Satellite-tracked movements of juvenile great egrets (Ardea alba) and plumed egrets (Ardea plumifera) from the Macquarie Marshes in the Murray–Darling Basin, Australia
Heather M. McGinness A * , Micha V. Jackson A , Luke R. Lloyd-Jones B , Xinyu Hou A , Louis O’Neill A , Shoshana Rapley A and Freya Robinson C
A * , Micha V. Jackson A , Luke R. Lloyd-Jones B , Xinyu Hou A , Louis O’Neill A , Shoshana Rapley A and Freya Robinson C
A
B
C
Abstract
Understanding waterbird movements is critical for conserving populations and protecting habitats.
To provide knowledge of where, when, and how quickly dispersing juvenile egrets move and support identification of critical routes and sites for habitat management.
We deployed GPS transmitters on 18 juvenile egrets of two species: great egret (Ardea alba; n = 10) and plumed egret (Ardea plumifera; n = 8) at natal sites in the Macquarie Marshes, Australia. We tracked dispersal movements, including timing, directions, and distances, as well as post-dispersal daily movement timing and distances travelled between roosts and foraging sites.
Dispersals of great egrets occurred in multiple directions (27–257 km in the first 72 h); all plumed egrets flew north (136–797 km in the first 72 h). Post-dispersal foraging movements from roosts were short for both species (1–2 km). One plumed egret was tracked flying from Australia to Papua New Guinea, completing a non-stop flight of approximately 38 h.
This is the first time that GPS telemetry has been used to track egret movements in Australia. It is also the first GPS record of a precise movement path between Australia and New Guinea for any large aggregate-nesting wader species. Tracking revealed key wetland sites and routes and highlighted use of small spatial areas post-dispersal.
Movement patterns suggest that juvenile great egrets may benefit more from wetland management in the Murray–Darling Basin than juvenile plumed egrets, at least during dispersal movements and their first year.
Keywords: dispersal, egret, environmental water, migration, movement, stopover, telemetry, waterbird.
Introduction
Egrets (Ardea spp.) and related wading waterbird species in the Ardeidae family are highly dependent on wetlands and water for breeding, foraging, and roosting habitats. Consequently, many egrets are highly mobile, particularly in regions like inland Australia with large fluctuations in water availability and weather. Understanding egret movements is critical for conserving their populations, protecting their habitats, and ensuring the overall health of ecosystems they inhabit. Around the world, use of movement tracking has greatly expanded knowledge about multiple egret species and their conservation and management requirements. For example, it has clarified the movement and nesting ecology of North American species (Brzorad et al. 2015; Koczur and Ballard 2024), documented critical routes, sites, and habitat use for habitat protection and resource prioritisation in China (Huang et al. 2022); quantified habitat use by post-fledging juveniles in South Korea, which is a critical stage for recruitment (Son et al. 2021), and identified potential threats and risks such as habitat loss, pollution, human disturbance, or climate change impacts (e.g. Huang et al. 2022). Such knowledge supports decision-making, effective management practices, and the development of targeted conservation strategies (Richardson and Taylor 2003; Arthur 2011).
Recently, a major satellite tracking effort has significantly expanded available knowledge about the movement ecology of Threskiornithidae species (ibis and spoonbills) in inland Australia that breed at the same sites as multiple egret species (McGinness et al. 2024a). This research has shown that ibis and spoonbills use common inland routes with identifiable characteristics (McGinness et al. 2024b). This information has allowed for detailed consideration of water and wetland management strategies; for example, suggesting resource requirements across the life cycle (McGinness et al. 2024a) and identifying where resource direction may be most beneficial (McGinness et al. 2024b). However, similar detailed knowledge about egret movements in Australia is lacking, hampering analogous efforts for understanding and conserving egret species across the continent.
Australia hosts 26 species in the Ardeidae family (herons, egrets and bitterns). Six of these are commonly known as egrets, with the great egret (Ardea alba), plumed egret (Ardea plumifera), cattle egret (Bulbulcus ibis), little egret (Egretta garzetta), and eastern reef egret (Egretta sacra) breeding residents, and the intermediate egret (Ardea intermedia) vagrant in the north and west. The great egret (A. alba) is an extremely widespread and well-studied species occurring across northern and southern America, Africa, southern and central Europe, and Australasia (Marchant and Higgins 1990; Del Hoyo et al. 1992; Pratt 2011). The plumed egret (A. plumifera) was previously regarded as a subspecies of the intermediate egret (A. intermedia plumifera), together with two other subspecies, the Asian intermediate egret (A. intermedia intermedia) and the African yellow-billed egret (A. intermedia brachyrhyncha; Walsh and Chafer (2022)). These three species were previously placed in the genus Mesophoyx (Del Hoyo et al. 2014) and grouped as Mesophoyx intermedia following Sibley and Monroe (1990). However in 2023, the International Ornithological Congress recognised all three subspecies as species, which were split into the ‘plumed egret’, ‘medium egret’, and ‘yellow-billed egret’ (Walsh and Chafer 2022; IOC 2024). In courtship and breeding, plumage the three species are easily distinguishable; however, in non-breeding plumage, they are difficult to separate in the field and immature individuals look very similar to adults (Marchant and Higgins 1990; Walsh and Chafer 2022). The plumed egret is mainly found in Australia, New Guinea, eastern Indonesia, and Timor-Leste. The IUCN Red List of Threatened Species currently describes the population as ‘least concern’, assumed decreasing, and ‘not a migrant’ (BirdLife International 2016), but these classifications likely need reassessment following the taxonomic revisions described above.
Internationally, great egrets, plumed egrets, and intermediate egrets are known to use a range of movement strategies, including migration, nomadism, and residency (Marchant and Higgins 1990; Del Hoyo et al. 1992; Melvin et al. 1999; Mužinić 2011; Brzorad et al. 2015; Hayes et al. 2023). Local on-ground counts and tracked movements of egrets are known to fluctuate with season, weather, and water levels, and vary from a few kilometres to thousands of kilometres, suggesting that movements are influenced by a range of factors (Geering et al. 1998; Weseloh et al. 2014; Brzorad et al. 2021; Lumpkin et al. 2023).
In Australia, the movement strategies, routes and staging sites of egret species are relatively poorly understood (Marchant and Higgins 1990; McKilligan 2005). However, it is known from leg-band and patagial tag recoveries and re-sightings that these species can move significant distances (Table 1), including as juveniles, even crossing from Australia to other landmasses (Geering et al. 1998; Chambers and Loyn 2006). Geering et al. (1998) used a combination of Australian Bird and Bat Banding Scheme (ABBBS) banding and patagial tag data to infer movement distances and patterns for great, intermediate (i.e. plumed, refer to the recent taxonomic split detailed above) and little egrets. They found that all three egret species undertake movements in all directions but suggested that the location of the natal heronry may affect the predominant direction of dispersal movements, with birds from inland nesting sites tending to move north, and birds from coastal sites moving in any direction. With few banded or tagged individuals found spending the winter near known nesting sites, and regular seasonal fluctuations in egret species abundance recorded in both Australia and nearby countries, it has been suggested that a partial seasonal migration may occur, with egrets spending late autumn and winter in New Guinea and returning to Australia to breed in spring and summer (Halse et al. 1996; Geering et al. 1998). For more information from ABBBS records, see the Supplementary Material.
| Great egret | Intermediate (plumed) egret | ||
|---|---|---|---|
| Number banded | 1075 | 1796 | |
| Banding period | 1959–2023 | 1958–2002 | |
| Distinct recoveries | 57 | 26 | |
| % recovered overall | 5.3 | 1.4 | |
| Recoveries after >1 month | 43 | 16 | |
| % recovered after >1 month | 4 | 0.89 | |
| Recoveries after >1 year | 14 | 8 | |
| % recovered after >1 year | 1.3 | 0.45 | |
| Max elapsed time (years) | 10 | 8 | |
| Max distance (km) | 3366 | 3992 |
While these insights and observations are intriguing, recovery and resighting data for banded birds remain limited (Table 1), and the precise timing and routes of movements by these egret species in Australia remain unknown, including juvenile dispersal movements from natal sites. Movement information for juvenile egrets is particularly important because mortality rates can be high and they may not mature or successfully breed until they are at least 2 years old, with implications for population dynamics and conservation management at appropriate scales (Marchant and Higgins 1990; Shirai 2013; Weseloh et al. 2014; Włodarczyk et al. 2020). Knowledge of the directions, distances, and timing of movements made by both juveniles and adults is essential for spatially and temporally appropriate and efficient policy and management for their conservation (Allen and Singh 2016; Cottee-Jones et al. 2016; Ogburn et al. 2017).
Across inland temperate and semi-arid breeding sites of eastern Australia, egret breeding events now occur less frequently than they did historically (Arthur 2011). Population modelling for egrets in Australia has found that: (1) most egrets must breed every 1–2 years for populations to persist; and (2) population growth is sensitive to adult survival (Arthur 2011). Modelling also showed that juvenile survival rates averaging 25–35% per year are probably required for persistence (Arthur 2011), emphasising the importance of habitat and food availability for both juveniles and adults after breeding events. There is a need to determine over what spatial scale egret populations function so that suitable foraging habitats and sufficient frequency of breeding events are enabled and supported at the appropriate scale by managers. To begin filling these knowledge gaps, we used Global Positioning System (GPS) satellite telemetry to track the natal dispersal movements of 18 juvenile great egrets and plumed egrets from the Macquarie Marshes, NSW, an important inland breeding site where these species have only been leg-banded in the past.
