A comparison of abundance and distribution model outputs using camera traps and sign surveys for feral pigs
Derek R. Risch A E , Jeremy Ringma A B , Shaya Honarvar C D and Melissa R. Price
A E , Jeremy Ringma A B , Shaya Honarvar C D and Melissa R. Price  A
A
A University of Hawai‘i at Mānoa, 1910 East-west Road, Honolulu, HI 96822, USA.
B Present address: Royal Melbourne Institute of Technology University, GPO Box 2476V, Melbourne, Vic. 3001, Australia.
C Department of Land and Natural Resources, Division of Forestry and Wildlife, 1151 Punchbowl Street, Honolulu, HI 96822, USA.
D Present address: University of Hawai‘i at Mānoa, School of Life Sciences, 3190 Maile Way, Honolulu, HI 96822, USA.
E Corresponding author. Email: drisch@hawaii.edu
Pacific Conservation Biology 27(2) 186-194 https://doi.org/10.1071/PC20032
Submitted: 8 April 2020 Accepted: 20 September 2020 Published: 14 October 2020
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
Species distribution models play a central role in informing wildlife management. For models to be useful, they must be based on data that best represent the presence or abundance of the species. Data used as inputs in the development of these models can be obtained through numerous methods, each subject to different biases and limitations but, to date, few studies have examined whether these biases result in different predictive spatial models, potentially influencing conservation decisions. In this study, we compare distribution model predictions of feral pig (Sus scrofa) relative abundance using the two most common monitoring methods: detections from camera traps and visual surveys of pig sign. These data were collected during the same period using standardised methods at survey sites generated using a random stratified sampling design. We found that although site-level observed sign data were only loosely correlated with observed camera detections (R2 = 0.32–0.45), predicted sign and camera counts from zero-inflated models were well correlated (R2 = 0.78–0.88). In this study we show one example in which fitting two different forms of abundance data using environmental covariates explains most of the variance between datasets. We conclude that, as long as outputs are produced through appropriate modelling techniques, these two common methods of obtaining abundance data may be used interchangeably to produce comparable distribution maps for decision-making purposes. However, for monitoring purposes, sign and camera trap data may not be used interchangeably at the site level.
Keywords: abundance index, feral pig, invasive species, monitoring, Pacific region, species distribution, Sus scrofa, ungulates, wild pig, wildlife management.
Introduction
Feral pigs (Sus scrofa) are among the most problematic mammalian invasive species due to their impacts on biodiversity and agricultural and livestock production (Massei and Genov 2004). Feral pigs occupy six continents, having been intentionally introduced by humans for food provisioning and game recreation (Barrios-Garcia and Ballari 2012). They are often referred to as ecosystem engineers, fundamentally altering ecosystems through a suite of impacts on soil properties, plant and animal communities, and hydrological processes (Hone 2002; Nogueira-Filho et al. 2009; Cole and Litton 2014; Hess 2016; Wehr 2018). Additionally, feral pigs negatively impact agriculture and livestock production through predation, competition, habitat disturbance and disease transmission (Gentle et al. 2015). In the United States alone, damages from feral pigs cost approximately US$1.5 billion per year (Pimental 2007) and globally threaten native species in over a quarter of the world’s countries (IUCN 2017).
These impacts have been particularly severe throughout the Pacific region where native and endemic species are naive to the threats from introduced ungulates (Parker et al. 2006; Desurmont et al. 2011). Feral pigs throughout the Pacific have effects across trophic levels ranging from disturbing the soil microbiome (Wehr et al. 2018) to creating habitat for mosquitoes and thus facilitating the spread of avian malaria that has led to the precipitous decline and extinction of numerous endemic avifauna (LaPointe et al. 2012; Wehr et al. 2018). Furthermore, feral pigs have proved to be effective predators on many island ecosystems, including the Galapagos, where they contributed to the decline of an endemic rail via egg predation and nest disturbance (Donlan et al. 2007).
Monitoring of impacts of feral pig populations has become a growing concern among non-government organisations, government entities, and private land owners (Engeman et al. 2013). Estimates of distribution and abundance are important for this purpose, enabling managers to locate areas of high feral pig abundance for targeting and evaluating the effectiveness of management actions (Wilson et al. 2006). Monitoring data for feral pigs are commonly collected using two methods: detection rate using remotely triggered camera traps (Holtfreter et al. 2008; Rovero and Marshall 2009; Bondi et al. 2010; Bengsen et al. 2011; Chauvenet et al. 2017; Keuling et al. 2018; Massei et al. 2018) and visual surveys of signs unique to feral pigs (Engeman et al. 2001, 2013; Massei et al. 2018). These two methods of data collection each have their own limitations and their use is often dependent on the amount of resources and personnel available to an agency, researcher or private landowner (Engeman et al. 2013). Camera traps have been widely used due to their appeal in remote monitoring of abundance, providing greater data resolution for reduced time in the field compared with sign surveys (Silveira et al. 2003; Rowcliffe et al. 2008; Bengsen et al. 2011). A disadvantage is the considerable up-front costs of purchasing cameras, but Rovero and Marshall (2009) found that reduced labour costs over time significantly decrease the overall cost of camera trapping compared with transect surveys. Cost of cameras may also limit sample size due to limitations on the number of cameras purchased, and the volume of photographs recorded will influence the cost and time required to process camera trap data. Sign surveys or tracking plots have been widely adopted as an index of monitoring the abundance and distribution of feral pigs by recording the presence or abundance of physical signs of feral pigs activity (Engeman et al. 2013). As detection probability can change through time (Watson et al. 2008; Southwell and Low 2009), sites may need to be visited on multiple occasions to confirm presence or absence of the focal species (Kéry et al. 2006), resulting in significant labour costs varying on the basis of the frequency of site visits and distance to field sites (Field et al. 2005; Hauser and McCarthy 2009). Reliability of this method is also influenced by observer ability and environmental factors such as precipitation, vegetation, and soil type (Fitzpatrick et al. 2009; Moore et al. 2011).
