Effects of an island-wide rodent eradication programme on two threatened bird species
Richard D. Segal A B * , Rachel Whitsed
A B * , Rachel Whitsed  B , Nicholas Carlile
B , Nicholas Carlile  C and Melanie Massaro
C and Melanie Massaro  A B
A B
A School of Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, Albury, NSW, Australia.
B Institute for Land, Water and Society, Charles Sturt University, Albury, NSW, Australia.
C Department of Planning, Industry and Environment NSW, Parramatta, NSW, Australia.
Pacific Conservation Biology 29(3) 253-266 https://doi.org/10.1071/PC21068
Submitted: 5 November 2021 Accepted: 24 April 2022 Published: 17 May 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: For the past 50 years, rodent eradications have been conducted worldwide to reverse the devastating impacts of introduced rodents on island species. However, few studies have quantitatively measured the effects of rodent eradications on native species.
Aims: This study investigated the effects of a rodent eradication on Lord Howe Island on two native birds.
Methods: To mitigate the risk of Lord Howe currawongs being poisoned during baiting operations, 30–40% of the population were taken into captivity during baiting, while the remaining currawongs were left in the wild. We studied currawong survival, nesting density and breeding success pre- and post-eradication to test how the baiting, a period in captivity, and the removal of rodents affected currawongs. We also investigated breeding success of white terns as they were expected to benefit from the eradication due to predator reduction.
Key results: We found that many currawongs left in the wild disappeared during the baiting period and nesting densities in one part of the island were significantly lower after the eradication. These currawongs likely died of poisoning as they were not resighted for 2 years post-eradication. White tern breeding success did not increase after the rodent eradication, although their predators were largely eliminated.
Conclusions: The captive management of currawongs mitigated the adverse effects of the baiting. As those currawongs that survived had high breeding success, we predict that the population will soon recover to pre-eradication size.
Implications: Our study reinforces the necessity of integrating ecological monitoring as part of future eradications on islands.
Keywords: breeding success, captive management, common white tern, Gygis alba, Lord Howe currawong, Lord Howe Island, rodent eradication, Strepera graculina crissalis.
Introduction
Rodents have been introduced to many islands worldwide by seafaring peoples and island colonists, resulting in severe population declines and extinctions of island species and thus altering many ecosystems permanently (Howald et al. 2007; Jones et al. 2008; Massaro et al. 2008). Several aspects of the biology and behaviour of rodents make them ideal invaders. Rodents can reproduce rapidly to colonise new areas or take advantage of increased food resources (Jones et al. 2008). Many species reach breeding age within months, have large litter sizes, and raise several litters per year (Parkes et al. 2007; Cory et al. 2011). Moreover, their varied diet allows them to survive in novel environments. They consume fruits and seeds and are also formidable predators, preying on invertebrates, reptiles and the eggs and chicks of nesting terrestrial and marine birds (Campbell and Atkinson 1999; Courchamp et al. 2003; Howald et al. 2007; Cory et al. 2011). Many island plant and animal species have evolved in the absence of rodents and therefore have not developed any chemical, anatomical or behavioural defences against these invaders (Blackburn et al. 2004; Massaro et al. 2008; Tershy et al. 2015). Hence, island plants and animals are particularly vulnerable to population declines and extinction when rodents are introduced (Campbell and Atkinson 1999; Blackburn et al. 2004; Howald et al. 2007).
For the past 50 years, rodent eradications have been conducted worldwide to reverse the impacts of these introduced rodents, restore island ecosystems and allow the recovery of remaining island species. Islands have offered opportunities to eradicate rather than control populations of invasive rodents because their isolation limits the risk of reinvasion by rodents after an eradication attempt (Bomford and O’Brien 1995; Cromarty et al. 2002). Dispersal of toxic cereal-based baits by air and on the ground has been the primary strategy to eradicate rodents on islands. The most commonly used toxins are anticoagulants that affect the blood clotting mechanisms in vertebrates (Howald et al. 2007; Campbell et al. 2015). Warfarin was the most frequently used toxin, but second-generation anticoagulants such as brodifacoum have replaced it. This is primarily due to rodents developing resistance to warfarin (Billing 2000; Howald et al. 2007; Campbell et al. 2015).
Due to the non-specificity of the toxins commonly used in rodent eradications, bait application on islands poses a lethal risk to existing vertebrates (Howald et al. 2007; Campbell et al. 2015; Croll et al. 2016). Some species may consume the baits directly (primary poisoning), and others may be indirectly exposed by consuming poisoned rodents (secondary poisoning). Several strategies exist to reduce non-target mortalities including using vivid bait colouration, conducting baiting operations when susceptible species are absent from the island and using bait stations to exclude certain species (Howald et al. 2007; Campbell et al. 2015). Another strategy is to capture endemic or endangered species at risk of poisoning and care for the entire or a percentage of the population during the baiting operations and until the poison has disintegrated within the environment (McClelland 2002; Campbell et al. 2015; Herrera-Giraldo et al. 2019). Such captive management is very labour intensive and expensive as it requires specialist veterinary personnel and facilities. Hence, the use of captive management as a mitigation strategy to reduce non-target mortality remains uncommon. However, captive management during rodent eradications has been successfully undertaken to benefit several endemic island species, including the Henderson rail (Porzana atra), Seychelles magpie-robin (Copsychus sechellarum) and Galapagos hawk (Buteo galapagoensis) (Merton et al. 2002; Towns and Broome 2003; Brooke 2019; Rueda et al. 2019). Although all these mitigation measures significantly minimise the number of non-target mortalities (e.g. Brooke 2019), they are not perfect and hence it is important to measure the effects of a rodent eradication on native species and ecosystems.
The publication of quantified ecological outcomes following invasive mammal eradications, including rodents on islands, remains uncommon. This is despite the success of rodent eradications worldwide (Towns et al. 2006; Bellingham et al. 2010; Jones et al. 2016; Brooke et al. 2018). In their review of global invasive mammal eradications on islands, Jones et al. (2016) found that 236 native species benefitted from eradicating invasive mammals on 181 islands. However, only 22 studies of 63 species documented specific responses to eradications such as trends in abundance or reproductive success and few of these contained quantitative information (Jones et al. 2016). Reporting of the ecological benefits of island-wide rodent eradications is also lacking in Australia, where ecological monitoring was undertaken for less than 20% of all rodent eradications (Segal et al. 2021a). Moreover, the failure to collect pre-eradication data prevents any quantitative comparison with post-eradication data (Buxton et al. 2016; Croll et al. 2016), limiting the ability to assess the ecological benefits and consequences of eradication programmes.
Australia’s Lord Howe Island provides an example of the devastating consequences of introduced rodents for native biota. House mice (Mus musculus) were introduced to Lord Howe Island in the 1860s, while black rats (Rattus rattus) arrived when a ship ran aground in 1918. Since then, introduced rats are thought to be responsible for the extinction of five endemic bird species, at least 13 species of endemic invertebrates and two plant species (Hutton et al. 2007; Wilkinson and Priddel 2011). The estimated 210 000 mice and 150 000 rats on Lord Howe Island continued to threaten the island’s flora and fauna (Gillespie and Bennett 2017) and thus, to conserve the remaining native species, an island-wide rodent eradication programme was conducted in the austral winter of 2019 (Walsh et al. 2019; Harper et al. 2020). A combination of aerial bait drops on unpopulated areas and a grid of bait stations in the settled areas were used to bait the entire island (Walsh et al. 2019; Harper et al. 2020). The last rodent was detected in September 2019 and the island remained rodent free for the duration of this study.
