Identifying hypotheses for drivers of decline of the bogong moth (Agrotis infusa)
Peter Caley A *
A *
A Commonwealth Scientific and Industrial Research Organisation, GPO Box 1700, Canberra, ACT 2601, Australia.
Pacific Conservation Biology 29(5) 429-444 https://doi.org/10.1071/PC22036
Submitted: 16 September 2022 Accepted: 27 November 2022 Published: 22 December 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Migratory populations of bogong moths in Australia are in decline. Numbers decreased after European settlement in the 1800s, and were stable before declining again from about 1980. Numerous hypothesised drivers for the decline have been postulated, and Caughley’s declining population paradigm provides a systematic approach to diagnosing which of these are important, and hence the knowledge to guide recovery actions.
Aims: This paper aims to assess which of the hypothesised drivers remain as candidate hypotheses for further investigation.
Methods: Within the context of known bogong moth life history and the timing of observed declines, hypothesised drivers of the two decline phases were assessed with respect to their potential impact on larval recruitment and adult survival during migration and aestivation.
Key results: Changes in vegetation composition and availability arising from the spread of pastoralism stand out as a likely driver of the early decline, with the herbivorous moth larva facing competition with introduced livestock, feral herbivores, and increased densities of native macropods. Many of the numerous postulated drivers of the most recent decline (e.g. changes in rainfall, rising temperatures in aestivation sites, increasing fire frequency) appear to have little support to be retained. Postulated drivers that remain as working hypotheses include increasing soil temperatures, increased cropping areas, and changed cropping practices and area. The effect of some drivers, such as artificial light pollution, is unclear and may warrant further investigation.
Conclusions: Inference on the drivers of bogong moth population decline is wanting.
Implications: Designed experiments are needed.
Keywords: Agrotis infusa, bogong moth, conservation, migration, monitoring, predation, recovery, survival.
Introduction
The bogong moth (Agrotis infusa) is a species of great cultural and ecological significance. As well as having totemic significance to First Nations peoples of Australia, the annual spring migration of bogong moths has become a feature on the calendar among Australian society more broadly, and is detectable on social media (Welvaert et al. 2017). Significant cultural events involving bogong moths include their springtime invasion of the newly commissioned Australian parliament house during the 1980s (McCormick 2005), and their disruption of the closing ceremony of the Sydney 2000 Olympics.
It appears that migratory populations of the bogong moth have been in decline since around 1980, following a period of a fluctuating though stable population from the mid 20th century (Green et al. 2021). If maintained, this decline will be accompanied by cascading negative impacts on species that are heavily dependent on the moths as a food source, such as the mountain pygmy possum (Burramys parvus) (Broome 2001), and alpine environments more generally that are adapted to the regular nutrient input that the annual influx of bogong moths brings (Green 2011). The post 1980s decline documented by Green et al. (2021) was followed by a very sharp decline that became evident in 2017, when numbers were the lowest recorded over 70 years of records. The recent sharp decline was attributed to the severe drought affecting eastern Australia at that time, though the underlying drivers of the medium-term decline were considered unclear Green et al. (2021). In response to the recent decline, the species has recently been added to the International Union for Conservation of Nature’s (IUCN) Red List of Threatened Species (Warrant et al. 2021).
The recent decline in the population of migrating bogong moths is seen as emblematic of systemic problems in the environmental health of the world we live in. Heightening concerns around a possible entrenched negative trajectory for bogong moth populations are reports of insects being in widespread decline across the globe (e.g. Sánchez-Bayo and Wyckhuys 2019; Wagner 2020), although the strength of this inference has been queried (e.g. Macgregor et al. 2019; Thomas et al. 2019). Indeed, a recent large study of trends in breeding North American monarch butterflies found no evidence for a ubiquitous decline (Crossley et al. 2022).
Implementing successful actions to save such a declining species requires that the causal underlying driver(s) of the decline are correctly inferred (cf. assumed) and effectively mitigated. Within conservation biology terminology, the current bogong moth situation currently sits within the ‘declining-population paradigm’ as described by Caughley (1994) that ‘focuses on ways of detecting, diagnosing and halting a population decline’. Despite their numbers being much reduced, the bogong moth currently remains abundant and widespread – far from a small population where the specific fortunes of individuals matter, and the ‘small-population paradigm’ (sensu Caughley 1994) may have currency.
The work of Caughley and Gunn (1996) is extremely useful in framing an approach to diagnosing the underlying factor(s) driving the decline of bogong moths. After observing that for the majority of historical extinctions we are either uncertain or ignorant as to the cause, they stress that successful diagnosis depends on a logical series of steps (adapted from Caughley 1994). These are:
-
Confirm that the species is presently in decline or that previously it was more widely distributed or more abundant.
-
Study the species’ natural history for knowledge of and a feel for its ecology, context and status.
-
When confident that this background knowledge is adequate to avoid silly mistakes, list all conceivable agents of decline.
-
For each agent, measure its level where the species now is and where the species used to be in time and space. For example, has a harvest increased? Does the species overlap with an introduced predator? Test one set against the other. Any contrast in the right direction identifies a putative agent of decline and thus a hypothesis to be tested. Do not assume that the answer is already provided by folk wisdom, whether lay or scientific. [my emphasis]
-
Test the hypothesis by experiment to confirm that the putative agent is causally linked to the decline, not simply associated with it. Treatments can often be used for this.
To further our understanding of the underlying drivers of bogong moth population dynamics as a precursor to developing testable hypotheses for their apparent decline, this paper seeks to address the first three steps, and partially addresses the fourth. First I review evidence for a decline during two post-European settlement phases. I then explore how many moths would have been present prior to the invasion of Europeans with the associated herbivores and land-use changes. The next section explores life history strategies of the Australian biota that utilise the inland areas considered important as larval recruitment areas for the bogong moth. I then explore the possible drivers of decline affecting larval recruitment, adult migration and adult aestivation. Two phases of putative decline are considered: the first following European settlement and the spread of pastoralism and cropping to the bogong breeding grounds, and the second during the recent 40 years (post-1980). Finally, I discuss experimental approaches to making better inference on which drivers are most important. Also considered is the importance of the putative drivers should the bogong moth population become biologically ‘small’.
History and evidence of decline of bogong moths
Early and pre-European period – Phase I decline
There are no published quantitative data on the abundance of bogong moths at the time of European settlement of Australia or for the subsequent ~150 years. We are thus constrained to inferring abundance during this early period from ethnography and the observations of the early settlers and explorers. The bogong moth was a very important source of food for First Nations people who incorporated the annual migration of bogong moths to Australia’s high country into their yearly foraging activity and culture (Flood 1973, 1980, 2010). Flood (1973) notes that ‘the ethnographic evidence suggests that in the protohistoric period their numbers were both large and reliable’. To support this, Flood (2010) notes that (albeit sparse) sources of information from the time were consistent in indicating that the number of first peoples gathering to feed on moths was large (many hundreds), the duration of their stay in the mountains feeding on moths was long (>2 months), and the number of moths immense (e.g. ‘millions’). For example, there was a report of 500 First Nations people camped on Mount Gudgenby station during the 1850’s who were most likely preparing to ascend the nearby peaks to collect moths (Flood 2010). One of the sources (Jardine 1901), reports of moths forming ‘a dark cloud’ and ravens being observed by the thousand around the rocks where millions of moths congregated (Helms 1890).