Materials and methods
Study area
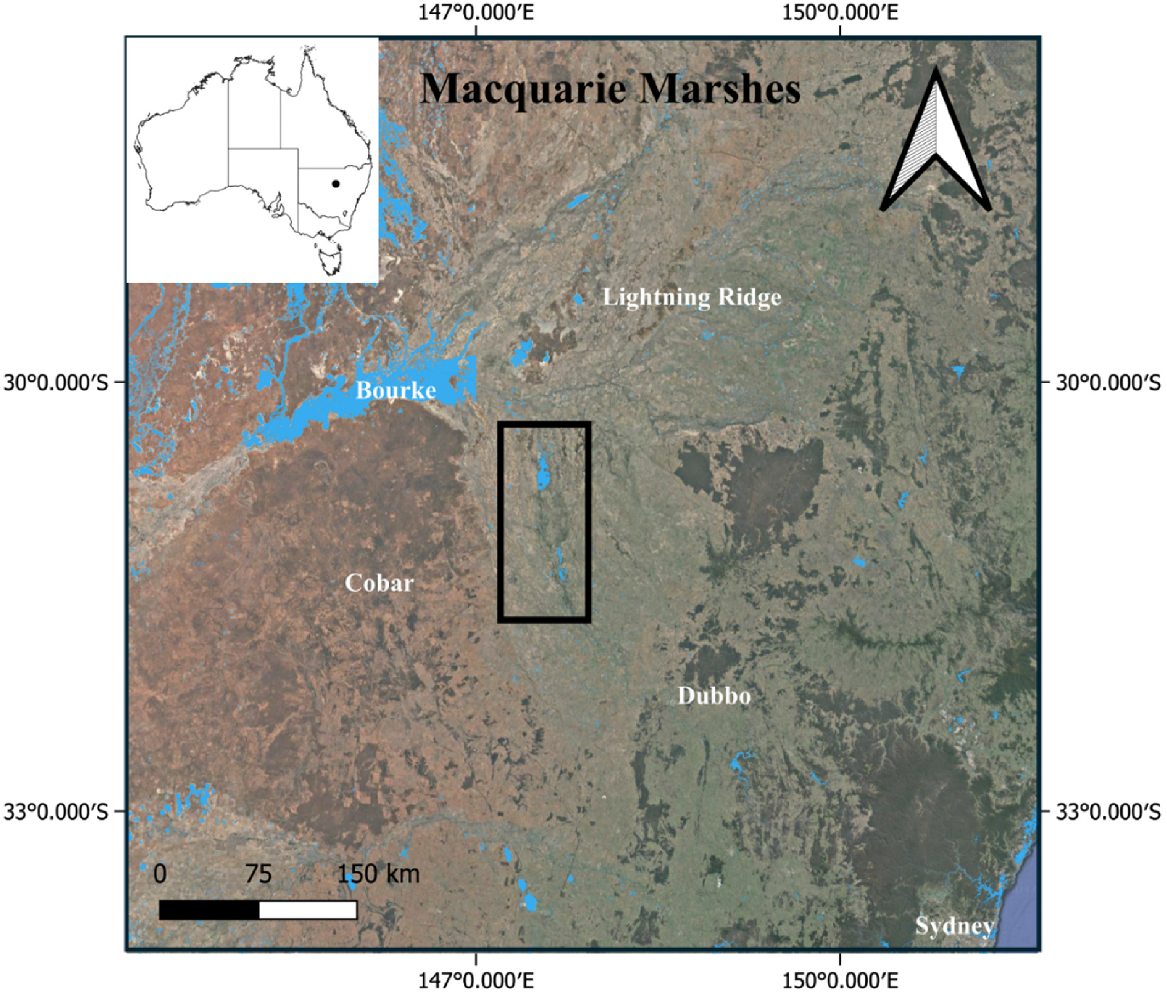
The Macquarie Marshes is a semi-arid floodplain wetland in central western New South Wales, Australia (Fig. 1), covering an area of at least 200,000 ha, depending on weather and river flows (Thomas et al. 2011). It is one of the most important wetlands in Australia for aggregate-nesting waterbirds both in terms of size (i.e. number of individuals breeding) and breeding frequency (Kingsford and Auld 2005). Breeding data collected between 1986 and 2001 indicate that great and plumed egrets breed regularly in the Macquarie Marshes, with more than 4000 nests of plumed egret recorded three times over this period (Kingsford and Auld 2005).
Map showing the Macquarie Marshes, New South Wales, Australia (black rectangle shows approximate extent), the location of capture sites of great egrets (n = 10) and plumed egrets (n = 8) tracked in 2023–2024.
During the 2022–2023 breeding season (austral summer), there was widespread flooding in the Macquarie Marshes for two consecutive years, which inundated approximately 232,000 ha, following above-average rainfall and river flows during 2021 and 2022 (Brandis et al. 2023). In October–November 2022, three large waterbird nesting aggregations (dominant species were straw-necked ibis (Threskiornis spinicollis), Australian white ibis (Threskiornis molluca), and royal spoonbill (Platelea regia) were established within the Macquarie Marshes (Brandis et al. 2023). Egrets, Nankeen Night Herons (Nycticorax caledonicus), and cormorants (Phalacrocoracidae) also nested within the extent of these nesting aggregations, and an additional 27 heronries comprising primarily egrets, herons, and cormorants were also recorded (Brandis et al. 2023). Combined with the relatively unusual occurrence that some egret breeding occurred low in shrubs rather than high in trees, this extensive breeding event provided an unusually good opportunity to catch juvenile egrets for GPS tracking of dispersal movements.
Transmitter deployment
Transmitters were deployed at two heronries within the Macquarie Marshes on 14–16 February 2023 (Fig. 1; see Supplementary Table S1). A total of 18 devices (n = 15 GeoTrak solar powered GPS Argos 12 g transmitters; n = 3 Druid solar powered GPS GSM 3G 10.6 g transmitters) were fitted on great egrets (n = 10) and plumed egrets (n = 8). Geotrak Argos transmitters were programmed to collect one location fix once every 3 h during long day-length months of the year (any fixes between August and April), transmitting every 2 days, and one fix every 6 h during months of the year with short day-lengths (any fixes between May and July), transmitting every 4 days. This was done to conserve battery during periods of reduced solar recharge. Sensor data collected by these transmitters included temperature, voltage, and activity. Druid GSM transmitters were programmed to collect a minimum of one fix per hour, with a ‘boost’ function to collect as many fixes as possible and transmit as often as possible when battery status and GSM reception was good, resulting in portions of each track with fixes only seconds apart. These transmitters also collected altitude and speed data, and sensor data including temperature, light intensity, air pressure, and voltage.
Transmitters recorded height above mean sea level in addition to longitude, latitude, and timestamp, with horizontal accuracy of <26 m or better, based on manufacturer’s accuracy values and on-ground testing of stationary units (Chelak et al. 2025). Vertical height errors can range from 1 to 33 m in GPS transmitters and there is greater variation associated with low-frequency fixes than high-frequency fixes, partly because of the greater number of satellites used per fix in high-frequency data (Byrne et al. 2017; Lato et al. 2022; Schaub et al. 2023; Chelak et al. 2025). Transmitters that use large numbers of satellites can have horizontal errors of only 6.5–8 m (Lato et al. 2022; Ferraz et al. 2024) and vertical errors of only 3.2 m (Lato et al. 2022). Schaub et al. (2023) reported a mean error for low-frequency transmitters of 6.3 ± 4.6 (s.d.) m compared to a mean of 2.4 ± 1.0 (s.d.) m for high-frequency data. The Druid transmitters used here typically recorded high-frequency data in continuous bursts when battery levels and reception are good, which is associated with use of higher numbers of satellites and higher accuracy (Schaub et al. 2023). The use of additional satellite systems such as GNSS or GLONASS in addition to GPS in Druid transmitters also likely reduces error (Schaub et al. 2023). We considered that horizontal errors were negligible compared to the scales at which these species move (i.e. the range of flight heights recorded for these species, and the long distances and fast rates that they travel) and that inferences regarding flight routes are reasonable; however, it is difficult to predict the effects of error variation on results (Poessel et al. 2018; Péron et al. 2020) and we recommend caution when interpreting these data, particularly for flight heights (Schaub et al. 2023).
Transmitters were 1.35% ± 0.06% (mean ± s.e.) and 2.69% ± 0.09% of the bird’s body weight for great and plumed egrets, respectively (Table S1). All egrets fitted with transmitters were juveniles that were nearly full-grown and were flapping or undertaking short flights but could still be caught by hand. Transmitters were attached with ‘backpack’ harnesses using leg loops made of Teflon ribbon or Spectra ribbon (Bally Ribbon Mills). Because the birds were juveniles, some harnesses were fitted with gathers in the ribbon using dissolvable sutures to allow adjustment in fit as birds grew or fitted less closely than for an adult. Back feathers deemed likely to cover the solar panel were trimmed for one great egret and four plumed egrets. Basic biometrics were recorded and feather and swab samples taken for future analysis. Feathers were extracted from the breast area and DNA was later extracted from these feather tips for sexing.
Dispersal distance and timing
We defined commencement of individual dispersal as a movement >1 km from the location of the nest, after which the individual did not return to within a 1-km radius of the nest location. We calculated geographic distance and cumulative distance travelled in the first 72 h of dispersal as: (1) the straight line distance between the last pre-dispersal fix and the fixes that occurred 24, 48, and 72 h later; and (2) the sum of straight line distances between every fix (3-h intervals for Geotrak tags, 1- h intervals for Druid tags) starting from the last pre-dispersal fix.
Post-dispersal movements
We explored the daily timing of post-dispersal movement activity by dividing fixes into those with distances to the next fix of >100 m and <100 m and calculating the proportion of >100 m and <100 m fixes per time period. Datasets with different location fix schedules were summarised separately (e.g. August–April, 3-h interval fixes; May–July, 6-h interval fixes) and data from transmitters with high-frequency fixes were simplified to one fix per hour. We also explored post-dispersal foraging distances. First, for each bird with post-dispersal fixes, we determined periods of residency by calculating the distance travelled between midnight fixes for each day. If this distance was <10 km, we classified this daily period as a residency period. Second, for all birds where there were at least three daily residency periods, we calculated the maximum distance travelled from the midnight (00:00 hours) fix to any other fix during that daily period and interpreted this as the maximum daily distanced travelled from the roost to forage. Finally, we visually explored possible associations between weather events and bird mortality by plotting the times that transmitters stopped transmitting with local minimum and maximum temperatures and rainfall data sourced from the Australian Bureau of Meteorology.
Ethics statement
All research protocols were approved by an authorised Animal Care and Ethics Committee, according to the Australian code of practice for the care and use of animals for scientific purposes (CSIRO Animals Ethics Committee permit 2019-13). On-ground fieldwork activities were conducted under New South Wales and Victoria Scientific Licences 102180 and 10010534.
Results
Overview
Juvenile egret dispersals from the heronries occurred 9–44 days after transmitter deployment (mean 23 ± 9 days; Table S1). Eight of 10 great egrets fitted with transmitters successfully dispersed (four female and four male; Table S1). Of the two great egrets that did not leave the heronries (both male), one (239909; Fig. S2) left the nest 30 days after transmitter deployment, but data ceased 2 days later, with no evidence in either GPS locations or transmitter sensor data to suggest transmitter detachment or mortality. The other egret (239913; Fig. S6) did not leave the nest location and sensors indicated a sudden drop in activity, voltage and solar recharge 13 days after deployment, suggesting that either the transmitter detached, or the bird died.
Five of eight plumed egrets fitted with transmitters successfully dispersed from the heronries (three female and two male; Table S1). Of the three that did not (two female, one male), one (239903; Fig. S11) left the nest 25 days after transmitter deployment and was foraging around the heronries, but data ceased 7 days later. There was no evidence of mortality in either GPS locations or activity sensor data, but transmitter voltage and temperature dropped 2 days after nest departure. Neither of the remaining two left the nest; one (239906; Fig. S13) stopped transmitting after a plunge in sensor temperature, voltage, and activity 22 days after deployment, suggesting that either the transmitter detached, or the bird died. The other (043E00; Fig. S9) ceased transmitting 19 days after deployment, with no evidence from GPS locations or sensor data of mortality or transmitter detachment.
There were some commonalities in the dates when transmitters ceased or when significant sensor changes were observed (Fig. 2). For the five individuals that did not disperse from the heronries, cease dates co-occurred with a heatwave of successive days of >40°C maximum temperatures: 27–8 February, 6–8 March, and 17–20 March (Fig. 2). An individual that had dispersed from the heronries but was still within the Macquarie Marshes area (239912; Fig. S5) also stopped transmitting during the heatwave on 17–20 March. Across northern NSW, maximum and minimum temperatures plunged on 9–11 March, immediately following the heatwave on 6–8 March. This change correlated with the cease dates of three individuals that had dispersed from the Macquarie Marshes (Fig. 2), one great egret and two plumed egrets (239911, Fig. S4; 239907, Fig. S14; 044300; Fig. S10).
