Conservation in the wake of myrtle rust – a case study on two critically endangered Australian rainforest plants
K. D. Sommerville A D , P. Cuneo A , G. Errington A , R. O. Makinson B , S. Pederson C , G. Phillips A , A. Rollason A , V. Viler A and C. A. Offord A
A D , P. Cuneo A , G. Errington A , R. O. Makinson B , S. Pederson C , G. Phillips A , A. Rollason A , V. Viler A and C. A. Offord A
A The Australian PlantBank, The Royal Botanic Gardens and Domain Trust, Mount Annan, NSW 2567, Australia.
B Australian Network for Plant Conservation, Canberra, ACT 2601, Australia.
C Botanic Gardens, Booderee National Park, Parks Australia, Jervis Bay, NSW 2540, Australia.
D Corresponding author. E-mail: karen.sommerville@rbgsyd.nsw.gov.au
Pacific Conservation Biology 26(3) 218-229 https://doi.org/10.1071/PC19026
Submitted: 27 June 2019 Accepted: 1 December 2019 Published: 23 December 2019
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
We investigated ex situ conservation options for two Australian rainforest species severely affected by myrtle rust in the wild – Rhodamnia rubescens (Benth.) Miq. and Rhodomyrtus psidioides (G.Don) Benth. Both species were successfully initiated into tissue culture though the rate of contamination was high and not significantly improved by the disinfection techniques tested. Explants surviving initiation grew well on Murashige and Skoog medium (MS; pH 6.0) with 30 g L−1 sucrose, 1 µm benzyl adenine and 0.2 µm indole-3-butyric acid. Culture of R. rubescens for eight weeks on MS with 0, 5, 10 or 20 µm indole-3-butyric acid resulted in root production for some plantlets, and successful transfer to potting mix; no significant differences in root production among treatments were detected. Both species were successfully propagated by semi-hardwood cuttings with strike rates of 0–67% for R. rubescens and 0–75% for R. psidioides. For R. rubescens, pretreatment of cuttings with Zaleton® and incubation in Preforma® plugs reduced the time to root development and significantly improved the strike rate (P = 0.001). R. rubescens seed proved to be orthodox and suitable for standard seedbanking; R. psidioides seed proved to be freezing sensitive but suitable for storage at 4°C. As the two species now produce few viable seeds in the wild, however, conservation by seedbanking will first require the establishment of a seed orchard from vegetatively propagated plants. We recommend swift action to conserve species in the Pacific similarly affected by myrtle rust before their growth and reproductive capacity are seriously diminished.
Additional keywords: Austropuccinia psidii, ex situ conservation, propagation, Rhodamnia, Rhodomyrtus, seed banking, tissue culture.
Introduction
Myrtle rust is a disease caused by the invasive fungus Austropuccinia psidii (formerly Puccinia psidii: Beenken 2017). The disease affects the growth, reproductive capacity and survival of plants in the family Myrtaceae. The pathogen was first described in Brazil in 1884 (Stewart et al. 2018) but, over the past 13 years, has been recorded in an increasing number of locations in the Pacific including Hawaii (Uchida et al. 2006), Japan (Kawanishi et al. 2009), Australia (Carnegie et al. 2010), Hainan Province in China (Zhuang and Wei 2011), New Caledonia (Giblin 2013), Indonesia (McTaggart et al. 2016), Singapore (du Plessis et al. 2017) and New Zealand (du Plessis et al. 2019). Recent genetic analyses of samples from all these locations (excluding Japan) have confirmed that the pathogen in each belongs to the same pandemic strain (Machado et al. 2015; McTaggart et al. 2016; Stewart et al. 2018; du Plessis et al. 2019).
Myrtle rust poses a severe threat to natural biodiversity in the Pacific and to horticulture and forestry industries based on myrtaceous species (Cannon 2009; McDonald 2012). The greatest spread and impact recorded to date appears to have been in Australia, where the 2253 currently accepted Myrtaceae taxa (Makinson 2018) make up more than 12% of the country’s flowering plants (based on estimates in Chapman 2009). At least 358 of these taxa have already been observed to be susceptible to myrtle rust infection, and an estimated 45 species are known or suspected to be in decline (Makinson 2018).
Two genera particularly susceptible to myrtle rust are Rhodamnia Jack and Rhodomyrtus (DC.) Rchb. Rhodamnia is a genus of rainforest trees and shrubs, with 40 species occurring naturally from the south-west Pacific (New Caledonia, Australia and Melanesia) to South-east Asia and Indo-China (WCSP 2019). Rhodomyrtus has a similar distribution, with 21 species occurring naturally from the south-west Pacific to South-east Asia, southern China, India and Sri Lanka (WCSP 2019). In Australia, 16 species of Rhodamnia, and 7 species and one subspecies of Rhodomyrtus, have been recorded as hosts for A. psidii. Thirteen of the Rhodamnia species and all the Rhodomyrtus taxa have been categorised as moderately to extremely susceptible to infection (Makinson 2018).
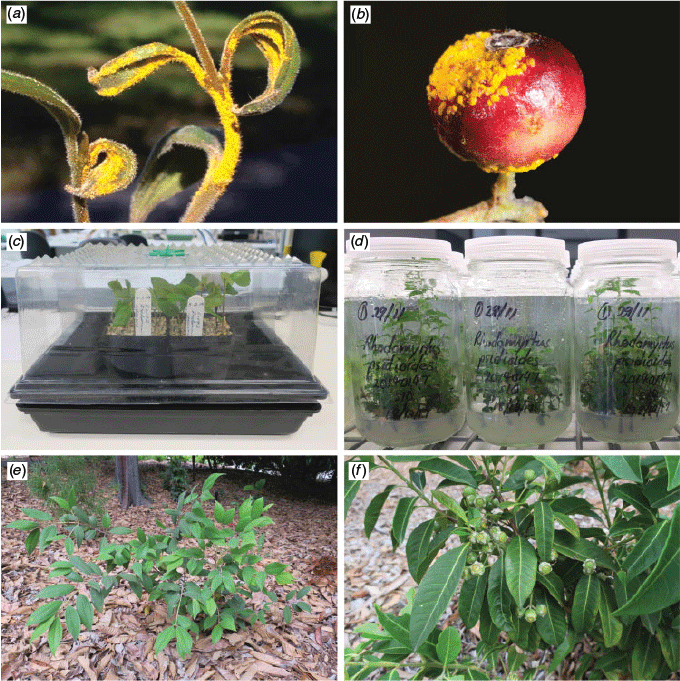
Two species that have been greatly affected by the disease in the past decade are Rhodamnia rubescens (Benth.) Miq. and Rhodomyrtus psidioides (G.Don) Benth. Both species are endemic to rainforest and rainforest margins in eastern Australia (Fig. 1) (Harden et al. 2006) and neither were regarded as threatened before 2010 (Benson and McDougall 1998; Harden et al. 2006; Floyd 2008), when myrtle rust was first discovered on living plants in the country (Carnegie et al. 2010). R. rubescens has been classified as ‘highly to extremely susceptible’ and R. psidioides as ‘extremely susceptible’ to infection, the disease affecting stems, leaves, flowers and fruits in plants of all age classes (Fig. 2a, b) (Pegg et al. 2014; Carnegie et al. 2016). Field assessments of both species have shown that the incidence of infection is widespread and severe. In a 2014 survey of R. rubescens, tree mortality attributable to myrtle rust (ranging from a few dead trees to 75% of the population) was identified at 18 of 43 sites (Carnegie et al. 2016). A similar survey of R. psidioides found 50–100% mortality at eight of 15 sites (Carnegie et al. 2016). Recent reports from Pegg et al. (2017), New South Wales (NSW) National Parks and Wildlife rangers, seed collectors from the Australian PlantBank and private property holders indicate that plant decline in both species is continuing, and seed production is rare.
