Invasive rodent eradications in French Pacific islands: a critical analysis of past efforts
Wilfried Weiss A B * , Fabrice Brescia A , Julien Baudat-Franceschi C , Tehani Withers D , Baudouin Des Monstiers E , Simon Ducatez F , Jean-Yves Meyer F G , Thomas Ghestemme D and Eric Vidal B
A B * , Fabrice Brescia A , Julien Baudat-Franceschi C , Tehani Withers D , Baudouin Des Monstiers E , Simon Ducatez F , Jean-Yves Meyer F G , Thomas Ghestemme D and Eric Vidal B
A
B
C
D
E
F
G
Abstract
The French Pacific Island territories are home to an exceptional terrestrial biodiversity that is threatened by invasive alien rodents causing drastic ecological damage, including the decline of endemic species.
This study compiled and analysed rodent eradication efforts in the French Pacific Island territories from 1982 to 2022, focusing on methods, challenges, and results.
We compiled data from the Database of Island Invasive Species Eradications and local reports. The dataset included a total of 85 eradication attempts conducted on 77 islands, and for each attempt, we extracted information on island size, targeted species, eradication techniques, and biosecurity measures. An attempt was considered successful if a monitoring carried out at least 2 years after the eradication failed to detect any rodent.
In New Caledonia and Wallis, 90% of rodent eradications attempts were successful and concerned quite small island (<60 ha). Eradication attempts in French Polynesia targeted larger islands but showed a lower success rate (56%). Brodifacoum was used in 97% of the operations, and biosecurity measures were uniformly applied, particularly in New Caledonia, where 76% of operations lacked re-invasion prevention protocols.
Our findings suggest that future eradication efforts should follow a global or territorial strategy prioritising islands for conservation. A more rigorous protocol, based on reliable data, is essential to success. Improved local stakeholder capacities is vital to safeguard the unique biodiversity of the French Pacific Island territories.
Keywords: biosecurity, conservation, eradication, French territory, invasive rodents, Pacific, sustainable strategy, tropical Island.
Introduction
Islands are one of the primary focal points in the current extinction crisis caused by invasive alien species worldwide (Samaniego et al. 2020; Roy et al. 2023). Island ecosystems have experienced early and severe anthropogenic extinction events and currently harbour a disproportionate number of globally threatened or recently extinct plant and animal species (Ricketts et al. 2005; Tershy et al. 2015). This is particularly true for tropical island ecosystems where invasive alien species are considered as the main threat to biodiversity (Blackburn et al. 2004; Lockwood et al. 2013), especially mammalian predator species, which are the principal drivers of native biodiversity loss on islands (Doherty et al. 2016). Among them, introduced rodents (rats, Rattus sp; mice, Mus sp.), which have colonised 80% of the world’s archipelagos (see Atkinson 1985), are the group that has had the most severe and widespread impacts on island birds, mammals, and reptiles (Doherty et al. 2016).
In recent decades, the eradication of invasive rodent species has become a major lever of action implemented for the conservation and restoration of native biodiversity on islands, with very positive effects and major successes on the island biota (Spatz et al. 2022). Indeed, since the first successful eradications of rodents on islands, whether deliberate or accidental, in mainland France and New Zealand in the 1950s and 1960s, eradication methods, protocols, and toxins have gradually improved (Howald et al. 2007; Campbell et al. 2015). This has resulted in a rapid and marked increase in the number and the surface area of islands freed from rodents in most regions of the world; for example, 596 successful island rodent eradications worldwide out of 871 attempts reported in 2019 (Spatz et al. 2022). However, marked differences have been observed in the success rates of operations, which were significantly lower in the tropics (Harper and Bunbury 2015; Russell and Holmes 2015).
In the tropical and subtropical Pacific, islands are home to five major invasive rodent species (Harper and Bunbury 2015; Samaniego et al. 2021). The Pacific rat (Rattus exulans) was introduced by the Austronesian/Polynesian settlers on several waves of introduction about 3000 years ago (Barnes et al. 2006). The other four species (black rat, Rattus rattus; brown rat, Rattus norvegicus; tanezumi rat, Rattus tanezumi; and house mouse, Mus musculus) were introduced in several waves (Harper and Bunbury 2015) with the European colonisers from the 17th century (Matisoo-Smith and Robins 2004; Drake and Hunt 2009). The introduction of these species had disastrous consequences for local ecosystems, leading to the decline or extinction of many endemic species, the disruption of island food chains, and the alteration of natural habitats (Fukami et al. 2006; Holdaway et al. 2007). The impacts of these rodents extend beyond biodiversity, also affecting local economies through the destruction of crops and the transmission of diseases, highlighting the urgency of developing effective and context-adapted eradication strategies.
These eradication strategies can be decided based on information from syntheses of operations, both successful or unsuccessful, which have already been conducted. Such syntheses already exist, covering eradication efforts at a global level or focusing on leading countries in terms of rodent eradication, such as Australia and New Zealand (Segal et al. 2022; Spatz et al. 2022). The advantage of these syntheses is that they enable feedback and knowledge to be integrated into decision-making, facilitating the implementation of optimal protocols or strategies (Pullin et al. 2020). They favour the assessment of the effectiveness of different eradication methods, and their adaptation to the unique context encountered on each island.
Here, we aimed to critique past rodent eradication efforts in the French territories of the Pacific. No compilation of experiences focused on these areas is available (e.g. see Russell and Broome 2016; Segal et al. 2022). The four French tropical Pacific territories (French Polynesia, New Caledonia, Wallis and Futuna, and Clipperton atoll) constitute a collection of more than 3600 islands and rocky or sandy islets according to the census by the UN Environment Programme World Conservation Monitoring Centre (UNEP-WCMC 2010), (~2900 islands in French Polynesia and ~700 in New Caledonia) distributed over a maritime surface area of ~6.8 million km2, 19 islands in Wallis and Futuna, and one in Clipperton. This large set of islands is home to an exceptional terrestrial biodiversity, falling within three of the World’s biodiversity hotspots (Myers et al. 2000), encompassing 113 Important Bird and Biodiversity Areas and home to a high number of endemic species with for example, ~3000 endemic vascular plants, 166 endemic land snail species, ~100 endemic lizard species, and >50 endemic bird species (Beauvais et al. 2006; Pippard 2012; Soubeyran et al. 2015). In such a context of vibrant yet threatened island biodiversity, combined with the significant threats posed by introduced rodents, compiling data on and analysing rodent eradication operations carried out in the French territories of the Pacific appears as an important task for future eradication programs. It would also help raising awareness among governmental agencies as well as environmental and conservation organisations on the feasibility of such programs. In this study, we reviewed and summarised all documented rodent eradication attempts conducted in these areas in the past 40 years (1982–2022).
In particular, we aimed to answer the following three questions: (1) how many attempts have been made to eradicate rodents and which species were targeted? (2) what are the outcomes of these operations in the different territories? How large are the treated areas, and did this size change over time? and (3) what methods and biosecurity strategies have been used? We also aimed to compare the results with those of other Pacific countries where such syntheses are already available from New Zealand and Australia. The data analysed will be used to draw up recommendations for future eradication operations. This synthesis will also serve as a basis for decision-making regarding the prioritisation of islands to be treated and the methodologies to be adopted in similar tropical insular environments.
