Reptiles on the brink: identifying the Australian terrestrial snake and lizard species most at risk of extinction
Hayley M. Geyle A , Reid Tingley
A , Reid Tingley  B , Andrew P. Amey C , Hal Cogger D , Patrick J. Couper C , Mark Cowan E , Michael D. Craig F G , Paul Doughty H , Don A. Driscoll I , Ryan J. Ellis H J , Jon-Paul Emery F , Aaron Fenner K , Michael G. Gardner K L , Stephen T. Garnett
B , Andrew P. Amey C , Hal Cogger D , Patrick J. Couper C , Mark Cowan E , Michael D. Craig F G , Paul Doughty H , Don A. Driscoll I , Ryan J. Ellis H J , Jon-Paul Emery F , Aaron Fenner K , Michael G. Gardner K L , Stephen T. Garnett  A , Graeme R. Gillespie M , Matthew J. Greenlees N , Conrad J. Hoskin O , J. Scott Keogh P , Ray Lloyd Q , Jane Melville
A , Graeme R. Gillespie M , Matthew J. Greenlees N , Conrad J. Hoskin O , J. Scott Keogh P , Ray Lloyd Q , Jane Melville  R , Peter J. McDonald S , Damian R. Michael T , Nicola J. Mitchell
R , Peter J. McDonald S , Damian R. Michael T , Nicola J. Mitchell  F , Chris Sanderson U V , Glenn M. Shea
F , Chris Sanderson U V , Glenn M. Shea  W X , Joanna Sumner
W X , Joanna Sumner  R , Erik Wapstra Y , John C. Z. Woinarski
R , Erik Wapstra Y , John C. Z. Woinarski  A and David G. Chapple
A and David G. Chapple  B Z
B Z
A Threatened Species Recovery Hub, National Environmental Science Program, Research Institute for the Environment and Livelihoods, Charles Darwin University, NT 0909, Australia.
B School of Biological Sciences, Monash University, Clayton, Vic. 3800, Australia.
C Biodiversity Program, Queensland Museum, South Brisbane, Qld 4101, Australia.
D Australian Museum, Sydney, NSW 2010, Australia.
E Department of Biodiversity, Conservation and Attractions, Kensington, WA 6151, Australia.
F School of Biological Sciences, The University of Western Australia, Crawley, WA 6009, Australia.
G School of Veterinary and Life Sciences, Murdoch University, Murdoch, WA 6150, Australia.
H Department of Terrestrial Zoology, Western Australian Museum, Welshpool, WA 6106, Australia.
I Centre for Integrative Ecology, School of Life and Environmental Sciences, Deakin University, Burwood, Vic. 3125, Australia.
J Biologic Environmental Survey, East Perth, WA 6004, Australia.
K College of Science and Engineering, Flinders University, Bedford Park, SA 5042, Australia.
L Evolutionary Biology Unit, South Australian Museum, Adelaide, SA 5000, Australia.
M Flora and Fauna Division, Department of Environment and Natural Resources, Palmerston, NT 0830, Australia.
N Department of Biological Sciences, Macquarie University, North Ryde, NSW 2109, Australia.
O College of Science and Engineering, James Cook University, Townsville, Qld 4811, Australia.
P Division of Ecology and Evolution, Research School of Biology, The Australian National University, Canberra, ACT 0200, Australia.
Q FaunaTrack, Denmark, WA 6333, Australia.
R Sciences Department, Museums Victoria, Carlton, Vic. 3053, Australia.
S SPREP Pacific Environment, Samoa.
T Institute for Land, Water and Society, Charles Sturt University, Albury, NSW 2640, Australia.
U School of Biological Sciences, University of Queensland, St Lucia, Qld 4072, Australia.
V Research School of Biology, The Australian National University, 46 Sullivans Creek Road, Acton, ACT 2601, Australia.
W Sydney School of Veterinary Science, B01, Faculty of Science, University of Sydney, NSW 2006, Australia.
X Australian Museum Research Institute, Australian Museum, 1 William Street, Sydney, NSW 2010, Australia.
Y School of Biological Sciences, University of Tasmania, Hobart, Tas. 7005, Australia.
Z Corresponding author. Email: David.Chapple@monash.edu
Pacific Conservation Biology - https://doi.org/10.1071/PC20033
Submitted: 8 April 2020 Accepted: 16 July 2020 Published online: 3 September 2020
Abstract
Australia hosts approximately 10% of the world’s reptile species, the largest number of any country. Despite this and evidence of widespread decline, the first comprehensive assessment of the conservation status of Australian terrestrial squamates (snakes and lizards) was undertaken only recently. Here we apply structured expert elicitation to the 60 species assessed to be in the highest IUCN threat categories to estimate their probability of extinction by 2040. We also assessed the probability of successful reintroduction for two Extinct in the Wild (EW) Christmas Island species with trial reintroductions underway. Collation and analysis of expert opinion indicated that six species are at high risk (>50%) of becoming extinct within the next 20 years, and up to 11 species could be lost within this timeframe unless management improves. The consensus among experts was that neither of the EW species were likely to persist outside of small fenced areas without a significant increase in resources for intense threat management. The 20 most imperilled species are all restricted in range, with three occurring only on islands. The others are endemic to a single state, with 55% occurring in Queensland. Invasive species (notably weeds and introduced predators) were the most prevalent threats, followed by agriculture, natural system modifications (primarily fire) and climate change. Increased resourcing and management intervention are urgently needed to avert the impending extinction of Australia’s imperilled terrestrial reptiles.
Additional keywords: anthropogenic mass extinction crisis, Australia, biodiversity conservation, Delphi, expert elicitation, IDEA, lizard, reptile, snake, squamate, terrestrial, threatening processes.
Introduction
The rate of ecological change is escalating as human impacts become more pervasive and intensive, and consequently, much of the world’s biodiversity has suffered marked declines (Johnson et al. 2017). A recent review by the United Nations estimated that up to one million species are threatened by extinction as a result of human impacts (IPBES 2019), with Australia having one of the worst track records globally for recent biodiversity loss (Ritchie et al. 2013). Along with signatories to the Convention on Biological Diversity, the Australian government has committed to avoiding further extinctions (United Nations 2015; Department of Environment and Energy 2016), a task that first requires identification of the species at most immediate risk. Typically, this is achieved using threatened species lists, such as the International Union for Conservation of Nature (IUCN) Red List of Threatened Species. While the IUCN Red List has been instrumental for establishing global conservation priorities (Rodrigues et al. 2006), it is not designed to distinguish species on a rapid trajectory towards extinction from those with very small populations that may persist for long periods (Geyle et al. 2018). This is because the threat categories conflate declining populations with small populations, so that counts of threatened species in a given category do not always translate directly into extinction risk (Dirzo et al. 2014). It is also far from comprehensive: about a quarter of recognised terrestrial vertebrate species have not been evaluated against IUCN Red List criteria (Tingley et al. 2019), and in many cases, existing assessments are out of date.