Great egrets
Juvenile great egrets travelled a mean distance of 30 ± 26 km (range 4–70 km) in the first 24 h of dispersal from the nest site, 63 ± 52 km (range 22–163 km) in the first 48 h of dispersal from the nest site, and 100 ± 93 km (range 27–257 km) in the first 72 h of dispersal from the nest site (Table S2). Mean cumulative distance travelled in the first 72 h was 108 ± 98 km (range 36–278 km; Table S2). The last fix prior to dispersal for all great egrets was from either ~3 am (n = 2) or ~6 am (n = 5) with one exception of an individual with last fix prior to dispersal at ~6 pm (Table S3), suggesting that post-natal dispersal movements in the early morning are most common for this species.
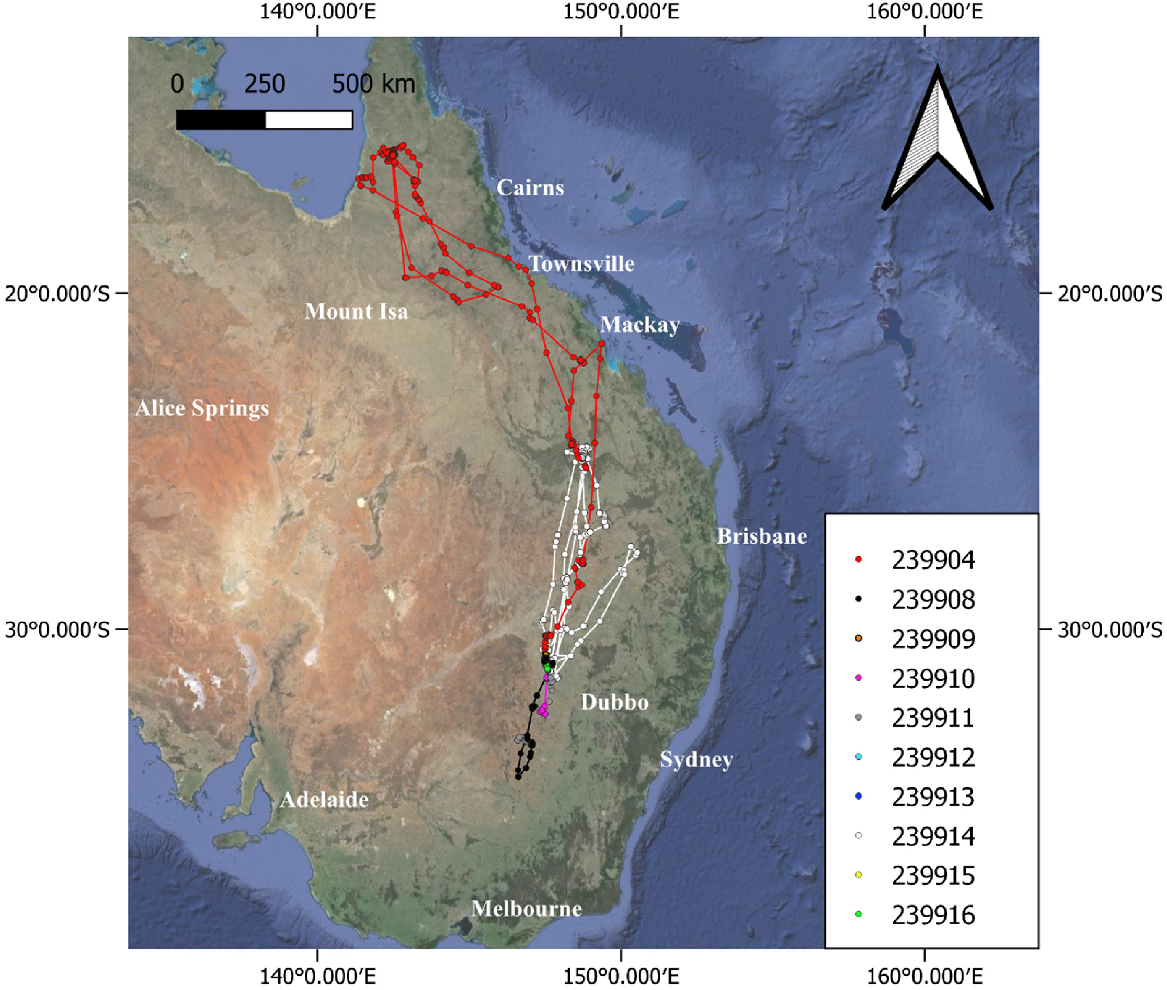
Two great egrets dispersed rapidly north (Fig. 3); these two individuals were still mobile and actively transmitting data at the time of writing (n = 715 and 714 days, Figs 4, 5). Of the other six that dispersed from the heronries, four dispersed rapidly south (Figs 3, S1, S3, S4, S8), while two moved to areas only slightly west or north of the Macquarie Marshes heronries (Figs S5, S7). Data ceased for two of the birds that few south with no evidence in either GPS locations or sensor data to indicate mortality, 24 and 36 days after deployment (Tables S1, S4). Four transmitters became stationary with corresponding temperature and battery sensor changes; the last fix locations of three of these were checked (the fourth could not be accessed), with two transmitters recovered showing evidence of predation and the third not found.
Great egrets (n = 10) tracked from the Macquarie Marshes in 2023–2024. Clustering of fixes at the natal site makes it difficult to see the four individuals (239909, 239912, 239913, 239915) that either did not disperse or were only tracked for a very short time post-dispersal.
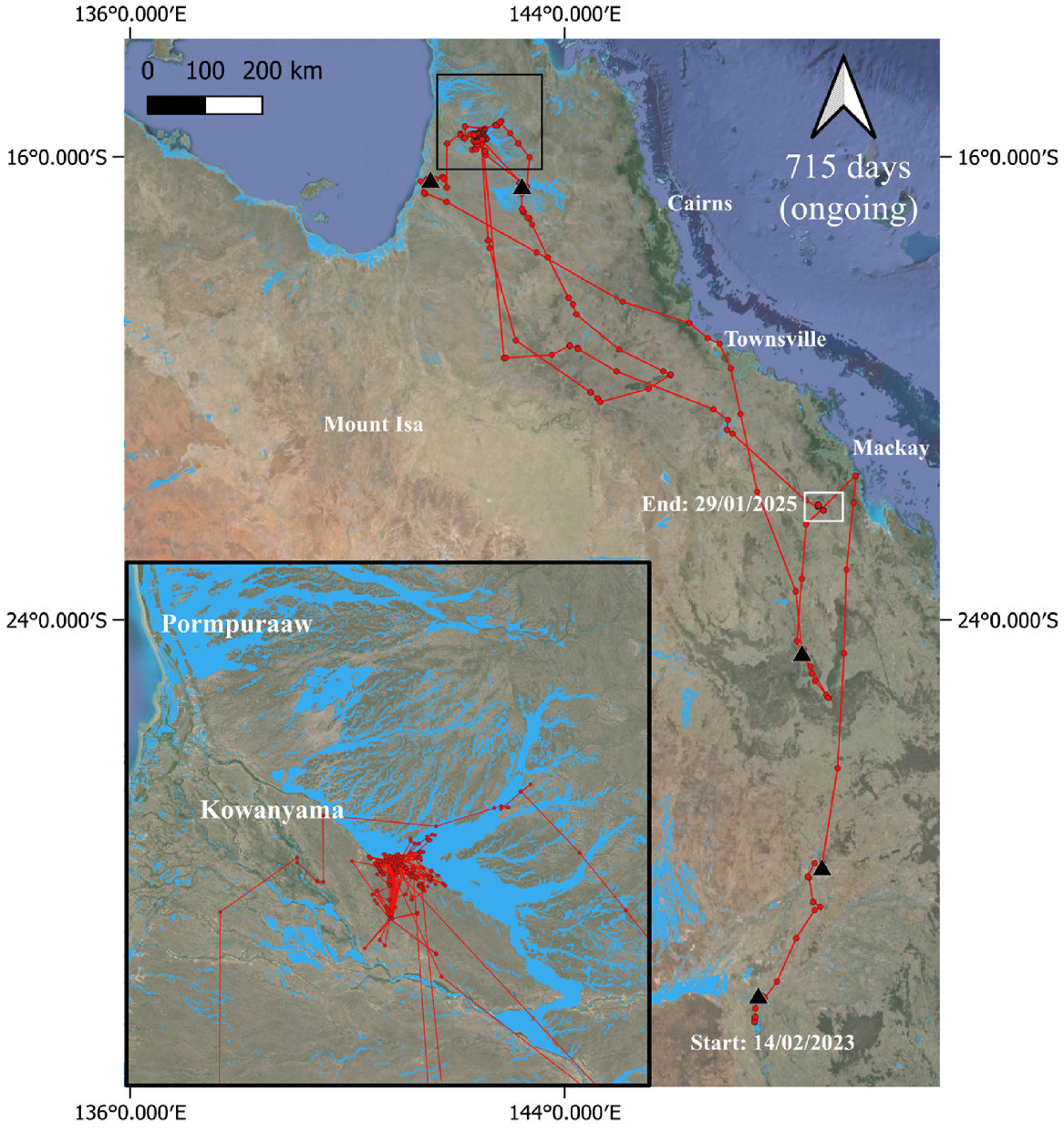
Female great egret (239904) tracked from the Macquarie Marshes 2023–2025. ‘Days’ under the north arrow = number of days bird was tracked to 29 January 2025 (‘ongoing’ = the individual was still transmitting at the cut-off date). Inset shows the location where the bird spent the most time. Black triangles indicate stopover areas. Animations of tracks can be viewed at https://doi.org/10.25919/g596-3b73.
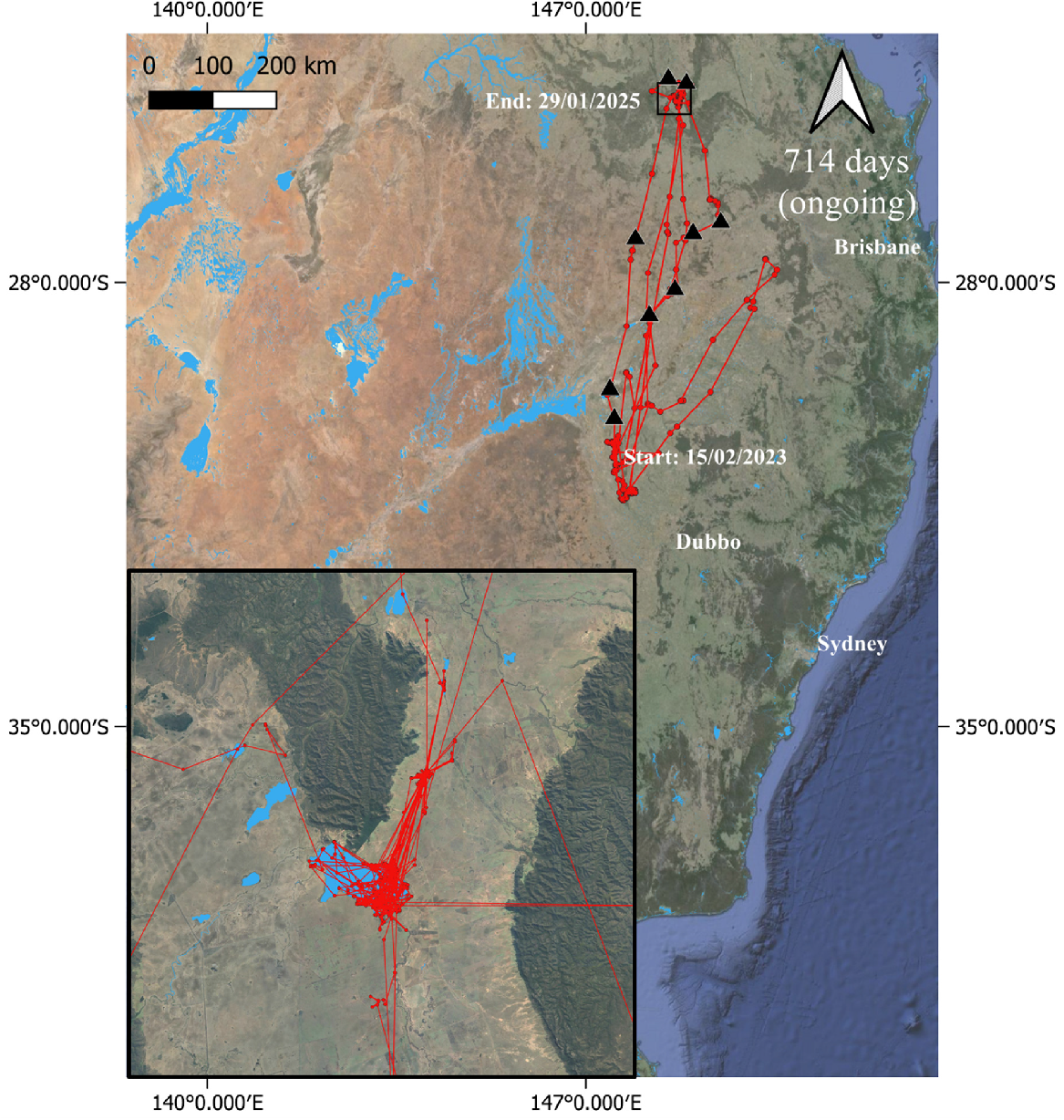
Male great egret (239914) tracked from the Macquarie Marshes in 2023–2025. ‘Days’ under the north arrow = number of days bird was tracked to 29 January 2025 (‘ongoing’ = the individual was still transmitting at the cut-off date). Inset shows the location where the bird spent the most time. Black triangles indicate stopover areas. Animations of tracks can be viewed https://doi.org/10.25919/g596-3b73.
