Distribution and foraging by non-breeding Caspian Terns on a large temperate estuary of south-western Australia – preliminary investigations
Susie Stockwell A B D , Claire N. Greenwell A B , James N. Dunlop C and Neil R. Loneragan A B
A B D , Claire N. Greenwell A B , James N. Dunlop C and Neil R. Loneragan A B
A Environmental and Conservation Sciences, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
B Centre for Sustainable Aquatic Ecosystems, Harry Butler Institute, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
C Conservation Council of Western Australia, Lotteries West House, 2 Delhi Street, West Perth, WA 6150, Australia.
D Corresponding author. Email: susiestockwell1@gmail.com
Pacific Conservation Biology 28(1) 48-56 https://doi.org/10.1071/PC20082
Submitted: 25 October 2020 Accepted: 29 March 2021 Published: 27 April 2021
Journal Compilation © CSIRO 2022 Open Access CC BY
Abstract
This study investigates the distribution, abundance, and foraging ecology of Caspian Terns, Hydroprogne caspia, during 5 months of their non-breeding season, in the Peel-Harvey Estuary, south-western Australia. Observations were carried out at 20 sites around the estuary and 6 main areas (13 sites) where terns were abundant. Terns were observed every hour over 5 h time-blocks in the morning, midday, and afternoon, and the number of birds, number of birds foraging and time spent foraging were recorded for 10 min on the hour. From the 760 h of observation, a single overnight roosting site was identified in November, where a maximum of 147 birds were counted in February, after which time the roosting site appeared to shift. The total number of terns, foragers and proportion of time foraging varied amongst the six areas and foraging activity differed amongst times of day. Two areas, both characterised by large, sandy spits adjacent to shallow water, one adjacent to a river mouth and one near an ocean channel, were particularly important for terns and their foraging. Foraging activity was higher in the morning than at other times of day. Although salinity, air temperature, water temperature and wind speed were correlated with the total terns, foragers and proportion of time foraging, the correlations accounted for <25% of the total variation explained. The results of this study provide information for evaluating the use of Caspian Terns as bio-indicators of the Peel-Harvey Estuary and highlights the importance of this system during the non-breeding period.
Keywords: Caspian Terns, coastal seabirds, community composition, ecological health, estuarine system, foraging, Hydroprogne caspia, non-breeding season, Peel-Harvey Estuary, Ramsar, roost site, south-western Australia.
Introduction
Coastal environments are highly productive and dynamic, often supporting diverse assemblages of avifauna worldwide. Among them are the coastal seabirds – long-lived, often high-order, predators who feed on the rich forage-fish and invertebrate communities within these environments (Gochfeld and Burger 1996; Balance et al. 2008). The daily return of these conspicuous birds to terrestrial habitats for resting and nesting makes them reliable and accessible sentinel candidates, enabling changes in community composition and ecological health to be tracked over time (Cairns 1988; Burger and Gochfeld 2004).
The Caspian Tern (Hydroprogne caspia) is the largest tern species, with a cosmopolitan distribution and increasing population trend worldwide (Gochfeld and Burger 1996; Birdlife International 2019). However, relatively few published studies have focused on this species, among them, a large breeding colony of approximately 9700 pairs on the Columbia River Estuary in the United States of America. This research examined aspects of nesting (Collis et al. 2012), chick growth and development (Lyons and Roby 2011), fledging success (Collar et al. 2017), as well as foraging ecology during breeding periods (Lyons et al. 2005). Research investigating heavy metal contamination and diet during breeding periods has also been carried out at a colony in south-western Australia (Dunlop and McNeill 2017). Studies at other colonies have examined social attraction (Hartman et al. 2019), population genetics (Boutilier et al. 2013), nutritional stress indicators (Patterson et al. 2015), and contaminants (Su et al. 2017) in birds during breeding periods. In contrast with studies on breeding colonies, little or no information is available on the ecology of Caspian Terns during their non-breeding periods.
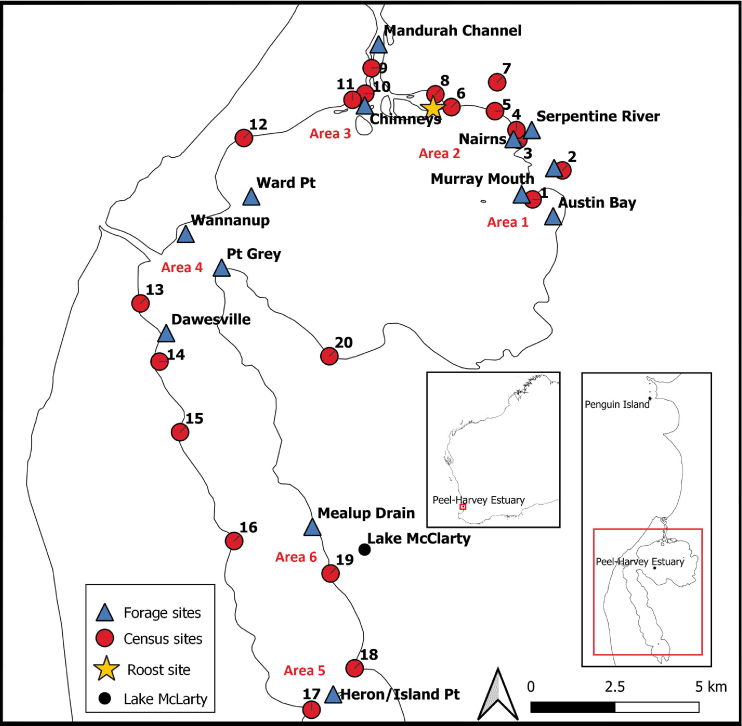
In Australia, Caspian Terns have a wide but scattered distribution across the coastlines, estuaries, wetlands, river systems, and ephemeral lakes such as Lake Eyre (Serventy et al. 1971; Birdlife International 2019). Although most breeding colonies are limited to several pairs, there are some larger, monospecific colonies that form on rocky offshore islands and sand spits near river mouths (Gochfeld and Burger 1996; Dunlop and McNeill 2017). In south-western Australia, the largest breeding colony of approximately 60 pairs is currently found on a rocky, nearshore island – Penguin Island, in Shoalwater Bay (Fig. 1; Dunlop and McNeill 2017), between August and November each year. Following egg incubation (~26–28 days) and chick fledging (~35–45 days after hatching; Gochfeld and Burger 1996), adults travel with their fledglings to non-breeding foraging sites such as the Peel-Harvey Estuary (–32.40S, 115.40E), ~30 km south of the breeding colony (Fig. 1; Dunlop and McNeill 2017). The Peel-Harvey Estuary is the largest and most diverse estuary in south-western Australia and forms part of the greater Ramsar-listed Peel-Yalgorup system (Hale and Butcher 2007).
