New information on site occupancy and detection rate of Mixophyes iteratus and implications for management
Gregory W. Lollback A C , Michele A. Lockwood B and David S. Hannah A
B and David S. Hannah A
A Engineering, Tweed Shire Council, PO Box 816, Murwillumbah, NSW 2484, Australia.
B School of Environment, Science and Engineering, Southern Cross University, PO Box 157, Lismore, NSW 2480, Australia.
C Corresponding author. Email: glollback@tweed.nsw.gov.au
Pacific Conservation Biology 27(3) 244-250 https://doi.org/10.1071/PC20075
Submitted: 22 September 2020 Accepted: 17 December 2020 Published: 19 January 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Mixophyes iteratus (giant barred frog) is listed as Endangered in state and federal legislation, but there have been only two previous studies in scientific literature that have focused on habitat preferences of this species. This study aimed to shed light on habitat selection of M. iteratus by conducting surveys during the 2019–2020 breeding season within the Tweed Valley, northern New South Wales, Australia. Detection rate was also quantified, which is a first for the species. A nested survey method was used in conjunction with a conditional survey design. There were 118 detections of M. iteratus over 31 habitat sites, all beside permanent stream pools >10 m in length. Occupancy modelling suggested that frogs were more likely found in sites with ≥12 m of undercut bank. Over three survey rounds, detection rate was 0.54–0.65. Surveys at historical sites showed that the species can survive severe drought, which was experienced through 2018 and 2019. Findings suggest that at least two surveys for M. iteratus should be conducted at favourable sites or when targeting the species, especially if land management at the site is proposed to change. Riparian management that retains undercut banks and pools is encouraged for the conservation of this species.
Keywords: detectability, frog, giant barred frog, habitat selection, Mixophyes iteratus, presence.
Introduction
There are 1798 Threatened species listed under the Environment Protection and Biodiversity Conservation Act 1999 and 934 Threatened species listed under the Biodiversity Conservation Act 2016. It can be argued that there is a lack of ecological knowledge for many of these species. Detailed knowledge about distribution, abundance, habitat preferences and other ecological aspects of Threatened species is critical for their conservation (Pilgrim et al. 2008; Scheele et al. 2018; Camino et al. 2020; Vermeiren et al. 2020). Without ecological knowledge providing the structural support for applied conservation decisions, there is often a greater chance that a Threatened species will further decline towards extinction (Gilchrist et al. 2005; Scheele et al. 2019).
Understanding habitat preferences of a species and the probability of detecting a species during a survey can help to save time and costs when designing a survey and assist the precision of statistical estimates after the data has been collected. Ecological knowledge can also help build a suite of a priori models (Burnham and Anderson 2002), can be used to estimate probabilities in modelling (McCarthy and Masters 2005) or aid in informing species distribution modelling (Vermeiren et al. 2020).
Mixophyes iteratus (giant barred frog) is listed as Endangered under the Environment Protection and Biodiversity Conservation Act 1999 and the Biodiversity Conservation Act 2016. There is limited information available on the ecology of this species, but there is a recovery plan to preserve this and other stream frog species in south-east Queensland (Hines 2002). This recovery plan has even been adopted and currently used by the Federal Government. Mixophyes iteratus has been described as residing adjacent to rainforest, wet sclerophyll (Meyer et al. 2001) and farmland streams that are slow moving with steep banks from lowlands to 100 m in elevation (Covacevich and MacDonald 1993; Hines 2002). It has been stated that the species breeds in permanent streams (Lemckert and Brassil 2000) due to the tadpole phase lasting 9 months or longer (Anstis 2013).
