Managing for cultural harvest of a valued introduced species, the Pacific rat (Rattus exulans) in Aotearoa New Zealand
Priscilla M. Wehi A E , Deborah J. Wilson
A E , Deborah J. Wilson  A , Clive Stone B , Hayley Ricardo A , Chris Jones C , Richard Jakob-Hoff D and Phil O’B. Lyver C
A , Clive Stone B , Hayley Ricardo A , Chris Jones C , Richard Jakob-Hoff D and Phil O’B. Lyver C
A Manaaki Whenua – Landcare Research, Private Bag 1930, Dunedin 9054, New Zealand.
B Ngātiwai Trust Board, 129 Port Road, Whangārei 0140, New Zealand.
C Manaaki Whenua – Landcare Research, PO Box 69040, Lincoln 7608, New Zealand.
D New Zealand Centre for Conservation Medicine, Auckland Zoo, Private Bag 78700, Grey Lynn, Auckland 1245, New Zealand.
E Corresponding author. Email: priscilla.wehi@otago.ac.nz
Pacific Conservation Biology 27(4) 432-441 https://doi.org/10.1071/PC20094
Submitted: 30 November 2020 Accepted: 1 July 2021 Published: 3 August 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Indigenous peoples’ relationships with biodiversity are often poorly recognised in conservation decision-making, but are critical to Indigenous identity and lifeways. These relationships extend to introduced species that are rarely protected under legislation. Kiore (Rattus exulans, Pacific rat) is a species introduced to Aotearoa New Zealand (hereafter Aotearoa) by Māori (the Indigenous people of Aotearoa) as a food source and bio-indicator of ecosystem state. Once common, kiore are now restricted in numbers and range, and widely considered an unwanted organism by conservation managers and some Māori. However, tribal group Ngātiwai wish to safeguard cultural access to remaining kiore on Mauitaha Island. Therefore, the goals of our study were to assess body condition and the reproductive and disease status of kiore on Mauitaha. Of 16 kiore caught, body condition based on body length to mass ratio was similar to that recorded on other islands in Aotearoa. Subcutaneous fat levels were moderate, but lower in individuals with disease inflammation. The results suggest satisfactory population health, but regular monitoring to identify temporal trends in kiore abundance and condition is important for cultural harvesting and long-term population survival. Planning for harvesting by future generations requires transforming conservation biology through Indigenous perspectives, through further assessment of methods, management and agency, examining how Indigenous knowledge and conventional science can be used to balance ecological and cultural trade-offs. Further consideration of ecological habitat and risk is also required for kiore, because the reserve is a single small island, and national conservation priorities focus on native species protection in ecosystems that exclude humans.
Keywords: biocultural diversity, biodiversity indicator, cultural harvesting, Indigenous knowledge, Indigenous people, kiore, Mauitaha Island, national park management, socioecological systems, traditional ecological knowledge.
Introduction
The philosophies of many Indigenous peoples emphasise relationships with, and the protection of, biodiversity for future generations (Watene 2016). These philosophies arise from Indigenous knowledge systems that embed time-deep ecological knowledge together with living cultural practice, including assessments of relationship health (Russell 2004; deKoninck 2005; Pfeiffer and Voeks 2008; Trigger 2008; Lyver et al. 2019; Wehi et al. 2021). However, such cultural relationships and practices are often contested in legislation. Protected areas globally are generally managed to maintain native biodiversity and improve ecosystem health for native species in the absence of humans (Poirier and Ostergren 2002; Dovers et al. 2015; Johnson et al. 2017). In Aotearoa New Zealand (henceforth referred to as Aotearoa), the Indigenous Māori people have been largely excluded from both the occupation and management of protected areas. As well, the ability of Māori to achieve management aspirations for native biodiversity is diminished under current law (Ruru et al. 2017; Wehi and Lord 2017). Nonetheless, some government legislative actions recognise that Indigenous knowledge systems, known in Aotearoa as mātauranga, are key for retention and regeneration of biodiversity and are thus recognised in cultural heritage and co-governance arrangements both in Aotearoa (e.g. Rakiura Titi (Muttonbird) Island Regulations, Te Awa Tupua (Whanganui River) Settlement Act 2017) and internationally (e.g. Studley and Bleisch 2018).
Worldwide, growing impetus for co-management with Indigenous groups has resulted in a range of agreements and plans that recognise Indigenous harvesting rights and application of Indigenous and local knowledge systems within protected areas (Parks Canada 2010; Muhumuza and Balkwill 2013; Dovers et al. 2015), transforming conservation biology through Indigenous perspectives. Issues arise, however, for Indigenous relationships with introduced species that may be critical to the vitality of Indigenous identity and lifeways (e.g. Brook et al. 2006; Bradshaw and Gorman 2007). Introduced species are often actively targeted for eradication if conservation managers identify detrimental effects on native species or ecosystems (Barbour and Schlesinger 2012). Similarly, enhancing modified ecological systems to support and maintain Indigenous cultural-ecological linkages may be a low priority for managers.
Scientific initiatives to conserve native species may also result in action that decouples Indigenous cultural-ecological systems (Miskelly 2014; Malone 2016). For example, the legal land designation of protected ecosystems may lead to constraints on harvesting. Additionally, operational management of conservation lands such as the monitoring of individual species and wider ecosystem health may rely heavily on conventional scientific advice that can be inconsistent with Indigenous knowledge systems and focus on species protection rather than biocultural relationships which frequently incorporate the concept of harvesting (e.g. Dowsley and Wenzel 2008). An alternative approach is to use scientific techniques and tools that support and complement both biodiversity conservation and cultural values (Bennett et al. 2007; Muhumuza and Balkwill 2013).
In this study we focus on the management of an island ecosystem by Ngātiwai, a northern Māori tribe in Aotearoa, and the associated monitoring of a species, kiore (Pacific rat, Rattus exulans) introduced to Aotearoa by the Polynesian ancestors of Māori in ~1280 AD (Wilmshurst et al. 2008). Once established, kiore were widely harvested for food and pelts across the country (Best 1908; Haami 1994). However, they have now largely disappeared from the mainland of Aotearoa, displaced by other rats (R. rattus, R. norvegicus) introduced to Aotearoa by Europeans (Wilmshurst and Ruscoe 2021). Kiore are also considered a pest species by the Department of Conservation (the government agency in Aotearoa responsible for conservation) because of their ecological impacts and, as such, have now been eradicated from many of Aotearoa’s offshore islands (Wilmshurst and Ruscoe 2021). Despite such policies, Ngātiwai wish to ensure that kiore harvesting remains a viable tribal activity for future generations because of their high cultural value, particularly for pelts and ceremonial use (Kapa 2003) and have sought to prevent the eradication of kiore from their tribal area (Roberts 1994; Craig 2002; Kapa 2003; DOC 2010a). Haami (1994) highlights kiore as indicators of fruit ripening, depending on condition and pelage colour, also noting that their flavour is strongly influenced by the dietary fruit and berries available. Although relationships with kiore are recorded in oral tradition from many different iwi (Haami 1994), and indeed inhabit many Pacific islands, Ngātiwai are the only group to date that we know of who have sought to specifically protect this species. Accordingly, kiore are currently are managed on two small islands for cultural harvest within the territory of Ngātiwai, and as a traditional indicator of ecosystem state (e.g. tawapou (Planchonella costata) fruit abundance; Haami 1994; H. Parata, Ngātiwai elder, pers. comm., 2017).