Of the two great egrets that dispersed north and remain active at the time of writing, the first individual (female; Fig. 4) was captured on 14 February 2023 and remained in the nesting area until 29 February 2023. Between early March and late April, it moved northwards, first remaining inland of the Great Dividing Range and then moving to a coastal area of north Queensland between Rockhampton and Mackay before returning southward to an area around Carnarvon National Park. It then made a major north-west movement and from mid-May 2023 to January 2025, it spent most of its time around the Alice River to the east of Kowanyama in far north Queensland (Fig. 4; inset), with the exception of one short trip ~600 km south-east in December 2023. While in the Alice River area, it regularly moved ≤500 m per day to and from the same locations, with a mean distance travelled from roost to foraging sites across all post-dispersal movements of 2.09 km ± 3.28 km (Table S6). Transmissions stopped briefly between 4 and 13 January 2025, after which the bird reappeared about 400 km to the south. Between 13 and 17 January, it made a major movement to the south-west. From 17 January to our cut-off date for analysis (29 January 2025), it frequented several small dams in the area around Coppabella (Queensland), about 80 km inland from the coast.
The second individual (male; Fig. 5) was captured on 15 February 2023 and remained in the nesting area and a floodplain immediately to the west of the nesting area until 1 April 2023. It then made a major northward movement between 1 and 5 April 2023 to the Lake Nuga Nuga area (Queensland). It remained there for over 18 months until early December 2024, with the exception of one trip between 21 February and 9 March 2024 when it went a short distance west and then north of Lake Nuga Nuga before travelling south ~400 km for a several days before returning. While in the Lake Nuga Nuga area, it frequently spent long periods at a small farm dam (Fig. 5). Like the great egret discussed previously, this individual regularly moved ≤500 m per day, to and from the same location, with a mean distance travelled from roost to foraging sites across all post-dispersal movements of 1.10 km ± 1.78 km (Table S6). On 4 December 2024, it began a major movement southward and by 7 December, it had reached the Macquarie Marshes, its natal site. Between 7 and 21 December, it explored the entirety of the Macquarie Marshes (approximately 100 km north to south) before moving northward, eventually crossing the Queensland border, with a northern-most location near the town of Moonie on 25 December. It then turned southward again and spent time from 25 December to 13 January in an agricultural area just to the east of the southern-most part of the Macquarie Marshes. On 13 January, it commenced a major northward movement and by 17 January, it had returned to the Lake Nuga Nuga area, where it remained until our cut-off date for analysis (29 January 2025).
Plumed egrets
Juvenile plumed egrets travelled a mean distance of 115 ± 110 km (range 36–302 km) in the first 24 h of dispersal from the nest site, 263 ± 194 km (range 106–500 km) in the first 48 h of dispersal from the nest site, and 402 ± 305 km (range 136–797 km) in the first 72 h of dispersal from the nest site (Table S2). Mean cumulative distance travelled in the first 72 h was 457 ± 278 km (range 178–817 km; Table S2). In contrast to great egrets, the last fix in the breeding area prior to dispersal for all plumed egrets was from either the afternoon or evening (n = 1 at ~23:00 hours; n = 2 at ~21:00 hours; n = 1 at ~15:00 hours) with one exception of an individual with last fix prior to dispersal at ~06:00 hours (Table S3), suggesting that post-natal dispersal movements in the evening and overnight are most common for this species. All five of the plumed egrets that dispersed from the Macquarie Marshes initially flew north (Fig. 6). Two plumed egret individuals (043A00 and 239917) took a similar route toward St Lawrence and Broad Sound on the north-east coast (Fig. 6), though at different times. The first individual (043A00; male; Fig. 7) was captured on 16 February 2023 and remained in the nesting area until 19 March 2023. It first travelled to the Narran Lakes where it remained until 22 March 2023 and then travelled north-east following the Lower Balonne River system upstream. It settled in the St George area and remained there for about 3 weeks before moving north-east on 13 April 2023. It then foraged in a small area adjacent to Broad Sound approximately 15 km west of Ogmore for an additional 3 weeks. At approximately 07:00 hours (timezone, UTC + 10) on 8 May 2023, it departed Australia and flew northward, arriving in Papua New Guinea the next evening at around 21:00 hours (timezone, UTC + 10), an approximately 38-h non-stop flight. During this flight, the boost function of the Druid tag resulted in high frequency GPS fixes between 07:00 hours and 08:00 hours on 8 May and between ~00:00 hours and 08:00 hours on 9 May, with altitude expected to be more accurate during these periods than during less frequent fixes. For all fixes of any frequency during the overseas flight with a VDOP value < 4 (n = 486 of 504 total fixes), mean altitude was 58 m and maximum altitude was 448 m. For high frequency fixes with a VDOP value <4, mean altitude was 56 m and maximum altitude was 348 m. On reaching Papua New Guinea after this overseas flight, the egret travelled south-east, first moving inland and passing Port Moresby and then moving back to the coast. By 11 May, it reached a floodplain area around the Kemp River near the town of Kalo, north of Hood Bay. It mostly remained within an area of about 20 km2 for the next 2 months before ceasing to transmit on 19 July 2023. Data ceased with no evidence of change in either GPS locations or sensor data to indicate mortality, 153 days after deployment.
Plumed egrets (n = 8) tracked from the Macquarie Marshes in 2023–2025. Clustering of fixes at the natal site makes it difficult to see the three individuals (043E00, 239903, 239906) that did not disperse.
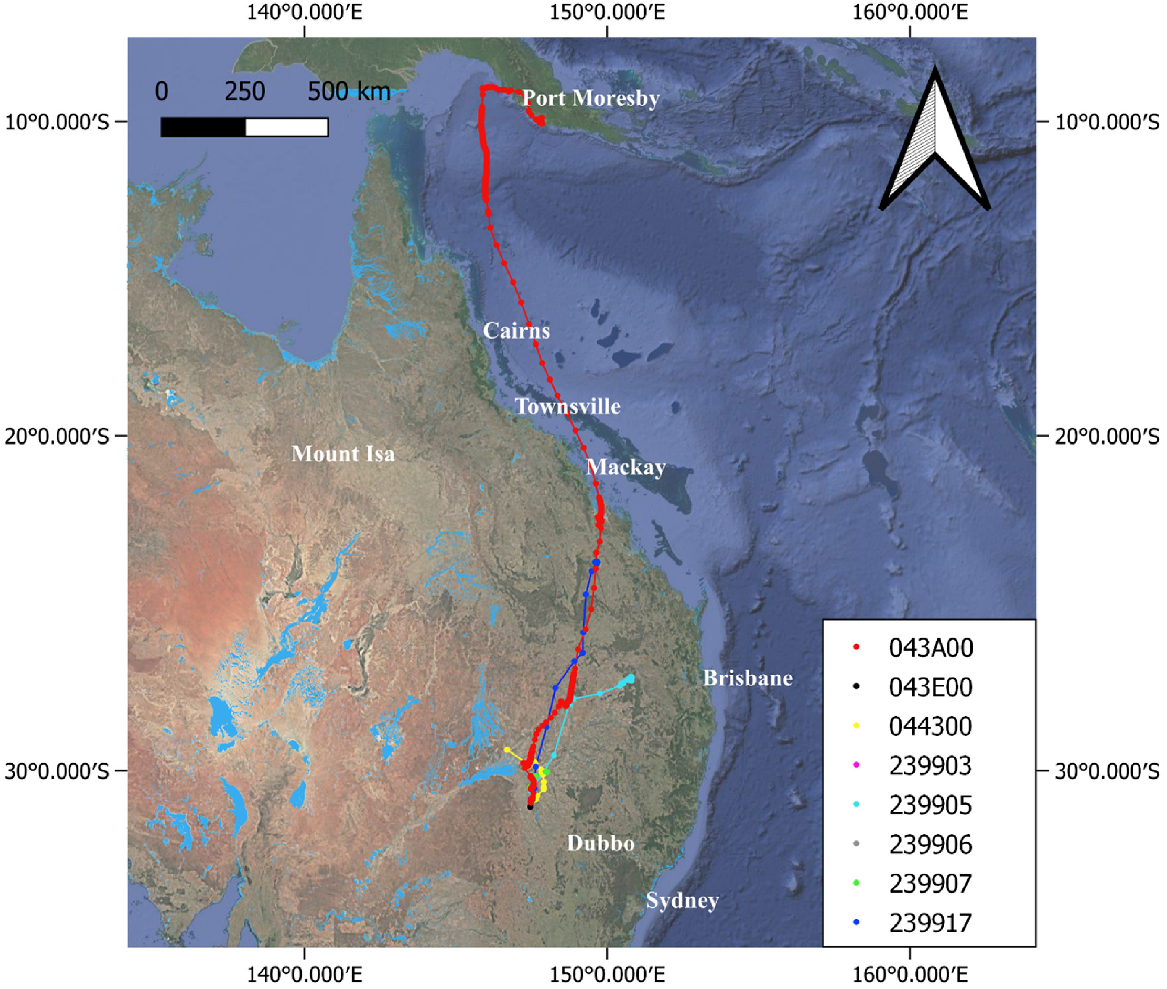
Male plumed egret (043A00) tracked from the Macquarie Marshes in 2023–2025. ‘Days’ under the north arrow = number of days bird was tracked to 29 January 2025 (‘ongoing’ = the individual was still transmitting at the cut-off date). Inset shows the location where the bird spent the most time. Black triangles indicate stopover areas. Animations of tracks can be viewed at https://doi.org/10.25919/g596-3b73.
