Predation on the threatened Carnaby’s cockatoo (Zanda latirostris) by feral cats (Felis catus)
Peter R. Mawson A * , Rick Dawson A , Brooke S. Richards A and Denis A. Saunders
A * , Rick Dawson A , Brooke S. Richards A and Denis A. Saunders  B
B
A
B
Abstract
Feral cats (Felis catus) prey on a wide range of Australian native bird species, with most records related to smaller, ground-dwelling or ground-nesting species.
This study examined the impact of feral cat predation on endangered Carnaby’s cockatoo (Zanda latirostris), a large obligate hollow-nesting species.
Cat predation was measured during a study into the breeding ecology of Carnaby’s cockatoo at a long-term study site in the northern wheatbelt of Western Australia.
Feral cats predated Carnaby’s cockatoo adult females in their nest hollow, their nestlings, and eggs in at least three out of 38 years. When it occurred in those 3 years, the feral cat predation impact, expressed as a percentage of breeding attempts on cockatoo nesting were 5.2%, 11.6%, and 24.1%. The increase in predation rate in the year with the highest recorded rate coincided with the breeding season following an intense 8-month drought in mid-western Western Australia, which likely had an adverse impact on prey species more often consumed by feral cats.
The results suggest that predation by cats can be a significant threat to Carnaby’s cockatoo because it reduces the survival of adult breeding females and recruitment of fledglings.
Control of feral cats by local-scale shooting and cage trapping have both been used to achieve short-term success, offering a possible means of mitigating the long-term impact of feral cats at the study site, and potentially elsewhere.
Keywords: Carnaby’s cockatoo (Zanda latirostris), drought, Felis catus, feral cat, predation.
Introduction
Feral cats (Felis catus) have been recorded preying on a wide range of terrestrial vertebrate and invertebrate prey species around the world (Lowe et al. 2000; Lepczyk et al. 2023). They have a greater impact on island species or ecosystems than those in continental locations (Medina et al. 2011). Within Australia, Doherty et al. (2015) identified a large number of widespread, and abundant Australian native species that were predated by cats. Woinarski et al. (2017) expanded Doherty et al.’s (2015) list of avian species to 357 (43.1% of Australian avian fauna), including 24 species listed as threatened or extinct by the IUCN (2016), and 71 of the 117 bird species then listed as threatened under Australian legislation. The majority of species identified were ground dwelling or ground nesting species, with few arboreal species listed. Woinarski et al. (2017) and Saunders et al. (2020a) reported on cat predation of Carnaby’s cockatoo (Zanda latirostris Carnaby, 1948). One other species of black cockatoo, the Western red-tailed cockatoo (Calyptorhynchus escondidus), has also been reported as being preyed on by feral cats (Saunders 2023), and there is also a single reported case of cat predation of a nestling Glossy-black cockatoo (Calyptorhynchus lathami) from Kangaroo Island, South Australia (Joseph 1982) that was not included in the list produced by Woinarski et al. (2017).
Feral cats have broad diets and usually select prey based on their availability (Jones and Coman 1981; Fitzgerald 1988; Doherty et al. 2015), but some individual cats have also been shown to be capable of highly selective hunting of relatively rare prey (Moseby et al. 2015; Hardman et al. 2016). Comparative studies of the diet of feral cats during ‘boom’ and ‘bust’ periods associated with periods of above average rainfall and dry or drought periods respectively, indicate that feral cats do not lose body condition when local environmental conditions deteriorate, but maintain body condition by expanding the breadth of prey species targeted (Yip et al. 2015).
Carnaby’s cockatoo is endemic to south-western Australia (Saunders and Pickup 2023). It is a large (660 g), black bird, with a distinctive white sub-terminal tail band. Sexes are the same size. They reach sexual maturity at 3 or 4 years of age, form pair bonds that last the life of one of the partners, breed in the late austral winter through to early summer in large hollows in mature eucalypt trees. One to two eggs are laid, on average 8 days apart, from early June to late December with the timing of the commencement of egg laying each season strongly influenced by rainfall in the first half of autumn. Both eggs usually hatch after 29 days incubation, with the second nestling usually dying within 24–48 h of hatching; occasionally both nestlings fledge. Once they have fledged their nestling(s), the family moves away from the breeding area and spends the non-breeding season (February–May) in flocks that mainly forage on native vegetation, particularly seeds of the Proteaceae (Banksia, Grevillea, and Hakea) (information from Saunders 1974, 1980, 1982; Saunders and Ingram 1998; Saunders et al. 2013, 2018, 2024a; Saunders and Dawson 2018; D. A. Saunders, P. R. Mawson, and R. Dawson, unpubl. data).
This paper describes the incidence and quantifies the level of predation by feral cats of breeding females, nestlings, and eggs of a population of the endangered Carnaby’s cockatoo over the course of a long-term study (1969–2024) including one breeding season preceded by a short but intense 8-month long drought in south-western Western Australia. Notes are also provided on cat predation on another obligate hollow nesting bird species, and discussion is provided on conservation implications for Carnaby’s cockatoo and allied species from this previously unreported threat.
Methods
The observations reported in this paper were made at Coomallo Creek (30°12′S; 115°30′E) located in the central northern wheatbelt of south-western Australia. Since 1969, this site has been used to study the breeding ecology and behaviour of Carnaby’s cockatoo. The study area consists of a 9-km long belt of wandoo woodland surrounded by cleared agricultural land and remnant native heathland vegetation.
The mixture of natural habitat found in the study area, crop, and pasture paddocks, along with stock water sources, dams, creek-lines, covered hay storage, and various machinery sheds and retired machinery dumps provide abundant and diverse habitat for feral cats and their main prey sources.
In this paper, we use the term feral cat to refer to cats that are are unowned, unsocialised, have no relationship with or dependence on humans, and reproduce in the wild (RSPCA 2018). These also meet all the criteria for feral cat status described by Lepczyk and Calver (2022) who differentiated owned/pet/companion, stray/lost/semi-feral, and feral cats.
The study site receives an average annual rainfall of 552 mm, with most (52.7%) falling during the austral winter (Bureau of Meteorology 2024a). The period encompassing the breeding season of 2023 (October 2023–May 2024) was very dry, receiving only 60 mm of rain compared to the long-term average of 186 mm for the same period (Bureau of Meteorology 2024b). That lower rainfall was also accompanied by 57 days of high day time maximum temperature (>35°C), of which 14 days were >40°C.