It appears certain that the abundance of bogong moths reported in these early records dwarfs anything seen in recent times. Why this would be the case is central to this paper, but bears touching on briefly now. During the mid-1800s when these observations of abundant bogong moths were made, the effect of modification of the vegetation on the inland plains by the invading European culture would have been negligible: sheep were yet to arrive in numbers, and the typical accounts of early explorers were that kangaroos were rare (Caughley et al. 1980) and ground vegetation suitable for pastoralism plentiful (except in times of drought).
Recent (post ~1980) times – Phase II decline
There are little published data on long-term trends of bogong moth numbers, either in their breeding grounds or during aestivation in the Australian High Country. The earliest published data comes from Common (1954), who measured the area of aestivating moths in a single boulder-formed cave on Mount Gingera during the summers of 1951/52 and 1952/53, and undertook light trapping over the period 1951–53 in Canberra. Green et al. (2021) collated and synthesised as far as practical, observations of bogong moth numbers from disparate sources and locations across the Australian Alps over the period 1951–2020. They documented an apparent stability in numbers from 1951 up until c.1980, followed by variable though declining numbers, with a severe crash in numbers in response to the 2017–19 severe drought in south-eastern Australia. The inference of stability over the period 1951–80 was based on the lack of comment of a declining trend by Common (1981) despite annual visits to the main aestivation sites on Mount Gingera, rather than quantitative data as such. Over the period 1994–2010, a standardised light-trapping index of abundance of bogong moths during spring at Charlotte Pass and Mount Blue Cow was highly variable, but showed no apparent trend over this reasonably short period (Gibson et al. 2018). Likewise, Gregg et al. (1993) provided detailed information on captures of bogong moths in tower-mounted light traps from Mount Dowe (within the Nandewar Ranges in northwest New South Wales) and Point Lookout (on the crest of the Great Dividing Range in the New England Tablelands) during the period 1985–90. Again, no trend was apparent in this short window of time, given the high year-to-year variation. Furthermore, the correlation between the two sites is low (r = −0.25), suggesting the population dynamics of the locations being sampling are not synchronised.
Most recently, there were no bogong moths aestivating in the northern part of their aestivation range (Mt Gingera, Mt Morgan, Bogong Peaks) during the 2021/22 summer, and only small populations in a restricted number of locations high on the Main Range (Ken Green, pers. comm.). These low numbers were lower than the preceding 2020/21 season, and occurred despite an expected rebound in numbers (admittedly from a very low base) arising from the onset of La Niña conditions in the putative breeding grounds during early 2020. Either we do not fully understand the migration behaviour (e.g. migration to may be triggered by temperature and/or food conditions not exceeded during the cool and wet La Niña conditions) or the recent severe decline has become entrenched.
Life-history strategies for surviving Australia’s harsh inland environment
General
Australia, and its inland areas in particular, are subject to highly variable rainfall (Robertson et al. 1987; Morton et al. 2011). This variability occurs over both short (within season) and longer (yearly to decadal) time scales, driven by phenomena such as El Niño and La Niña events in the Pacific Ocean, and the Indian Ocean Dipole (Ashok et al. 2003). Drought conditions may persist over multiple years. Summers in inland regions are typically very hot and dry. Away from the refuges of tree-lined watercourses, the plains are particularly inhospitable, although they may be highly productive following rains. The Australian biota, large and small, has evolved many strategies to cope with the extreme climate variation. Despite these adaptive responses to cope with environmental variation, the population dynamics of much of the biota is still characterised by ‘booms’ and ‘busts’ that largely follow the cycles of La Niña and El Niño-driven rainfall patterns (Morton 2022). Some examples follow for species that utilise areas of inland Australia similar to those preferred by the bogong moth for breeding.
Waterbirds
Australian waterbird species, such as the grey teal (Anas gracilis) are highly mobile, noted for their nomadism, and able to utilise shallow ephemeral wetlands across the inland (Roshier 2009). They can detect far away rainfall events, and may travel during good times to learn about the spatial layout of their environment, and use this to develop internal maps of wetland locations (Roshier 2009). This strategy seems to be working, with numbers estimated in the millions and little evidence they are undergoing long-term decline after correcting for seasonal conditions (Caley et al. 2022). Compared to bogong moths, waterbird species have the advantage of much longer life spans, such that older birds may play an important role navigating to faraway resources (Reid 2009), with the mobility to utilise favourable conditions virtually anywhere in the eastern half of Australia. During prolonged times of good conditions, waterbirds can also capitalise by having multiple clutches.
Small mammals
Species of small mammals that share the bogong moth breeding range have experienced variable fates. The long-haired rat (Rattus villosissimus) preferentially feeds on green food – grass, herbs and the roots of ephemeral plants (Morton 2022). It survives inter-boom periods in hard-to-detect refuges, with eruptive dynamics following some (but not all) wet years. Their generation interval is short enough to result in multiple generations within a boom period. However, they appear to have suffered a major range contraction, particularly from the eastern part of their former range that overlaps with bogong moth breeding grounds (Bonyhardy 2019). Indeed, species of small native rodents within the ‘critical weight range’ (35–5500 g) have fared particularly poorly, with many now extinct from the region (Burbidge and McKenzie 1989). Habitat modification through increased grazing pressure was a major driver of decline long before the introduced predators arrived (Bonyhardy 2019), with predation by the introduced red fox Vulpes vulpes, supported by a thriving European rabbit Oryctolagous cuniculus population, probably the final nail in the coffin for many. In contrast, the self-introduced, largely granivorous house mouse (Mus musculus) has adapted well to the cereal cropping regions of south-eastern Australia where populations show aperiodic outbreaks [termed ‘irruptions’ by Leopold (1943)] over large areas, that are often triggered by antecedent rainfall conditions (Singleton et al. 2005).
Large mammals
Several large herbivorous mammals maintain a high abundance in inland areas despite a largely sedentary existence, of which the red kangaroo (Osphranter rufus) is arguably the most highly adapted and widespread species. Euros (Osphranter robustus) exhibit behavioural thermoregulation (e.g. retreating to rock crevices in the heat of the day). Though originally restricted somewhat by the availability of rocky outcrops, with the provision of water they can now simply use the shade of vegetation. Wild pigs (Sus scrofa), although locally abundant, are constrained in their ability to utilise the pastures of inland Australia by access to shade and water (Choquenot and Ruscoe 2003).
Amphibians
Amphibians from the semi-arid regions of the bogong breeding range are remarkable in their ability to survive extended dry periods. For example, painted burrowing frogs (Neobatrachus sudellae) survive multi-year dry periods by aestivating deep underground within cocoon structures (Withers 1995).
Insects
Insects commonly use diapause to avoid hot, dry summers and drought periods. For example, eggs of the Australian Plague Locust (Chortoicetes terminifera Walker) are capable of surviving several months of drought conditions (Wardhaugh 1986).