A common application of monitoring data is the creation of species distribution models. These models are used to map the predicted suitability, species occurrence probability, or relative abundance of a focal species over a region of interest (Guisan and Thuiller 2005; Sarre et al. 2013). For invasive species, these distribution models are often used in conservation decision making by overlaying the distribution of the invasive species on the distribution of the target conservation asset to determine the level of threat posed by invasive species to species of conservation concern (Evans et al. 2011; Tulloch et al. 2015). However, model outputs may differ depending on the type of input data used and thereby influence management decisions made from perceived threats.
Given the extensive impacts of feral pigs and the widely available monitoring methods, it remains difficult to accurately predict the abundance of their populations. Most studies focus on the probability of presence, and a few studies have attempted to quantify the abundance of feral pig populations, but none within the Pacific region (Engeman et al. 2001) or at a resolution appropriate for informing management actions within an island or large conservation area (e.g. National Park). Recognising the importance of monitoring abundance for the management of this species, and the lack of conformity across approaches to monitoring feral pig populations, we developed this study with two objectives in mind. First, our study introduces methodology to extrapolate estimates of relative feral pig abundance across diverse landscapes using common forms of monitoring data. Second, we compare the effect each monitoring data type has on the respective estimates of relative abundance and speculate on how these differences may influence management decisions. We compare the relatability between these two data sources using regressions of observed data and data fitted to predictive models. The results from this study provide individuals with the methodology to monitor feral pig abundances across islands throughout the Pacific region and areas of similar scale and complexity (e.g. National Parks, agricultural lands, or other nature reserves). Furthermore, this study elucidates the similarities and differences between two of the most common approaches to monitoring ungulates and provides discussion regarding which approach may be most beneficial to agencies and individuals with varying access to resources.
Materials and methods
Study area
The island of O‘ahu is one of the eight main Hawaiian Islands and has a land area of 1546 km2. There are two main mountain ranges, the Ko‘olau range of eastern O‘ahu, with elevations up to 960 m, and the Wai‘anae range of western O‘ahu with a maximum elevation of 1227 m. Mean annual rainfall varies greatly across the island from 200 mm to 7000 mm but is highly correlated with elevation and temperature (Giambelluca et al. 2012). Additionally, windward systems receive a higher mean annual rainfall than their leeward counterparts (Giambelluca et al. 2012). Community assemblages of vegetation can range from dry coastal shrubland to high-elevation wet forests, all of which can vary between native, mixed, and non-native assemblages.
For access reasons, all survey sites were restricted to forest reserves or privately held land with pre-established access permission. There are 240 km2 of forest reserves comprising nearly 16% of O‘ahu’s land area with 101 km of existing ungulate-proof fencing within these reserves. These fences separate feral pigs from species of concern such as threatened and endangered plants, nesting seabirds, and forest birds. Areas with ungulate-exclusion fencing were excluded from the site selection process.
Site selection
Research sites to record feral pig abundance data were allocated using a random stratified sampling design. Several environmental factors are known to strongly correlate with altitude on the Hawaiian islands, hence research sites were divided equally into three altitudinal bands (0–333 m, 334–666 m and 667–1200 m) to prevent disproportionate sampling of the more frequent, low-altitude habitats and ensure thorough sampling across ecological gradients. An equal number (n = 15) of research sites were randomly drawn from these three altitudinal bands, resulting in 45 total research sites across O‘ahu. Potential sites were allocated by drawing from a 500 m by 500 m (25 ha) raster grid of O‘ahu, created using R packages ‘raster’ (Hijmans et al. 2017) and ‘rgdal’ (Bivand et al. 2018). Sites were located at the centroid of each raster cell and drawn from land recognised as a reserve using the Hawai‘i state government reserve outline and privately held land with pre-established access permission. To ensure research sites were spatially independent of each other, separate sites were generated using this process for each year of data collection. Extensive areas of fallow agricultural land and urban areas were excluded from this study due to the diversity of ownership and relatively small parcel sizes of privatised agricultural land in Hawai‘i. When a randomly selected site could not be reached due to safety reasons (slope, topography), the site was moved to the closest analogous location within 500 m that could be safely accessed, or else was excluded from the study.
Survey design
Research sites were surveyed for a 2-week period between the months of June and November of 2016 and 2017 using camera traps and sign surveys. Each research site consisted of an array of six cameras (Bushnell Trophy Cams, Bushnell, Overland Park, KS) distributed at regular 50-m intervals. Cameras were programmed to take two consecutive images for each trigger and reset after 3 s. Cameras were deployed at each research site for a 2-week period under one of two configurations: (1) a rectangular array, with cameras deployed in two parallel lines of three; or (2) a linear array, with all six cameras deployed along a transect. Linear arrays were deployed only on areas where topography did not allow for a rectangular array, such as on ridge crests with steep receding slopes on either side. Cameras were deployed in a manner that maximised the probability of detection, such as focused on a clearing, trail or area with obvious previous pig activity within a 10-m radius of randomly pre-selected GPS coordinates. Based on previous experience deploying camera traps throughout diverse landscapes in Hawai‘i, we chose 10 m as a reasonable radius that would allow us to set up cameras to maximise detection probability while retaining the randomness of our site selection process. Cameras were attached to vegetation at approximately waist height and angled on a level to slightly downward facing trajectory with the ground. Camera data were reviewed manually using Irfanview photo-editing software (www.irfanview.com) so that photos containing images of feral pigs were filtered into a final database for analysis.