Consequently, recognised IUCN conservation status (i.e. Critically Endangered, Endangered or Vulnerable) may not be the most sensitive means to identify priorities for halting further extinctions. Indeed, the Christmas Island forest skink (Emoia nativitatis) – the only documented extinction of an Australia squamate to date – became extinct in the wild before it was assigned any conservation status, and the few captive individuals died soon after it was listed as Critically Endangered in 2010 (Woinarski et al. 2017). The long interval between the demonstration of a significant decline in this species (Cogger and Sadlier 1999) and its listing as threatened meant that it was not afforded any particular priority for research or conservation management until it was far too late (Woinarski et al. 2017). Similarly, for two other endemic Christmas Island species that currently exist only in captivity, the blue-tailed skink (Cryptoblepharus egeriae) and Lister’s gecko (Lepidodactylus listeri), there was either no formal assessment of conservation status (blue-tailed skink), or the status was outdated (Lister’s gecko), prior to their extinction in the wild (in 2010 and 2012 respectively). It is possible that more Australian reptiles may follow a similar trajectory, given the general susceptibility of reptiles to climate change (Kearney et al. 2009; Sinervo et al. 2010), and the fact that species that are highly vulnerable to climate change impacts do not always overlap in range with species that have been assessed as threatened (Böhm et al. 2016a; Meng et al. 2016).
Australia is a hotspot for reptile diversity, hosting the largest number of species of any country in the world, and approximately 10% of all known species globally (Tingley et al. 2019). The Australian reptile fauna is very distinctive (>90% of species are endemic) (Chapman 2009), but poorly resolved, in part due to the existence of many cryptic lineages (Donnellan et al. 1993; Oliver et al. 2009). By global standards, there is a very high ongoing rate of description of new species, many of which have traits that make them susceptible to extinction (Meiri 2016). For example, a recent taxonomic review of a wide-ranging agamid species (genus Tympanocryptis) resulted in formal recognition of several species with very restricted ranges, including one species that may already be extinct (Melville et al. 2019).
Despite mounting evidence of ongoing global declines of reptile species (Gibbons et al. 2000; Huey et al. 2010; Tingley et al. 2016), reptiles are typically neglected in conservation planning. This is primarily because many species are poorly known, there is limited understanding of population trends, and in many cases detection is difficult, making monitoring unfeasible (Tingley et al. 2016; Woinarski 2018). The lack of, or limited, monitoring for most threatened reptiles is a major impediment to conservation recovery (Woinarski 2018; Scheele et al. 2019; Gillespie et al. 2020). Without adequate monitoring, the impacts of threats are poorly understood, and managers may lose opportunities to prevent extinctions because precipitous declines are not detected with sufficient time to respond (Woinarski 2018).
In 2016, mounting concerns among experts that reptiles were underassessed and under-represented in conservation planning led to a special journal issue of Biological Conservation, aiming to address some of the knowledge gaps in reptile conservation (Tingley et al. 2016). Following recommendations developed as part of that work, and the IUCN’s efforts to complete their Global Reptile Assessment, two workshops were held in 2017 to undertake assessments of the conservation status of all Australian terrestrial squamates (snakes and lizards) against IUCN categories and criteria (Chapple et al. 2019; Tingley et al. 2019). Here we extend and complement this work by identifying which Australian terrestrial squamates are most likely to go extinct in the next 20 years, an arbitrary period over which change might reasonably be assessed, and which might reasonably be influenced by policy changes made today. We used structured expert elicitation to forecast which, and how many, Australian terrestrial squamates are at imminent risk of extinction, with the aim of improving prioritisation, direction and resourcing of management that could prevent future extinctions. This approach follows estimates of imminent extinction risk among Australian birds, mammals (Geyle et al. 2018) and freshwater fish (Lintermans et al. 2020). Note that this assessment preceded the 2019–20 wildfires in Australia, which are likely to have severely worsened the conservation outlook for many species.
Materials and methods
Initial selection of species
We considered all Australian terrestrial squamates listed as Critically Endangered (CR), Endangered (EN), or Vulnerable (VU) under Criterion D2 (i.e. restricted area of occupancy or number of locations with a plausible future threat that could drive the species to CR or Extinct in a very short time) (IUCN 2012), based on a recent and comprehensive review using IUCN criteria (Chapple et al. 2019; Tingley et al. 2019) (a total of 51 species). An additional nine species were added as a consequence of recent revisions of taxonomy and descriptions of new species (Amey et al. 2019a, 2019b; Hoskin et al. 2019; Melville et al. 2019) to prevent overlooking any species for which a threatened status may be warranted. Note that we do not consider taxonomic revisions or descriptions made after May 2019. In total, 60 of the ~1000 Australian terrestrial squamate species were included in our elicitation. A list of the nine additional species considered, along with justification for their inclusion, is provided as Supplementary Material (see Supplementary Material S1).
Extinct in the wild species
Two Extinct in the Wild (EW) species (Lister’s gecko (Lepidodactylus listeri) and the blue-tailed skink (Cryptoblepharus egeriae)) were also assessed as part of this study. Both species persist in captive colonies, but trial reintroductions into predator-free exclosures on Christmas Island are underway (Andrew et al. 2018) and might allow re-establishment of populations within the 20-year timeframe of interest. Following the IUCN definition for successful re-establishment of a wild population, we assume that these species would meet criteria for no longer being EW if (1) reintroductions occur and populations are established within the former range of the species, and (2) individuals persist beyond small fenced exclosures (IUCN 2012). For these two species, we consider the probability that there will be no wild populations in 20 years’ time, considering this to be the same, conceptually, as the reverse probability of successful re-establishment.
Expert selection
More than 50 key researchers were invited to participate in this study based on their contributions to a recent review of the conservation status of Australia squamates (Chapple et al. 2019; Tingley et al. 2019). This included individuals from academic institutions, state and federal government offices and agencies, consulting agencies, museums, zoos, and non-government organisations. Just over half (~51%) of those invited agreed to be involved, making up an expert panel of 26 people (all of whom are listed as authors here). All participants had worked with Australian terrestrial squamates and had relevant knowledge of their distributions, ecology and threatening processes.
Structured expert elicitation
We used a structured expert elicitation approach for obtaining estimates of extinction probability (Burgman et al. 2011; McBride et al. 2012). This approach has been developed in an attempt to reduce the incidence of some commonly encountered biases in expert elicitation processes (McBride et al. 2012; Hemming et al. 2018). Our adapted elicitation procedure involved four main steps, all of which were conducted remotely via email or phone:
Participants were provided with a summary of the available information on ecology, threats and trends (based largely on the material collated during the recent Red List assessment). This ensured that everyone had the same information available to them when judging a given species’ extinction risk. All participants were then asked to estimate the probability of extinction in the wild (or in the case of the two EW Christmas Island species, the probability that there will be no wild populations) in 20 years’ time assuming current levels and direction of management (Round 1 scores). We also asked participants for an associated level of confidence in their estimates (i.e. very low, low, moderate, high or very high). Participants were able to use additional resources to inform their estimates; however, they were asked not to discuss their scores with any others participating in the expert elicitation (as each individual assessment was to be treated as independent).