|
R. rubescens and R. psidioides have now been listed as Critically Endangered under the NSW Biodiversity Conservation Act 2016 (NSW Threatened Species Scientific Committee 2019a, 2019b). The process from nomination to final determination took almost three years; in the interim, both species underwent considerable decline, but the lack of formal listing meant that no State Government funding was available for work on conservation of either species. The species have also been nominated for listing under the national Environmental Protection and Biodiversity Act 1999; however, assessment of these nominations is not scheduled for completion till 30 April 2020 (https://www.environment.gov.au/sprat-public/action/report-fpal). Access to threatened species funding through the Commonwealth Government has thus been likewise difficult to obtain.
Given the level of threat to both species in the natural environment, ex situ conservation of any remaining genetic diversity was seen to be imperative; however, no information was available on the suitability of the few seeds available for seedbanking (Sommerville et al. 2017; Royal Botanic Gardens Kew 2019) and there were no published accounts of techniques to propagate the species by cuttings or tissue culture. To remedy this lack, and in anticipation of official recognition of the species as threatened, the Australian PlantBank (PlantBank) began developing tissue culture protocols for R. rubescens in 2013 using material from a single garden-grown specimen. From 2015, this work was extended to R. psidioides and expanded to include an investigation of the potential for seedbanking and propagation by cuttings of wild material (for both species at PlantBank and for R. rubescens at the Botanic Gardens, Booderee National Park (BBG). In this paper, we describe the methods used to conserve the species to date and their relative success. We suggest a strategy for future conservation efforts for R. rubescens and R. psidioides and suggest a protocol for handling species in similar situations in Australia and other Pacific nations in the future.
Methods
Materials
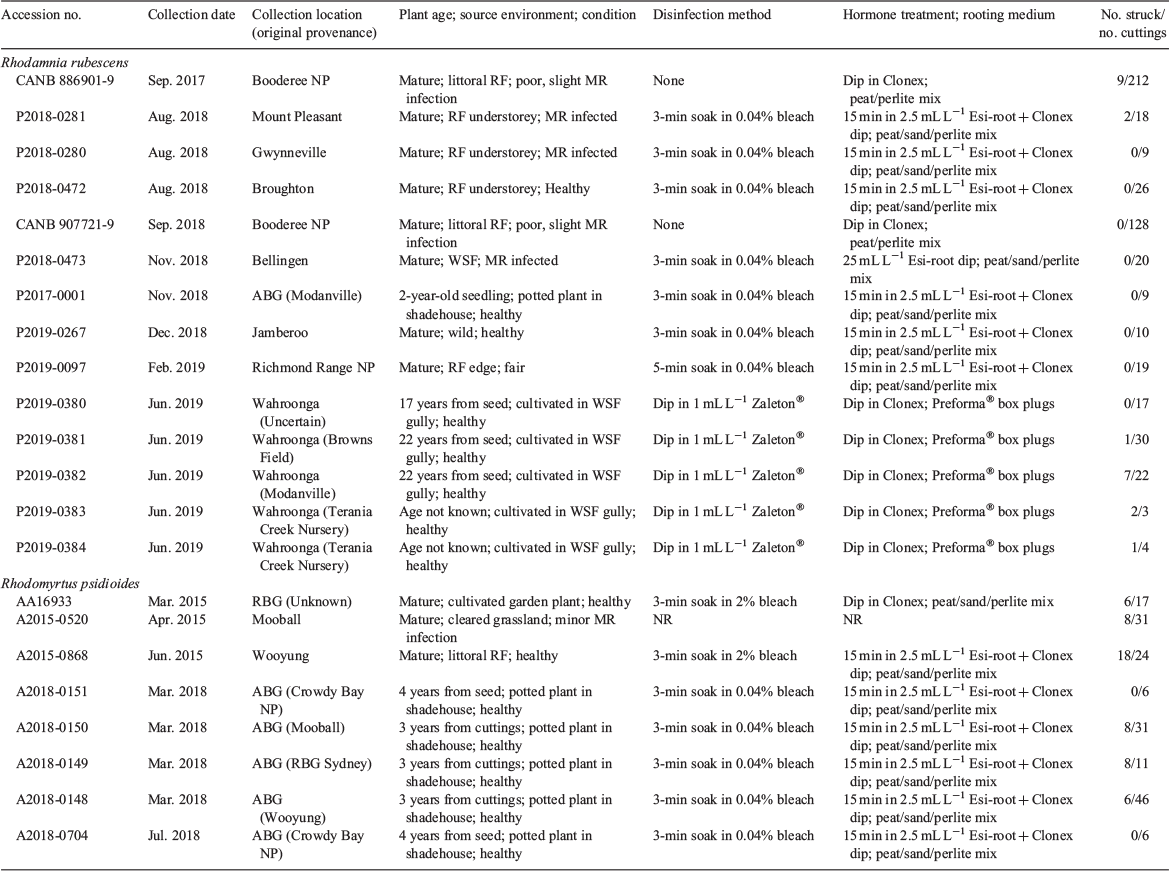
Material for initial propagation of Rhodamnia rubescens was obtained from a single plant growing at the Australian Botanic Garden, Mount Annan (ABG). The plant was donated to the Garden by Terania Rainforest Nursery in 1986 and had been propagated from a wild plant occurring in roadside vegetation in Modanville, NSW. Seed was obtained from the same plant in January 2017. Material for initial propagation of Rhodomyrtus psidioides was obtained from a 1-year-old potted seedling (original provenance Crowdy Bay National Park, NSW) held at the ABG and a single plant growing at The Royal Botanic Garden, Sydney (original provenance not recorded). Material for further propagation experiments on both species was obtained from wild plants growing in littoral rainforest or cleared areas in northern NSW (Tables 1–3).
Propagation by tissue culture
Attempts to initiate tissue cultures from cultivated plants were made in April and July 2013 for Rhodamnia rubescens and March 2015 for Rhodomyrtus psidioides. Tip cuttings of recently matured semi-hardwood were collected from the outer canopy of each source plant and processed as described below.
Pretreatments
R. rubescens cuttings were subjected to one of two pretreatment experiments. In Experiment 1 (April 2013), the cutting material was divided into 45 × 1-cm-long explants, each containing a single leaf node. The leaves were removed from each node and the explants were subjected to one of three in-field treatments: (1) no treatment (explants wrapped in damp paper towels); (2) immersion in 4% Plant Preservative Mixture™ (PPM™; Plant Cell Technology, Washington) in liquid Murashige and Skoog medium (MS, Murashige and Skoog 1962) prepared from powdered MS basal salts with vitamins (Product M519, Phytotechnology Laboratories, Kansas; pH of PPM/MS solution unadjusted, as recommended by supplier); or (3) immersion in 70% ethanol for 45 s followed by immersion in sterile deionised water. The explants were then transported to PlantBank and those in a pretreatment solution were placed on an orbital shaker at 220 rpm for 4.5 h before washing and disinfecting as described below.