At each of the six camera locations within each research site, signs of feral pig presence were recorded in four quadrats (10 m by 10 m), resulting in 24 quadrats and a total surveyed area of 2400 m2 per research site. Surveys of sign were standardised at a 2-min search period for each quadrat. Quadrats were structured in a square configuration around the camera location except in areas where steep terrain prevented access to more than 10 m surrounding the camera location, in which case quadrats were run along a linear transect similar to tracking plots (Engeman et al. 2013; Massei et al. 2018). In each quadrat we recorded the presence or absence of old and new signs of tracks, scat, digging and browsed vegetation. New sign was defined as having likely occurred no later than 2 weeks earlier, based on leaf fall on top of sign, desiccation of soil, layered disturbances, or other visual cues of time since the sign was produced. Sign surveys were conducted both upon the deployment and recovery of cameras from each site.
Model development
Total counts of camera-captured observations of feral pigs at each research site over the standardised 2-week sampling period were averaged by the number of cameras deployed at each site to estimate average counts of camera-captured observations of feral pigs per site. Presence and absence data from the sign surveys at each quadrat were collated across the 24 quadrats per research site to quantify the total abundance of all recorded sign per site. The sign survey data were also filtered by the type of sign recorded to obtain the total abundance of tracks, scat, digging and browsed vegetation per research site. Previous studies identify that these types of abundance data gathered from camera traps and surveys of pig sign have been shown to accurately reflect the abundance and distribution of feral pigs and so were used as the response in separate models of species abundance (Rovero and Marshall 2009; Bengsen et al. 2011; Chauvenet et al. 2017; Massei et al. 2018). The abundance data used in model building included: average counts of camera-captured observations per site (camera), abundance of all observed sign per site (all sign), abundance of observed tracks per site (all track), abundance of observed scat per site (all scat), abundance of observed digging per site (all dig), and the abundance of observed browsed vegetation per site (all vegetation). A small number of covariates were used in the modelling process because of a limited number of survey sites (n = 42) and inherent autocorrelation among many environmental covariates in Hawaiian systems. Collinearity between predictors was considered using pairwise Pearson coefficients (R2) and any predictors with R2 greater than 0.75 were excluded (Elith et al. 2010; Dormann et al. 2013). Covariates were selected based on an a priori hypothesis of response–covariate relationship. These hypotheses were developed based on an understanding that our covariates must include biotic and abiotic factors that adequately describe the quality of the habitat and the ecological requirements of feral pigs (Diong 1982). In doing so, we identified four covariates that best represented these requirements, which included: mean annual rainfall as a proxy for available water and habitat quality, vegetation height as protective and thermal cover and available forage, vegetation density as habitat quality and accessibility, and percentage native vegetative cover to distinguish between plant community composition.
We used generalised linear models (GLM) with Poisson and negative binomial distributions from the ‘stats’ and ‘MASS’ (Venables and Ripley 2002) packages respectively and zero-inflated models with the same distributions from the ‘pscl’ package (Zeileis et al. 2008) within the program R (R Core Team 2017). This method was chosen over commonly adopted modelling techniques using presence-only data such as Maxent (Elith et al. 2011) due to the nature of occupancy by feral pigs at a site on O‘ahu. Feral pigs are broadly distributed across O‘ahu and are present in nearly all areas; however, their abundance may vary by several orders of magnitude. Hence, our adopted modelling method was required to discriminate between relative abundance of feral pigs between sites, not simply the probability of presence.
Models were constructed using the average number of camera detections (camera) and frequency of sign (all sign, all track, all scat, all dig, and all vegetation). These six forms of abundance data were treated as the response variable in the model-fitting process using different configurations and interactions between each of the covariates. The same covariate configurations were used with each of the six abundance data types and were fitted to models of increasing complexity to identify best-fit models. These models were: GLM Poisson, GLM negative binomial, zero-inflated Poisson, and zero-inflated negative binomial. Zero-inflated models are two-component mixture models that estimate the distribution output based on two separate model components, the first component modelling the likelihood of excess zeroes (zero component) and the second component modelling the positive counts (count component) (Zeileis et al. 2008). We included covariates for the zero component that might explain excess zero counts inherent in environmental observation data. The covariate configurations with terms for the zero component were excluded when fitted to GLMs as these models do not provide a means of separating these two processes. A stepwise process was used to consider first- and second-order relationships for the four environmental covariates. Best-fit models were chosen based on Akaike Information Criteria (AIC) and the predicted outputs of each model were visually inspected for any signs of model overfitting.
Regression analysis
Linear regressions were used to determine directionality and correlative strength between the different forms of abundance data collected for this study in Program R 3.3.2 (R Core Team 2017). Program R package ‘ggplot2’ was used to generate regressions of sign data (all sign, all track, and all dig) against camera data for both our observed data and predicted data from zero-inflated distribution models (Wickham 2016). Variance and significance for each regression were reported using R2-values and P-values, respectively.
Results
Data were collected from 42 sites over a 2-year period between the months of June and November of 2016 and 2017. Feral pigs were detected by camera traps at 21 of the 42 sites. For sites where feral pigs were detected, an average of 31 ± 6 (s.e.) photographs of feral pigs per camera were captured over the 2-week sample period, with a maximum of 311 captures at a single site. In comparison, sign was detected at 27 of 42 sites; feral pigs were detected by camera traps at 18 of these 27 sites. Approximately seven times more sign was detected at sites where feral pigs were captured on cameras, compared with sites where no feral pigs were detected by cameras (camera detection 7.19 ± 1.26 (s.e.), no camera detections 1.14 ± 0.42 (s.e.)).