Individual estimates of extinction probability and their associated confidence were compiled, and then modelled using a linear mixed-effects model (‘lme’ in package ‘nlme’) in R 3.6.0 (R Core Team 2019), where estimates were logit-transformed prior to analysis. We controlled for individual experts consistently underestimating or overestimating likelihood of extinction by specifying their identity as random intercepts. We specified a variance structure in which the variance increased with the level of uncertainty associated with each estimate of likelihood of extinction. Confidence classes of ‘very low’, ‘low’, ‘moderate’, ‘high’ and ‘very high’ were converted to uncertainty scores of 90, 70, 50, 30 and 10% respectively. This model allowed us to predict the probability of extinction (with 95% confidence intervals) for each taxon. Summary statistics (including mean, median, range and outliers) were also calculated, and participants were provided with figures displaying both the summary statistics and their individual estimates so that they could see where their estimates lay relative to the rest of the group (an example is provided in Supplementary Material S2).
Participants were asked to review the results, while noting any concerns about the spread of estimates given for a particular species, outliers or the rankings of extinction probability. Where concerns were raised, participants were invited to provide an anonymous written statement (which was then distributed to the rest of the group). Participants were then encouraged to take part in a teleconference, during which a facilitator drew attention to any marked discrepancies in the draft scores and individual concerns, triggering a general conversation about the interpretation and context of species background information. Each participant was given the opportunity to clarify information about the presented data, introduce further relevant information that may justify either a greater or lesser risk of extinction, and to cross-examine new information. A recording of the teleconference and detailed minutes was provided to all participants, including nine participants who were unable to attend the teleconference.
Participants were then asked to provide a second, final assessment of the probability of extinction (and associated confidence) for each species from which the results were finalised (Round 2 scores).
Estimating the number of species likely to become extinct in the next 20 years
The predicted probabilities of extinction for each of the 60 extant terrestrial squamates (assessed by the experts) were summed to estimate the number of species (from this subset of terrestrial squamates) likely to become extinct in the next 20 years (as per Geyle et al. 2018).
Testing for concordance among expert assessments
We measured the level of agreement among experts in the relative ranking of the most imperilled terrestrial squamates using Kendall’s Coefficient of Concordance (W) (Kendall and Babinton Smith 1939). This test allows for comparison of multiple outcomes (i.e. assessments made by multiple experts), whilst making no assumptions about the distribution of data. Average ranks were used to correct for the large number of tied values in the dataset, and ranks were compared only for experts who assessed all 60 species (n = 15).
Geographic distribution of the most imperilled terrestrial squamates
We mapped the distribution of the most imperilled terrestrial squamates according to their presence in each Interim Biogeographic Regionalisation for Australia (IBRA) subregion (South Australia Department of Environment Water and Natural Resources 2015) using data compiled as part of the recent review (Chapple et al. 2019; Tingley et al. 2019). Occurrence data were collated from various sources including museums, State and Federal Government Departments, citizen science programs and academic researchers (Tingley et al. 2019).
Threatening processes
Threat information was obtained from IUCN (2019) to determine the number and proportion of species threatened by various threat types. We compared these figures with those reported in Tingley et al. (2019) to determine if there were any differences in the prevalence of threats affecting the most imperilled terrestrial squamates compared with all IUCN-listed squamates (including those in the Least Concern and Near Threatened categories). Note that this comparison does not consider the relative importance of threats, but rather the total number of species affected by a given threat type. Where threat information was not available (i.e. for the newly described or redefined species listed in Supplementary Material S1), threat information was derived from the published literature and validated by experts. Threat information for Anilios obtusifrons, Lampropholis bellendenkerensis and L. elliotensis was derived from Chapple et al. (2019), who prepared draft assessments for these newly described species, as they lacked IUCN profiles (as of January 2020). Note that threat information was also included for the two EW Christmas Island species.
Results
Expert elicitation, extinction probabilities, and the number of species likely to go extinct
On average, 19 estimates were received for each species (ranging from 16 to 21). Fifteen experts provided estimates for all 60 species, while others chose only to assess species for which they had first-hand experience. Several participants adjusted their Round 1 scores following discussions (including many who did not partake in the teleconference), resulting in changes to the modelled probabilities for every species under consideration (a comparison of Round 1 and 2 modelled outputs is provided in Supplementary Material S3). For most species (~82%), the predicted probability of extinction decreased following discussion, and in some cases by a considerable amount; on average, there was a 4.3% decrease in modelled probability of extinction (ranging from 0.2% for Saproscincus saltus to 32.5% for Tympanocryptis lineata). The predicted probability of extinction of 11 species (~18%) increased by an average of 8% (ranging from 0.1% for T. pinguicolla to 20% for Saltuarius eximius) following discussions and re-estimation.
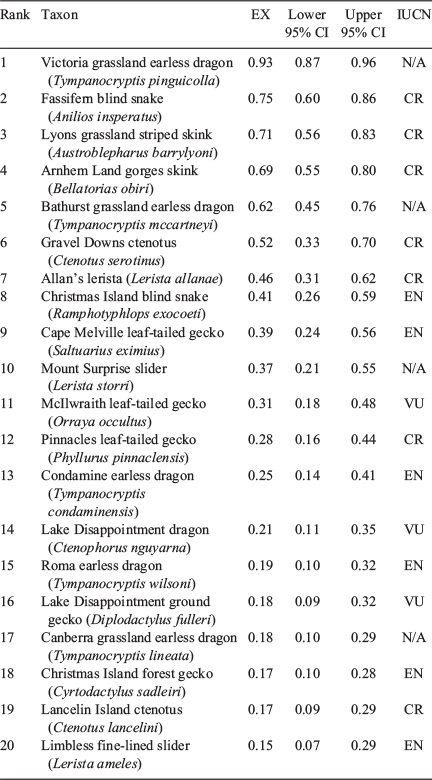
Collation and analysis of expert opinion (Round 2 scores) indicated that six of 60 species are at high risk (likelihood >50%) of becoming extinct within the next 20 years (Table 1, Supplementary Material S4). The six species at highest risk included two agamids (the Victoria and Bathurst grassland earless dragons (Tympanocryptis pinguicolla and T. mccartneyi)), one blind snake (the Fassifern blind snake (Anilios insperatus)) and three skinks (the Lyons grassland striped skink (Austroblepharus barrylyoni), the Arnhem Land gorges skink (Bellatorias obiri) and the Gravel Downs ctenotus (Ctenotus serotinus)). Summing across the extinction risk values assigned by experts to the 60 species assessed, we estimated that 11 species could become extinct in the wild in the next 20 years unless management improves. There was a reasonable and highly significant degree of conformity among experts (of those who provided estimates for all 60 species, n = 15) in their assessments of extinction risk (W = 0.56, P < 0.001).