Materials and methods
Study sites
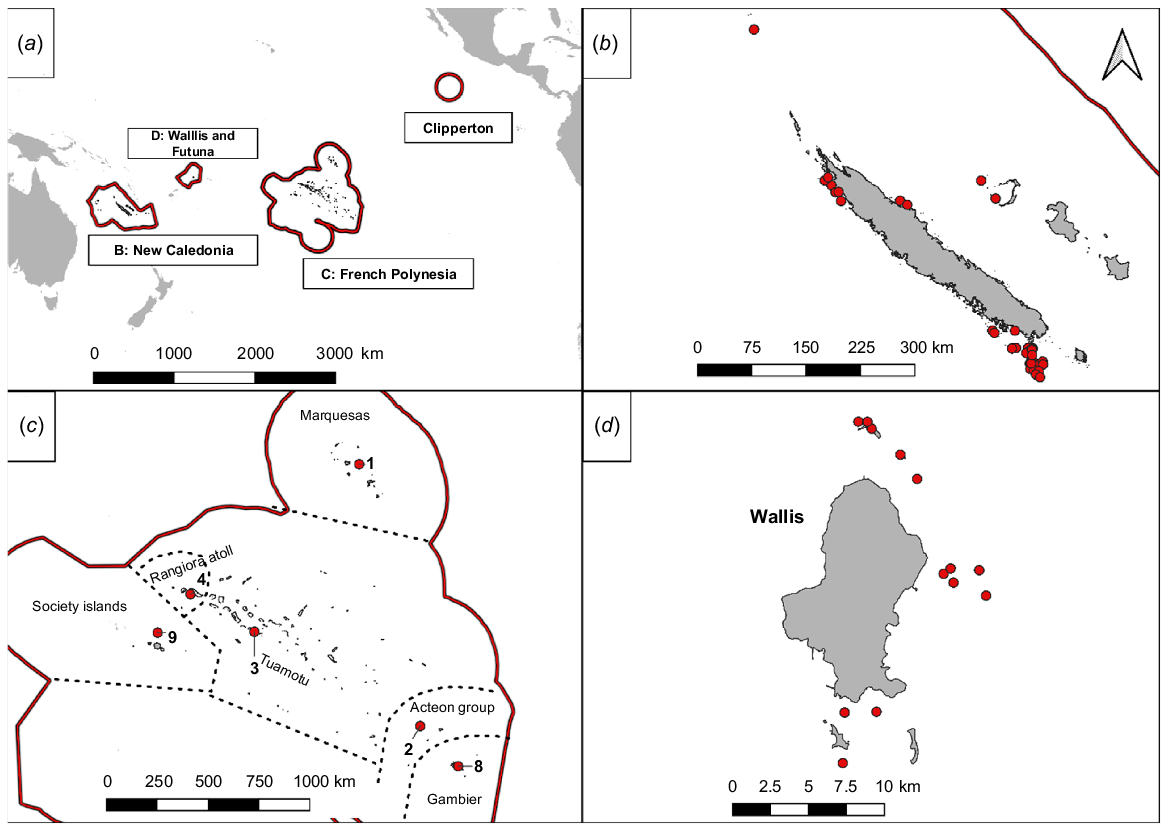
The study was conducted in four French tropical island territories in the South Pacific: (1) New Caledonia (~267,000 inhabitants); (2) French Polynesia (~308,000 inhabitants); (3) Wallis and Futuna (~11,000 inhabitants); and (4) the uninhabited Clipperton atoll (Fig. 1). Due to their geographical position, these four territories are characterised by a tropical to subtropical climate. However, New Caledonia exhibits a slightly milder climate, influenced by the trade winds (Maitrepierre et al. 2012).
(a) Location of the four French territories in the Pacific. (b) New Caledonia, (c) French Polynesia, and (d) Wallis. The red dot represents the islands where rodent eradication attempts have been made. The numbers correspond to the number of islands where rodent eradication attempts have been made in French Polynesia. The red lines represent the limits of the Exclusive Economic Zones. For mapping, we used QGIS 3.16.10 open source software.
Data collection
We carried out a search using the Database of Island Invasive Species Eradications (DIISE 2019). This database lists invasive vertebrate species eradications carried out on islands worldwide. We selected the eradication operations either completed or currently underway in the considered geographical areas. The selection focuses exclusively on rodent eradication and excludes control (i.e. local limitation of rodent population) projects. Each operation is described in detail in the database, along with the outcome of the operation. In some cases, a report is attached to the operations. However, some operations may still be missing, due to the scarcity of publications or reports with regard to eradication attempts (Spatz et al. 2022). Moreover, failed operations may not be fully reported, despite the information they could provide in terms of conservation strategies and methodologies (Catalano et al. 2019). To address this bias, a complementary ‘grey’ literature search was conducted to maximise the information included in our database (see Supplementary Tables S1–S4). For this purpose, we contacted a total of 19 individuals belonging to the various institutions, organisations, and associations involved. These included local environmental management services and affiliated agencies (e.g. Agence Néo-Calédonienne de la Biodiversité (ANCB) in New Caledonia), as well as international organisations such as the Secretariat of the Pacific Regional Environment Programme (SPREP) and the Pacific Regional Invasive Species Management Support Service (PRISMSS) program. We also consulted archives related to eradication campaigns from research institutes such as the IRD, and reached out to non-governmental organisations (NGOs) (e.g. Conservation International, BirdLife) as well as local associations involved in island ecosystem and invasive species management, including Société d’Ornithologie de Polynésie Française (SOP) Manu, Société Calédonienne d’Ornithologie (SCO), Association pour la Sauvegarde de la Biodiversité d’Ouvéa (ASBO), and Hô-üt. They were asked if they were aware of any operations and if so, what documents or details they could provide. Local newspapers were also searched using keywords such as ‘eradication,’ ‘rodents’, ‘rats’, and ‘mice’. Requests for more information and documentation were sent to local managers, NGOs and operators who had carried out the listed eradication operations.
Compilation and analysis
By compiling and analysing all these documents, we were able to provide an overview of the eradication attempts and efforts deployed in the past 40 years, the methods used, the characteristics of the islands treated, and the results achieved. In many cases, the documents describe eradication programs covering multiple islands. For the purpose of our analysis, we treated these as distinct operations. Operations are listed in the database according to the name of the island and the date of eradication attempt in the form ‘name_year.’ The characteristics of the islands include their geographical region, location, island category (atoll or high island), distance from the main island or nearest island, surface area and rodent species targeted by the eradication. We also examined whether each operation was preceded by a feasibility study; i.e. whether prior visits had been made to the island to assess the ecological and invasion context on the island and adapt the eradication protocol. All data collected, along with their source, and analysed for each operation are in Tables S1–S4. We also considered what type of organisation initiated these eradication attempts, which organisations carried them out, as well as what the motivations and objectives were to address the following questions: what were the main species or ecosystems targeted for conservation or restoration?; which eradication method was used? and in the case of poison, which active ingredient was used and what was the application method (e.g. manual spraying, drones, helicopters, station) and the quantity of poison used (kg/ha)? In addition, we set out to establish how many bait applications there had been and what the interval time was between applications. Eradication outcomes were classified into six categories: successful; to be confirmed; failed; unknown; pre-status unknown; and re-invaded. An operation is considered ‘completed’ if it is classified as either ‘successful’ or ‘failed’. In the case of a ‘failed’ operation at 1 year after treatment, a new operation may be carried out the same year. An operation was considered ‘successful’ when no rodent had been detected on the island 2 years after the eradication attempt (Howald et al. 2007). Operations classified as ‘to be confirmed’ are operations where the treatment phase of the eradication operation has been completed, but the absence of rodents at least 2 years after the operation has yet to be confirmed. We considered an operation as ‘failed’ if rodents had been detected within the first 2 years following the operation. This differs to ‘re-invaded’ islands where rodents have recolonised the island after a successful eradication (Pichlmueller et al. 2020). Re-invasion can occur as early as the year following the treatment phase. Of course, without genetic sampling, it is not possible to distinguish between a failure and a re-invasion within the 2 years following the treatment (Abdelkrim et al. 2007; Russell et al. 2010). We assume that an eradication operation should incorporate biosecurity measures and immediate post-treatment monitoring to minimise the risk of re-invasion (Russell et al. 2008a, 2010). Operations classified as ‘unknown’ are those where no verification has been conducted since the eradication attempt. Finally, operations classified as ‘pre-status unknown’ are those where an eradication operation has been attempted without knowing for certain whether or not (or which species) invasive rodents were present.
The success rate (Srate) was calculated based on ‘successful’ or ‘failed’ operations only, excluding ‘to be confirmed,’ ‘unknown’, or ‘pre-status unknown’ operations:
Temporal trends from 1982 to 2022 were examined following two types of cumulative metric: the number of eradication operations conducted per year; and the total surface treated per year. We compared the total number of eradication operations, and the surface treated for each region (New Caledonia, French Polynesia, and Wallis) in the period 1982–2022. Temporal trends were compared with data from two other large island countries, Australia and New Zealand. To do so, we extracted information on rodent eradication operations carried out on the islands of New Zealand and Australia from the Database of Island Invasive Species Eradications 2018 database. It should be noted that this database has not been updated since 2019. Therefore, operations with the status ‘to be confirmed’ or ‘progressing’ that could not be supplemented with additional documents have been listed as ‘unknown status’. In addition, when several operations were carried out at the same time on the same island, they were considered as a single eradication operation targeting several rodent species. Finally, if at least one of the species targeted in an operation was not successfully eradicated, the operation was classified as a ‘failure’.