This study investigates the abundance, distribution and foraging ecology of the Caspian Tern population on the Peel-Harvey Estuary in south-western Australia during 5 months of their ~ 9-month non-breeding season. Direct observations were undertaken to determine: (1) the location of potential night roost site(s); (2) the number and distribution of birds around the entire estuary; and (3) the foraging activity of birds in areas of high abundance to understand how foraging patterns varied with time of day and among locations.
Methods
Study region
The Peel-Harvey Estuary is a large, micro-tidal system comprising two shallow connected basins; the Peel Inlet and the Harvey Estuary, which cover an area of ~136 km2 (Fig. 1). The depth of the estuary ranges from 0 to 3.9 m at the lowest water level (Department of Transport 2006), with a daily tidal fluctuation of ≤1 m (Bureau of Meteorology 2019). The system is fed by three tributary rivers; the Serpentine and Murray enter from the north-east, and the Harvey from the south (Fig. 1). It has two openings to the Indian Ocean in the north-western region; a natural channel in the north and an engineered channel to the south, that was opened in 1994 (Fig. 1). The Peel-Harvey Estuary is surrounded by an extensive network of lakes and wetlands to the north, south, and east, which comprise the greater Peel-Yalgorup system (O’Malley and Willmott 2015). The region experiences a Mediterranean climate, with hot, dry summers (December–February) and cool, wet winters (June–August) with moderate rainfall (mean annual rainfall = 880 mm) and persistent winds year-round (average wind speed = 12–16 km h−1) (Hale and Butcher 2007; Bureau of Meteorology 2019).
Environmental data
The time of first and last light was recorded for each day of monitoring (Bureau of Meteorology 2019) in order to calculate the proportion of each time block available to terns for foraging. Abiotic data for tide height, air temperature, rainfall and wind speed, from the Mandurah Channel (station 009977) were downloaded from the Bureau of Meteorology and means were calculated for each monitoring block from five readings taken at hourly intervals (Bureau of Meteorology 2019). Salinity, water temperature, and chlorophyll a data were obtained from the Department of Water and Environmental Regulation (DWER) from monitoring sites in close proximity to all six main foraging areas (DWER 2019).
Caspian Terns
Data on Caspian Tern numbers and foraging activities were collected at sites across the Peel-Harvey Estuary in south-western Australia between October 2018 and February 2019 (Fig. 1), immediately following the terns’ annual breeding season at Penguin Island (Dunlop and McNeill 2017). In addition to these data, colour-banded Caspian Terns sighted at monitoring sites within the Peel-Harvey Estuary were recorded. Note that these birds were banded at the breeding colony between 2012 and 2018 under an approved Australian Bird and Bat Banding Scheme project (J. N. Dunlop, unpubl. data).
Roost counts
Historical data and large counts of Caspian Terns on the Peel-Harvey Estuary in the very early morning suggested that birds were night roosting in the area. A search was initiated to locate potential night roosting sites for Caspian Terns on the estuary, primarily by watching and listening for birds as they left foraging areas in the late afternoon and following their general direction of travel. These observations were made in the late afternoon until 15 November 2018 when a location was found where all birds appeared to roost (Fig. 1). All birds were counted at this location by one observer at approximately fortnightly intervals from 28 November 2018 until mid-March 2019. Three counts were conducted by a concealed observer in the 10-min period before last light, once all birds had assembled for the night. These average roost counts were later compared with counts from all monitoring sites around the estuary during the same fortnight to understand whether birds were likely to be aggregating at a single roost site at night.
Estuary-wide census
Caspian Tern numbers and their foraging activities were recorded at 20 accessible sites across six main areas around the perimeter of the estuary, fortnightly, between 5 October 2018 and 17 February 2019 (Fig. 1). Sites were selected based on being accessible and relatively equally spaced around the estuary. The total number of Caspian Terns (total terns) and the number of birds foraging (foragers) were counted over a 10-min period at each site. The time the terns collectively spent foraging during this period, i.e. from the time at least one bird started foraging until all birds ceased foraging, was recorded using a stopwatch. The proportion of time that any bird within the group was observed foraging within the 10-min period was then calculated as a percentage, i.e. % time foraging = (foraging time/10) × 100. Each site was surveyed on 10 occasions at varying times of day from early morning to early evening, i.e. 04:30 to 19:30 hours. Note that the rapid movement of individuals prevented the use of focal follows to obtain data on the foraging of individuals.