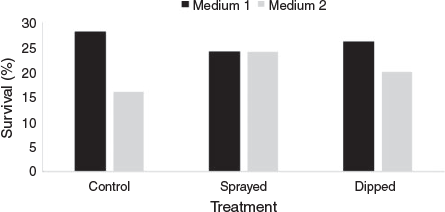
In Experiment 2 (July 2013), randomly selected branchlets of the source plant were subjected to one of three pretreatments: (1) no fungicide application (control); (2) branchlets sprayed two days before collection with 0.32 mL L−1 Tilt® 250 EC (Syngenta, North Ryde; active constituent 250 g L−1 propiconazole); (3) detached branchlets dipped in 0.32 mL L−1 Tilt® 250 EC for 1 h. The material was then washed and disinfected as described below.
No pretreatments were applied to R. psidioides.
Culture initiation
All explants were washed and disinfected to remove surface fungi and bacteria. If not previously removed for pretreatment, leaves were removed from each stem, the petioles were trimmed to ~4 mm in length and the stems were cut into 1-cm lengths, each containing a single leaf node. The explants were then cleaned by gentle scrubbing with a toothbrush (R. rubescens) or by shaking for 30 min (R. psidioides) in a solution of 2% w/v Alconox® (Sigma Aldrich, Castle Hill). Following washing, the explants were rinsed in deionised water then placed on an orbital shaker at 220 rpm for 20–30 min (Table 2) in dilute household bleach (50% v/v White King®, Pental, Shepparton; active constituent 4% NaOCl when fresh). Several drops of Tween 20 surfactant were added to the bleach solution to aid wetting and improve contact between the bleach and the plant material. The disinfected explants were then transferred to a laminar flow cabinet and rinsed three times in sterile deionised water.
The basal end of each explant was recut with a sterile scalpel and the explant transferred to a test tube containing sterile initiation medium. Most explants were placed into half-strength MS (basal medium with vitamins, pH 6.0); however, for R. rubescens in Experiment 2, half the pretreated explants were placed into half-strength MS and the remainder into half-strength MS with 1 µm benzyl adenine (BA). Both media types contained 30 g L−1 sucrose and were solidified with 9 g L−1 agar (product code A1296, Sigma-Aldrich, Castle Hill). All explants were incubated for six weeks at 23 ± 2°C under cool white fluorescent light (PPFD ~45 µmol m−2 s−1) with a 16/8 h light/dark cycle, and were monitored weekly for survival and evidence of fungal or bacterial contamination. For R. rubescens Experiments 1 and 2, differences in survival and contamination among treatments were tested by binary logistic regression in Minitab® 16 Statistical Software; categorical treatments were entered as factors in the analysis.
Multiplication
Following the six-week initiation period, explants remaining alive and uncontaminated were transferred to one of two types of media: MS (pH 6.0) with 30 g L−1 sucrose, 1 µm BA and 0.2 µm indole-3-butyric acid (IBA); or Woody Plant Medium (Lloyd and McCown 1980) (pH 5.75) with 20 g L−1 sucrose, 1 µm BA and 0.2 µm IBA. Both media types were solidified with 9 g L−1 agar. The explants were incubated at 23 ± 2°C under cool white fluorescent light (PPFD 45–50 µmol m−2 s−1) with a 16/8 h light/dark cycle. The resulting plantlets were subcultured every 2–3 months till sufficient multiplication had occurred to enable an exflasking experiment.
Exflasking
In May 2015, October 2015 and August 2018, R. rubescens plantlets were subcultured onto MS (pH 6.0, 30 g L−1 sucrose) containing 0, 5, 10 or 20 µm IBA to determine the best concentration of IBA for encouraging root growth without detrimental effects on plantlet health. Five replicates of five plantlets per treatment were incubated for eight weeks at 23 ± 2°C under cool white fluorescent light (PPFD 45–50 µmol m−2 s−1) with a 16/8 h light/dark cycle. Following the incubation period, the plantlets were removed from the agar and observations of growth (plant height, number of leaf nodes) and root production (number of roots, total root length) were made.
Plantlets with and without roots were transferred into sterile potting mix consisting of 70% fine perlite (Chillagoe Perlite, Mareeba), 20% vermiculite (L & A Fazzini, Greenacre), 10% peat moss (Sun Gro Horticulture, Canada), 2 g L−1 Nutricote® Total TE 70-day slow-release fertiliser (Yates, Padstow), 0.64 g L−1 Micromax® micronutrients (ICL, Bella Vista), 0.24 g L−1 iron sulphate and 0.20 g L−1 dolomite. Each treatment was represented in one or two blocks of plantlets randomly positioned in each of three 48-cell potting trays. The plantlets were watered in with a solution of 8 g L−1 Aquasol® (Yates, Padstow) and 1 mL L−1 Auxinone (Barmac, Darra; active constituents 0.075 g L−1 naphthalene acetic acid and 0.075 g L−1 indole acetic acid). Each tray was suspended over a reservoir of tap water in a mini plastic greenhouse and incubated at 25°C for four weeks under cool white fluorescent light (PPFD 45 µmol m−2 s−1) with a 12/12 h light/dark cycle.
Following incubation, plantlets were removed from the potting mix and observed again for root production. Differences among treatments in terms of plant height, number of leaf nodes and number of roots were analysed by one-way analysis of variance in GenStat® 11.1 (VSN International, Hemel Hempstead). Assumptions of normality and homoskedasticity were tested by plotting residuals against expected and fitted values, respectively. Data not meeting the assumptions of normality and equal variance were analysed by a non-parametric one-way analysis of variance (Kruskal and Wallis 1952).
All rooted plantlets were transferred to pots containing a proprietary potting mix consisting of pine bark, coir peat, coarse sand and fertiliser (Grange Growing Solutions, Ebenezer).
Propagation by cuttings
Semi-hardwood cuttings of both species were collected from garden-grown or wild-sourced material in autumn, winter or spring (Table 2). Stems and attached foliage were disinfected (Table 2) by soaking for 3–5 min in dilute household bleach (White King®) then rinsing in tap water or by dipping the material briefly in 1 mL L−1 Zaleton® (Yates, Padstow NSW; active constituents 200 g L−1 tebuconazole and 100 g L−1 trifloxystrobin). After disinfection, the stems were reduced in size to 1–4 leaf nodes (depending on the quality of the material) and any lower leaves were removed. The cuttings were then subjected to one of three growth regulator treatments: (1) dipping of the basal end in Clonex Rooting Hormone Gel (Yates, Padstow NSW; active constituent 3 g L−1 indole butyric acid); (2) dipping of the basal end in 25 mL L−1 Ezi-root (Garden City Plastics, Somersby, NSW; active constituents 1.6 g L−1 indolebutyric acid and 1.6 g L−1 naphthylacetic acid); or (3) soaking the entire cutting in 2.5 mL L−1 Ezi-root for 15 min then dipping the basal end in Clonex Gel (Table 2).
