Does the grey nurse shark (Carcharias taurus) exhibit agonistic pectoral fin depression? A stereo-video photogrammetric assessment off eastern Australia
Kirby R. Smith A C , Carol Scarpaci A , Brett M. Louden B and Nicholas M. Otway BA College of Engineering and Science, Victoria University, PO Box 14428, Melbourne, Vic. 8001, Australia.
B Port Stephens Fisheries Institute, New South Wales Department of Primary Industries, Locked Bag 1, Nelson Bay, NSW 2315, Australia.
C Corresponding author. Email: kirby.smith1@live.vu.edu.au; kirbysmith84@hotmail.com
Pacific Conservation Biology 22(1) 3-11 https://doi.org/10.1071/PC15024
Submitted: 14 July 2015 Accepted: 6 December 2015 Published: 11 March 2016
Abstract
Underwater stereo-video photogrammetry was used to document the pectoral fin positions of various life-history stages of the critically endangered east Australian population of the grey nurse shark (Carcharias taurus) during normal swimming behaviour at multiple aggregation sites. A wide range in pectoral fin positions was recorded with dihedral pectoral fin angles ranging from –25 to 88°. Pectoral fin angles varied significantly among sites and this was attributed to the differing navigational and energetic requirements of the sharks. There was no significant relationship between pectoral fin angles and distances separating the shark and scuba diver. The wide range in pectoral fin angles, interactive use of the fins during swimming, low-energy behaviours of the sharks at aggregation sites and absence of ‘fight’ response agonistic behaviour indicated that the species does not exhibit agonistic pectoral fin depression. Reports of agonistic pectoral fin depression in the grey nurse shark obtained with visual estimates should be treated as preliminary observations requiring further testing using accurate sampling methods such as stereo photogrammetry. It is important that diver compliance with existing management guidelines that prohibit divers from chasing or harassing grey nurse sharks and blocking cave and gutter entrances is maintained.
Additional keywords: agonistic behaviour, critically endangered, pectoral fin angle, stereo photogrammetry.
Introduction
Agonistic behaviour encompasses all fearful, threatening and aggressive actions exhibited by animals (Brown and Hunsperger 1963; Hill et al. 2014). Animals present the fight or flight response when exposed to a perceived risk (Suresh et al. 2014), with the threat of injury (e.g. exposing the teeth) and actual physical aggression (e.g. biting) considered ‘fight’ reactions whereas fearful behaviours (e.g. fleeing) represent ‘flight’ responses (Hill et al. 2014). Stimuli that can induce the fight or flight response include predators, other species, conspecifics and unfamiliar objects. The motivation for responding to such stimuli may be self-defence, protection of young, territory or food, maintaining or challenging dominance within a social hierarchy, or competition for mates.
The agonistic behaviour of sharks has been documented for a variety of species with that of the grey reef shark (Carcharhinus amblyrhynchos) most known (Johnson and Nelson 1973; Nelson 1981–82; Nelson et al. 1986). A conspicuous threat display described for this species, and later termed ‘hunch’ (Myrberg and Gruber 1974), was exhibited in response to rapid approaches by a scuba diver (Johnson and Nelson 1973). The display incorporated a laterally exaggerated swimming motion, rolling, snout elevation, sustained pectoral fin depression, back arching and lateral bending (Johnson and Nelson 1973). More recently, a review described the agonistic behaviours putatively exhibited by 23 shark species including the grey reef shark (Martin 2007). Twenty-nine behaviours were reported with pectoral fin depression evident across all 23 species (Martin 2007). Agonistic pectoral fin depression was defined as the sustained (>5 s), bilateral lowering of the pectoral fins more than during normal swimming behaviour (Martin 2007) and the pectoral fin angle (PFA) was measured as the dihedral angle (i.e. the position of the pectoral fin below the horizontal plane) in accordance with laboratory studies of the North American leopard shark (Triakis semifasciata) (Wilga and Lauder 2000; Maia et al. 2012). Underwater visual estimates were previously used to quantify the PFA of sharks (Johnson and Nelson 1973; Martin 2007), but more recent research (Harvey et al. 2004) has shown that there are substantial errors and observer subjectivity inherent in this method. In contrast, stereo photogrammetry is not a new technique and provides much greater accuracy and precision when measuring lengths and angles (Harvey and Shortis 1995; Harvey et al. 2004). For example, stereo photogrammetry with still photographs was used to document lengths, orientations and nearest-neighbour distances of sharks to describe the three-dimensional schooling behaviour of scalloped hammerhead sharks (Sphyrna lewini) (Klimley 1981–82, 1985; Klimley and Brown 1983).
Underwater visual estimates and rapid scuba diver approaches were used by researchers to document agonistic pectoral fin depression in the grey nurse (sandtiger, ragged-tooth) shark (Carcharias taurus Rafinesque, 1810) at two shipwrecks off North Carolina, USA (Martin 2007). Underwater visual estimates were also used to report a single instance of agonistic pectoral fin depression in a grey nurse shark during interactions with approaching tourist scuba divers in a cave at Fish Rock off New South Wales (NSW), Australia (Barker et al. 2011). In contrast, stereo photogrammetry with video was recently used to quantify the swimming behaviours of various life-history stages of the grey nurse shark at multiple aggregation sites along the Australian east coast and included the measurement of PFA (Smith et al. 2015). This research showed that a wide range of PFA was evident across the sites sampled and attributed it to variation in navigational and energetic requirements associated with the depth, topography and water movement (Smith et al. 2015). The sharks did not exhibit any other threatening/aggressive (‘fight’ response) agonistic behaviours in the presence of the researchers (Smith et al. 2015).