Data on which avian species were using nest hollows suitable for use by Carnaby’s cockatoos at Coomallo Creek were gathered for 22 years between 1969 and 1996 (Saunders 1982; Saunders and Ingram 1998), and for every year from 2009 to 2024 (Saunders et al. 2014a, 2014b, 2024b; Saunders and Dawson 2018). Natural nest hollows used by Carnaby’s cockatoos were in decline at this site after 1996, and remaining natural hollows were repaired during the period 2009–2021 (Saunders et al. 2023) and through into 2024. A total of 82 artificial nest hollows were added over the period 2011–2023. Most artificial hollows have an internal diameter of 375–400 mm, a depth of 1.0–1.2 m, and are supplied with internal galvanised 50 mm mesh ladder, a sacrificial chewing post made from wandoo, tuart (Eucalyptus gomphocephala), or jarrah (Eucalyptus marginata), and a eucalypt woodchip substrate on the hollow floor. A small number of artificial nest hollows were constructed with a 450-mm diameter and a depth of 1.2–1.5 m. Natural nest hollow entrances at the study site are at an average height of 4.7 ± 1.3 m (range 2.3–9.9 m; Saunders et al. 2014b), and artificial nest hollow entrances are at an average of 4.0–5.2 m (Saunders et al. 2020a). Adult Carnaby’s cockatoos roost in tree canopies higher above the ground than nest hollow entrances, and in trees that do not contain nest hollows (P. R. Mawson, R. Dawson and D. A. Saunders, unpubl. obs.).
Since 1969, the study site has been monitored during field trips of 3–5 days in September and November most years with all known nesting hollows examined and contents recorded. Since 2009, shorter trips (1–2 days) were also made in October and January to band nestlings prior to fledging, and 3-day trips in March were made in the following year to repair damaged hollows and band any late season nestlings.
Over the course of this study, predation events involving Carnaby’s cockatoos were observed in both natural and artificial nest hollows. South-west carpet pythons (Morelia spilota imbricata) were observed preying on adult female and nestling Carnaby’s cockatoos (Dawson et al. 2011; Saunders et al. 2020b) and Western corellas (Cacatua pastinator butleri) regularly destroy a small number of Carnaby’s cockatoo eggs during each breeding season with and without associated usurpation of the Carnaby’s cockatoo nests (D. A. Saunders, R. Dawson and P. R. Mawson, unpubl. data). Claw marks have also been observed on the trunks of some nest trees, which we have attributed to Gould’s monitor (Varanus gouldii), but we have not been able to confirm that this species also preys on eggs, nestlings, or adult female Carnaby’s cockatoos as any predation by monitors would be likely to leave no trace (diagnostic remains of eggs or birds) in the nest hollow. We note that only female Carnaby’s cockatoo brood, so predation of adults at the nest-site is confined to females.
For the purposes of this paper, we have inferred feral cat predation events by the presence of: cat claw scratches on the trunk of the tree where the hollow occurred (or on an adjacent tree where the canopy connects with that of the nest tree; Fig. 1); the presence of one or more sets of wings (but usually no other body parts; adult and older nestling (7–10 week) primary flight feathers, that can be distinguished based on length, colour, and shape of the end of the feather, dismembered from the remainder of the cockatoo body; the presence of a Carnaby’s cockatoo head with clear signs of having been chewed by a mammal (Fig. 2); or the presence of Carnaby’s cockatoo egg shell with clear evidence of mammalian tooth marks consistent with the dentition of feral cats (Fig. 3). Destruction of Carnaby’s cockatoo eggs by Western corellas was differentiated from feral cat predation by the presence of a triangular-shaped perforation of the eggshell by the corellas’ upper mandible, usually around the transverse mid-line of the egg (Fig. 4), with or without a larger rhomboid shaped perforation nearly opposite the smaller perforation and consistent with the lower mandible. Cat claw marks were differentiated from those made by varanids on the basis of the depth of the claw marks (deeper for cats), the spacing between individual claw marks from the same foot (wider for varanids), and the orientation and spacing of the claw marks (offset at a 45° angle relative to the central orientation of the trunk and individual sets of claw marks not at the same height for varanids).
Feral cat claw scratches on the trunk of a wandoo supporting an artificial nest hollow from which a Carnaby’s cockatoo egg was predated in November 2024 (photograph: Rick Dawson).

Remains of an adult female Carnaby’s cockatoo predated by a feral cat in artificial nest hollow #57 in November 2024. Note the wing and tail feathers and the partial skull and mandibles at the bottom centre (photograph: Rick Dawson).

Carnaby’s cockatoo egg predated by a feral cat in November 2024 (photograph: Teagan Johnston).

Carnaby’s cockatoo eggs destroyed by Western corellas. Note the triangular shape to the perforation of the egg at the bottom of the image showing upper mandible imprint and the broader lower mandible imprint at the top of the perforation (photograph: Rick Dawson).

Animal ethics
Field work and animal handling were conducted under appropriate ethics approvals from the Western Australian Department of Biodiversity, Conservation and Attractions Animal Ethics Committee project approval numbers 2011/30, 2014/23, 2017/21, 2020/10C and 2023-09G for the period 2009–2024, and bird banding approvals for the same periods (Australian Bird and Bat Banding Authority #418 held by DAS and #1862 held by PRM).
Results
Although we did not systematically survey cat abundance, during the period 1969–1996, feral cats were observed in the study area but there were no recorded predation events attributable to cats. The first predation events attributable to feral cats were recorded in 2014 (Table 1), with five events recorded that year in natural nest hollows in the wandoo woodlands and six in artificial hollows. They included eight nestlings and one adult female. This represented 11.6% of the total breeding attempts for that year. No further feral cat predation events were recorded until the austral spring of 2023 when six events were recorded, one in a natural nest hollow and five in artificial nest hollows. The predation events included five nestlings (each single nestlings in separate hollows) and one adult female; 5.2% of the total breeding attempts. In 2024, there were 20 predation events recorded, three in natural hollows, and 17 in artificial hollows. Those 20 events included 10 eggs from seven clutches, four nestlings, and seven adult females; 24.1% of the total breeding attempts. Some cats took more than one life stage from some nests, and more than one egg, nestling or adult female and egg or nestling combination (Table 2). In 2024, we also recorded the predation of two wood ducks (Chenonetta jubata) by feral cats in natural nest hollows during late season breeding attempts by that species.
| 2014 | 2023 | 2024 | ||
|---|---|---|---|---|
| Natural nest hollows | ||||
| 8 | 1 N | |||
| 10 | 1 N | |||
| 12 | 1 N | |||
| 43 | 1 AdF | |||
| 63 | Wood Duck | |||
| 90 | 1 N | |||
| 164 | 1 N | |||
| 191 | 1 N | |||
| 304 | 1 N | |||
| 358 | Wood Duck | |||
| 361 | 1 N | |||
| Artificial nest hollows | ||||
| 6A | 1 AdF | |||
| 9A | 1 N | |||
| 10A | 1 N | |||
| 12 | 1 N | |||
| 15 | 1 N | |||
| 18A | 2 eggs | |||
| 20A | 1 AdF | |||
| 21A | 1 egg | |||
| 22A | 1 AdF | 2 eggs + 1 AdF | ||
| 24A | 1 N | |||
| 27A | 1 N | 1 egg | ||
| 42 | 1 N | |||
| 57 | 1 N | 1 AdF | ||
| 58 | 2 N | |||
| 59 | 1 N | |||
| 60 | 1 N | 1 N | ||
| 61 | 1 N | |||
| 62 | 1 egg | |||
| 65 | 1 AdF | |||
| 69 | 2 eggs + 1 AdF | |||
| 70 | 2 eggs | |||
| 71 | 1 AdF | |||
| 77 | 1 N | |||
| 78 | 1 N |
Data in parentheses are the percentage of the total breeding attempts made by Carnaby’s cockatoos in each year predated by feral cats, including re-laying events after early season failures.