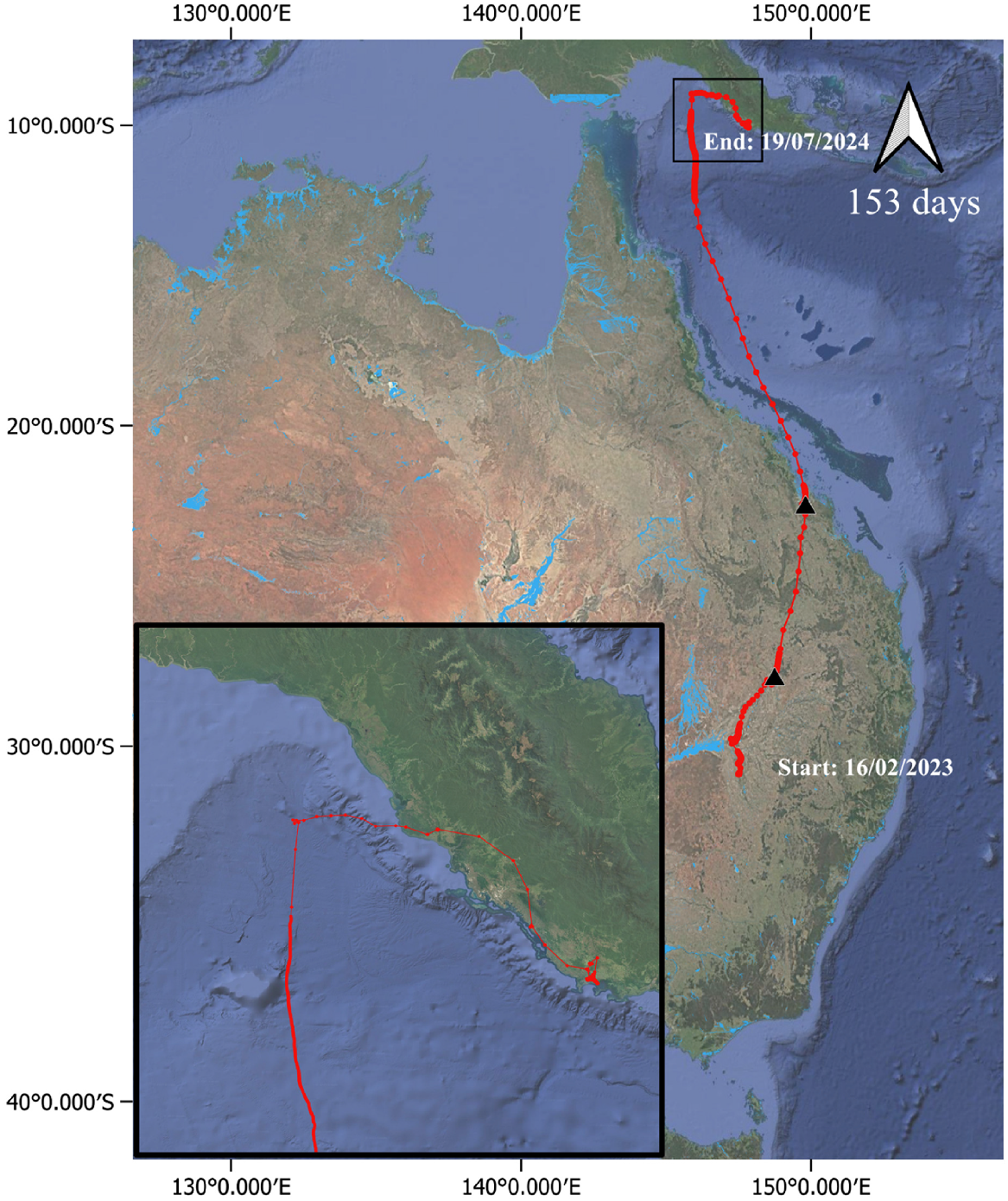
A second individual (239917; Fig. S15) was captured on 16 February 2023 and remained in the nesting area until 13 March 2023. It then made a major north-east movement, following a similar route to the first individual, and last transmitted near Baralaba, about 100 km south-west of Rockhampton. Data ceased with no evidence of change in either GPS locations or sensor data to indicate mortality, 41 days after deployment.
A third plumed egret (239905; Fig. S12) also flew north toward St George but then turned east to an area surrounded by forest 30 km east of Tara, Qld. Data ceased with no evidence of change in either GPS locations or sensor data to indicate mortality after 38 days of tracking, however the transmitter restarted approximately 1 month later, stationary on the shore of the farm dam where it last transmitted. A similar route was initially taken by a fourth individual (239907; Fig. S14). However, it became stationary approximately 170 km from the heronries after 38 days of tracking, and there was evidence of predation when the transmitter was recovered.
The only individual that did not continue flying north or north-east (044300; Fig. S10) turned north-west toward the Narran Lakes after resting at the Barwon-Darling River overnight on 7 March 2023. It stayed a night and a day at Narran Lakes, then flew further north-west on the evening of 9 March 2023, stopping to rest again overnight at the Culgoa River. The next morning, it flew north-west again until 09:00 hours but turned south-west and then south between 09:00 hours nd 10:00 hours, arriving at the Barwon-Darling River at Bourke at ~15:00 hours on 10 March 2023. It was mobile in the area of the river until 00:00 hours,when transmissions ceased, with no evidence of change in either GPS locations or sensor data to indicate mortality after 23 days of tracking.
Post-dispersal movements
Post-dispersal, for both species there was relatively little movement at night, with 72–93% of night fixes <100 m from subsequent fixes (Fig. 8; Tables S4, S5). Movements >100 m mostly occurred in the early morning around dawn (80–87% of fixes). During the day, similar proportions of movements >100 m and movements <100 m were recorded (51–64%; Fig. 8; Tables S4, S5).
The number of post-dispersal ‘resting’ (dark grey, distance between fixes <100 m) and ‘movement’ (dark grey, distance between fixes <100 m) fixes per time block for (a) great egrets, August–April (3-h interval fixes); (b) great egrets, May–July (6-h interval fixes); (c) plumed egrets, all months tracked (3-h interval fixes), and (d) plumed egrets, all months tracked (1-h interval fixes).
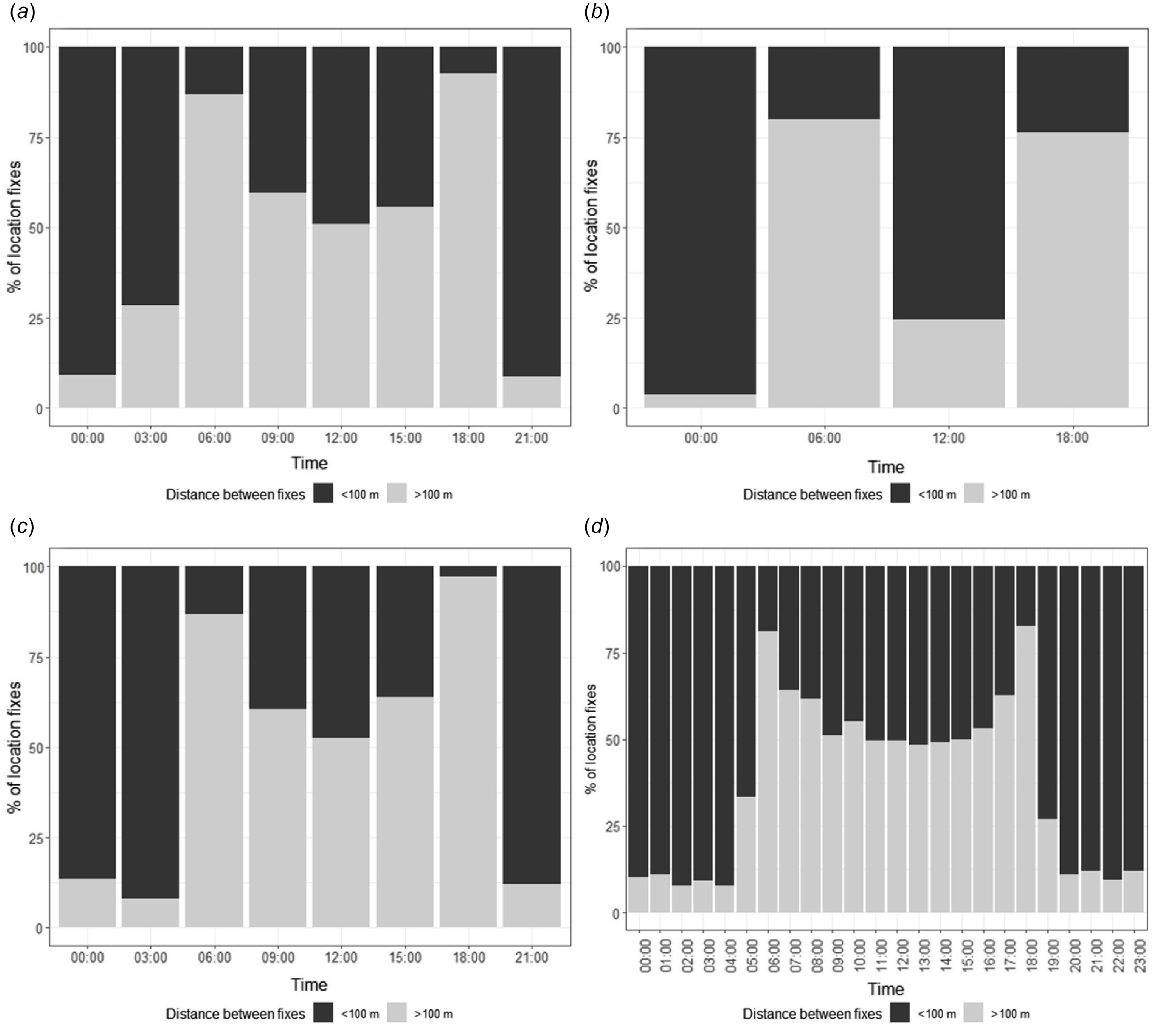
Post-dispersal distances travelled between roosts and foraging sites (maximum distance travelled from roost per day) were similar for great (1.61 km ± 2.68 km) and plumed egrets (2.02 km ± 1.73 km) and among individual birds (Table S6).
Discussion
Dispersal directions and distances
While dispersals of great egrets occurred in multiple directions including south, all dispersals of plumed egrets were to the north. Plumed egrets also travelled further and faster than great egrets. These data correspond to indications from banding and patagial tag data that plumed egrets tend to fly north and undertake bigger movements than those of great egrets in Australia (Geering et al. 1998). However, these movements have never been tracked showing precise routes and timing until now.
The individuals of each species that were tracked for longest flew rapidly north to sub-tropical and tropical zones outside of the Murray–Darling Basin. It has been suggested previously that egrets dispersing from temporary semi-arid inland wetlands dependent on natural flooding in Australia may undertake more extensive movements to such zones, particularly compared to egrets dispersing from more predictable or permanent coastal wetlands (Marchant and Higgins 1990; Geering et al. 1998). Sub-tropical and tropical zones provide conditions that are warmer, more predictable and more productive during winter than semi-arid and temperate zones (Kirby et al. 2008); conditions that are likely to be particularly important for juvenile growth and survival. Juveniles in the family Ardeidae (herons, egrets, and bitterns) are often subject to high and variable mortality rates, rarely move back to their natal sites in their first year, if they return at all, and often do not breed until they are at least 2 years old (Marchant and Higgins 1990; Shirai 2013; Weseloh et al. 2014; Włodarczyk et al. 2020), although the age of first breeding can very between individuals and cohorts (Hafner et al. 1998). Consequently, habitat conditions in non-breeding sites hosting juveniles are critical for recruitment.