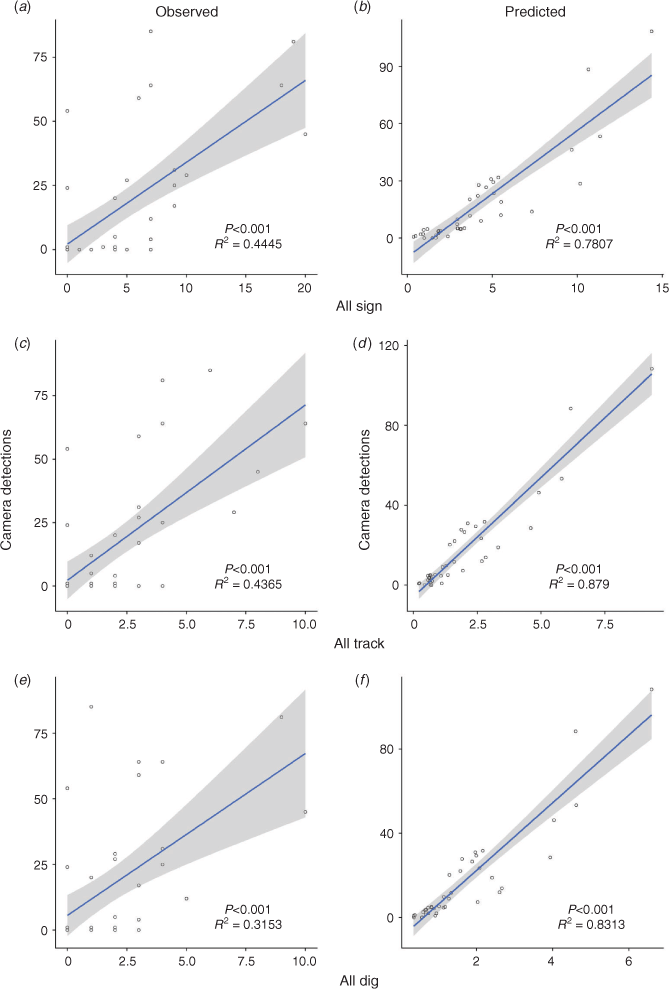
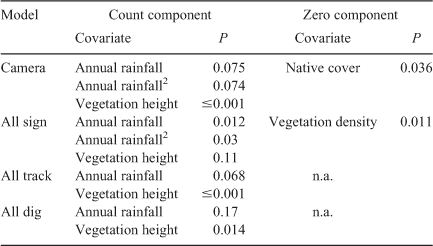
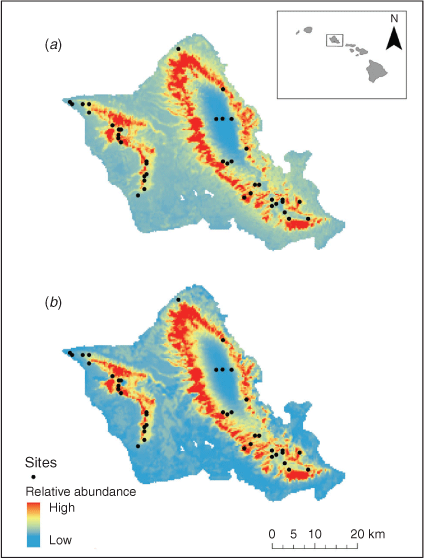
Zero-inflated negative binomial models performed best for the camera and all sign models (Table 1). Model summary results from Poisson and negative binomial GLMs are excluded from Table 1 due to AIC suggesting a considerably worse fit than their zero-inflated counterparts. Vegetation density and native vegetative cover were excluded as covariates for the count component of these models as AIC suggested a better model fit without them (Table 1). However, vegetation density and native cover were included as covariates for the zero component of the sign and camera models, respectively, as they were found to be significant predictors for the likelihood of zero counts (all sign, P = 0.011; camera, P = 0.036). All sign, all dig, and all track formed similar yet weaker relationships while all scat and all vegetation did not produce significant relationships (Table 2). Scat and browsed vegetation data collected for this study were included in the all sign analysis but lacked the replicates required to be analysed individually. Scat and browsed vegetation were observed at only 5 and 10 sites respectively. The camera and all sign models were taken as the best species distribution model of relative feral pig abundance (Fig. 1). However, all track and all dig produced similar distribution maps and formed similar regressions.
|
|
Spatial maps resulting from the best-fit models from camera and all sign data depict subtle differences in the allocation of relative feral pig abundance (Fig. 1). Both models show higher relative feral pig abundances constrained along an altitudinal band with very low abundance predicted above a threshold elevation in the Ko‘olau mountain range. The resulting map from camera data shows similar patterns in the distribution of relative feral pig abundance but is more diffuse across the landscape primarily throughout the lower elevations. The general trend between both maps shows the highest abundance of feral pigs at mid-elevation in both the Wai‘anae (west) and Ko‘olau (east) mountain ranges.
All forms of observed data showed weak correlations with high variance (Fig. 2). Much of the variance of the observed data was explained by fitting the data to zero-inflated models. After model fit, all predicted data exhibited significant strong correlations with low variance (all sign: P < 0.001, R2 = 0.7807; all track: P < 0.001, R2 = 0.879; all dig: P < 0.001, R2 = 0.8313).
Discussion
Despite only weak correlative relationships between the different forms of abundance data (Fig. 2a, c, e), the predicted outputs from models constructed with these different data types formed much stronger correlations (Fig. 2b, d, f). This is partially due to the zero-inflation process correcting for false negatives, but also because some of the variance between raw count data is explained by environmental covariates. As a result, the final species distribution models built using different forms of abundance data are largely analogous (Fig. 1) in their output and would likely result in similar management recommendations.
