larger crabs. We quantify and report the abundance and selectivity of these traps, relative to co-located samples obtained using beam trawls and standard commercial round traps, to establish whether these small-mesh traps may offer any improvements over existing survey methods. Comparison and evaluation of these small-mesh traps against other existing survey gear, in different places and at different times, showed that the traps are more effective at catching smaller crabs when they are present, and equally or more effective at catching larger size classes of crabs. The beam trawl appeared to be effective at capturing a reasonable size range of crabs; however, the number of crabs caught (using a similar investment of time) was substantially lower than that caught in traps. This novel small-mesh research trap appears suitable for fisheries-independent surveys of portunid crab species.
Keywords: crablet, crustacean, decapod, estuarine, estuary, fisheries, fishing gear, Portunidae.
Introduction
Portunid crabs support valuable fisheries throughout the world (Food and Agriculture Organization of the United Nations 2020). This taxonomic group includes numerous species of varying distribution and habitat associations, and portunid crab fisheries occur across estuaries, marine embayments and coastal areas. Blue swimmer crab (BSC, Portunus armatus) is an important portunid crab that supports valuable commercial and recreational fisheries (Johnston et al. 2019). Inter-annual variation in portunid fisheries is observed throughout its range, which is thought to be driven by environmental variation (Loneragan 1999; de Lestang et al. 2003; Meynecke et al. 2012; Alberts-Hubatsch et al. 2014; Harris et al. 2016). Despite their general abundance, substantial variation in density is observed in both heavily exploited and unfished populations, and environmental effects on reproduction and recruitment are an important factor contributing to this variability (Chandrapavan et al. 2019). Predicting and responding to this variability represents a significant challenge for management of the sustainability of exploited populations.
Surveying free-ranging crab populations is necessary for monitoring the health of exploited stocks, predicting future productivity, and quantifying factors that contribute to stock variability. Effective sampling methods generally need to capture the desired size range of individuals, as well as support the derivation of estimates of relative abundance with reasonable accuracy and precision. However, no sampling method is perfect, and it is important to consider how different methods perform and elements of bias that may be specific for different sampling approaches. Crabs are captured using a range of different approaches, such as cylindrical wire traps, beach seines, trawls, pots, gill nets and lift nets (Butcher et al. 2012). The nuances of these different gear types, configurations, and the mesh sizes used, mean that many of these may select for different life-history stages or exploit different behaviours to catch crabs. Knowledge of how different sampling gear perform is important both for selecting the most appropriate approach for the question of interest, and for understanding comparative differences among data sets collected using different approaches.
For blue swimmer crab, various studies have reported benthic trawling (Sumpton et al. 2003; Harris et al. 2012) as a suitable approach for surveying exploited crab populations. Beam trawling is often used in these surveys, because a precise estimate of swept area can be calculated, and the gear can capture both juveniles and adults (Bacheler et al. 2013). However, beam trawling is time, labour, and fuel intensive, and can have environmental impacts on sensitive habitats (McConnaughey et al. 2020). Also, nocturnal surveys are often required for BSC, which can exacerbate labour costs. Crab traps provide an alternate approach to efficiently capture crabs, but have their own set of biases, including the following: the sampled area may be uncertain owing to variable bait plume dispersal; antagonistic behaviour may affect trap efficiency; and catch saturation (i.e. declining catch rate as fishing time increases) can decouple catch and abundance. Nonetheless, traps still present a useful means of surveying crab populations, and are used in observer surveys of commercial fishing operations (Harris et al. 2012) or research surveys that are independent of commercial fishing effort (Scandol and Kennelly 2002). Various design specifications of traps can affect their size selectivity and efficacy. In particular, mesh size and escape gaps can be used to control size selectivity (e.g. Broadhurst et al. 2019). Furthermore, these variables can interact; crabs have been shown to be more motivated to find the entrance funnels of traps with a smaller mesh size, because crabs are less able to access the bait by inserting their chelipeds through the mesh (Zhou and Shirley 1997); hence, a greater number of funnels may improve efficacy. Thus, choosing appropriate design features that are most effective for the particular objectives under investigation is important when using traps in independent surveys.
Standard commercial traps are typically designed to avoid capture of smaller blue swimmer crab, so may have limited suitability for juvenile surveys (Butcher et al. 2012; de Lestang et al. 2012; Broadhurst et al. 2020). Modifying the trap design with smaller mesh may improve selectivity for smaller (juvenile) crabs, where sampling of these size classes is important. Here, we present a novel research trap design for independent surveys of BSC populations, incorporating smaller mesh sizes than those of conventional commercial round traps. We evaluate the catch numbers and size structures sampled by these novel traps, and compare with co-located samples obtained using beam trawls and standard commercial round traps, to establish whether these traps may offer any advantages (e.g. wider size ranges of sampled crabs) over existing survey methods. We also compare samples collected using the novel traps with those collected with commercial round traps across an entire fishing season, to ascertain how they perform as the size structure of the population changes through time. Specifically, the study addressed the following two hypotheses: (1) spatial and temporal patterns in crab catch will be consistent among sampling methods, although catch magnitudes may vary among these, reflecting different sampling efficiency; and, (2) novel small-mesh traps will sample smaller crabs more effectively than do the other methods, while sampling larger crabs with similar efficacy.