Dispersal distances and directions can differ between subpopulations and individuals of the same species (Hawkes 2009). The individual variation in great egret movements found here reflects that seen in great egret populations in the northern hemisphere (Todte et al. 2010; Włodarczyk et al. 2020; Brzorad et al. 2021; Jennings et al. 2021; Lumpkin et al. 2023). Movements from hundreds to thousands of kilometres are common in this species, particularly post-breeding as birds move to foraging areas away from nesting areas (Włodarczyk et al. 2020). In Europe, post breeding movements are mainly to foraging sites within 800–950 km from breeding areas, and juveniles move further than adults (Włodarczyk et al. 2020). In north America, post breeding movements from Californian breeding sites are generally 100–500 km (Weseloh et al. 2014; Lumpkin et al. 2023).
Dispersal timing
With dispersal dates extending into late March, egret juveniles were clearly still dependent on local resources within and surrounding the Macquarie Marshes heronries into early autumn. This timing suggests that additional provision of water and associated food resources in and around these heronries as late as March could benefit juvenile survival in these species. A previous study of the breeding success of four egret species across two nesting sites in New South Wales over six seasons (from 1982/83 to 1987/88) showed that the mean number of young fledged per successful nest fluctuated, with the greatest success for all species occurring in wet years (Maddock and Baxter 1991). Great and plumed egrets are dependent on aquatic prey (such as fish, frogs, and invertebrates) and associated foraging habitats, and consequently are at risk of reduced breeding success and starvation of juveniles where resources are limited or change (Marchant and Higgins 1990; Maddock and Baxter 1991; Richardson and Taylor 2003). Juvenile birds are inexperienced foragers, and starvation can increase the risk of mortality from other causes such as toxins, diseases, predation, and weather (Recher and Recher 1969; Marchetti and Price 1989; Lindström 1999; Daunt et al. 2007; Bates and Ballard 2014). Following dispersal away from breeding areas, egrets also face reduced prey availability in the cooler months (Geary et al. 2015). Consequently, juvenile mortality rates after leaving the nest and during the first year are typically high (Hafner et al. 1998; Geary et al. 2015; Henny et al. 2017). For example, in western Europe, the estimated annual survival rate of juvenile great egrets in one study was only half that of adults (Włodarczyk et al. 2020). Hence this age group is a prime target for strategic management impact on population persistence.
Given great egrets and plumed egrets typically begin nesting in established sites during spring and early summer (Marchant and Higgins 1990) and juveniles may not depart until autumn, targeted management can theoretically plan well ahead in terms of supplying sufficient water and wetland habitats during and after breeding in and around known breeding areas (Briggs and Thornton 1999; Richardson and Taylor 2003; Arthur et al. 2012). However, prioritising management actions to support juvenile egrets during dispersal and post-dispersal until they reach adulthood is more complicated, given the relative lack of information on species movements and the variability in movement distances, directions and destinations found to-date. Our data indicate that juvenile plumed egrets that have dispersed from the Macquarie Marshes are likely to be more reliant on resources in sub-tropical and tropical locations and less likely to be reliant on resources within the Murray–Darling Basin than great egret juveniles. Therefore, juvenile great egrets may benefit more from water and wetland management in the Murray–Darling Basin than juvenile plumed egrets, both during dispersal movements and their first year.
Nevertheless, both species use stopover sites within the Murray–Darling Basin during dispersal to feed and rest, and these stopover sites can be supported and protected. There were some notable areas used by multiple individuals and both species as stopover sites to rest and forage during dispersal. The Narran Lakes in northern NSW, approximately 80 km north of the Macquarie Marshes, was visited by two plumed egrets (043A00; Fig. 7; 044300; Fig. S10) and a great egret (239914). The plumed egrets arrived at the Narran Lakes (NSW) within 2 days of leaving their nest sites, and stayed for 1–2 days before moving on, one to the north and the other to the west. The great egret arrived at the Narran Lakes approximately 4 days after leaving its nest and stayed for 1 day before departing again, flying north-north-east. Both of the great egrets and two of the plumed egrets travelling north stopped to rest in the Balonne River floodplains in the St George area (Qld). Other areas used outside the heronries included northern parts of Macquarie River and its floodplain, the Castlereagh (NSW), Lachlan and Barwon-Darling rivers (NSW), and the Broad Sound/St Lawrence area (Qld).
Water conditions in stopover sites during dispersal periods are critical, and can affect dispersal patterns and distances travelled (Henny et al. 2017). Water depth and changes in water extent are particularly important, influencing food availability for egrets and the suitability of foraging habitats (Beerens et al. 2015; Calle et al. 2018; Jennings et al. 2021). These factors can in turn affect juvenile survival (Borkhataria et al. 2012). In semi-arid and temperate areas inland, water resources can be limited, and water quality can be poor during late summer and autumn when egret juveniles are dispersing, and these stressors can be further influenced by weather extremes such as high maximum temperatures, storms, and low overnight minimum temperatures. There is considerable potential for managers to support juvenile survival through provision of resources such as water or other actions such as predator control or disturbance in stopover sites, and satellite tracking bird movements to locate these sites and when they are used is a useful way to inform these resource management decisions.
Post-dispersal movements
Post-dispersal, foraging, and roosting movement behaviour also varied between species and individuals. The two great egrets tracked for longest both settled down by winter and spent multiple consecutive months in the same areas, interspersed with occasional exploratory movements and returns (Figs 4, 5). In contrast, post-dispersal none of the plumed egrets were tracked using small areas for extended periods in this way. In general, our results suggest more mobile behaviour by juvenile plumed egrets than great egrets.
Flying to and from foraging sites is costly in terms of energy use for birds, and must be balanced against energy intake factors such as prey availability, size, and quality (Bryan et al. 1995; Brzorad et al. 2004, 2015; Maccarone et al. 2012; Jennings et al. 2021). Therefore, birds can be expected to choose foraging sites that are closer to their nest or roosting sites if other factors are equal. The short distances moved between foraging and roosting sites by the egrets tracked for longest in our study suggest that food availability remained sufficient over long periods, and the benefits of the chosen roosting and foraging sites outweighed any risks associated with moving. In a recent north American study, breeding great egrets tracked foraging from nests averaged maximum distances of 4.3 ± 0.1 km, while non-breeding birds (including juveniles) foraging from roosts averaged 3.3 ± 0.1 km (Brzorad et al. 2021). Juvenile little egrets (E. garzetta) in east Asia and juvenile reddish egrets in north America have also been shown to have small post-dispersal winter foraging distances and home ranges (Geary et al. 2015; Pang et al. 2020). However egret foraging distances can vary significantly among individuals, sexes, and years (Maccarone et al. 2008; Brzorad et al. 2015, 2021).
As far as we are aware, this is the first study quantifying the foraging movement distances of plumed egrets (or intermediate egrets) from their roosts. Closely related species of similar size and ecology such as the purple heron (Ardea purpurea) have been tracked, with findings similar to those for great egrets, in that distances travelled by adults from roosts post-breeding are shorter than those travelled from nests during breeding, and juveniles post-dispersal travel shorter distances to forage than adults (e.g. 0.4 ± 0.1 km for juvenile purple herons (Winden et al. 2012)). Similar patterns have been found for colonial-nesting seabirds (Corbeau et al. 2020). It is likely that the shorter distances travelled to forage from roosts by juveniles maximise energy efficiency and foraging time as birds mature, which would be particularly important given the inexperience and vulnerability of juveniles in their early years. Juvenile birds are often less adept at flight and spend more time foraging than adults (Daunt et al. 2007; Rotics et al. 2016; Corbeau et al. 2020).
The fact that the longest-tracked egrets were the individuals that moved to sub-tropical and tropical areas of northern Australia and Papua New Guinea suggests that conditions in these areas are particularly beneficial for juvenile survival. Increases in on-ground numbers of egrets in coastal northern Australia and Papua New Guinea frequently co-occur with decreases in numbers in southern and inland Australia (Marchant and Higgins 1990; Halse et al. 1996; Jaensch and Richardson 2013). In these northern regions, the monsoonal wet season results in flooding and significant increases in aquatic productivity in late summer and autumn, increasing habitat and food availability around the time that juveniles arrive from southern breeding sites (Jaensch and Richardson 2013). The coasts of northern Australia also host a large number of breeding and foraging sites for great egrets and plumed egrets, with the plumed egret the most abundant breeding species in many locations and one of the most abundant large wading species in the region (Jaensch and Richardson 2013).
All three egrets tracked beyond the natal site for >2 weeks transitioned into periods of residency at non-breeding sites. For comparison, a recent analysis of 122 individuals of three aggregate-nesting species satellite-tracked from the Murray–Darling Basin found that 32% of straw-necked ibis and 17% of royal spoonbills had no period of residency in any month, while all Australian white ibis had multiple or extended periods of residency (McGinness et al. 2024a). Consistent with results from that study that 65% of identified residency areas used by these species were not associated with wetlands formally listed by the Ramsar Convention on Wetlands of International Importance (McGinness et al. 2024c), neither of the areas in Australia used by the two great egrets with extended residency periods are recognised as such.
The plumed egret that we tracked for longest eventually flew to Papua New Guinea in May. Between mid-May and mid-July 2023, it used a floodplain area approximately 35 km2 in size. The timing of this individual’s arrival in Papua New Guinea (Fig. 7) corresponded with the timing of historical leg band recoveries of two juvenile plumed (‘intermediate’) egrets in Papua New Guinea, when both birds were banded as nestlings in the Macquarie Marshes in January 1994 and then seen in Papua New Guinea in May 1994 (Geering et al. 1998). While in some egret populations post-breeding migration involves more juveniles than adults (Bates et al. 2015), it is unknown what proportion of the population of plumed egrets in Australia migrates to Papua New Guinea and when they return, or if there are differences between adults and juveniles in these movements (Geering et al. 1998; Walsh and Chafer 2022). This is complicated by the fact that immature individuals of this species are visually indistinguishable from non-breeding adults (Marchant and Higgins 1990). Long-term satellite-tracking of multiple individuals is essential to fully understanding movements of juveniles compared with adults. Unfortunately, the individual that we satellite-tracked was carrying a transmitter using the GSM 3G network, which has limited coverage in Papua New Guinea and is being progressively shut down in Australia. Given the data showed no sign of detachment or mortality, we assume that this individual flew out of network range, and we are unlikely to receive further data from it if it does return to Australia, given the 3G network shut down in 2024. Hence, we recommend that future movement tracking research for egrets from Australia use transmitters able to communicate with long-term international satellite networks such as the Argos network.
While one of the great egrets we tracked returned to its natal site in the Macquarie Marshes at approximately 2 years old, it only stayed in the area for a few days and then returned to the site in Queensland where it had spent most of its time since dispersal. This shows fidelity to a non-breeding site, and perhaps some fidelity to a natal site as an immature individual, but we still do not know if or when egrets hatched inland commonly return to their natal sites to breed in Australia. Some insight into these questions could still be gained if existing satellite-tagged individuals continue to be tracked and survive to become breeding adults, or additional individuals are tagged. In other regions, adult egrets and related herons show fidelity to breeding sites, particularly in coastal areas, but less is known about fidelity to intermittent inland breeding sites and non-breeding areas, for juveniles or adults (Lumpkin et al. 2023).