We developed our models based on an understanding of feral pig habitat suitability and utilisation (Ballari and Barrios-García 2014). Covariate sets were constructed from this understanding and although some covariates were not significant predictors for the count component of our zero-inflated models, they were found to be significant predictors for the zero component (Table 2). We had predicted that vegetation density would likely be an important covariate for feral pig abundance but only found it to be significant for the likelihood of zero counts of feral pig sign (Table 2). This supports the idea that the observer ability to detect sign is influenced by the amount of vegetation (i.e. likelihood of zeroes increases with increasing vegetation density). Dense vegetation may be influencing presence or absence of sign of feral pigs in two ways. Sign of feral pigs may be obscured by thick vegetation, and/or feral pigs may keep to pre-established game or hiking trails in densely vegetated areas in contrast to roaming more freely in areas with lower vegetation density. In areas of high-density vegetation, camera traps may provide higher-resolution data by capturing feral pigs moving through areas in which their sign would otherwise be difficult to detect, providing more useful data as inputs into a model of species relative abundance. Similarly, native cover was found to be a significant covariate for the zero component of our game camera model (i.e. likelihood of zero counts increases with increasing proportion of native to non-native cover). Standardised sign survey methods likely provide more useful data for modelling the relative abundance of feral pigs within these environments since the disturbance of this vegetation by feral pigs is typically easily observed due to the tunnelling through, trampling or rooting of understorey ferns.
Feral pigs are present in over a quarter of the world’s countries (IUCN 2017) and there is substantial variation across these regions with respect to two of the most limiting factors to collecting adequate data: availability of funds and accessibility of labour (Engeman 2005; Thomas et al. 2013). In regions limited by funds, sign survey methods of collecting data on feral pig abundance might be more feasible if field personnel incorporated a systematic methodology of acquiring data during their typical field operations. In regions limited by field personnel, data collected from remote camera traps may be more feasible due to their ability to be deployed over extended periods with reduced labour when compared with sign survey methods (Silveira et al. 2003; Rovero and Marshall 2009). However, the volume of photographs recorded on camera traps will also influence the cost and feasibility of this approach. Recent advancements in utilising artificial intelligence and machine learning algorithms to identify unique photographs of species captured by camera traps suggest that costs associated with processing data from camera traps may be greatly reduced in the near future, making this monitoring method more amenable to agencies with limited funds (Norouzzadeh et al. 2018). Regardless, we propose that researchers and agencies across these regions may prioritise the method of data collection based on these limiting factors with confidence that either data type will yield comparable results for wildlife management, as long as data collection is distributed at appropriate scales to capture environmental variation associated with distribution.
This study provides an example in which these two common types of monitoring data result in comparable spatial outputs of species abundance. However, future studies may further explore the relatability between these two data types, particularly in continental landscapes where environmental gradients may occur over much larger distances. The methods employed in this study can likely be applied to any area with abundant widespread populations of feral pigs where it is important to know the relative abundance of feral pigs across the landscape, as opposed to their occupancy (presence or absence). Examples of these regions could be islands throughout the Pacific region, national parks or other natural areas throughout Australia, New Zealand, and the United States, or any other region with high feral pig abundance. From a management standpoint, occupancy models in these areas generated from presence-only or presence-absence data provide little information as to the perceived benefit of a management action if the output estimates a uniformly distributed high probability of feral pig presence across the study area. However, models of relative abundance enable managers to weight the benefit of management actions and target areas of high feral pig abundance. We propose that our method of modelling relative abundance using either form of abundance data might be a better alternative in these regions where occupancy models might not provide adequate information to inform management decisions. Finally, we reiterate that standardised survey designs with survey locations stratified across environmental gradients are imperative for ensuring that data are comparable and representative (Guisan and Zimmermann 2000; Hirzel and Guisan 2002; Maggini et al. 2002; Vaughan and Ormerod 2003).
Although the results from this study indicate strong relatedness across data types for distribution modelling, each data type may provide key information that may be beneficial to managers, landowners, or researchers at individual sites. For example, camera detections may provide additional data on the quantity of individuals in each frame, and their behavioural patterns; they have also been useful in mark–recapture studies for density estimates (Silver et al. 2004; Holtfreter et al. 2008; Rovero and Marshall 2009). Likewise, sign data have been widely adopted as a metric for direct impact, allowing researchers to quantify extent of impact from the focal species (Engeman et al. 2013). Nonetheless, we provide an example in which two of the most commonly collected abundance data for monitoring and modelling purposes are highly correlated in their spatial outputs.
Management implications
Our comparison of two methods of monitoring feral pig abundance demonstrates that, given an appropriate sampling design and model development to minimise bias, the two data types can yield comparable results. Environmental agencies, researchers, or non-governmental organisations are typically limited by either their ability to have personnel in the field or the funding available for data collection. As such, the feasibility of each method may vary across agencies and regions. For organisations with personnel consistently in the field, the additional collection of data on the abundance of sign requires minimal additional cost given they can survey a randomly generated site during their normal operations. For organisations with limited field personnel, the deployment of game cameras provides long-term, passive data collection for conservation decision making. Our results indicate that resource managers, researchers and private landowners may prioritise methods of data collection based on resources or personnel available with confidence that spatial outputs will be significantly correlated at the landscape scale, resulting in analogous wildlife management decisions.
Conflicts of interest
The authors declare no conflicts of interest.