Materials and methods
Description of sampling gear
The new research traps were simply a ‘small-mesh trap’ adapted from the standard commercial round traps (termed ‘large-mesh traps’) employed in commercial and recreational fisheries in south-eastern Australia (Leland et al. 2013). The traps were the same overall dimensions (900-mm diameter, 300-mm height), with two 10-mm stainless-steel rings to give structure to the trap. The research traps had a 25-mm diamond mesh of green–grey net (polyethylene, PE) tied with chaff rope to the top and bottom ring. In addition, the research traps had four entry funnels instead of two (funnels were 300 × 200 mm on the outside and tapering to 200 × 50 mm). The large-mesh traps had a 55-mm diamond mesh of black net (PE), and only two entry funnels, but the top and bottom ring were threaded through the mesh rather than tied with chaff rope (Fig. 1). The beam trawl design employed has been described in detail in Rotherham et al. (2008). This consisted of a stainless-steel beam trawl frame (3 × 0.8 m), and trawl body, including a 3.7-m headrope, 4.1-m ground rope, with diamond-shaped 26 mm mesh made from twisted three-strand ~1.1-mm diameter green PE.
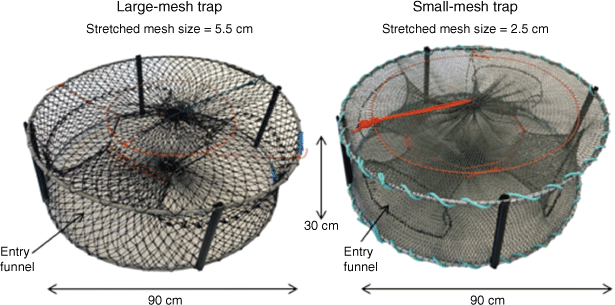
|
Sampling design and collection
This study was conducted in the following three estuaries that support BSC fisheries in south-eastern Australia: Wallis Lake (32°18′S, 152°30′E), Port Stephens (32°44′S, 152°3′E) and Lake Macquarie (33°05′S, 151°35′E, Fig. 2). Sampling was conducted in two phases. Phase 1 compared samples captured using the three different gear collected over four nights spread across 2 months (October and November 2018), in two estuaries (two nights in each of Lake Macquarie and Port Stephens). Phase 2 compared small-mesh and large-mesh traps at five sites within Wallis Lake, Lake Macquarie and Port Stephens estuary, for a period of 6 months (December–May, 2018–2019). Each estuary was sampled during two nights within each month.
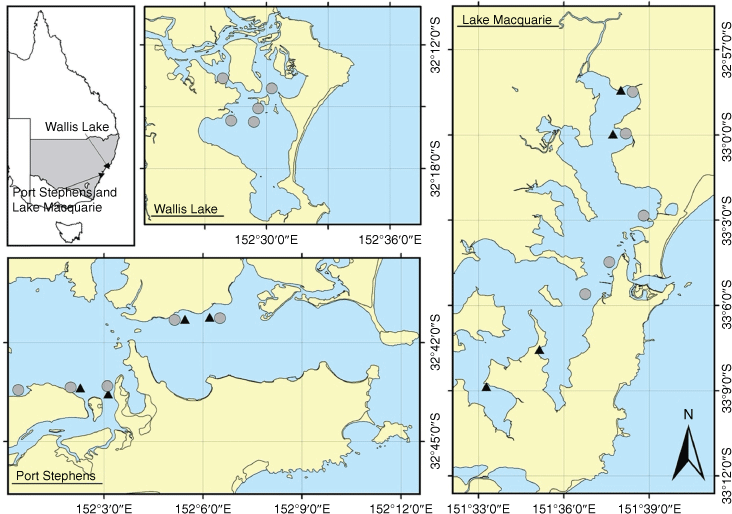
|
For Phase 1 sampling, five large-mesh traps, five small-mesh traps, and five trawl replicates (trawls were of 5-min duration at a speed of 1–2 knots (~0.51–1.03 m s−1), which covered the distance of ~150 m) were deployed within each of four sites within each estuary. Sampling occurred at depths of 2–4 m adjacent to shallow seagrass habitats, and different gear were fished simultaneously, with traps deployed in the afternoon, trawling occurring in the evening, and traps being collected the following day (i.e. ~18 h sets). Gear deployments were separated by >50 m. Traps were baited similarly, with two thawed sea mullets per trap, chopped in half, with the two tails placed inside a 20 × 20-cm bait bag of 1-cm mesh, and the two heads skewered on a metal hook, with the baits secured to the centre of the trap. GPS waypoints were used to mark the location (including start and finish for trawls) of each gear deployment. Traps were deployed and retrieved in the same order to ensure similar soak times. During Phase 2, the same procedures were followed except that four large-mesh traps and four small-mesh traps were deployed at each site in three estuaries, and there was no trawling. Temperature and salinity were measured at each site to ensure that conditions were unlikely to affect the main comparisons being made, and ranged from 17 to 25°C and from 32 to 36.
On retrieval of gear, crabs were placed into an ice slurry for 30 s or less to calm them so that they could be identified and measured (Bellchambers et al. 2005). Blue swimmer crabs were counted and sexed, and carapace length (the distance between the frontal notch and the posterior carapace margin) was measured using vernier callipers, and the crabs were then released. Crabs were returned to the water at the location of capture following data collection.
Sample collection was conducted under a Section 37 Scientific Collection Permit (permit P01/0059), and Animal Research Authority 13-08 issued by NSW Department of Primary Industries.
Statistical analyses
All statistical analyses were conducted in R (ver. 4.0.2, R Foundation for Statistical Computing, Vienna, Austria), and several approaches were used to evaluate efficacy of small-mesh traps, relative to large-mesh traps and beam trawl. First, the total number of crabs captured using different gear at each site during Phase 1 was evaluated using a generalised linear mixed model (GLMM) with a Poisson error distribution. This model included fixed factors of Estuary (Lake Macquarie and Port Stephens) and Gear (small-mesh traps, large-mesh traps and beam trawl) and an interaction term of estuary × gear. To account for dependency structure in the sampling, we included random intercept effects of site (nested within estuary) and date. We note that trawls and traps have very different units of effort, but we considered a single trap or trawl a replicate ‘sampling unit’, because the sampling unit reflected how these gear are generally used (we elaborate on this within the Discussion). The model was fit using the glmmTMB package (see https://cran.r-project.org/package=glmmTMB; Brooks et al. 2017), residuals and assumptions of the model were checked using the DHARMa package (see https://cran.r-project.org/web/packages/DHARMa/), and the pairwise comparisons were conducted using the emmeans package (see https://cran.r-project.org/package=emmeans). The results of this model were visualised with marginal effects plots and post hoc tests were conducted using adjusted P-values.