Most statements in literature about M. iteratus ecology appear to be extrapolation from occurrence records, however, two studies have quantified habitat selection of the species: Lemckert (1999) and Lewis and Rohweder (2005). The former study measured broad-scale variables (variables exhibiting little change over small distances, such as rainfall, elevation, altitude and logging disturbance) at 52 stream sites and related these variables to the presence of many frog species, including M. iteratus. Lewis and Rohweder (2005) surveyed for M. iteratus at 70 sites across the Bungawalbin catchment in northern New South Wales and measured fine-scale habitat variables (variables that change over a small distance, such as the presence of undercut banks and leaf litter cover) and broad-scale variables and related these to M. iteratus presence. Additional information on the species ecology includes: Lemckert and Brassil (2000) and Streatfield (1999) studied movement of the species in relation to the river bank; Koch and Hero (2007) looked at how abiotic factors influenced detection (but they did not estimate detection rate); and Knowles et al. (2015) studied aspects of the breeding biology of M. iteratus and other Mixophyes species. However, detailed knowledge on habitat selection remains very limited and the detectability of the species during surveys has rarely been investigated.
Due to the lack of support from quantified data for habitat selection of M. iteratus and considering that the species is listed as Endangered under Commonwealth and state legislation, the aim of this study was to measure and to determine critical habitat elements selected by this species. This study also attempted to estimate the detection probability of the species within its breeding season.
Materials and methods
Study area
The Tweed Valley is located in northeast New South Wales, Australia in the northern part of M. iteratus’ distribution. The Tweed Valley, which is an erosion caldera in a subtropical climate, has only six historical locations for M. iteratus from the New South Wales Government’s species data collection, BioNet (https://www.environment.nsw.gov.au/wildlifeatlas/about.htm). Goldingay et al. (1999) found the species at nine sites within Mebbin National Park and Tweed Shire Council (TSC) has confirmed records from seven additional locations that were collected during environmental investigations. Most records are in the western half of the shire, where residential development is less intense.
The study area encompassed approximately 236 km2, which is demarcated by the boundaries of the Tweed Shire and the erosion caldera and ocean (Fig. 1).
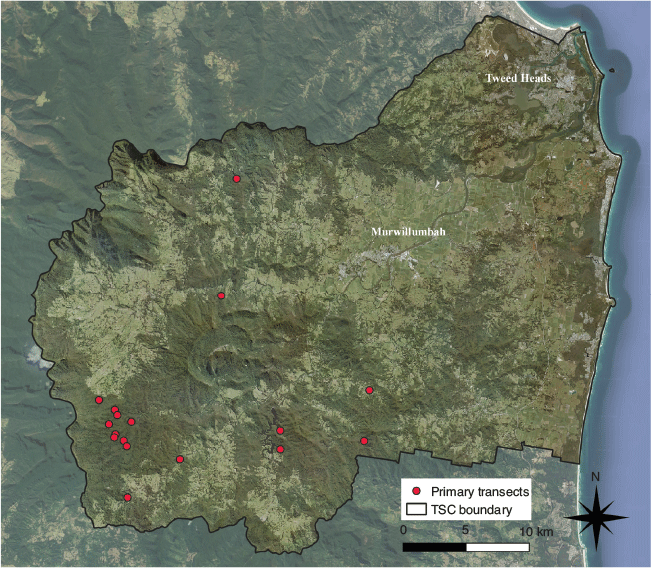
|
Weather within the Tweed Valley is warm and temperate, with a mean minimum and maximum temperature of 14.5°C and 25.8°C respectively. Mean yearly rainfall is 1571 mm. Long-term weather statistics were taken from the Murwillumbah (Bray Park) weather station and can be accessed at www.bom.gov.au. Prior to surveys commencing, the Tweed Valley was in drought with 2019 recording only 716.7 mm of rainfall at the Murwillumbah station, which is the lowest on record since the station became operational in 1972. The nearby weather station at Condong Sugar Mill, located approximately 6 km from the Bray Park station, opened in 1887 and closed in 1972. The lowest annual rainfall recorded from this station was 693.6 mm in 1902 (www.bom.gov.au).
Surveys
Before commencing frog surveys, three reference sites within the study area were visited at least four times from 25 November 2019 to 17 December 2019 to determine species activity. Frog surveys began on the 17 December 2019 and concluded on 3 April 2020. Surveys were nested and consisted of three types: primary, secondary and tertiary transects. Primary transects initially explored stream reaches and varied in length from 200 to 1500 m. The purpose of these surveys was to find individuals in streams where they were likely to occur. Primary transects occurred within third-order streams (Strahler system) that were mostly permanent. Many reaches surveyed had previous records of M. iteratus ranging from 1999 in Mebbin National Park (Goldingay et al. 1999) to more recent TSC records ranging from 2004 to 2017.