N, nestling; AdF, adult female.
| Year | Natural hollows | Artificial hollows | # of predation events attributed to feral cats | |
|---|---|---|---|---|
| 2009 | 0 | 0 | 0 | |
| 2010 | 0 | 0 | 0 | |
| 2011 | 0 | 0 | 0 | |
| 2012 | 0 | 0 | 0 | |
| 2013 | 0 | 0 | 0 | |
| 2014 | 5 | 6 | 11 (11.6%) | |
| 2015 | 0 | 0 | 0 | |
| 2016 | 0 | 0 | 0 | |
| 2017 | 0 | 0 | 0 | |
| 2018 | 0 | 0 | 0 | |
| 2019 | 0 | 0 | 0 | |
| 2020 | 0 | 0 | 0 | |
| 2021 | 0 | 0 | 0 | |
| 2022 | 0 | 0 | 0 | |
| 2023 | 1 | 5 | 6 (5.2%) | |
| 2024 | 2A | 17 | 20 (24.1%) |
The predation events involving adult female Carnaby’s cockatoos in 2024 were notable for the fact that the heads had been removed from the body, the rear of the skull was chewed, and the brain tissue consumed. The heads were then left in the nest hollow. This method of consuming specific body parts, had not been observed in the other 2 years that single predation events of adult female cockatoos occurred, although the sample size in 2014 and 2023 was limited to a single predation event in each year.
Over the 3 years that it occurred, feral cat predation was recorded in 35 different nests: 11 natural hollows; and 24 artificial hollows. Predation events were mostly recorded in those hollows as single events across the 3 years (Table 2). Only four individual nests had two predation events, and half of those were in nests where predation events were separated by 9 years. The geographic spread of the nests subjected to predation by feral cats was not random (Fig. 5). There was a clear pattern of predated nests occurring along linear corridors of native vegetation or in closely spaced trees situated in cleared paddocks.
Map of the Coomallo Creek study site showing the locations of the Carnaby’s cockatoo nest hollows where predation events by feral cats were recorded. Predation events in 2014 (green circles), 2023 (yellow triangles), and 2024 (orange pentagons).
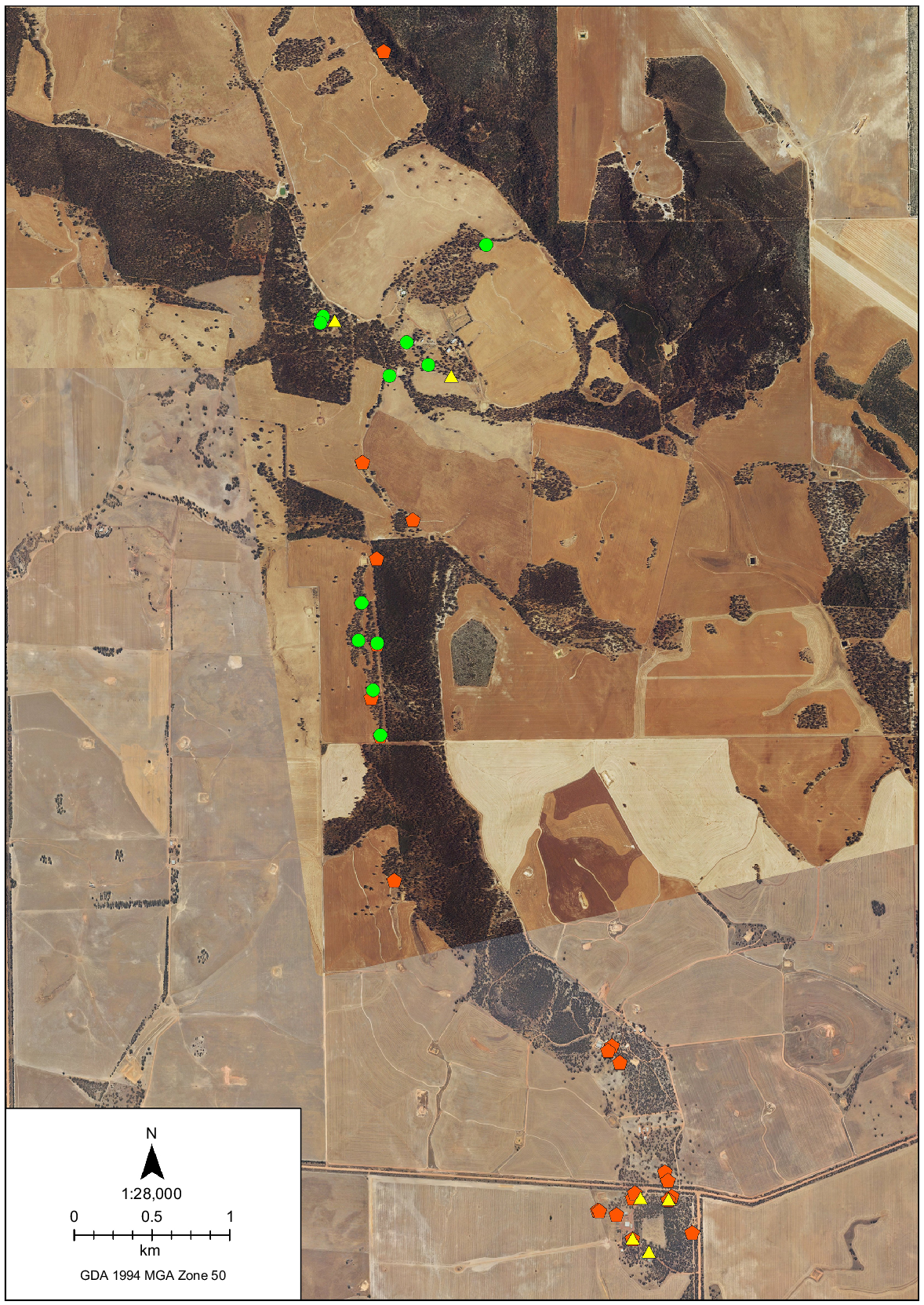
The contribution that natural and artificial hollows made to the total number of monitored nests varied across the years. The number of natural hollows declined due to natural attrition, but ranged from 79 to 94, and was at a low of 79 in 2024. The number of artificial hollows increased from zero in 2010 to a maximum of 82 by 2024. In 2024, 52 of the 82 artificial hollows (63.4%) were used by cockatoos. The feral cat predation events in the 20 hollows in 2024 amounted to predation in 9.7% (3/31) of the natural hollows used by Carnaby’s cockatoos, and 32.7% (17/52) of the artificial hollows. The dimensions of the entrances to natural and artificial hollows, and the height aboveground to the entrance of the hollows where feral cat predation events were recorded were examined. The smallest dimension of the entrance to natural hollows that feral cats entered was 150 mm wide, while the smallest diameter measurement for the tubular open-topped artificial nest hollows was 375 mm (Fig. 2). The mean height aboveground of the artificial hollows (4.41 ± 0.4 m; mean ± s.d.) was significantly greater than the natural nest hollows (3.85 ± 0.7 m; Student’s unpaired t-test: t = 2.80, d.f. = 28, P = 0.0091). However, there was no significant difference between the height to the hollow entrance in natural hollows that suffered predation events and those that did not (Student’s t-test: t = 1.11, d.f. = 53, P = 0.62), and also for artificial nest hollows (t = 0.34, d.f. = 77, P = 0.74).