Because of the small number of crabs collected across beam trawl samples (n = 19), length structures of crabs sampled during Phase 1 were pooled and presented as kernel density estimates (KDE) by gear type, and differences in length structure were assessed visually. A more detailed breakdown of length structures by sex, estuary and gear type is presented in the Supplementary Information (Supplementary Fig. S1, S2). To compare the relative length-dependent catch efficiency of the three methods (beam trawl, small-mesh and large-mesh traps), we applied pairwise comparisons using the binomial generalised linear model (with a 3° polynomial for length) method of Herrmann et al. (2017). We calculated the length-dependent catch-comparison ratios (ccl) and catch-comparison rates (crl) where l is a specific length class, ccl is the ratio of crabs in a size class l caught in one type of gear to the total number of crabs in a size class l caught in both types of gear, and crl is a direct relative value of the catch efficiency between two gear types for length l. If the catch efficiency is equal to 1, then the efficiency of the gear is equal. A full description of the modelling can be found in Herrmann et al. (2017) and Savina et al. (2017), which fully derive the equations for comparable experiments. We applied a double-bootstrapping method (1000 replications, Herrmann et al. 2017; Savina et al. 2017) to calculate 95% confidence intervals and incorporate the uncertainty in the estimation resulting from between-deployment (trawl or trap) variation in catch efficiency and availability of crabs, as well as uncertainty associated with the size structure of the catch across individual deployments. This procedure was implemented with the ‘selfisher’ R package (see https://github.com/mebrooks/selfisher; Brooks et al. 2020) and because of the double bootstrapping procedure inherently controlling for variation which would traditionally be simulated with a random effect of deployment or trap, we did not include any random effects. During this analysis, we initially included estuary as a factor in the models but this was removed and the estuaries pooled, because likelihood ratio tests showed the models including estuary gave no improvements over the simpler model for any of the pairwise comparisons (P > 0.6).
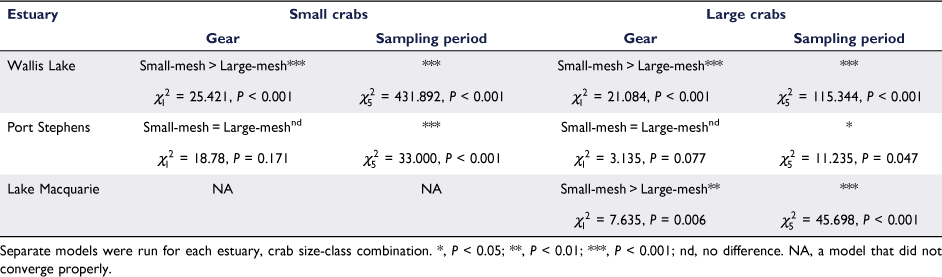
Abundance data (number of crabs per trap) from Phase 2 was also analysed using GLMMs following the above method but with a negative binomial error distribution (which was found to be better than Poisson when the model residuals were inspected). Each estuary was analysed separately to simplify the interpretation. The GLMMs included gear (small-mesh trap and large-mesh traps) and month (December–May) as fixed factors and included random slope effects of site and date to account for dependency structure in the data collection. To account for sampling effort, the soak time of each trap was included in the models as an offset (note that 12 traps of the 1440 deployments were lost and were not included in the analysis). Because our hypothesis concerned the performance of gear type over the fishing season, the key term in the model was the interaction between month and gear, which, if significant, would suggest that the two gear types have differing efficiencies over the fishing season. This analysis was also repeated for abundance data split into size categories above and below 60 mm CL, to quantify the effects of trap type on relative abundance above and below the size of full selectivity (~60 mm) for the large-mesh traps.
Catch selectivity of small- and large-mesh traps over the 6-month fishing season was assessed in a way similar to the initial 2-month, three-way gear comparison, through the use of binomial GLMMs. To assess whether there was any variation in catch selectivity among months or estuaries, we conducted a model-selection process, starting with the base selectivity model (all estuaries and months pooled), and compared this with more complicated models including estuary or month variables. As part of these hypothesis testing models, we incorporated random effects of date and site (nested within estuary) to account for dependency in sampling structure. The best-performing model was then used to estimate the confidence intervals by using the bootstrapping methods described above (after removing the random effects, which the double-bootstrapping naturally incorporates). As the major driver of temporal variability (within a fishing season) in sampling is likely to be the progression of size classes through the population, we considered the monthly size structure visually with KDE estimates produced for each month, gear and estuary.
Results
Overall, 7768 BSC were caught across Phases 1 and 2 of the sampling period. During Phase 1, the small-mesh traps captured 434 BSC individuals, the large-mesh traps captured 362 BSC individuals and the beam trawl captured 19 BSC individuals. During Phase 2, 3834 BSC were captured in the small-mesh traps, and 3119 BSC were captured in the large-mesh traps.