The grey nurse shark, unlike the grey reef shark, has been consistently referred to as a docile species (Pollard et al. 1996; Compagno 2001; Otway and Ellis 2011) although it is known to take fish from spearfishers (Compagno 2001). This relatively large shark (males and females grow to ~3.00 and 3.20 m, respectively) has a widespread but fragmented distribution in warm-temperate and tropical coastal waters across the globe (Compagno 2001; Goldman et al. 2006; Last and Stevens 2009). It is slow to reach sexual maturity (50.0% sexual maturity: males = 2.10 m at 6–7 years, females = 2.59 m at 10–12 years: Goldman et al. 2006; Otway and Ellis 2011), has low fecundity (maximum of two pups biennially) and has experienced global population declines from targeted and indirect commercial and recreational fishing (Cavanagh et al. 2003; Otway et al. 2004). Consequently, the species is listed globally as ‘Vulnerable’ on the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species (IUCN 2015). In Australia, there are two genetically distinct grey nurse shark populations that occur off the east and west coasts (Stow et al. 2006; Ahonen et al. 2009). The eastern population occurs along the Queensland and NSW coasts, and following numerous anthropogenic impacts (Otway and Ellis 2011) is currently estimated to comprise 1146–1662 individuals (Lincoln Smith and Roberts 2010). Accordingly, the population is listed as ‘Critically Endangered’ by the IUCN (Cavanagh et al. 2003; IUCN 2015) and under Commonwealth and state (Queensland and NSW) legislation (Smith et al. 2014). The east Australian population exhibits annual (male) and biennial (female) migrations (≤4500 km), which are associated with the reproductive cycle and punctuated with visits to numerous aggregation sites (Bansemer and Bennett 2009; Otway et al. 2009; Otway and Ellis 2011).
The tendency of grey nurse sharks to aggregate combined with their docile nature (Pollard et al. 1996; Compagno 2001; Otway and Ellis 2011) has enabled the operation of a successful wildlife tourism industry involving passive interactions between scuba divers and the sharks (Smith et al. 2014). To mitigate potential adverse impacts on the sharks, the activities of this tourism sector are managed via a voluntary code of conduct and federal and state legislation (Smith et al. 2014). Management guidelines prohibit the feeding of grey nurse sharks whereas other marine wildlife tourism industries rely on provisioning target species including the bull (Carcharhinus leucas, e.g. Brunnschweiler and Barnett 2013), Caribbean reef (Carcharhinus perezi, e.g. Maljković and Côté 2011), Galapagos (Carcharhinus galapagensis, e.g. Meyer et al. 2009), sandbar (Carcharhinus plumbeus, e.g. Meyer et al. 2009), sicklefin lemon (Negaprion acutidens, e.g. Clua et al. 2010), silky (Carcharhinus falciformis, e.g. Clarke et al. 2011), tiger (Galeocerdo cuvier, e.g. Meyer et al. 2009; Hammerschlag et al. 2012), white (Carcharodon carcharias, e.g. Laroche et al. 2007) and whitetip reef (Triaenodon obesus, e.g. Fitzpatrick et al. 2011) sharks. Shark provisioning tourism may induce the sharks to exhibit aggressive agonistic behaviour towards humans because of the association with food (Laroche et al. 2007) or towards conspecifics due to increased competition for the food (e.g. Semeniuk and Rothley 2008).
The reports of agonistic pectoral fin depression in grey nurse sharks relative to decreasing diver distances (i.e. Martin 2007; Barker et al. 2011) contrast with the wide range of PFA documented for the species during typical swimming behaviour (i.e. hovering, milling and active swimming) (Smith et al. 2015). The aim of this study was to quantify grey nurse shark PFA in relation to the distances of scuba divers across multiple life-history stages (i.e. time) and aggregation sites (i.e. space). This research will contribute to the developing ethogram for the species (Smith et al. 2015) and to the assessment and management of this tourism industry.
Materials and methods
Study sites
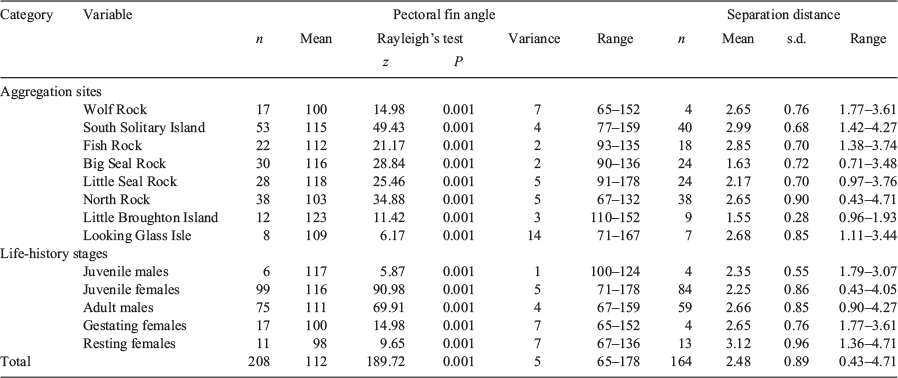
Sampling of the PFA of grey nurse sharks was done by a maximum of three scuba divers in the absence of commercial and recreational fishing and tourist scuba divers (Smith et al. 2014) over eight aggregation sites along ~800 km of the east Australian coast during the austral autumn of 2010 (Fig. 1). These sites were selected as they are inhabited differentially by five life-history stages (juvenile males, juvenile females, adult males, gestating females and resting females) of the grey nurse shark (Bansemer and Bennett 2009; Otway and Ellis 2011). The sites are influenced by a 1–2-m south-easterly swell, onshore winds, the 1–4-kn East Australian Current and sea surface temperatures varying annually between 19.0 and 28.0°C (Otway and Ellis 2011; Smith et al. 2014, 2015). The sites vary physically (topography, substratum, water movement) and biologically (presence of kelp, invertebrate and vertebrate fauna) to differing degrees (Smith et al. 2015).