In partnership with Ngātiwai, we conducted a first scientific assessment of kiore body condition on the island of Mauitaha (26 ha; 35.9°S, 174.7°E; also known as West Chicken Island) since the signing of an island co-management agreement between Ngātiwai and Aotearoa’s Department of Conservation (DOC) in 2010. We also examined kiore for signs of disease, an indicator used within Indigenous frameworks to assess harvesting potential and population health, together with body condition (Lyver and Gunn 2004; Moller et al. 2004). We then considered the future usefulness of this monitoring approach with particular emphasis on how it aligns with culturally based monitoring approaches and Ngātiwai’s desire to maintain and revitalise their relationship with the islands and kiore. We highlight how current reserve classifications in Aotearoa constrain Indigenous resource management of kiore, and future considerations for partnership.
Methods
People, relationships and co-management
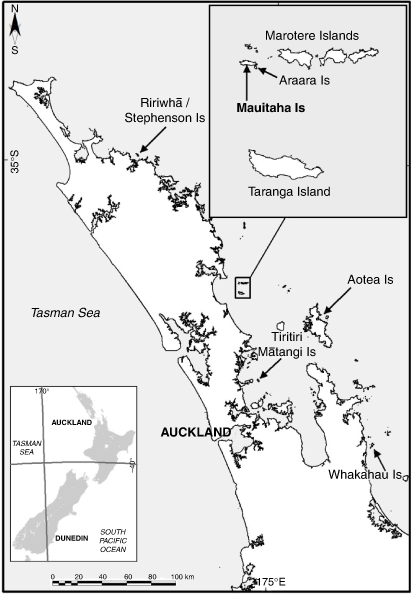
Ngātiwai people have occupied the east coast of Te Tai Tokerau (Northland) since the beginning of human occupation in Aotearoa (www.ngatiwai.iwi.nz). Ngātiwai tribal territory stretches over land and sea for a latitudinal distance of approximately 180 km, encompassing the eastern seaboard and all offshore islands (Te Iwi o Ngatiwai 2007). Signs of past agricultural practice and human settlement are present on many of these islands (Prickett 1984). Because of their historical significance and diverse flora and fauna, most of these islands are classified as Protected Areas under the jurisdiction of the DOC. As such, island management plans include eradication or control of introduced animals, including kiore, and weeds (e.g. Towns et al. 2003; DOC 2010b). In contrast, although Ngātiwai also wish to protect native biodiversity, their tribal policy highlights the importance of kiore and protection of this introduced species for cultural harvest as a priority. It also suggests the potential for cultural harvesting as a management tool (Te Iwi o Ngatiwai 2007). Ngātiwai are therefore required to assess risk to a range of species and ecosystems, including kiore, resulting in the unique management of islands based on critical trade-offs in decision-making.
In 2010, a Memorandum of Agreement between the Minister of Conservation and the Ngātiwai Trust Board appointed Ngātiwai to control and manage the offshore islands Mauitaha and Araara (2 ha), two of the Hen and Chicken Islands Nature Reserve (Reserves Act 1977; DOC 2010b). The agreement is globally unique in establishing management of a nature reserve for an introduced species (kiore), formally acknowledging Indigenous relationships with that species and allowing for its cultural harvest. In return, Ngātiwai agreed to support kiore eradication from the much larger Taranga (Hen Island; 470 ha) in the same island group. Nature reserves are set aside for the protection and preservation of flora and fauna in their ‘natural state’, and access is strictly by permit only (Reserves Act 1977, section 20). Ngātiwai harvesting practices on Mauitaha were therefore constrained for many years prior to the 2010 agreement, and contact between Ngātiwai and kiore on the island is still limited. Nevertheless, Ngātiwai wish to actively manage kiore on Mauitaha to ensure the persistence of this species for future generations (Te Iwi o Ngatiwai 2007).
This research was a partnership between conventional scientists and Ngātiwai. Scientists and tribal representatives met prior to sampling to decide on research direction and activity. Kiore data collection took place under the guidance of a Ngātiwai expert and co-author, and pelts were returned to Ngātiwai post-trapping. Our findings were presented back to the Ngātiwai Trust Board and others for comment and discussion.
Study site
Mauitaha and Araara are the only two islands in the 15-island Marotere group, ~8 km off the east coast of Te Tai Tokerau (Towns et al. 2003), where kiore are still extant (DOC 2010a; Fig. 1). They are separated by approximately 40 m of sea at high tide but connected at low tide. Kiore were eradicated from most of the Marotere islands in 1994–1997 (Towns et al. 2003), and from the main island Taranga in 2011.
|
Mauitaha rises steeply from sea level to 125 m at its highest point. The cliffs are dominated by harakeke (Phormium tenax), ngaio (Myoporum laetum), māhoe (Melicytus ramiflorus) and Coprosma spp., with pōhutukawa (Metrosideros excelsa), māpou (Myrsine australis) and kānuka (Kunzea ericoides) at higher elevation (McCallum et al. 1984). Other tree and shrub species include houpara (Pseudopanax lessonii), whauwhaupaku (P. arboreum), karo (Pittosporum crassifolium), kohekohe (Dysoxylum spectabile) and puka (Meryta sinclairii) (Atkinson 1971). The extent of kānuka and māpou, coupled with the scarcity of mature vegetation, suggests that Mauitaha was burnt within historic times (Atkinson 1971; McCallum et al. 1984).
Trapping
Working with Ngātiwai experts, we designed our study to precede the traditional kiore harvesting period in late autumn and early winter when kiore were described as both ‘large’ and ‘fat’ from foraging ripe fruit, such as tawapou (Haami 1994; Best 1908, 2005). This harvest time also exploits a period when kiore are abundant following the spring birth pulse. The customary approach of targeting a species in its prime condition and when juveniles or sub-adults are available to buffer harvest impacts on the population is also utilised by other tribes in Aotearoa (see Lyver et al. 2009).
To obtain individuals for examination, we set traps for 4 nights in late March 2016 (i.e. in the austral autumn) when kiore abundance was expected to be relatively high following summer breeding (Wilmshurst and Ruscoe 2021). We placed trap stations ~21 m apart along a winding line (830 m) that traversed the island from east to west (Fig. 1) to achieve a representative sample of potential kiore habitats present on the island. The line began in low-elevation shrub-land and forest, crossed harakeke-dominated slopes, and followed a small track through higher-elevation forest and scrub. To augment our trap numbers, we placed two additional trap stations in forest on a short 35 m line, perpendicular to and 50 m south of the main line. We trapped kiore with Victor Professional rat snap traps (‘snap traps’; Woodstream Corporation, Lancaster PA, USA) and Elliott live-capture box traps (‘live traps’; Elliott Scientific Equipment, Upwey, Vic., Australia). At each trap station we set a pair of snap traps (27 pairs on the first night and 39 pairs on the second night). Paired traps were placed back-to-back inside a corrugated plastic (Corflute) tunnel (500 × 120 × 120 mm), with plastic baffles restricting entrance holes to 40 × 40 mm to reduce the risk of by-catch of non-target species, primarily birds.