Total catch, size structure and selectivity among trap types and trawls
The abundance of crabs caught varied substantially among gear and estuaries (Fig. 3). There was a greater number of crabs caught in Port Stephens than Lake Macquarie 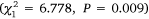 , and there were differences in the number of crabs using different gear
, and there were differences in the number of crabs using different gear 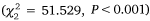 . There was no evidence of an interaction between Estuary and Gear Type
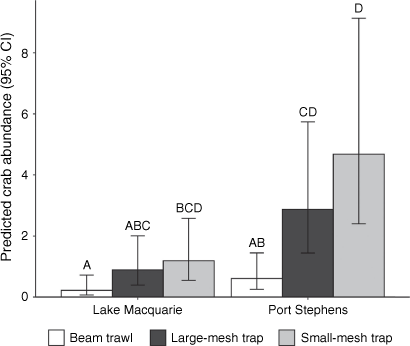
. There was no evidence of an interaction between Estuary and Gear Type 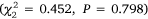 . Post hoc tests showed that more crabs were caught in small-mesh (P < 0.001) and large-mesh (P < 0.001) traps than in the beam trawl, but with no difference between the trap types (P = 0.117, Fig. 3).
. Post hoc tests showed that more crabs were caught in small-mesh (P < 0.001) and large-mesh (P < 0.001) traps than in the beam trawl, but with no difference between the trap types (P = 0.117, Fig. 3).
|
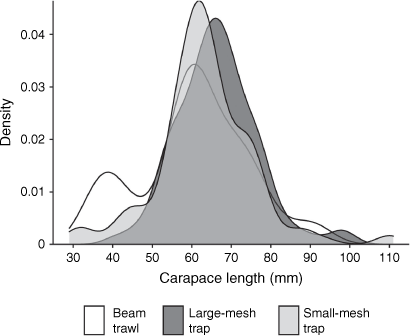
There were minor differences between the length structures of crabs sampled by different gear, either when considering both sexes pooled (Fig. 4), or male and female independently (Supplementary Fig. S1). The overall size structure of crabs in Lake Macquarie appeared to be larger than in Port Stephens (Supplementary Fig. S2). In Port Stephens, small-mesh traps appeared to capture more smaller crabs than did large-mesh traps, and there was some evidence for multiple smaller modes that were not evident for large-mesh traps (Supplementary Fig. S2). In some instances, smaller size classes appeared to be better represented in beam trawl samples than in small-mesh traps in terms of the percentage of the harvest within the gear type, but this was likely to be due to the small sample numbers caught in beam trawls, potentially not being representative (Fig. 4).
|
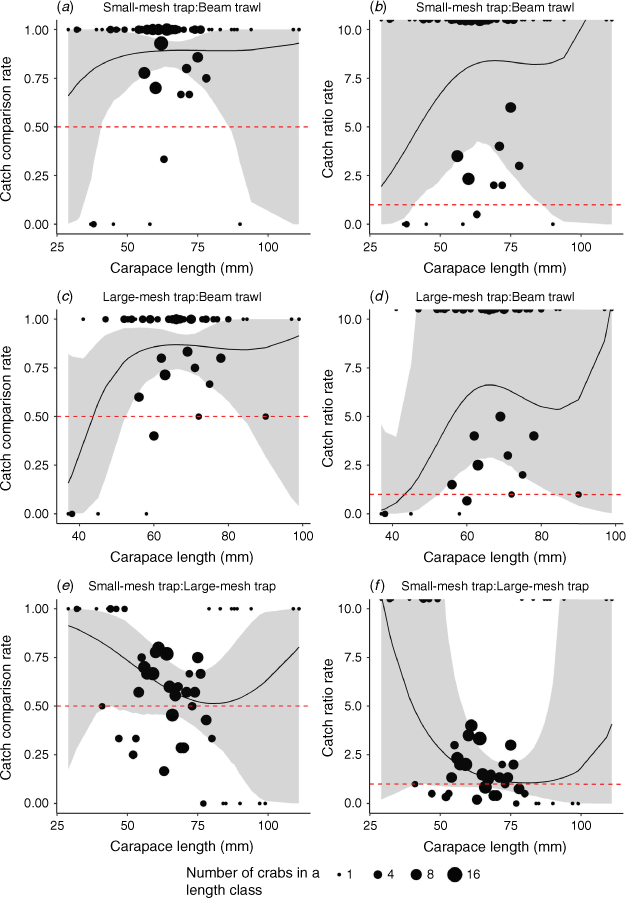
The pairwise comparisons of selectivity for Phase 1 samples are visualised in Fig. 5. Beam trawls were found to catch fewer moderate-size crabs than were both types of traps. Small-mesh traps had a significantly higher catch comparison rate for crabs between 40 and 85 mm CL (ccr 95% CI does not overlap 0.5; Fig. 5a), whereas large-mesh traps had a significantly higher catch comparison rate for crabs between 53 and 82 mm CL (ccr 95% CI does not overlap 0.5; Fig. 5c). These patterns were reflected in the catch ratio rate, with moderate-sized crabs being caught ~5× more in traps than in the trawls (Fig. 5b, d). There was no evidence of differing selectivity between the two types of traps (95% CI of catch comparison rate overlaps 0.5 at all sizes), although there was a trend towards small-mesh traps being more efficient at sampling small (<50 mm CL) and large (>95 mm CL) crabs (Fig. 5e). This was reflected in the catch ratio rate, with small traps being likely to capture much greater numbers of small crabs (Fig. 5f).