|
Underwater stereo-video photogrammetry system
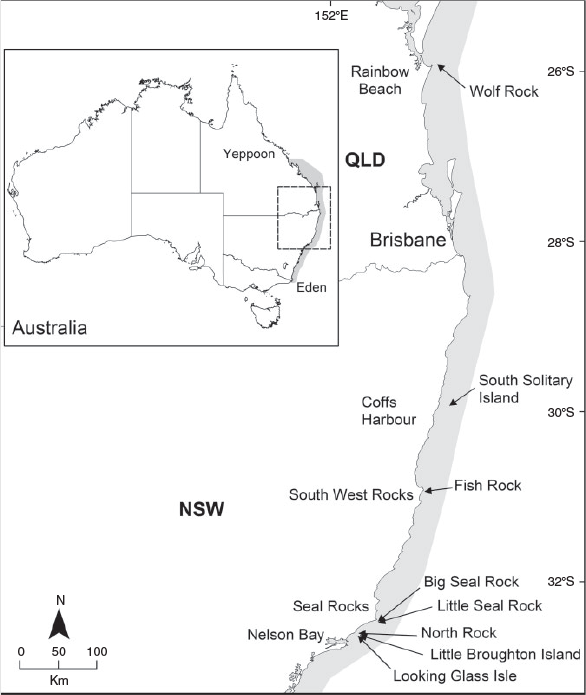
The underwater stereo-video photogrammetry system (USVPS) used to capture videos of grey nurse sharks comprised two digital video cameras attached with an inward angle of 4° (to produce some overlap of the left and right images) to an aluminium base bar (further details: Otway et al. 2008; Smith et al. 2015) (Fig. 2). An image synchronisation unit was affixed to the end of an aluminium rod that was mounted at a right angle to the middle of the base bar (further details: Otway et al. 2008; Smith et al. 2015) (Fig. 2). The USVPS was calibrated before sampling, recorded 24 frames s–1 and allowed stereo images of sharks that were 3.00 m in total length (TL) at a minimum distance of 3.00 m from the base bar of the system (further details: Otway et al. 2008; Smith et al. 2015) (Fig. 2). The videos were then downloaded with Adobe Premiere (ver. 6.0) and analysed using EventMeasure (©SeaGIS Pty Ltd, further details: Smith et al. 2015). This software enabled length measurements (mm) to be quantified with great accuracy (±0.2%) and precision (±0.3–1.2%) irrespective of the shark’s three-dimensional position in the water column (Otway et al. 2008).
|
Life-history stages of the grey nurse shark
The precaudal length (PCL) of each shark (i.e. tip of the snout to the precaudal pit: Compagno 2001) was measured (Fig. 2) rather than TL due to reduced error (Francis 2006) and the varying elevation and oscillations of the caudal fin (Smith et al. 2015). A significant linear regression relationship developed from necropsies of 150 grey nurse sharks (Otway et al. 2008; Otway 2015) was used to calculate TL from PCL (i.e. TL = 1.368PCL + 0.069, with TL and PCL in metres, n = 66, R2 = 0.99, P < 0.001). The life-history stage of each shark was then determined from its TL, sex (i.e. males were identified by the presence of claspers) and maturity ogives (Otway and Ellis 2011).
Pectoral fin angles in the grey nurse shark
Individual grey nurse sharks were identified using 20 standard morphometric measurements (Bass et al. 1975; Compagno 2001; Otway 2015) obtained with the USVPS (to the nearest mm) when quantifying swimming behaviour (Smith et al. 2015) and PFA. Retained fishing gear (i.e. hooks and line), tail ropes and obvious spots and/or scars (Bansemer and Bennett 2009; Barker et al. 2011; Otway 2015) were used to augment the morphometric measurements for individual identification.
Additional morphometric length measurements were quantified and used together with significant regression relationships and standard geometric equations to calculate the PFA of grey nurse sharks. Continuous observation (Altmann 1974) was used to select sharks suitable for measuring the left or right PFA during normal swimming behaviour (i.e. hovering, milling and active swimming) (Smith et al. 2014, 2015) which was recorded for each shark measured. The PFA represented the position of the pectoral fin in the water column relative to the trunk (Fig. 3a) and fins that were perpendicular to the trunk, raised or lowered had a PFA of 90°, <90° and >90°, respectively. The PFA was defined as the angle subtended by a point (point A) at the top of a straight imaginary line equal in length to the fifth gill slit and perpendicular to the pectoral fin insertion, the pectoral fin insertion itself (point B) and the pectoral fin apex (point C) (Fig. 3b, c). The USVPS was used to measure the lengths between: (1) the top (point D) and the bottom (point E) of the fifth gill slit (line DE = AB); (2) the pectoral fin apex (point C) and the top of the fifth gill slit (point D) (line CD); and (3) the pectoral fin length (PFL) from the pectoral fin origin at the bottom of the fifth gill slit (point E) to the pectoral fin free rear tip (point F) (line EF) (Fig. 3b, c). The pectoral fin height (PFH) (line BC: Fig. 3b, c) was calculated from PFL using a regression (PFH = 1.212PFL – 22.752, n = 53, R2 = 0.96, P < 0.001). As the pectoral fin insertion was not always visible, the pectoral fin base length (PFBL) (line BE = AD) was determined from PFL via a regression (PFBL = 0.671PFL – 25.537, n = 53, R2 = 0.94, P < 0.001). The length between the top of the imaginary line and the pectoral fin apex (line AC) was then calculated from lines AD and CD using Pythagoras’ theorem. The PFA (angle ABC) was calculated with the law of cosines (De Sapio 1976) where ABC = arcos [(BC2 + AB2 – AC2)/2(BC × AB)]. EventMeasure also provided the range (mm) of the shark relative to the base bar (Fig. 2). This enabled the separation distance, defined as the minimum distance between each individually identified shark and the diver with the USVPS, to be calculated once as the range minus the length of the synchronisation element (1150 mm) for each PFA measurement.

|
Statistical analyses
The timing and duration of occupation of aggregation sites by grey nurse sharks is dependent on several environmental and biological (e.g. sexual segregation, reproductive cycle and migratory behaviour) factors resulting in unavoidable confounding of sites and life-history stages. Nevertheless, separate statistical analyses among sites and life-history stages were done to determine whether more general patterns existed. All statistical analyses were conducted with a Type I (α) error rate of P = 0.05. The mean and angular variance of grey nurse shark PFA among and across all aggregation sites and life-history stages were calculated using tests associated with the Von Mises (circular normal) distribution (Batschelet 1981; Zar 2010). Rayleigh tests examined for significant mean PFA directions and Watson–Williams multisample F tests for significant differences among mean PFA according to sites, life-history stages and swimming behaviours (Batschelet 1981; Zar 2010). If grey nurse sharks exhibit agonistic pectoral fin depression then previous research (Johnson and Nelson 1973; Nelson et al. 1986) predicts that the PFA would increase with decreasing distance between the approaching diver and the shark. To test this hypothesis, the PFA of each grey nurse shark was examined against separation distance at the eight sites, among the five life-history stages and the pooled data were plotted. The respective angular-linear correlation coefficients were then calculated and their significance tested (Batschelet 1981; Zar 2010).