The Lord Howe currawong (Strepera graculina crissalis) is a large, omnivorous, forest-dwelling bird endemic to Lord Howe Island, which is considered a subspecies of the pied currawong (Strepera graculina) found in eastern mainland Australia. Due to its restricted range and small population, the Lord Howe currawong is classified as vulnerable within the state of New South Wales (Office of Environment and Heritage NSW 2020a). The baiting programme was expected to pose a considerable risk to Lord Howe currawongs, as brodifacoum, the rodenticide used during the rodent eradication on Lord Howe Island, is also toxic to birds (Campbell et al. 2015; Lohr and Davis 2018). Currawongs are naturally curious and thus, were expected to consume both poison pellets and poisoned rodents. To mitigate this risk and minimise the impact of the baiting programme on the Lord Howe currawong, 129 currawongs (an estimated 30–40% of the population) were taken into captivity on the island for the duration of the baiting programme, while the remaining population was left in the wild.
The removal of rodents from the island was also expected to directly affect the currawongs. While rodents were not a direct threat to the currawong population, currawongs and rodents were competitors for shared food resources (seabird offspring, invertebrates and fruit). Therefore, the removal of rodents was expected to benefit the currawong population in the long term, despite rodents occasionally being part of the currawong’s diet prior to the eradication (Carlile and Priddel 2007).
The population of common white terns (Gygis alba) on Lord Howe Island was also expected to benefit from the rodent eradication. While rodent predation of white tern nests on Lord Howe Island has not been recorded, it does occur on islands elsewhere (Grant et al. 1981), and thus, the removal of rodents from the island was expected to directly benefit white tern nesting success. Additionally, the poisoning of rodents on the island was expected to eliminate the population of masked owls (Tyto novaehollandiae) (Milledge et al. 2019), which are known predators of white terns on the island (Carlile and Priddel 2015). Several owl species were introduced to the island in the 1920s as an attempt to reduce rat numbers following their invasion of the island, but only the masked owl survives today (Milledge et al. 2019). As masked owls were expected to be eradicated during the baiting programme, white tern nesting success was expected to increase after the rodent eradication.
Finally, the island’s white tern population is also affected by currawongs, which have been observed to feed on eggs and chicks of white terns. Therefore, a reduction in currawongs due to non-target mortality during the baiting programme was expected to increase tern breeding success, at least in the first few years after the rodent eradication. However, as currawongs foraged to some small extent on rodents (2.5%) prior to the eradication (Carlile and Priddel 2007), there was also the possibility that the removal of rodent prey may actually increase currawong predation of tern nests after the eradication.
This study investigated the effects of the island-wide rodent eradication programme on Lord Howe Island on the Lord Howe currawong and the common white tern. First, we measured the effect of the baiting programme on currawong survival and nesting densities. We expected currawong survival to be lower during the baiting programme (2018–2019) than before (2017–2018) or after the eradication (2019–2020). If the baiting programme had a significant effect on currawong survival, we also expected nesting densities to be lower immediately after the eradication than before the eradication. Second, we measured how the removal of rodents affected currawong breeding success. As rodents were primarily competitors for food resources, we expected currawong breeding success to increase after the rodent eradication. Third, we investigated how the captive management may have affected currawongs by comparing captive currawongs’ breeding success and body condition with those of currawongs left in the wild. Finally, we investigated whether the removal and reduction of white tern predators (rodents, owls and currawongs) during the rodent eradication benefitted white tern nesting success.
Materials and methods
Study site
Lord Howe Island is located 760 km north-east of Sydney, New South Wales in the Tasman Sea between Australia and New Zealand (31.54°S, 159.08°E). Lord Howe Island was listed as a UNESCO World Heritage Site in 1982 for its spectacular natural and physical features and range of unique, endemic animals and plants (Hutton et al. 2007; United Nations Educational, Scientific and Cultural Organisation 2022). The island is approximately 11 km long and up to 3 km wide, rising to 875 m at its highest point, Mount Gower (Department of Environment and Climate Change (NSW) 2007). The island’s 1455 ha are covered mainly by native vegetation, with approximately 75% of the island’s land area inside a Permanent Park Preserve (Department of Environment and Climate Change (NSW) 2007). The island experiences a mild maritime climate, with cool, wet winters 13–18°C and drier, warmer summers 13–25°C (Department of Environment and Climate Change (NSW) 2007). The average annual rainfall is 1650 mm with high cloud cover and humidity throughout the year (>60% relative humidity) (Department of Environment and Climate Change (NSW) 2007).
The baiting programme for the eradication of rodents on Lord Howe Island consisted of three parts: (1) aerial baiting; (2) bait stations; and (3) hand-broadcasting of baits. The baits used were 2 g cereal pellets containing 0.02 g/kg of brodifacoum (Harper et al. 2020). Aerial baiting using a helicopter occurred over the uninhabited parts of the island on 8–12 June 2019 and 1–5 July 2019. Bait was distributed at a density of 12 kg/ha during the first baiting operation and 8 kg/ha during the second baiting operation (Lord Howe Island Board 2017; Harper et al. 2020). In the settled areas of the island, approximately 19 000 bait stations were deployed in a 10 × 10 m square grid between 22 May 2019 and 5 November 2019. Each bait station held a maximum of 80 g of bait (Harper et al. 2020). An additional 9500 hand-broadcasting points were located in the areas of overlap between the aerial application and bait station network. Bait pellets were hand-broadcast at the same time as the aerial bait drops and at the same density (Harper et al. 2020).
Study species
Adult Lord Howe currawongs form breeding pairs and defend their nesting territories in forested gullies and creek lines year-round (Segal et al. 2021b). Currawongs on Lord Howe Island build their nests and raise their young between September and January each year. The female builds an open-cup nest and incubates the eggs, while both adults feed the nestlings and defend the nest. Incubation lasts for 21 days and nestlings take a further 35 days to fledge (56 days or 8 weeks in total). Juvenile currawongs stay with their parents for up to 2 months after fledging (Wood 1998). In the absence of large, resident diurnal raptors and corvids, the currawong is a significant predator of the island’s vertebrates and invertebrates. Eggs and chicks of other nesting birds on the island are a considerable part of the currawong’s diet, especially during the chick rearing stage. It is likely that before rodents were introduced to Lord Howe Island, invertebrates were an important part of the currawong’s diet, but as many invertebrates disappeared due to the invasion of rodents (Hutton et al. 2007; Wilkinson and Priddel 2011), currawongs had to change their diet. Before the rodent eradication, currawongs occasionally provisioned their offspring with rodents (Carlile and Priddel 2007). However, rodents primarily competed with currawongs for food resources, rather than being an important prey item for currawongs.
White terns have a broad distribution across the Pacific and nest on many oceanic islands, from Hawai‘i and the Galapagos to Micronesia and Polynesia (Higgins and Davies 1996). The species only relatively recently (in 1968) started to nest on Lord Howe Island, and due to its restricted range and small population within New South Wales, they are considered vulnerable (Carlile and Priddel 2015; Office of Environment and Heritage NSW 2020b). The population grew 12% per year until 2006, but growth then declined to 2.7% per year (2006–2014; Carlile and Priddel 2015). The breeding season of the common white tern on Lord Howe Island starts in September and can extend to March in the following year (Carlile and Priddel 2015). This tern species lays its single egg in a notch or depression on a horizontal branch (Hart et al. 2016). Incubation duties are shared between adults and lasts for around 35 days (Dorward 1963; Carlile and Priddel 2015). Both adults also provision nestlings for about 68 days, but the nestling period can range from 49 to 75 days (Hutton 1991; Carlile and Priddel 2015). Fledglings remain dependent on their parents for another 3–4 weeks (Carlile and Priddel 2015). Pairs on the island readily re-nest after losing an egg or chick (Carlile and Priddel 2015).