More detailed studies of foraging
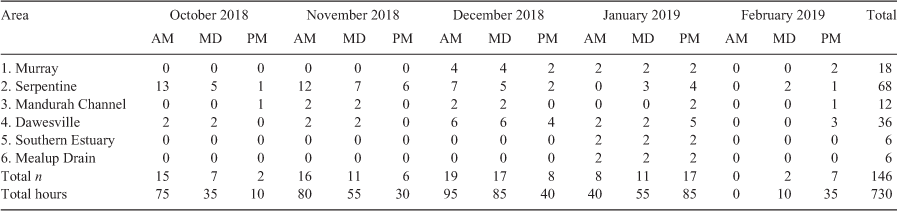
During the estuary-wide surveys, 13 sites in 6 main foraging areas, with high levels of foraging activity were identified (Fig. 1). The influence of time of day on foraging activity in these areas was investigated by dividing the diurnal period into three 5-h blocks: ‘morning’ (04:30–09:30 hours), ‘midday’ (09:30–14:30 hours) and ‘afternoon’ (14:30–19:30 hours). Observations were made from a kayak or the shoreline, depending on site accessibility. The total terns, number of foragers and the proportion of time spent foraging (‘tern indices’) were recorded during each observation block, light conditions permitting. A stopwatch was started at the first sign of foraging activity and stopped when no terns were foraging at the site. All 13 sites were surveyed at least twice in each time block between October 2018 and February 2019 (Table 1). However, the intensity of sampling varied between sites, with a greater number of observations taken at the mouth of the Serpentine River (68 observation periods) and the Dawesville region (36 observation periods). Overall, a total of 730 observation hours in 146 time blocks were completed (Supplementary material Table S1). Caspian Terns were identified in situ according to their physical and behavioural characteristics by an experienced observer, and their activity was described following an ethogram, developed from Nye and Dickman (2005). Three main categories of foraging activity were recognised and recorded – scanning, hovering and plunge diving (Nye and Dickman 2005).
Data analyses
Two-way analyses of variance (ANOVA) were used to test whether the total number of terns (total terns), number of foragers (foragers) and proportion of time spent foraging (time) differed amongst areas (six areas) and times of day (three blocks: morning, midday, afternoon), pooling data across months. Sites within an area were treated as replicates in these analyses. The data for the total number of terns and foragers were square root transformed to normalise the data, while no transformation was necessary for the proportion of foraging time. Where significant differences were found, post-hoc Tukey’s Honestly Significant Difference (HSD) tests were used to investigate which treatments differed significantly (P < 0.05) (Rogers et al. 2019).
Correlation analyses were undertaken to investigate the relationship between the total number of terns, foragers, and the proportion of time spent foraging by terns, and environmental conditions (salinity, water temperature, air temperature, tidal height, wind speed, chlorophyll a, and rainfall). Additional correlation analyses examined the influence of the same abiotic factors over each tern-based index within the two most frequently sampled areas: Serpentine and Dawesville. All statistical analyses and graphical displays were carried out using the ‘dplyr’, ‘agricolae’ and ‘stats’ R software packages (R Core Team 2019).
Results
Roost counts and census
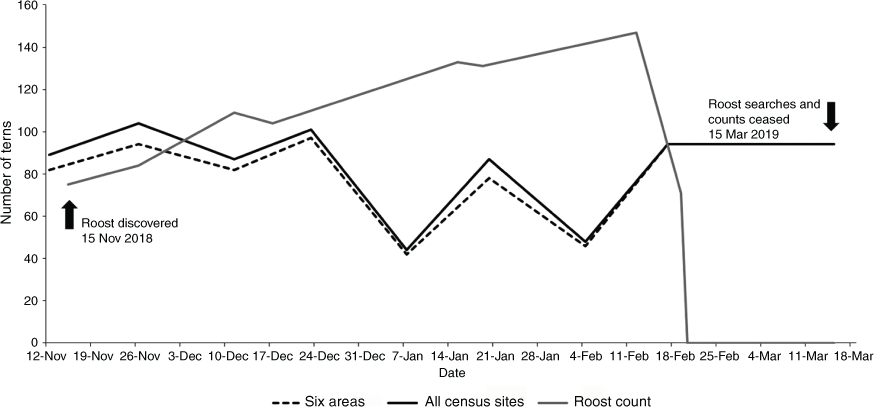
Surveys at the night roost site showed an increase in the mean number of Caspian Terns from 84 birds on 28 November to 147 on 11 February 2019, ~4 months after the end of their breeding season (Fig. 2). However, on 21 February, the roost count declined from 147 birds to 71 birds, and from 22 February 2019, no birds were recorded at that roost (Fig. 2). Searches of this site and other potential roosts continued until mid-March 2019 but no alternate sites were uncovered. The number of terns counted at the roost site was similar to the total number of birds counted from the total estuary census sites and the combined counts from the six main study areas, from November to 24 December (range = 80 to 104 for census sites and 82 to 97 for focus areas; Fig. 2). Roost counts were markedly higher than those from the census and focus areas from 7 January to 17 February 2019 (roost = 131–147; census and focus areas = 42–94) (Fig. 2). Although the roost counts declined greatly in late February, the number of birds censused around the estuary remained high during the day, indicating that the birds had shifted their roosting site to another location close to the estuary.