Bogong moths
Bogong moths have evolved a different strategy to exploit the resources of the inland plains. Following Common (1954), during autumn adult moths breed and lay their eggs focusing on the dicotyledonous annual plants. Despite records of breeding elsewhere, inland regions of south-eastern Australia that possess grey/black self-mulching cracking clays are considered to be the preferred breeding grounds (Common 1954). It is thought that the cracks and/or friable nature of these soils provide refuge for the larval stage (common cutworm), allowing them to shelter in the soil during the day, emerge at night and chew off a young plant at ground level (hence their name), before consuming the material from the safety of the soil (Zborowski and Edwards 2007). After the completion of several larval stages, pupation occurs during early springtime after which the majority of the newly hatched adult moths from this generation depart from the plains before conditions become too harsh, and begin a migration to aestivation sites in the higher parts of the Australian Alps. No aestivating populations have been reported elsewhere on the mainland.
It is believed that adult bogong moths cannot withstand the summer hot temperatures typically experienced on the inland plains, unlike other sympatric species such as Helicoverpa armigera (Bawa et al. 2021), suggesting that rising temperatures are the trigger that initiates migration from the breeding areas, and additionally drives them upwards to higher elevations after arrival at lower elevations in the mountains. Once in the Australian Alps they congregate in rock crevices and aestivate, save for semi-regular flights during the evening, of unknown purpose. During aestivation they are subject to facultative diapause. From around February onwards, moths start the return journey to their breeding grounds and the cycle repeats. This migratory behaviour is unique to the insect world of the southern hemisphere. The migration is highly directed, using visual landmarks, the night sky, and the Earth’s magnetic field (Dreyer et al. 2018). In the northern hemisphere, the North American monarch butterfly (Danaus plexippus) is the only other moth or butterfly whose migratory behaviour is at a similar scale (Warrant et al. 2016).
Bogong moths are long-lived by moth standards (~8 months), but short-lived compared to many of the biota utilising the inland. They must attempt to breed each year regardless of conditions – with no option of either waiting out the drought in situ (as is the case for red kangaroos), or elsewhere (e.g. coastal wetlands in case of waterbirds), or in local refugia (e.g. underground in the case of some amphibians and reptiles), or in a state of diapause within their breeding range (as many insects do). Neither can they move any meaningful distance during the larval stage to track spatiotemporal changes in resource availability. In comparison to mammals and birds, and in their favour, is potentially much higher annual fecundity, with females reportedly capable of laying up to 2000 eggs [Common (1981) cited within Warrant et al. (2016)]. Their reproductive strategy is largely r-selected, though with a twist of having migratory behaviour that avoids having to survive the harsh inland summers.
Exploring/diagnosing the causative/proximal factors driving population decline
Influences on larval recruitment in breeding grounds
Reduction in autumn rainfall
The breeding cycle is thought to rely heavily on autumn rainfall producing a pre-winter flush of palatable vegetation (particularly dicotyledonous annuals) for the early stages of the moth larva to feed on. The larvae also find seedlings of many monocotyledonous plants palatable. There is, however, little obvious evidence that trends in either autumn (Fig. 1) or winter (Fig. 2) rainfall in south-eastern Australia per se has changed sufficiently to cause the observed longer-term changes in bogong moth abundance (although see interaction with temperature below). This is confirmed by applying the Mann–Kendall trend test to 10-year block-bootstrapped (to remove serial dependence) samples of these time series (i.e. no significant trend detected).
|
|
|
|
Increasing heat stress
As mentioned previously, bogong moth adults are susceptible to high temperatures, although the effect of high temperatures on their larva is largely unknown. Rising temperatures arising from increasing atmospheric CO2 concentration were comparatively small during the 19th century, suggesting little or no role to play in the Phase I decline. However, both the mean and maximum temperatures having been steadily rising from around the beginning of the Phase II decline (Fig. 3). Rising temperatures can reduce available soil moisture for a given level of rainfall (Cai et al. 2009), thus potentially impacting on vegetation growth and larval survival.
Predation
Wild (feral) pigs
Wild pigs are capable of predating on cutworms and pupae, and now occur throughout the breeding range. The expected low density of larvae (Green 2008) may not preclude pigs targeting them using their highly developed sense of smell. Indeed, wild pigs are observed to make the effort to dig for native budworm pupae (Helicoverpa punctigera) within cropping systems (Tony Lockrey, pers. comm.). Wild pigs have been present within the breeding range for many years, and although they were still expanding their range in the 1970s (Hone and Waithman 1979), they had colonised most of the black-soil habitats by then (Pullar 1950). The colonisation of wild pigs was thus too late to have contributed to the Phase I decline, and largely complete before the Phase II decline.
Mice (Mus musculus)
Through the provision of refuge and reduced disturbance to burrows, the adoption of conservation agriculture farming practices appears to increase the density of mice within crops (Ruscoe et al. 2022), whereas under traditional cultivation methods, mice breeding was restricted mainly to the edges of crops (Singleton 1989). This could result in increased levels of predation on cutworm larva and/or pupae, as mice are known to predate on native budworms. The uptake of conservation tillage practices started in 1990, with near complete uptake by 2019, coinciding with the Phase II decline.
Changed fire regimes
It is argued (see Mansergh et al. 2022) that the cessation of Traditional Owner land management, and in particular their deliberate firing of grasslands, was an important component of the habitat modification that caused the demise of the critical weight range mammal species prior to the arrival of exotic predators. A postulated beneficial effect of the firing was the promotion of murnong (Yam daisies, Microseris spp.) that were more palatable to bogong larva than the grasses that largely replaced them under heavy grazing pressure (Mansergh et al. 2022).
Competition with herbivores
Functionally, bogong moth larvae are simply small herbivores, and hence compete for palatable vegetation with other herbivores both small and large. Since European settlement there have been major changes to grazing pressure across the entirety of the moth breeding grounds, and the impact of large herbivores on the larva can be reasoned to occur on multiple fronts. First, the extent and intensity of grazing pressure has increased greatly through the provision of additional watering points for domestic stock (Fig. 4). This has enabled both domestic stock and native herbivores to continue grazing in areas where formerly a natural shortage of water would have caused either their departure or demise. Second, across most of the bogong breeding grounds, the cessation of indigenous hunting by First Nations peoples and the removal of dingoes (Canis familiaris dingo) has released macropod populations from predation, and they now occur at much higher (5–10-fold) densities (Caughley et al. 1980). Third, the dominant vegetation remaining under intensive grazing pressure will be less palatable to bogong moth larvae (who prefer the more palatable dicotyledonous annuals), as the introduction of sheep was followed by the near-removal of such highly palatable species (Caughley 1987a). Being forced to eat less palatable vegetation has potentially impacted on bogong moth larvae more than the larger herbivores, due to the latter possessing performance enhancing digestive features such as grinding teeth and large-scale gut fermentation. Pastoralism had started in earnest across most of the breeding grounds by the mid-1800s, with peak population irruptions, associated with large-scale and severe vegetation damage apparent by the time of the Federation Drought around 1900 (Caughley 1987a). The timing fits with the Phase I decline.