Secondary transects were 100 m in length, with the location of detections during primary transect surveys becoming the centre of the secondary transect. Secondary transects were conducted after all primary transects were completed. There were two additional visits to each secondary transect. Secondary transects were situated at least 225 m apart to ensure independence from one another. This distance was based on data collected by Lemckert and Brassil (2000), who found that the average nightly distance moved by radio-tracked M. iteratus was 21.5 m, with a maximum nightly movement of 200 m. The purpose of secondary transects was to estimate detection rate and to inform the placement of tertiary (or habitat) transects. Tertiary transects were 50 m in length. These transects were at least 225 m apart and included reaches and reach sections where frogs were detected as well as where frogs were not detected. The centre of the habitat transect was located where frog sightings were most dense. Tertiary transects that did not contain frog detections were located along primary transects, were systematic in placement, and started and finished at least 100 m from features such as bridges, houses or unpassable pools that determined primary transect start and finish locations.
Repeat visits to locations within primary transects followed the conditional design described by Specht et al. (2017). By revisiting locations where frogs were sighted and not revisiting sites where frogs were not initially sighted, the efficiency of estimating a detection rate was increased.
Primary transect length and resampling frequency and design within Mebbin National Park was different to the hierarchical structure used at other survey locations. This is because the work by Goldingay et al. (1999) is historically significant for scientific knowledge of the species within the Tweed Shire and allows for a direct comparison between the two studies. Since these primary transects were only 200 m long, three complete surveys were executed at these primary transects, regardless of detection history. Secondary and tertiary transects at this location were consistent with the other locations.
Primary and secondary transects involved two observers searching for frogs from 0 to 20 m from both stream edges, as determined by Lemckert and Brassil (2000) as M. iteratus’ preferential range. Surveys commenced at sunset. Frogs were often detected by eyeshine using a Ledlenser H14R.2 headlamp or by vocalisation. Each 100 m survey took roughly 15 min to traverse. Once frogs were found: an in situ geotagged photograph was taken and added as a QGIS mapping layer as an identification reference for each individual frog; each frog was measured with callipers to determine its sex and snout–vent length (SVL). Juveniles (<40 mm SVL), sub-adults (40–59 mm SVL), adult males (60–97 mm SVL) exhibiting nuptial pads and/or calling and adult females (65–120 mm SVL) without nuptial pads were identified accordingly; the sex of each individual was noted based on call behaviour or the presence/absence of nuptial pads; the location was recorded using a Garmin GPSmap 64s; the distance from the stream was estimated; and the substrate the frog was sitting on was also noted. Following national frog hygiene protocols set out by Murray et al. (2011), clean gloves were used to briefly and carefully handle frogs to further determine their sex, palm colour and presence or absence of nuptial pads. Individuals were quickly returned to their exact point of detection.
Abiotic conditions were measured with a pocket Kestrel 3500 Weather Meter and were recorded at the start and end of each of the primary and secondary survey and were concurrent with methods used by Koch and Hero (2007). The average value of each condition was used in the analysis. These environmental conditions included air temperature, relative humidity, barometric pressure, moon visibility, cloud cover, current rain activity and the occurrence of rainfall within the 24-h period, as provided by the Bureau of Meteorology and confirmed by evidence on site. The number of Mixophyes fasciolatus detected per 100 m was also recorded during each survey.
Tertiary transects were located 2.5 m from the stream bank, which was close to the average distance frogs were located after two survey rounds (n = 69, mean = 2.7 m). Transects ran parallel to the stream edge, on both sides of the stream. Tertiary surveys commenced after the completion of the second round of surveys, however, most were completed after all the secondary transect surveys were finished.