Cuttings processed at PlantBank were placed into punnets of steam-sterilised propagation mix (one part coir peat, two parts coarse sand and four parts perlite) or Preforma® box plugs (Jiffy Products International, Zwijndrecht; plug size 30/50, soil mix VECO1). Cuttings free of myrtle rust were placed on a greenhouse bench heated to 25°C under an automatic fogging system that maintained humidity at 85–90%. The punnets were given additional light watering by hand if required. Cuttings affected by myrtle rust were placed in a mini plastic greenhouse (Fig. 2c) suspended over a reservoir of tap water in a 25°C walk-in incubator. Cuttings processed at BBG were placed into punnets of propagation mix consisting of one part coir peat and four parts perlite. The punnets were placed on a greenhouse bench heated to 20–22°C under an automatic fogging system that maintained humidity at 60% or above. Automated overhead irrigation was applied every second day, for 5 min at a time, to keep the growing media moist.
Cuttings at both locations were monitored regularly for leaf drop and development of myrtle rust; affected material was removed immediately and remaining material treated with fungicide if deemed necessary. Root development was checked after 6–8 weeks and periodically thereafter until roots appeared or the material deteriorated. For R. rubescens, the strike rate of cuttings disinfected with bleach and placed in peat/sand/perlite mix was compared to that of cuttings disinfected with fungicide and placed in Preformer® plugs using binary logistic regression in Minitab 16.
Seed storage behaviour
Fruits of R. rubescens were collected at maturity from a cultivated plant in January 2017 (Accession P2017-0001: Table 3) and held at 15°C for ten days before processing. Seed storage behaviour was investigated by comparing the germination of fresh seeds with seeds dried to 4.9 ± 0.5% moisture content (wet weight basis) and stored for three months in vacuum-sealed foil packets at 4°C or −20°C. Seeds from each treatment were sown in five replicates of 10 seeds on 0.7% agar in glass Petri dishes. The dishes were sealed with plastic wrap and incubated at a constant 25°C with a 12/12 h light/dark cycle.
Fruits of R. psidioides were collected at maturity from cultivated plants in April–May 2019 (Accessions P2015-0426 and P2015-0429: Table 3). Seeds were extracted by hand and sown immediately, after drying to 3.7 ± 0.3% and 3.5 ± 0.3% moisture content, respectively, and after drying and storage in vacuum-sealed foil packets at 15°C and −20°C for one month. Three replicates of 10 seeds per treatment were sown on 0.9% agar in glass Petri dishes and incubated at 25/15°C with a 12/12 h light/dark cycle.
Germination for both species was monitored weekly. Seeds were considered to have germinated when a healthy radicle protruded at least 2 mm from the seed coat, but germinants were observed further to ensure the subsequent emergence of cotyledons. The tests were terminated when there was no further germination for at least two weeks. Any seeds that failed to germinate were then dissected with a scalpel to determine whether they remained viable. Differences in germination percentages between treatments were analysed by a non-parametric one-way analysis of variance (Kruskal and Wallis 1952). A seed storage behaviour category was assigned following Hong and Ellis (1996).
Results
Propagation by tissue culture
Initiation
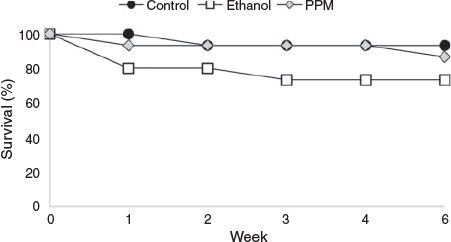
Initial survival rates (i.e. material alive and uncontaminated) for R. rubescens explants initiated into tissue culture in April 2013 ranged from 73 to 93%. Pretreatment with ethanol or PPM™ did not significantly improve survival compared to the control (Fig. 3; P = 0.305). Bacterial or fungal contamination continued to appear on surviving explants following the initial six-week observation period and all explants were eventually discarded due to contamination or death.
|
Initial survival rates of R. rubescens explants initiated into tissue culture in June 2013 were comparatively low (20–26%). Pretreatment with fungicide gave no significant improvement in survival over the control (P = 0.740) (Fig. 4). Untreated explants incubated on media with BA appeared to have a higher survival rate than those incubated on media without BA; however, this effect was not significant (P = 0.740) (Fig. 4).
|
Multiplication
Surviving explants transferred to growth media began to shoot within 2–3 weeks. For R. rubescens, initial culture on MS media led to greater proliferation of new shoots than culture on WPM (data not presented) therefore further culture was on MS only. Sufficient material (>100 plantlets) was available for exflasking experiments on R. rubescens after ~12 months of culture; R. psidioides cultures (Fig. 2d) grew more slowly and had just multiplied sufficiently to enable exflasking experiments at the time of writing this manuscript.
Exflasking
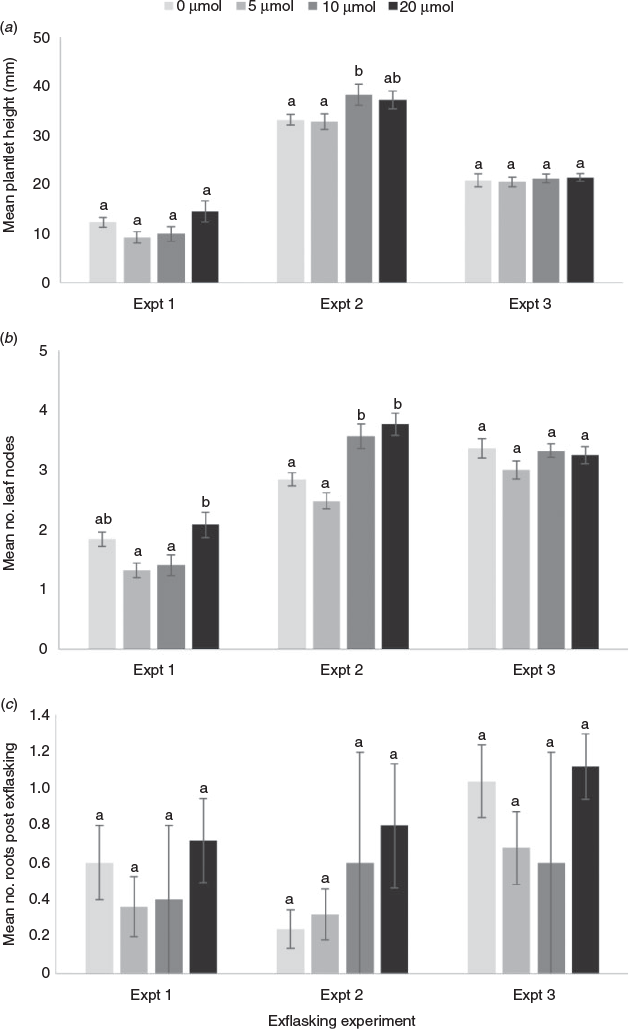
The effect of IBA on growth and root development of R. rubescens varied among exflasking experiments. In Experiment 1, there was no significant difference among treatments in terms of plant height (Fig. 5a), but 0 and 20 µm IBA resulted in a significantly greater number of leaf nodes than 5 µm IBA (P = 0.003) (Fig. 5b). In Experiment 2, 10 µm IBA resulted in significantly greater plant height than 0 or 5 µm IBA (P = 0.045) (Fig. 5a) while both 10 and 20 µm IBA resulted in a significantly greater number of leaf nodes (P < 0.001) (Fig. 5b). No significant differences among treatments were observed in either plant height or number of leaf nodes in Experiment 3. Differences among the three experiments in terms of mean plant height and number of leaf nodes were attributed to differences in size of the material at the commencement of each experiment and were not analysed (Figs 5a, b).
|
The mean proportion of plants producing roots following eight weeks culture with IBA ranged from 0 to 0.12 in Experiments 1 and 2, and 0 to 0.48 in Experiment 3. No roots were produced at an IBA level of 0 µm in any of the experiments. Variation within each of the other treatment levels was great and no significant differences among treatments were detected (P = 0.193, 0.193 and 0.149 for Experiments 1, 2 and 3, respectively).