In the first 2 nights, the snap traps caught one kiore and one lizard. We then replaced most pairs of snap traps with single live traps on nights 3 and 4 to increase our kiore capture success and prevent additional lizard mortality. We placed one live trap in each of 39 tunnels and removed the plastic baffles that had restricted tunnel entrance sizes. We retained two pairs of snap traps (one pair at the site of the first night’s successful snap trap capture) to maximise captures. On the final night, we added two more live traps based on the presence of kiore sign. All traps were baited with a mixture of peanut butter and rolled oats and checked each morning. Kiore captured in live traps were euthanased by cervical dislocation. With all snap traps and live traps combined, we completed 220 trap-nights (i.e. number of traps × number of nights set). Additional information on capture rates and use of camera traps and tracking tunnels to monitor kiore activity is included in the Supplementary Material (Table S1).
Kiore measurements
We weighed all trapped kiore to the nearest gram, and measured head and body length (HBL) and total length including tail (mm). We measured HBL in three ways to enable comparison with results of other studies: (1) nose to pelvic girdle (known as the new British Museum method, Jewell and Fullager 1966; Moller and Craig 1987); (2) nose to anus, and (3) nose to end of furred part of upper tail (J. C. Russell, pers. comm.). We cleaned kiore skulls by soaking them in water for several days and assigned kiore to age classes on the basis of tooth-wear indices on upper molars. We assigned age classes on a scale from 1 (young animal with third molars not fully emerged) to 7 (old animal with molar crowns eroded almost to the roots; Karnoukhova 1972), based on the most-worn side of the jaw (Moller and Tilley 1986). All teeth were aged independently by two experts, and any differences resolved by re-inspection.
Kiore body condition (subcutaneous fat) and reproductive status
We scored subcutaneous fat on a scale from 1 (no fat deposits) to 5 (very heavy fat deposits; Wirtz 1972), and recorded indicators of reproductive status. For females we recorded: whether or not the vagina was perforate (an indicator of maturity and oestrus condition, where a perforated vagina indicates a receptive female); uterus size (an indicator of present and previous pregnancies, recorded as undeveloped, moderately developed, or enlarged (Moller and Craig 1987); numbers of embryos present, and placental scars, as an indicator of past births. For males, we recorded testes position (scrotal or abdominal) and length (mm). Abdominal testes position can indicate that a young animal is not yet mature, and the testes of mature animals can retract from the scrotum after the breeding season (Moller and Craig 1987).
Kiore disease status
We examined kiore livers for the presence of pale regions indicating possible pathology. We took liver samples from each animal, storing them in ethanol for histopathological analysis (carried out at NZ Veterinary Pathology, Auckland Zoo, Auckland, NZ). We also collected 14 faecal pellets, which we cooled and then froze before inspecting them for parasites. Faeces were suspended in saturated zinc sulfate solution and examined by light microscopy at 100× and 400× magnification following Hendrix and Sirois (2007).
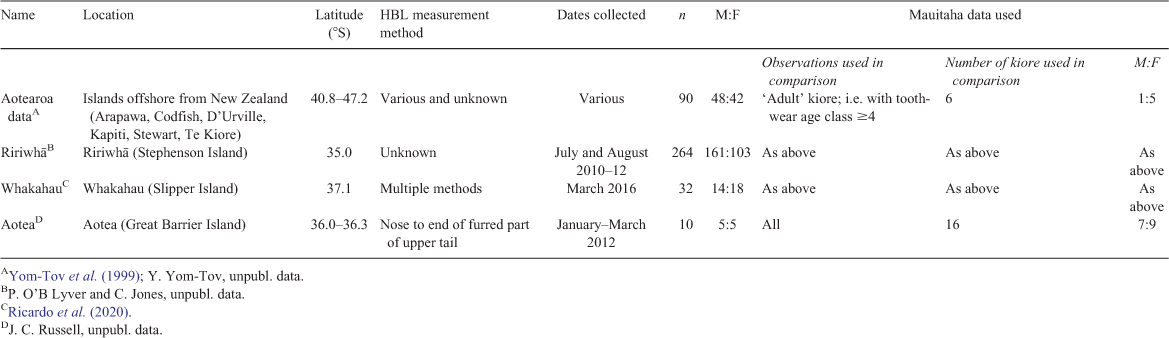
Kiore body condition (mass–HBL relationship) and effect of disease
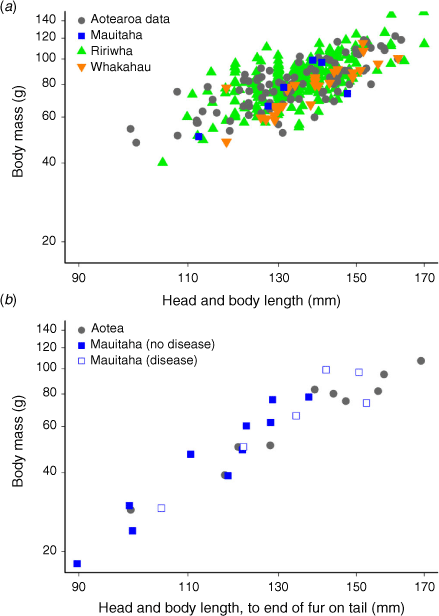
We used the relationship between mass and HBL as an index of body condition (Krebs and Singleton 1993), and tested for differences between the kiore we captured on Mauitaha and those from six other offshore islands (henceforth ‘Aotearoa data’, assembled by Yom-Tov et al. 1999; Table 1). The Aotearoa data included only kiore classified as ‘adults’ on the basis of tooth-wear age class 4 and higher (n = 90). We therefore selected Mauitaha kiore with tooth-wear age classes ≥4 (n = 6; Table 1) for comparison with the Aotearoa data. Simultaneously (in the same model) we compared body condition of kiore from Ririwhā and Whakahau with the Aotearoa data. The Whakahau dataset (n = 32) was collected at a similar time of year to our Mauitaha data (March), but the Ririwhā dataset (n = 264) was collected in winter. We included all Ririwhā and Whakahau kiore in this comparison, as their HBL measurements were within the range of the Aotearoa data, although we lacked tooth-wear age classes for them. Analyses were done in program R ver. 3.4.4 (R Core Team 2018).