Variation in terns among areas and times of day
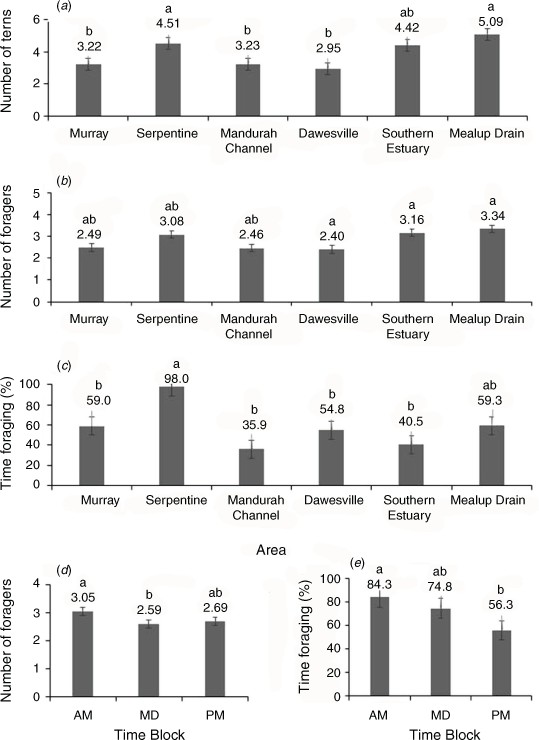
Two-way ANOVAs of the data from the six focus areas showed that total tern counts differed significantly amongst areas of the estuary (F5,128 = 10.52, P < 0.0001), but not times of day (F2,128 = 5.68, P = 0.48) and that the area × time interaction was not significant (F10,128 = 4.65, P = 0.81). A post-hoc Tukey’s HSD test showed that total tern counts were significantly greater at Mealup Drain (5.1) and Serpentine (4.5) than Mandurah Channel (3.2), Murray (3.2), and Dawesville (3.0), whereas no significant differences were found between these latter areas and the Southern Estuary (4.4; Fig. 3a).
|
The mean number of foragers and mean proportion of time spent foraging, like the total number of terns, differed significantly amongst areas (F5,128 ≥ 4.54, P < 0.001). However, they also different amongst times of day (F2,128 ≥ 3.15, P ≤ 0.046), and the area × time interactions were not significant (F10,128 ≤ 1.11, P ≥ 0.36). The mean number of foragers was significantly greater at Mealup Drain (3.34), Southern Estuary (3.16) and Dawesville (3.4) than the Serpentine (3.08), Murray (2.49), and Mandurah Channel (2.46; Fig. 3b). The proportion of time spent foraging was far greater at the Serpentine (98%) than all other areas (35–59%; Fig. 3c).
Tukey’s HSD test showed that the mean number of foragers and mean percentage of time spent foraging were significantly higher in the morning (F2,128 ≥ 3.15, P ≤ 0.046 and F2,128 ≥ 3.53, P ≤ 0.032 respectively) than at other times: foragers in the morning (3.1 birds) were greater than at midday (2.6) but not the afternoon (2.7; Fig. 3d), whereas the percentage of time foraging was greater in the morning (84.3%) than afternoon (56.4%), but not midday (74.8%; Fig. 3e).
Monthly variation in tern indices
In the Serpentine area, the mean total number of terns increased from 18.1 birds in October to 29.2 in November, as did the mean number of foragers (October = 7.4, November = 12.7 birds). The proportion of time spent foraging was greater in October and November (>61%) than in December and January (<46%). In Dawesville, the mean total number of terns fluctuated between months, with the most birds present in December 2018 (mean = 13.3) and the least in February 2019 (3.3 birds), whereas the mean number of foragers was lowest in October 2018 (4.8 birds), and highest in January 2019 (8.7 birds). The mean proportion of time spent foraging at Dawesville was highest in November (62.4%) and lowest in January (14.7%).
Abiotic influences on foraging patterns
The strongest correlations were found between time spent foraging and both salinity (R2 = 0.25, P < 0.001, n = 138) and water temperature (R2 = 0.22, P < 0.001, n = 138); and air temperature and the number of foragers (R2 = 0.11, P < 0.001, n = 147; Table S2). In almost all cases, the proportion of variation explained by environmental variables accounted for less of the total variation in tern indices than for those for the full data set (Table S3).