Currawong survival
We banded currawongs on the island between September and December each year in 2017–2020. We captured birds individually using a spring-loaded net trap on their territories, at a feeding table or where they congregated (e.g. in orchards, top of Mt Gower). Each bird was fitted with a numbered metal band and a unique combination of three coloured plastic bands. We also recorded the colour combination of all birds seen on the island throughout the breeding season each year. To estimate year-to-year survival, we conducted a Cormack-Jolly-Seber mark/recapture analysis in R (ver. 4.0.2) using RMARK ver. 2.2.7 (Laake 2013; R Core Team 2020).
Currawong nest locations and breeding success
We located currawong nests by searching the forested areas of the island systematically during the currawong breeding season (September–January) in 2017, 2018, 2019 and 2020. The three main regions searched were the Northern Hills, the Transit Hill area and the area around Intermediate Hill to the base of Mount Lidgbird. Due to the steepness of the terrain, we were only able to search a portion of the southern part of the island around Mt Lidgbird and Mt Gower. We used the island’s forest walking tracks to access these areas, and any gullies or creek lines favoured by currawongs for nesting (Segal et al. 2021b) were followed from top to bottom to find currawong territories. In addition, we investigated any locations where currawongs were heard calling or seen from a distance. Once located, we followed birds displaying breeding behaviours including territorial defence, carrying nesting material or carrying food to their nest. Any nestling or fledgling begging calls heard were also investigated to locate nests. We recorded GPS locations of nests to an accuracy of less than 5 m. The areas where we found nests in 2017 were also searched for nests in subsequent years. Increased knowledge about the locations of established currawong territories from year to year and the employment of a field assistant in 2019 and 2020 allowed us to search more areas from year to year. However, this increased search effort did not influence our nest density analyses (see below), because we only included areas that were searched in all 3 years (2018, 2019 and 2020).
We estimated currawongs’ nest density changes from year to year by conducting kernel density analyses using nest locations. Maps of kernel densities were generated in ArcGIS 10.7.1 using the Kernel Density tool with nests as the input and using the default cell size and search radius (ESRI Inc. 2019). The reduced sampling effort in 2017 means the nests found in that year were not a representative sample; therefore, we only produced kernel densities for 2018, 2019 and 2020, using the 2018 search area as the reference area (i.e. we excluded additional areas searched in 2019 and/or 2020). We also conducted average nearest neighbour analyses in ArcGIS 10.7.1 using the Average Nearest Neighbour tool (ESRI Inc. 2019) to test whether there was a significant difference between the clustering of nests each year. We again included only nests found in areas searched during all 3 years to determine whether clustering was significantly different between years (2018, 2019 and 2020).
We visited the nests every 4 days during the breeding season until the nestlings had fledged or the nests had failed. Nests were observed from a distance of 10–30 m with 10 × 42 binoculars to minimise disturbance to the nesting birds. We could not observe nest contents directly because Lord Howe currawongs vigorously defend their nests and most nests were situated very high in the forest canopy (up to 20 m). The nesting status was determined by observing the adults’ behaviour at the nest (nest building, incubating, brooding, feeding nestlings) and the presence of nestlings or their begging calls.
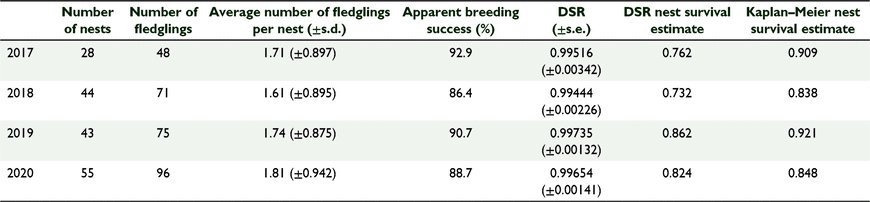
For each year, we measured nesting success for currawongs by calculating the average number of fledglings per nest and the apparent breeding success. The apparent breeding success was the number of nests that produced at least one fledgling each year divided by the total number of nests each year, expressed as a percentage. However, the apparent breeding success underestimates failure rates because not all nests are found before they fail (Mayfield 1961; Rotella et al. 2004). Consequently, we also measured nesting success for each year by calculating the daily survival rate (DSR) using the modified Mayfield method (Mayfield 1961; Rotella et al. 2004; Shaffer 2004). We calculated DSR for the nesting period in R (ver. 4.0.2) using RMARK ver. 2.2.7 (Laake 2013) and calculated the overall nest survival for each year by raising the DSR to the power of 56, the total number of days for the nesting period. We also generated Kaplan–Meier survival curves for currawong nests in each year in R using the survival and survminer packages (Kassambara et al. 2021; Therneau 2021). For pairs that re-nested because their first nest failed, we used data on the nest that we monitored for the longest. Additional nests by the same pair were not considered to be independent nesting attempts. Re-nesting currawong pairs were identified by their coloured leg bands.
Effects of the captive management on currawongs
A total of 129 currawongs were taken into captivity on the island in May 2019 (M. Shiels and F. Hulst, pers. comm.) and managed by staff from Taronga Zoo to act as insurance in the event that the wild population would decline severely due to poisoning. The remaining birds were left in the wild during the baiting programme. Before the eradication, the aim was to manage 50% of the currawong population in captivity during the baiting programme (Wilkinson and Priddel 2011), using a population estimate of 240 individuals (O’Dwyer and Carlile 2017). However, based on currawong numbers seen during this study, we now suggest that this was an underestimate of the population size of the currawongs prior to the eradication. Instead, we estimate that between 30 and 40% of the currawong population were in captivity during the baiting programme.
Of the currawongs taken into captivity during baiting, 125 were released into the wild at their capture location in September 2019. One bird was severely ill when captured and was consequently euthanised, while three further birds died in captivity. To assess the impacts of the captive management programme on the currawong population, we identified the pairs that had nested in 2018 and were subsequently taken into captivity (hereafter referred to as captive birds). We also identified those that were left in the wild during the baiting programme (hereafter referred to as wild birds). We then compared the apparent breeding success, the average number of fledglings produced and the DSR for each group after the period of captivity. The period of captive management extended into the start of the currawongs’ breeding season, so we estimated the average laying dates in 2019 for the captive and wild pairs. We also compared the occupied territories in 2018 and in 2019 to assess whether any pairs were lost during the rodent eradication.
To evaluate the effects of the rodent eradication and captive management programme on the currawongs’ condition, we captured 50–70 different birds annually (2018, 2019 and 2020) between October and December and weighed them using a digital balance. Using data from all 3 years, we first conducted a two-way ANOVA in R to test whether year, age or the interaction between year and age influenced currawong weights. To test whether the captive management period influenced currawong weights in 2019, we used a one-way ANOVA to compare weights of birds that had been in captivity for 4 months and those that had been left in the wild. The majority of birds weighed in 2019 were adults (93%); thus, age was not included as a predictor variable in the model. As birds were weighed several months after those birds in captivity had been released, colour combinations of leg bands of currawongs were used to determine whether birds had been in captivity during the baiting programme (n = 27) or had been left in the wild (n = 29). For both analyses, we visually inspected the residual plots to ensure the data were normally distributed and the variance across groups was homogeneous.