References
Ballari, S. A., and Barrios‐García, M. N. (2014). A review of wild boar Sus scrofa diet and factors affecting food selection in native and introduced ranges. Mammal Review 44, 124–134.| A review of wild boar Sus scrofa diet and factors affecting food selection in native and introduced ranges.Crossref | GoogleScholarGoogle Scholar |
Barrios-Garcia, M. N., and Ballari, S. A. (2012). Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biological Invasions 14, 2283–2300.
| Impact of wild boar (Sus scrofa) in its introduced and native range: a review.Crossref | GoogleScholarGoogle Scholar |
Bengsen, A. J., Leung, L. K.-P., Lapidge, S. J., and Gordon, I. J. (2011). Using a general index approach to analyze camera-trap abundance indices. The Journal of Wildlife Management 75, 1222–1227.
| Using a general index approach to analyze camera-trap abundance indices.Crossref | GoogleScholarGoogle Scholar |
Bivand, R., Keitt, T., and Rowlingson, B. (2018). rgdal: bindings for the geospatial data abstraction library. R package version 1.4.3. Available at https://CRAN.R-project.org/package=rgdal
Bondi, N. D., White, J. G., Stevens, M., and Cooke, R. (2010). A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities. Wildlife Research 37, 456–465.
| A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities.Crossref | GoogleScholarGoogle Scholar |
Chauvenet, A. L. M., Gill, R. M. A., Smith, G. C., Ward, A. I., and Massei, G. (2017). Quantifying the bias in density estimated from distance sampling and camera trapping of unmarked individuals. Ecological Modelling 350, 79–86.
| Quantifying the bias in density estimated from distance sampling and camera trapping of unmarked individuals.Crossref | GoogleScholarGoogle Scholar |
Cole, R. J., and Litton, C. M. (2014). Vegetation response to removal of non-native feral pigs from Hawaiian tropical montane wet forest. Biological Invasions 16, 125–140.
| Vegetation response to removal of non-native feral pigs from Hawaiian tropical montane wet forest.Crossref | GoogleScholarGoogle Scholar |
Desurmont, G. A., Donoghue, M. J., Clement, W. L., and Agrawal, A. A. (2011). Evolutionary history predicts plant defense against an invasive pest. Proceedings of National Academy of Science 108, 7070–7074.
| Evolutionary history predicts plant defense against an invasive pest.Crossref | GoogleScholarGoogle Scholar |
Diong, C. H. (1982). Population biology and management of the feral pig (Sus scrofa L.) in Kipahulu Valley, Maui. Ph.D. Thesis, University of Hawaii.
Donlan, C. J., Campbell, K., Cabrera, W., Lavoie, C., Carrion, V., and Cruz, F. (2007). Recovery of the Galápagos rail (Laterallus spilonotus) following the removal of invasive mammals. Biological Conservation 138, 520–524.
| Recovery of the Galápagos rail (Laterallus spilonotus) following the removal of invasive mammals.Crossref | GoogleScholarGoogle Scholar |
Dormann, C. F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Carré, G., Marquéz, J. R. G., Gruber, B., Lafourcade, B., Leitão, P. J., Münkemüller, T., McClean, C., Osborne, P. E., Reineking, B., Schröder, B., Skidmore, A. K., Zurell, D., and Lautenbach, S. (2013). Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46.
| Collinearity: a review of methods to deal with it and a simulation study evaluating their performance.Crossref | GoogleScholarGoogle Scholar |
Elith, J., Kearney, M., and Phillips, S. (2010). The art of modelling range-shifting species. Methods in Ecology and Evolution 1, 330–342
Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E., and Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Diversity and Distributions 17, 43–57.
| A statistical explanation of MaxEnt for ecologists.Crossref | GoogleScholarGoogle Scholar |
Engeman, R. M. (2005). Indexing principles and a widely applicable paradigm for indexing animal populations. Wildlife Research 32, 203–210.
| Indexing principles and a widely applicable paradigm for indexing animal populations.Crossref | GoogleScholarGoogle Scholar |
Engeman, R. M., Constantin, B., Nelson, M., Woolard, J., and Bourassa, J. (2001). Monitoring changes in feral swine abundance and spatial distribution. Environmental Conservation 28, 235–240.
| Monitoring changes in feral swine abundance and spatial distribution.Crossref | GoogleScholarGoogle Scholar |
Engeman, R. M., Massei, G., Sage, M., and Gentle, M. N. (2013). Monitoring wild pig populations: a review of methods. Environmental Science and Pollution Research 20, 8077–8091.
| Monitoring wild pig populations: a review of methods.Crossref | GoogleScholarGoogle Scholar | 23881593PubMed |
Evans, M. C., Possingham, H. P., and Wilson, K. A. (2011). What to do in the face of multiple threats? Incorporating dependencies within a return on investment framework for conservation. Diversity and Distributions 17, 437–450.
| What to do in the face of multiple threats? Incorporating dependencies within a return on investment framework for conservation.Crossref | GoogleScholarGoogle Scholar |
Field, S. A., Tyre, A. J., and Possingham, H. P. (2005). Optimizing allocation of monitoring effort under economic and observational constraints. The Journal of Wildlife Management 69, 473–482.
| Optimizing allocation of monitoring effort under economic and observational constraints.Crossref | GoogleScholarGoogle Scholar |
Fitzpatrick, M. C., Preisser, E. L., Ellison, A. M., and Elkinton, J. S. (2009). Observer bias and the detection of low-density populations. Ecological Applications 19, 1673–1679.
| Observer bias and the detection of low-density populations.Crossref | GoogleScholarGoogle Scholar | 19831062PubMed |
Gentle, M., Speed, J., and Marshall, D. (2015). Consumption of crops by feral pigs (Sus scrofa) in a fragmented agricultural landscape. Australian Mammalogy 37, 194–200.
| Consumption of crops by feral pigs (Sus scrofa) in a fragmented agricultural landscape.Crossref | GoogleScholarGoogle Scholar |
Giambelluca, T. W., Chen, Q., Frazier, A. G., Price, J. P., Chen, Y.-L., Chu, P.-S., Eischeid, J. K., and Delparte, D. M. (2012). Online rainfall atlas of Hawai‘i. Bulletin of the American Meteorological Society 94, 313–316.