Results
Life-history stages of the grey nurse shark
Across the eight study sites, 273 grey nurse sharks were individually identified from morphometric measurements and retained fishing gear, tail ropes, spots and/or scars when present. Grey nurse sharks at Wolf Rock comprised only gestating females (n = 18) 2.55–2.80 m TL. The population at South Solitary Island (n = 22) included mostly adult males (72.7%) with some adult females and juveniles of both sexes, and ranged from 1.77 to 2.63 m TL. At Fish Rock, the population (n = 15) comprised juvenile and adult males and females 1.88–2.56 m TL. The sharks at Big Seal Rock (n = 79) were juveniles and adults of both sexes that ranged from 1.50 to 2.70 m TL. At Little Seal Rock, the shark population (n = 33) comprised primarily juvenile females (81.8%) with some juvenile males and adults, and ranged from 1.44 to 2.45 m TL. The sharks at North Rock (n = 67) were mainly juvenile females (89.6%) but also included juvenile males and adults, and ranged from 1.52 to 2.92 m TL. The population at Little Broughton Island (n = 30) comprised juvenile males and females including some pups, and ranged from 1.27 to 1.86 m TL. The sharks at Looking Glass Isle (n = 9) were all juvenile males and females 1.42–1.78 m TL.
Pectoral fin positions in the grey nurse shark
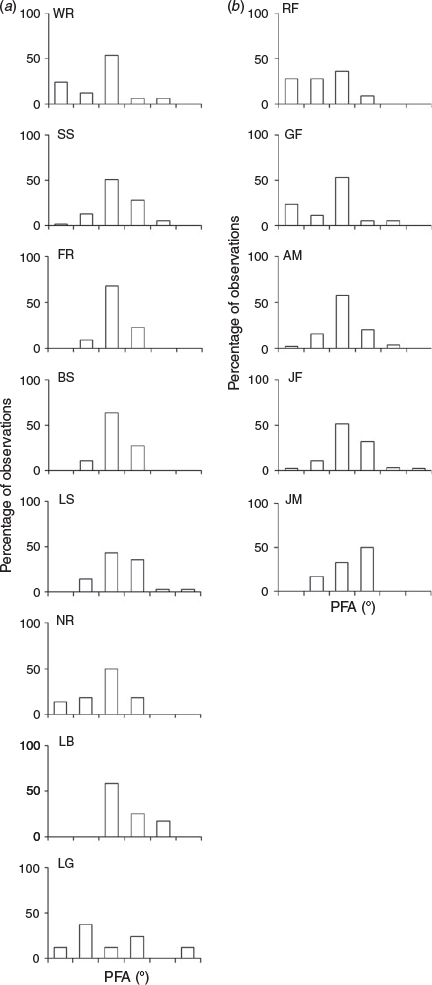
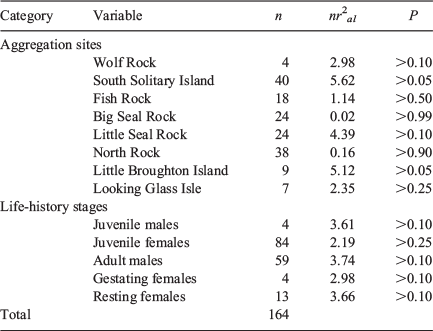
The mean PFA of grey nurse sharks differed significantly among aggregation sites (Watson–Williams test: F7,200 = 4.83, P < 0.0005) but not life-history stages (Watson–Williams test: F4,203 = –0.54, P > 0.25) or the three swimming behaviours exhibited (Watson–Williams test: F2,205 = 0.33, P > 0.25). Generally, pectoral fins were held lowest at Little Broughton Island and highest at Wolf Rock and North Rock (Table 1, Fig. 4). While not significant, there was a trend towards greater mean PFA for juvenile sharks (Table 1).
|
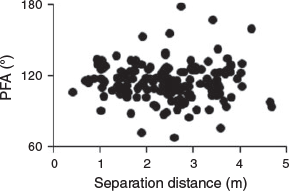
Samples of PFA and separation distances at Wolf Rock, Little Broughton Island and Looking Glass Isle were small (n ≤ 9) (Table 2) due to a combination of few individual grey nurse sharks and environmental constraints with a likely lack of power in the respective angular-linear correlation analyses. Sample sizes at the remaining five sites were reasonable (n ≥ 18) (Table 2), but there were still no significant correlations between the PFA and separation distances of sharks from the diver with the USVPS among aggregation sites (Table 2). Sample sizes for juvenile males, gestating females (only present at Wolf Rock) and resting females were small (n ≤ 13) (Table 2) and also likely resulted in low power in the respective angular-linear correlation analyses. Larger sample sizes were obtained for juvenile females and adult males (n ≥ 59) (Table 2), but again there were no significant correlations between the PFA and separation distance among life-history stages (Table 2, Fig. 5). Combining the data across all sites and life-history stages (n = 164) showed that there was no relationship between PFA and separation distance (Fig. 5).
|
|
Discussion
The occupation of Wolf Rock by gestating female grey nurse sharks (i.e. sexually segregated from males) during autumn was entirely consistent with previous research (Bansemer and Bennett 2009; Smith et al. 2014). South Solitary Island, Fish Rock, Big Seal Rock, Little Seal Rock and North Rock were occupied by varying numbers of grey nurse sharks in the remaining life-history stages and these patterns were also similar to those found in earlier studies (Otway et al. 2009; Otway and Ellis 2011; Smith et al. 2014). In contrast, Little Broughton Island and Looking Glass Isle were inhabited only by juvenile male and female sharks as previously documented (Otway et al. 2009).