The extent to which herbivores can reduce available vegetation can be illustrated by applying the interactive plant–herbivore model of Caughley (1987b) to the vegetation of the Menindee Lakes region within Kinchega National Park over the period 1998–2020. Despite the highly variable rainfall over this period (Fig. 5), in the hypothetical absence of large herbivores, a rainfall–vegetation only model effectively smooths out much of the variation (Fig. 6). The addition of a herbivore to the system (in this case red kangaroos) changes the available vegetation biomass considerably – the peaks in vegetation biomass are attenuated somewhat, but the troughs are deepened considerably (Fig. 6). The modelled red kangaroo population decreases nearly 10-fold from a 2017 peak following the Indian Dipole-induced wet winter of 2016 (Fig. 6). The effect of kangaroos on vegetation during drought is striking: in their absence, vegetation biomass rarely drops below 200 kg ha−2, whereas in their presence the biomass can effectively hit zero during drought (Fig. 6), when many plants persist in the landscape as seeds alone. The most recent drought (2017–19) was particularly severe, with estimated vegetation biomass near zero during 2018 and 2019, prior to the drought breaking in 2020 with the onset of La Niña conditions (Fig. 6).
|
|
|
|
There is also evidence that competition from large herbivores may be increasing on a number of fronts. There has been an ongoing increase in the distribution of farm dams (Malerba et al. 2021) that has enabled livestock to graze more widely and intensively (Landsberg et al. 2003). The impact of domestic livestock on vegetation is visible from space (Fig. 7). Increased competition with herbivores has thus been occurring during both phases of decline. Potentially offsetting this ongoing intensification in grazing is the reversion of pastoral lands to conservation use. Recent examples include the Paroo-Darling National Park (178 053 ha) and Narriearra Caryapundy Swamp National Park (153 415 ha). These parks encompass considerable areas of the grey/black soil areas (although a very small percentage of the total black-soil area). Being ‘inside’ the dingo fence and hence largely dingo-free due to ongoing suppression of dingo populations, they represent a ‘laissez-faire’ plant–herbivore system (Caughley 1987b), as described previously.
Changing dryland cropping systems
Overlay and scale
The major cropping areas of south-eastern Australia lie within the moth breeding grounds. From the mid-1860s and up until the early 2000s, the area of dryland cropping has increased at an annual rate of 3.2% (Angus and Good 2004).
Pest status
Although Common (1954) noted the preference of bogong moth larvae to feed on dicotyledonous annuals, the range of crops recorded as suffering from cutworm damage is broad, including monocotyledonous species (Hassan 1977). The degree to which broadscale crops are suitable for bogong moths can be gleaned from their reported pest status (Table 1). Being a summer crop makes cotton less suitable as a host than one would first expect. Summer pastures are also considered a poor host. A common theme is that bogong moth larvae are often feeding on the weeds (if present) within the crop.
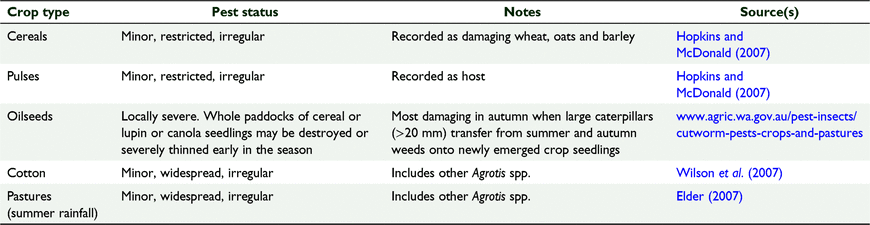
|
It appears that damage can be more severe than indicated in Table 1. For example, during 2014 there were widespread reports of cutworm damage over a 1000 km long band of the cropping region from the Central West Slopes and Plains district of New South Wales to southwest Victoria (see https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2015/02/serial-pests-wrap-up). The damage was recorded from May until July and included extensive damage to newly emerged canola and cereal crops, with near total crop destruction in some instances. It was considered almost certain that these cutworms were A. infusa, and it preceded a good season for aestivating bogong moth numbers on Mount Gingera as reported by Caley and Welvaert (2018). There were independent reports corroborating the extensive nature of the infestation and damage that cutworms caused to seedling canola crops in particular, over a large region from southern to central western New South Wales (www.abc.net.au/news/rural/2014-05-30/cutworms/5489922). Paddocks that had been rotated out of pasture or containing weedy areas were considered particularly at risk, and included a range of soil types, although areas of heavier soils were more heavily impacted. Curiously, reports of cutworm damage in the autumn/winter of 2015 were near non-existent yet the number of aestivating moths in the mountains the following summer was similar (Caley and Welvaert 2018), pointing to uncertainty in the contribution of cropping areas to overall bogong moth numbers.
Intensification
Until the 1980s, almost all Australian dryland crops were cereals grown in rotation with annual pastures and fallows, in conjunction with running sheep. This has become much less common, with continual cropping (including crop rotation with crops such as canola) much more prevalent.
Insecticidal use
Bogong moth larvae can be effectively controlled using insecticides where they are causing damage to crops. In addition, where they are present, although not in numbers sufficient to cause significant damage, they may become collateral damage of insecticide applications targeting other pests (e.g. Helicoverpa spp.). The treatment of seeds with systemic insecticides (e.g. neonicotinoids), mainly targeted at aphids attacking seedlings, would also be effective for up to several weeks post germination in killing young cutworms feeding on the seedling. Neonicotinoids were first used in Australia in 1994 and are now used widely (Maino et al. 2021).
In-crop tillage changes
Conservation agriculture (including ‘minimum-’, ‘zero-’ or ‘no-till’) was developed as a method of improving soil structure through retained stubble (cf. burning), soil productivity, and to minimise soil erosion. Uptake started around 1990, and grew rapidly such that by 2019 it accounted for greater than 90% of dryland cropping in south-eastern Australia (Rochecouste et al. 2022). It is plausible that fine soil tillage (as practised prior to the adoption of conservation agriculture) could have provided suitable habitat for bogong moth larvae beyond the self-mulching clay soil type, by providing a loose soil that the larvae could burrow into.
In-crop weed management
Controlling the ‘green bridge’ between successive crops has become central to the management of pests (including cutworms) and diseases. The conservation agriculture systems rely heavily on the use of herbicides to manage in-crop weeds (cf. controlling by mechanical cultivation), and modern spraying equipment has enabled this to be achieved at broad scales. Under this cropping system, the control of in-crop green material over the late summer period and into autumn (when bogong moths are returning to laying eggs) is near complete. Herbicide resistance is an issue, although the species involved are mainly grasses (e.g. rye grass), and of limited palatability to the bogong larva.
Paddock amalgamation
There has been considerable rationalisation of the cropping industry. The amalgamation of properties, often involving the merging of paddocks has reduced the extent of intercrop vegetation (including broadleaf weed species), enabling the use of larger and more efficient farming equipment. The move away from running sheep has resulted in less need for fencing, and hence less intercrop fence line habitat.
Increase in irrigated agriculture
The area of preferred breeding habitat used for irrigated agriculture has steadily increased during Phase II of the decline, particularly cotton on black-soil habitats. The timing (summer) and intensive control of weeds within irrigated cotton renders them unsuitable for cutworm development, however the percentage of these preferred habitats used for cotton remains in single digits (Green et al. 2021).
Summary of potential drivers of changes in larval recruitment
The initial invasion of introduced herbivores and predator-release of native herbivores occurred across the entire breeding range, with temporal overlap with both the Phase I and Phase II declines. The change in methods and intensification of cropping systems has occurred largely since the 1980s, coinciding with Phase II of the decline. Although this modern-day crop management appears to coincide with reducing infestations of bogong moths, this coincidence could also result from bogong moth numbers being reduced by other unrelated factors.