Discussion
Our data suggest that feral cats may not be regular predators of obligate hollow nesting species such as Carnaby’s cockatoos (Table 1). However, when they do prey on this species, they can have a significant impact on the total reproductive output in a single year at a particular location. The relative significance of the predation effort would appear to be low in most years; however, in 2024, the impact on the reproductive output of Carnaby’s cockatoo was exacerbated by the lower-than-normal number of breeding attempts by the birds at this site (83 breeding attempts in 2024 compared with 142 in 2023). The reduced breeding effort was a function of the extremely low autumn rainfall in 2024 (when drought conditions were still in effect), which delayed the onset of the breeding season to the latest recorded (Saunders et al. 2020c, 2024b), resulting in fewer Carnaby’s cockatoos attempting to breed at Coomallo Creek. Feral cats were not particular about which life stage they consumed (eggs, nestlings, or adult females; Table 2), but their capacity to catch and subdue adult females while they are incubating eggs or brooding nestlings poses a serious threat to the conservation of the species, as increased adult mortality has previously been identified as a key threat to the species’ conservation (Williams et al. 2017; Saunders et al. 2024b).
The habit of feral cats preying on a series of nests in close proximity suggests that one, or a small number of feral cats were likely responsible for the predation events in 2014 and 2023 (Fig. 5). Published studies involving predation by feral cats on burrowing mammals such as bilbies (Macrotis lagotis) and burrowing bettongs (Bettongia lesueur) (Short and Turner 2000; Moseby et al. 2011) and cave-dwelling bats (e.g. Pilbara leaf-nosed bats, Rhinonicteris aurantia; Moyses et al. 2024) and probably also ghost bats (Macroderma gigas; Bat Call WA Pty Ltd 2021), and substantial aboveground structures such as those made by greater stick-nest rats (Leporillus conditor; Short et al. 2018) demonstrate that feral cats readily return to fixed habitat structures when hunting preferred prey. Moseby and McGregor (2022) also concluded that mammal species with conspicuous burrows, latrine sites, and foraging diggings are more likely to be susceptible to cat predation.
In a similar result, selective predation by a single feral cat was responsible for most mortality in a reintroduced population of western quolls (Dasyurus geoffroii) in a study in South Australia (Moseby et al. 2015) and in a reintroduced population of rufous hare wallabies (Lagorchestes hirsutus) in a study in Western Australia (Hardman et al. 2016). Domestic cats have been shown to exhibit individual, or between-phenotype, variation in hunting behaviour, and will hunt specific prey types even when those prey become scarce (Dickman and Newsome 2015).
The density of available nest hollows within the study area varied from 11.2 per km2 in 2009 to 53.2 per km2 in 2021, due largely to the addition of 82 artificial nest hollows to the site commencing in 2011 (Saunders et al. 2018, 2020a; P. R. Mawson, R. Dawson and D. A. Saunders, unpubl. data). The addition of artificial nest hollows and repairs to natural nest hollows (Saunders et al. 2023) meant that the average distance between nest hollows declined over the duration of the study. The much higher predation effort, both in terms of the total number of events (n = 19) and the relative impact (24.1% of Carnaby’s cockatoo breeding attempts) recorded in 2024 may be because more feral cats were involved, but the proximity of the affected hollows (Fig. 5) suggests that the observed outcome may have been a function of selective predation by a single feral cat rather than the number of feral cats engaging in the predation. Such a hunting strategy by feral cats could mean that Carnaby’s cockatoos that are nesting in isolated woodland patches within an agricultural landscape could be at greater risk from cat predation than they would be if nest trees were more widely spaced.
At Coomallo Creek, artificial hollows were intentionally placed approximately 4 m above the ground, as this was calculated to be the average height to natural hollow entrances at this site (Saunders et al. 2020a). The fact that feral cats were able to access both natural and artificial hollows around 4 m above the ground indicates that climbing to this height poses no difficulties for some cats. In fact, a cat leaving a hollow 9 m above the ground after preying on an adult female Western red-tailed cockatoo at Nereeno Hill, 100 km north-east of Coomallo Creek, has been recorded (D. A. Saunders, unpubl. data). While it appears that feral cats focused on artificial hollows when preying on Carnaby’s cockatoos, it is worth noting that in southern part of the Coomallo Creek study area, where most of the predation events were recorded, Carnaby’s cockatoo have nested in nearly all the 36 artificial nest hollows in this part of the study site, but have used only three of the 10 natural nest hollows remaining in this part of the study site. Artificial hollows are now the dominant hollow type due to the loss of most of the natural hollows in this part of the study site due to wildfire in December 2010 (Saunders et al. 2020a).
The focused nature of the cat predation events on hollows located within the same small paddock or woodland remnants is consistent with the findings in other published studies on feral cat habitat use in Australia (Graham et al. 2012). Moseby and McGregor (2022) reported that feral cats in arid central Australia spent significantly more time at microsites with high vegetation cover including single shrubs and trees, suggesting that they use prominent prey cues and patches of thick cover to increase their probability of encountering prey. Moseby and McGregor (2022) also considered that feral cats conceal themselves during hunting or feeding activity and suggested that prey species with conspicuous cues are at higher risk of predation and this vulnerability could increase over time as resident cats learn to identify the location of prey cues within their home range. Carnaby’s cockatoo attendance at nest hollows at or just after sunset to feed or brood their young would certainly provide both auditory and visual cues to feral cats hunting around sunset, but the lack of evidence of widespread predation of Carnaby’s cockatoo nests across all the Coomallo Creek study area and in more than just a few years, suggests that not all feral cats at Coomallo Creek were hunting in this manner. This is further supported by the observation that once a single adult male feral cat was culled at the study site in late September 2014 no further predation events were recorded that breeding season.