Further incubation for four weeks in potting mix greatly improved the proportion of plantlets producing roots; however, the mean number of roots produced did not differ significantly among IBA treatments in any of the experiments (P = 0.762, 0.772 and 0.242 for Experiments 1, 2 and 3, respectively).
Rooted plantlets grew well in potting media under 70% shade cloth. Individuals planted out in garden beds at the ABG also established well (Fig. 2e). These were observed to be infected with myrtle rust in March 2019 and were treated with fungicide accordingly.
Propagation by cuttings
The cutting strike rate for R. rubescens ranged from 0 to 67% (Table 2). Cuttings disinfected with bleach and placed in peat/sand/perlite, or not disinfected and placed in peat/perlite propagation mix, had a mean strike rate of 2 ± 1% and took six months to begin producing roots. Cuttings disinfected with Zaleton® and placed in Preforma® box plugs had a mean strike rate of 25 ± 12% and began producing roots within six weeks. Binary logistic regression comparing ‘bleach + peat/sand/perlite’ with ‘Zaleton® + Preforma® plugs’ detected a significant difference between the methods (P = 0.001); the Zaleton® + Preforma® method improved the odds of root production by a factor of 9.2.
Minor infestations of myrtle rust were subsequently observed on material propagated from untreated cuttings and cuttings disinfected with bleach; these were controlled by removal of infected material and application of an appropriate fungicide. No outbreaks of myrtle rust were observed on cuttings disinfected with Zaleton. Rooted cuttings transferred to potting media at BBG have grown well under shade and have provided material for initiation of further tissue cultures. Rooted cuttings at PlantBank have only recently been transferred to potting media but are growing well at present.
The cutting strike rate for R. psidioides ranged from 0 to 75% (mean 31 ± 10%: Table 2) and root production was observed within six weeks. Rooted cuttings transferred to potting media have grown well and three accessions planted out in close proximity at the ABG in February 2018 produced fruit with viable seed in April–May 2019 (Fig. 2f). A minor infestation of myrtle rust observed on the plants in March 2019 was controlled as described above.
Seed collection and storage behaviour
Seeds were collected from three wild locations and one cultivated plant for R. rubescens (Table 3). The number of viable seeds in the wild collections was small (0–132 seeds per collection); however, sufficient seeds were collected from the cultivated plant to test storage behaviour. There was no significant difference (P = 0.895) in germination percentage between fresh (78 ± 4%), dried (77 ± 7%) and freezer-stored (77 ± 7%) seeds. On the basis of this result, the seeds of R. rubescens may be categorised as ‘probably orthodox’ in storage behaviour and suitable for seedbanking at subzero temperatures.
Three seed collections were made from one wild location of R. psidioides (Table 3). More than 90% of the seeds in each collection were empty. Seed collected from two cultivated plants at ABG had a lower proportion of empty seeds (54–76%: Table 3) and contained sufficient viable seeds to test storage behaviour. For accession P2015-0868, the germination percentage of dried seed (100 ± 0%) was significantly greater than germination of fresh seed (89 ± 0.3%) (P = 0.034 adjusted for ties). Storage of dry seed at −20°C resulted in significantly less germination (82 ± 3% radicle emergence and 74 ± 4% shoot emergence) than storage at 15°C (100 ± 0% for both radicle and shoot emergence). For P2015-0520, the germination percentage of dried seed (97 ± 3%) was greater than, but not significantly different from, the germination of fresh seed (87 ± 9%) (P = 0.346 adjusted for ties). However, storage of dry seed at −20°C resulted in considerably less germination (50 ± 6% for radicle emergence and 23 ± 9% for shoot emergence) than storage at 15°C (96 ± 4%) (P = 0.052 adjusted for ties). Because of an apparent sensitivity to freezing, the seeds of R. psidioides may be categorised as ‘intermediate’ in storage behaviour and are suitable for seed banking at temperatures above zero.
Discussion
Myrtle rust poses an enormous threat to plant biodiversity in the Pacific region. The large number of species already found to be susceptible to the pathogen, and the rapid rate at which some of those species have declined (Makinson 2018), suggest that myrtle rust has the potential to have an impact as severe as the pandemic outbreaks of Dutch elm disease in the last century. Dutch elm disease, which affects many trees in the genus Ulmus, is caused by the fungi Ophiostoma ulmi and Ophiostoma novo-ulmi and is spread by bark beetles (Brasier 1991). The disease is difficult to contain and treat and has killed millions of elms in Europe and North America (Karnosky 1979), including 95% of the North American population of Ulmus americana (Shukla et al. 2012). The wind-dispersed myrtle rust pathogen is also difficult to contain and likewise difficult to treat in the wild. Its ability to infect many different genera in the same family presages an even more detrimental effect on the environment than that caused by Dutch elm disease. For species highly susceptible to the rust, ex situ conservation offers the best solution for preventing extinction and, potentially, for preserving disease-resistant material that can be returned to the wild.
Effective conservation of plants affected by disease depends on the development of techniques that make the most of what is often a small amount of poor-quality plant material remaining. Seed banking is the most efficient conservation option for preserving high levels of diversity providing the seed is tolerant of drying and storage at subzero temperatures (Offord and Makinson 2009). R. rubescens seed has been shown here to be suitable for banking; however, the seed is now scarce in the wild. R. psidioides seed is tolerant of drying but is sensitive to storage at −20°C. The seed for this species could be safely stored at temperatures above zero; however, as with R. rubescens, viable seed is scarce in the wild. Collections of seed for both species will remain a conservation goal, but this is likely to require the establishment of a seed orchard from vegetatively propagated material. The recent fruiting of R. psidioides propagated from cuttings and planted at the ABG (Fig. 2f) indicates that this is an achievable goal.
While the level of genetic diversity in a vegetatively propagated collection is often low compared to a seed collection, vegetative propagation will maintain desirable features in the parent plant that might be lost in sexual reproduction. The most desirable feature to maintain in this situation, of course, is resistance to myrtle rust. The results of vegetative propagation of R. rubescens and R. psidioides to date have been somewhat encouraging, though considerable challenges remain. Propagation by cuttings was possible for both species and for R. psidioides the strike rate in peat/sand/perlite propagation mix was up to 75% within 6–8 weeks. Cuttings of R. rubescens in the same propagation mix were very slow to strike and the number of cuttings successfully producing roots was very low (≤11%). In contrast, material treated with Zaleton® and placed in coir-based propagation plugs began to produce roots within six weeks and had a strike rate of up to 67%.