White tern breeding success
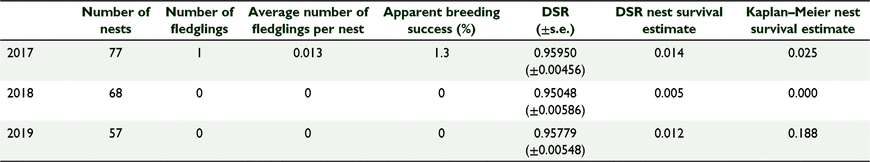
We located common white tern nests by searching the settlement area of the island, where they nest predominantly in Norfolk Island Pines (Araucaria heterophylla), during their breeding season in 2017, 2018 and 2019. We visited each nest every 4 days between October and January until the nestling had fledged or the nest had failed. Nests were observed from 5 to 20 m with 10 × 42 binoculars to minimise disturbance to the nesting birds. We measured nesting success for white terns for each year as we did for the currawongs. On some occasions, the first nest of a tern pair failed and was abandoned. For pairs that re-nested, we used data of the nest that we monitored for the longest. We assumed that a tern nest in the same location as a failed nest was a re-nesting attempt, as terns are known for their nest site fidelity (Dorward 1963; Niethammer and Patrick 1998).
Results
Currawong survival
In our population surveys during the breeding season (September–January), we recorded 228, 253, 154 and 178 currawongs in 2017, 2018, 2019 and 2020, respectively. The currawongs recorded alive in 2019 included birds that had been in captivity earlier that year and those that had remained in the wild. Currawong survival probability (ϕ) was considerably lower in the period when the baiting for rodents occurred (2018–2019) (ϕ = 0.2783, Table 1) than before the eradication (2017–2018: ϕ = 0.6140) or after the eradication (2019–2020: ϕ = 0.8429). The probability of detecting a marked currawong was high (>0.84) throughout the entire study period.

|
Currawong nesting density
We found 45 nests in 2018, 45 nests in 2019 and 61 nests in 2020. As some of these nests were re-nesting attempts (after the failure of the first nesting attempt), we included only one nest per pair (44, 43, 55 nests in 2018, 2019 and 2020, respectively) in further analyses. For comparing nesting densities across years, we only included nests within the areas that were searched during all 3 years (2018–2020; small square in Fig. 1). The kernel density maps of the currawong nest locations were similar across years, except for a cluster of nests on the south-eastern side of the island (Fig. 1). In 2018 (before the rodent eradication), there were 17 occupied territories in this part of the island, while there were only six occupied territories in 2019 (immediately after the rodent eradication) and seven occupied territories in 2020 in this area (Fig. 1). Across the entire island area that was searched during all 3 years (small square in Fig. 1), 17 territories occupied by currawong pairs in 2018 were not occupied in 2019, after the rodent eradication. In contrast, only one territory that was occupied by a currawong pair in 2019 was not occupied in 2020. The nearest neighbour analysis confirmed significant clustering of nests in the 2018 breeding season before the rodent eradication. Nests were more dispersed and less significantly clustered in 2019 and 2020 (post eradication) (Table 2).

|
Currawong breeding success
In total, 48, 71, 75 and 96 fledglings were raised by 28, 44, 43 and 55 currawong pairs in 2017, 2018, 2019 and 2020, respectively (Table 3). Despite a number of empty territories in 2019, we found a similar number of nests in 2019 than in 2018, because we searched extensively other areas of the island in 2019 that we did not search in 2018. There was no significant difference in the number of fledglings raised per nest between years (F = 0.39, P = 0.76, d.f. = 3) and the apparent breeding success was also similar across years (Table 3). Nest survival probability of currawongs was very high (>0.83) before and after the rodent eradication (Fig. 2a) and there was no significant difference in nest survival among years (χ2 = 1.9, P = 0.6, d.f. = 3) (Fig. 2a).
|
|
Effects of captive management on currawongs
Of the 125 currawongs taken into captivity for the duration of the baiting operations and released, at least 55% were adult birds, while the remainder were juveniles. Of the adult birds taken into captivity, 16 pairs had nested in 2018 before the captive period (Fig. 3b). After release from captivity, 26 pairs from captivity nested in 2019 (Fig. 3c): the remaining, previously captive adults may have bred in locations not surveyed in 2019. Six nesting pairs from 2018 that were not taken into captivity were found to be nesting in the same locations in 2019. No pairs from the Rocky Run and Boat Harbour areas of the island were taken into captivity and 70% of the territories in this area of the island were unoccupied in 2019.
The last captive currawongs were released on 24 September 2019, several weeks into their usual breeding season. As a result, the estimated average laying date for birds that had been in captivity was 15 October 2019. This date was 3 weeks later than the estimated average laying date of those breeding birds that had not been in captivity (24 September 2019) and slightly later than in 2018 (10 October 2018) and 2020 (3 October 2020). Additionally, for breeding birds that had been in captivity, the average number of fledglings per nest (1.61 ± 1.01), the apparent breeding success (83.3%) and the DSR for nests (0.99638 ± 0.00181) was lower than for those breeding birds that had remained in the wild during baiting operations (1.84 ± 0.60; 100%; 1.000 ± 0.000, respectively). However, the average number of fledglings per nest was not statistically significantly different between birds that were in captivity and those left in the wild (F = 1.007, P = 0.322, d.f. = 1).
Currawongs were significantly heavier before the rodent eradication in 2018 than those weighed after the rodent eradication in 2019 and 2020 (F = 15.811, P < 0.001, d.f. = 2, Fig. 4a). Juveniles were also significantly heavier than adults as we weighed birds during the breeding season when adults are busy with parental care duties (F = 5.278, P = 0.023, d.f. = 1). However, the interaction between year and age was not significant (F = 1.283, P = 0.280, d.f. = 2). Of the currawongs weighed during the breeding season in 2019, those birds that had not been in captivity (n = 29) were significantly heavier than those in captivity (n = 27) (F = 4.675, P = 0.0351, d.f. = 1, Fig. 4b). As most of the birds weighed in 2019 were adults, the significant difference in weight between captive and wild birds was not due to age.
White tern nesting success
We located 77, 68 and 57 common white tern nests during the 2017, 2018 and 2019 breeding seasons, respectively (111, 116 and 111 nests, respectively, including re-nesting attempts and nests not followed to completion). In these nests, we observed that 17, 5 and 10 nestlings hatched in 2017, 2018 and 2019, respectively. However, only one nestling survived until fledging in all three breeding seasons (Table 4). We assume that the other hatched nestlings were predated, abandoned or had fallen from their nest tree. The DSR for each year were similar but there was a significant difference between the nest survival curves (χ2 = 9.8, P = 0.007, d.f. = 2) (Table 4, Fig. 2b). The highest nest survival probability occurred after the rodent eradication (0.188).
Discussion
This study has shown that the baiting operations during the island-wide rodent eradication immediately affected the Lord Howe currawong population. A significant number of currawongs that were not taken into captivity for the baiting period disappeared and were not resighted after the rodent eradication in 2019 or 2020. Our analysis clearly showed that survival rates of currawongs was significantly lower when the baiting occurred (from 2018 to 2019) than in the period before (2017–2018) or after the rodent eradication (2019–2020). Furthermore, we identified 11 and 10 unoccupied nesting territories on the island’s south eastern side (Rocky Run and Boat Harbour area) in 2019 and 2020, respectively, and this resulted in an overall lower nesting density in this area after the rodent eradication. None of the birds nesting in this area in 2018 were taken into captivity during the baiting programme. As birds from those territories were not resighted in 2019 or 2020, despite considerable searching effort, we conclude that these missing birds died from primary or secondary poisoning. Moreover, 25 currawongs were found dead in the forest during and shortly after the baiting programme and necropsies of 15 of those birds showed evidence of poisoning in all those currawongs (R. Segal, N. Carlile, pers. obs.). The remaining 10 birds were too decomposed (only bones remained) to conduct necropsies. There were likely more currawong deaths but due to the terrain and density of the forest, their carcases were not found before complete decomposition.