|
Temporal comparison between trap types
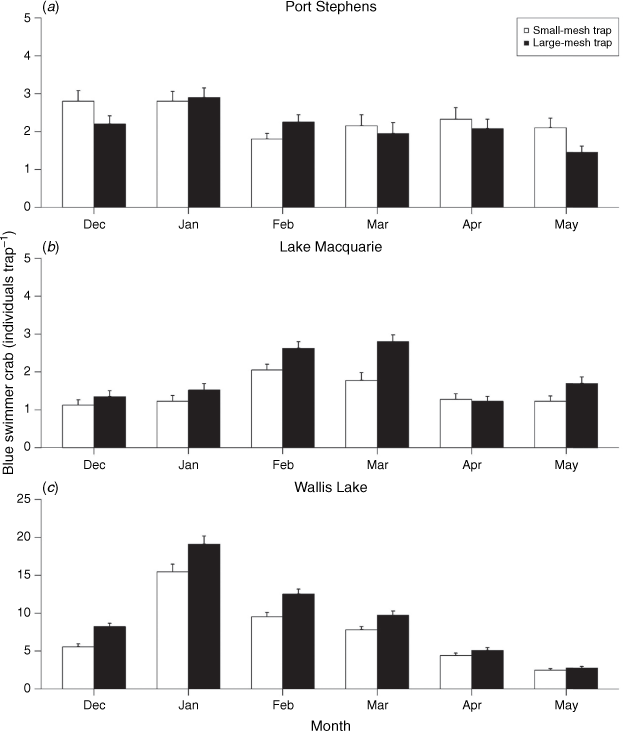
The average number of individuals per trap varied among estuaries during Phase 2, with the highest catches in Wallis Lake, followed by Port Stephens, and then Lake Macquarie. Small-mesh traps consistently captured a greater amount of crabs than did large-mesh traps in Lake Macquarie 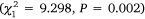 and Wallis Lake
and Wallis Lake 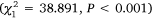 , but there was no difference between gear in Port Stephens
, but there was no difference between gear in Port Stephens 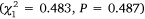 and no significant interactions between gear and month for any estuary (P > 0.6). Overall abundance varied through time for Port Stephens
and no significant interactions between gear and month for any estuary (P > 0.6). Overall abundance varied through time for Port Stephens 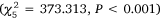 , Lake Macquarie
, Lake Macquarie 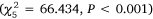 and Wallis Lake
and Wallis Lake 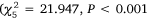 , Fig. 6), showing a minor downward trend through time in Port Stephens (Fig. 6a), as opposed to clear peaks in Lake Macquarie (February and March, Fig. 6b) and Wallis Lake (January and February, Fig. 6c). Similar patterns were resolved when abundance data was split into groups above and below 60 mm CL (Table 1), with small-mesh traps catching a greater number of both small (<60 mm CL) and large (>60 mm CL) crabs in Wallis Lake, and small-mesh traps catching a greater number of large crabs in Lake Macquarie (very few small crabs were caught in this estuary). The small crab model in Lake Macquarie did not converge, likely because of the very low number of crabs (72 crabs over 472 traps). Again, there was no interaction between gear and month for any estuary (P > 0.5).
, Fig. 6), showing a minor downward trend through time in Port Stephens (Fig. 6a), as opposed to clear peaks in Lake Macquarie (February and March, Fig. 6b) and Wallis Lake (January and February, Fig. 6c). Similar patterns were resolved when abundance data was split into groups above and below 60 mm CL (Table 1), with small-mesh traps catching a greater number of both small (<60 mm CL) and large (>60 mm CL) crabs in Wallis Lake, and small-mesh traps catching a greater number of large crabs in Lake Macquarie (very few small crabs were caught in this estuary). The small crab model in Lake Macquarie did not converge, likely because of the very low number of crabs (72 crabs over 472 traps). Again, there was no interaction between gear and month for any estuary (P > 0.5).
|
|
Temporal variation in trap selectivity
Our model selection process identified that the most parsimonious model was the base selectivity model plus a fixed effect of estuary (Supplementary Table S1), suggesting that although the patterns of selectivity between the traps were similar among estuaries, there were still some differences among estuaries (Fig. 7a). In all estuaries, small-mesh traps were more effective at sampling the smaller crabs and there was no indication of differing selectivity for larger crabs (Fig. 7). Although it was clear that there was some progression through the size classes in the population over time (Supplementary Fig. S3), selectivity models that included month did not perform well (Supplementary Table S1), giving no indication that the selectivity of traps varied over the fishing season. When the three estuaries were pooled to estimate a global selectivity comparison of small- and large-mesh traps, the small-mesh traps were more efficient at catching crabs less than 47 mm CL (ccr 95% CI does not overlap 0.5), with some evidence that small traps also caught more crabs of all sizes (Fig. 7c, d).
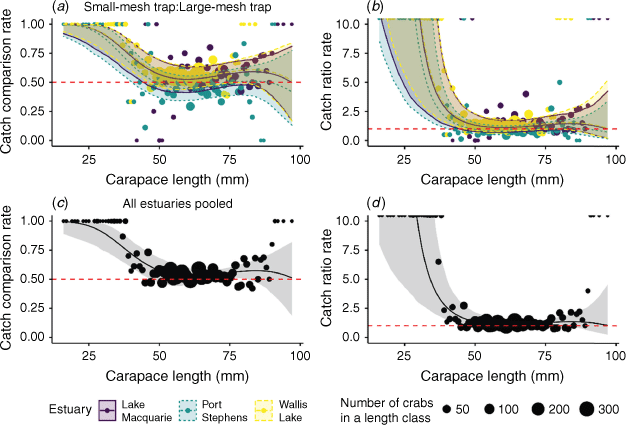
|
Discussion
Our evaluation showed that small-mesh traps represent a suitable gear for fisheries-independent surveys for portunid crabs. Although the small-mesh traps were designed specifically for targeting BSC, this gear should be similarly applicable for other species of swimming crabs found in other regions. Comparison of the small-mesh traps against other survey gear in different places and times suggested that the traps can catch more crabs, and are more effective at catching smaller crabs when they are present, but also equally or more effective at catching larger size classes. The beam trawl appeared to be effective at capturing a reasonable size range of crabs; however, the number of crabs caught during 5-min trawl shots was substantially lower than that caught in traps (which sample overnight). The five beam trawl replicates took roughly a similar amount of time that it takes, to bait, deploy and retrieve five round traps. Therefore, small-mesh traps could be considered to provide a good alternative survey method to more active methods such as beam trawling, with some advantages, including potentially greater catch per unit effort of manpower, minimal bycatch, minimal effect on sensitive habitats, and no interaction with other set fishing gear in commercially fished estuaries. It is important to reiterate that all fishing gear have inherent biases, and these are discussed below, with an emphasis on the small-mesh traps.