Grey nurse sharks at five different life-history stages and at eight aggregation sites off eastern Australia exhibited a wide range of PFA during usual swimming behaviour (hovering, milling and active swimming) with dihedral angles (sensu Wilga and Lauder 2000) from 25° above the horizontal to 88° below. There was also significant variation in the mean PFA among aggregation sites but the pectoral fins were held in a depressed position with the mean dihedral angles between 10 and 33° below the horizontal during normal swimming. The mean PFA at Little Broughton Island was significantly greater than those at other sites and this was likely due to the pectoral fins being used by the juvenile sharks to maintain station (sensu Wilga and Lauder 2000; Maia et al. 2012) in this dynamic site with variable currents and surge from breaking waves that reach the shallow, complex seabed topography (Smith et al. 2015).
The ranges in PFA documented in this study encompassed previous observations of putative agonistic pectoral fin depression (30–50°) in grey nurse sharks at two shipwrecks off North Carolina, USA, in 2002 following rapid approaches by scuba divers (Martin 2007). The well-documented agonistic ‘hunch’ display exhibited by grey reef sharks (Johnson and Nelson 1973; Nelson et al. 1986) comprising pectoral fin depression with laterally exaggerated swimming, rolling, snout elevation, back arching and lateral bending was elicited by divers charging to within 4.00 m (on average). However, this display did not occur during passive interactions with divers at an average separation distance of 2.00 m (Johnson and Nelson 1973). Grey nurse sharks did not exhibit agonistic pectoral fin depression during any approaches by divers to within <0.5 m of the shark and this was clearly evidenced by the absence of significant relationships between PFA and separation distances across all of the sites and life-history stages sampled. Moreover, none of the other elements of the ‘hunch’ display were exhibited by grey nurse sharks in this study or in previous research along the east coast of Australia (Smith et al. 2010, 2015; Barker et al. 2011). These findings are also consistent with the absence of changes in normal swimming behaviour (hovering, milling and active swimming) during interactions with up to 10 tourist divers participating in two consecutive dives over a period of three hours (Smith et al. 2014).
The above results contrasted greatly with the increased intensity of agonistic pectoral fin depression as separation distance decreased reported in a previous study (Martin 2007) and this may have been attributable to differences in diver behaviour. However, the degree of diver influence cannot be assessed as the other study (Martin 2007) did not quantify separation distances nor were any of the observations analysed statistically. Moreover, details of the methods used to quantify the PFA, sample sizes and the life-history stages of the sharks observed were not provided. Recent sampling of grey nurse sharks using underwater stereo photogrammetry has shown that there are substantial inaccuracies in visual estimates of shark angles (unpubl. data). It is probable that there were inaccuracies in previous observations (Martin 2007; Barker et al. 2011) of agonistic pectoral fin depression in grey nurse sharks as the angle between the pectoral fin and an imaginary, horizontal plane in three-dimensional space was visually estimated. Importantly, accurate measurement of lengths and angles of a three-dimensional object (i.e. a shark) in three-dimensional space requires the use of a calibrated stereo photogrammetry system (Harvey and Shortis 1995; Harvey et al. 2004) and fixed reference points that are easily and routinely discerned.
Descriptions of non-agonistic behaviours (e.g. swimming, feeding and reproduction) are also necessary for the appropriate identification of agonistic pectoral fin depression and associated behaviours. The swimming behaviours of the grey reef shark were described which enabled clear recognition of the agonistic ‘hunch’ display (Johnson and Nelson 1973). Whilst earlier work (Martin 2007) that reported agonistic pectoral fin depression in grey nurse sharks acknowledged the similarities between the positions of pectoral fins during signalling (i.e. agonistic display) and swimming, neither descriptions nor reference to previous studies of normal swimming and/or non-swimming behaviours in the species were provided. In contrast, this study and previous work (Smith et al. 2015) has shown that grey nurse sharks actively engaged their pectoral fins for navigation (i.e. ascending, descending and turning) and stabilisation (i.e. maintaining position) akin to the North American leopard shark (Wilga and Lauder 2000; Maia et al. 2012) and the grey reef shark (Barlow 1974) with PFA varying according to physical and energetic parameters.
The review of shark agonistic behaviours noted that the presence of other agonistic behaviours can also aid identification of agonistic pectoral fin depression (Martin 2007). The absence of ‘fight’ response agonistic behaviours exhibited by grey nurse sharks along the east coast of Australia together with their low-energy swimming behaviours (Smith et al. 2015) indicated that this species adopts energy-conservation measures when at aggregation sites. These findings strongly suggested that grey nurse sharks are unlikely to expend energy on agonistic pectoral fin depression display, instead opting to flee from perceived threats (i.e. rapid withdrawal) as previously documented (Smith et al. 2015) and are in line with prior descriptions of its placid temperament (Pollard et al. 1996; Compagno 2001; Otway and Ellis 2011). Rapid withdrawal in response to the presence of a submersible has also been recorded for blackfin reef, silvertip and whitetip reef sharks (Nelson et al. 1986).
The results of this study indicated that the grey nurse shark does not exhibit agonistic pectoral fin depression in contrast to previous work (Martin 2007; Barker et al. 2011). Instead, grey nurse sharks exhibit the flight response (Smith et al. 2015) rather than the overt, aggressive agonistic behaviour displayed by grey reef sharks (Johnson and Nelson 1973; Nelson 1981–82; Nelson et al. 1986) when exposed to perceived threats. Consequently, tourism management guidelines that stipulate that scuba divers must not chase or harass grey nurse sharks or block cave and gutter entrances (EA 2002; NSWG 2010) are appropriate for minimising disturbance to the sharks. Moreover, it is important that existing satisfactory diver compliance with these management guidelines (Smith et al. 2010, 2014) is maintained to effectively protect the sharks from behavioural stressors.