Discussion and conclusion
This study has attempted to address the first four of five steps outlined by Caughley and Gunn (1996) as being needed to correctly diagnose and reverse the decline of bogong moth populations. The first step in the process, namely confirming that the species is in decline or was once more widely distributed, revealed a lack of data from the early period following the arrival of Europeans (early 19th century); hence the reliance on inference from ethnography and the observations of early settlers and explorers. More recent data on changes in the abundance of aestivating adults is becoming more quantitative and the apparent trend more alarming.
Examination of the life-history strategies of other animal groups that utilise similar habitats is revealing. Indeed, there are similarities between the predicament of the bogong moths with that of the small herbivorous marsupials that were driven to extinction during the late 19th century. Most of these were dependent on the ground-level vegetation for food and shelter. The addition of introduced predators, particularly the red fox, supported largely by a burgeoning European rabbit population, was also no doubt an important driver. With respect to the extinctions of small mammal species from the inland plains, Caughley (1987a, p. 4) noted:
The wallabies, bandicoots, dasyures and rodents that disappeared were all small animals that lived on the ground and were dependent on the thin layer of herbs and grasses for cover. The main cause of the extinctions seems to have been modification of the habitat by the sheep and cattle, …
The identified candidate list of hypothesised drivers considered here is probably by no means exhaustive, and should be updated as new knowledge comes to hand. Such knowledge could take different forms, but preferably include deductive reasoning as to why an avenue of research should be pursued. This is important. Steps 4 and 5 outlined by Caughley and Gunn (1996) are essentially the hypothetico–deductive (H–D) method of scientific learning championed by Popper (1962). Wildlife science, however, often goes no further than the method of retroduction, whereby an explanation (hypothesis) is generated for a repeatable phenomenon: the problem is that there are often multiple competing explanations (Romesburg 1981). Ultimately, we would like to identify the underlying causal mechanism(s) driving the decline, however, there is also no doubt that the underlying processes that are generating bogong moth abundance may be complex or not easily observable. Cox and Wermuth (2004) stress the importance of appreciating the possibility of effect modifiers, be they intermediate variables, background variables, or unobserved confounders when trying to infer causality.
There are also hypothesised drivers that are expected to be so infrequent as to be considered unimportant within the current management horizon (e.g. asteroid collisions), although one of particular relevance to the bogong moth is the reversal of the Earth’s magnetic poles, given their reliance on the magnetic field for navigation (Dreyer et al. 2018). The last reversal occurred ~41 000 years ago (Cooper et al. 2021).
The current parlous state of the bogong moth population was predicted nearly 50 years ago by Flood (1973) who wrote (p. 91) ‘It begins to look as if the coming of the European is proving as disastrous for the moths as for the men in the Monaro’; where the ‘men in the Monaro’ refer to the First Nations Peoples of the Southern Tablelands region of New South Wales. The dramatic changes wrought on the vegetation composition of the western plains and other pastoral regions following European settlement remain ‘standing’ as a hypothesis central to the Phase I decline. The logic and evidence for competition from herbivores, and changed vegetation composition, is strong. Some of the drivers that I have dismissed as playing a role in the Phase I decline include predation of adults by introduced species such as wild pigs, rising temperatures, and light pollution. Healthy bogong moth populations have clearly withstood what appears to have been considerable human predation for many millennia. That is not to say that these drivers would not become important should the bogong moth population reduce such that the ‘small-population paradigm’ becomes relevant. Once a population becomes small, all manner of sources of reduced fecundity and increased mortality become potentially important, as well as potential management distraction(s). It remains important, however, to identify what driver(s) made the population small in the first place, as the first order management response is to ameliorate the effect of these drivers.
Regarding Phase II of the decline, and looking to the future, the underlying components of the putative drivers of decline are not easily obtainable with the available information, hence considerable uncertainties remain within Step 4 of the approach of Caughley and Gunn (1996). This area needs effort. For example, it is not clear whether the increase in the area under dryland cropping is of benefit or detriment to bogong moth recruitment, as the effect of changing cropping practices (e.g. reduced tillage, increased herbicide use) are unclear. Similarly, for changes in the type and application of insecticides, both the impact and usage statistics are hard to obtain. The complexity of estimating the use of neonicotinoids is a case in point (see Maino et al. 2021).
Testing the various remaining hypotheses will require methods for monitoring the different life cycle phases. In particular, there is a need to develop methods for monitoring the bogong moth recruitment from different locations with sufficient precision to make useful inferences on the effects of the different drivers at play. Ground-level light traps can be configured to target moths moving within the local vicinity (cf. undertaking migratory flight). In the context of investigating recruitment, light trapping has inherent challenges including (1) seasonal variation in the timing of moth emergence; (2) catch-rate strongly influenced by weather conditions (temperature, wind and lunar phase), (3) uncertain provenance as to the spatial location from which the captured moths have emerged, and (4) trap specificity (a risk of traps being overwhelmed with non-target species). A specific (e.g. pheromone-based) trap would be useful in overcoming the last issue only. Sifting soil for larvae (see Green 2008) has a number of advantages from an inferential viewpoint. These include a lesser risk of mistiming the sampling, no confounding by weather, and known provenance of larvae detected. Knowing the exact location of detected pupae (give or take limited movement during the cutworm life stage) should enable fine-scale preferences to be elucidated. The major drawback of this approach is the logistics of sieving large volumes of soil, and the inability to distinguish A. infusa from the other members of the genus based on morphology alone. The logistical challenges of soil-sifting can be addressed to some extent by mechanisation (e.g. small portable mechanical diggers and mechanical sifting machines), and the identification challenge solved with appropriate genetic probes (given the moderate numbers of individuals involved this should not be prohibitively expensive).
Critically testing the influence of putative land-use drivers will require the use of experimental design. For estimating the effects of predation by wild pigs on larval recruitment, the appropriate treatment would be exclosure plots versus control plots (e.g. Mitchell et al. 2007). The expected intrinsic plot-to-plot variation will be high owing to the expected vagaries of moth oviposition, in addition to high year-to-year variation arising from variation in the population of adults. Sufficient replication will overcome plot-to-plot variation, while design (e.g. paired plots) and appropriate analysis (e.g. use of random effects and/or state-space models) can help deal with year-to-year variation. Should the impacts of ALAN be deemed potentially important, quantifying its effect should be possible from the sampling and survey of households. The approach would be to build a sampling frame of houses associated with the migration path (using stratification if necessary). From a sample of houses from this frame, spring surveys of dead moths around the exterior would be undertaken, and a standard design-based estimator (e.g. Horvitz–Thompson) used to estimate the number of moths waylaid during migration for all houses.
The bogong moth is not yet lost, and there are possibly actions we could take to avoid its ongoing demise. This study has not completed the fifth step in the diagnosis of decline as outlined by Caughley and Gunn (1996), and critical experimentation is needed to ensure that the underlying reason(s) for the decline are correctly identified. Conserving a species in decline would not progress without this knowledge – noted by Caughley and Gunn (1996) as a self-evident fact that is often lost in a crisis.
Data availability
All data used in this study is publicly available and can be accessed through links provided within the text or in the reference list.
Conflicts of interest
The author has no conflicts of interest to declare.