Measured habitat variables were based on those used by Lewis and Rohweder (2005). Variables included: stream morphology (bend, straight or sweep); flow type in metres (riffle, run or pool); the number of snags; the length (m) of undercut bank; the length (m) of overhanging vegetation <1 m high and also >5 m high; canopy cover (%); litter cover (%); stream width (m); area of bench (m2); disturbance level (low, moderate or high). Canopy cover, litter cover and stream width were measured at the 5, 15, 25, 35 and 45 m mark along the transect. Canopy cover was measured by taking a photo 1 m above the ground and then estimating cover. Litter cover was measured using a 1 × 1-m quadrat. The maximum width of bank bench was limited to 20 m.
Analysis
The aim of the analysis was to estimate what variables were associated with M. iteratus occupancy and detection rate. This was achieved using a single season model explained by MacKenzie et al. (2002). Before occupancy modelling was undertaken, correlations between habitat variables were quantified using a Spearman’s rank correlation, with correlated variables (ρ ≥ 0.7, P-value < 0.05) excluded from the analysis. Abiotic variables collected during primary and secondary surveys were used as covariates to estimate detection rate. Habitat variables collected during the tertiary survey were used as covariates to estimate occupancy. Counts of the congeneric species, M. fasciolatus were also used as a covariate when modelling detection rate because mass calling by M. fasciolatus could possibly mask the detection of calling male M. iteratus.
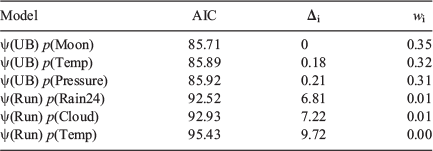
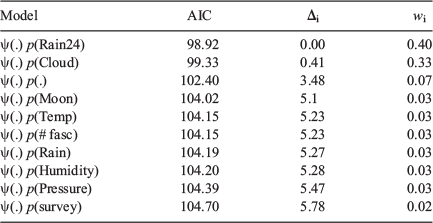
Akaike’s information criterion (AIC) was used for the selection of models. Within this section, all AIC information is referenced to Burnham and Anderson (2002). Models with an AIC difference (Δi) ≤2 indicate significant support that it is the best suited model within the suite. Models with Δi ranging between four and seven are less supported and models with Δi >10 are not competitive and thus omitted. The Akaike weight (wi) was also calculated. All occupancy analysis was completed in Program Presence 2.12.39 (Hines 2006).
Results
A total of 17 reaches were surveyed (primary transects), with an average length of 544 m (s.d. = 460 m) and minimum and maximum length of 200 and 1620 m respectively. Primary transects included nine historic sites that were surveyed by Goldingay et al. (1999), three reaches known to TSC since 2004 (Lewis 2004) and 2016, three anecdotal sites that date back to 2017 and one site known to an author since 2017. The remaining primary transect was in a third order stream on private property that was adjacent to Wollumbin National Park, but did not have any prior records of M. iteratus.
There were 13 independent (>225 m apart) locations with M. iteratus after all primary transects were surveyed. These 13 locations then became the midpoint of secondary transects. There were an additional four independent locations where frogs were detected after the second round of surveys (secondary transects) and after three rounds of surveys there was one additional independent location with frogs. Overall, there were 41 surveys conducted at 21 secondary transects. There were 13 locations where M. iteratus was not detected. There was a total of 31 tertiary transects where habitat surveys were conducted, with M. iteratus occupying 18 of these transects. Mixophyes iteratus was detected at seven of the nine sites surveyed by Goldingay et al. (1999), all the reaches known to contain the species by TSC, two of the three reaches with anecdotal accounts of the species and one site known to an author. Transect identification and nesting, as well as detection history is available as Supplementary Material.
There were 118 detections of M. iteratus in total, comprised of 45 adult males, 32 adult females, 11 subadults, 19 juveniles, 10 adults where the sex was not identified and three frogs where neither sex nor size were recorded. Individual frogs were resighted across the survey period and two detections were of frogs in amplexus (Fig. 2). One pair of amplecting frogs was observed one night before the last survey (2 April 2020), indicating frog surveys finished within the breeding season. Adult female SVL ranged from 70 to 116 mm with an average of 98 mm and a standard deviation of 10 mm (n = 24). Adult male SVL ranged from 61 to 98 mm with an average of 74 mm and a standard deviation of 9 mm (n = 38). Sub-adult SVL ranged from 40 to 58 mm with an average of 45 mm and a standard deviation of 5 mm (n = 11). Juvenile SVL ranged from 29 to 39 mm with an average of 34 mm and a standard deviation of 3 mm (n = 24). Note that SVL statistics most likely include pseudo replication, as it is highly likely individual frogs were detected and measured more than once over the three survey rounds.