|
Extinct in the Wild species
A total of 21 experts assessed the probability that there will be no wild populations of Lister’s gecko and the blue-tailed skink in 20 years’ time, with most experts having little confidence that re-establishment attempts (within their natural range) would be successful. While both species had high probabilities of extinction (suggesting a very low probability of successful re-establishment: Table 2), efforts for the blue-tailed skink were considered slightly less likely to fail by some experts. This was attributed to perceived lower susceptibility to predation compared with Lister’s gecko, or due to greater difficulties in establishing populations of Lister’s gecko (because of dispersal behaviour and more specialised habitat preferences). Nevertheless, the consensus among experts was that neither species is likely to persist on Christmas Island outside of predator-free exclosures without a significant increase in resources for intense threat management.

|
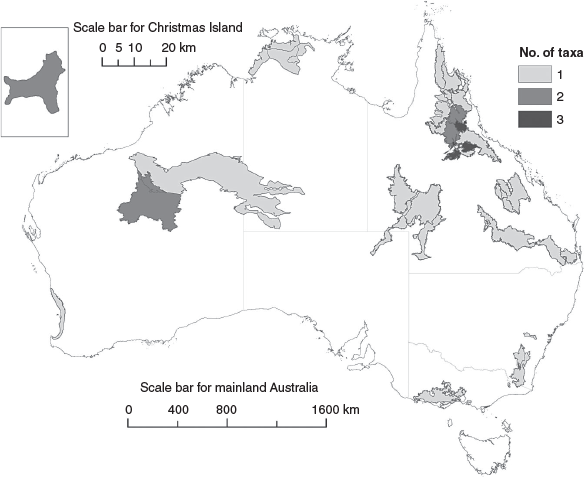
Geographic distribution of the most imperilled terrestrial squamates
Three of the terrestrial squamates with highest extinction risk (i.e. those ranking in the top 20: Table 1) occur only on islands: two on Christmas Island, and one on Lancelin Island off the coast of Western Australia (a tiny low-lying sand island <1 km2 in size). All of the remaining reptiles are endemic to a single state, with more than half (55%) occurring only in Queensland (north-eastern Australia), mostly in the Einasleigh Uplands, Brigalow Belt, Cape York Peninsula and Channel Country biogeographic regions (Fig. 1). The top 20 most imperilled species are restricted in range, with a maximum Area of Occupancy (AOO) of 56 km2 and an average AOO of ~17 km2, with most (65%) having an AOO ≤16 km2 (Chapple et al. 2019; J. Melville, unpubl. data). The current distribution for one species (the Bathurst grassland earless dragon) is unknown; it has been recorded from only two locations (with records >20 years old) (J. Melville, unpubl. data). Several species are known only from a single location (i.e. the Fassifern blind snake, the Lyons grassland striped skink, the Cape Melville leaf-tailed gecko (Saltuarius eximius), the Mount Surprise slider (Lerista storri), the Pinnacles leaf-tailed gecko (Phyllurus pinnaclensis), and the Lake Disappointment dragon (Ctenophorus nguyarna) and ground gecko (Diplodactylus fulleri): Chapple et al. 2019).
|
Threatening processes
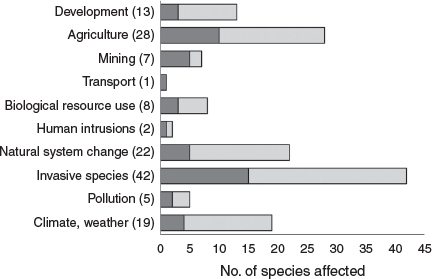
Invasive and other problematic species (i.e. overabundant native species) and diseases were the most prevalent threats to the most imperilled terrestrial squamates, affecting 67.7% (n = 42) of the 62 species considered as part of this study (Fig. 2). Within this broader category, weeds (including buffel grass (Cenchrus ciliaris), gamba grass (Andropogon gayanus) and hawkweed (Hieracium spp.), among others) impacted the highest number of species (40%, n = 25), followed by the feral cat (Felis catus) (29%, n = 18) and the red fox (Vulpes vulpes) (16%, n = 10) (Supplementary Material S5). Approximately 21% of the terrestrial squamate species considered here (n = 13) were also impacted directly or indirectly (through habitat degradation or predation) by other invasive species, including black rats (Rattus rattus), feral pigs (Sus scrofa), deer (Rusa unicolor and Cervus elaphus), feral horses (Equus caballus), invasive invertebrates (Solenopsis invicta, Anoplolepis gracilipes and Scolopendra subspinipes), Oriental wolf snakes (Lycodon capucinus) and cane toads (Rhinella marina), while one species was impacted by the native eastern grey kangaroo (Macropus giganteus) (through overgrazing of grasslands: Chapple et al. 2019). Other notable threats included agriculture (45.2%, n = 28), natural system modifications (35.5%, n = 22, with 94% of this factor related to inappropriate fire regimes), and climate change and severe weather (30.6%, n = 19) (Fig. 2). This ranking of threats was broadly analogous to the threats facing all Australian squamates identified in Tingley et al. (2019).
|
Of the top 20 most imperilled species (Table 1), a higher proportion was impacted by invasive species (75%, n = 15) and agriculture (50%, n = 10) compared with all 62 species considered (including the two EW Christmas Island species), while a smaller proportion were impacted by fire (25%, n = 5) and climate change (20%, n = 4) (Fig. 2). Notably, of the seven squamate species considered that are affected by energy production and mining, five ranked in the top 20 most imperilled (Fig. 2).
Discussion
The status of Australian terrestrial squamates has deteriorated over the past 25 years, with the proportion of species assessed as threatened nearly doubling since 1993 (Cogger et al. 1993; Tingley et al. 2019). The last decade has also seen the first documented extinction of an Australian squamate (the Christmas Island forest skink), with two other endemic Christmas Island species becoming extinct in the wild (the blue-tailed skink and Lister’s gecko) (Andrew et al. 2018; Woinarski 2018). In the wake of continued decline and increasing pressures associated with ongoing threatening processes, it is imperative that extinction risk is recognised in a timely manner to allow for implementation of effective management responses aimed at preventing extinctions (Woinarski et al. 2017). Here we used structured expert elicitation to forecast which, and how many, Australian terrestrial squamates are in imminent danger of extinction.