The higher and faster strike rate of the latter technique may be attributable to the method itself but may also reflect the vigour of the source plants. Though originally collected from a variety of provenances (Table 2), these source plants were growing in the same forested gully and had been hand-watered and fertilised regularly from the moment they were planted out (A. Jennings, Northern Beaches Council, pers. comm). At the time the cuttings were collected, most individuals were in very good condition, showing active growth and only minor evidence of myrtle rust. However, material with a similar healthy appearance taken from three other locations and propagated in peat/sand/perlite mix failed to strike, suggesting that the technique used also has a strong influence on propagation success. In all other populations of R. rubescens sampled to date, the living plants remaining have had sparse, disease-affected foliage and little new growth. Such material is difficult to propagate regardless of the technique used (Hartmann and Kester 1975).
The ability of both R. rubescens and R. psidioides to sucker (Benson and McDougall 1998) suggests that root cuttings or sucker transplants may offer alternative ways to propagate individuals when the above-ground parts of the parent plant are in poor condition. Root cuttings would perhaps be a last resort as they respond to plant age and health in a similar way to stem cuttings (Hartmann and Kester 1975). Suckers with healthy shoots, however, may be readily conserved by severing the connection to the parent plant with a sharp spade and transferring the plantlet (with root system intact) to a pot. Regeneration from suckers has not been reported recently for R. rubescens, but R. psidioides suckers with healthy foliage have been observed in several locations in northern NSW (C. Stehn, NSW Department of Planning, Infrastructure and Environment, pers. comm). Some of these suckers have now been successfully transplanted to pot culture at PlantBank.
R. rubescens and R. psidioides also proved amenable to tissue culture, which has the advantage of providing a pathogen-free environment for conservation. In these preliminary experiments, the proportion of explants surviving the initiation process without fungal or bacterial contamination was relatively low − 0–23% for R. rubescens and 11–55% for R. psidioides – even though the material was obtained from cultivated plants (Table 1). The incidence of contamination would likely have been greater if material collected from the wild had been used as wild plants growing in warm, moist habitats are inclined to carry high levels of exogenous and endogenous microorganisms (Drew 2013) which make it difficult to produce the sterile plant material required for in vitro culture. Plant Preservative MixtureTM (a broad-spectrum microbicide) is sometimes used in tissue culture media to reduce contamination but this mixture is not always effective in controlling endogenous microbes and can be phytotoxic (Thomas et al. 2017). The use of antibiotics in culture can also lead to changes in gene expression and regulation (Ryu et al. 2017).
The microbial load of wild-sourced plant material can be reduced by first establishing mother plants in a clean environment, such as by growing plants propagated by seed or cuttings in a greenhouse, and then selecting only actively growing material from those mother plants for initiation (Offord et al. 2009; Drew 2013). If material suitable for establishing those potted collections is not available, however, then direct culture of wild-collected material may be the only viable option.
Techniques for initiating tissue cultures in the field have been described for commercially important species such as Musa, Coffea, Citrus and Theobroma (Pence 2005) but do not seem to be in routine use for wild plant conservation. Pence (2005) tested two methods for in vitro collecting that could potentially be applied to a broad range of species. The author found that small amounts of material withdrawn from the pith and vascular tissues of green stems (using a syringe and needle) resulted in very low rates of contamination. Observations of contamination were made after 7–14 days, however, and it has been noted in this laboratory that contamination may continue to appear for many weeks after initiation. Shukla et al. (2012), however, successfully initiated cultures of American elm using fresh and dormant buds from a hundred-year-old tree and Sugii (2011) described three disinfestation methods used to successfully initiate cultures from contamination-prone seed or embryos of endangered Hawaiian plants. Initiation of tissue cultures from a small amount of wild-collected material may therefore be possible for myrtle-rust-affected species but is likely to require some experimentation to find the best method of disinfecting the material without killing it.
On the basis of our conservation attempts to date, the best approach for conserving R. rubescens and R. psidioides will be to first establish potted plant collections by propagating semi-hardwood cuttings or transplanting suckers. If feasible (e.g. for accessible plants on private property), the vigour of the parent plants could be improved beforehand by applying fertiliser and hand-watering. Cuttings should be collected at a time of year when new growth has just hardened off and myrtle rust is not active. The best timing will vary depending on latitude and altitude but propagation responses to date suggest that early winter is suitable for R. rubescens and early spring for R. psidioides, in NSW. Cuttings should be treated with Zaleton® (or an equivalent fungicide), dipped in Clonex gel and placed in Preforma® box plugs for rooting. Suckers may be collected at any time of year if the shoots appear healthy. Any soil clinging to the roots should be gently washed off and the entire plantlet treated with Zaleton® before planting in sterilised potting mix. Successfully propagated plants can then be used to establish seed orchards to generate seed for banking. The plants can also be used to generate living conservation collections for distribution to Botanic Gardens in areas less affected by myrtle rust, and as a source of material for initiating tissue cultures to use as back-up collections. Tissue cultures, in turn, could be used for research into long-term preservation of shoot tips by cryopreservation.
This paper has concentrated on two Australian rainforest species that were relatively common in 2010 and are now listed as Critically Endangered in NSW. Other Australian host species that didn’t appear to be severely affected by myrtle rust initially – such as Archirhodomyrtus beckleri and Decaspermum humile – are now showing signs of serious decline (Pegg et al. 2017; Dianne Brown, NSW Department of Planning, Infrastructure and Environment, pers. comm) and a further 41 species have the potential to follow suit (Makinson 2018). Given the speed of decline observed in Australia, we recommend pre-emptive ex situ conservation for any species in the Pacific observed to be highly susceptible to the rust or deemed to be at risk of severe impact. The availability of funding will, of course, influence what can be achieved but conservation of affected species will be far easier and less expensive if action can be taken before reproductive capacity is affected. In countries where many species are at risk, as in Australia, priority could first be given to those occurring in habitats most conducive to myrtle rust infection (Berthon et al. 2018; Makinson 2018).
For species with seed potentially suitable for banking, collecting seed from a range of provenances while the species is still capable of fruiting will ensure that the greatest level of diversity is conserved. For those species with seed sensitive to drying or freezing – such as Syzygium species – seed can be collected and germinated while fresh to provide parent plants for further propagation. If seed production is rare, material for vegetative propagation should be collected, if possible, while plants are still healthy and capable of active growth. Where a species is in serious decline, however, we recommend attempting to propagate both affected individuals and individuals showing signs of disease resistance; collections with a wide range of genotypes will offer the greatest potential for future breeding for disease resistance and production of plants for rewilding programs. Once established, a collection can be duplicated by vegetative propagation and distributed to Botanic Gardens for conservation in regions where the rust is not as active or can be effectively controlled. To aid in control of the rust, potted or planted individuals should be maintained at a height that facilitates treatment with fungicide, and should be kept in a location that limits dispersal of the pathogen to other hosts.
While spread of the pandemic strain of myrtle rust appears to be out of our control, protection of affected species is not. Effective conservation of those species, however, will require swift action and collaboration among Botanic Gardens and other organisations engaged in plant conservation.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank the sponsors of the Rainforest Conservation Project – the Arcadia Fund, the Greatorex Foundation, the HSBC Bank Australia and several private benefactors – for funding the work of KDS and AR. We thank Richard Johnstone, Lotte von Richter, Andrew Jennings, Craig Ward, the NSW Office of Environment and Heritage and several private property owners for collection assistance, and Carmen Laidlaw for laboratory assistance. We also thank Eric Bunn and two anonymous reviewers for helpful comments on the manuscript.