The assembly of a boxplot of the surfaces treated by territory was generated in RStudio 2023.0.1 (R Core Team 2023) using the ggplot2 (Wickham 2016) package.
Results
We found information on 85 invasive rodent eradication attempts that were carried out in the French Pacific territories between 1982 and 2022, on 77 different islands, covering a total area of 2773.36 ha (Table 1). A total of 38 attempts were carried out in New Caledonia (total area of 290.50 ha), 34 in French Polynesia (total area of 2386.81 ha), and 13 operations were recently (2021) conducted in Wallis and Futuna (total area of 96.05 ha, all around Wallis). No operation was conducted in Clipperton. These eradications focused on three different rodent species (R. rattus, Rr; R. exulans, Re; M. musculus, Mm). Most involved a single species but sometimes two co-occurring species were targeted (Rr and Re, Rr and Mm).
| No. operations | Total area (ha) | ||
|---|---|---|---|
| New Caledonia | 38 | 290.50 | |
| French Polynesia | 34 | 2386.81 | |
| Wallis | 13 | 96.05 | |
| Clipperton | 0 | 0 | |
| Total | 85 | 2773.36 |
A total of 50% of all operations conducted in New Caledonia were considered successful while only 5.26% failed (Table 2). However, 14 of the 38 operations were implemented prior to a clear confirmation of rodent presence on the islands and therefore have an unknown pre-status. In French Polynesia, the success rate was 41.18%, with a 32.35% failure rate and 26.47% of operations still awaiting formal confirmation of their outcome. For Wallis, 79.92% of operations were considered successful while only 7.69% failed. However, 2 of the 13 were implemented despite no evidence of rodent presence on these islets and are therefore categorised as having an unknown pre-status.
| N operations | SuccessA (%) | FailedB (%) | To be confirmedC (%) | Unknown pre-statusD (%) | UnknownE (%) | ||
|---|---|---|---|---|---|---|---|
| New Caledonia | 38 | 50.00 | 5.26 | 0.00 | 36.84 | 7.89 | |
| French Polynesia | 34 | 41.18 | 32.35 | 26.47 | 0.00 | 0.00 | |
| Wallis | 13 | 79.92 | 7.69 | 0 | 15.38 | 0.00 | |
| Clipperton | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 85 | 50.59 | 16.47 | 10.59 | 3.53 | 16.47 |
Focusing on operations that were monitored for at least 2 years post-treatment, with a known outcome, and where the presence of rodents prior to the operation had been clearly established (i.e. 21 operations in New Caledonia, 25 in French Polynesia and 11 in Wallis), success rates reached 90.48% in New Caledonia, 56% in French Polynesia, and 90.91 in Wallis (Table 3).
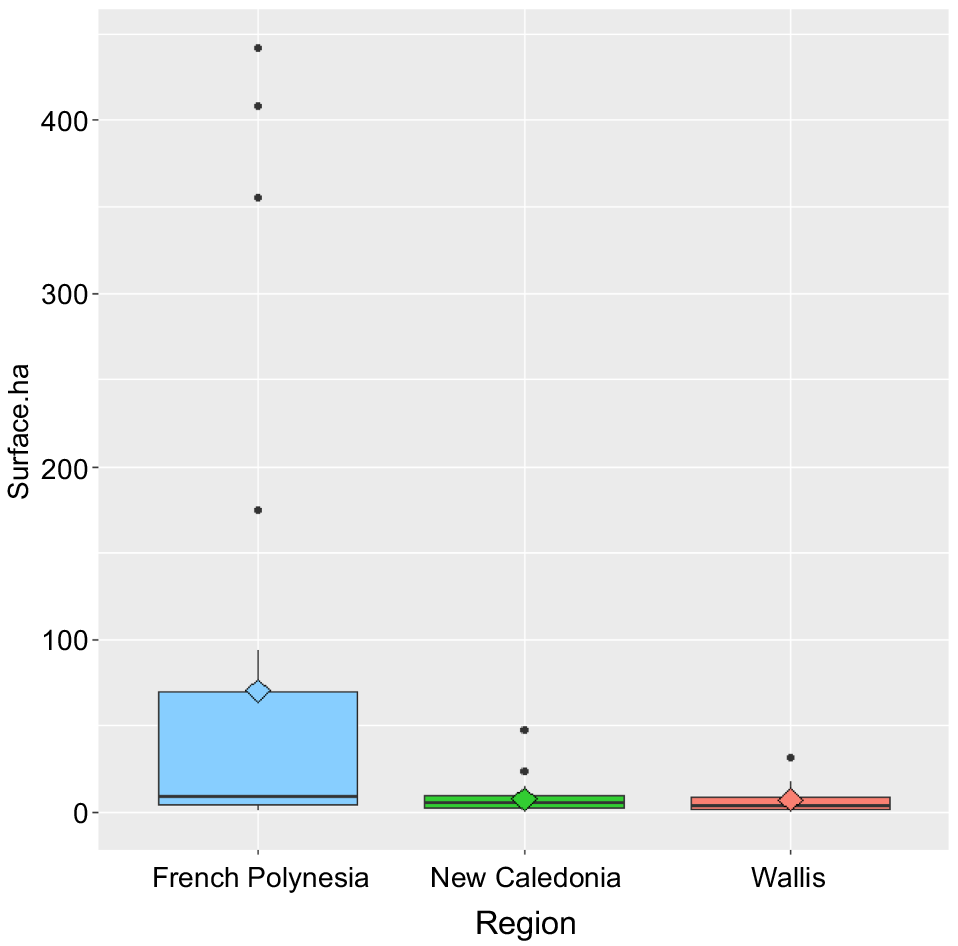
There is a 10-fold difference in the size of treated islands between French Polynesia and New Caledonia or Wallis (Fig. 2 and Table 4). The average surface area of treated islands was around 7 ha in Wallis (7.39 ± 9.34 ha; min, 0.34; max, 31.55) and New Caledonia (7.64 ± 8.60 ha; min, 0.25; max, 47.83) compared with 72 ha (72.33 ± 126.37 ha; min, 0.70; max, 442.12) in French Polynesia. In New Caledonia and Wallis, the eradication operations carried out concerned small islands, with a maximum surface area of 47.83 ha for New Caledonia and 31.55 ha for Wallis, whereas in French Polynesia the eradication attempts concerned larger islands, reaching up to 442.12 ha.
Surface areas of islands treated for rodent eradications in the French Pacific territories. Blue, French Polynesia; green, New Caledonia; orange, Wallis.