Moseby and McGregor (2022) also reported that the feral cats they monitored moved on average 7 km (6.6–7.6 km 95% CI and min 0.1 km max 16.2 km) each night. Our entire study area only extends 9 km in length and 1–1.5 km in width, so it is conceivable that only one feral cat was responsible for the predation in nest hollows. Jansen et al. (2021) report on similar 7–9 km per day movements of radio-collared cats from their study site in arid and semi-arid South Australia, and which included a long-distance dispersal of one cat into more mesic coastal woodland habitat. Most published studies on feral cat movements in Australia come from tropical, arid or semi-arid environments, or insular locations. Tiller et al. (2021) recorded daily movements by feral cats in a range of environments including arid, semi-arid, and coastal heathlands and eucalypt (Eucalyptus occidentalis) woodlands that varied from 3.2 to 6.8 km per day depending on habitat type and sex of the feral cats, with male cats travelling further than females. This suggests that the typical daily (or nightly) movements of feral cats are similar across all habitats and latitudes in Australia.
Since 2009, we observed low impact from feral cats at Coomallo Creek most years. The irregular occurrence of breeding seasons in which feral cat predation occurred warrants further examination, and in particular what options feral cats had at Coomallo Creek for food sources. Potential prey species available include: house mice (Mus musculus), rabbits, Western banjo frogs (Limnodynastes dorsalis), wood ducks, Australian shelduck (Tadorna tadornoides), grey teal (Anas gracilis), Pacific black duck (Anas superciliosa), galah (Eolophus roseicapilla), and black rat (Rattus rattus) (first recorded by us at the study site in September 2024). Native mammals recorded in the study site are limited to two large species of macropod (western grey kangaroo, Macropus fuliginosus; euro, Osphranter robustus) and short-beaked echidna (Tachyglossus aculeatus). There are few records of any native mammal species in the Coomallo Creek area, but species including possums, dunnarts, bandicoots, and native rodents that might have been present have become locally extinct within the wider region as they have in most other parts of the south-western Australia, and well before this long-term study site was established in 1969 (Abbott 2009).
Dickman (1996) stated that prey size influences the impacts of feral cat predation, and on the Australian mainland this impact falls most heavily on species weighing less than 200 g, and that prey availability, comprising local abundance, density, naivety, body size, and species composition is inextricably linked to diet selection by feral cats. Woinarski et al. (2017) reported that cats preyed preferentially on birds in the weight range 60–300 g. Adult Carnaby’s cockatoos weigh 560–790 g (Saunders 2009), but the portion that cats consume would be a lesser mass, and nestling cockatoos have a range of body mass depending on their age at the time they were predated. Domestic cats are primarily opportunistic hunters and ‘choosey’ only secondarily (Barratt 1997), and similarly diet selection by feral cats appears driven primarily by what to hunt rather than what to eat (Bradshaw 2006). In combination, these two traits have been observed to manifest themselves in feral cats killing more than they can eat (Hardman et al. 2016), and in some studies as much as 28% of prey killed by feral cats goes uneaten (McGregor et al. 2015). We observed only the indigestible parts of Carnaby’s cockatoo remains in nest hollows predated by feral cats (i.e. wing bones and flight feathers, partial skull, mandibles, egg shells, and tail feathers) suggesting that the feral cat(s) responsible for the predation were consuming most of the prey taken each time, giving further support for the notion that food was in short supply for the cats in 2024.
A short but severe drought occurred in the northern wheatbelt of Western Australia from October 2023 to the end of May 2024 (Bureau of Meteorology 2024b). This drought appeared to have a significant impact on house mice numbers at Coomallo Creek, which in 2021 and 2022, had supported large populations of boobook owls (Ninox boobook) and barn owls (Tyto alba) (Mawson et al. 2024). Observations of rabbits living in roadside vegetation, larger remnants of native vegetation, and within redundant farm machinery stockpiles indicated that their populations also fell to near zero due to the drought. With the two main staples in diet of feral cats depleted or completely gone, the next or only alternative food sources were Port Lincoln ringnecks (Barnardius z. zonarius), galahs, western corellas, and Carnaby’s cockatoos. All four species of parrot and cockatoo could be caught by feral cats around stock water troughs, but only hollows containing western corellas and Carnaby’s cockatoos have entrances large enough to allow feral cats to gain entry (Saunders et al. 1982).
In early 2024, one of the landowners at Coomallo Creek reported shooting a large male feral cat that was hiding under the over-hang of a water trough. This feral cat had killed five of the landowner’s poultry and was using an artificial hollow (#27A; Table 2) for its day time refuge. When we examined this hollow in March 2024, it contained the wing feathers of galahs and Carnaby’s cockatoos (Fig. 6), which we presume were captured at the water trough and carried back to the artificial nest hollow to be consumed. The Carnaby’s cockatoo remains found in this nest hollow were not included in the tally of predation events in Tables 1 and 2, as these predation events occurred after the completion of the surveys for the 2023 breeding season, but this evidence indicates that predation can also occur away from the hollow where no evidence would be expected to persist, meaning that our calculations of predation rates by feral cats are likely to be conservative.
Remains of galahs and Carnaby’s cockatoos in an artificial nest hollow (#27A) attributed to predation by a feral cat known to be utilising the hollow as a daytime refuge in March 2024 (photograph: Rick Dawson).

While there was a significant difference in the average height aboveground between the entrances to the natural and artificial hollows where feral cat predation was recorded, this finding is of no conservation significance. All it demonstrates is that feral cats can readily access hollows at this site. We also conduct studies of Carnaby’s cockatoo nesting ecology at other sites within the south-west of Western Australia (Saunders et al. 2014a) where nest entrance heights range from 2 m to >10 m. We have only observed evidence in the form of prey remains or cat claw scratches on nest tree trunks at one other site (66 km east-north-east of Coomallo Creek), also located in the northern wheatbelt but subject to annual pest control, and where 50 feral cats were culled over 21 years (Saunders and Doley 2019). The only other reported predation of large cockatoo species by cats in south-western Western Australia is that by Saunders (1991, 2023) who reported feral cat predation of Western red-tailed cockatoos in 5.8% of 503 breeding attempts in salmon gum (Eucalyptus salmonophloia) woodland over 14 breeding seasons. One cat was responsible for killing 10 nestlings in the spring of 1978; 16.7% of breeding attempts that season. The similar overall rate of predation by cats of Western red-tailed cockatoos to what we recorded on Carnaby’s cockatoos during 2014 and 2023 suggests that this level of predation may be typical, but not necessarily regular. Two of the authors (PRM and RD) have also monitored Carnaby’s cockatoo nest hollows on a rail reserve south-east of this study site from 1996 to 2024, and during that time we have monitored 96 Carnaby’s cockatoo nesting attempts during 16 different years and have not recorded a single case of predation by feral cats despite some parts of the monitored rail reserve running through and either side of a rural community.