Colour-banded individuals
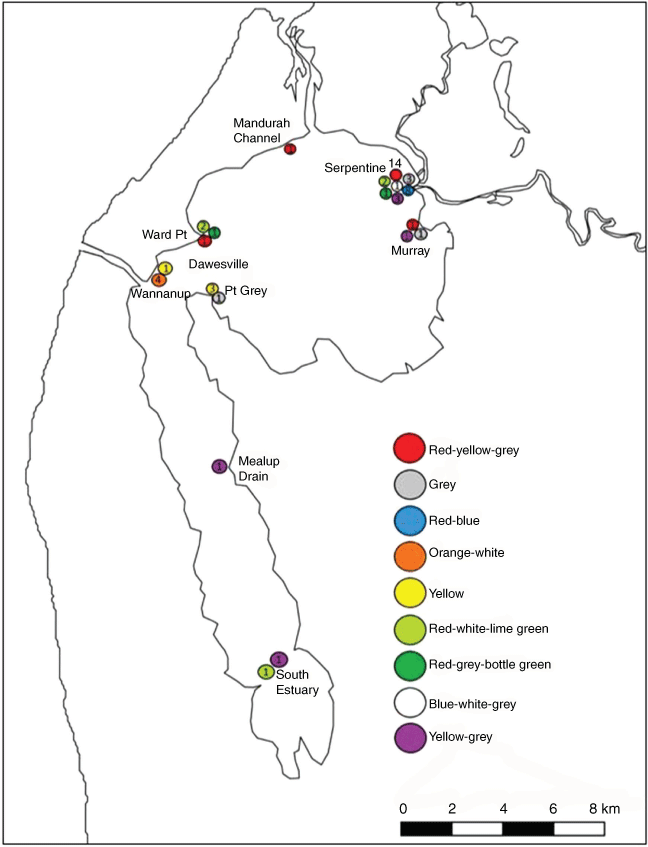
Nine individual birds were recognised by their colour bands at sand spits across the estuary between October and February (Fig. 4). Some birds were re-sighted frequently in a limited number of locations. The most frequently re-sighted individual, red-yellow-grey (17 sightings) was sighted only in the Peel Inlet and most frequently at the mouths of the Murray and Serpentine rivers (14 sightings). The orange-grey-white individual and its fledged chick were sighted four times but only at Wannanup spit in the Dawesville area (Fig. 4) – they appeared reluctant to leave even when disturbed by dogs and kite surfers (S. Stockwell, pers. obs.).
|
Discussion
Roost counts and census
A single night roost site was identified where terns assembled during the evenings for much of the study period. To our knowledge, this is the first report of a night roost location during the non-breeding season. A maximum of 147 birds were recorded at the night roost in mid-February, matching estimates of the total number of adult birds at their Penguin Island breeding colony (i.e. ~60 pairs in 2017 and 2018) combined with an approximation of successfully fledged juveniles from the breeding colony (Dunlop and McNeill 2017; J. N. Dunlop, unpubl. data). One year later in February 2020, opportunistic observations found a Caspian Tern roost site close to the previous location, on dried mudflats at Creery Island, near the Mandurah Channel (Fig. 1). Although the new roost site was not discovered before the end of the current study, similar numbers of terns were observed at regular foraging sites around the estuary during the day. Therefore, the decline in night roost attendance does not appear to reflect a change in the number of birds utilising the estuary and suggests that the roost site had shifted to another location.
The identification of night roost sites is very significant for the monitoring and management of terns utilising the Peel-Harvey as risks to the birds, e.g. predators and disturbance by people and pets (see, for example, Greenwell et al. 2019a), can be assessed and managed, thus enhancing the conservation of the population. The putative roost site for the Caspian Terns could be managed in a similar way to areas used to enhance the conservation of Fairy Terns, Sternula nereis nereis (Greenwell et al. 2019a, 2019b), e.g. fencing the roost area(s), restricting access by walkers and dogs, video monitoring to detect predators such as cats and foxes. These management measures would likely benefit other species in the area such as resident and migratory shorebirds.
Regular estuary censuses throughout the study period consistently found the highest tern counts in six main areas of the estuary. These sites are characterised by exposed sandy spits and large expanses of shallow water. These observations were consistent with those from intensive focal sampling around the estuary. The smaller number of birds counted at the census sites within these six areas from early January until mid-February compared with roost counts indicates that the terns may be foraging in wetland areas adjacent to the estuary such as Lake McLarty that were not surveyed. During this time, water levels in these wetlands declined, concentrating forage fish in reduced volumes of water, thus creating ideal foraging conditions. The dispersal of foraging terns to nearby wetlands, observed later in summer, could account for reduced tern counts in the census but the higher roost counts at night.
Variation in foraging patterns
Frequent resights of nine colour-banded individuals provide some insight into individual foraging patterns (Shiomi et al. 2015). These observations show large variation between individual foraging patterns with some birds appearing to prefer specific forage sites, while others were seen in widely separated locations (Fig. 4). This suggests some birds show a degree of forage site fidelity and make decisions on where to forage based on past experience, returning to areas with which they are familiar. No other studies of Caspian Tern populations elsewhere document variation in individual foraging patterns or interactions between birds within a population. However, social learning is thought to play a critical role in maximising feeding efficiency and the identification of productive foraging sites among gregarious birds (Turner 1964; Emlen and Demong 1975). Satellite tracking studies of Caspian Terns in southwestern Australia would be valuable for gaining more detailed knowledge of individual foraging patterns and their energetic requirements (e.g. Brisson-Curadeau et al. 2017; Fijn et al. 2017). Such studies have provided valuable information on the post-breeding migration pathways for Caspian Terns in the western United States of America and the use of Salton Sea in southern California as an important stop-over site (Lyons et al. 2018).
Population-level foraging patterns
The more comprehensive observations from the six focus areas revealed significant variation in the counts of total terns and foragers, as well as the proportion of time spent foraging by terns among areas and times of day. Total tern counts were significantly greater in two areas, Mealup Drain and Serpentine, which have expansive sandspits above the high tide mark where large numbers of terns were frequently observed ‘loafing’ between foraging bouts at all times of day. The ready availability of fish in shallow waters are ideal conditions for fledglings as they learn to forage independently, and no adult terns were observed providing fish to fledglings after the end of November. Forager counts were significantly greater at Mealup Drain, Southern Estuary, and Dawesville, all of which are located at the mouths of tributaries where nutrient inflows could attract higher abundances of forage fish. The southern Harvey Estuary and Dawesville both contain tidal sandspits which are not accessible for roosting at higher tides. This probably explains why these areas were significant for foragers but not for total tern counts. The number of foragers and proportion of time spent foraging were greatest in the morning, possibly because of the need to replenish energy reserves that become depleted throughout the previous night and to meet their daily energetic requirements (Bonter et al. 2013; Fijn et al. 2017).