Declaration of funding
This research did not receive any specific funding. Support for writing was provided by the CSIRO.
Acknowledgements
The author acknowledges the First Nation’s people of the land on which they live and work, for whom the bogong moth holds special significance – the Ngunnawal and Ngambri people of the Canberra region. Peter Gregg participated in useful discussions. The comments and ideas of Jim Hone, Eric Warrant and an anonymous referee greatly improved the manuscript.
References
Angus JF, Good AJ (2004) Chapter 10: Dryland cropping in Australia. In ‘Challenges and strategies of dryland agriculture’. (Eds SC Rao, J Ryan) pp. 151–166. (Scientific Publishers: Jodhpur, India)Ashok, K, Guan, Z, and Yamagata, T (2003). Influence of the Indian Ocean Dipole on the Australian winter rainfall. Geophysical Research Letters 30, 1821.
| Influence of the Indian Ocean Dipole on the Australian winter rainfall.Crossref | GoogleScholarGoogle Scholar |
Bawa, SA, Gregg, PC, Del Soccoro, AP, Miller, C, and Andrew, NR (2021). Estimating the differences in critical thermal maximum and metabolic rate of Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) across life stages. PeerJ 9, e12479.
| Estimating the differences in critical thermal maximum and metabolic rate of Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) across life stages.Crossref | GoogleScholarGoogle Scholar |
Birtchnell MJ, Gibson M (2008) Flowering ecology of honey-producing flora in south-east Australia. Rural Industries Research and Development Corporation, Canberra.
Bonyhardy T (2019) ‘The enchantment of the long-haired rat: a rodent history of Australia.’ (Text Publishing Company: Melbourne)
Broome, LS (2001). Density, home range, seasonal movements and habitat use of the mountain pygmy-possum Burramys parvus (Marsupialia: Burramyidae) at Mount Blue Cow, Kosciuszko National Park. Austral Ecology 26, 275–292.
| Density, home range, seasonal movements and habitat use of the mountain pygmy-possum Burramys parvus (Marsupialia: Burramyidae) at Mount Blue Cow, Kosciuszko National Park.Crossref | GoogleScholarGoogle Scholar |
Burbidge, AA, and McKenzie, NL (1989). Patterns in the modern decline of western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50, 143–198.
| Patterns in the modern decline of western Australia’s vertebrate fauna: causes and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Cai, W, Cowan, T, Briggs, P, and Raupach, M (2009). Rising temperature depletes soil moisture and exacerbates severe drought conditions across southeast Australia. Geophysical Research Letters 36, L21709.
| Rising temperature depletes soil moisture and exacerbates severe drought conditions across southeast Australia.Crossref | GoogleScholarGoogle Scholar |
Caley, P, and Welvaert, M (2018). Aestivation dynamics of bogong moths (Agrotis infusa) in the Australian Alps and predation by wild pigs (Sus scrofa). Pacific Conservation Biology 24, 178–182.
| Aestivation dynamics of bogong moths (Agrotis infusa) in the Australian Alps and predation by wild pigs (Sus scrofa).Crossref | GoogleScholarGoogle Scholar |
Caley, P, Reid, JRW, Colloff, MJ, and Barry, SC (2022). On inferring population trends of mobile waterbirds from aerial transect surveys in variable environments. Environmental and Ecological Statistics 29, 3–31.
| On inferring population trends of mobile waterbirds from aerial transect surveys in variable environments.Crossref | GoogleScholarGoogle Scholar |
Caughley G (1987a) Chapter 1: Introduction to the sheep rangelands. In ‘Kangaroos: their ecology and management in the sheep rangelands of Australia’. (Eds G Caughley, N Shepherd, J Short) pp. 1–13. (Cambridge University Press: Cambridge)
Caughley G (1987b) Chapter 10: Ecological relationships. In ‘Kangaroos: their ecology and management in the sheep rangelands of Australia’. (Eds G Caughley, N Shepherd, J Short) pp. 159–187. (Cambridge University Press: Cambridge)
Caughley, G (1994). Directions in conservation biology. Journal of Animal Ecology 63, 215–244.
| Directions in conservation biology.Crossref | GoogleScholarGoogle Scholar |
Caughley G, Gunn A (1996) ‘Conservation biology in theory and practice.’ (Blackwell Science: Cambridge)
Caughley, G, Grigg, GC, Caughley, J, and Hill, GJE (1980). Does dingo predation control the densities of kangaroos and emus? Wildlife Research 7, 1–12.
| Does dingo predation control the densities of kangaroos and emus?Crossref | GoogleScholarGoogle Scholar |
Choquenot, D, and Ruscoe, WA (2003). Landscape complementation and food limitation of large herbivores: habitat-related constraints on the foraging efficiency of wild pigs. Journal of Animal Ecology 72, 14–26.
| Landscape complementation and food limitation of large herbivores: habitat-related constraints on the foraging efficiency of wild pigs.Crossref | GoogleScholarGoogle Scholar |
Clarke M, Le Feuvre D (2021) Size and scope of the Australian honey bee and pollination industry — a snapshot. AgriFutures Australia, Canberra.
Common, IFB (1954). A study of the ecology of the adult bogong moth Agrotis infusa (Boisd) (Lepidoptera: Noctuidae), with special reference to its behaviour during migration and aestivation. Australian Journal of Zoology 2, 223–263.
| A study of the ecology of the adult bogong moth Agrotis infusa (Boisd) (Lepidoptera: Noctuidae), with special reference to its behaviour during migration and aestivation.Crossref | GoogleScholarGoogle Scholar |
Common, IFB (1981). The bogong moth. Bogong 2, 4–5.
Common IFB (1990) ‘Moths of Australia.’ (Melbourne University Press: Collingwood, Vic.)
Cooper, A, Turney, CSM, Palmer, J, Hogg, A, McGlone, M, Wilmshurst, J, et al. (2021). A global environmental crisis 42,000 years ago. Science 371, 811–818.
| A global environmental crisis 42,000 years ago.Crossref | GoogleScholarGoogle Scholar |
Cox, DR, and Wermuth, N (2004). Causality: a statistical view. International Statistical Review 72, 285–305.
| Causality: a statistical view.Crossref | GoogleScholarGoogle Scholar |
Crossley, MS, Meehan, TD, Moran, MD, Glassberg, J, Snyder, WE, and Davis, AK (2022). Opposing global change drivers counterbalance trends in breeding North American monarch butterflies. Global Change Biology 28, 4726–4735.
| Opposing global change drivers counterbalance trends in breeding North American monarch butterflies.Crossref | GoogleScholarGoogle Scholar |
Dreyer, D, Frost, B, Mouritsen, H, Günther, A, Green, K, Whitehouse, M, Johnsen, S, Heinze, S, and Warrant, E (2018). The Earth’s magnetic field and visual landmarks steer migratory flight behavior in the nocturnal Australian bogong moth. Current Biology 28, 2160–2166.e5.
| The Earth’s magnetic field and visual landmarks steer migratory flight behavior in the nocturnal Australian bogong moth.Crossref | GoogleScholarGoogle Scholar |
Elder RJ (2007) Chapter 13: Pastures—summer rainfall. In ‘Pests of field crops and pastures: identification and control’. (Ed. PT Bailey) pp. 355–369. (CSIRO Publishing: Melbourne)
Flood J (1973) The Moth-hunters: investigations towards a prehistory of the southeastern highlands of Australia. PhD thesis, The Australian National University, Canberra.