Factors affecting trap efficacy
There are several design characteristics of the small-mesh traps that may have contributed to improvements in capture efficacy, including mesh size, trap shape, and the type and number of entrances to the trap. The effect of mesh sizes, trap type and shape have been assessed previously, particularly in the context of escape gaps as a strategy to reduce capture of undersized crabs (Guillory and Prejean 1997; Guillory 1998; Bellchambers and de Lestang 2005; Rotherham et al. 2013; Broadhurst et al. 2017, 2019, 2020). Previous studies have shown reduced effectiveness of finer mesh traps owing to clogging of the mesh with weed (Miller 1980); however, this work mostly investigated traps with two entrances, and these problems were not encountered in our study. Previous work on the congeneric Portunus pelagicus also showed that round traps tended to encourage greater searching time around the trap, which enhanced the probability of finding an entrance leading to the bait (Vazquez Archdale 2012). Leland et al. (2013) also found round-shaped traps to be more effective in catching BSC than were rectangular, wire and hoop nets.
Funnel-type entrances tend to have higher catch efficiencies than have other types of trap entrances (Vazquez Archdale et al. 2006, 2007; Bergshoeff et al. 2019). Previous experiments on the portunid Charybdis japonica (Asian Paddle Crab) found that funnel-type entrances had higher capture efficacies than did slit entrances because they were easier to enter without the chance of entanglement (Vazquez Archdale et al. 2006). In addition, Broadhurst et al. (2020) found that small meshed funnel-type entrances had no effect on the escape of BSC. Additional entry funnels may increase the catch of BSC (Smith and Sumpton 1989), on the basis of observations of antagonistic behaviour around entry funnels hindering the ingress of individuals into the trap. Portunid crabs generally advance towards the bait plume from down current and move around the side of the trap until they locate a funnel (Vazquez Archdale et al. 2006). These factors all suggest that a greater number of funnel-type entrances will aid ingress of crabs into the trap, and the decreased chance of antagonistic interaction while entering the trap is likely to particularly benefit smaller crabs.
Comparative biases in sampling gear
In our experiment, we conducted an extended survey throughout the austral summer and autumn to compare the efficacy of the large- and small-mesh traps as size classes progressed through the estuary during warmer months. There is some evidence for the appearance of smaller size classes in Wallis Lake over the 6 months of data collection, when new recruits were more abundant earlier in the summer. Smith et al. (2004) reported that the capture of BSC using passive fishing methods such as traps tended to capture more mature crabs than did active methods such as otter trawling and seine netting, which better represent the range of size classes present. The small-mesh traps showed no differences in size structure to beam trawl, suggesting that this bias may be reduced for the small-mesh trap design, although we note that few crabs were caught in the beam trawls.
Despite the promising results observed for small-mesh traps, any trap survey generally suffers from imprecise knowledge of the areal measure of sampling effort. By contrast, beam trawl surveys have a known or controlled swept area, such that numbers can be expressed per unit area covered (i.e. crabs ha−1). However, traps rely on the use of bait to attract animals in the surrounding area to encounter and enter the trap. Although soak time for traps can be easily determined and used to standardise catches among deployments (i.e. crabs h−1), the spatial extent of the bait plume can vary, and this is difficult to quantify. Bait plume is affected by several abiotic factors, including benthic features, tide, wind and rainfall, all of which can contribute variable ‘sampling effort’ (Taylor et al. 2013) and non-circular attraction zones (Winger and Walsh 2011). This is particularly relevant where soak time transcends tidal cycles or weather events. Although drogues and current meters can be used to provide a comparative measure of current movement during deployment (Taylor et al. 2013), this is useful really only for very short-term trap deployments. Another potential issue is the decline in the strength of the olfactory stimulus throughout the deployment, which means that attraction of crabs may taper off before traps are retrieved. This would be expected to occur at similar rates among traps, so is unlikely to affect relative measures of crab abundance. Even though common across all trap surveys, these issues are difficult to overcome, although there is some evidence to suggest that biological factors such as variable movement rates introduce much greater variation than does plume size (Brêthes et al. 1985). There is also a possibility of some interaction between co-located gear in our study (such as crabs startled by the beam trawl then encountering traps), which may have influenced the patterns resolved. Although we could not specifically examine this in our study, we suggest it is unlikely to have had a large effect because gear deployments were separated by a minimum of 50 m, a separation distance previously used to ensure independence in blue swimmer crab sampling experiments (Leland et al. 2013), and trawling occurred for only a short period following trap deployment (and early in the period of trap soaking), with all animals returned to the water at the place where they were captured. If any competitive sampling or displacement effects did occur, the size selectivity results of the study are also unlikely to be affected.
When used to generate an index of relative abundance, it is important that independent survey data are representative of the actual abundance across population densities at the spatial scale of interest (Addison and Bell 1997; Bacheler et al. 2013). However, the cumulative impacts of the design and deployment of traps may create biases that affect this relationship (Miller 1980). For example, a decline in catch rate as soak-time and accumulated catch increase (i.e. saturation) is a potential source of bias in trap surveys (Bacheler et al. 2013). In the small-mesh traps, the observed increase in the abundance of BSC both above and below 60 mm CL, particularly in Wallis Lake (the estuary with the greatest abundance), suggests that saturation of the traps did not occur at the population densities at which crabs were sampled, and the presence of larger crabs in traps did not inhibit small crabs from entering the trap.
As a final note, even though detailed data were not recorded in this study, some bycatch was encountered in both trap types. Bycatch taxa included yellowfin bream (Acanthopagrus australis), tarwhine (Rhabdosargus sarba), eastern striped grunter (Pelates sexlineatus), as well as non-swimming crabs, prawns, and very low numbers of eel, octopus, and small elasmobranchs (n < 6 in total). Observations suggested that small-mesh traps tended to retain larger numbers of eastern striped grunter (only in Lake Macquarie where this species is particularly abundant) and prawns (only during periods when prawns were abundant) than did large-mesh traps, with negligible differences for the other species.