|
Habitat analysis
There were 31 tertiary transects that were used as replicates for the occupancy analysis. Mixophyes iteratus was detected on 18 of these transects.
Litter cover was significantly correlated with canopy cover (ρ = 0.85, P = 0.00) and with overhanging vegetation >5 m tall (ρ = 0.74, P = 0.00). Therefore, canopy cover and overhanging vegetation >5 m tall were excluded from the occupancy analysis. Overhanging vegetation <1 m tall (ρ = –0.71, P = 0.00) was used in the analysis because its correlation with litter cover was marginal and does not serve the same ecological function as leaf litter.
Frogs were detected with varying leaf litter cover (11–99%, 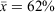 ), metres of vegetation cover <1 m tall (3–95 m,
), metres of vegetation cover <1 m tall (3–95 m, 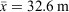 ), number of snags (0–11,
), number of snags (0–11, 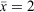 ), metres of riffle (0–27 m,
), metres of riffle (0–27 m, 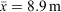 ), stream width (0.4–10.2 m,
), stream width (0.4–10.2 m, 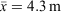 ) and bench area (0–2000 m2,
) and bench area (0–2000 m2, 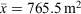 ). Frogs were only found where there was >10 m length of pool and when there was a minimum of 12 m of undercut bank. Frogs were not found when there was >27 m of run in a 50 m transect.
). Frogs were only found where there was >10 m length of pool and when there was a minimum of 12 m of undercut bank. Frogs were not found when there was >27 m of run in a 50 m transect.
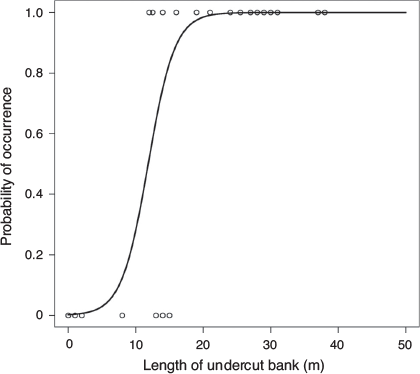
From a large suite of models with different combinations of habitat and abiotic variables used to model occupancy and detection rate, there were only six models with an AIC Δi <10 (Table 1). Within this refined suite of models, only models with undercut banks had substantial support (Δi <2) for being the ‘best’ model to explain M. iteratus occupancy. The relationship between occupancy and length of undercut bank was positive (Fig. 3). The remaining models from the refined suite contained a negative relationship between the length of stream run and M. iteratus occupancy. The top model formula was:
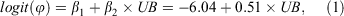
where UB = length of undercut bank on both sides of the stream (m). β1 and β2 are model coefficients. The standard error of β1 = 4.60 and the standard error of β2 = 0.35.
|
|
Model averaging produced an occupancy rate estimate of 0.62 (s.d. = 0.43) and detection rate of 0.65 (s.d. = 0.00), 0.64 (s.d. = 0.00) and 0.65 (s.d. = 0.00) for survey rounds one, two and three, respectively.
Detection rate analysis
Surveys were conducted in mild conditions in regard to temperature (18.7–27.3°C, x¯ = 23.4°C) and rainfall, with drizzling rain occurring during 26 out of 72 tertiary site surveys. Humidity ranged from 80.0 to 100% ( ), barometric pressure ranged from 991.5 to 1016.8 hPa (
), barometric pressure ranged from 991.5 to 1016.8 hPa ( ), cloud cover ranged from 0 to 100% (
), cloud cover ranged from 0 to 100% (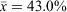 ), moon visibility ranged from 0 to 75% (
), moon visibility ranged from 0 to 75% (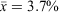 ) and the number of M. fasciolatus ranged from 0 to 7 frogs per 100 m2 (
) and the number of M. fasciolatus ranged from 0 to 7 frogs per 100 m2 (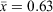 frogs per 100 m2). There were 32 survey events where it had rained in the previous 24 h.
frogs per 100 m2). There were 32 survey events where it had rained in the previous 24 h.