| Mean area (ha) | s.d. (ha) | Min. area (ha) | Max. area (ha) | Median (ha) | ||
|---|---|---|---|---|---|---|
| New Caledonia | 7.64 | 8.60 | 0.25 | 47.83 | 5.66 | |
| French Polynesia | 72.33 | 126.37 | 0.70 | 442.12 | 10.00 | |
| Wallis | 7.39 | 9.34 | 0.34 | 31.55 | 3.86 | |
| Total | 33.30 | 84.47 | 0.25 | 442.12 | 5.88 |
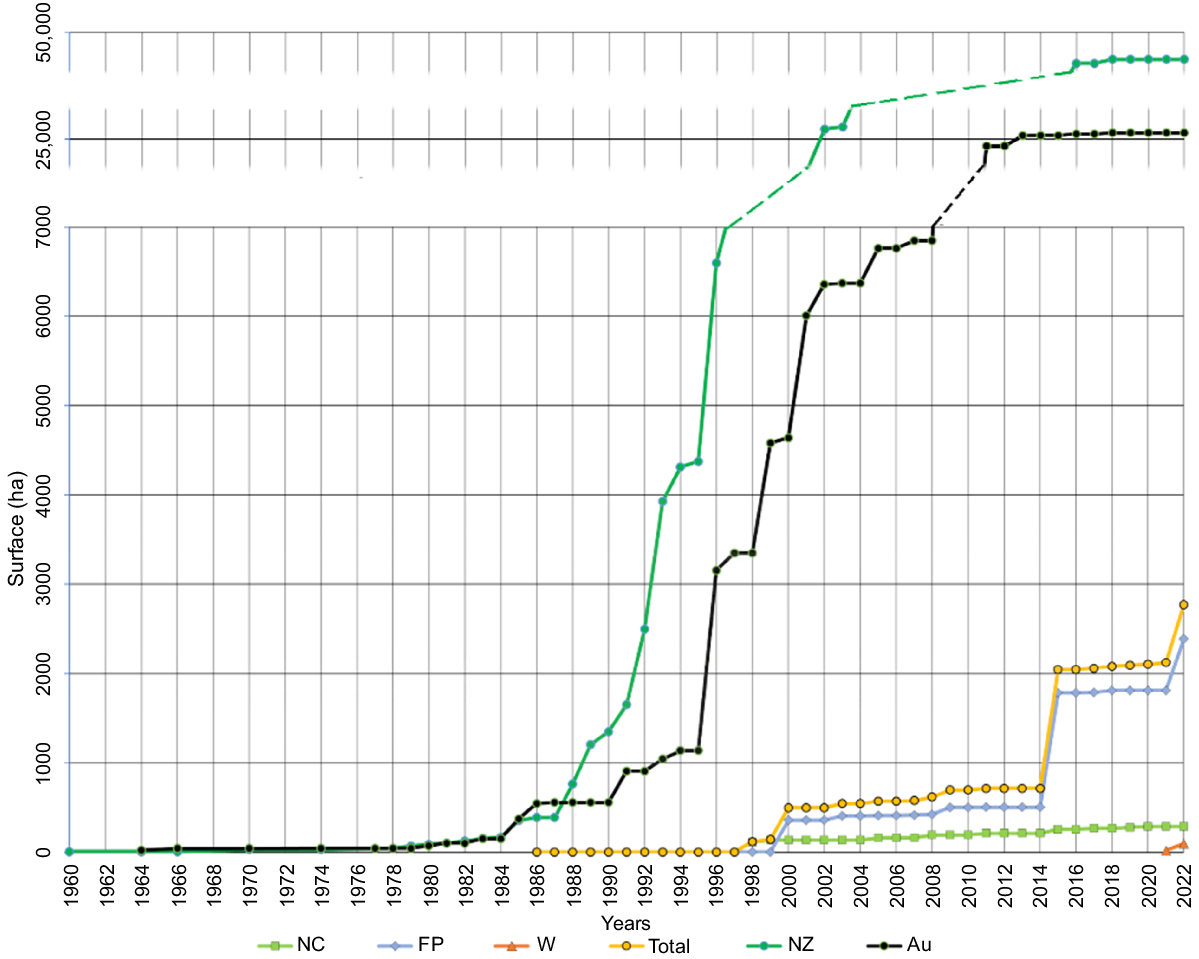
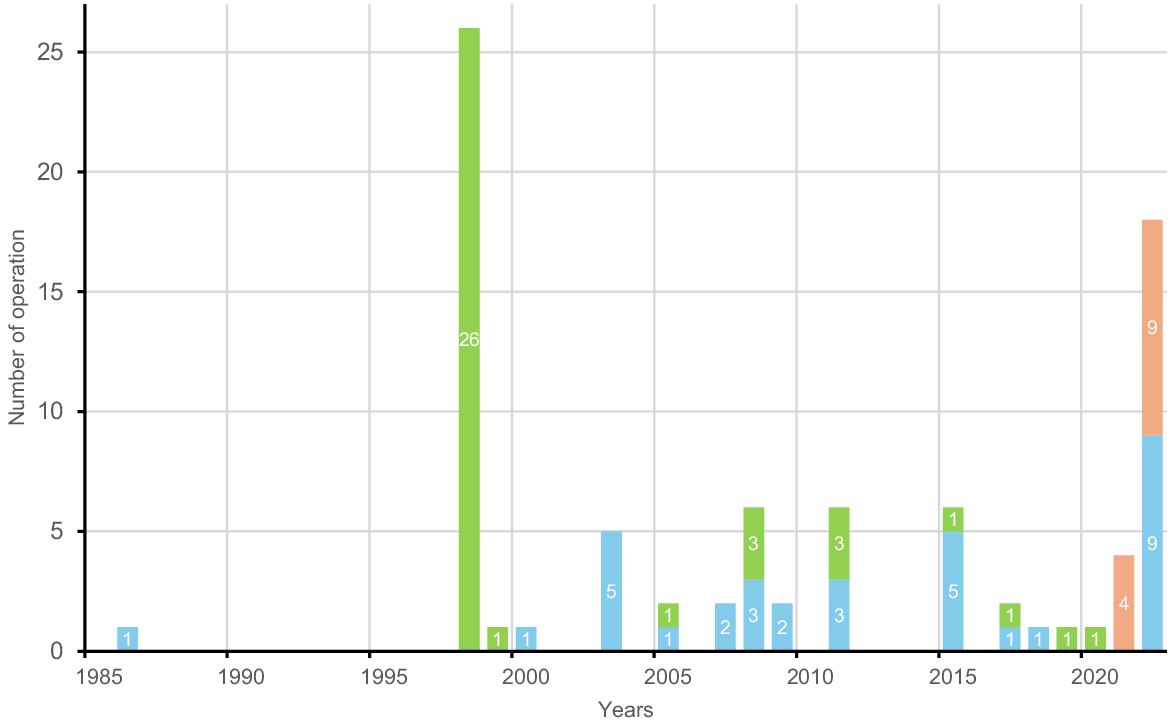
Eradication attempts of introduced rodents began at roughly the same period in New Caledonia and French Polynesia; i.e. the late 1990s and early 2000s (Fig. 3). In New Caledonia, the eradication campaign got off to a flying start, with 26 operations conducted the same first year (Fig. 4). Eradication attempts then continued in a scattered pattern, targeting small and medium-sized islands without a subsequent increase in the number or size of projects planned.
Cumulative surface area of islands treated in the past 60 years. Green square, New Caledonia; blue diamond, French Polynesia; orange triangle, Wallis; yellow circle, French Pacific Archipelago; dark green circle, New Zealand; black circles, Australia.
Number of eradications carried out annually in French Pacific territories inthe past 40 years. Green, New Caledonia; blue, French Polynesia; orange, Wallis.
In French Polynesia, however, the number of projects has increased steadily over time, as has the surface area treated, progressing in stages, depending on the new methods used to spread baiting.
All recorded eradication attempts have been carried out by poisoning, mainly distributed by hand broadcast (70.59% in French Polynesia), sometimes combined with bait stations (68.42% in New Caledonia) or more recently by aerial broadcasting (helicopter or drone) (Table 5). Brodifacoum at least 0.02 g/kg was used in 97% of eradication attempts. Out of 85 operations, 83 use two baiting sessions, with bait quantities (per ha) varying depending on the method: in French Polynesia, this was 12 kg/ha for bait stations, 2–27.5 kg/ha for hand broadcast, 21.1–32.3 kg/ha for aerial broadcast, and 25–27.5 kg/ha for drones combined with hand broadcast; in New Caledonia, this was 2–7 kg/ha for bait stations, 3.5–25 kg/ha for hand broadcast, and 7–13 kg/ha for hand broadcast with bait stations; and in Wallis, this was 25–48 kg/ha for hand broadcast and 25 kg/ha for drones.
| Methods | French Polynesia (%) | New Caledonia (%) | Wallis (%) | Clipperton (%) | All (%) | |
|---|---|---|---|---|---|---|
| Aerial broadcast | 14.71 | 0 | 0 | 0 | 5.88 | |
| Bait stations | 5.88 | 68.42 | 0 | 0 | 32.94 | |
| Station and hand broadcast | 2.94 | 23.68 | 0 | 0 | 11.76 | |
| Drone broadcast | 0 | 0 | 61.54 | 0 | 9.41 | |
| Drone and hand broadcast | 2.94 | 0 | 0.00 | 0 | 1.18 | |
| Hand broadcast | 70.59 | 7.89 | 38.46 | 0 | 37.65 | |
| Fumigation | 2.94 | 0 | 0 | 0 | 1.18 | |
| Total | 100 | 100 | 100 | 0 | 100 |
Although often mentioned in the documents reviewed for all the recorded eradication attempts, biosecurity protocols were not necessarily carried out. In New Caledonia, 76.32% of eradication operations were not accompanied by any protocol or biosecurity measures, 18.42% included the implementation of early detection systems combined with communication campaign, while 5.26% included only communication. In French Polynesia, 100% of operations included the implementation of early detection systems combined with communication, while in Wallis, 100% of operations were accompanied by communication campaigns (but none by early detection systems). All (100%) eradications were motivated by the conservation of island biodiversity, particularly seabird colonies. 72 operations aimed to conserve seabird colonies and/or potential nesting habitats, covering a total of 2711 ha across all territories. However, very few targeted a specific endangered species, except for example, the Fairy Tern (Sternula nereis) in the northern Important Bird Area of New Caledonia, which involved six operations treating a total of 49 ha. In French Polynesia, the Polynesian Ground Dove (Pampusana erythroptera) was the focus of four operations across two islands, treating 11 ha, and the Tuamotu Sandpiper (Prosobonia parvirostris) in the Tuamotu Archipelago was the focus of three operations across three islands, treating a total of 7 ha.