The Serpentine area was the most consistently observed area and was one where foraging by terns, including recently fledged juveniles, was almost continuous (98%). This area is characterised by extensive sand spits above the high-tide line and shallow areas with sand and seagrass substrate, and is located at the mouth of a tributary river close to the roost site (Fig. 1). The total number of terns and foragers in the Serpentine area were highest in November. Most adults and their newly fledged juveniles had arrived from the breeding colony by this time. Possibly, the adults teach fledglings to forage at this site as it is close to their roost site and is characterised by shallow, clear, productive waters. In November, water levels across the estuary and surrounding wetlands were higher, which could have concentrated the terns in optimal foraging areas such as the Serpentine. As water levels declined later in the summer other exposed areas, adjacent to the estuary, are likely to have become accessible, such as Lake McLarty.
Abiotic influences on foraging patterns
Although salinity, air temperature, water temperature and wind speed were correlated with the tern-based indices overall, the correlations accounted for <25% of the total variation explained. No single abiotic factor was significant for all tern-based indices. Further investigation could be warranted to identify optimal foraging conditions for Caspian Terns on the estuary and elsewhere.
Estuaries are productive environments, which create ideal conditions for rapid growth of fish (Lenanton et al. 1984; Elliott et al. 2015) and the Peel-Harvey Estuary supports a commercial haul and gill-net fishery of mainly mullet (Mugil cephalus and Aldrichetta forsteri) and whiting (Sillago schomburgkii). During the late spring and summer, many marine species move into the Serpentine and Murray rivers (Potter et al. 1983; Loneragan et al. 1986, 1987; Potter et al. 2016), providing a reliable food source for piscivorous predators such as Caspian Terns, the likely motivation for their annual return during the non-breeding period.
Caspian Terns as ecological indicators
Both marine and estuarine environments are increasingly recognised as a global priority for conservation, although these vast systems are often logistically challenging and expensive to monitor. Seabirds can be used as sentinel species to provide indicators of ecological health for these systems (Cairns 1988), as many aspects of their behaviour and life history can be used to monitor changes in their environments over time (Burger and Gochfeld 2004). This Caspian Tern population consistently spends its time between Penguin Island and Peel-Harvey Estuary in their breeding and non-breeding seasons, respectively, a distance of ~30 km (Dunlop and McNeill 2017). This consistent, short movement coupled with their ecology as top predators makes Caspian Terns potential candidates for biological indicators of the condition of the Ramsar-listed Peel-Harvey Estuary and the marine environment surrounding Penguin Island. Further studies that explore patterns of Caspian Tern foraging ecology in the second half of the non-breeding season (March–July) and individual time-activity budgets could be undertaken to gain further insights into the drivers and interactions associated with movement and habitat usage of this potentially important indicator species within the estuary. Information obtained from Caspian Terns could play an important role in supporting monitoring and conservation of both these ecosystems and the terns themselves.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This project was funded by Murdoch University and completed under the animal ethics permit R3079/18. A heartfelt thank you to community members Bob Patterson, Dave Martin, Jesse Steele and Robert Wroth for their local knowledge, and to Katherine Stockwell and Kate Born for their time and enthusiasm with the GIS components of the project. We are appreciative of Dr Frances Loneragan from the Department of Water and Environmental Regulation, Western Australia, who provided data on water quality. We are grateful to and acknowledge the Bindjarup Noongar people as Traditional Custodians of Peel-Harvey Estuary, and pay respects to all Elders past, present and emerging.
References
Balance L. T., Ainley D. G., and Hunt G. L. (2008). Seabird Foraging Ecology. In ‘Encyclopedia of Ocean Science’, 2nd edn. (Eds J. Steele, S.A. Thorpe, K.K. Turekian.) pp. 2636–2644. (Academic Press: London.)Birdlife International (2019). Hydroprogne caspia (amended version of 2018 assessment). The IUCN Red List of Threatened Species 2019: e.T22694524A155509311. Available at https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T22694524A155509311.en [Accessed 15 December 2019].
Bonter, D. N., Zuckerberg, B. Z., Sedgwick, C. W., and Hochachka, W. M. (2013). Daily foraging patterns in free-living birds: exploring the predation-starvation tradeoff. Proceedings of the Royal Society B: Biological Sciences 280, 20123087.
| Daily foraging patterns in free-living birds: exploring the predation-starvation tradeoff.Crossref | GoogleScholarGoogle Scholar | 23595267PubMed |
Boutilier, S. T., Taylor, S. A., Morris-Pocock, J. A., Lavoie, R. A., and Friesen, V. L. (2013). Evidence for genetic differentiation among Caspian Tern (Hydroprogne caspia) populations in North America. Conservation Genetics 15, 275–281.
| Evidence for genetic differentiation among Caspian Tern (Hydroprogne caspia) populations in North America.Crossref | GoogleScholarGoogle Scholar |
Brisson-Curadeau, E., Patterson, A., Whelan, S., Lazarus, T., and Elliott, K. H. (2017). Tracking cairns: biologging improves the use of seabirds as sentinels of the sea. Frontiers in Marine Science 4, 1–7.
| Tracking cairns: biologging improves the use of seabirds as sentinels of the sea.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2019). Climate data for Mandurah Channel (station 009977). Available at http://www.bom.gov.au/climate/averages/tables/cw_009977.shtml (Accessed 27 February 2019.)