To test for differences in condition, we first fitted a linear mixed-effects model in R package lme4 (Bates et al. 2015) to the Aotearoa data, with the response variable loge(mass) and predictor variable loge(HBL) (Krebs and Singleton 1993). Because methods used to measure HBL in the Aotearoa data varied (Yom-Tov et al. 1999), for Mauitaha and Whakahau kiore we used HBL measured from nose to anus, which was intermediate between our two other HBL measurement methods. The method used to measure Ririwhā kiore was undocumented. Island was fitted as a random effect to account for the collection of multiple individuals from each of six islands. A condition index for each kiore from Mauitaha, Ririwhā and the Aotearoa data was then calculated as the ratio of its predicted loge(mass) (based on this fitted model) and its actual loge(mass) (Krebs and Singleton 1993). The condition index was 1 for an animal whose loge(mass) and loge(HBL) were on the fitted line, and the mean index for kiore in the Aotearoa data was 1. We then used a linear model to compare condition indices of kiore from Mauitaha, Ririwhā, and Whakahau with the Aotearoa data.
We also tested for differences in the relationship between body mass and HBL between kiore from Mauitaha and Aotea (Great Barrier Island; Table 1), and for effects on condition of disease identified in the Mauitaha kiore. The Aotea dataset was collected during summer and early autumn (January–March), with HBL measured from nose to end-of-fur-on-tail. For this comparison, we used all the Mauitaha data, with HBL measurements made using this same method (Table 1). We fitted a linear model to the Aotea data, similar to the above mixed-effects model but, omitting the random effect ‘island’. We then calculated condition indices as above for (1) all Mauitaha kiore and (2) Mauitaha kiore with disease identified (see below), and compared these with Aotea kiore condition, with two separate linear models.
We expected that the Ririwhā kiore captured in winter would have ceased reproduction, include relatively few juveniles and subadults, and hence be heavier on average than the Mauitaha kiore captured in early autumn. We tested this prediction by comparing kiore mass between the two datasets, using a linear model with the explanatory variables location, sex and their interaction. Finally, we used an additional linear model to test whether subcutaneous fat scores of Mauitaha kiore (response variable) were related to the categorical variables disease, sex and their interaction.
Results
Kiore size and condition
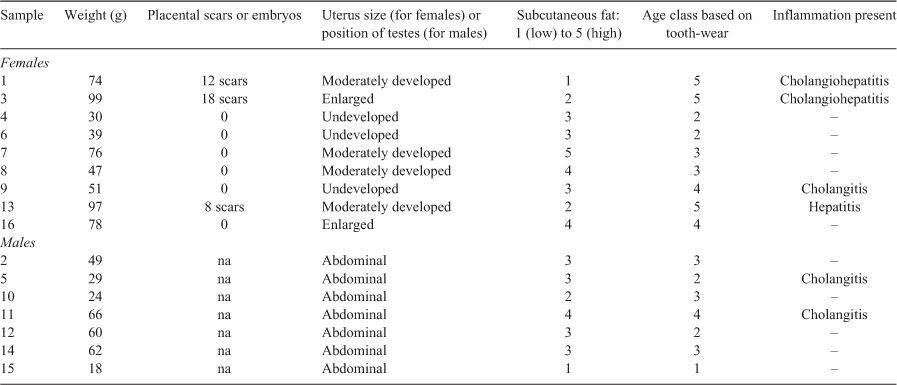
We captured 16 kiore; 9 females and 7 males (Table 2). The body condition of ‘adult’ Mauitaha kiore (those with tooth-wear age class ≥4; Table 1) did not differ from kiore on other islands in Aotearoa, based on relationships between body mass and length (mean Mauitaha condition index 0.96, n = 6, t392 = 0.7, P = 0.48, Aotearoa data index 1.0, n = 90; Fig. 2a, see Supplementary Material Table S2 for all necropsy details). This was despite five of the six Mauitaha kiore used in this comparison having signs of disease, i.e. inflamed livers and/or bile ducts. The condition of Ririwhā kiore was also similar to the Aotearoa data (index 1.03, n = 264, t392 = 1.5, P = 0.13), but Whakahau kiore had lower body condition than kiore in the Aotearoa data (0.92, n = 32, t392 = 2.9, P = 0.004). In the separate analysis, Mauitaha kiore body condition was also similar to that of Aotea kiore (Mauitaha index 1.07, n = 16, t24 = 1.1, P = 0.3, Aotea index 1.0, n = 10; Fig. 2b). As expected, kiore captured on Mauitaha in autumn weighed less than kiore captured in winter in the Ririwhā study (mean body mass on Mauitaha 56.2 ± 6.2 g s.e. (range 18–99 g; t276 = 2.5, P = 0.013, R2 = 0.21); on Ririwhā (84.2 ± 0.9 g; 40–151 g)). Mauitaha adult and subadult kiore had ‘moderate’ subcutaneous fat (Table 2), based on Wirtz’s (1972) scale (adult mean 3.1, n = 12 (adults classed as >38 g based on 95% CIs in Wirtz 1972); subadult mean 3.0, n = 2 (subadults classed as 25–38 g).
|
Kiore reproductive status
Of the nine females trapped, three had never reproduced, based on their undeveloped uteri (see Table S2 for reproductive details). Three others had placental scars, showing that they had reproduced previously. The remaining three females with moderately developed or enlarged uteri were probably in early stages of pregnancy. All seven trapped male kiore had abdominal testes; the largest (66 g) had a developed scrotum indicating that its testes had been scrotal but had since retracted.
Kiore disease status
Of the 16 necropsied kiore, 4 had visible pale patches on the liver indicating possible lesions (all from females; Table S2). Histopathological analysis confirmed inflammation of the liver or the liver and bile ducts in three of these kiore, and inflammation of the bile ducts was identified in three additional kiore. Faecal samples contained only low numbers of parasites. The faecal samples were in good condition indicating little if any degeneration between collection and processing. Five of the six kiore with inflamed livers and/or bile ducts were large or mature animals: three were the longest (HBL) kiore caught, and five had tooth-wear age class ≥4. Their mean subcutaneous fat scores were lower than in non-diseased kiore (2.5 vs 3.1, t12 = 3.2, P < 0.01; R2 = 0.52). Male kiore had lower mean subcutaneous fat scores than females (2.7 vs 3.0, t12 = 2.6, P < 0.05) and the relationship between disease and subcutaneous fat was weaker in males (interaction between sex and disease, t12 = 3.2, P < 0.01).
Discussion
Kiore body condition on Mauitaha (based on the relationship of body mass to HBL) was consistent with data from other islands in Aotearoa, and levels of disease were typical of rodent populations (Hsu 1979; Roberts et al. 2013; Fuehrer 2014). Further, the reproductive condition of kiore captured on Mauitaha was consistent with data from other forested islands in Aotearoa, for both females and males.