Overall, experts were pessimistic about the state of the species under consideration, with average extinction probabilities estimated to be approximately 20%, and with six species considered to have extinction probabilities greater than 50% in the next 20 years. Additionally, our results suggest that up to 11 species could be lost within this timeframe, a figure that is markedly higher than the already large trajectory of change reported over the previous two decades. While fewer extinctions have been documented for Australian squamates than for other vertebrate groups (i.e. birds, mammals, frogs) (Woinarski et al. 2019), the high level of cryptic diversity present in Australian terrestrial squamates, coupled with extensive clearing of key habitat types that may have supported small, narrow-range endemics, and the very restricted ranges of many recently discovered species (Amey et al. 2019a, 2019b; Hoskin et al. 2019; Melville et al. 2019), suggests that there may have been earlier undetected extinctions. Five of the nine species that we evaluated in addition to the list of species threatened according to IUCN criteria (i.e. those described or revised recently: Supplementary Material S1) ranked in the top 10 most imperilled, further supporting this observation.
There is greater uncertainty associated with the conservation status of squamates in Australia relative to other terrestrial vertebrate groups, primarily due to high levels of data deficiency. For example, 61 of the 1020 squamate species (~6%) considered in the Australian review were categorised as Data Deficient (Chapple et al. 2019), a far higher rate than for comparable reviews of Australian birds (none) (Garnett et al. 2011) and terrestrial mammals (~0.9%) (Woinarski et al. 2014). It is also possible that the two species ranked with highest extinction risk here are already extinct. The Victoria grassland earless dragon has not been seen for several decades despite extensive survey effort (Robertson and Evans 2009; Banks et al. 2017); however, as some potential habitat in its range in western Victoria remains unsurveyed, it is possible that one or more small populations persist in remnant grasslands (Banks et al. 2017; Melville et al. 2019). The Fassifern blind snake is known only from the holotype (collected in 1992), despite several attempts to locate additional specimens (Venchi et al. 2015). If not extinct, then this species is likely to be of extreme conservation concern, as the type locality is close to the large and expanding urban areas of Brisbane and Ipswich, and the single site from which it is known has been extensively cleared (Venchi et al. 2015). Further surveys are required to determine whether either of these species are extant (Venchi et al. 2015; Melville et al. 2019).
A notable feature of our results is the generally higher risk of extinction predicted for the most-at-risk terrestrial squamates relative to a previous study conducted on Australian mammals using the same methods, but the comparatively similar results for Australian birds (Geyle et al. 2018). This pattern may be because many of the squamates considered in this study are persisting in remnant pockets of vegetation adjacent to highly developed areas (e.g. the Bathurst grassland earless dragon and Allan’s lerista (Lerista allanae)), similarly to the most imperilled birds, and consequently also face a high risk of extinction due to habitat loss, fragmentation, and edge effects (Haddad et al. 2015). By contrast, many mammals have already been lost from these areas, with future extinctions predicted to occur in the less developed parts of central and northern Australia (Geyle et al. 2018). Another contributing factor may be that many squamates occupy extremely restricted ranges (~68% of the species assessed have an estimated Area of Occupancy <100 km2), making them particularly vulnerable to stochastic events (Murray et al. 2017). Furthermore, squamates generally lack the public and political appeal that helps catalyse recovery support for other Australian threatened vertebrates, leading to relatively little resourcing for conservation (Woinarski 2018). By contrast, there are generally more well-established and coordinated management efforts for mammals and birds, with many mammal species that were previously highly imperilled showing substantial recent recovery as result of predator exclusion and translocation (Kanowski et al. 2018; Moseby et al. 2018; Read et al. 2018).
Our analysis of threats facing the most imperilled terrestrial squamates was consistent with that reported for all terrestrial squamate species in Tingley et al. (2019), and with other studies that have identified invasive species, habitat loss or modification (i.e. through agriculture, urbanisation, altered fire regimes and mining) and climate change as major threats (Sinervo et al. 2010; Böhm et al. 2016b ). A substantial suite of threatened reptiles are closely associated with habitats that are currently being cleared at a high rate (notably temperate grasslands of south-eastern Australia and brigalow woodlands of central Queensland), providing indirect evidence of substantial declines for those species (Woinarski 2018). For several other species persisting in already highly modified landscapes, changing land-use is likely to contribute further to declines. For example, a shift from mixed-crop farms to broadacre monocultures (often irrigated cotton) in the Condamine River floodplains has led to the destruction of critical habitat for the Condamine earless dragon (Tympanocryptis condaminensis) (Melville 2018).
This suggests that an increase in the projected number of extinctions over the next two decades is plausible. An important lesson may be learnt from Christmas Island: despite evidence of decline in at least four of the island’s six native squamates from the 1970s to the 1990s (Cogger and Sadlier 1999), relatively few resources were invested for management and monitoring. Consequently, the rate and scale of decline (and its cause) was not appreciated in time to prevent extinctions (Woinarski et al. 2017), in an alarming parallel to the recent extinction of the Christmas Island pipistrelle (Pipistrellus murrayi) (Martin et al. 2012).
The probability of further extinctions of Australian squamate species is high, particularly in the face of increasing pressures associated with climate change, which are not yet well understood, and may have been underestimated here. Notably, at least 17 squamate species (including five considered as part of this study) have been substantially affected by the widespread and catastrophic wildfires that devastated eastern and southern Australia in late 2019 and early 2020 (Department of Environment and Energy 2020; Department of Environment, Land, Water and Planning 2020). Our assessment was undertaken before these fires, and it is possible that they may have added to the list of species that should have been considered. It is still too early to determine the impact (both short- and long-term) of the fires at a species level. Nevertheless, and notwithstanding potential fire impacts, our results suggest that up to 11 species could become extinct by 2040 under current management regimes. A more strategic, better-resourced conservation response is urgently required if we are to avert future extinctions of Australia’s terrestrial squamates.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The preparation of this paper, including data collation and analysis, was supported by the Australian Government’s National Environmental Science Program through the Threatened Species Recovery Hub.