The results from this study also suggests that at most other sites, and in most years at Coomallo Creek, traditional prey species such as house mice and rabbits are available in great enough numbers that most feral cats do not need expend the effort of climbing several metres aboveground and risking injury from a protective adult female Carnaby’s cockatoo. However, in drought years when those normal mammal prey species are not available, feral cats represent a serious threat to the conservation of Carnaby’s cockatoos. This risk would be greater in small woodlands where cockatoo nests are concentrated, and in isolated woodlands where the Carnaby’s cockatoo breeding population is either already small, or is becoming so due to a decline in the number of available nest hollows due to natural attrition (Saunders 1979; Saunders et al. 2014b, 2020a).
Nest boxes are widely used for the conservation of imperilled hollow-nesting birds and mammals in Australia. A previous study reported cat predation on the critically endangered Leadbeater’s possum (Gymnobelideus leadbeateri), with cats stalking possums at nest boxes (McComb et al. 2019).
The conservation implications of the findings set out above are that feral cats should be considered a key threat to Carnaby’s cockatoos and other large, cockatoo species, and that this new threat should be recognised in the recovery plan that is currently being developed for three species of threatened black cockatoos, endemic to south-west Western Australia.
Controlling feral cats is a challenge under most conditions, but in broadacre farming systems with low human populations and a largely nocturnal predator, shooting alone is unlikely to provide sufficient control of cat populations, except possibly around farm buildings where familiarity with people and machinery may lead to complacency in some cats. At Coomallo Creek, the landowners culled eight feral cats (five shot and three trapped; Fig. 7) within a 21-month period to April 2025, but this was insufficient to prevent substantial predation on cockatoos through the 2024 breeding season.
Feral cat trapped in January 2025 at the southern end of the Coomallo Creek study site, Western Australia using canned tuna as bait (photograph: S. Barrett).

Trapping feral cats can be difficult and not all cats appear equally susceptible to trapping (Short et al. 2002) and require as many as 98 nights trapping effort per cat trapped (Augusteyn and Nolan 2022). We have also trapped one adult female feral cat and four kittens residing in a shed adjacent to our onsite accommodation. However, if food for feral cats is at times a limiting factor in later summer through to winter, then it is possible that dedicated trapping programs commencing at the start of the cockatoo breeding season may provide adequate levels of control to mitigate the threat posed by feral cats. Similarly, shooting programs during the nesting season and focused around the woodland areas containing nest hollows may provide sufficient control to reduce predation by cats to a level where it is no more significant than any other cause of breeding failure.
Both shooting and trapping have high social acceptability in rural farming landscapes, and are considered more effective in woodland habitats than other habitat types (Dorph et al. 2024). If applied to specific cats that target nesting hollows then a good outcome is likely (DCCEEW 2024). Measuring the success of any cat trapping and shooting programs is reliant on the assumption that removing cats while recording annual breeding success rates comparable to those recorded in years with no cat predation events were recorded is in some way a useful, albeit indirect measure the efficacy of the cat control.
One other management option that is likely to reduce cat predation on Carnaby’s cockatoo at this site (or other hollow-nesting species at other discrete sites) is to fix metal guards around the trunks of trees that contain nesting hollows. Such exclusion devices have been highly successful at reducing predation by brush-tailed possums on threatened glossy black-cockatoos on Kangaroo Island (Berris et al. 2018). Our limited experience using this method at Coomallo Creek indicates that it can only reliably be applied to trees that have no, or no close, canopy contact with any other trees.
Conflicts of interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions
Peter R. Mawson: conceptualisation, methodology, formal analysis, investigation, writing – original draft, writing – review and editing. Rick Dawson: conceptualisation, methodology, formal analysis, investigation, writing – review and editing. Brooke S. Richards: conceptualisation, methodology, spatial mapping, writing – original draft, writing – review and editing. Denis A. Saunders: conceptualisation, methodology, formal analysis, investigation, writing – original draft, writing – review and editing.
Acknowledgements
We are grateful to John Ingram for technical support 1972–1996; the Hayes, McAlpine, Paish, Barrett and Raffan families for supporting this research on their properties; the Raffan family for providing accommodation during the field work; Drs Manda Page, Juanita Renwick, and Jacqui Richards for support; Drs John Woinarski and Jeff Short for critical comment on a pre-submission draft of our manuscript, and two anonymous reviewers for their contributions.
References
Abbott I (2009) Historical perspectives of the ecology of some conspicuous vertebrate species in south-west Western Australia. Conservation Science Western Australia 6(3), 1-214 Available at https://library.dbca.wa.gov.au/static/Journals/080559/080559-06.009.pdf.
| Google Scholar |
Augusteyn J, Nolan B (2022) Evaluating methods for controlling feral cats that minimise non-target impacts at Taunton National Park (Scientific). Ecological Restoration and Management 23, 43-52.
| Crossref | Google Scholar |
Barratt DG (1997) Predation by house cats, Felis catus (L.), in Canberra, Australia. I. Prey composition and preference. Wildlife Research 24, 263-277.
| Crossref | Google Scholar |
Bat Call WA Pty Ltd (2021) A review of ghost bat ecology, threats and survey requirements. Report prepared for the Department of Agriculture, Water and the Environment, Canberra. CC BY-NC-ND 4.0. Available at https://www.agriculture.gov.au/sites/default/files/documents/review-ghostbat-ecology-threats.pdf [accessed 22 April 2025]
Berris K, Barth M, Mooney T, Paton D, Kinloch M, Copley P, Maguire A, Crowley G, Garnett ST (2018) From the brink of extinction: successful recovery of the glossy black-cockatoo on Kangaroo Island. In ‘Recovering Australian threatened species: a book of hope’. (Eds S Garnett, P Latch, D Lindenmayer, J Woinarski) pp. 75–84. (CSIRO Publishing: Melbourne)
Bradshaw JWS (2006) The evolutionary basis for the feeding behavior of domestic dogs (Canis familiaris) and cats (Felis catus). Journal of Nutrition 136, 1927S-1931S.
| Crossref | Google Scholar | PubMed |
Bureau of Meteorology (2024a) Monthly rainfall data. Badgingarra Research Stations #009037. Available at http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=139&p_display_type=dataFile&p_startYear=&p_c=&p_stn_num=009037 [accessed 25 February 2025]
Bureau of Meteorology (2024b) Drought – Rainfall deficiencies and water availability. Issued 6 June 2024. Available at http://www.bom.gov.au/climate/drought/archive/20240606.archive.shtml#tabs=Rainfall-deficiencies [accessed 26 February 2025]
Dawson R, Saunders DA, Mawson P (2011) The vulnerable and the endangered: Carpet Python predation on a breeding female Carnaby’s Black Cockatoo. Australian Zoologist 35, 679-680.