Body condition is a key harvesting indicator for many Indigenous people globally (Kofinas et al. 2003; Lyver and Gunn 2004) and, in kiore, may act as an indicator of other ecosystem processes such as fruiting (Haami 1994; H. Parata, Ngātiwai elder, pers. comm., 2017). Other customary harvests by Māori in Aotearoa are informed by indicators of body condition, and especially fat levels (e.g. kererū, Hemiphaga novaeseelandiae, Lyver et al. 2009). Body fat is often a seasonal resource; the Adi people of north-east India similarly prefer to harvest rodents (Rattus spp., Bandicota spp., and Mus musculus) in winter (Meyer-Rochow et al. 2015). In autumn and winter, the proportion of large adult kiore in the population increases as reproduction ceases and juveniles and subadults develop, as observed on Kure Atoll, Hawaii (28°N c.f. Mauitaha at 35°S; Wirtz 1972). Our finding that kiore from the Ririwhā study trapped in winter were heavier on average than Mauitaha kiore trapped in early autumn is consistent with this dynamic. Similarly, on Tiritiri Mātangi Island in north-eastern Aotearoa, kiore were heaviest in spring before young animals entered the trappable population (Moller and Craig 1987).
The presence of at least three pregnant females (those with moderately developed or enlarged uteri) shows that the breeding season on Mauitaha continues into early autumn. The high numbers of placental scars in some females indicate multiple prior pregnancies, as the litter size of kiore in Aotearoa is usually less than nine (Wilmshurst and Ruscoe 2021). The larger Mauitaha males may also still have been in reproductive condition in early autumn, despite none having scrotal testes. Long kiore breeding seasons extending into autumn are typical on forested islands in Aotearoa (Wilmshurst and Ruscoe 2021). In contrast, on islands with extensive exotic grass cover, kiore bred only when grass seed was present in spring and early summer (Moller and Craig 1987; Atkinson and Towns 2005). Moller and Craig (1987) found that only 10–35% of male kiore live-trapped in autumn months in forest habitat on Tiritiri Mātangi had scrotal testes, although the cauda epididymis of snap-trapped males showed that the larger males continued to be reproductive in April or May when pregnant females were no longer found (Moller and Craig 1987).
Future monitoring of Mauitaha’s kiore population
Ngātiwai’s aim to manage kiore for cultural harvest to ensure an abundance of resources for cultural harvesting and to sustain kiore for future generations (Te Iwi o Ngatiwai 2007) requires a biocultural approach to management to succeed. Cultural objectives include recognising and reinforcing the customary value and status of kiore as a valued species (Kapa 2003; Te Iwi o Ngatiwai 2007; DOC 2010b), transmitting associated traditions and stories to new generations (Haami 1994), and fulfilling harvesting needs (DOC 2010b). Retaining mātauranga contributes to cultural and linguistic vitality (Maffi 2001; Wilder et al. 2016), but questions remain about best practice ways to partner with government agencies and/or use conventional management approaches to achieve both biological and cultural objectives. Indigenous knowledge and practice are key to protection of both cultural and biological diversity (Pfeiffer and Voeks 2008), but the relationship of Indigenous systems with conventional scientific tools is not straightforward. Periodic monitoring to assess population trends and health, and accordingly modify long-term management as required, would help secure the future of Mauitaha’s kiore population. This could, for example, be done by integrating aspects of monitoring, such as inspecting livers for signs of disease, into cultural harvest practices.
As part of the ongoing collaborative management of Mauitaha, the conventional science methods used here to assess kiore condition could be applied within a tribal program that includes monitoring alongside other cultural indicators, which would fit with sustainable harvesting practices that maintain Ngātiwai’s relationship with kiore in future generations (A. Monks, C. Stone and P. Wehi, unpubl. data). Close interactions between trappers and kiore contribute to knowledge of the species and develop and maintain cultural-ecological connections. However, we highlight that too often knowledge integration ignores the power relations between Indigenous people and the state (Bohensky and Maru 2011) and does not address structural issues around agency of Indigenous peoples. Ngātiwai’s goals and objectives, and how Ngātiwai prefer to engage with kiore, are foundational to future monitoring and action, and for social justice to be enacted. Any knowledge integration needs to be cognisant of the links between culture and knowledge, of issues in legislation, and policy, and of the appropriate distribution of power (Bohensky and Maru 2011). To go forward from the assessment presented here, we suggest that any future monitoring methods by external agencies should take an explicit biocultural partnership approach with Ngātiwai (Gavin et al. 2015; Sterling et al. 2017) that overcomes current barriers such as visiting restrictions and difficult physical access.
Despite the ability of kiore to utilise diverse foods and environments (Wilmshurst and Ruscoe 2021), and strong advocacy from Ngātiwai for their protection, the long-term viability of kiore populations is not assured. This is, in part, because of the divergent priorities of government agencies and Ngātiwai, and, in part, because of a range of biological issues. Kiore populations in New Zealand are much reduced from previous times, so now occupy only a small proportion of their former range (Wilmshurst and Ruscoe 2021). As well, Ricardo et al.’s (2020) study of kiore on Whakahau suggests that kiore may benefit from commensal environments, as is also recorded in Māori oral tradition, but these environments are no longer present within the network of protected areas managed by DOC, including Mauitaha. Although our data show that kiore are present and breeding on Mauitaha, with body condition comparable to populations on other New Zealand islands, populations on small islands are vulnerable to deleterious effects of in-breeding (Keller and Waller 2002) and to variable food availability. Perhaps most importantly, population decreases (as well as increases) are to be expected in any small population in a fluctuating natural environment, and there is no ‘insurance’ population (e.g. on another island) that is not under threat of eradication. Mauitaha is small, relatively dry, dominated by scrub and early successional forest and with little grassland (Atkinson 1971; McCallum et al. 1984). It is unclear whether Mauitaha provides enough suitable habitat as the sole location for managing and protecting kiore long-term, and the forest and other habitat on Mauitaha are not currently managed to sustain kiore. Further work to identify baseline ‘natural states’ and to actively manage kiore habitat, potentially including restoration planting or increased human activity that may benefit kiore (Ricardo et al. 2020), could be useful points of discussion between Ngātiwai and its governmental partners. Indicators of relative kiore abundance that can be used to help assess populations in a straightforward and cost-effective way may be critical for management and sustainability of these cultural-ecological systems. Moreover, harvesting that uses preferred Ngātiwai methods (such as cervical dislocation rather than other methods of euthanasia) are to be supported. Further, future planning that values the expertise of knowledge holders might also explicitly support tribal priorities and methodologies that address the trade-offs between cultural benefits and potential damage to other native species.
There are many ironies in conducting a trapping study to examine the condition and health of a culturally valued introduced species that impacts native biota on an island that has Nature Reserve status and is largely inaccessible to its traditional owners. Ongoing management will require compromises between the conflicting drivers of protecting native biological diversity while also supporting cultural traditions and practices. The integrity of Indigenous knowledge relies on continued interaction and relationship with both species and the wider biophysical environment (Wehi et al. 2021), but legal restrictions such as those that apply here on the movements of both Ngātiwai and kiore set boundaries on these interactions, with consequences for management. Indeed, managing a Nature Reserve for the sustainability of an introduced species is contrary to conservation and reserve legislation in Aotearoa, suggesting that there is a lag in legislation with regard to the needs of Indigenous peoples that needs to be addressed. Potential options lie in the reclassification and realignment of conservation priorities of some Nature Reserves, like Mauitaha, to cultural-ecological priorities within a novel (within Aotearoa) ‘cultural reserve’ classification.