A suite of models was run using abiotic variables as covariates, while keeping occupancy constant, as a way to determine what conditions influenced detectability. The analysis indicated that detection was better without cloud cover and when it had not rained within the 24-h period prior to surveying (Table 2). Although there was less support for unvarying detection rate over the survey period, this model performed relatively well. The influence of these factors – cloud cover and rain – was slight, but held some weight when compared with other measured factors such as barometric pressure and moon visibility that held no influence over M. iteratus detectability. Model averaging using the suite of models gave a detection rate for the first, second and third survey rounds of 0.57 (0.10 s.d.), 0.65 (0.08 s.d.) and 0.54 (0.10 s.d.), respectively.
|
The estimated detection rates can be used to find the probability of detecting M. iteratus given a certain number of repeat visits. Using the lower estimated detection rate of 0.54, the probability of detecting the species at least once at an occupied site is 0.54, 0.79 and 0.90 given one, two and three visits, respectively. Using the highest estimated detection rate of 0.65, the probability of detecting the species at an occupied site at least once given one, two and three visits is 0.65, 0.88 and 0.96, respectively.
Discussion
Frog surveys undertaken in late 2019 and early 2020 within the Tweed Shire determined that M. iteratus favoured sites with undercut banks. When occupancy rate was held constant across survey sites, the estimated detection rate of the species was 0.54–0.65. This study is one of only three (the others being Lemckert 1999 and Lewis and Rohweder 2005) that have systematically documented habitat selection of this Endangered species and is the only study to estimate the species’ detection rate.
Although there is little published research quantifying the habitat requirements of M. iteratus, Lewis and Rohweder (2005) suggested a specific set of environmental conditions and habitat requirements for this species’ foraging and breeding needs, which included a wide forested riparian zone with leaf litter cover, permanent water and undercut banks. Litter cover is essential for overwintering hibernation, diurnal sheltering and general concealment. However, in this study it was not directly associated with M. iteratus occupancy as it was a common feature of forested areas and not a unique attribute to sites that are specifically occupied by this species.
Unlike Lemckert (1999), this study did not have sites that had been recently logged and all sites had riparian vegetation >10 m wide. That is, sites surveyed in the Tweed exhibited disturbance in the form of weeds and human and stock traffic, but was not to the extent of the dramatic disturbance measured by Lemckert (1999). In fact, disturbance was a poor predictor of occurrence in the Tweed Valley, which contrasts with findings by Lewis and Rohweder (2005). This is likely because the upper sub/catchments associated with survey sites within the Tweed tend to be heavily forested and consist of rocky banks and beds, which may limit heavy downstream sediment loads in comparison to the Lewis and Rohweder (2005) study within the Bungawalbin catchment, where sedimentation may have led to the infilling of streams.
Our modelling suggests that habitat features which support M. iteratus selective breeding needs outweigh other environmental factors for this species. All sites where M. iteratus was present had a permanent pool. Permanent surface flows support the species’ long larval stage. This is illustrated by the significant occupancy of M. iteratus at sites with permanent pools >10 m in length that also featured undercut banks >10 m that provide a terrestrial substrate on which to deposit eggs (Anstis 2013; Knowles et al. 2015). In turn, sites with deep permanent pools that lacked undercut banks, were not occupied by M. iteratus, proving that pools alone do not support the species’ long-term recruitment. The key necessity for this species’ survival is the presence of undercut banks. This habitat component is a requirement for M. iteratus to successfully breed, as the females kick their eggs onto the vertical face of the bank’s undercut where they remain protected above the water level until the tadpole hatchlings drop into the stream and eventually develop into frogs (Anstis 2013; Knowles et al. 2015).
The data has shown that as the length of run increases, the probability of M. iteratus occupancy diminishes. This negative correlation strongly suggests the run’s characteristic of swiftly moving water would likely inhibit the species reproductive success. As obligate stream dwellers, these frogs demonstrate a clear preference for permanent pools (in combination with undercut banks) over long lengths of running streams. This is due to the aforementioned ovipositional processes, which if attempted in a swift flowing stream, would likely prove unsuccessful.