Conclusion
This study using stereo photogrammetry showed that agonistic pectoral fin depression was not exhibited by grey nurse sharks at various life-history stages in the presence of scuba divers off eastern Australia. Instead, pectoral fin depression occurred during normal swimming behaviour and was attributed to the differing navigational and energetic requirements of the sharks in habitats with varying physical conditions. Moreover, at aggregation sites grey nurse sharks adopted energy-conservation regimes and exhibited ‘flight’ rather than ‘fight’ responses to perceived threats. The contrasting results in two earlier studies were derived from visual estimates of PFA and anecdotal reports of agonistic pectoral fin depression in grey nurse sharks. When the three studies are considered together, the weight of quantitative evidence suggests that the grey nurse shark does not exhibit agonistic pectoral fin depression in the absence of aggressively approaching divers. Reports of agonistic pectoral fin depression in the grey nurse shark obtained with visual estimates should be considered preliminary observations in need of further testing using rigorous scientific methodology such as stereo photogrammetry. It is important that diver compliance with current tourism management guidelines is maintained to protect the sharks from behavioural stressors.
Acknowledgements
This work was done whilst the senior author was in receipt of an Australian Postgraduate Award and funding from Victoria University and the NSW Department of Primary Industries (DPI). The research was conducted under a NSW DPI scientific research permit (P01/0059(A)-2.0) and animal research ethics committee approval (ACEC 99/14 Port Stephens). We thank J. Seager of ©SeaGIS Pty Ltd for his invaluable support with using EventMeasure, J. Gilligan for his assistance in the field and G. West for his help with the figures.
References
Ahonen, H., Harcourt, R. J., and Stow, A. J. (2009). Nuclear and mitochondrial DNA reveals isolation of imperilled grey nurse shark populations (Carcharias taurus). Molecular Ecology 18, 4409–4421.| Nuclear and mitochondrial DNA reveals isolation of imperilled grey nurse shark populations (Carcharias taurus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFaitrbE&md5=3809ae207d488071066a4c883914a3b3CAS | 19804378PubMed |
Altmann, J. (1974). Observational study of behaviour: sampling methods. Behaviour 49, 227–266.
| Observational study of behaviour: sampling methods.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2c7mtlWmsQ%3D%3D&md5=a288ef4cb1b3f374509656df6d32db9aCAS | 4597405PubMed |
Bansemer, C. S., and Bennett, M. B. (2009). Reproductive periodicity, localised movements and behavioural segregation of pregnant Carcharias taurus at Wolf Rock, southeast Queensland, Australia. Marine Ecology Progress Series 374, 215–227.
| Reproductive periodicity, localised movements and behavioural segregation of pregnant Carcharias taurus at Wolf Rock, southeast Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Barker, S. M., Peddemors, V. M., and Williamson, J. E. (2011). A video and photographic study of aggregation, swimming and respiratory behaviour changes in the grey nurse shark (Carcharias taurus) in response to the presence of scuba divers. Marine and Freshwater Behaviour and Physiology 44, 75–92.
| A video and photographic study of aggregation, swimming and respiratory behaviour changes in the grey nurse shark (Carcharias taurus) in response to the presence of scuba divers.Crossref | GoogleScholarGoogle Scholar |
Barlow, G. W. (1974). Derivation of threat display in the gray reef shark. Marine Behaviour and Physiology 3, 71–81.
| Derivation of threat display in the gray reef shark.Crossref | GoogleScholarGoogle Scholar |
Bass, A. J., D’Aubrey, J. D., and Kistnasamy, N. (1975). Sharks of the east coast of southern Africa. IV. The families Odontaspididae, Scapanorhynchidae, Isuridae, Cetorhinidae, Alopiidae, Orectolobidae, and Rhiniodontidae: investigational report No. 39. The Oceanographic Research Institute, Durban.
Batschelet, E. (1981). ‘Circular Statistics in Biology.’ (Academic Press: London.)
Brown, J. L., and Hunsperger, R. W. (1963). Neurothology and the motivation of agonistic behaviour. Animal Behaviour 11, 439–448.
| Neurothology and the motivation of agonistic behaviour.Crossref | GoogleScholarGoogle Scholar |
Brunnschweiler, J. M., and Barnett, A. (2013). Opportunistic visitors: long-term behavioural response of bull sharks to food provisioning in Fiji. PLoS One 8, e58522.
| Opportunistic visitors: long-term behavioural response of bull sharks to food provisioning in Fiji.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXks1Wnsb4%3D&md5=98bcebe9a23b8fd1e93fafb732daee25CAS | 23516496PubMed |
Cavanagh, R. D., Kyne, P. M., Fowler, S. L., Musick, J. A., and Bennett, M. B. (Eds) (2003). The conservation status of Australasian chondrichthyans: report of the IUCN Shark Specialist Group Australia and Oceania Regional Red List Workshop. The University of Queensland, School of Biomedical Sciences, Brisbane.
Clarke, C., Lea, J. S. E., and Ormond, R. F. G. (2011). Reef-use and residency patterns of a baited population of silky sharks, Carcharhinus falciformis, in the Red Sea. Marine and Freshwater Research 62, 668–675.
| Reef-use and residency patterns of a baited population of silky sharks, Carcharhinus falciformis, in the Red Sea.Crossref | GoogleScholarGoogle Scholar |
Clua, E., Buray, N., Legendre, P., Mourier, J., and Planes, S. (2010). Behavioural response of sicklefin lemon sharks Negaprion acutidens to underwater feeding for ecotourism purposes. Marine Ecology Progress Series 414, 257–266.
| Behavioural response of sicklefin lemon sharks Negaprion acutidens to underwater feeding for ecotourism purposes.Crossref | GoogleScholarGoogle Scholar |
Compagno, L. J. V. (2001). ‘Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date. Vol. 2. Bullhead, Mackerel and Carpet Sharks (Heterodontiformes, Lamniformes and Orectolobiformes).’ (Food and Agriculture Organization of the United Nations: Rome.)
De Sapio, R. (1976). ‘Calculus for the Life Sciences.’ (W.H. Freeman and Co.: San Francisco, CA.)
Environment Australia [EA] (2002). Recovery plan for the grey nurse shark (Carcharias taurus) in Australia. Commonwealth of Australia, Canberra, ACT.