| Crossref | Google Scholar |
DCCEEW (2024) Threat abatement plan for predation by feral cats 2024. Department of Climate Change, Energy, the Environment and Water. Available at https://www.dcceew.gov.au/sites/default/files/documents/tap-for-predation-feral-cats-2024.pdf [accessed 23 April 2025]
Dickman CR (1996) ‘Overview of the impacts of feral cats on Australian native fauna.’ (Australian Nature Conservation Agency: Canberra, Australia) Available at https://www.dcceew.gov.au/sites/default/files/documents/impacts-feral-cats.pdf
Dickman CR, Newsome TM (2015) Individual hunting behaviour and prey specialisation in the house cat Felis catus: implications for conservation and management. Animal Behaviour and Science 173, 76-87.
| Crossref | Google Scholar |
Doherty TS, Davis RA, van Etten EJB, Algar D, Dickman CR, Edwards G, Masters P, Palmer R, Robinson S (2015) A continental-scale analysis of feral cat diet in Australia. Journal of Biogeography 42, 964-975.
| Crossref | Google Scholar |
Dorph A, Ballard G, Legge S, Algar D, Basnett G, Buckmaster T, Dunlop J, Edwards AM, Hine A, Knight AR, Marshall E, McColl-Gausden SC, Pauza MD, Penman TD (2024) Current and emerging feral cat management practices in Australia. Wildlife Research 51, WR23107.
| Crossref | Google Scholar |
Graham CA, Maron M, McAlpine CA (2012) Influence of landscape structure on invasive predators: feral cats and red foxes in the brigalow landscapes, Queensland, Australia. Wildlife Research 39, 661-676.
| Crossref | Google Scholar |
Hardman B, Moro D, Calver M (2016) Direct evidence implicates feral cat predation as the primary cause of failure of a mammal reintroduction programme. Ecological Management and Restoration 17, 152-158.
| Crossref | Google Scholar |
IUCN (2016) The IUCN Red List of Threatened Species. Version 2016-3. Available at www.iucnredlist.org [accessed 25 January 2025]
Jansen J, McGregor H, Axford G, Dean AT, Comte S, Johnson CN, Moseby KE, Brandle R, Peacock DE, Jones ME (2021) Long-distance movements of feral cats in semi-arid South Australia and implications for conservation management. Animals 11, 3125.
| Crossref | Google Scholar | PubMed |
Jones E, Coman BJ (1981) Ecology of the feral cat, Felis catus (L.), in south-eastern Australia I. Diet. Australian Wildlife Research 8, 537-547.
| Crossref | Google Scholar |
Joseph L (1982) The glossy black-cockatoo on Kangaroo Island. Emu 82, 46-49.
| Crossref | Google Scholar |
Lepczyk CA, Calver MC (2022) Cat got your tongue? The misnomer of ‘community cats’ and its relevance to conservation. Biological Invasions 24, 2313-2321.
| Crossref | Google Scholar |
Lepczyk CA, Fantle-Lepczyk JE, Dunham KD, Bonnaud E, Lindner J, Doherty TS, Woinarski JCZ (2023) A global synthesis and assessment of free-ranging domestic cat diet. Nature Communications 14, 7809.
| Crossref | Google Scholar | PubMed |
Lowe S, Browne M, Boudjelas S (2000) 100 of the world’s worst invasive alien species: A selection from the global invasive species database. Invasive Species Specialist Group, International Union for Conservation of Nature. Available at https://portals.iucn.org/library/sites/library/files/documents/2000-126.pdf
Mawson PR, Dawson R, Saunders DA (2024) Breeding by Barn Owls Tyto alba in artificial nest hollows established for an endangered black cockatoo in the northern wheatbelt of Western Australia. Australian Zoologist 44, 26-34.
| Crossref | Google Scholar |
McComb LB, Lentini PE, Harley DKP, Lumsden LF, Antrobus JS, Eyre AC, Briscoe NJ (2019) Feral cat predation on Leadbeater’s possum (Gymnobelideus leadbeateri) and observations of arboreal hunting at nest boxes. Australian Mammalogy 41, 262-265.
| Crossref | Google Scholar |
McGregor H, Legge S, Jones ME, Johnson CN (2015) Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS ONE 10, e0133915.
| Crossref | Google Scholar | PubMed |
Moseby KE, McGregor HM (2022) Feral cats use fine scale prey cues and microhabitat patches of dense vegetation when hunting prey in arid Australia. Global Ecology and Conservation 35, e02093.
| Crossref | Google Scholar |
Moseby KE, Read JL, Paton DC, Copley P, Hill BM, Crisp HA (2011) Predation determines the outcome of 10 reintroduction attempts in arid South Australia. Biological Conservation 144, 2863-2872.
| Crossref | Google Scholar |
Moseby KE, Peacock DE, Read JL (2015) Catastrophic cat predation: a call for predator profiling in wildlife protection programs. Biological Conservation 191, 331-340.
| Crossref | Google Scholar |
Moyses J, Grabham C, Armstrong KN, Knuckey CG, D’Rozario B (2024) Feral cat predation of the threatened Pilbara leaf-nosed bat – a key threatening process. Australian Mammalogy 46, AM23049.
| Crossref | Google Scholar |
Medina FM, Bonnaud E, Vidal E, Tershy BR, Zavaleta ES, Donlan CJ, Keitt BS, Le Corre M, Horwath SV, Nogales M (2011) A global review of the impacts of invasive cats on island endangered vertebrates. Global Change Biology 17, 3503-3510.
| Crossref | Google Scholar |
RSPCA (2018) Identifying best practice domestic cat management in Australia: Summary of findings and recommendations. Australian Government and National Landcare Program. Available at https://kb.rspca.org.au/wp-content/uploads/2019/01/Identifying-Best-Practice-Domestic-Cat-Management-in-Australia-RSPCA-Research-Report-May-2018.pdf
Saunders DA (1974) Subspeciation in the White-tailed Black Cockatoo, Calyptorhynchus baudinii, in Western Australia. Australian Wildlife Research 1, 55-69.
| Crossref | Google Scholar |
Saunders DA (1979) The availability of tree hollows for use as nest sites by White-tailed Black Cockatoos. Australian Wildlife Research 6, 205-216.
| Crossref | Google Scholar |
Saunders DA (1980) Food and movements of the short-billed form of the White-tailed Black Cockatoo. Australian Wildlife Research 7, 257-69.
| Crossref | Google Scholar |
Saunders DA (1982) The breeding behaviour and biology of the short-billed form of the White-tailed Black Cockatoo Calyptorhynchus funereus. Ibis 124, 422-455.