| Online rainfall atlas of Hawai‘i.Crossref | GoogleScholarGoogle Scholar |
Guisan, A., and Zimmermann, N. E. (2000). Predictive habitat distribution models in ecology. Ecological Modelling 135, 147–186.
| Predictive habitat distribution models in ecology.Crossref | GoogleScholarGoogle Scholar |
Guisan, A., and Thuiller, W. (2005). Predicting species distribution: offering more than simple habitat models. Ecology Letters 8, 993–1009.
| Predicting species distribution: offering more than simple habitat models.Crossref | GoogleScholarGoogle Scholar |
Hauser, C. E., and McCarthy, M. A. (2009). Streamlining ‘search and destroy’: cost-effective surveillance for invasive species management. Ecology Letters 12, 683–692.
| Streamlining ‘search and destroy’: cost-effective surveillance for invasive species management.Crossref | GoogleScholarGoogle Scholar | 19453617PubMed |
Hess, S. C. (2016). A tour de force by Hawaii’s invasive mammals: establishment, takeover, and ecosystem restoration through eradication. Mammal Study 41, 47–60.
| A tour de force by Hawaii’s invasive mammals: establishment, takeover, and ecosystem restoration through eradication.Crossref | GoogleScholarGoogle Scholar |
Hijmans, R. J., Van Etten, J., Cheng, J., Mattiuzzi, M., Sumner, M., Greenberg, J. A., and Ghosh, A. (2017). Package ‘raster’: Geographic data analysis and modeling. Available at https://cran.r-project.org/web/packages/raster/raster.pdf
Hirzel, A., and Guisan, A. (2002). Which is the optimal sampling strategy for habitat suitability modelling. Ecological Modelling 157, 331–341.
| Which is the optimal sampling strategy for habitat suitability modelling.Crossref | GoogleScholarGoogle Scholar |
Holtfreter, R. W., Williams, B. L., Ditchkoff, S. S., and Grand, J. B. (2008). Feral pig detectability with game cameras. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 62, 17–21.
Hone, J. (2002). Feral pigs in Namadgi National Park, Australia: dynamics, impacts and management. Biological Conservation 105, 231–242.
| Feral pigs in Namadgi National Park, Australia: dynamics, impacts and management.Crossref | GoogleScholarGoogle Scholar |
IUCN (2017). The IUCN Red List of Threatened Species. Version 2017-3. Available at http://www.iucnredlist.org (accessed 18 May 2018).
Kéry, M., Spillmann, J. H., Truong, C., and Holderegger, R. (2006). How biased are estimates of extinction probability in revisitation studies?. Journal of Ecology 94, 980–986.
| How biased are estimates of extinction probability in revisitation studies?.Crossref | GoogleScholarGoogle Scholar |
Keuling, O., Sange, M., Acevedo, P., Podgorski, T., Smith, G., Scandura, M., Apollonio, M., Ferroglio, E., and Vicente, J. (2018). Guidance on estimation of wild boar population abundance and density: methods, challenges, possibilities. EFSA Supporting Publications 15, 1449E.
| Guidance on estimation of wild boar population abundance and density: methods, challenges, possibilities.Crossref | GoogleScholarGoogle Scholar |
LaPointe, D. A., Atkinson, C. T., and Samuel, M. D. (2012). Ecology and conservation biology of avian malaria: ecology of avian malaria. Annals of the New York Academy of Sciences 1249, 211–226.
| Ecology and conservation biology of avian malaria: ecology of avian malaria.Crossref | GoogleScholarGoogle Scholar | 22320256PubMed |
Maggini, R., Guisan, A., and Cherix, D. (2002). A stratified approach for modeling the distribution of a threatened ant species in the Swiss National Park. Biodiversity and Conservation 11, 2117–2141.
| A stratified approach for modeling the distribution of a threatened ant species in the Swiss National Park.Crossref | GoogleScholarGoogle Scholar |
Massei, G., and Genov, P. (2004). The environmental impact of wild boar. Galemys 16, 135–145.
Massei, G., Coats, J., Lambert, M. S., Pietravalle, S., Gill, R., and Cowan, D. (2018). Camera traps and activity signs to estimate wild boar density and derive abundance indices. Pest Management Science 74, 853–860.
| Camera traps and activity signs to estimate wild boar density and derive abundance indices.Crossref | GoogleScholarGoogle Scholar |
Moore, J. L., Hauser, C. E., Bear, J. L., Williams, N. S. G., and McCarthy, M. A. (2011). Estimating detection–effort curves for plants using search experiments. Ecological Applications 21, 601–607.
| Estimating detection–effort curves for plants using search experiments.Crossref | GoogleScholarGoogle Scholar | 21563589PubMed |
Nogueira-Filho, S. L. G., Nogueira, S. S. C., and Fragoso, J. M. V. (2009). Ecological impacts of feral pigs in the Hawaiian Islands. Biodiversity and Conservation 18, 3677.
| Ecological impacts of feral pigs in the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar |
Norouzzadeh, M. S., Nguyen, A., Kosmala, M., Swanson, A., Palmer, M. S., Packer, C., and Clune, J. (2018). Automatically identifying, counting, and describing wild animals in camera-trap images with deep learning. Proceedings of the National Academy of Sciences 115, E5716–E5725.
| Automatically identifying, counting, and describing wild animals in camera-trap images with deep learning.Crossref | GoogleScholarGoogle Scholar |
Parker, J. D., Burkepile, D. E., and Hay, M. E. (2006). Opposing effects of native and exotic herbivores on plant invasions. Science 311, 1459–1461.
| Opposing effects of native and exotic herbivores on plant invasions.Crossref | GoogleScholarGoogle Scholar | 16527979PubMed |
Pimental, D. (2007). Environmental and economic costs of vertebrate species invasions into the United States. In ‘Managing Vertebrate Invasive Species: Proceedings of an International Symposium, National Wildlife Research Center, Fort Collins, CO’. (Eds G. W. Witmer, W. C. Pitt, and K. A. Fagerstone.).