Fitzpatrick, R., Abrantes, K. G., Seymour, J., and Barnett, A. (2011). Variation in depth of whitetip reef sharks: does provisioning ecotourism change their behaviour? Coral Reefs 30, 569–577.
| Variation in depth of whitetip reef sharks: does provisioning ecotourism change their behaviour?Crossref | GoogleScholarGoogle Scholar |
Francis, M. P. (2006). Morphometric minefields – towards a measurement standard for chondrichthyan fishes. Environmental Biology of Fishes 77, 407–421.
| Morphometric minefields – towards a measurement standard for chondrichthyan fishes.Crossref | GoogleScholarGoogle Scholar |
Goldman, K. J., Branstetter, S., and Musick, J. A. (2006). A re-examination of the age and growth of sand tiger sharks, Carcharias taurus, in the western North Atlantic: the importance of ageing protocols and use of multiple back-calculation techniques. Environmental Biology of Fishes 77, 241–252.
| A re-examination of the age and growth of sand tiger sharks, Carcharias taurus, in the western North Atlantic: the importance of ageing protocols and use of multiple back-calculation techniques.Crossref | GoogleScholarGoogle Scholar |
Hammerschlag, N., Gallagher, A. J., Wester, J., Luo, J., and Ault, J. S. (2012). Don’t bite the hand that feeds: assessing ecological impacts of provisioning ecotourism on an apex marine predator. Functional Ecology 26, 567–576.
| Don’t bite the hand that feeds: assessing ecological impacts of provisioning ecotourism on an apex marine predator.Crossref | GoogleScholarGoogle Scholar |
Harvey, E., and Shortis, M. (1995). A system for stereo-video measurement of sub-tidal organisms. Marine Technology Society Journal 29, 10–22.
Harvey, E., Fletcher, D., Shortis, M. R., and Kendrick, G. A. (2004). A comparison of underwater visual distance estimates made by scuba divers and a stereo-video system: implications for underwater visual census of reef fish abundance. Marine and Freshwater Research 55, 573–580.
| A comparison of underwater visual distance estimates made by scuba divers and a stereo-video system: implications for underwater visual census of reef fish abundance.Crossref | GoogleScholarGoogle Scholar |
Hill, H. M., Artz, S., and Lopez, M. (2014). Sex, love, or war? A representation of 20 years of research on the social interactions of animals. International Journal of Comparative Psychology 27, 50–68.
International Union for Conservation of Nature (IUCN) (2015). ‘The IUCN Red List of Threatened Species: version 2015–3.’ Available at: www.iucnredlist.org [accessed 7 October 2015].
Johnson, R. H., and Nelson, D. R. (1973). Agonistic display in the gray reef shark, Carcharhinus menisorrah, and its relationship to attacks on man. Copeia 1973, 76–84.
| Agonistic display in the gray reef shark, Carcharhinus menisorrah, and its relationship to attacks on man.Crossref | GoogleScholarGoogle Scholar |
Klimley, A. P. (1981–82). Grouping behavior in the scalloped hammerhead. Oceanus 24, 65–71.
Klimley, A. P. (1985). Schooling in Sphyrna lewini, a species with low risk of predation: a non-egalitarian state. Zeitschrift für Tierpsychologie 70, 297–319.
Klimley, A. P., and Brown, S. T. (1983). Stereophotography for the field biologist: measurement of lengths and three-dimensional positions of free-swimming sharks. Marine Biology 74, 175–185.
| Stereophotography for the field biologist: measurement of lengths and three-dimensional positions of free-swimming sharks.Crossref | GoogleScholarGoogle Scholar |
Laroche, R. K., Kock, A. A., Dill, L. M., and Oosthuizen, W. H. (2007). Effects of provisioning ecotourism activity on the behaviour of white sharks Carcharodon carcharias. Marine Ecology Progress Series 338, 199–209.
| Effects of provisioning ecotourism activity on the behaviour of white sharks Carcharodon carcharias.Crossref | GoogleScholarGoogle Scholar |
Last, P. R., and Stevens, J. D. (2009). ‘Sharks and Rays of Australia.’ 2nd edn. (CSIRO Publishing: Melbourne.)
Lincoln Smith, M., and Roberts, C. (2010). Development and implementation of a population estimation protocol to provide an estimate of east coast population numbers for grey nurse sharks (Carcharias taurus): prepared for the Department of the Environment, Water, Heritage and the Arts. Cardno Ecology Lab, Brookvale.
Maia, A. M. R., Wilga, C. A. D., and Lauder, G. V. (2012). Biomechanics of locomotion in sharks, rays, and chimaeras. In ‘Biology of Sharks and Their Relatives’. 2nd edn. (Eds J. Carrier, J. Musick, and M. Heithaus.) Chapter 5, pp. 125–151. (CRC Press: Boca Raton, FL.)
Maljković, A., and Côté, I. M. (2011). Effects of tourism-related provisioning on the trophic signatures and movement patterns of an apex predator, the Caribbean reef shark. Biological Conservation 144, 859–865.
| Effects of tourism-related provisioning on the trophic signatures and movement patterns of an apex predator, the Caribbean reef shark.Crossref | GoogleScholarGoogle Scholar |
Martin, R. A. (2007). A review of shark agonistic displays: comparison of display features and implications for shark–human interactions. Marine and Freshwater Behaviour and Physiology 40, 3–34.
| A review of shark agonistic displays: comparison of display features and implications for shark–human interactions.Crossref | GoogleScholarGoogle Scholar |
Meyer, C. G., Dale, J. J., Papastamatiou, Y. P., Whitney, N. M., and Holland, K. M. (2009). Seasonal cycles and long-term trends in abundance and species composition of sharks associated with cage diving ecotourism activities in Hawaii. Environmental Conservation 36, 104–111.
| Seasonal cycles and long-term trends in abundance and species composition of sharks associated with cage diving ecotourism activities in Hawaii.Crossref | GoogleScholarGoogle Scholar |
Myrberg, A. A., and Gruber, S. H. (1974). The behavior of the bonnethead shark, Sphyrna tiburo. Copeia 1974, 358–374.
| The behavior of the bonnethead shark, Sphyrna tiburo.Crossref | GoogleScholarGoogle Scholar |
Nelson, D. R. (1981–82). Aggression in sharks: is the gray reef shark different? Oceanus 24, 45–55.