In New Caledonia, 68% of the operations were carried out by a foreign private operator, 26% by local NGOs supported by international NGOs (BirdLife International) and local institutions and 6% by a research team. In French Polynesia, 97% of operations were carried out by local NGOs supported by international NGOs (BirdLife International and Island Conservation), while all eradication attempts in Wallis were carried out by a cooperation between local institutions and the international NGO Island Conservation.
Discussion
French territories in the Pacific represent not only a unique set of thousands of islands and islets spread over considerable ocean surfaces, but also a remarkable concentration of endemic and endangered plant and animal species. Preserving this exceptional ecological heritage must include significant actions to counter the impacts of invasive species alongside a resolute strategy to restore island environments by removing pest species, among which introduced rodents are species that should be targeted as a priority.
Our study showed that at least 85 different rodent removal projects have been attempted in the French territories in the Pacific in the past 40 years on 77 different islands representing 2773.36 ha of eradication surfaces. Compared with the 193 and 91 offshore islands where rodent eradication attempts have been carried out in New Zealand and Australia, respectively (Segal et al. 2022), these numbers indicate significant efforts devoted to the eradication of invasive rodents in the French Pacific islands. However, compared to these two leading Pacific countries, the eradications carried out in the French Pacific territories had started later, were less ambitious in terms of surface areas treated, and experienced weaker growth over time, particularly in New Caledonia. Indeed, in Australia and New Zealand, rodent eradication began in the late 1960s and early 1970s. The number of operations and the size of treated areas grew rapidly in the mid-1980s. The ramp-up in eradication operations has been exponential, reaching 25,000 ha for Australia in 2014, and 47,000 ha in New Zealand in 2018 (Segal et al. 2022; Innes et al. 2024; Fig. 3).
Interestingly, the dynamics of eradication projects are markedly different between French Polynesia and New Caledonia. While projects began around the same period, we observed a gradual build-up in French Polynesia only, whereas operations in New Caledonia remained small-scale and limited in number. This clear difference in the scale, complexity, and frequency of eradication programs may partly reflect the earlier and broader adoption of aerial baiting techniques, either by helicopter or, more recently, by drone in French Polynesia. However, other factors such as funding levels and institutional frameworks likely also played a role (Holmes et al. 2015a). Also, projects there have benefit from consistent efforts and involvement of two major international NGOs (BirdLife International and Island Conservation) in close support of a local organisation, the Polynesian Ornithological Society (also known as ‘Manu’) deeply committed to an ambitious rodent eradication effort. The structured approach adopted by these specialised organisations, focusing on the prioritisation, design, and implementation of eradication programs, is more likely to deliver sustained efforts and long-term success compared to successes achieved when projects are implemented solely by governmental management agencies without a clearly defined strategy (e.g. Tershy et al. 2012; Samaniego et al. 2021). We note that where most of the eradication attempts are recent (2021 and 2022) in Wallis, the eradication campaign was commissioned by the local authorities to the Island Conservation NGO, which then relied on local staff for on-site operations.
Global eradication attempts have a success rate of 87% (N = 516) (Holmes et al. 2015b), while in Australia and New Zealand, leaders in this field in the region, the respective success rates were of 58% (N = 126) and 54% (N = 306), respectively (e.g. Segal et al. 2022), this is likely due to the fact that Australia and New Zealand have conducted numerous early trial attempts and pioneering projects compared to other territories, which focused more on applying established methodologies (Towns and Broome 2003; Rauzon 2007; Holmes et al. 2015b). In contrast, eradications carried out in New Caledonia and Wallis showed a success rate of 90.48% and 90.91%, respectively, indicating high efficiency, although most operations there involved relatively small islands. In French Polynesia, the overall eradication success rate was closer to those observed in New Zealand and Australia. This is likely because, as in these two countries, eradications have also been attempted on larger islands (on average eight times larger than in New Caledonia), which typically makes eradications more challenging and thus reduces the overall success rate compared to smaller islands. The likely causes of failure in tropical environments are not yet fully understood. Even under theoretically unfavourable conditions, such as a high abundance of crabs, which compete for access to the bait, abundant food availability, and significant rodent populations, an operation can sometimes be successful (Samaniego et al. 2020). Nevertheless, identifying the real reasons for failure often remains challenging, especially several years later.
Various factors may contribute to the failure of an operation, including the lack of detailed reports. Possible causes are due to operational or human errors such as incorrect bait application or inadequate preparation (e.g. the absence of preliminary studies). Adverse weather conditions (Holmes et al. 2015b) such as heavy rainfall washing away bait or excessive delays between baiting rounds, can also allow rodent populations to recover. Operations must be properly budgeted, with adequate financial reserves for unforeseen expenses (Kappes et al. 2019). Ultimately, the success of an operation largely depends on thorough preparation and meticulous execution (Samaniego et al. 2021), including precise handling, appropriate monitoring supported by effective biosecurity measures (Kennedy and Broome 2019), and the production of comprehensive reports. However, reports of failed projects are often lacking or only available in limited detail (Catalano et al. 2019; Samaniego et al. 2021).
The virtually systematic use of Brodifacoum at 0.02 g/kg (97% of the operations examined) underlines that this has been accepted as an efficient bait for rodent eradication (Howald et al. 2007; Broome et al. 2010; Parkes et al. 2011). The diversity of bait application methods, ranging from simple manual spreading to more advanced techniques such as the use of drones, illustrates an adaptation to new technologies and local specificities (Segal et al. 2022) allowing larger surfaces or steeper islands to be treated (Broome et al. 2017; Hoffmann et al. 2023). Drones have expanded the possibilities for effective aerial baiting methods on small remote islands, particularly in French Polynesia, where expertise was limited and helicopters would be expensive (e.g. drone projects for the islets of Ua Pou and Kamaka). However, as an innovative technology, it remains a very expensive solution compared to ground-based methods. Additionally, ground-based methods allow project managers to employ more local staff and actively involve them in the project. This is advantageous as it helps to raise awareness of biosecurity rules within local communities and encourages their participation in the project. One of the main drawbacks of using drones is their limited capacity to operate under challenging weather conditions. On Rapa Island, where wind and sea conditions are rarely optimal, drone technology would not have been cost-effective. Adapting methods according to the budget is essential. Another specificity of the French Pacific islands is a level of social acceptance that is probably lower as compared to Australia and New Zealand. New Zealand conducts a significant amount of research on social acceptance, for example, in the context of the Predator Free 2050 program (Russell 2014; Dickie and Medvecky 2023).
The documents and reports we reviewed rarely mentioned the education and awareness of local populations to biosecurity protocols. Collaboration between managers, NGOs, local communities, and the private sector is crucial for the successful implementation of biosecurity measures. It allows for integrated management and better social acceptance (Harper 2020). A better integration of local populations early in these projects, with regards to both biosecurity and eradication operations, is clearly needed to improve their success, but also to prioritise projects that are also meaningful for local communities. However, it can be challenging to change the habits of populations, especially when cultural aspects are involved, which requires sustained communication efforts over time. Local social acceptance, as well as institutional acceptance, is not easy to achieve (Crowley et al. 2017) in a tense and ‘fragmented’ context in terms of decision-making and political positioning on biodiversity and its conservation challenges, as is the case in New Caledonia (Rodary 2024). This situation is further complicated by deep cultural differences. Gaining the support of local customary authorities at various levels, as well as that of institutional and administrative authorities, is complex and takes time. It is also essential to understand how local communities perceive invasive species, as these perceptions can significantly influence the success or failure of eradication projects (Kapitza et al. 2019).
This synthesis also revealed that, although biosecurity was systematically mentioned in the feasibility studies, anti-reinvasion measures were not systematically implemented nor maintained over time, particularly in New Caledonia compared with French Polynesia. In New Caledonia and Wallis, only limited communication and public information actions appear to have been systematically implemented. Although communication campaigns can be useful (Haley et al. 2023), they are generally not sufficient in order to efficiently detect and prevent the natural or human-induced reinvasion of islands by rodents; specific surveillance, maintained operational over time to guarantee an early detection of re-invasion, is essential (e.g. Russell et al. 2008b; Davis et al. 2023).