Common routes
Although representing a small sample from only one site and only young birds, our findings suggest some potential commonalities and differences in movement strategy between egrets and other aggregate-nesting waterbirds that breed in inland eastern Australia. McGinness et al. (2024b) analysed an extensive dataset of 73 straw-necked ibis and 42 royal spoonbills from breeding sites in the Murray Darling Basin, including from the Macquarie Marshes, and identified an ‘inland flyway’ comprising common routes used by these two species characterised by flat, low-elevation areas with mid to high rainfall (McGinness et al. (2024b); refer to Figs 2–6). When compared with results presented here (refer to McGinness et al. (2024b)Figs 2, 3, 6 of this study), juvenile egrets appeared to use routes most similar to that of royal spoonbills (McGinness et al. (2024b)Fig. 2b), another heavily wetland-dependent species. Like spoonbills, tracked egrets stayed west of Australia’s Great Dividing Range (a >300-m elevation contour that runs north-south along the east of the continent; refer to McGinness et al. (2024b)). The one egret tracked to the coast (Fig. 7) visited a similar area to multiple spoonbills, and multiple royal spoonbills have been tracked to the western part of Cape York like the great egret tracked in this study (Fig. 4), while no straw-necked ibis have been tracked to this area (McGinness et al. (2024b)Fig. 2). Although limited conclusions should be drawn from the small sample size in this study, these preliminary findings suggest that habitat provisioning may be mutually beneficial for royal spoonbills and egrets that nest in the MDB.
Considerations for future research
The timing of capture and tagging is an important consideration in avian movement research, particularly for juveniles. Late in the breeding season, risk factors for bird mortality could be increased, including starvation disease, parasites, predation, and abandonment. Reproductive success monitoring from the Macquarie Marshes has shown that earlier cohorts had a higher average reproductive success rate across non-mobile chick stages than later ones, and that mortality rates were significantly higher at multiple rookeries later in the season (Brandis et al. 2023). Juveniles hatched early in the breeding season may therefore be more likely to survive than those hatched late in the season. During this study, heronry access restrictions resulted in egret capture and transmitter deployment occurring very late in the breeding season. Very few adult egrets were seen, even where juveniles were still in nests; most adults and juveniles had already dispersed. Many of the remaining juveniles were clearly weak and there was evidence of disease and starvation in the heronry. While effort was made to capture and fit harnesses only to apparently healthy individuals, there was no way to be completely sure of bird health status in the field. Given these factors, together with the generally higher mortality rates for juveniles than for adults, and a paucity of knowledge regarding adult movements and survival rates, we recommend that future capture and tracking efforts occur earlier in the breeding season and include adults where accessible to maximise the chances of obtaining quality movement data. The movements of adult egrets are of particular interest, because population modelling for egrets suggests that adult survival is critical for population maintenance and growth, and most adults must breed every 1–2 years for populations to persist (Arthur 2011). Knowledge of the spatial and temporal scales of populations function is critical for conservation of these species (Arthur 2011).
It is possible that some harness failure occurred during this study. Leg loop harnesses rather than chest/wing harnesses were used in this study to reduce chances of potential bill entanglement, which was thought to be a risk due to the long bills and flexible necks of egrets. However, leg loop harnesses have a greater chance of falling off birds by sliding backward if not correctly fitted, or if the bird loses significant weight, or actively preens the unit backward. In addition, we included dissolvable suture gathers in the harnesses of some individuals to allow for further body growth, but it is possible that these sutures may have snapped early, again resulting in the harness falling off. Finally, egrets have long back feathers and insufficient feather trimming for some individuals could have resulted in obstruction of the solar panel that powers the transmitters, leading to battery failure. The latter is a particular problem for very small solar panels. We recommend that future tracking of egrets and similar species use flexible medical-grade silicone tubing leg-loop harnesses, which are soft, rounded, and elastic, allowing a better fit and eliminating the need for dissolvable suture gathers in juvenile harnesses, and that trimming of back feathers that may cover solar panels be used as standard procedure.
Conclusions
This is the first time that GPS satellite telemetry has been used to track egret movements in Australia. As far as we are aware, it is also the first GPS-tracked record of a precise movement path between Australia and New Guinea for an egret or any other large aggregate-nesting wader. This information is useful for species conservation and water and wetland management efforts because it helps us to understand the drivers of on-ground species responses and associated metrics (e.g. abundance and breeding) at relevant spatial and temporal scales. It thereby assists in evaluation of responses and in prioritisation of species life stages and critical sites for management. Identification of movement timing, distances, and stopover sites during dispersal is useful for decision making regarding the location and timing of management resource allocations. Future research including tracking of both adults and juveniles, ideally captured early in the breeding season, should further improve understanding of the importance of natal sites and natal site fidelity in these species.
Data availability
The data supporting the conclusions of this article are available at https://doi.org/10.25919/g596-3b73.
Declaration of funding
The original research that formed the basis of this article was co-funded by the Commonwealth Environmental Water Holder (CEWH) and the Commonwealth Scientific and Industrial Research Organisation (CSIRO) through the CEWH Monitoring, Evaluation and Research project (2019–2025), administered by the Department of Climate Change, Energy, the Environment and Water and its precursors.
Author contributions
Heather McGinness conceived the idea and led the project, data collection, data processing, planning and interpretation of analyses, and wrote the manuscript. Micha Jackson and Luke Lloyd-Jones co-wrote the manuscript. Micha Jackson, Luke Lloyd-Jones, Freya Robinson and Xinyu Hu contributed to data processing and analysis. Heather McGinness, Freya Robinson, Louis O’Neill, Shoshana Rapley, and Micha Jackson led the fieldwork and data collection.
Acknowledgements
The authors are grateful for the assistance of colleagues, collaborators, and volunteers with fieldwork, and the support of program leaders.
References
Allen AM, Singh NJ (2016) Linking movement ecology with wildlife management and conservation. Frontiers in Ecology and Evolution 3, 155.
| Crossref | Google Scholar |
Arthur AD (2011) Using an age-structured population model to define management requirements for conservation of egrets in the Murray–Darling Basin, Australia. Emu – Austral Ornithology 111(3), 191-196.
| Crossref | Google Scholar |
Arthur AD, Reid JRW, Kingsford RT, McGinness HM, Ward KA, Harper MJ (2012) Breeding flow thresholds of colonial breeding waterbirds in the Murray–Darling Basin, Australia. Wetlands 32(2), 257-265.
| Crossref | Google Scholar |
Bates EM, Ballard BM (2014) Factors influencing behavior and success of foraging reddish egrets (Egretta rufescens). Waterbirds 37(2), 191-202.
| Crossref | Google Scholar |
Bates EM, Koczur LM, Ballard BM (2015) Post-Fledging survival and dispersal of juvenile reddish egrets (Egretta rufescens). Waterbirds 38(4), 401-406.
| Crossref | Google Scholar |
Beerens JM, Noonburg EG, Gawlik DE (2015) Linking dynamic habitat selection with wading bird foraging distributions across resource gradients. PLoS ONE 10(6), e0128182.
| Crossref | Google Scholar | PubMed |
BirdLife International (2016) Ardea plumifera. The IUCN Red List of Threatened Species 2016, e.T22727683A94956915.
| Crossref | Google Scholar |
Borkhataria RR, Frederick PC, Keller RA, Collazo JA (2012) Temporal variation in local wetland hydrology influences postdispersal survival of juvenile Wood Storks (Mycteria americana). The Auk 129(3), 517-528.
| Crossref | Google Scholar |
Brandis K, Hewitt S, Smith M, Stewart J, Juilliard L, McEvoy T, Francis RJ (2023) Macquarie Marshes Breeding Waterbirds Reproductive Success Monitoring 2022–23. Centre for Ecosystem Science, UNSW, UoNS Wales, Sydney. Available at https://www.dcceew.gov.au/sites/default/files/documents/macquarie-marshes-breeding-waterbirds-reproductive-success-monitoring-2022-23.pdf
Briggs SV, Thornton SA (1999) Management of water regimes in River Red Gum Eucalyptus camaldulensis wetlands for waterbird breeding. Australian Zoologist 31(1), 187-197.
| Crossref | Google Scholar |
Bryan AL, Jr, Coulter MC, Pennycuick CJ (1995) Foraging strategies and energetic costs of foraging flights by breeding wood storks. The Condor: Ornithological Applications 97(1), 133-140.
| Crossref | Google Scholar |
Brzorad JN, Maccarone AD, Conley KJ (2004) Foraging energetics of great egrets and snowy egrets. Journal of Field Ornithology 75(3), 266-280.
| Crossref | Google Scholar |
Brzorad JN, Maccarone AD, Stone HM (2015) A telemetry-based study of great egret (Ardea alba) nest-attendance patterns, food-provisioning rates, and foraging activity in Kansas, USA. Waterbirds 38(2), 162-172.
| Crossref | Google Scholar |
Brzorad JN, Allen MC, Jennings S, Condeso E, Elbin S, Kays R, Lumpkin D, Schweitzer S, Tsipoura N, Maccarone AD (2021) Seasonal patterns in daily flight distance and space use by great egrets (Ardea alba). Waterbirds 44(3), 343-355.
| Crossref | Google Scholar |
Byrne ME, Holland AE, Bryan AL, Beasley JC (2017) Environmental conditions and animal behavior influence performance of solar-powered GPS-GSM transmitters. The Condor: Ornithological Applications 119(3), 389-404.
| Crossref | Google Scholar |
Calle L, Green L, Strong A, Gawlik DE (2018) Time-integrated habitat availability is a resource attribute that informs patterns of use in intertidal areas. Ecological Monographs 88(4), 600-620.
| Crossref | Google Scholar |
Chambers LE, Loyn RH (2006) The influence of climate variability on numbers of three waterbird species in Western Port, Victoria, 1973–2002. International Journal of Biometeorology 50(5), 292-304.
| Crossref | Google Scholar | PubMed |
Chelak MS, Kohl MT, Small JR, Smith KT, Pratt AC, Beck JL, Backen CR, Flack MB, Wayment HP, Wood JA, Howell R, Strange TD, McDonald LR, Manlove KR, Frey SN, Larsen RT, Maxfield BA, Dahlgren DK, Messmer TA, Stoner DC (2025) Refurbishing used GPS transmitters improves performance for subsequent deployments on greater sage-grouse. Wildlife Society Bulletin 49(1), e1566.
| Crossref | Google Scholar |
Corbeau A, Prudor A, Kato A, Weimerskirch H (2020) Development of flight and foraging behaviour in a juvenile seabird with extreme soaring capacities. Journal of Animal Ecology 89(1), 20-28.
| Crossref | Google Scholar | PubMed |
Cottee-Jones HEW, Matthews TJ, Whittaker RJ (2016) The movement shortfall in bird conservation: accounting for nomadic, dispersive and irruptive species. Animal Conservation 19(3), 227-234.
| Crossref | Google Scholar |
Daunt F, Afanasyev V, Adam A, Croxall JP, Wanless S (2007) From cradle to early grave: juvenile mortality in European shags Phalacrocorax aristotelis results from inadequate development of foraging proficiency. Biology Letters 3(4), 371-374.