The captive management of the currawongs clearly mitigated the adverse effects of the baiting programme on the Lord Howe currawong population. Of the 125 currawongs that were in captivity during the baiting programme, 44 and 60 bred and raised offspring successfully in 2019 and 2020, respectively. As around 45% of the captive birds were juveniles, some of these juveniles only reached maturity and started to breed in 2020. The period in captivity delayed egg laying for those birds in 2019 and breeding success was slightly lower in captive currawongs than wild birds. The lower breeding success and weights of captive versus wild birds in 2019 may indicate that there were considerable energetic costs placed on released captive birds to re-establish their territories, many of which were filled during their absence. Overall, breeding success was as high in 2019 and 2020 as prior to the rodent eradication (2017 and 2018). Furthermore, several pairs of currawongs were observed raising second broods in the 2019 breeding season (L. Brice, pers. comm.) after we had left the island. The consistently high currawong breeding success after the eradication will over time compensate for the immediate loss of individuals during the eradication.
The high breeding success after the rodent eradication suggests that currawongs did not suffer from a food shortage due to the loss of rodents from their diet. The reduced competition with rodents for other food resources, such as native fruits, invertebrates and vertebrates, may have compensated for any loss of rodent prey that the currawongs may have experienced. Details of how the composition of the currawongs’ diet has changed following the removal of rodents as prey and competitors requires further investigation. Regardless of these detailed changes in diet composition, the higher amount of food resources available to currawongs after the rodent eradication is likely to affect other aspects of the currawongs’ ecology. For example, prior to the rodent eradication, suitable breeding habitat for currawongs was limited to creek lines within the forest. This restricted habitat prevented many currawongs from breeding (Segal et al. 2021b). Based on spatial modelling before the rodent eradication, we estimated that the island can support a maximum of 84 breeding pairs (Segal et al. 2021b). Due to the difficult terrain on Lord Howe Island, the number of breeding pairs we found during each of our annual surveys was lower than the estimated carrying capacity. Increased food resources resulting from reduced competition with rodents may have the effect of upgrading marginal habitat to suitable habitat. Several species of terrestrial birds have expanded their range following rodent eradications on islands, including kaka (Nestor meridionalis) and bellbirds (Anthornis melanura) on Great Mercury Island, New Zealand, and pipits (Anthus novaeseelandiae aucklandicus) on Campbell Island, New Zealand (Bellingham et al. 2010; Towns et al. 2018). An increase in the amount of habitat suitable for breeding currawongs, in turn, would allow more currawongs to breed each year. Alternatively, increased resources after the rodent eradication may enable currawongs to breed successfully on smaller territories and thus, in higher densities (Adams 2001; Segal et al. 2021b). Many populations of invertebrate and vertebrate prey species have been found to increase on islands elsewhere following rodent eradications (Bellingham et al. 2010; Priddel et al. 2011). Regardless of the mechanisms, overall, the rodent eradication is likely to benefit the currawong population in the long-term.
Although the captive management of currawongs was a success, there was a clear bias from which locations currawongs were taken into captivity. All juveniles and some adult currawongs were captured at a site where they have been fed prior to the rodent eradication. Other adults were taken from territories if their territories could be easily accessed on foot (northern hills, Transit Hill and Intermediate Hill areas). Adults from more remote areas (Rocky Run, Boat Harbour and Erskine Valley) were not captured, due to the long transportation times from the point of capture to the captive facility. The omission of birds from these areas may have long-term implications for the genetic diversity of the island’s currawong population for two reasons. First, despite their small distribution, bird populations on small islands can host genetically distinct subpopulations, as has been recently shown to be the case in the endemic Lord Howe woodhen (Hypotaenidia sylvestris) (Major et al. 2021). In this flightless species, woodhens from Mt Gower are genetically distinct from the lowland population (Major et al. 2021). Genetic distinction at a 1 km scale is uncommon in birds, however it has been observed at a 6–10 km scale in other island bird species (white-breasted thrasher, Ramphocinclus brachyurus (Temple et al. 2006); Réunion grey white-eye, Zosterops borbonicus (Bertrand et al. 2014)). Second, the loss of a significant number of currawongs during the rodent eradication has reduced an already small population further, making this population even more susceptible to the loss of allelic diversity through genetic drift (Ballou et al. 2010). In combination, genetic drift and inbreeding can lead to small island populations becoming genetically different in relatively short time periods (Funk et al. 2016; Forsdick et al. 2017). Two island populations of black robins were found to be genetically different after only 26 years apart because they had lost different alleles during this period (Forsdick et al. 2017). Similarly, the sub-populations of the Lord Howe woodhen are genetically different because of a loss of allelic richness in the lowland subpopulation from inbreeding following a severe population bottleneck in the 1980s and subsequent genetic drift (Major et al. 2021). The loss of a significant number of currawongs combined with the location bias when currawongs were captured may have implications for the genetic health of the currawong population in the future. Further research is underway to test whether genetic diversity has been lost in the currawong population due to the rodent eradication.
The island’s common white terns have not benefitted from the rodent eradication as was expected. Although rodents were removed entirely from the island, introduced masked owls were almost completely eliminated from the island (H. Bower, pers. comm.) and the currawong population was considerably reduced (shown here), white tern nesting success remained zero after the rodent eradication. Our nest survival curves show that white tern offspring survived for longer in the nest after the rodent eradication (Fig. 2b), but regardless of this initial increased survival of offspring in the nest, we did not observe a single chick that survived until fledging in 2019. On other islands in the Pacific, white tern breeding success ranged widely, from 9% on Christmas Island to 74% in Hawai‘i (Ashmole 1968; VanderWerf 2003). On Lord Howe Island, it is possible that some ‘rogue’ currawongs were responsible for the high nest failure in white terns. On Big South Cape Island, New Zealand, a few weka (Gallirallus australis) were responsible for killing a large number of sooty shearwater (Ardenna griseus) chicks at one site, while other sites remained unaffected (Harper 2007). The loss of eggs and chicks due to poor weather, such as strong winds and heavy rain, may also be a contributing cause for the low breeding success of white terns on Lord Howe Island.
Globally, many different species of seabirds have been the greatest beneficiaries of rodent eradications on islands due to reduced predation of eggs and chicks (e.g. Lavers et al. 2010; Springer 2012; Croll et al. 2016). Rodents have the greatest impact on ground- and burrow-nesting seabirds (Jones et al. 2008; Lavers et al. 2010). For example, the nests of black-winged petrels (Pterodroma nigripennis) were heavily predated by rats on Lord Howe Island prior to the rodent eradication (T. O’Dwyer, pers. comm.). It is likely that ground-nesting seabirds have benefitted considerably more from the rodent eradication than white terns on Lord Howe Island.
This study is one of few that assessed quantitatively the ecological effects of an island-wide rodent eradication on native birds, including one terrestrial bird species, using pre- and post-eradication data (Jones et al. 2016; Segal et al. 2021a). As well as informing stakeholders and demonstrating a return on investment for funding bodies, measuring the ecological benefits and consequences of rodent eradications may contribute to the planning and management of future eradications. Results of this study highlight the importance of carefully planned and executed captive management of threatened native species and the necessity of integrating ecological monitoring as part of future rodent eradications on islands.
Data availability
The data used in this study is not currently available to other parties.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of funding
This research was funded by the Australia & Pacific Science Foundation (grant number 18/4) and Charles Sturt University. RDS was supported financially by an Australian Government Research Training Programme and an Institute for Land, Water and Society scholarship. MM was supported by a research fellowship by Charles Sturt University in 2019–2020 and a grant by the Australian Research Council (DP200101281). RDS was funded by the Lord Howe Island Board and Office of Environment and Heritage NSW for the 2017 field season. These organisations that financially supported this research were not involved in the preparation of the data or manuscript or the decision to submit for publication.