References
Amey, A. P., Couper, P. J., and Wilmer, J. W. (2019a). A new species of Lerista Bell, 1833 (Reptilia: Scincidae) from Cape York Peninsula, Queensland, belonging to the Lerista allanae clade but strongly disjunct from other members of the clade. Zootaxa 4613, 161–171.| A new species of Lerista Bell, 1833 (Reptilia: Scincidae) from Cape York Peninsula, Queensland, belonging to the Lerista allanae clade but strongly disjunct from other members of the clade.Crossref | GoogleScholarGoogle Scholar |
Amey, A. P., Couper, P. J., and Wilmer, J. W. (2019b). Two new species of Lerista Bell, 1833 (Reptilia: Scincidae) from north Queensland populations formerly assigned to Lerista storri Greer, McDonald and Lawrie, 1983. Zootaxa 4577, 473–493.
| Two new species of Lerista Bell, 1833 (Reptilia: Scincidae) from north Queensland populations formerly assigned to Lerista storri Greer, McDonald and Lawrie, 1983.Crossref | GoogleScholarGoogle Scholar |
Andrew, P., Cogger, H., Driscoll, D., Flakus, S., Harlow, P., Maple, D., Misso, M., Pink, C., Retallick, K., Rose, K., Tiernan, B., West, J., and Woinarski, J. C. Z. (2018). Somewhat saved: a captive breeding programme for two endemic Christmas Island lizard species, now extinct in the wild. Oryx 52, 171–174.
| Somewhat saved: a captive breeding programme for two endemic Christmas Island lizard species, now extinct in the wild.Crossref | GoogleScholarGoogle Scholar |
Banks, C. B., Robertson, P., Magrath, M. J. L., and Harley, D. (2017). Searching for the grassland earless dragon ‘Tympanocryptis pinguicolla’ in Western Victoria. Victorian Naturalist 134, 187–198.
Böhm, M., Cook, D., Ma, H., Davidson, A. D., García, A., Tapley, B., Pearce-Kelly, P., and Carr, J. (2016a). Hot and bothered: using trait-based approaches to assess climate change vulnerability in reptiles. Biological Conservation 204, 32–41.
| Hot and bothered: using trait-based approaches to assess climate change vulnerability in reptiles.Crossref | GoogleScholarGoogle Scholar |
Böhm, M., Williams, R., Bramhall, H. R., McMillan, K. M., Davidson, A. D., Garcia, A., Bland, L. M., Bielby, J., and Collen, B. (2016b). Correlates of extinction risk in squamate reptiles: the relative importance of biology, geography, threat and range size. Global Ecology and Biogeography 25, 391–405.
| Correlates of extinction risk in squamate reptiles: the relative importance of biology, geography, threat and range size.Crossref | GoogleScholarGoogle Scholar |
Burgman, M. A., McBride, M., Ashton, R., Speirs-Bridge, A., Flander, L., Wintle, B., Fidler, F., Rumpff, L., and Twardy, C. (2011). Expert status and performance. PLOS ONE 6, e22998.
| Expert status and performance.Crossref | GoogleScholarGoogle Scholar | 21829574PubMed |
Chapman, A. D. (2009). Numbers of Living Species in Australia and the World. Department of the Environment, Water, Heritage and the Arts. Canberra, Australia.
Chapple, D. G., Tingley, R., Mitchell, N. J., Macdonald, S. L., Keogh, J. S., Shea, G. M., Bowles, P., Cox, N. A., and Woinarski, J. C. Z. (2019). ‘The Action Plan for Australian Lizards and Snakes 2017.’ (CSIRO Publishing: Melbourne.)
Cogger, H. G., Cameron, E. E., Sadlier, R. A., and Eggler, P. (1993). ‘The Action Plan for Australian Reptiles.’ (The Australian Nature Conservation Agency: Canberra, Australia.)
Cogger, H. G., and Sadlier, R. A. (1999). ‘The Terrestrial Reptiles of Christmas Island: A Reappraisal of Their Status.’ (The Australian Museum: Sydney, Australia.)
Department of Environment and Energy. (2016). The National Threatened Species Strategy. Department of the Environment and Heritage. Canberra, Australia.
Department of Environment and Energy. (2020). Wildlife and threatened species bushfire recovery research and resources. Available at http://www.environment.gov.au/biodiversity/bushfire-recovery/research-and-resources [accessed January 2020].
Department of Environment, Land, Water and Planning. (2020). Victoria’s bushfire emergency: biodiversity response and recovery. Preliminary report – Version 1. Department of Environment, Land, Water and Planning. Melbourne.
Dirzo, R., Young, H. S., Galetti, M., Ceballos, G., Isaac, N. J. B., and Collen, B. (2014). Defaunation in the Anthropocene. Science 345, 401–406.
| Defaunation in the Anthropocene.Crossref | GoogleScholarGoogle Scholar | 25061202PubMed |
Donnellan, S. C., Adams, M., Hutchinson, M., and Baverstock, P. R. (1993). The identification of cryptic species in the Australian herpetofauna: a high research priority. In ‘Herpetology in Australia: A Diverse Discipline’. (Eds D. Lunney, and D. Ayres.) pp. 121–126. (Surrey Beatty: Sydney.)
Garnett, S. T., Szabo, J., and Dutson, G. (2011). ‘The Action Plan for Australian Birds 2010.’ (CSIRO Publishing: Melbourne.)
Geyle, H. M., Woinarski, J. C. Z., Baker, G. B., Dickman, C. R., Dutson, G., Fisher, D. O., Ford, H., Holdsworth, M., Jones, M. E., Kutt, A., Legge, S., Leiper, I., Loyn, R., Murphy, B. P., Menkhorst, P., Reside, A. E., Ritchie, E. G., Roberts, F. E., Tingley, R., and Garnett, S. T. (2018). Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions. Pacific Conservation Biology 24, 157–167.
| Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions.Crossref | GoogleScholarGoogle Scholar |
Gibbons, J. W., Scott, D. E., Ryan, T. J., Buhlmann, K. A., Tuberville, T. D., Metts, B. S., Greene, J. L., Mills, T., Leiden, Y., Poppy, S., and Winne, C. T. (2000). The global decline of reptiles, déjà vu amphibians: reptile species are declining on a global scale. Six significant threats to reptile populations are habitat loss and degradation, introduced invasive species, environmental pollution, disease, unsustainable use, and global climate change. BioScience 50, 653–666.
Gillespie, G. R., Fukuda, Y., and McDonald, P. (2020). Using non-systematically collected data to evaluate the conservation status of elusive species: a case study on Australia’s Oenpelli python. Wildlife Research 47, 146–157.
| Using non-systematically collected data to evaluate the conservation status of elusive species: a case study on Australia’s Oenpelli python.Crossref | GoogleScholarGoogle Scholar |
Haddad, N. M., Brudvig, L. A., Clobert, J., Davies, K. F., Gonzalez, A., Holt, R. D., Lovejoy, T. E., Sexton, J. O., Austin, M. P., Collins, C. D., Cook, W. M., Damschen, E. I., Ewers, R. M., Foster, B. L., Jenkins, C. N., King, A. J., Laurance, W. F., Levey, D. J., Margules, C. R., Melbourne, B. A., Nicholls, A. O., Orrock, J. L., Song, D.-X., and Townshend, J. R. (2015). Habitat fragmentation and its lasting impact on Earth’s ecosystems. Science Advances 1, e1500052.
| Habitat fragmentation and its lasting impact on Earth’s ecosystems.Crossref | GoogleScholarGoogle Scholar | 26601154PubMed |
Hemming, V., Burgman, M. A., Hanea, A. M., McBride, M. F., and Wintle, B. C. (2018). A practical guide to structured expert elicitation using the IDEA protocol. Methods in Ecology and Evolution 9, 169–180.
| A practical guide to structured expert elicitation using the IDEA protocol.Crossref | GoogleScholarGoogle Scholar |
Hoskin, C. J., Bertola, L. V., and Higgie, M. (2019). A new species of Phyllurus leaf-tailed gecko (Lacertilia: Carphodactylidae) from the Pinnacles, north-east Australia. Zootaxa 4576, 127–139.
| A new species of Phyllurus leaf-tailed gecko (Lacertilia: Carphodactylidae) from the Pinnacles, north-east Australia.Crossref | GoogleScholarGoogle Scholar |
Huey, R. B., Losos, J. B., and Moritz, C. (2010). Are lizards toast? Science 328, 832.
| Are lizards toast?Crossref | GoogleScholarGoogle Scholar | 20466909PubMed |
IPBES (2019). Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. IPBES secretariat. Bonn, Germany.