Conclusions
The small-mesh trap presented here shows promise for fisheries-independent surveys of portunid crabs, particularly where sampling of smaller recruits is important. Although the choice of gear for research surveys is context- and question-specific, these traps represent a useful stand-alone sampling gear for quantifying relative abundance and size structure in crab populations. Furthermore, these traps may also be a good complementary approach to surveys of crab populations using beam trawl surveys. As with any gear, it is important to understand sources of bias that affect their efficacy, and, in particular, whether this varies across different sampling conditions, different sampling locations or different size structures in the target population. The data presented here suggest that these novel small-mesh traps perform in a reasonably consistent fashion across estuaries and different conditions. Their application in broader surveys will help better understand their utility and improve confidence in the resulting abundance and size-structure data.
Data availability
The authors declare that the data used here are available upon request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of funding
This project was supported by the Fisheries Research and Development Corporation on behalf of the Australian Government (project 2017/006) and the NSW Recreational Fishing Saltwater Trust. RH was supported by the Australian Government (RTP) and NSW DPIE top up scholarship. HTS was supported by a NSW Research Attraction and Acceleration Program grant. Funding bodies had no role in the design, data collection, analysis or interpretation of data.
Supplementary material
Supplementary material is available online.
Acknowledgements
The authors thank B. Leach, M. Burns, L. Bidgood, A. Wiltshire, A. Pidd, and T. New for assistance in collecting samples. This is SIMS publication #288.
References
Addison, JT, and Bell, MC (1997). Simulation modelling of capture processes in trap fisheries for clawed lobsters. Marine and Freshwater Research 48, 1035–1044.Alberts-Hubatsch, H, Yip Lee, S, Diele, K, Wolff, M, and Nordhaus, I (2014). Microhabitat use of Early Benthic Stage mud crabs, Scylla serrata (Forskål, 1775), in eastern Australia. Journal of Crustacean Biology 34, 604–610.
Bacheler, NM, Schobernd, ZH, Berrane, DJ, Schobernd, CM, Mitchell, WA, and Geraldi, NR (2013). When a trap is not a trap: converging entry and exit rates and their effect on trap saturation of black sea bass (Centropristis striata). ICES Journal of Marine Science 70, 873–882.
Bellchambers, LM, and de Lestang, S (2005). Selectivity of different gear types for sampling the blue swimmer crab, Portunus pelagicus L. Fisheries Research 73, 21–27.
Bellchambers, LM, Smith, KD, and Evans, SN (2005). Effect of exposure to ice slurries on nonovigerous and ovigerous blue swimmer crabs, Portunus pelagicus. Journal of Crustacean Biology 25, 274–278.
Bergshoeff, JA, McKenzie, CH, and Favaro, B (2019). Improving the efficiency of the Fukui trap as a capture tool for the invasive European green crab (Carcinus maenas) in Newfoundland, Canada. PeerJ 7, e6308.
| 30713818PubMed |
Brêthes, J-C, Bouchard, R, and Desrosiers, G (1985). Determination of the area prospected by a baited trap from a tagging and recapture experiment with snow crabs (Chionoecetes opilio). Journal of Northwest Atlantic Fishery Science 6, 37–42.
Broadhurst, MK, Millar, RB, and Hughes, B (2017). Performance of industry-developed escape gaps in Australian Portunus pelagicus traps. Fisheries Research 187, 120–126.
Broadhurst, MK, Smith, TM, Millar, RB, Hughes, B, Raoult, V, and Gaston, TF (2019). Cumulative selectivity benefits of increasing mesh size and using escape gaps in Australian Portunus armatus traps. Fisheries Management and Ecology 26, 319–326.
Broadhurst, MK, Tolhurst, DJ, Hughes, B, Raoult, V, Smith, TM, and Gaston, TF (2020). Optimising mesh size with escape gaps in a dual-species portunid-trap fishery. Aquaculture and Fisheries 5, 308–316.
Brooks, ME, Kristensen, K, Van Benthem, KJ, Magnusson, A, Berg, CW, Nielsen, A, Skaug, HJ, Machler, M, and Bolker, BM (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9, 378–400.
Brooks, ME, Melli, V, Savina, E, Santos, J, Millar, R, O’Neill, FG, Veiga-Malta, T, Krag, LA, and Feekings, JP (2020). Introducing selfisher: open source software for statistical analyses of fishing gear selectivity. bioRxiv. , .
Butcher, PA, Leland, JC, Broadhurst, MK, Paterson, BD, and Mayer, DG (2012). Giant mud crab (Scylla serrata): relative efficiencies of common baited traps and impacts on discards. ICES Journal of Marine Science 69, 1511–1522.
Chandrapavan, A, Caputi, N, and Kangas, MI (2019). The decline and recovery of a crab population from an extreme marine heatwave and a changing climate. Frontiers in Marine Science 6, 510.
de Lestang, S, Hall, NG, and Potter, IC (2003). Reproductive biology of the blue swimmer crab (Portunus pelagicus, Decapoda: Portunidae) in five bodies of water on the west coast of Australia. Fishery Bulletin 101, 745–757.
de Lestang S, Caputi N, How J, Melville-Smith R, Thomson A, Stephenson P (2012) Stock assessment for the west coast rock lobster fishery. Fisheries Research Report 217, Department of Fisheries, Perth, WA, Australia. Available at https://www.fish.wa.gov.au/Documents/research_reports/frr217.pdf
Food and Agriculture Organization of the United Nations (2020) ‘Sustainability in action. State of world fisheries and aquaculture.’ (FAO: Rome, Italy)
Guillory, V (1998). Blue crab, Callinectes sapidus, retention rates in different trap meshes. Marine Fisheries Review 60, 35–37.