Acknowledgements
This study was conducted under Charles Sturt University animal ethics permits A17066 and A18047 and New South Wales scientific licences SL101935 and SL102102. We thank Joanne Heathcote and Terry O’Dwyer for their assistance in nest searching, trapping, banding and compilation of banding and nesting data. We also thank Bruce and Fiona Robertson, Jaclyn Pearson and Helen Fairlamb for their assistance in nest searching. We thank the following LHIB staff for their assistance during our time on the island: Hank and Sue Bower, Andrew Walsh and Darcie Bellanto. We also thank Leon Brice and Ian Fitzgerald for their support during our work on the island and for allowing access to their properties for trapping and nest searches. Michael Shiels and Dr. Frances Hulst from Taronga Zoo provided the list of captive currawongs. Thanks to Deanna Duffy and Simon McDonald (SPAN, CSU) for their assistance with spatial data analysis and two anonymous reviewers for their comments that greatly improved the paper.
References
Adams, ES (2001). Approaches to the study of territory size and shape. Annual Review of Ecology and Systematics 32, 277–303.| Approaches to the study of territory size and shape.Crossref | GoogleScholarGoogle Scholar |
Ashmole, NP (1968). Breeding and molt in the white tern (Gygis alba) on Christmas Island, Pacific Ocean. The Condor 70, 35––55.
Ballou JD, Lees C, Faust LJ, Long S, Lynch C, Bingaman Lackey L, Foose TJ (2010) Demographic and genetic management of captive populations. In ‘Wild mammals in captivity: principles and techniques for zoo management’. (Eds DG Kleiman, KV Thompson, CK Baer) pp. 219–252. (University of Chicago Press: Chicago)
Bellingham, PJ, Towns, DR, Cameron, EK, Davis, JJ, Wardle, DA, Wilmshurst, JM, and Mulder, CPH (2010). New Zealand island restoration: seabirds, predators and the importance of history. New Zealand Journal of Ecology 34, 115–136.
Bertrand, JAM, Bourgeois, YXC, Delahaie, B, Duval, T, García-Jiménez, R, Cornuault, J, Heeb, P, Milá, B, Pujol, B, and Thébaud, C (2014). Extremely reduced dispersal and gene flow in an island bird. Heredity 112, 190–196.
| Extremely reduced dispersal and gene flow in an island bird.Crossref | GoogleScholarGoogle Scholar |
Billing, J (2000). The control of introduced Rattus rattus L. on Lord Howe Island. II. The status of warfarin resistance in rats and mice. Wildlife Research 27, 659–661.
| The control of introduced Rattus rattus L. on Lord Howe Island. II. The status of warfarin resistance in rats and mice.Crossref | GoogleScholarGoogle Scholar |
Blackburn, TM, Cassey, P, Duncan, RP, Evans, KL, and Gaston, KJ (2004). Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958.
| Avian extinction and mammalian introductions on oceanic islands.Crossref | GoogleScholarGoogle Scholar | 15448269PubMed |
Bomford, M, and O’Brien, P (1995). Eradication or Control for Vertebrate Pests? Wildlife Society Bulletin 23, 249–255.
Brooke MdL (2019) Rat eradication in the Pitcairn Islands, South Pacific: a 25-year perspective. In ‘Island invasives: scaling up to meet the challenge’. (Eds CR Veitch, MN Clout, AR Martin, JC Russell, CJ West) pp. 95–99. (IUCN: Gland, Switzerland)
Brooke, MdL, Bonnaud, E, Dilley, BJ, Flint, EN, Holmes, ND, Jones, HP, Provost, P, Rocamora, G, Ryan, PG, Surman, C, and Buxton, RT (2018). Seabird population changes following mammal eradications on islands. Animal Conservation 21, 3–12.
| Seabird population changes following mammal eradications on islands.Crossref | GoogleScholarGoogle Scholar |
Buxton, R, Taylor, G, Jones, C, Lyver, POB, Moller, H, Cree, A, and Towns, D (2016). Spatio-temporal changes in density and distribution of burrow-nesting seabird colonies after rat eradication. New Zealand Journal of Ecology 40, 88–99.
| Spatio-temporal changes in density and distribution of burrow-nesting seabird colonies after rat eradication.Crossref | GoogleScholarGoogle Scholar |
Campbell, DJ, and Atkinson, IAE (1999). Effects of kiore (Rattus exulans Peale) on recruitment of indigenous coastal trees on northern offshore islands of New Zealand. Journal of the Royal Society of New Zealand 29, 265–290.
| Effects of kiore (Rattus exulans Peale) on recruitment of indigenous coastal trees on northern offshore islands of New Zealand.Crossref | GoogleScholarGoogle Scholar |
Campbell, KJ, Beek, J, Eason, CT, Glen, AS, Godwin, J, Gould, F, Holmes, ND, Howald, GR, Madden, FM, Ponder, JB, Threadgill, DW, Wegmann, AS, and Baxter, GS (2015). The next generation of rodent eradications: innovative technologies and tools to improve species specificity and increase their feasibility on islands. Biological Conservation 185, 47–58.
| The next generation of rodent eradications: innovative technologies and tools to improve species specificity and increase their feasibility on islands.Crossref | GoogleScholarGoogle Scholar |
Carlile N, Priddel D (2007) ‘Population size and distribution of the Lord Howe Currawong (Strepera graculina crissalis).’ (Department of Environment and Climate Change: Hurstville, NSW)
Carlile, N, and Priddel, D (2015). Establishment and growth of the White Tern Gygis alba population on Lord Howe Island, Australia. Marine Ornithology 43, 113–118.
Cory, F, Wilson, A, Priddel, D, Carlile, N, and Klomp, N (2011). Eradication of the House Mouse (Mus musculus) from Montague Island, New South Wales, Australia. Ecological Management & Restoration 12, 102–109.
| Eradication of the House Mouse (Mus musculus) from Montague Island, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Courchamp, F, Chapuis, J-L, and Pascal, M (2003). Mammal invaders on islands: impact, control and control impact. Biological Reviews 78, 347–383.
| Mammal invaders on islands: impact, control and control impact.Crossref | GoogleScholarGoogle Scholar | 14558589PubMed |
Croll, DA, Newton, KM, McKown, M, Holmes, N, Williams, JC, Young, HS, Buckelew, S, Wolf, CA, Howald, G, Bock, MF, Curl, JA, and Tershy, BR (2016). Passive recovery of an island bird community after rodent eradication. Biological Invasions 18, 703–715.
| Passive recovery of an island bird community after rodent eradication.Crossref | GoogleScholarGoogle Scholar |
Cromarty P, Broome K, Cox A, Empson RA, Hutchinson WM, McFadden I (2002) Eradication planning for invasive alien species on islands - the approach developed by the New Zealand Department of Conservation. In ‘Turning the tide: the eradication of invasive species’. (Eds CR Veitch, MN Clout) pp. 85–91. (IUCN: Gland, Switzerland)
Department of Environment and Climate Change (NSW) (2007) ‘Lord Howe Island Biodiversity Management Plan.’ (Department of Environment and Climate Change (NSW): Sydney, Australia)
Dorward, DF (1963). The fairy tern Gygis Alba on Ascension Island. IBIS 103b, 365–378.