Despite the recent severe drought, this species has been able to persist at sites for at least two decades (Goldingay et al. 1999) as these streams continue to provide permanent pools and surface flows. Additionally, mating adults, juveniles and subadults were detected during this study, indicating that the species can survive severe drought without a major contraction of occurrence, as long as some water remains at breeding sites.
An important source of variation to be considered in survey design for rare species is its ability to be detected (MacKenzie et al. 2002). Detection can be influenced by an array of factors including habitat, species behavioural characteristics and abundance levels. Our findings outline that fluctuations in abiotic and meteorological factors do not appear to be significantly correlated with M. iteratus detectability during their breeding season. Further, this long breeding season, spanning from September to May (Spring to Autumn) would require persistence through a range of fluctuating temperatures, levels of rainfall, humidity and barometric pressure. This is illustrated by consistent detection rates produced by the model averages for all three survey rounds.
The findings of Lemckert and Brassil (2000) on the movement of M. iteratus report that adult frogs exhibited restricted movement of ~20 m from the stream edge and <10 m along the stream bank per night. Limited vagility is considered an additional factor that may contribute to the likelihood of detecting M. iteratus. Covariates such as age, sex or reproductive state have been found to influence behaviour, movements and therefore, detection (Lemckert and Brassil 2000; Koch and Hero 2007).
Crypsis, the ability of an animal to avoid observation or detection by other animals or predators (Allaby 2015), may be influenced by a predator’s angle of vision (Toledo and Haddad 2009). Depending on the species, some frogs may prove more cryptic from a dorsal view than a lateral one. Such was the case with eyeshine detection of M. iteratus within this study. It was our experience that individuals were more successfully spotted from a lateral perspective achieved by spotlighting from a distance on the opposite stream bank that provided a direct angle and less vegetative impediment to catch their eyeshine. When viewed dorsally in close proximity, the tendency of M. iteratus to camouflage with their environment would often prevent detection.
Implications for management
Habitat clearing (Lemckert 1999) and riparian degradation likely have played a significant role (Lewis and Rohweder 2005) in the reduction of population size and the findings of this study indicate that the presence of permanent water and undercut banks are an important combination for the preservation of the species. In addition to permanent water, sites with undercut banks are crucial for M. iteratus reproductive processes and therefore their sustained recruitment levels. The probability of detecting the species is considerably higher after conducting two or more surveys. Results demonstrate that detectability after two surveys ranged between 79 and 88%. Hence, prior to undertaking any land management activities, new and historic sites that exhibit the specialist habitat features of permanent pools and undercut banks, require two surveys within the breeding season to determine M. iteratus occupancy. Although three survey visits resulted in a higher probability of detection, time constraints and costs must be taken into account. Our results also suggest conducting surveys in fine, clear weather. It is encouraged to incorporate the findings of Koch and Hero (2007) and not survey for M. iteratus when ambient temperature is <18°C.
Measures that protect undercut banks on private and public land where the species have been identified should be utilised, and stream rehabilitation should focus on favourable habitat when possible. Further, the creation and reinforcement of undercut banks at sites have the potential to mitigate habitat loss through fortifying the structure of oviposition sites that are integral to the long-term survival of M. iteratus. Retainment of undercut banks could be achieved through limiting trampling from human and stock traffic, and by planting vegetation with root systems that hold soil together along riparian areas where erosion is likely (Rutherford et al. 2007).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Thank you to two anonymous reviewers and the editors at Pacific Conservation Biology for improving the manuscript. Thank you to Kathleen Hellmann and NSW National Parks and Wildlife Service for allowing the study to include sites within Mebbin National Park and Mount Jerusalem National Park. Thank you to Tai O’Connor and Mark Pepper for allowing us onto their properties to search for frogs. Matthew Bloor and Michael Corke provided valuable information on historic Mixophyes iteratus sites. Thanks to Liam Thompson who provided valuable assistance with frog surveys. Lastly, thank you to our patient families for supporting this work. Fieldwork was conducted under NSW Department of Planning, Industry and Environment Scientific Licence SL100540. This research did not receive any specific funding.