R Core Team (2017). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rovero, F., and Marshall, A. R. (2009). Camera trapping photographic rate as an index of density in forest ungulates. Journal of Applied Ecology 46, 1011–1017.
| Camera trapping photographic rate as an index of density in forest ungulates.Crossref | GoogleScholarGoogle Scholar |
Rowcliffe, J. M., Field, J., Turvey, S. T., and Carbone, C. (2008). Estimating animal density using camera traps without the need for individual recognition. Jouurnal of Applied Ecology 45, 1228–1236.
| Estimating animal density using camera traps without the need for individual recognition.Crossref | GoogleScholarGoogle Scholar |
Sarre, S. D., MacDonald, A. J., Barclay, C., Saunders, G. R., and Ramsey, D. S. L. (2013). Foxes are now widespread in Tasmania: DNA detection defines the distribution of this rare but invasive carnivore. Journal of Applied Ecology 50, 459–468.
| Foxes are now widespread in Tasmania: DNA detection defines the distribution of this rare but invasive carnivore.Crossref | GoogleScholarGoogle Scholar |
Silveira, L., Jácomo, A. T. A., and Diniz-Filho, J. A. F. (2003). Camera trap, line transect census and track surveys: a comparative evaluation. Biological Conservation 114, 351–355.
| Camera trap, line transect census and track surveys: a comparative evaluation.Crossref | GoogleScholarGoogle Scholar |
Silver, S. C., Ostro, L. E. T., Marsh, L. K., Maffei, L., Noss, A. J., Kelly, M. J., Wallace, R. B., Gómez, H., and Ayala, G. (2004). The use of camera traps for estimating jaguar Panthera onca abundance and density using capture/recapture analysis. Oryx 38, 148–154.
| The use of camera traps for estimating jaguar Panthera onca abundance and density using capture/recapture analysis.Crossref | GoogleScholarGoogle Scholar |
Southwell, C., and Low, M. (2009). Black and white or shades of grey? Detectability of Adélie penguins during shipboard surveys in the Antarctic pack-ice. Journal of Applied Ecology 46, 136–143.
| Black and white or shades of grey? Detectability of Adélie penguins during shipboard surveys in the Antarctic pack-ice.Crossref | GoogleScholarGoogle Scholar |
Thomas, J. F., Engeman, R. M., Tillman, E. A., Fischer, J. W., Orzell, S. L., Glueck, D. H., Felix, R. K., and Avery, M. L. (2013). Optimizing line intercept sampling and estimation for feral swine damage levels in ecologically sensitive wetland plant communities. Environmental Science and Pollution Research 20, 1503–1510.
| Optimizing line intercept sampling and estimation for feral swine damage levels in ecologically sensitive wetland plant communities.Crossref | GoogleScholarGoogle Scholar | 22707203PubMed |
Tulloch, V. J., Tulloch, A. I., Visconti, P., Halpern, B. S., Watson, J. E., Evans, M. C., Auerbach, N. A., Barnes, M., Beger, M., Chadès, I., Giakoumi, S., McDonald-Madden, E., Murray, N. J., Ringma, J., and Possingham, H. P. (2015). Why do we map threats? Linking threat mapping with actions to make better conservation decisions. Frontiers of Ecology and the Environment 13, 91–99.
| Why do we map threats? Linking threat mapping with actions to make better conservation decisions.Crossref | GoogleScholarGoogle Scholar |
Vaughan, I. P., and Ormerod, S. J. (2003). Improving the quality of distribution models for conservation by addressing shortcomings in the field collection of training data. Conservation Biology 17, 1601–1611.
| Improving the quality of distribution models for conservation by addressing shortcomings in the field collection of training data.Crossref | GoogleScholarGoogle Scholar |
Venables, W. N., and Ripley, B. D. (2002). ‘Modern Applied Statistics with S.’ (Springer: New York.)
Watson, C. A., Weckerly, F. W., Hatfield, J. S., Farquhar, C. C., and Williamson, P. S. (2008). Presence–nonpresence surveys of golden-cheeked warblers: detection, occupancy and survey effort. Animal Conservation 11, 484–492.
| Presence–nonpresence surveys of golden-cheeked warblers: detection, occupancy and survey effort.Crossref | GoogleScholarGoogle Scholar |
Wehr, N. H. (2018). Responses of soil invertebrate and bacterial communities to the removal of nonnative feral pigs (Sus scrofa) from a Hawaiian tropical montane wet forest. M.S. Thesis, University of Hawai’i at Manoa, Hawaii.
Wehr, N. H., Hess, S. C., and Litton, C. M. (2018). Biology and impacts of Pacific islands invasive species. 14. Sus scrofa, the feral pig (Artiodactyla: Suidae). Pacific Science 72, 177–198.
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag: New York.)
Wilson, K. A., McBride, M. F., Bode, M., and Possingham, H. P. (2006). Prioritizing global conservation efforts. Nature 440, 337–340.
| Prioritizing global conservation efforts.Crossref | GoogleScholarGoogle Scholar | 16541073PubMed |
Zeileis, A., Kleiber, C., and Jackman, S. (2008). Regression models for count data in R. Journal of Statistical Software 27, 1–25.
| Regression models for count data in R.Crossref | GoogleScholarGoogle Scholar |