IUCN (2012). ‘IUCN Red List Categories and Criteria: Version 3.1.’ 2nd edn. (Gland, Switzerland and Cambridge, UK.)
IUCN (2019). The IUCN Red List of Threatened Species. Version 2019-2. Available at: http://www.iucnredlist.org [accessed July 2019].
Johnson, C. N., Balmford, A., Brook, B. W., Buettel, J. C., Galetti, M., Guangchun, L., and Wilmshurst, J. M. (2017). Biodiversity losses and conservation responses in the Anthropocene. Science 356, 270–275.
| Biodiversity losses and conservation responses in the Anthropocene.Crossref | GoogleScholarGoogle Scholar | 28428393PubMed |
Kanowski, J., Roshier, D., Smith, M. A., and Fleming, A. (2018). Effective conservation of critical weight range mammals: reintroduction projects of the Australian Wildlife Conservancy. In ‘Recovering Australian Threatened Species. A Book of Hope’. (Eds S. Garnett, P. Latch, D. Lindenmayer, and J. Woinarski.) pp. 269–279. (CSIRO Publishing: Melbourne.)
Kearney, M., Shine, R., and Porter, W. P. (2009). The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proceedings of the National Academy of Sciences 106, 3835–3840.
| The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming.Crossref | GoogleScholarGoogle Scholar |
Kendall, M. G., and Babinton Smith, B. (1939). The problem of m rankings. The Annals of Mathematical Statistics 10, 275–287.
| The problem of m rankings.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M., Geyle, H. M., Beatty, S., Brown, C., Ebner, B., Freeman, R., Hammer, M. P., Humphreys, W. F., Kennard, M. J., Kern, P., Martin, K., Morgan, D., Raadik, T. A., Unmack, P. J., Wager, R., Woinarski, J. C. Z., and Garnett, S. T. (2020). Big trouble for little fish: Australian freshwater fishes in imminent risk of extinction. Pacific Conservation Biology , .
| Big trouble for little fish: Australian freshwater fishes in imminent risk of extinction.Crossref | GoogleScholarGoogle Scholar |
Martin, T. G., Nally, S., Burbidge, A. A., Arnall, S., Garnett, S. T., Hayward, M. W., Lumsden, L. F., Menkhorst, P., McDonald-Madden, E., and Possingham, H. P. (2012). Acting fast helps avoid extinction. Conservation Letters 5, 274–280.
| Acting fast helps avoid extinction.Crossref | GoogleScholarGoogle Scholar |
McBride, M. F., Garnett, S. T., Szabo, J. K., Burbidge, A. H., Butchart, S. H. M., Christidis, L., Dutson, G., Ford, H. A., Loyn, R. H., Watson, D. M., and Burgman, M. A. (2012). Structured elicitation of expert judgments for threatened species assessment: a case study on a continental scale using email. Methods in Ecology and Evolution 3, 906–920.
| Structured elicitation of expert judgments for threatened species assessment: a case study on a continental scale using email.Crossref | GoogleScholarGoogle Scholar |
Meiri, S. (2016). Small, rare and trendy: traits and biogeography of lizards described in the 21st century. Journal of Zoology 299, 251–261.
| Small, rare and trendy: traits and biogeography of lizards described in the 21st century.Crossref | GoogleScholarGoogle Scholar |
Melville, J. (2018). Conservation genetics of eastern Australian herpetofauna in a rapidly changing landscape: a perspective on conservation management and policy implementation. Pacific Conservation Biology 24, 310–317.
| Conservation genetics of eastern Australian herpetofauna in a rapidly changing landscape: a perspective on conservation management and policy implementation.Crossref | GoogleScholarGoogle Scholar |
Melville, J., Chaplin, K., Hutchinson, M., Sumner, J., Gruber, B., MacDonald, A. J., and Sarre, S. D. (2019). Taxonomy and conservation of grassland earless dragons: new species and an assessment of the first possible extinction of a reptile on mainland Australia. Royal Society Open Science 6, 190233.
| Taxonomy and conservation of grassland earless dragons: new species and an assessment of the first possible extinction of a reptile on mainland Australia.Crossref | GoogleScholarGoogle Scholar | 31218062PubMed |
Meng, H., Carr, J., Beraducci, J., Bowles, P., Branch, W. R., Capitani, C., Chenga, J., Cox, N., Howell, K., Malonza, P., Marchant, R., Mbilinyi, B., Mukama, K., Msuya, C., Platts, P. J., Safari, I., Spawls, S., Shennan-Farpon, Y., Wagner, P., and Burgess, N. D. (2016). Tanzania’s reptile biodiversity: distribution, threats and climate change vulnerability. Biological Conservation 204, 72–82.
| Tanzania’s reptile biodiversity: distribution, threats and climate change vulnerability.Crossref | GoogleScholarGoogle Scholar |
Moseby, K., Copley, P., Paton, D. C., and Read, J. L. (2018). Arid Recovery: a successful conservation partnership. In ‘Recovering Australian Threatened Species. A Book of Hope’. (Eds S. Garnett, P. Latch, D. Lindenmayer, and J. Woinarski.) pp. 259–268. (CSIRO Publishing: Melbourne.)
Murray, N. J., Keith, D. A., Bland, L. M., Nicholson, E., Regan, T. J., Rodríguez, J. P., and Bedward, M. (2017). The use of range size to assess risks to biodiversity from stochastic threats. Diversity and Distributions 23, 474–483.
| The use of range size to assess risks to biodiversity from stochastic threats.Crossref | GoogleScholarGoogle Scholar |
Oliver, P. M., Adams, M., Lee, M. S. Y., Hutchinson, M. N., and Doughty, P. (2009). Cryptic diversity in vertebrates: molecular data double estimates of species diversity in a radiation of Australian lizards (Diplodactylus, Gekkota). Proceedings of the Royal Society B: Biological Sciences 276, 2001–2007.
| Cryptic diversity in vertebrates: molecular data double estimates of species diversity in a radiation of Australian lizards (Diplodactylus, Gekkota).Crossref | GoogleScholarGoogle Scholar | 19324781PubMed |
R Core Team (2019). R: a language and environment for statistical computing. R foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/ [accessed December 2019].