It would be relevant to complete the database by including information such as costs (largely missing information in the analysed documents here) and the number of local people involved. Additional data on cases of multi-invasion, vegetation type and density, rodent abundance, crab abundance, allocated budget, baiting grid layout, quantity of bait used, accessibility challenges of the islands, and the types of islets, could also be incorporated. This would enable multifactorial analyses to identify the causes of both success and failure in these territories.
Recommendations for the future
Given the exceptional biodiversity of the French Pacific islands and the threats posed by invasive rodents, both metropolitan France and the local overseas territorial authorities in charge of environmental issues have an important national and global responsibility to pursue and intensify rodent eradication efforts on these islands (Meyer et al. 2018). This includes stepping up eradication attempts and expanding the area and complexity of islands treated. This can be notably achieved by devoting more resources to these operations, obtaining public and decision-maker support for such operations, having local staff and structures trained by partnership with international specialists, and using innovative emerging technologies such as bait dispersal techniques by drone, for instance (see Campbell et al. 2015). The eradication strategies deployed in the past 15 years in French Polynesia is going in that direction, with significant conservation successes (e.g. (Aguirre-Muñoz et al. 2011; Griffiths et al. 2019; Samaniego et al. 2020). These successes support the interest of funding and developing similar programs on the numerous islands and islets with high conservation values that are still invaded by rodents, which continue to threaten endangered fauna and flora. In other French territories of the Pacific, major efforts are still required, especially in New Caledonia where conservation issues on the set of 700 islands and islets are considerable and eradication programs relatively rare, despite evidence of the conservation gains they can bring, including locally (e.g. see Philippe-Lesaffre et al. 2023).
The relative lag of New Caledonia’s biodiversity hotspots in efforts to eradicate invasive rodents is undoubtedly due to a combination of several factors. First, unlike the other French territories in the Pacific, New Caledonia has already had to deal with the catastrophic consequences of the introduction of Rusa deer (Cervus timorensis) (De Garine-Wichatitsky et al. 2003; Frantz et al. 2024), which subsequently led to the implementation of major efforts and large-scale mobilisation of already limited resources by local authorities over a period of several decades. Second, the administrative complexity of the territorial management of the environment and biodiversity in New Caledonia, split between several distinct administrations and political authorities, has probably delayed the emergence of a coherent and proactive rodent eradication strategy on the territory’s islands. Third, it is more challenging to secure funding due to the lack of seabird species with a high-concern IUCN status. The bird species with the highest conservation value, particularly in terms of endemism, are mainly located on Grande Terre and, to a lesser extent, on the Loyalty Islands.
It is therefore important to develop inventories and research on taxa other than birds (Baudat-Franceschi et al. 2011) in these territories to broaden the range of taxa that could benefit from the eradication of introduced rodents, provided their negative impact on the taxa in question is demonstrated.
Without a strategy defined by the competent authorities at the territory level, the other territories would at least need a structure similar to SOP Manu in French Polynesia to uphold the objective of rodent eradication and to implement sustained and long-term eradication actions. It is worth noting that each operation provided an opportunity for operators to learn, with accumulated experience playing a key role (Samaniego et al. 2021). In New Caledonia, however, the lack of institutional continuity has resulted in a loss of expertise, meaning that each new operation often has to start from scratch. This loss of cumulative knowledge underscores the need for a coordinated and territory-wide strategy led by a small number of dedicated agencies that cut across different operations. These agencies should be committed to clearly defined biodiversity goals and capable of sustaining long-term rodent control efforts something that has been lacking so far. A territory-wide eradication strategy is now required, with the identification of priority islands and islets to be treated along with planning for future larger-scale eradication attempts. Because of the multitude of situations with high extinction risks and necessarily limited resources, the identification of the most important and achievable rodent removal projects is necessary (e.g. Holmes et al. 2019). Many different parameters can be used separately or concurrently in order to decide whether to eradicate rodents on one island as opposed to another. For islands deemed technically feasible, the first priority is generally given to expected conservation gains; for example, saving highly threatened species from extinction (Holmes et al. 2019), restoring important seabird colonies (see Capizzi et al. 2010), and/or the ecological services they provide to adjacent reefs (Graham et al. 2018). Our study has shown that up to now in the French Pacific territories, introduced rodent eradications have mainly been carried out to conserve or restore seabird colonies, and secondarily to safeguard certain remnant populations of highly endangered species. Prioritisation of the possible candidate eradication operations is also generally modulated by the risk of re-invasion (Harris et al. 2012), and by sociopolitical feasibility and acceptability (Crowley et al. 2017; Holmes et al. 2019). This prioritisation exercise has only been partially carried out for the French Pacific territories and their thousands of islands and would benefit from being completed and finalised so as to provide a clear roadmap for each territory. It would also be worthwhile to consider the impacts of tourism activities in prioritisation efforts. Tourism can increase rodent abundance by enhancing food availability and facilitating human-assisted dispersal and could therefore provide an additional incentive to focus eradication efforts on certain islands, particularly where threatened or emblematic species are present (Radley et al. 2021).
However, we should point out that several significant rodent eradication campaigns are currently ongoing or under consideration, both in New Caledonia (e.g. Walpole island (170 ha) is home to a strictly endemic skink species (Epibator insularis) and of important seabird colonies), French Polynesia (e.g. on islets off Rapa, in the Austral islands, home to the only colonies of the critically endangered Rapa shearwater, Puffinus myrtae), and Clipperton atoll (180 ha, breeding site for the world’s largest colony of masked boobies, Sula dactylatra).
Strategies involving the use of poison often raise concerns among populations, particularly regarding the persistence of chemical residues in the environment or the risks of secondary poisoning (Dickie and Medvecky 2023) and the emergence of poison resistance in rodent populations (Russell and Broome 2016; Sran et al. 2022). These concerns can impact the reputation of the responsible managers, making them less inclined to develop dedicated strategies. This highlights the importance of careful management and communication. Given the significant ecological uncertainties, integrating all stakeholders would promote better acceptance of poison use in eradication actions, especially concerning primary and secondary poisoning risk (Eason et al. 1999). This would also strengthen local capacities and continually improve eradication and biosecurity methods across each territory.
With this synthesis, we hope to motivate and facilitate future eradication projects in the French Pacific territories, empower public and private agencies, and raise the standards of future operations in line with international rodent eradication guidelines (Keitt et al. 2015).
The next step is to develop a comprehensive strategy for the French Pacific territories or, more realistically, a specific strategy for each territory, considering their unique characteristics. These strategies should identify the islets that should be prioritised for conservation purposes and those that are invaded by rodents. It should also draw on the experiences of previous operations described in this manuscript to determine which islands are most likely to achieve successful eradication. These strategies must take into account the costs associated with each intervention on the different islets and propose a rigorous methodology for carrying out the eradications, relying on the database created for this manuscript.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Declaration of funding
This study was partly supported by the Fonds Pacifique-Fonds de coopération économique, social et culturel pour le Pacifique sud (Project RASIL 2022/2237), the Province Sud of New Caledonia (Convention C980.22). WW was supported by two scholarships (Province Nord of New Caledonia, and New Caledonia Agronomic Institute (IAC)).
Acknowledgements
We are grateful to ASSNC (Association pour la Sauvegarde de la Nature Néo-Calédonienne), the Hô-üt association, ANCB (Agence Néo-Calédonienne de la Biodiversité), SOP Manu (Société d’Ornithologie de Polynésie Française, Manu), ASBO (Association pour la Sauvegarde de la Biodiversité d’Ouvéa), Island Conservation, as well as the South and North Provinces of New Caledonia for providing data on rodent eradication.