References
Beenken, L. (2017). Austropuccinia: a new genus name for the myrtle rust Puccinia psidii placed within the redefined family Sphaerophragmiaceae (Pucciniales). Phytotaxa 297, 53–61.| Austropuccinia: a new genus name for the myrtle rust Puccinia psidii placed within the redefined family Sphaerophragmiaceae (Pucciniales).Crossref | GoogleScholarGoogle Scholar |
Benson, D., and McDougall, L. (1998). Ecology of Sydney plants. Part 6: Dicotyledon family Myrtaceae. Cunninghamia 5, 809–987.
Berthon, K., Esperon-Rodriguez, M., Beaumont, L. J., Carnegie, A. J., and Leishman, M. R. (2018). Assessment and prioritisation of plant species at risk from myrtle rust (Austropuccinia psidii) under current and future climates in Australia. Biological Conservation 218, 154–162.
| Assessment and prioritisation of plant species at risk from myrtle rust (Austropuccinia psidii) under current and future climates in Australia.Crossref | GoogleScholarGoogle Scholar |
Brasier, C. M. (1991). Ophiostoma novo-ulmi sp. nov., causative agent of current Dutch elm disease pandemics. Mycopathologia 115, 151–161.
| Ophiostoma novo-ulmi sp. nov., causative agent of current Dutch elm disease pandemics.Crossref | GoogleScholarGoogle Scholar |
Cannon, A. M. (2009). Myrtle rust – forest industries issues paper. Forest and Wood Products Australia, Melbourne. Available at: https://www.fwpa.com.au/resources/resources/127-myrtle-rust-forest-industry-issues-paper.html [accessed 13 June 2019].
Carnegie, A. J., Lidbetter, J. R., Walker, J., Horwood, M. A., Tesoriero, L., Glen, M., and Priest, M. J. (2010). Uredo rangelii, a taxon in the guava rust complex, newly recorded on Myrtaceae in Australia. Australasian Plant Pathology 39, 463–466.
| Uredo rangelii, a taxon in the guava rust complex, newly recorded on Myrtaceae in Australia.Crossref | GoogleScholarGoogle Scholar |
Carnegie, A. J., Kathuria, A., Pegg, G. S., Entwistle, P., Nagel, M., and Giblin, F. R. (2016). Impact of the invasive rust Puccinia psidii (myrtle rust) on native Myrtaceae in natural ecosystems in Australia. Biological Invasions 18, 127–144.
| Impact of the invasive rust Puccinia psidii (myrtle rust) on native Myrtaceae in natural ecosystems in Australia.Crossref | GoogleScholarGoogle Scholar |
Chapman, A.D. (2009). Numbers of living species in Australia and the World. 2nd edn. Australian Government, Canberra. Available at: https://www.environment.gov.au/system/files/pages/2ee3f4a1-f130-465b-9c7a-79373680a067/files/nlsaw-2nd-complete.pdf [accessed 18 June 2019].
Drew, R. A. (2013). Micropropagation of tropical tree species. Acta Horticulturae , 57–64.
| Micropropagation of tropical tree species.Crossref | GoogleScholarGoogle Scholar |
du Plessis, E., McTaggart, A. R., Granados, G. M., Wingfield, M. J., Roux, J., Ali, M. I. M., Pegg, G. S., Makinson, J., and Purcell, M. (2017). First report of myrtle rust caused by Austropuccinia psidii on Rhodomyrtus tomentosa (Myrtaceae) from Singapore. Plant Disease 101, 1676.
| First report of myrtle rust caused by Austropuccinia psidii on Rhodomyrtus tomentosa (Myrtaceae) from Singapore.Crossref | GoogleScholarGoogle Scholar |
du Plessis, E., Granados, G. M., Barnes, I., Ho, W. H., Alexander, B. J. R., Roux, J., and McTaggart, A. R. (2019). The pandemic strain of Austropuccinia psidii causes myrtle rust in New Zealand and Singapore. Australasian Plant Pathology , .
| The pandemic strain of Austropuccinia psidii causes myrtle rust in New Zealand and Singapore.Crossref | GoogleScholarGoogle Scholar |
Floyd, A. G. (2008). ‘Rainforest Trees of Mainland South-eastern Australia.’ (Terania Rainforest Publishing: Lismore, NSW.)
Giblin, F. (2013). Myrtle rust report: New Caledonia. University of the Sunshine Coast, Maroochydore, Queensland.
Harden, G. J., McDonald, W. J. F., and Williams, J. B. (2006). ‘Rainforest Trees and Shrubs: a Field Guide to their Identification.’ (Gwen Harden Publishing: Nambucca Heads, NSW.)
Hartmann, H. T., and Kester, D. E. (1975). ‘Plant Propagation – Principles and Practices.’ 3rd edn. (Prentice Hall: New Jersey.)
Hong, T. D., and Ellis, R. H. (1996). ‘A Protocol to Determine Seed Storage Behaviour.’ (International Plant Genetic Resources Institute: Rome.)
Karnosky, D. F. (1979). Dutch elm disease: a review of the history, environmental implications, control and research needs. Environmental Conservation 6, 311–322.
| Dutch elm disease: a review of the history, environmental implications, control and research needs.Crossref | GoogleScholarGoogle Scholar |
Kawanishi, T., Uemastu, S., Kakishima, M., Kagiwada, S., Hamamoto, H., Horie, H., and Namba, S. (2009). First report of rust disease on ohia and the causal fungus, Puccinia psidii, in Japan. Journal of General Plant Pathology 75, 428–431.
| First report of rust disease on ohia and the causal fungus, Puccinia psidii, in Japan.Crossref | GoogleScholarGoogle Scholar |
Kruskal, W. H., and Wallis, W. A. (1952). Use of ranks in one-criterion variance analysis. Journal of the American Statistical Association 47, 583–621.
| Use of ranks in one-criterion variance analysis.Crossref | GoogleScholarGoogle Scholar |
Lloyd, G., and McCown, B. (1980). Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. International Plant Propagators’ Society Proceedings 30, 421–427.
Machado, P. da S., Alfenas, A. C., Alfenas, R. F., Mohammed, C. L., and Glen, M. (2015). Microsatellite analysis indicates that Puccinia psidii in Australia is mutating but not recombining. Australasian Plant Pathology 44, 455–462.
| Microsatellite analysis indicates that Puccinia psidii in Australia is mutating but not recombining.Crossref | GoogleScholarGoogle Scholar |
Makinson, R. O. (2018). Myrtle rust reviewed: the impacts of the invasive plant pathogen Austropuccinia psidii on the Australian environment. Plant Biosecurity Cooperative Research Centre, Canberra. Available at: https://www.anpc.asn.au/myrtle-rust/ [accessed 13 June 2019].
McDonald, J. (2012). Australian nursery industry myrtle rust management plan. Nursery and Garden Industry Australia, Sydney. Available at: https://www.ngiq.asn.au/wp-content/uploads/Myrtle-Rust-Management-Plan-2012-Final.pdf [accessed 13 June 2019].