| Crossref | Google Scholar |
Saunders DA (1991) The effect of land clearing on the ecology of Carnaby’s Cockatoo and the inland Red-tailed Black-Cockatoo in the wheatbelt of Western Australia. In ‘Acta XX Congressus Internationalis Ornithologici’, 2–9 December 1990, Christchurch, New Zealand. vol. 1, pp. 658–665. (New Zealand Ornithological Congress Trust Board)
Saunders DA (2009) Body weights and egg dimensions of Australian parrots (Aves: Psittaciformes): compiled 1980–1987. 136 p. CSIRO Sustainable Ecosystems, Canberra. Available at https://www.researchgate.net/publication/264534702_Body_Weights_and_Egg_Dimensions_of_Australian_Parrots_Aves_Psittaciformes_compilerm_te87_INTRODUCTION
Saunders DA (2023) The breeding biology of the Western Red-tailed Cockatoo Calyptorhynchus escondidus in the wheatbelt of Western Australia. Australian Zoologist 43, 199-219.
| Crossref | Google Scholar |
Saunders DA, Dawson R (2018) Cumulative learnings and conservation implications of a long-term study of the endangered Carnaby’s Cockatoo Calyptorhynchus latirostris. Australian Zoologist 39, 591-609.
| Crossref | Google Scholar |
Saunders DA, Doley A (2019) Culling farm wildlife for conservation and production on “Koobabbie”, a cereal and sheep growing property, in the northern wheatbelt of Western Australia. Australian Zoologist 40, 203-217.
| Crossref | Google Scholar |
Saunders DA, Ingram JA (1998) Twenty-eight years of monitoring a breeding population of Carnaby’s Cockatoo. Pacific Conservation Biology 4, 261-270.
| Crossref | Google Scholar |
Saunders DA, Pickup G (2023) A review of the taxonomy and distribution of Australia’s endemic Calyptorhynchinae black cockatoos. Australian Zoologist 43, 145-191.
| Crossref | Google Scholar |
Saunders DA, Smith GT, Rowley I (1982) The availability and dimensions of tree hollows that provide nest sites for cockatoos (Psittaciformes) in Western Australia. Wildlife Research 9, 541-556.
| Crossref | Google Scholar |
Saunders DA, Wintle BA, Mawson PR, Dawson R (2013) Egg-laying and rainfall synchrony in an endangered bird species: implications for conservation in a changing climate. Biological Conservation 161, 1-9.
| Crossref | Google Scholar |
Saunders DA, Mawson PR, Dawson R (2014a) One fledgling or two in the endangered Carnaby’s Cockatoo (Calyptorhynchus latirostris): a strategy for survival or legacy from a bygone era? Conservation Physiology 2, cou001.
| Crossref | Google Scholar |
Saunders DA, Mawson PR, Dawson R (2014b) Use of tree hollows by Carnaby’s Cockatoo and the fate of large hollow-bearing trees at Coomallo Creek, Western Australia 1969–2013. Biological Conservation 177, 185-193.
| Crossref | Google Scholar |
Saunders DA, White NE, Dawson R, Mawson PR (2018) Breeding site fidelity, and breeding pair infidelity in the endangered Carnaby’s Cockatoo Calyptorhynchus latirostris. Nature Conservation 27, 59-74.
| Crossref | Google Scholar |
Saunders DA, Dawson R, Mawson P, Cunningham RB (2020a) Artificial hollows provide an effective short-term solution to the loss of natural nesting hollows for Carnaby’s Cockatoo Calyptorhynchus latirostris. Biological Conservation 245, 108556.
| Crossref | Google Scholar |
Saunders DA, Mawson PR, Dawson R (2020b) Predation by Southwestern Carpet Python Morelia spilota imbricata of Carnaby’s Cockatoo Calyptorhynchus latirostris in a breeding hollow. Australian Zoologist 41, 54-57.
| Crossref | Google Scholar |
Saunders DA, Dawson R, Mawson PR, Nicholls AO (2020c) Factors affecting nestling condition and timing of egg-laying in the endangered Carnaby’s Cockatoo Calyptorhynchus latirostris. Pacific Conservation Biology 26, 22-34.
| Crossref | Google Scholar |
Saunders DA, Dawson R, Mawson PR (2023) Artificial nesting hollows for the conservation of Carnaby’s cockatoo Calyptorhynchus latirostris: definitely not a case of erect and forget. Pacific Conservation Biology 29, 119-129.
| Crossref | Google Scholar |
Saunders DA, Mawson PR, Dawson R, Beswick H, Pickup G, Usher K (2024a) Movements of adult and fledgling Carnaby’s cockatoos (Zanda latirostris Carnaby, 1948) from eleven breeding areas throughout their range. Pacific Conservation Biology 30, PC24042.
| Crossref | Google Scholar |
Saunders DA, Mawson PR, Dawson R, Pickup G (2024b) A challenging future for Carnaby’s Cockatoo (Zanda latirostris) under a changing climate. Pacific Conservation Biology 30, PC24065.
| Crossref | Google Scholar |
Short J, Turner B (2000) Reintroduction of the burrowing bettong Bettongia lesueur (Marsupialia: Potoroidae) to mainland Australia. Biological Conservation 96, 185-196.
| Crossref | Google Scholar |
Short J, Turner B, Risbey D (2002) Control of feral cats for nature conservation. III. Trapping. Wildlife Research 29, 475-487.
| Crossref | Google Scholar |
Short J, Richards JD, O’Neill S (2018) Reintroduction of the greater stick-nest rat (Leporillus conditor) to Heirisson Prong, Shark Bay: an unsuccessful attempt to establish a mainland population. Australian Mammalogy 40, 269-280.
| Crossref | Google Scholar |
Tiller C, Fletcher J, Comer S, Algar D (2021) Using activity and movement patterns to improve the rate of bait encounter during large-scale aerial baiting for feral cats. Australasian Journal of Environmental Management 28, 220-235.
| Crossref | Google Scholar |
Williams MR, Yates CJ, Saunders DA, Dawson R, Barrett GW (2017) Combined demographic and resource models quantify the effects of potential land-use change on the endangered Carnaby’s cockatoo (Calyptorhynchus latirostris). Biological Conservation 210, 8-15.
| Crossref | Google Scholar |
Woinarski JCZ, Woolley LA, Garnett ST, Legge SM, Murphy BP, Lawes MJ, Comer S, Dickman CR, Doherty TS, Edwards G, Nankivill A, Palmer R, Paton D (2017) Compilation and traits of Australian bird species killed by cats. Biological Conservation 216, 1-9.
| Crossref | Google Scholar |
Yip SJS, Rich MS, Dickman CR (2015) Diet of the feral cat, Felis catus, in central Australian grassland habitats during population cycles of its principal prey. Mammal Research 60, 39-50.
| Crossref | Google Scholar |