Flood J (1980) ‘The Moth hunters: Aboriginal prehistory of the Australian Alps.’ (Australian Institute of Aboriginal Studies: Canberra)
Flood J (2010) ‘Moth hunters of the Australian Capital Territory: Aboriginal traditional life in the Canberra Region.’ 2nd edn. (Gecko Books: Marleston, South Australia)
Gibson, RK, Broome, L, and Hutchinson, MF (2018). Susceptibility to climate change via effects on food resources: the feeding ecology of the endangered mountain pygmy-possum (Burramys parvus). Wildlife Research 45, 539–550.
| Susceptibility to climate change via effects on food resources: the feeding ecology of the endangered mountain pygmy-possum (Burramys parvus).Crossref | GoogleScholarGoogle Scholar |
Green, K (2008). Migratory bogong moths (Agrotis infusa) transport arsenic and concentrate it to lethal effect by estivating gregariously in alpine regions of the Snowy Mountains of Australia. Arctic, Antarctic, and Alpine Research 40, 74–80.
| Migratory bogong moths (Agrotis infusa) transport arsenic and concentrate it to lethal effect by estivating gregariously in alpine regions of the Snowy Mountains of Australia.Crossref | GoogleScholarGoogle Scholar |
Green, K (2010). The aestivation sites of bogong moths, “Agrotis infusa” (Boisduval) (Lepidoptera: Noctuidae), in the Snowy Mountains and the projected effects of climate change. The Australian Entomologist 37, 93–104.
Green, K (2011). The transport of nutrients and energy into the Australian Snowy Mountains by migrating bogong moths Agrotis infusa. Austral Ecology 36, 25–34.
| The transport of nutrients and energy into the Australian Snowy Mountains by migrating bogong moths Agrotis infusa.Crossref | GoogleScholarGoogle Scholar |
Green, K, and Osborne, WS (1981). The diet of foxes, Vulpes vulpes (L.), in relation to abundance of prey above the winter snowline in New South Wales. Wildlife Research 8, 349–360.
| The diet of foxes, Vulpes vulpes (L.), in relation to abundance of prey above the winter snowline in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Green, K, Caley, P, Baker, M, Dreyer, D, Wallace, J, and Warrant, E (2021). Australian bogong moth Agrotis infusa (Lepidoptera: Noctuidae), 1951–2020: decline and crash. Austral Entomology 60, 66–81.
| Australian bogong moth Agrotis infusa (Lepidoptera: Noctuidae), 1951–2020: decline and crash.Crossref | GoogleScholarGoogle Scholar |
Gregg, PC, Fitt, GP, Coombs, M, and Henderson, GS (1993). Migrating moths (Lepidoptera) collected in tower-mounted light traps in northern New South Wales, Australia: species composition and seasonal abundance. Bulletin of Entomological Research 83, 563–578.
| Migrating moths (Lepidoptera) collected in tower-mounted light traps in northern New South Wales, Australia: species composition and seasonal abundance.Crossref | GoogleScholarGoogle Scholar |
Hassan E (1977) ‘Major insect and mite pest of Australian crops.’ (Ento Press: Gatton, Qld.)
Helms, R (1890). Report of a collecting trip to Mount Kosciusko. Records of the Australian Museum 1, 11–17.
| Report of a collecting trip to Mount Kosciusko.Crossref | GoogleScholarGoogle Scholar |
Hone, J (1999). On rate of increase (r): patterns of variation in Australian mammals and the implications for wildlife management. Journal of Applied Ecology 36, 709–718.
| On rate of increase (r): patterns of variation in Australian mammals and the implications for wildlife management.Crossref | GoogleScholarGoogle Scholar |
Hone, J (2002). Feral pigs in Namadgi National Park, Australia: dynamics, impacts and management. Biological Conservation 105, 231–242.
| Feral pigs in Namadgi National Park, Australia: dynamics, impacts and management.Crossref | GoogleScholarGoogle Scholar |
Hone, J, and Waithman, J (1979). Feral pigs are spreading. Agricultural Gazette of New South Wales 90, 12–13.
Hopkins DC, McDonald G (2007) Chapter 2: Cereals — pests and beneficials in the field. In ‘Pests of field crops and pastures: identification and control’. (Ed. PT Bailey) pp. 5–39. (CSIRO Publishing: Melbourne)
Jardine, W (1901). Customs of the Currak-da-bidgee tribe, New South Wales. Science of Man 4, 53–54.
Landsberg, J, James, CD, Morton, SR, Müller, WJ, and Stol, J (2003). Abundance and composition of plant species along grazing gradients in Australian rangelands. Journal of Applied Ecology 40, 1008–1024.
| Abundance and composition of plant species along grazing gradients in Australian rangelands.Crossref | GoogleScholarGoogle Scholar |
Leopold, A (1943). Deer irruptions. Wisconsin Conservation Bulletin 8, 3–11.
Macgregor, CJ, Williams, JH, Bell, JR, and Thomas, CD (2019). Moth biomass has fluctuated over 50 years in Britain but lacks a clear trend. Nature Ecology & Evolution 3, 1645–1649.
| Moth biomass has fluctuated over 50 years in Britain but lacks a clear trend.Crossref | GoogleScholarGoogle Scholar |
Maino, JL, Cushen, A, Valavi, R, and Umina, PA (2021). Spatial variation in Australian neonicotinoid usage and priorities for resistance monitoring. Journal of Economic Entomology 114, 2524–2533.
| Spatial variation in Australian neonicotinoid usage and priorities for resistance monitoring.Crossref | GoogleScholarGoogle Scholar |
Malerba, ME, Wright, N, and Macreadie, PI (2021). A continental-scale assessment of density, size, distribution and historical trends of farm dams using Deep Learning Convolutional Neural Networks. Remote Sensing 13, 319.
| A continental-scale assessment of density, size, distribution and historical trends of farm dams using Deep Learning Convolutional Neural Networks.Crossref | GoogleScholarGoogle Scholar |
Mansergh, IM, Cheal, DC, Burch, JW, and Allen, HR (2022). Something went missing: cessation of Traditional Owner land management and rapid mammalian population collapses in the semi-arid region of the Murray–Darling Basin, southeastern Australia. Proceedings of the Royal Society of Victoria 134, 45–84.
| Something went missing: cessation of Traditional Owner land management and rapid mammalian population collapses in the semi-arid region of the Murray–Darling Basin, southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
McCormick B (2005) Bogong moths and Parliament House. Parliamentary Library, Canberra. Available at https://apo.org.au/sites/default/files/resource-files/2005-10/apo-nid1411.pdf
Mitchell, J, Dorney, W, Mayer, R, and McIlroy, J (2007). Ecological impacts of feral pig diggings in north Queensland rainforests. Wildlife Research 34, 603–608.
| Ecological impacts of feral pig diggings in north Queensland rainforests.Crossref | GoogleScholarGoogle Scholar |
Morton S (2022) ‘Australian deserts: ecology and landscapes.’ (CSIRO Publishing: Melbourne)
Morton, SR, Stafford Smith, DM, Dickman, CR, Dunkerley, DL, Friedel, MH, McAllister, RRJ, Reid, JRW, Roshier, DA, Smith, MA, Walsh, FJ, Wardle, GM, Watson, IW, and Westoby, M (2011). A fresh framework for the ecology of arid Australia. Journal of Arid Environments 75, 313–329.