More broadly, active management is a vital component of Indigenous stewardship worldwide that requires reciprocal ongoing relationships between people and valued species (e.g. Wheeler et al. 2020). Such management must take account of relationships with introduced species that are culturally important, whether kiore or other habitat-modifying species such as pua’a or pigs in Hawai’i (Pejchar and Mooney 2009), or even the introduced kiawe now used as firewood because native woody plants are unavailable in subsistence communities in many parts of Hawai’i (Kamelamela 2012). Cross-cultural monitoring that supports the vitality of Indigenous knowledge systems, and allows opportunities for Indigenous communities to prioritise metrics and trade-offs, while helping to future-proof ecological monitoring is required (Lyver et al. 2019). However, although the integration of Indigenous knowledge and conventional science has benefits, such as strengths in understanding causal links and fast responses to new information, it is also clear that national laws and policies need to make space for Indigenous forms of cultural practice and assessment, and evaluation processes need to distribute power more equally across knowledge producers (Bohensky and Maru 2011). Indeed, recent assessments of Indigenous communities in the Arctic and elsewhere concur with Ngātiwai’s desire not only for inclusive research and decision-making, but also structural change in institutions, policies, and power and agency in technologies, approaches and priorities (Wheeler et al. 2020).
Assessments such as the one undertaken here, as a response to new and challenging circumstances, can be used to provide a foundation for equitable partnership that supports cross fertilisation of different knowledges, and an integrated approach for the benefit of communities and ecosystems. Such an approach includes co-production of research by communities and scientists, in ways that value and incorporate Indigenous knowledge.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We are grateful to Ngātiwai for support of this research and permission to study kiore on Mauitaha Island. We especially thank Hori Parata for contributing his knowledge of kiore biology and traditional management. We thank Whangārei DOC staff and Gretchen Brownstein for logistical support, Yoram Yom-Tov for use of the Aotearoa kiore data, James Russell for the Aotea kiore data, Grace Yee for laboratory work, Mike Fitzgerald, John Innes and Claire Kilner for advice and discussions about tooth-wear aging, Craig Pauling for information about former kiore harvesting methods, and Shona Hall for assistance to access archives. This research was approved by the Animal Ethics Committee of Manaaki Whenua – Landcare Research (approval no. 16/01/02). It was funded by a Rutherford Discovery Fellowship (RDF 14-LCR-001) awarded to PMW by the Royal Society of New Zealand, and by the Strategic Science Investment Fund awarded to Manaaki Whenua by the Ministry of Business, Innovation and Employment (MBIE). The NZ Department of Conservation Biodiversity Capability Fund and MBIE (Te Hiringa Tangata Ki Tai Pari Ki Tai Timu – Bicultural restoration of coastal forest ecosystems; C09X0908) funded the Ririwhā research.
References
Atkinson, I. A. E. (1971). Report of the flora and vegetation of the Western Chicken Islands and Marotiri Stacks. Unpublished report, Botany Division DSIR, Lower Hutt.Atkinson, I. A. E., and Towns, D. R. (2005). Kiore. In ‘The handbook of New Zealand mammals’, 2nd edn. (Ed. C. M. King). pp. 159–174. (Oxford University Press: Melbourne, Vic., Australia.)
Barbour, W., and Schlesinger, C. (2012). Who’s the boss? Post‐colonialism, ecological research and conservation management on Australian Indigenous lands. Ecological Management and Restoration 13, 36–41.
| Who’s the boss? Post‐colonialism, ecological research and conservation management on Australian Indigenous lands.Crossref | GoogleScholarGoogle Scholar |
Bates, D., Machler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bennett, E. L., Blencowe, E., Brandon, K., Brown, D., Burn, R. W., Cowlishaw, G., Davies, G., et al. (2007). Hunting for consensus: reconciling bushmeat harvest, conservation, and development policy in West and Central Africa. Conservation Biology 21, 884–887.
| Hunting for consensus: reconciling bushmeat harvest, conservation, and development policy in West and Central Africa.Crossref | GoogleScholarGoogle Scholar | 17531066PubMed |
Best, E. (1908). Maori Forest Lore. Part 2. Transactions of the New Zealand Institute 41, 231–285.
Best, E. (2005). ‘Forest lore of the Māori.’ (First published in 1942 as Dominion Museum Bulletin 14 and Polynesian Society Memoir 18.) (Te Papa, Wellington, New Zealand.)
Bohensky, E. L., and Maru, Y. (2011). Indigenous knowledge, science, and resilience: what have we learned from a decade of international literature on “integration”? Ecology and Society 16, 4.
| Indigenous knowledge, science, and resilience: what have we learned from a decade of international literature on “integration”?Crossref | GoogleScholarGoogle Scholar |
Bradshaw, C. J. A. and Gorman, J. T. (2007). A case for indigenous feral pest management. In ‘Investing in Indigenous natural resource management’. (Eds M. K. Luckert, B. M. Campbell, J. T. Gorman, and S. T. Garnett.) pp. 31–37. (Charles Darwin University Press: Darwin, NT, Australia.)
Brook, B. W., Bowman, D. M. J. S., Bradshaw, C. J. A., Campbell, B. M., and Whitehead, P. J. (2006). Managing an endangered Asian bovid in an Australian national park: the role and limitations of ecological-economic models in decision-making. Environmental Management 38, 463–469.
| Managing an endangered Asian bovid in an Australian national park: the role and limitations of ecological-economic models in decision-making.Crossref | GoogleScholarGoogle Scholar | 16736298PubMed |
Craig, J. L. (2002). Eradicating kiore. Forest and Bird 306, 3.
deKoninck, V. (2005). Joint management of banteng (Bos javanicus) in a contested cultural landscape: observations and implications. Human Dimensions of Wildlife 10, 123–135.
| Joint management of banteng (Bos javanicus) in a contested cultural landscape: observations and implications.Crossref | GoogleScholarGoogle Scholar |
Department of Conservation. (2010a). DOC and Ngatiwai Trust Board sign management agreement for two of the Marotere (Chicken) Islands. Available at http://www.doc.govt.nz/news/media-releases/2010/doc-and-ngatiwai-trust-board-sign-management-agreement-for-two-of-the-marotere-chicken-islands
Department of Conservation. (2010b). ‘Memorandum of agreement for the control and management of an area of nature reserve.’ (Department of Conservation: Whangarei, New Zealand.)
Dovers, S., Feary, S., Martin, A., McMillan, L., Morgan, D., and Tollefson, M. (2015). Engagement and participation in protected area management: who, why, how and when? In ‘Protected area governance and management’. (Eds G. L. Worboys, M. Lockwood, A. Kothari, S. Feary, and I. Pulsford.) pp. 413–440. (ANU Press: Canberra, ACT, Australia.)