| The fairy tern Gygis Alba on Ascension Island.Crossref | GoogleScholarGoogle Scholar |
ESRI Inc. (2019) ‘ArcGIS desktop 10.7.1.’ (ESRI: Redlands, CA)
Forsdick, NJ, Cubrinovska, I, Massaro, M, and Hale, ML (2017). Genetic diversity and population differentiation within and between island populations of two sympatric Petroica robins, the Chatham Island black robin and tomtit. Conservation Genetics 18, 275–285.
| Genetic diversity and population differentiation within and between island populations of two sympatric Petroica robins, the Chatham Island black robin and tomtit.Crossref | GoogleScholarGoogle Scholar |
Funk, WC, Lovich, RE, Hohenlohe, PA, Hofman, CA, Morrison, SA, Ghalambor, CK, Maldonado, JE, Rick, TC, Day, MD, Polato, NR, Fitzpatrick, SW, Coonan, TJ, Crooks, KR, Dillon, A, Garcelon, DK, King, JL, Boser, CL, Gould, N, and Andelt, WF (2016). Adaptive divergence despite strong genetic drift: genomic analysis of the evolutionary mechanisms causing genetic differentiation in the island fox (Urocyon littoralis). Molecular Ecology 25, 2176–2194.
| Adaptive divergence despite strong genetic drift: genomic analysis of the evolutionary mechanisms causing genetic differentiation in the island fox (Urocyon littoralis).Crossref | GoogleScholarGoogle Scholar | 26992010PubMed |
Gillespie, R, and Bennett, J (2017). Costs and benefits of rodent eradication on Lord Howe Island, Australia. Ecological Economics 140, 215–224.
| Costs and benefits of rodent eradication on Lord Howe Island, Australia.Crossref | GoogleScholarGoogle Scholar |
Grant, GS, Pettit, TN, and Whittow, GC (1981). Rat predation on Bonin petrel eggs on Midway Atoll. Journal of Field Ornithology 52, 336–338.
Harper, GA (2007). Detecting predation of a burrow-nesting seabird by two introduced predators, using stable isotopes, dietary analysis and experimental removals. Wildlife Research 34, 443–453.
| Detecting predation of a burrow-nesting seabird by two introduced predators, using stable isotopes, dietary analysis and experimental removals.Crossref | GoogleScholarGoogle Scholar |
Harper GA, Pahor S, Birch D (2020) The Lord Howe Island rodent eradication: lessons learnt from an inhabited island. In ‘Proceedings of the vertebrate pest conference Vol. 29’, pp. 1–11. (UC Agriculture & Natural Resources)
Hart, LA, Gane, J, Olivier, I, Downs, CT, and Brown, M (2016). A helping hand: artificial nest site provisioning increases breeding success of a tropical seabird. African Journal of Marine Science 38, 233–239.
| A helping hand: artificial nest site provisioning increases breeding success of a tropical seabird.Crossref | GoogleScholarGoogle Scholar |
Herrera-Giraldo JL, Figuerola-Hernández CE, Holmes ND, Swinnerton K, Bermúdez-Carambot EN, González-Maya JF, Gómez-Hoyos DA (2019) Survival analysis of two endemic lizard species before, during and after a rat eradication attempt on Desecheo Island, Puerto Rico. In ‘Island invasives: scaling up to meet the challenge’. (Eds CR Veitch, MN Clout, AR Martin, JC Russell, CJ West) pp. 191–195. (IUCN: Gland, Switzerland)
Higgins PJ, Davies SJJF (Eds) (1996) ‘Handbook of Australian, New Zealand and Antarctic birds: snipe to pigeons. Vol. 3.’ pp. 825–833. (Oxford University Press: Melbourne, Vic.)
Howald, G, Donlan, CJ, Gálvan, JP, Russell, JC, Parkes, J, Samaniego, A, Wang, Y, Veitch, D, Genovesi, P, Pascal, M, Saunders, A, and Tershy, B (2007). Invasive rodent eradication on islands. Conservation Biology 21, 1258–1268.
| Invasive rodent eradication on islands.Crossref | GoogleScholarGoogle Scholar | 17883491PubMed |
Hutton I (1991) ‘Birds of Lord Howe Island: past and present.’ (Ian Hutton: Coffs Harbour, NSW)
Hutton, I, Parkes, JP, and Sinclair, ARE (2007). Reassembling island ecosystems: the case of Lord Howe Island. Animal Conservation 10, 22–29.
| Reassembling island ecosystems: the case of Lord Howe Island.Crossref | GoogleScholarGoogle Scholar |
Jones, HP, Tershy, BR, Zavaleta, ES, Croll, DA, Keitt, BS, Finkelstein, ME, and Howald, GR (2008). Severity of the effects of invasive rats on seabirds: a global review. Conservation Biology 22, 16–26.
| Severity of the effects of invasive rats on seabirds: a global review.Crossref | GoogleScholarGoogle Scholar | 18254849PubMed |
Jones, HP, Holmes, ND, Butchart, SHM, Tershy, BR, Kappes, PJ, Corkery, I, Aguirre-Muñoz, A, Armstrong, DP, Bonnaud, E, Burbidge, AA, Campbell, K, Courchamp, F, Cowan, PE, Cuthbert, RJ, Ebbert, S, Genovesi, P, Howald, GR, Keitt, BS, Kress, SW, Miskelly, CM, Oppel, S, Poncet, S, Rauzon, MJ, Rocamora, G, Russell, JC, Samaniego-Herrera, A, Seddon, PJ, Spatz, DR, Towns, DR, and Croll, DA (2016). Invasive mammal eradications on islands results in substantial conservation gains. Proceedings of the National Academy of Sciences of the United States of America 113, 4033–4038.
| Invasive mammal eradications on islands results in substantial conservation gains.Crossref | GoogleScholarGoogle Scholar | 27001852PubMed |
Kassambara A, Kosinski M, Biecek P, Fabian S (2021) survminer: drawing survival curves using ‘ggplot2’. Available at https://CRAN.R-project.org/package=survminer
Laake J (2013) RMark: an r interface for analysis of capture-recapture data with MARK. AFSC Processed Report 2013-01. Alaska Fisheries Science Center, NOAA, National Marine Fisheries Service, Seattle, WA. Available at http://www.afsc.noaa.gov/Publications/ProcRpt/PR2013-01.pdf
Lavers, JL, Wilcox, C, and Donlan, JC (2010). Bird demographic responses to predator removal programs. Biological Invasions 12, 3839–3859.
| Bird demographic responses to predator removal programs.Crossref | GoogleScholarGoogle Scholar |
Lohr, MT, and Davis, RA (2018). Anticoagulant rodenticide use, non-target impacts and regulation: a case study from Australia. Science of the Total Environment 634, 1372–1384.
| Anticoagulant rodenticide use, non-target impacts and regulation: a case study from Australia.Crossref | GoogleScholarGoogle Scholar | 29710637PubMed |
Lord Howe Island Board (2017) Lord Howe Island Rodent Eradication Project – NSW species impact statement. Lord Howe Island Board, Lord Howe Island, Australia.
Major, RE, Ewart, KM, Portelli, DJ, King, A, Tsang, LR, O’Dwyer, T, Carlile, N, Haselden, C, Bower, H, Alquezar-Planas, DE, Johnson, RN, and Eldridge, MDB (2021). Islands within islands: genetic structuring at small spatial scales has implications for long-term persistence of a threatened species. Animal Conservation 24, 95–107.
| Islands within islands: genetic structuring at small spatial scales has implications for long-term persistence of a threatened species.Crossref | GoogleScholarGoogle Scholar |
Massaro, M, Starling-Windhof, A, Briskie, JV, and Martin, TE (2008). Introduced mammalian predators induce behavioural changes in parental care in an endemic New Zealand bird. PLoS ONE 3, e2331.
| Introduced mammalian predators induce behavioural changes in parental care in an endemic New Zealand bird.Crossref | GoogleScholarGoogle Scholar | 18523640PubMed |
Mayfield, HF (1961). Nesting success calculated from exposure. Wilson Bulletin 73, 255–261.