McTaggart, A. R., Roux, J., Granados, G. M., Gafur, A., Tarrigan, M., Santhakumar, P., and Wingfield, M. J. (2016). Rust (Puccinia psidii) recorded in Indonesia poses a threat to forests and forestry in South-East Asia. Australasian Plant Pathology 45, 83–89.
| Rust (Puccinia psidii) recorded in Indonesia poses a threat to forests and forestry in South-East Asia.Crossref | GoogleScholarGoogle Scholar |
Murashige, T., and Skoog, F. S. (1962). A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiologia Plantarum 15, 473–497.
| A revised medium for rapid growth and bio assays with Tobacco tissue cultures.Crossref | GoogleScholarGoogle Scholar |
NSW Threatened Species Scientific Committee (2019a). Final determination – Rhodamnia rubescens. Available at: https://www.environment.nsw.gov.au/news/nsw-threatened-species-scientific-committee-final-determination-rhodamnia-rubescens [accessed 4 February 2019].
NSW Threatened Species Scientific Committee (2019b). Final determination – Rhodomyrtus psidioides. Available at: https://www.environment.nsw.gov.au/news/nsw-threatened-species-scientific-committee-final-determination-rhodomyrtus-psidioides [accessed 18 March 2019].
Offord, C. A., and Makinson, R. O. (2009). Options and major considerations for germplasm conservation. In ‘Plant Germplasm Conservation in Australia: Strategies and Guidelines for Developing, Managing and Utilising ex situ Collections’. (Eds C. A. Offord, and P. F. Meagher.) Chapter 2, pp. 11–34. (Australian Network for Plant Conservation: Canberra.)
Offord, C. A., Bunn, E., Turner, S. R., Sommerville, K. D., Siemon, J., and Ashmore, S. E. (2009). Tissue culture. In ‘Plant Germplasm Conservation in Australia: Strategies and Guidelines for Developing, Managing and Utilising ex situ Collections’. (Eds C. A. Offord, and P. F. Meagher.) Chapter 6, pp. 109–128. (Australian Network for Plant Conservation: Canberra.)
Pegg, G. S., Giblin, F. R., McTaggart, A. R., Guymer, G. P., Taylor, H., Ireland, K. B., Shivas, R. G., and Perry, S. (2014). Puccinia psidii in Queensland, Australia: disease symptoms, distribution and impact. Plant Pathology 63, 1005–1021.
| Puccinia psidii in Queensland, Australia: disease symptoms, distribution and impact.Crossref | GoogleScholarGoogle Scholar |
Pegg, G., Taylor, T., Entwistle, P., Gordon, G., Giblin, F., and Carnegie, A. (2017). Impact of Austropuccinia psidii (myrtle rust) on Myrtaceae-rich wet sclerophyll forests in south east Queensland. PLoS One 12, e0188058.
| Impact of Austropuccinia psidii (myrtle rust) on Myrtaceae-rich wet sclerophyll forests in south east Queensland.Crossref | GoogleScholarGoogle Scholar | 29161305PubMed |
Pence, V. C. (2005). in vitro collecting (IVC). I. The effect of collecting method and antimicrobial agents on contamination in temperate and tropical collections. In Vitro Cellular & Developmental Biology. Plant 41, 324–332.
| in vitro collecting (IVC). I. The effect of collecting method and antimicrobial agents on contamination in temperate and tropical collections.Crossref | GoogleScholarGoogle Scholar |
Royal Botanic Gardens Kew (2019). Seed Information Database (Version 7.1). Available at: http://data.kew.org/sid/ [accessed 20 March 2019].
Ryu, A. H., Eckalbar, W. L., Kreimer, A., Yosef, N., and Ahituv, N. (2017). Use antibiotics in cell culture with caution: genome-wide identification of antibiotic-induced changes in gene expression and regulation. Scientific Reports 7, 7533.
| Use antibiotics in cell culture with caution: genome-wide identification of antibiotic-induced changes in gene expression and regulation.Crossref | GoogleScholarGoogle Scholar | 28790348PubMed |
Shukla, M. R., Maxwell, A., Jones, P., Sullivan, J. A., Liu, C., Gosling, S., and Saxena, P. K. (2012). in vitro conservation of American elm (Ulmus americana): potential role of auxin metabolism in sustained plant proliferation. Canadian Journal of Forest Research 42, 686–697.
| in vitro conservation of American elm (Ulmus americana): potential role of auxin metabolism in sustained plant proliferation.Crossref | GoogleScholarGoogle Scholar |
Sommerville, K. D., Clarke, B., Keppel, G., McGill, C., Newby, Z.-J., Wyse, S. V., James, S. A., and Offord, C. A. (2017). Saving rainforests in the south Pacific: challenges in ex situ conservation. Australian Journal of Botany 65, 609–624.
| Saving rainforests in the south Pacific: challenges in ex situ conservation.Crossref | GoogleScholarGoogle Scholar |
Stewart, J. E., Ross-Davis, A. L., Graça, R. N., Alfenas, A. C., Peever, T. L., Hanna, J. W., Uchida, J. Y., Hauff, R. D., Kadooka, C. Y., Kim, M.-S., Cannon, P. G., Namba, S., Simeto, S., Pérez, C. A., Rayamajhi, M. B., Lodge, D. J., Arguedas, M., Medel-Ortiz, R., López-Ramirez, M. A., Tennant, P., Glen, M., Machado, P. S., McTaggart, A. R., Carnegie, A. J., and Klopfenstein, N. B. (2018). Genetic diversity of the myrtle rust pathogen (Austropuccinia psidii) in the Americas and Hawaii: global implications for invasive threat assessments. Forest Pathology 48, e12378.
| Genetic diversity of the myrtle rust pathogen (Austropuccinia psidii) in the Americas and Hawaii: global implications for invasive threat assessments.Crossref | GoogleScholarGoogle Scholar |
Sugii, N. C. (2011). The establishment of axenic seed and embryo cultures of endangered Hawaiian plant species: special review of disinfestation protocols. In Vitro Cellular & Developmental Biology. Plant 47, 157–169.
| The establishment of axenic seed and embryo cultures of endangered Hawaiian plant species: special review of disinfestation protocols.Crossref | GoogleScholarGoogle Scholar |
Thomas, P., Agrawal, M., and Bharathkumar, C. B. (2017). Use of Plant Preservative MixtureTM for establishing in vitro cultures from field plants: experience with papaya reveals several PPMTM tolerant endophytic bacteria. Plant Cell Reports 36, 1717–1730.
| Use of Plant Preservative MixtureTM for establishing in vitro cultures from field plants: experience with papaya reveals several PPMTM tolerant endophytic bacteria.Crossref | GoogleScholarGoogle Scholar | 28748257PubMed |
Uchida, J., Zhong, S., and Kilgore, E. (2006). First report of a rust disease on Ohia caused by Puccinia psidii in Hawaii. Plant Disease 90, 524.
| First report of a rust disease on Ohia caused by Puccinia psidii in Hawaii.Crossref | GoogleScholarGoogle Scholar | 30786610PubMed |
WCSP (2019). World checklist of selected plant families. Facilitated by the Royal Botanic Gardens, Kew. Available at: http://apps.kew.org/wcsp/ [accessed 20 March 2019].
Zhuang, J.-Y., and Wei, S.-X. (2011). Additional materials for the rust flora of Hainan Province, China. Junwu Xuebao 30, 853–860.