Dowsley, M., and Wenzel, G. (2008). The time of the most polar bears: a co-management conflict in Nunavut. Arctic 2008, 177–189.
| The time of the most polar bears: a co-management conflict in Nunavut.Crossref | GoogleScholarGoogle Scholar |
Fuehrer, H. P. (2014). An overview of the host spectrum and distribution of Calodium hepaticum (syn. Capillaria hepatica): part 1 – Muroidea. Parasitology Research 113, 619–640.
| An overview of the host spectrum and distribution of Calodium hepaticum (syn. Capillaria hepatica): part 1 – Muroidea.Crossref | GoogleScholarGoogle Scholar | 24248632PubMed |
Gavin, M. C., McCarter, J., Mead, A., Berkes, F., Stepp, J. R., Peterson, D., and Tang, R. (2015). Defining biocultural approaches to conservation. Trends in Ecology and Evolution 30, 140–145.
| Defining biocultural approaches to conservation.Crossref | GoogleScholarGoogle Scholar | 25622889PubMed |
Haami, B. J. T. M. (1994). The kiore rat in Aotearoa: A Māori perspective. In ‘Science of the Pacific peoples – fauna, flora, food and medicine. Vol. 3’. (Eds J. Morrison, T. Geraghty, and L. Crowl.) pp. 65–76. (University of the South Pacific: Suva, Fiji.)
Hendrix, C. M., and Sirois, M. (2007). ‘Laboratory procedures for veterinary technicians’, 5th edn. (Mosby Elsevier: St Louis MS, USA.)
Hsu, C.-K. (1979). Parasitic diseases. In ‘The laboratory rat. Vol. I: Biology and diseases’. (Eds H. J. Baker, J. R. Lindsey, and S. H. Weisbroth.) pp. 307–331. (Academic Press: New York, USA.)
Jewell, P. A., and Fullagar, P. J. (1966). Body measurements of small mammals: sources of error and anatomical changes. Journal of Zoology 150, 501–509.
| Body measurements of small mammals: sources of error and anatomical changes.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. N., Balmford, A., Brook, B. W., Buettel, J. C., Galetti, M., Guangchun, L., and Wilmshurst, J. M. (2017). Biodiversity losses and conservation responses in the Anthropocene. Science 356, 270–275.
| Biodiversity losses and conservation responses in the Anthropocene.Crossref | GoogleScholarGoogle Scholar | 28428393PubMed |
Kamelamela, K. (2012). Aligning paradigm needs of conservation and culturally vibrant communities. Available at https://scholarspace.manoa.hawaii.edu/handle/10125/27118
Kapa, D. (2003). The eradication of kiore and the fulfilment of kaitiakitanga obligations. Auckland University Law Review 9, 1326–1352.
Karnoukhova, N. G. (1972). Age determination of brown and black rats. Soviet Journal of Ecology 2, 144–147.
Keller, L. F., and Waller, D. M. (2002). Inbreeding effects in wild populations. Trends in Ecology and Evolution 17, 230–241.
| Inbreeding effects in wild populations.Crossref | GoogleScholarGoogle Scholar |
Kofinas, G., Lyver, P., Russell, D., White, R., Nelson, A., and Flanders, N. (2003). Towards a protocol for community monitoring of caribou body condition. Rangifer 25, 43–52.
| Towards a protocol for community monitoring of caribou body condition.Crossref | GoogleScholarGoogle Scholar |
Krebs, C. J., and Singleton, G. R. (1993). Indices of condition for small mammals. Australian Journal of Zoology 41, 317–323.
| Indices of condition for small mammals.Crossref | GoogleScholarGoogle Scholar |
Lyver, P. O’. B., and Gunn, A. (2004). Calibration of hunters’ impressions with female caribou body condition indices to predict probability of pregnancy. Arctic 2004, 233–241.
| Calibration of hunters’ impressions with female caribou body condition indices to predict probability of pregnancy.Crossref | GoogleScholarGoogle Scholar |
Lyver, P. O’. B., Jones, C., and Doherty, J. (2009). Flavour or fore-thought: Tuhoe traditional management strategies for the conservation of kereru (Hemiphaga novaeseelandiae novaeseelandiae) in New Zealand. Ecology and Society 14, 40.
| Flavour or fore-thought: Tuhoe traditional management strategies for the conservation of kereru (Hemiphaga novaeseelandiae novaeseelandiae) in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Lyver, P. O’. B., Timoti, P., Davis, T., and Tylianakis, J. M. (2019). Biocultural hysteresis inhibits adaptation to environmental change. Trends in Ecology and Evolution 34, 771–780.
| Biocultural hysteresis inhibits adaptation to environmental change.Crossref | GoogleScholarGoogle Scholar |
Malone, K. (2016). Manitoba’s Sayisi Dene: forced relocation, racism, survival. CBC News, Canada, 16 August 2016.
Maffi, L. (Ed.) (2001). ‘On biocultural diversity: Linking language, knowledge, and the environment.’ (Smithsonian Institution Press: Washington DC.)
McCallum, J., Bellingham, P. J., Hay, J. R., and Hitchmough, R. A. (1984). The birds of the Chickens Islands, northern New Zealand. Tane 30, 105–124.
Meyer-Rochow, V. B., Megu, K., and Chakravorty, J. (2015). Rats: if you can’t beat them eat them! (Tricks of the trade observed among the Adi and other north-east Indian tribals). Journal of Ethnobiology and Ethnomedicine 11, 45.
| Rats: if you can’t beat them eat them! (Tricks of the trade observed among the Adi and other north-east Indian tribals).Crossref | GoogleScholarGoogle Scholar | 26024664PubMed |
Miskelly, C. M. (2014). Legal protection of New Zealand’s indigenous terrestrial fauna – an historical review. Tuhinga 25, 25–101.
Moller, H., and Tilley, J. A. V. (1986). Rodents and their predators in the eastern Bay of Islands. New Zealand Journal of Zoology 13, 563–572.
| Rodents and their predators in the eastern Bay of Islands.Crossref | GoogleScholarGoogle Scholar |
Moller, H., and Craig, J. L. (1987). The population ecology of Rattus exulans on Tiritiri Mātangi Island, and a model of comparative population dynamics in New Zealand. New Zealand Journal of Zoology 14, 305–328.
| The population ecology of Rattus exulans on Tiritiri Mātangi Island, and a model of comparative population dynamics in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Moller, H., Berkes, F., Lyver, P. O’. B., and Kislalioglu, M. (2004). Combining science and traditional ecological knowledge: monitoring populations for co-management. Ecology and Society 9, 2.
| Combining science and traditional ecological knowledge: monitoring populations for co-management.Crossref | GoogleScholarGoogle Scholar |
Muhumuza, M., and Balkwill, K. (2013). Factors affecting the success of conserving biodiversity in national parks: a review of case studies from Africa. International Journal of Biodiversity 2013, 1–20.
| Factors affecting the success of conserving biodiversity in national parks: a review of case studies from Africa.Crossref | GoogleScholarGoogle Scholar |
Parks Canada. (2010). ‘Tongait KakKasuangita SilakKijapvinga – Torngat Mountains National Park Canada Management Plan.’ (Parks Canada: Ottawa, Canada.)