Nelson, D. R., Johnson, R. R., McKibben, J. N., and Pittenger, G. G. (1986). Agonistic attacks on divers and submersibles by gray reef sharks, Carcharhinus amblyrhynchos: antipredatory or competitive? Bulletin of Marine Science 38, 68–88.
New South Wales Government [NSWG] (2010). ‘Fisheries Management (General) Regulation 2010: Fisheries Management Act 1994.’ Available at http://www.legislation.nsw.gov.au/sessionalview/sessional/sr/2010-475.pdf (accessed 10 April 2013).
Otway, N. M. (2015). Serum biochemical reference intervals for free-living sand tiger sharks (Carcharias taurus) from east Australian waters. Veterinary Clinical Pathology 44, 262–274.
| Serum biochemical reference intervals for free-living sand tiger sharks (Carcharias taurus) from east Australian waters.Crossref | GoogleScholarGoogle Scholar | 25865808PubMed |
Otway, N. M., and Ellis, M. T. (2011). Pop-up archival satellite tagging of Carcharias taurus: movements and depth/temperature-related use of south-eastern Australian waters. Marine and Freshwater Research 62, 607–620.
| Pop-up archival satellite tagging of Carcharias taurus: movements and depth/temperature-related use of south-eastern Australian waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnvFanu7s%3D&md5=ba6144db4655e9c0cfaa2ca147fbc766CAS |
Otway, N. M., Bradshaw, C. J. A., and Harcourt, R. G. (2004). Estimating the rate of quasi-extinction of the Australian grey nurse shark (Carcharias taurus) population using deterministic age- and stage-classified models. Biological Conservation 119, 341–350.
| Estimating the rate of quasi-extinction of the Australian grey nurse shark (Carcharias taurus) population using deterministic age- and stage-classified models.Crossref | GoogleScholarGoogle Scholar |
Otway, N. M., Louden, B. M., Storrie, M. T., and Gilligan, J. J. (2008). Construction of an underwater photogrammetry system for quantifying the population size-structure and rates of mortality of the critically endangered grey nurse shark in SE Australian waters. NSW Department of Primary Industries, Nelson Bay, NSW.
Otway, N. M., Storrie, M. T., Louden, B. M., and Gilligan, J. J. (2009). Documentation of depth-related migratory movements, behaviour movements at critical habitat sites and the effects of scuba diving for the east coast grey nurse shark population. Fisheries Final Report Series. Industry and Investment NSW, Nelson Bay, NSW.
Pollard, D. A., Lincoln Smith, M. P., and Smith, A. K. (1996). The biology and conservation status of the grey nurse shark (Carcharias taurus, Rafinesque 1810) in New South Wales, Australia. Aquatic Conservation: Marine and Freshwater Ecosystems 6, 1–20.
| The biology and conservation status of the grey nurse shark (Carcharias taurus, Rafinesque 1810) in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Semeniuk, C. A. D., and Rothley, K. D. (2008). Costs of group-living for a normally solitary forager: effects of provisioning tourism on southern stingrays Dasyatis Americana. Marine Ecology Progress Series 357, 271–282.
| Costs of group-living for a normally solitary forager: effects of provisioning tourism on southern stingrays Dasyatis Americana.Crossref | GoogleScholarGoogle Scholar |
Smith, K., Scarr, M., and Scarpaci, C. (2010). Grey nurse shark (Carcharias taurus) diving tourism: tourist compliance and shark behaviour at Fish Rock, Australia. Environmental Management 46, 699–710.
| Grey nurse shark (Carcharias taurus) diving tourism: tourist compliance and shark behaviour at Fish Rock, Australia.Crossref | GoogleScholarGoogle Scholar | 20872140PubMed |
Smith, K. R., Scarpaci, C., Scarr, M. J., and Otway, N. M. (2014). Scuba diving tourism with critically endangered grey nurse sharks (Carcharias taurus) off eastern Australia: tourist demographics, shark behaviour and diver compliance. Tourism Management 45, 211–225.
| Scuba diving tourism with critically endangered grey nurse sharks (Carcharias taurus) off eastern Australia: tourist demographics, shark behaviour and diver compliance.Crossref | GoogleScholarGoogle Scholar |
Smith, K. R., Scarpaci, C., and Otway, N. M. (2015). Behaviour of aggregated grey nurse sharks Carcharias taurus off eastern Australia: similarities and differences among life-history stages and sites. Endangered Species Research 27, 69–85.
| Behaviour of aggregated grey nurse sharks Carcharias taurus off eastern Australia: similarities and differences among life-history stages and sites.Crossref | GoogleScholarGoogle Scholar |
Stow, A., Zenger, K., Briscoe, D., Gillings, M., Peddemors, V., Otway, N., and Harcourt, R. (2006). Isolation and genetic diversity of endangered grey nurse shark (Carcharias taurus) populations. Biology Letters 2, 308–311.
| Isolation and genetic diversity of endangered grey nurse shark (Carcharias taurus) populations.Crossref | GoogleScholarGoogle Scholar | 17148390PubMed |
Suresh, A., Latha, S. S., Nair, P., and Radhika, N. (2014). Prediction of fight or flight response using artificial neural networks. American Journal of Applied Sciences 11, 912–920.
| Prediction of fight or flight response using artificial neural networks.Crossref | GoogleScholarGoogle Scholar |
Wilga, C. D., and Lauder, G. V. (2000). Three-dimensional kinematics and wake structure of the pectoral fins during locomotion in leopard sharks Triakis semifasciata. The Journal of Experimental Biology 203, 2261–2278.
| 1:STN:280:DC%2BD3cvntlWktA%3D%3D&md5=f70d401f0b2d31215dce19ffad4ab073CAS | 10887066PubMed |
Zar, J. H. (2010). ‘Biostatistical Analysis.’ 5th edn. (Prentice Hall, Inc.: Upper Saddle River, NJ.)