Burger, J., and Gochfeld, M. (2004). Marine Birds as Sentinels of Environmental Pollution. Ecohealth Journal Consortium 1, 263–274.
| Marine Birds as Sentinels of Environmental Pollution.Crossref | GoogleScholarGoogle Scholar |
Cairns, D. K. (1988). Seabirds as indicators of marine food supplies. Biological Oceanography 5, 261–271.
Collar, S., Roby, D. D., and Lyons, D. E. (2017). Top-down and bottom-up interactions influence fledging success at North America’s largest colony of Caspian Terns (Hydroprogne caspia). Estuaries and Coasts 40, 1808–1818.
| Top-down and bottom-up interactions influence fledging success at North America’s largest colony of Caspian Terns (Hydroprogne caspia).Crossref | GoogleScholarGoogle Scholar |
Collis, K., Roby, D. D., Larson, K. W., Adrean, L. J., Nelson, K., Evans, A. F., Hostetter, N., Battaglia, D., Lyons, D. E., Marcella, T., and Patterson, A. (2012). Trends in Caspian Tern nesting and diet in San Francisco Bay: Conservation implications for terns and salmonids. Waterbirds 35, 25–34.
| Trends in Caspian Tern nesting and diet in San Francisco Bay: Conservation implications for terns and salmonids.Crossref | GoogleScholarGoogle Scholar |
Department of Transport (2006). Cartographic Services, Department of Transport, Perth, Western Australia, Australia. WA848 Peel Inlet and Harvey Estuary e5-7. Available at transport.wa.gov.au
Department of Water and Environmental Regulation (DWER) (2019). Water Quality Data on Peel-Harvey Estuary from 2017–2019. Government of Western Australia. Perth, Western Australia. Available at http://wir.water.wa.gov.au/Pages/Water-Information-Reporting.aspx (Accessed 1 April 2019.)
Dunlop, J. N., and McNeill, S. (2017). Local movements, foraging patterns, and heavy metals exposure in Caspian Terns Hydroprogne caspia breeding on Penguin Island, Western Australia. Marine Ornithology 45, 115–120.
Elliott, M., Mander, L., Mazik, K., Simenstad, C., Valesini, F., Whitfield, A., and Wolanski, E. (2015). Ecoengineering and ecohydrology: successes and failures in estuarine restoration. Estuarine, Coastal and Shelf Science 176, 12–35.
| Ecoengineering and ecohydrology: successes and failures in estuarine restoration.Crossref | GoogleScholarGoogle Scholar |
Emlen, S., and Demong, N. (1975). Adaptive significance of synchronized breeding in a colonial bird: a new hypothesis. Science 188, 1029–1031.
| Adaptive significance of synchronized breeding in a colonial bird: a new hypothesis.Crossref | GoogleScholarGoogle Scholar | 1145188PubMed |
Fijn, R. C., de Jong, J., Courtens, W., Verstraete, H., Stienen, E. W. M., and Poot, M. J. M. (2017). GPS-tracking and colony observations reveal variation in offshore habitat use and foraging ecology of breeding Sandwich Terns. Journal of Sea Research 127, 203–211.
| GPS-tracking and colony observations reveal variation in offshore habitat use and foraging ecology of breeding Sandwich Terns.Crossref | GoogleScholarGoogle Scholar |
Gochfeld M., and Burger J. (1996). Family Sternidae (Terns). Pages 624–667 In ‘Handbook of the birds of the world.’ Eds J. del Hoyo, A. Elliott, J. Sargatal, J. Cabot.) pp 624–667. (Lynx Editions: Barcelona.)
Greenwell, C., Calver, M. J., and Loneragan, N. R. (2019a). Cat gets its tern: a case study of cat predation on a threatened coastal seabird. Animals 9, 1–15.
| Cat gets its tern: a case study of cat predation on a threatened coastal seabird.Crossref | GoogleScholarGoogle Scholar |
Greenwell, C., Dunlop, J. N., and Loneragan, N. R. (2019b). Nest desertion: an anti-predator strategy of the Australian Fairy Tern (Sternula nereis nereis). Marine Ornithology 47, 197–201.
Hale J., and Butcher R. (2007). Ecological character description of the Peel-Yalgorup Ramsar site. Report to the Department of Environment and Conservation and the Peel-Harvey Catchment Council. Perth, Western Australia.
Hartman, C. A., Ackerman, J. T., Herzog, M. P., Strong, C., and Trachtenberg, D. (2019). Social attraction used to establish Caspian Tern nesting colonies in San Francisco Bay. Global Ecology and Conservation 20, 1–11.
| Social attraction used to establish Caspian Tern nesting colonies in San Francisco Bay.Crossref | GoogleScholarGoogle Scholar |
Lenanton, R. C. J., Potter, I. C., Loneragan, N. R., and Chrystal, P. J. (1984). Age structure and changes in abundance of three important species of teleost in a eutrophic estuary (Pisces: Teleostei). Journal of Zoology 203, 311–327.
| Age structure and changes in abundance of three important species of teleost in a eutrophic estuary (Pisces: Teleostei).Crossref | GoogleScholarGoogle Scholar |
Loneragan, N. R., Potter, I. C., Lenanton, R. C. J., and Caputi, N. (1986). Spatial and seasonal differences in the fish fauna in the shallows of a large Australian estuary. Marine Biology: International Journal on Life in Oceans and Coastal Waters 92, 575–586.