Pejchar, L., and Mooney, H. A. (2009). Invasive species, ecosystem services and human well-being. Trends in Ecology & Evolution 24, 497–504.
| Invasive species, ecosystem services and human well-being.Crossref | GoogleScholarGoogle Scholar |
Pfeiffer, J. M., and Voeks, R. A. (2008). Biological invasions and biocultural diversity: linking ecological and cultural systems. Environmental Conservation 35, 281–293.
| Biological invasions and biocultural diversity: linking ecological and cultural systems.Crossref | GoogleScholarGoogle Scholar |
Poirier, R., and Ostergren, D. (2002). Evicting people from nature: Indigenous land rights and national parks in Australia, Russia, and the United States. Journal of Natural Resources 42, 331–351.
Prickett, N. (1984). An archaeological survey of the Chickens Islands (Marotere), New Zealand. Tane 30, 177–197.
R Core Team. (2018). R: A language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria.) Available at https://www.R-project.org/.
Reserves Act (1977 ). Available at http://legislation.govt.nz/act/public/1977/0066/58.0/DLM444305.html. [Accessed 23 May 2019].
Ricardo, H., Wilson, D. J., and Wehi, P. M. (2020). Kiore (Rattus exulans) distribution and relative abundance on a small highly modified island. New Zealand Journal of Zoology 47, 350–359.
| Kiore (Rattus exulans) distribution and relative abundance on a small highly modified island.Crossref | GoogleScholarGoogle Scholar |
Roberts, M. (1994). A pakeha view of the kiore rat in New Zealand. In ‘Science of Pacific Island Peoples (3) Flora, Fauna, Food and Medicine’. (Eds J. Morrison, P. Geraghty, and L. Crowl.) pp 124–141. (University of South Pacific: Suva, Fiji.)
Roberts, L. S., Janovy, J. Jr, and Nadler, S. (2013). ‘Foundations of Parasitology’, 9th edn. (McGraw-Hill: New York.)
Ruru, J., Lyver, P. O’. B., Scott, N., and Edmunds, D. (2017). Reversing the decline in New Zealand’s biodiversity: empowering Māori within reformed conservation law. Policy Quarterly 13, 65–71.
| Reversing the decline in New Zealand’s biodiversity: empowering Māori within reformed conservation law.Crossref | GoogleScholarGoogle Scholar |
Russell, J. C. (2004). Invading the Pacific: biological and cultural dimensions of invasive species in the Pacific region. Graduate Journal of Asia-Pacific Studies 2, 77–94.
Sterling, E. J., Filardi, C., Toomey, A., Sigouin, A., Betley, E., Gazit, N., Newell, J., et al. (2017). Biocultural approaches to well-being and sustainability indicators across scales. Nature Ecology & Evolution 1, 1798–1806.
| Biocultural approaches to well-being and sustainability indicators across scales.Crossref | GoogleScholarGoogle Scholar |
Studley, J., and Bleisch, W. (2018). Juristic personhood for sacred natural sites: a potential means for protecting nature. Parks 24, 81–96.
| Juristic personhood for sacred natural sites: a potential means for protecting nature.Crossref | GoogleScholarGoogle Scholar |
Te Iwi o Ngatiwai (2007). ‘Iwi environmental policy document.’ (Ngatiwai Trust Board Resource Management Unit: Whāngarei.)
Towns, D., Parrish, R., and Resource Management Unit Ngātiwai Trust Board. (2003). ‘Restoration of the principal Marotere Islands.’ (Department of Conservation: Wellington, New Zealand.)
Trigger, D. S. (2008). Indigeneity, ferality, and what ‘belongs’ in the Australian bush: Aboriginal responses to ‘introduced’ animals and plants in a settler-descendant society. Journal of the Royal Anthropological Institute 14, 628–646.
| Indigeneity, ferality, and what ‘belongs’ in the Australian bush: Aboriginal responses to ‘introduced’ animals and plants in a settler-descendant society.Crossref | GoogleScholarGoogle Scholar |
Watene, K. (2016). Valuing nature: Māori philosophy and the capability approach. Oxford Development Studies 44, 287–296.
| Valuing nature: Māori philosophy and the capability approach.Crossref | GoogleScholarGoogle Scholar |
Wehi, P. M., and Lord, J. M. (2017). Importance of including cultural practices in ecological restoration. Conservation Biology 31, 1109–1118.
| Importance of including cultural practices in ecological restoration.Crossref | GoogleScholarGoogle Scholar | 28233353PubMed |
Wehi, P. M., Whaanga, H., Watene, K., and Steeves, T. E. (2021). Mātauranga as knowledge, process and practice in Aotearoa New Zealand. In ‘Handbook of Indigenous environmental knowledge: global themes and practice’. (Eds T. Thornton, and S. Bhagwat). (Routledge: New York.)
Wheeler, H., Danielsen, F., Fidel, M., Hausner, V., Horstkotte, T., Johnson, N., Lee, O., Mukherjee, N., Amos, A., Ashthorn, H., and Ballari, O. (2020). The need for transformative changes in the use of Indigenous knowledge along with science for environmental decision-making in the Arctic. People and Nature 3, 544–56.
| The need for transformative changes in the use of Indigenous knowledge along with science for environmental decision-making in the Arctic.Crossref | GoogleScholarGoogle Scholar |
Wilder, B. T., O’Meara, C., Monti, L., and Nabhan, G. P. (2016). The importance of Indigenous knowledge in curbing the loss of language and biodiversity. Bioscience 66, 499–509.
| The importance of Indigenous knowledge in curbing the loss of language and biodiversity.Crossref | GoogleScholarGoogle Scholar |
Wilmshurst, J. M., and Ruscoe, W. A. (2021). Rattus exulans. In ‘The handbook of New Zealand mammals’, 3rd edn. (Eds C. M. King, and D. M. Forsyth.) pp. 161–240. (CSIRO Publishing: Melbourne, Vic., Australia.)
Wilmshurst, J. M., Anderson, A. J., Higham, T. F. G., and Worthy, T. H. (2008). Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proceedings of the National Academy of Sciences USA 105, 7676–7680.
| Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat.Crossref | GoogleScholarGoogle Scholar |
Wirtz, W. O. (1972). Population ecology of Polynesian rat, Rattus exulans, on Kure Atoll, Hawaii. Pacific Science 26, 433–464.
Yom-Tov, Y., Yom-Tov, S., and Moller, H. (1999). Competition, coexistence, and adaptation amongst rodent invaders to Pacific and New Zealand islands. Journal of Biogeography 26, 947–958.
| Competition, coexistence, and adaptation amongst rodent invaders to Pacific and New Zealand islands.Crossref | GoogleScholarGoogle Scholar |