Read, J., Copley, P., Ward, M., Dagg, E., Olds, L., Taggart, D., and West, R. (2018). Bringing back warru: return of the black-footed rock-wallaby to the APY Lands. In ‘Recovering Australian Threatened Species. A Book of Hope’. (Eds S. Garnett, P. Latch, D. Lindenmayer, and J. Woinarski.) pp. 237–247. (CSIRO Publishing: Melbourne.)
Ritchie, E. G., Bradshaw, C. J. A., Dickman, C. R., Hobbs, R., Johnson, C. N., Johnston, E. L., Laurance, W. F., Lindenmayer, D., McCarthy, M. A., Nimmo, D. G., Possingham, H. H., Pressey, R. L., Watson, D. M., and Woinarski, J. (2013). Continental-scale governance and the hastening of loss of Australia’s biodiversity. Conservation Biology 27, 1133–1135.
| Continental-scale governance and the hastening of loss of Australia’s biodiversity.Crossref | GoogleScholarGoogle Scholar | 24299077PubMed |
Robertson, P., and Evans, M. (2009). National recovery plan for the grassland earless dragon Tympanocryptis pinguicolla. Department of Territory and Municipal Services.
Rodrigues, A., Pilgrim, J., Lamoreux, J., Hoffmann, M., and Brooks, T. (2006). The value of the IUCN Red List for conservation. Trends in Ecology and Evolution 21, 71–76.
| The value of the IUCN Red List for conservation.Crossref | GoogleScholarGoogle Scholar | 16701477PubMed |
Scheele, B. C., Legge, S., Blanchard, W., Garnett, S., Geyle, H., Gillespie, G., Harrison, P., Lindenmayer, D., Lintermans, M., Robinson, N., and Woinarski, J. (2019). Continental-scale assessment reveals inadequate monitoring for threatened vertebrates in a megadiverse country. Biological Conservation 235, 273–278.
| Continental-scale assessment reveals inadequate monitoring for threatened vertebrates in a megadiverse country.Crossref | GoogleScholarGoogle Scholar |
Sinervo, B., Méndez-de-la-Cruz, F., Miles, D. B., Heulin, B., Bastiaans, E., Villagrán-Santa Cruz, M., Lara-Resendiz, R., Martínez-Méndez, N., Calderón-Espinosa, M. L., Meza-Lázaro, R. N., Gadsden, H., Avila, L. J., Morando, M., De la Riva, I. J., Sepulveda, P. V., Rocha, C. F. D., Ibargüengoytía, N., Puntriano, C. A., Massot, M., Lepetz, V., Oksanen, T. A., Chapple, D. G., Bauer, A. M., Branch, W. R., Clobert, J., and Sites, J. W. (2010). Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899.
| Erosion of lizard diversity by climate change and altered thermal niches.Crossref | GoogleScholarGoogle Scholar | 20466932PubMed |
South Australia Department of Environment, Water, and Natural Resources (2015). IBRA Subregion Australia Version 7.0 – ARC. Bioregional Assessment Source Dataset. Available at http://data.bioregionalassessments.gov.au/dataset/2ebdaa06-8dcc-4058-94d7-7399acbe6555 [accessed October 2019].
Tingley, R., Macdonald, S. L., Mitchell, N. J., Woinarski, J. C. Z., Meiri, S., Bowles, P., Cox, N. A., Shea, G. M., Böhm, M., Chanson, J., Tognelli, M. F., Harris, J., Walke, C., Harrison, N., Victor, S., Woods, C., Amey, A. P., Bamford, M., Catt, G., Clemann, N., Couper, P. J., Cogger, H., Cowan, M., Craig, M. D., Dickman, C. R., Doughty, P., Ellis, R., Fenner, A., Ford, S., Gaikhorst, G., Gillespie, G. R., Greenlees, M. J., Hobson, R., Hoskin, C. J., How, R., Hutchinson, M. N., Lloyd, R., McDonald, P., Melville, J., Michael, D. R., Moritz, C., Oliver, P. M., Peterson, G., Robertson, P., Sanderson, C., Somaweera, R., Teale, R., Valentine, L., Vanderduys, E., Venz, M., Wapstra, E., Wilson, S., and Chapple, D. G. (2019). Geographic and taxonomic patterns of extinction risk in Australian squamates. Biological Conservation 238, 108203.
| Geographic and taxonomic patterns of extinction risk in Australian squamates.Crossref | GoogleScholarGoogle Scholar |
Tingley, R., Meiri, S., and Chapple, D. G. (2016). Addressing knowledge gaps in reptile conservation. Biological Conservation 204, 1–5.
| Addressing knowledge gaps in reptile conservation.Crossref | GoogleScholarGoogle Scholar |
United Nations (2015). Transforming our world: the 2030 agenda for sustainable development. Resolution adopted by the General Assembly on 25 September 2015. UN General Assembly, New York.
Venchi, A., Wilson, S. K., and Borsboom, A. C. (2015). A new blind snake (Serpentes: Typhlopidae) from an endangered habitat in south-eastern Queensland, Australia. Zootaxa 3990, 272–278.
| A new blind snake (Serpentes: Typhlopidae) from an endangered habitat in south-eastern Queensland, Australia.Crossref | GoogleScholarGoogle Scholar | 26250233PubMed |
Woinarski, J. C. Z. (2018). The extent and adequacy of monitoring for Australian threatened reptile species. In ‘Monitoring Threatened Species and Ecological Communities’. (Eds S. Legge, D. B. Lindenmayer, N. M. Robinson, B. C. Scheele, D. M. Southwell, and B. Wintle.) pp. 69–84. (CSIRO Publishing: Melbourne.)
Woinarski, J. C. Z., Braby, M. F., Burbidge, A. A., Coates, D., Garnett, S. T., Fensham, R. J., Legge, S. M., McKenzie, N. L., Silcock, J. L., and Murphy, B. P. (2019). Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia. Biological Conservation 239, 108261.
| Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Burbidge, A. A., and Harrison, P. L. (2014). ‘The Action Plan for Australian Mammals 2012.’ (CSIRO Publishing: Melbourne.)
Woinarski, J. C. Z., Garnett, S. T., Legge, S. M., and Lindenmayer, D. B. (2017). The contribution of policy, law, management, research, and advocacy failings to the recent extinctions of three Australian vertebrate species. Conservation Biology 31, 13–23.
| The contribution of policy, law, management, research, and advocacy failings to the recent extinctions of three Australian vertebrate species.Crossref | GoogleScholarGoogle Scholar |