| Spatial and seasonal differences in the fish fauna in the shallows of a large Australian estuary.Crossref | GoogleScholarGoogle Scholar |
Loneragan, N. R., Potter, I. C., Lenanton, R. C. J., and Caputi, N. (1987). Influence of environmental variables on the fish fauna of the deeper waters of a large Australian estuary. Marine Biology 94, 631–641.
| Influence of environmental variables on the fish fauna of the deeper waters of a large Australian estuary.Crossref | GoogleScholarGoogle Scholar |
Lyons, D. E., and Roby, D. D. (2011). Validating growth and development of a seabird as an indicator of food availability: captive-reared Caspian Tern chicks fed ad libitum and restricted diets. Journal of Field Ornithology 82, 88–100.
| Validating growth and development of a seabird as an indicator of food availability: captive-reared Caspian Tern chicks fed ad libitum and restricted diets.Crossref | GoogleScholarGoogle Scholar |
Lyons, D. E., Roby, D. D., and Collis, K. (2005). Foraging ecology of Caspian Terns in the Columbia River estuary, USA. Waterbirds 28, 280–291.
| Foraging ecology of Caspian Terns in the Columbia River estuary, USA.Crossref | GoogleScholarGoogle Scholar |
Lyons, D. E., Patterson, A. G. L., Tennyson, J., Lawes, T. J., and Roby, D. D. (2018). The Salton Sea: critical migratory stopover habitat for Caspian Terns (Hydroprogne caspia) in the North American Pacific Flyway. Waterbirds 41, 154–165.
| The Salton Sea: critical migratory stopover habitat for Caspian Terns (Hydroprogne caspia) in the North American Pacific Flyway.Crossref | GoogleScholarGoogle Scholar |
Nye, E. R., and Dickman, C. R. (2005). Activity budgets and habitat use of the Green Pygmy-goose (Nettapus pulchellus) on dry-season refuges in Kakadu National Park, Northern Territory. Emu 105, 217–222.
| Activity budgets and habitat use of the Green Pygmy-goose (Nettapus pulchellus) on dry-season refuges in Kakadu National Park, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
O’Malley J., and Willmott A. (2015). Binjareb Boodja landscapes 2025, a strategy for natural resource management in the Peel-Harvey region. A report to the Peel-Harvey Catchment Council, Jane O’Malley & Andrew Del Marco, Mandurah, Western Australia.
Patterson, A. G. L., Kitaysky, A. S., Lyons, D. E., and Roby, D. D. (2015). Nutritional stress affects corticosterone deposition in feathers of Caspian Tern chicks. Journal of Avian Biology 46, 18–24.
| Nutritional stress affects corticosterone deposition in feathers of Caspian Tern chicks.Crossref | GoogleScholarGoogle Scholar |
Potter, I. C., Chrystal, P. J., and Loneragan, N. R. (1983). The biology of the Blue Manna Crab Portunus pelagicus in an Australian estuary. Marine Biology 78, 75–85.
| The biology of the Blue Manna Crab Portunus pelagicus in an Australian estuary.Crossref | GoogleScholarGoogle Scholar |
Potter, I. C., Veale, L., Tweedley, J. R., and Clarke, K. R. (2016). Decadal changes in the ichthyofauna of a eutrophic estuary following a remedial engineering modification and subsequent environmental shifts. Estuarine, Coastal and Shelf Science 181, 345–363.
| Decadal changes in the ichthyofauna of a eutrophic estuary following a remedial engineering modification and subsequent environmental shifts.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2019). R: A language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria.) Available at https://www.Rproject.org/
Rogers, T. A., Fowler, A. J., Steer, M. A., and Gillanders, B. M. (2019). Resolving the early life history of King George Whiting (Sillaginodes punctatus: Perciformes) using otolith microstructure and trace element chemistry. Marine & Freshwater Research 70, 1659–1674.
| Resolving the early life history of King George Whiting (Sillaginodes punctatus: Perciformes) using otolith microstructure and trace element chemistry.Crossref | GoogleScholarGoogle Scholar |
Serventy D. L., Serventy V., and Warham J. (1971). The Handbook of Australian Seabirds. (Eds A. H. Reed, A.W. Reed.) pp. 207–209. (A.H. & A.W. Reed Ltd: Sydney, Australia.)
Shiomi, K., Lotberg, U., and Akesson, S. (2015). Seasonal distributions of Caspian Terns Hydroprogne caspia from Swedish populations, revealed by recoveries and resightings of ringed birds. Ringing & Migration 30, 22–36.
| Seasonal distributions of Caspian Terns Hydroprogne caspia from Swedish populations, revealed by recoveries and resightings of ringed birds.Crossref | GoogleScholarGoogle Scholar |
Su, G., Letcher, R. J., Moore, J. N., Williams, L. L., and Grasman, K. A. (2017). Contaminants of emerging concern in Caspian Tern compared to Herring Gull eggs from Michigan colonies in the Great Lakes of North America. Environmental Pollution 222, 154–164.
| Contaminants of emerging concern in Caspian Tern compared to Herring Gull eggs from Michigan colonies in the Great Lakes of North America.Crossref | GoogleScholarGoogle Scholar | 28089466PubMed |
Turner, E. R. A. (1964). Social Feeding in Birds. Behaviour 24, 1–44.
| Social Feeding in Birds.Crossref | GoogleScholarGoogle Scholar |