References
Abdelkrim J, Pascal M, Samadi S (2007) Establishing causes of eradication failure based on genetics: case study of ship rat eradication in Ste. Anne Archipelago. Conservation Biology 21(3), 719-730.
| Crossref | Google Scholar | PubMed |
Aguirre-Muñoz A, Samaniego-Herrera A, Luna-Mendoza L, Ortiz-Alcaraz A, Rodríguez-Malagón M, Félix-Lizárraga M, Méndez-Sánchez F, González-Gómez R, Torres-García F, Hernández-Montoya JC, Barredo-Barberena JM, Latofski-Robles M (2011) Eradications of invasive mammals on islands in Mexico: the roles of history and the collaboration between government agencies, local communities and a non-government organisation. In ‘Island invasives: eradications and management’. (Eds CR Veitch, MN Clout, DR Towns) pp. 386–394. (IUCN: Gland, Switzerland)
Barnes S, Matisoo-Smith E, Hunt TL (2006) Ancient DNA of the Pacific rat (Rattus exulans) from Rapa Nui (Easter Island). Journal of Archaeological Science 33(11), 1536-1540.
| Crossref | Google Scholar |
Baudat-Franceschi J, Cromarty P, Golding C, Cranwell S, Breton JL, Butin JP, Boudjelas S (2011) Rodent eradication to protect seabirds in New Caledonia: the importance of baseline biological surveys, feasibility studies and community support. In ‘Island Invasives: eradication and management’. (Eds CR Veitch, MN Clout, DR Towns) pp. 26–31. (IUCN)
Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ (2004) Avian extinction and mammalian introductions on oceanic islands. Science 305(5692), 1955-1958.
| Crossref | Google Scholar | PubMed |
Broome K, Murphy E, Cunningham C, Eason CT (2010) New Zealand’s use of brodifacoum in eradication efforts and current investigation of new baits and toxins. Proceedings of the Vertebrate Pest Conference 24, 172-177.
| Crossref | Google Scholar |
Campbell KJ, Beek J, Eason CT, Glen AS, Godwin J, Gould F, Holmes ND, Howald GR, Madden FM, Ponder JB, Threadgill DW, Wegmann AS, Baxter GS (2015) The next generation of rodent eradications: innovative technologies and tools to improve species specificity and increase their feasibility on islands. Biological Conservation 185, 47-58.
| Crossref | Google Scholar |
Capizzi D, Baccetti N, Sposimo P (2010) Prioritizing rat eradication on islands by cost and effectiveness to protect nesting seabirds. Biological Conservation 143(7), 1716-1727.
| Crossref | Google Scholar |
Catalano AS, Lyons-White J, Mills MM, Knight AT (2019) Learning from published project failures in conservation. Biological Conservation 238, 108223.
| Crossref | Google Scholar |
Crowley SL, Hinchliffe S, McDonald RA (2017) Invasive species management will benefit from social impact assessment. Journal of Applied Ecology 54(2), 351-357.
| Crossref | Google Scholar |
Davis RA, Seddon PJ, Craig MD, Russell JC (2023) A review of methods for detecting rats at low densities, with implications for surveillance. Biological Invasions 25, 3773-3791.
| Crossref | Google Scholar |
De Garine-Wichatitsky M, Duncan P, Labbe A, Suprin B, Chardonnet P, Maillard D (2003) A review of the diet of Rusa Deer Cervus timorensis russa in New Caledonia: are the endemic plants defenceless against this introduced, eruptive ruminant? Pacific Conservation Biology 9(2), 136-143.
| Crossref | Google Scholar |
Dickie L, Medvecky F (2023) The attitudes of young adults towards mammalian predator control and Predator Free 2050 in Aotearoa New Zealand. Australasian Journal of Environmental Management 30(2), 170-187.
| Crossref | Google Scholar |
DIISE (2019) The database of Island invasive species eradications: developed by Island conservation. University of California Santa Cruz Coastal Conservation Action Lab, IUCN SSC Invasive Species Specialist Group, University of Auckland and Landcare Research New Zealand. Available at http://diise.islandconservation.org/ [28 June 2021]
Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR (2016) Invasive predators and global biodiversity loss. Proceedings of the National Academy of Sciences 113(40), 11261-11265.
| Crossref | Google Scholar |
Drake DR, Hunt TL (2009) Invasive rodents on islands: integrating historical and contemporary ecology. Biological Invasions 11, 1483-1487.
| Crossref | Google Scholar |
Eason CT, Milne L, Potts M, Morriss G, Wright GRG, Sutherland ORW (1999) Secondary and tertiary poisoning risks associated with brodifacoum. New Zealand Journal of Ecology 23, 219-224.
| Google Scholar |
Frantz AC, Luttringer A, Colyn M, Kazilas C, Berlioz E (2024) Landscape structure does not hinder the dispersal of an invasive herbivorous mammal in the New Caledonian biodiversity hotspot. European Journal of Wildlife Research 70(1), 6.
| Crossref | Google Scholar |
Fukami T, Wardle DA, Bellingham PJ, Mulder CPH, Towns DR, Yeates GW, Bonner KI, Durrett MS, Grant-Hoffman MN, Williamson WM (2006) Above- and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecology Letters 9(12), 1299-1307.
| Crossref | Google Scholar | PubMed |
Graham NA, Wilson SK, Carr P, Hoey AS, Jennings S, MacNeil MA (2018) Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 559(7713), 250-253.
| Crossref | Google Scholar | PubMed |
Griffiths R, Cranwell S, Derand D, Ghestemme T, Will D, Zito J, Hall T, Pott M, Coulston G (2019) Multi island, multi invasive species eradication in French Polynesia demonstrates economies of scale. In ‘Island invasives: scaling up to meet the challenge, Vol. 62.’ IUCN Occasional Paper SSC. (Eds C Veitch, M Clout, A Martin, J Russell, C West) pp. 611–617. (IUCN)
Haley AL, Lemieux TA, Piczak ML, Karau S, D’Addario A, Irvine RL, Beaudoin C, Bennett JR, Cooke SJ (2023) On the effectiveness of public awareness campaigns for the management of invasive species. Environmental Conservation 50(4), 202-211.
| Crossref | Google Scholar |
Harper GA (2020) Tropical Island rodent eradications: a proposed model to improve outcomes. Proceedings of the Vertebrate Pest Conference 29, 3.
| Google Scholar |
Harper GA, Bunbury N (2015) Invasive rats on tropical islands: their population biology and impacts on native species. Global Ecology and Conservation 3, 607-627.
| Crossref | Google Scholar |
Harris DB, Gregory SD, Bull LS, Courchamp F (2012) Island prioritization for invasive rodent eradications with an emphasis on reinvasion risk. Biological Invasions 14, 1251-1263.
| Crossref | Google Scholar |
Hoffmann BD, Tessmann A, Quinn G, Lawton F (2023) Quantification of flight times of aerial treatments targeting invasive species: the interplay of helicopter or drone with bait-delivery systems, flight speed and bait form. Pest Management Science 79(6), 2050-2055.
| Crossref | Google Scholar | PubMed |
Holdaway RN, Hawke DJ, Hyatt OM, Wood GC (2007) Stable isotopic (δ15N, δ13C) analysis of wood in trees growing in past and present colonies of burrow-nesting seabirds in New Zealand. I. δ15N in two species of conifer (Podocarpaceae) from a mainland colony of Westland petrels (Procellaria westlandica), Punakaiki, South Island. Journal of the Royal Society of New Zealand 37(2), 75-84.
| Crossref | Google Scholar |
Holmes ND, Campbell KJ, Keitt BS, Griffiths R, Beek J, Donlan CJ, Broome KG (2015a) Reporting costs for invasive vertebrate eradications. Biological Invasions 17, 2913-2925.
| Crossref | Google Scholar |
Holmes ND, Griffiths R, Pott M, Alifano A, Will D, Wegmann AS, Russell JC (2015b) Factors associated with rodent eradication failure. Biological Conservation 185, 8-16.
| Crossref | Google Scholar |
Holmes ND, Spatz DR, Oppel S, Tershy B, Croll DA, Keitt B, Genovesi P, Burfield IJ, Will DJ, Bond AL, Wegmann A, Aguirre-Muñoz A, Raine AF, Knapp CR, Hung C-H, Wingate D, Hagen E, Méndez-Sánchez F, Rocamora G, Yuan H-W, Fric J, Millett J, Russell J, Liske-Clark J, Vidal E, Jourdan H, Campbell K, Springer K, Swinnerton K, Gibbons-Decherong L, Langrand O, Brooke MdL, McMinn M, Bunbury N, Oliveira N, Sposimo P, Geraldes P, McClelland P, Hodum P, Ryan PG, Borroto-Páez R, Pierce R, Griffiths R, Fisher RN, Wanless R, Pasachnik SA, Cranwell S, Micol T, Butchart SHM (2019) Globally important islands where eradicating invasive mammals will benefit highly threatened vertebrates. PLoS ONE 14(3), e0212128.