| Crossref | Google Scholar | PubMed |
Ferraz G, Pacheco C, Fernández-Tizón M, Marques AT, Alves PC, Silva JP, Mougeot F (2024) Using GPS and accelerometer data to remotely detect breeding events in two elusive ground-nesting steppe birds. Animal Biotelemetry 12(1), 30.
| Crossref | Google Scholar |
Geary B, Green MC, Ballard BM (2015) Movements and survival of juvenile reddish egrets Egretta rufescens on the Gulf of Mexico coast. Endangered Species Research 28(2), 123-134.
| Crossref | Google Scholar |
Geering DJ, Maddock M, Cam GR, Ireland C, Halse SA, Pearson GB (1998) Movement patterns of great, intermediate and little egrets from Australian breeding colonies. Corella 22, 37-46.
| Google Scholar |
Hafner H, Kayser Y, Boy V, Fasola M, Julliard A-C, Pradel R, Cézilly F (1998) Local survival, natal dispersal, and recruitment in little egrets egretta garzetta. Journal of Avian Biology 29(3), 216-227.
| Crossref | Google Scholar |
Halse SA, Pearson GB, Jaensch RP, Kulmoi P, Gregory P, Kay WR, Storey AW (1996) Waterbird surveys of the middle fly river floodplain, Pap New Guinea. Wildlife Research 23(5), 557-569.
| Crossref | Google Scholar |
Hawkes C (2009) Linking movement behaviour, dispersal and population processes: is individual variation a key? Journal of Animal Ecology 78(5), 894-906.
| Crossref | Google Scholar | PubMed |
Hayes FE, Nakamura LH, Hiss NB, Capllonch P (2023) Seasonal distribution of the cocoi heron (Ardea cocoi) and great egret (Ardea alba) in Southern South America: evidence for partial migration. Journal of Heron Biology and Conservation 8(4), 1-9.
| Google Scholar |
Henny CJ, Hill EF, Grove RA, Chelgren ND, Haggerty PK (2017) Mercury and drought along the lower Carson River, Nevada: IV. Snowy egret post-fledging dispersal, timing of migration and survival, 2002–2004. Ecotoxicology and Environmental Safety 135, 358-367.
| Crossref | Google Scholar | PubMed |
Huang Z, Zhou X, Fang W, Chen X (2022) Migration and wintering of vulnerable adult Chinese Egrets (Egretta eulophotes) revealed by GPS tracking. Avian Research 13, 100055.
| Crossref | Google Scholar |
IOC (2024) Proposed splits/lumps IOC version 14.2 (DRAFT). International Ornithological Committee. Available at https://www.worldbirdnames.org/new/updates/proposed-splits/
Jaensch R, Richardson P (2013) Waterbird breeding colonies in the Gulf Plains, 2009–2013. The Sunbird: Journal of the Queensland Ornithological Society 43(2), 45-64.
| Google Scholar |
Jennings S, Lumpkin D, Warnock N, Condeso TE, Kelly JP (2021) Great egret (Ardea alba) habitat selection and foraging behavior in a temperate estuary: comparing natural wetlands to areas with shellfish aquaculture. PLoS ONE 16(12), e0261963.
| Crossref | Google Scholar |
Kingsford RT, Auld KM (2005) Waterbird breeding and environmental flow management in the Macquarie Marshes, arid Australia. River Research and Applications 21(2–3), 187-200.
| Crossref | Google Scholar |
Kirby JS, Stattersfield AJ, Butchart SHM, Evans MI, Grimmett RFA, Jones VR, O’Sullivan J, Tucker GM, Newton I (2008) Key conservation issues for migratory land- and waterbird species on the world’s major flyways. Bird Conservation International 18(S1), S49-S73.
| Crossref | Google Scholar |
Koczur LM, Ballard BM (2024) Drivers of partial migration in the reddish egret Egretta rufescens. Journal of Avian Biology 2024(1–2), e03133.
| Crossref | Google Scholar |
Lato KA, Stepanuk JEF, Heywood EI, Conners MG, Thorne LH (2022) Assessing the accuracy of altitude estimates in avian biologging devices. PLoS ONE 17(10), e0276098.
| Crossref | Google Scholar | PubMed |
Lindström J (1999) Early development and fitness in birds and mammals. Trends in Ecology & Evolution 14(9), 343-348.
| Crossref | Google Scholar | PubMed |
Lumpkin D, Jennings S, Warnock N, Condeso TE (2023) Partial migration by great egrets Ardea alba in Coastal California. Waterbirds 45(2), 150-158.
| Crossref | Google Scholar |
Maccarone AD, Brzorad JN, Stone HM (2008) Characteristics and energetics of great egret and snowy egret foraging flights. Waterbirds 31(4), 541-549.
| Crossref | Google Scholar |
Maccarone AD, Brzorad JN, Stone HM (2012) A telemetry-based study of snowy egret (Egretta thula) nest-activity patterns, food-provisioning rates and foraging energetics. Waterbirds 35(3), 394-401.
| Crossref | Google Scholar |
Maddock M, Baxter GS (1991) Breeding success of egrets related to rainfall: a six-year Australian study. Colonial Waterbirds 14(2), 133-139.
| Crossref | Google Scholar |
Marchetti K, Price T (1989) Differences in the foraging of juvenile and adult birds: the importance of developmental constraints. Biological Reviews 64(1), 51-70.
| Crossref | Google Scholar |
McGinness HM, Lloyd-Jones LR, Robinson F, Langston A, O’Neill LG, Rapley S, Jackson MV, Hodgson J, Piper M, Davies M, Martin JM, Kingsford R, Brandis K, Doerr V, Mac Nally R (2024a) Satellite telemetry reveals complex mixed movement strategies in ibis and spoonbills of Australia: implications for water and wetland management. Movement Ecology 12(1), 74.
| Crossref | Google Scholar |
McGinness HM, Jackson MV, Lloyd-Jones L, Robinson F, Langston A, O’Neill LG, Rapley S, Piper M, Davies M, Hodgson J, Martin JM, Kingsford R, Brandis K, Doerr V, Mac Nally R (2024b) Extensive tracking of nomadic waterbird movements reveals an inland flyway. Ecology and Evolution 14(12), e70668.
| Crossref | Google Scholar |
McGinness HM, Lloyd-Jones LR, Robinson F, Langston A, O’Neill LG, Rapley S, Jackson MV, Hodgson J, Piper M, Davies M, Martin JM, Kingsford R, Brandis K, Doerr V, Mac Nally R (2024c) Habitat use by nomadic ibis and spoonbills post-dispersal from breeding sites. Landscape Ecology 39(11), 189.
| Crossref | Google Scholar |
Melvin SL, Gawlik DE, Scharff T (1999) Long-term movement patterns for seven species of wading birds. Waterbirds: The International Journal of Waterbird Biology 22(3), 411-416.
| Crossref | Google Scholar |
Mužinić J (2011) Intermediate egret ardea intermedia in the Neretva River Valley (Croatia): evaluation of historical and recent data on its movements. Avian Biology Research 4(3), 118-121.
| Crossref | Google Scholar |
Ogburn MB, Harrison A-L, Whoriskey FG, Cooke SJ, Mills Flemming JE, Torres LG (2017) Addressing challenges in the application of animal movement ecology to aquatic conservation and management. Frontiers in Marine Science 4, 70.
| Crossref | Google Scholar |
Pang C-C, Sung Y-H, Chung Y-T, Ying H-K, Fong HHN, Yu Y-T (2020) Spatial ecology of little egret (Egretta garzetta) in Hong Kong uncovers preference for commercial fishponds. PeerJ 8, e9893.
| Crossref | Google Scholar |
Péron G, Calabrese JM, Duriez O, Fleming CH, García-Jiménez R, Johnston A, Lambertucci SA, Safi K, Shepard ELC (2020) The challenges of estimating the distribution of flight heights from telemetry or altimetry data. Animal Biotelemetry 8(1), 5.
| Crossref | Google Scholar |
Poessel SA, Duerr AE, Hall JC, Braham MA, Katzner TE (2018) Improving estimation of flight altitude in wildlife telemetry studies. Journal of Applied Ecology 55(4), 2064-2070.
| Crossref | Google Scholar |
Pratt HD (2011) Observations on species limits in the Great Egret (Ardea alba) complex. Journal of Heron Biology and Conservation 1(5), 1 5.
| Google Scholar |
Recher HF, Recher JA (1969) Comparative foraging efficiency of adult and immature little blue herons (Florida caerulea). Animal Behaviour 17(2), 320-322.
| Crossref | Google Scholar |
Richardson AJ, Taylor IR (2003) Are rice fields in southeastern Australia an adequate substitute for natural wetlands as foraging areas for egrets? Waterbirds 26(3), 353-363.
| Crossref | Google Scholar |
Rotics S, Kaatz M, Resheff YS, Turjeman SF, Zurell D, Sapir N, Eggers U, Flack A, Fiedler W, Jeltsch F, Wikelski M, Nathan R (2016) The challenges of the first migration: movement and behaviour of juvenile vs. adult white storks with insights regarding juvenile mortality. Journal of Animal Ecology 85(4), 938-947.
| Crossref | Google Scholar | PubMed |
Schaub T, Millon A, De Zutter C, Buij R, Chadoeuf J, Lee S, Mionnet A, Klaassen RHG (2023) How to improve the accuracy of height data from bird tracking devices? An assessment of high-frequency GPS tracking and barometric altimetry in field conditions. Animal Biotelemetry 11(1), 31.
| Crossref | Google Scholar |
Shirai T (2013) Breeding colony attachment and long-term reproductive history of individually identified grey herons. Yamashina Journal of Ornithology 44(2), 79-91.
| Crossref | Google Scholar |
Son S-J, Oh J-W, Hyun B-R, Kang J-H (2021) Home range of juvenile chinese egrets Egretta eulophotes during post-fledging stage in Chilsan Archipelago, Republic of Korea. Korean Journal of Environment and Ecology 35(2), 98-105.
| Crossref | Google Scholar |
Thomas RF, Kingsford RT, Lu Y, Hunter SJ (2011) Landsat mapping of annual inundation (1979–2006) of the Macquarie Marshes in semi-arid Australia. International Journal of Remote Sensing 32(16), 4545-4569.
| Crossref | Google Scholar |
Todte I, Kaatz M, Fiedler W (2010) Woher stammen in Deutschland auftretende Silberreiher Casmerodius albus? Erste hinweise aus der satellitentelemetrie eines vogels und aus neuen ringfunden. Vogelwarte 48, 269-273.
| Google Scholar |
Walsh AC, Chafer CJ (2022) Taxonomic revision, occurrence, and identification of intermediate egret Ardea intermedia in North Queensland, Australia. Australian Field Ornithology 39, 174-194.
| Google Scholar |
Weseloh D, Moore D, Knezevic T (2014) Wintering locations of ontario-banded great egrets: new jersey to the Caribbean. Ontario Birds 32(1), 2-11.
| Google Scholar |
Winden JVD, Horssen PWV, Poot MJM, Gyimesi A (2012) Pre-migratory behaviour of the purple heron in the Netherlands. Ardeola 59(1), 3-15.
| Crossref | Google Scholar |
Włodarczyk R, Szafara D, Kaczmarek K, Janiszewski T, Minias P (2020) Migratory behaviour and survival of great egrets after range expansion in Central Europe. PeerJ 8, e9002.
| Crossref | Google Scholar | PubMed |