McClelland P (2002) Eradication of Pacific rats (Rattus exulans) from Whenua Hou Nature Reserve (Codfish Island), Putauhinu and Rarotoka Islands, New Zealand. In ‘Turning the tide: the eradication of invasive species’. (Eds CR Veitch, MN Clout) pp. 173–181. (IUCN: Gland, Switzerland)
Merton D, Climo G, Laboudallon V, Robert S, Mander C (2002) Alien mammal eradication and quarantine on inhabited islands on the Seychelles. In ‘Turning the tide: the eradication of invasive species.’ (Eds CR Veitch, MN Clout) pp. 182–198. (IUCN: Gland, Switzerland)
Milledge, D, Bower, H, and Carlile, N (2019). Removing a threatened apex predator from an oceanic World Heritage island: the Masked Owls of Lord Howe Island. Australian Zoologist 40, 75–91.
| Removing a threatened apex predator from an oceanic World Heritage island: the Masked Owls of Lord Howe Island.Crossref | GoogleScholarGoogle Scholar |
Niethammer KR, Patrick LB (1998) White tern (Gygis alba). In ‘The birds of North America. Vol. 371’. (Eds A Poole, F Gill). (The Birds of North America Inc.: Philadelphia, Pennsylvania)
O’Dwyer T, Carlile N (2017) ‘Draft assessment of Lord Howe currawong Strepera graculina crissalis population numbers in preparation for proposed rodent eradication program.’ (Office of Environment and Heritage: Hurstville, NSW)
Office of Environment and Heritage NSW (2020a) Pied Currawong (Lord Howe Is. subsp.). Available at https://www.environment.nsw.gov.au/threatenedspeciesapp/profile.aspx?id=10897
Office of Environment and Heritage NSW (2020b) White Tern – profile. Available at https://www.environment.nsw.gov.au/threatenedspeciesapp/profile.aspx?id=10383
Parkes J, Fisher P, Russel J, Morris K (2007) ‘Exotic rodents on Australian islands: issue paper.’ (Department of Environment and Water Resources: Canberra, ACT)
Priddel D, Carlile N, Wilkinson I, Wheeler R (2011) Eradication of exotic mammals from offshore islands in New South Wales, Australia. In ‘Island invasives: eradication and management.’ (Eds CR Veitch, MN Clout, DR Towns) pp. 337–344. (IUCN: Gland, Switzerland)
R Core Team (2020) R: a language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org
Rotella, JJ, Dinsmore, SJ, and Shaffer, TL (2004). Modelling nest-survival data: a comparison of recently developed methods that can be implemented in MARK and SAS. Animal Biodiversity and Conservation 27, 187–205.
Rueda D, Carrion V, Castaño PA, Cunninghame F, Fisher P, Hagen E, Ponder JB, Riekena CA, Sevilla C, Shield H, Will D, Campbell KJ (2019) Preventing extinctions: planning and undertaking invasive rodent eradication from Pinzon Island, Galapagos. In ‘Island invasives: scaling up to meet the challenge.’ (Eds CR Veitch, MN Clout, AR Martin, JC Russell, CJ West) pp. 51–56. (IUCN: Gland, Switzerland)
Segal, RD, Massaro, M, Carlile, N, and Whitsed, R (2021b). Small-scale species distribution model identifies restricted breeding habitat for an endemic island bird. Animal Conservation 24, 959–969.
| Small-scale species distribution model identifies restricted breeding habitat for an endemic island bird.Crossref | GoogleScholarGoogle Scholar |
Segal, RD, Whitsed, R, and Massaro, M (2021a). Review of the reporting of ecological effects of rodent eradications on Australian and New Zealand islands. Pacific Conservation Biology 28, 4–14.
| Review of the reporting of ecological effects of rodent eradications on Australian and New Zealand islands.Crossref | GoogleScholarGoogle Scholar |
Shaffer, TL (2004). A unified approach to analyzing nest success. The Auk 121, 526–540.
| A unified approach to analyzing nest success.Crossref | GoogleScholarGoogle Scholar |
Springer K (2012) Ecological restoration of sub-Antarctic Macquarie Island. In ‘Proceedings of the vertebrate pest conference. Vol. 25’, pp. 34–37. (UC Agriculture & Natural Resources)
Temple, HJ, Hoffman, JI, and Amos, W (2006). Dispersal, philopatry and intergroup relatedness: fine-scale genetic structure in the white-breasted thrasher, Ramphocinclus brachyurus. Molecular Ecology 15, 3449–3458.
| Dispersal, philopatry and intergroup relatedness: fine-scale genetic structure in the white-breasted thrasher, Ramphocinclus brachyurus.Crossref | GoogleScholarGoogle Scholar | 16968282PubMed |
Tershy, BR, Shen, K-W, Newton, KM, Holmes, ND, and Croll, DA (2015). The importance of islands for the protection of biological and linguistic diversity. BioScience 65, 592–597.
| The importance of islands for the protection of biological and linguistic diversity.Crossref | GoogleScholarGoogle Scholar |
Therneau T (2021) A package for survival analysis in R. R package version 3.3-1. Available at https://CRAN.R-project.org/package=survival
Towns, DR, and Broome, KG (2003). From small Maria to massive Campbell: forty years of rat eradications from New Zealand islands. New Zealand Journal of Zoology 30, 377–398.
| From small Maria to massive Campbell: forty years of rat eradications from New Zealand islands.Crossref | GoogleScholarGoogle Scholar |
Towns, DR, Atkinson, IAE, and Daugherty, CH (2006). Have the harmful effects of introduced rats on islands been exaggerated? Biological Invasions 8, 863–891.
| Have the harmful effects of introduced rats on islands been exaggerated?Crossref | GoogleScholarGoogle Scholar |
Towns D, Broome K, Saunders A (2018) Ecological restoration on New Zealand islands: a history of shifting scales and paradigms. In ‘Australian Island Arks: conservation, management and opportunities.’ (Eds D Moro, D Bell, SL Bryant) pp. 205–220. (CSIRO Publishing: Clayton South, Vic.)
United Nations Educational, Scientific and Cultural Organisation (2022) Lord Howe Island Group. Available at https://whc.unesco.org/en/list/186/
VanderWerf, EA (2003). Distribution, abundance, and breeding biology of White Terns on Oahu, Hawaii. The Wilson Bulletin 115, 258–262.
Walsh A, Wilson A, Bower H, McClelland P, Pearson J (2019) Winning the hearts and minds – proceeding to implementation of the Lord Howe Island rodent eradication project: a case study. In ‘Island invasives: scaling up to meet the challenge’. (Eds CR Veitch, MN Clout, AR Martin, JC Russell, CJ West) pp. 522–530. (IUCN: Gland, Switzerland)
Wilkinson IS, Priddel D (2011) Rodent eradication on Lord Howe Island: challenges posed by people, livestock and threatened endemics. In ‘Island invasives: eradication and management.’ (Eds CR Veitch, MN Clout, DR Towns) pp. 508–514. (IUCN: Gland, Switzerland)
Wood, KA (1998). Seasonal changes in diet of pied currawongs Strepera graculina at Wollongong, New South Wales. Emu - Austral Ornithology 98, 157–170.
| Seasonal changes in diet of pied currawongs Strepera graculina at Wollongong, New South Wales.Crossref | GoogleScholarGoogle Scholar |