| Crossref | Google Scholar | PubMed |
Howald G, Donlan CJ, Galvan JP, Russell JC, Parkes J, Samaniego A, Wang Y, Veitch D, Genovesi P, Pascal M, Saunders A, Tershy B (2007) Invasive rodent eradication on islands. Conservation Biology 21(5), 1258-1268.
| Crossref | Google Scholar | PubMed |
Innes JG, Norbury G, Samaniego A, Walker S, Wilson DJ (2024) Rodent management in Aotearoa New Zealand: approaches and challenges to landscape-scale control. Integrative Zoology 19(1), 8-26.
| Crossref | Google Scholar | PubMed |
Kapitza K, Zimmermann H, Martín-López B, Von Wehrden H (2019) Research on the social perception of invasive species: a systematic literature review. NeoBiota 43, 47-68.
| Crossref | Google Scholar |
Kappes PJ, Bond AL, Russell JC, Wanless RM (2019) Diagnosing and responding to causes of failure to eradicate invasive rodents. Biological Invasions 21(7), 2247-2254.
| Crossref | Google Scholar |
Keitt B, Griffiths R, Boudjelas S, Broome K, Cranwell S, Millett J, Pitt W, Samaniego-Herrera A (2015) Best practice guidelines for rat eradication on tropical islands. Biological Conservation 185, 17-26.
| Crossref | Google Scholar |
Matisoo-Smith E, Robins JH (2004) Origins and dispersals of Pacific peoples: evidence from mtDNA phylogenies of the Pacific rat. Proceedings of the National Academy of Sciences 101(24), 9167-9172.
| Crossref | Google Scholar |
Meyer JY, Strasberg D, Vidal E, Jourdan H, Delnatte C, Muller S (2018) Quelle stratégie de recherche pour une meilleure conservation de la biodiversité terrestre dans les îles tropicales ultramarines françaises? Naturae 2018(4), 15 26.
| Google Scholar |
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403(6772), 853-858.
| Crossref | Google Scholar | PubMed |
Parkes J, Fisher P, Forrester G (2011) Diagnosing the cause of failure to eradicate introduced rodents on islands: brodifacoum versus diphacinone and method of bait delivery. Conservation Evidence 8, 100-106.
| Google Scholar |
Philippe-Lesaffre M, Thibault M, Caut S, Bourgeois K, Berr T, Ravache A, Vidal E, Courchamp F, Bonnaud E (2023) Recovery of insular seabird populations years after rodent eradication. Conservation Biology 37(3), e14042.
| Crossref | Google Scholar | PubMed |
Pichlmueller F, Murphy EC, MacKay JWB, Henderson J, Fewster RM, Russell JC (2020) Island invasion and reinvasion: Informing invasive species management with genetic measures of connectivity. Journal of Applied Ecology 57(11), 2258-2270.
| Crossref | Google Scholar |
Pullin AS, Cheng SH, Cooke SJ, Haddaway NR, Macura B, Mckinnon MC, Taylor JJ (2020) Informing conservation decisions through evidence synthesis and communication. In ‘Conservation research, policy and practice’. (Eds WJ Sutherland, PNM Brotherton, ZG Davies, N Ockendon, N Pettorelli, JA Vickery) pp. 114–128. (Cambridge University Press) 10.1017/9781108638210.007
R Core Team (2023) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. Available at https://www.R-project.org/
Radley P, Davis RA, Doherty TS (2021) Impacts of invasive rats and tourism on a Threatened Island bird: the palau micronesian scrubfowl. Bird Conservation International 31(2), 206-218.
| Crossref | Google Scholar |
Rauzon M (2007) Island restoration: exploring the past; anticipating the future. Marine Ornithology 35(2), 97-107.
| Crossref | Google Scholar |
Ricketts TH, Dinerstein E, Boucher T, Brooks TM, Butchart SH, Hoffmann M, Lamoreux JF, Morrison J, Parr M, Pilgrim JD, Rodrigues ASL, Sechrest W, Wallace GE, Berlin K, Bielby J, Burgess ND, Church DR, Cox N, Knox D, Loucks C, Luck GW, Master LL, Moore R, Naidoo R, Ridgely R, Schatz GE, Shire G, Strand H, Wettengel W, Wikramanayake E (2005) Pinpointing and preventing imminent extinctions. Proceedings of the National Academy of Sciences 102(51), 18497-18501.
| Crossref | Google Scholar |
Rodary E (2024) Which environmental policies for New Caledonia? In ‘Geographies of new caledonia-kanaky: environments, politics and cultures’. (Eds M Kowasch, SPJ Batterbury) pp. 13–20. (Springer)10.1007/978-3-031-49140-5_2
Roy HE, Pauchard A, Stoett P, Renard Truong T, Bacher S, Galil BS, Hulme PE, Ikeda T, Sankaran KV, McGeoch MA, Meyerson LA, Nuñez MA, Ordonez A, Rahlao SJ, Schwindt E, Seebens H, Sheppard AW, Vandvik V (2023) IPBES invasive alien species assessment: summary for policymakers. Zenodo. 10.5281/zenodo.10127924
Russell JC (2014) A comparison of attitudes towards introduced wildlife in New Zealand in 1994 and 2012. Journal of the Royal Society of New Zealand 44(4), 136-151.
| Crossref | Google Scholar |
Russell JC, Broome KG (2016) Fifty years of rodent eradications in New Zealand: another decade of advances. New Zealand Journal of Ecology 40(2), 197-204.
| Crossref | Google Scholar |
Russell JC, Holmes ND (2015) Tropical island conservation: rat eradication for species recovery. Biological Conservation 185, 1-7.
| Crossref | Google Scholar |
Russell JC, Beaven BM, MacKay JWB, Towns DR, Clout MN (2008b) Testing island biosecurity systems for invasive rats. Wildlife Research 35(3), 215-221.
| Crossref | Google Scholar |
Russell JC, Miller SD, Harper GA, MacInnes HE, Wylie MJ, Fewster RM (2010) Survivors or reinvaders? Using genetic assignment to identify invasive pests following eradication. Biological Invasions 12, 1747-1757.
| Crossref | Google Scholar |
Samaniego A, Griffiths R, Gronwald M, Holmes ND, Oppel S, Stevenson BC, Russell JC (2020) Risks posed by rat reproduction and diet to eradications on tropical islands. Biological Invasions 22, 1365-1378.
| Crossref | Google Scholar |
Samaniego A, Kappes P, Broome K, Cranwell S, Griffiths R, Harper G, McClelland P, Palmer R, Rocamora G, Springer K, Will D, Siers S (2021) Factors leading to successful island rodent eradications following initial failure. Conservation Science and Practice 3(6), e404.
| Crossref | Google Scholar |
Segal RD, Whitsed R, Massaro M (2022) Review of the reporting of ecological effects of rodent eradications on Australian and New Zealand islands. Pacific Conservation Biology 28(1), 4-14.
| Crossref | Google Scholar |
Soubeyran Y, Meyer JY, Lebouvier M, De Thoisy B, Lavergne C, Urtizberea F, Kirchner F (2015) Dealing with invasive alien species in the French overseas territories: results and benefits of a 7-year Initiative. Biological Invasions 17, 545-554.
| Crossref | Google Scholar |
Spatz DR, Holmes ND, Will DJ, Hein S, Carter ZT, Fewster RM, Keitt B, Genovesi P, Samaniego A, Croll DA, Tershy BR, Russell JC (2022) The global contribution of invasive vertebrate eradication as a key island restoration tool. Scientific Reports 12(1), 13391.
| Crossref | Google Scholar | PubMed |
Sran SPK, Gartrell BG, Fisher P, Armstrong DP (2022) Apparent resistance to brodifacoum in Rattus rattus in a New Zealand site with no history of anticoagulant-based rodent control. Wildlife Research 50(1), 28-38.
| Crossref | Google Scholar |
Tershy BR, Croll DA, Newton KM (2012) Accomplishments and impact of the NGO, Island Conservation, over 15 years (1994–2009). Biodiversity and Conservation 21, 957-965.
| Crossref | Google Scholar |
Tershy BR, Shen K-W, Newton KM, Holmes ND, Croll DA (2015) The importance of islands for the protection of biological and linguistic diversity. Bioscience 65(6), 592-597.
| Crossref | Google Scholar |
Towns DR, Broome KG (2003) From small Maria to massive Campbell: forty years of rat eradications from New Zealand islands. New Zealand Journal of Zoology 30(4), 377-398.
| Crossref | Google Scholar |