Guillory, V, and Prejean, P (1997). Blue crab, Callinectes sapidus, trap selectivity studies: mesh size. Marine Fisheries Review 59, 29.
Harris, D, Johnston, D, Sporer, E, Kangas, M, Felipe, N, and Caputi, N (2012). Biology and management of a multi-sector blue swimmer crab fishery in a subtropical embayment – Shark Bay, Western Australia. Marine and Freshwater Research 63, 1165–1179.
Harris D, Johnston D, Baker J, Foster M (2016) Adopting a Citizen Science approach to develop cost-effective methods that will deliver annual information for managing small-scale recreational fisheries: The Southwest Recreational Crabbing Project. Fisheries Research, No. 281, Department of Fisheries, WA, Australia.
Herrmann, B, Sistiaga, M, Rindahl, L, and Tatone, I (2017). Estimation of the effect of gear design changes on catch efficiency: methodology and a case study for a Spanish longline fishery targeting hake (Merluccius merluccius). Fisheries Research 185, 153–160.
Johnston D, Chandrapavan A, Garland A, Beckmann C, Johnson, DD (2019) Blue swimmer crab (Portunus armatus). In ‘Status of Key Australian Fish Stocks 2018’. (Fisheries Research and Development Corporation: Canberra, ACT, Australia) Available at http://fish.gov.au/report/179-Blue-Swimmer-Crab-2018 [Verified 2 December 2020]
Leland, JC, Butcher, PA, Broadhurst, MK, Paterson, BD, and Mayer, DG (2013). Relative trap efficiency for recreationally caught eastern Australian blue swimmer crab (Portunus pelagicus) and associated injury and mortality of discards. Fisheries Research 147, 304–311.
Loneragan, NR (1999). River flows and estuarine ecosystems: implications for coastal fisheries from a review and a case study of the Logan River, southeast Queensland. Australian Journal of Ecology 24, 431–440.
McConnaughey, RA, Hiddink, JG, Jennings, S, et al. (2020). Choosing best practices for managing impacts of trawl fishing on seabed habitats and biota. Fish and Fisheries 21, 319–337.
Meynecke, J-O, Grubert, M, and Gillson, J (2012). Giant mud crab (Scylla serrata) catches and climate drivers in Australia – a large scale comparison. Marine and Freshwater Research 63, 84–94.
Miller, RJ (1980). Design criteria for crab traps. ICES Journal of Marine Science 39, 140–147.
Rotherham, D, Broadhurst, MK, Gray, CA, and Johnson, DD (2008). Developing a beam trawl for sampling estuarine fish and crustaceans: assessment of a codend cover and effects of different sizes of mesh in the body and codend. ICES Journal of Marine Science 65, 687–696.
Rotherham, D, Johnson, DD, Macbeth, WG, and Gray, CA (2013). Escape gaps as a management strategy for reducing bycatch in net‐covered traps for the giant mud crab Scylla serrata. North American Journal of Fisheries Management 33, 307–317.
Savina, E, Krag, LA, Frandsen, RP, and Madsen, N (2017). Effect of fisher’s soak tactic on catch pattern in the Danish gillnet plaice fishery. Fisheries Research 196, 56–65.
Scandol, J, and Kennelly, S (2002). Using a fishery-independent survey to assess the status of a spanner crab Ranina ranina fishery: univariate analyses and biomass modelling. Crustaceana 75, 13–39.
Smith, GS, and Sumpton, WD (1989). Behaviour of the commercial sand crab Portunus pelagicus (L.) at trap entrances. Asian Fisheries Science 3, 101–113.
Smith, KD, Hall, NG, de Lestang, S, and Potter, IC (2004). Potential bias in estimates of the size of maturity of crabs derived from trap samples. ICES Journal of Marine Science 61, 906–912.
Sumpton W, Gaddes S, McLennan M, Campbell M, Tonks M, Good N, Hagedoorn W, Skilleter G (2003) Fisheries biology and assessment of the blue swimmer crab (Portunus pelagicus) in Queensland. Queensland Department of Primary Industries, FRDC Project, Qld, Australia.
Taylor, MD, Baker, J, and Suthers, IM (2013). Tidal currents, sampling effort and baited remote underwater video (BRUV) surveys: are we drawing the right conclusions? Fisheries Research 140, 96–104.
Vazquez Archdale, M (2012). Development of trapping gear and methods for swimming crabs. Crabs: Anatomy, Habitat and Ecological Significance , 27–47.
Vazquez Archdale, M, Añasco, CP, and Hiromori, S (2006). Comparative fishing trials for invasive swimming crabs Charybdis japonica and Portunus pelagicus using collapsible pots. Fisheries Research 82, 50–55.
Vazquez Archdale, M, Añasco, CP, Kawamura, Y, and Tomiki, S (2007). Effect of two collapsible pot designs on escape rate and behavior of the invasive swimming crabs Charybdis japonica and Portunus pelagicus. Fisheries Research 85, 202–209.
Winger, PD, and Walsh, PJ (2011). Selectivity, efficiency, and underwater observations of modified trap designs for the snow crab (Chionoecetes opilio) fishery in Newfoundland and Labrador. Fisheries Research 109, 107–113.
Zhou, S, and Shirley, TC (1997). Performance of two red king crab pot designs. Canadian Journal of Fisheries and Aquatic Sciences 54, 1858–1864.


 s.e.) blue swimmer crabs captured during each month across Port Stephens, Lake Macquarie and Wallis Lake in large-mesh and small-mesh traps.
s.e.) blue swimmer crabs captured during each month across Port Stephens, Lake Macquarie and Wallis Lake in large-mesh and small-mesh traps.