| A fresh framework for the ecology of arid Australia.Crossref | GoogleScholarGoogle Scholar |
Owens, ACS, and Lewis, SM (2018). The impact of artificial light at night on nocturnal insects: a review and synthesis. Ecology and Evolution 8, 11337–11358.
| The impact of artificial light at night on nocturnal insects: a review and synthesis.Crossref | GoogleScholarGoogle Scholar |
Popper KR (1962) ‘Conjectures and refutations.’ (Basic Books: New York)
Pullar, EM (1950). The wild (feral) pigs of Australia and their role in the spread of infectious diseases. Australian Veterinary Journal 26, 99–110.
| The wild (feral) pigs of Australia and their role in the spread of infectious diseases.Crossref | GoogleScholarGoogle Scholar |
Reid J (2009) Chapter 6: Australian pelican: flexible responses to uncertainty. In ‘Boom and bust: bird stories for a dry country’. (Eds L Robin, R Heinsohn, L Joseph) pp. 95–120. (CSIRO Publishing: Melbourne)
Robertson G, Short J, Wellard G (1987) Chapter 2: The environment of the Australian sheep rangelands. In ‘Kangaroos: their ecology and management in the sheep rangelands of Australia’. (Eds G Caughley, N Shepherd, J Short) pp. 14–34. (Cambridge University Press)
Rochecouste J-F, Baker J, Crabtree B (2022) Conservation agriculture in Australian dryland cropping and in New Zealand: the lessons of 70 years. In ‘Advances in conservation agriculture: adoption and spread. Vol. 3’. (Ed. A Kassam) (Burleigh Dodds Science Publishing Limited). https://doi.org/10.19103/AS.2021.0088.15
Romesburg, HC (1981). Wildlife science: gaining reliable knowledge. The Journal of Wildlife Management 45, 293–313.
| Wildlife science: gaining reliable knowledge.Crossref | GoogleScholarGoogle Scholar |
Roshier D (2009) Chapter 5: Grey teal: survivors in a changing world. In ‘Boom and bust: bird stories for a dry country’. (Eds L Robin, R Heinsohn, L Joseph) pp. 75–94. (CSIRO Publishing: Melbourne)
Ruscoe, WA, Brown, PR, Henry, S, van de Weyer, N, Robinson, F, Hinds, LA, and Singleton, GR (2022). Conservation agriculture practices have changed habitat use by rodent pests: implications for management of feral house mice. Journal of Pest Science 95, 493–503.
| Conservation agriculture practices have changed habitat use by rodent pests: implications for management of feral house mice.Crossref | GoogleScholarGoogle Scholar |
Sánchez-Bayo, F, and Wyckhuys, KAG (2019). Worldwide decline of the entomofauna: a review of its drivers. Biological Conservation 232, 8–27.
| Worldwide decline of the entomofauna: a review of its drivers.Crossref | GoogleScholarGoogle Scholar |
Short, J (1998). The extinction of rat-kangaroos (Marsupialia:Potoroidae) in New South Wales, Australia. Biological Conservation 86, 365–377.
| The extinction of rat-kangaroos (Marsupialia:Potoroidae) in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Singleton, GR (1989). Population dynamics of an outbreak of house mice (Mus domesticus) in the mallee wheatlands of Australia—hypothesis of plague formation. Journal of Zoology 219, 495–515.
| Population dynamics of an outbreak of house mice (Mus domesticus) in the mallee wheatlands of Australia—hypothesis of plague formation.Crossref | GoogleScholarGoogle Scholar |
Singleton, GR, Brown, PR, Pech, RP, Jacob, J, Mutze, GJ, and Krebs, CJ (2005). One hundred years of eruptions of house mice in Australia – a natural biological curio. Biological Journal of the Linnean Society 84, 617–627.
| One hundred years of eruptions of house mice in Australia – a natural biological curio.Crossref | GoogleScholarGoogle Scholar |
Thomas, CD, Jones, T, and Hartley, SE (2019). “Insectageddon”: a call for more robust data and rigorous analyses. Global Change Biology 25, 1891–1892.
| “Insectageddon”: a call for more robust data and rigorous analyses.Crossref | GoogleScholarGoogle Scholar |
Wagner, DL (2020). Insect declines in the anthropocene. Annual Review of Entomology 65, 457–480.
| Insect declines in the anthropocene.Crossref | GoogleScholarGoogle Scholar |
Wardhaugh KG (1986) Chapter 6: Diapause strategies in the Australian plague locust (Chortoicetes terminifera Walker). In ‘The evolution of insect life cycles’. (Eds F Taylor, R Karban) pp. 89–104. (Springer US: New York)
Warrant, E, Frost, B, Green, K, Mouritsen, H, Dreyer, D, Adden, A, Brauburger, K, and Heinze, S (2016). The Australian bogong moth Agrotis infusa: a long-distance nocturnal navigator. Frontiers in Behavioral Neuroscience 10, 77.
| The Australian bogong moth Agrotis infusa: a long-distance nocturnal navigator.Crossref | GoogleScholarGoogle Scholar |
Warrant EJ, Whitehouse MEA, Green KP, Wallace JRA, Caley P, Tomlinson S, Umbers K (2021) Agrotis infusa. The IUCN Red List of Threatened Species, e.T190513532A196183274. Available at https://dx.doi.org/10.2305/IUCN.UK.2021-3.RLTS.T190513532A196183274.en [Accessed 8 December 2022]
Welch, HE (1963). Amphimermis bogongae sp.nov. and Hexamermis cavicola sp.nov. from the Australian bogong moth, Agrotis infusa (Boisd.), with a review of the genus Amphimermis Kaburaki & Imamura, 1932 (Nematoda: Mermithidae). Parasitology 53, 55–62.
| Amphimermis bogongae sp.nov. and Hexamermis cavicola sp.nov. from the Australian bogong moth, Agrotis infusa (Boisd.), with a review of the genus Amphimermis Kaburaki & Imamura, 1932 (Nematoda: Mermithidae).Crossref | GoogleScholarGoogle Scholar |
Welvaert, M, Al-Ghattas, O, Cameron, M, and Caley, P (2017). Limits of use of social media for monitoring biosecurity events. PLoS ONE 12, e0172457.
| Limits of use of social media for monitoring biosecurity events.Crossref | GoogleScholarGoogle Scholar |
Wilson LJ, Fitt GP, Deutscherr S, Kahn M, Pyke BA (2007) Chapter 3: Cotton. In ‘Pests of field crops and pastures: identification and control’. (Ed. PT Bailey) pp. 63–101. (CSIRO Publishing: Melbourne)
Withers, PC (1995). Cocoon formation and structure in the estivating Australian desert frogs, Neobatrachus and Cyclorana. Australian Journal of Zoology 43, 429–441.
| Cocoon formation and structure in the estivating Australian desert frogs, Neobatrachus and Cyclorana.Crossref | GoogleScholarGoogle Scholar |
Zborowski P, Edwards T (2007) ‘A guide to Australian moths.’ (CSIRO Publishing: Melbourne)