References
Allaby, M. (Ed.) (2015). ‘A dictionary of ecology’, 5th edn. (Oxford University Press: Oxford, UK.)Anstis, M. (2013). ‘Tadpoles and frogs of Australia.’ (New Holland: Sydney, NSW.)
Burnham, K. P., and Anderson, D. R. (2002). ‘Model selection and multimodal inference’, 2nd edn. (Springer-Verlag: New York, USA.)
Camino, M., Thompson, J., Andrade, L., Cortez, S., Matteucci, S. D., and Altrichter, M. (2020). Using local ecological knowledge to improve large terrestrial mammal surveys, build local capacity and increase conservation opportunities. Biological Conservation 244, 1–8.
| Using local ecological knowledge to improve large terrestrial mammal surveys, build local capacity and increase conservation opportunities.Crossref | GoogleScholarGoogle Scholar |
Covacevich, J. A., and MacDonald, K. R. (1993). Distribution and conservation of frogs and reptiles of Queensland rainforests. Memoirs of the Queensland Museum 34, 189–199.
Gilchrist, G., Mallory, M., and Merkel, F. (2005). Can local ecological knowledge contribute to wildlife management? Case studies of migratory birds. Ecology and Society 10, .
| Can local ecological knowledge contribute to wildlife management? Case studies of migratory birds.Crossref | GoogleScholarGoogle Scholar |
Goldingay, R., Newell, D., and Graham, M. (1999). The status of rainforest stream frogs in north-eastern New South Wales: decline or recovery? In ‘Declines and disappearances of Australian frogs’. (Ed. A. Campbell.) pp. 64–71. (Environment Australia: Canberra, ACT.)
Hines, H. (2002). ‘Recovery plan for stream frogs of south-east Queensland 2001–2005.’ (Queensland Parks and Wildlife Service: Brisbane, Qld.)
Hines, J. E. (2006). ‘PRESENCE – software to estimate patch occupancy and related parameters.’ (USGS-PWRC.) Available at http://www.mbr-pwrc.usgs.gov/software/presence.html
Knowles, R., Thumm, K., Mahony, M., Hines, H., Newell, D., and Cunningham, M. (2015). Oviposition and egg mass morphology in barred frogs (Anura: Myobatrachidae: Mixophyes Günther, 1864), its phylogenetic significance and implications for conservation management. Australian Zoologist 37, 381–402.
| Oviposition and egg mass morphology in barred frogs (Anura: Myobatrachidae: Mixophyes Günther, 1864), its phylogenetic significance and implications for conservation management.Crossref | GoogleScholarGoogle Scholar |
Koch, A. J., and Hero, J.-M. (2007). The relationship between environmental conditions and activity of the giant barred frog (Mixophyes iteratus) on the Coomera River, south-east Queensland. Australian Journal of Zoology 55, 89–95.
| The relationship between environmental conditions and activity of the giant barred frog (Mixophyes iteratus) on the Coomera River, south-east Queensland.Crossref | GoogleScholarGoogle Scholar |
Lemckert, F. (1999). Impacts of selective logging on frogs in a forested area of northern New South Wales. Biological Conservation 89, 321–328.
| Impacts of selective logging on frogs in a forested area of northern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Lemckert, F., and Brassil, T. (2000). Movements and habitat use of the endangered giant barred river frog (Mixophyes iteratus) and the implications for its conservation in timber production forests. Biological Conservation 96, 177–184.
| Movements and habitat use of the endangered giant barred river frog (Mixophyes iteratus) and the implications for its conservation in timber production forests.Crossref | GoogleScholarGoogle Scholar |
Lewis, B. D. (2004). Proposed replacement of five timber bridges on Kyogle Road: Microchiropteran bat survey. Report prepared for Tweed Shire Council. (Lewis Ecological Surveys: Forster, NSW, Australia.)
Lewis, B. D., and Rohweder, D. A. (2005). Distribution, habitat, and conservation status of the giant barred frog Mixophyes iteratus in the Bungawalbin catchment, northeastern New South Wales. Pacific Conservation Biology 11, 189–197.
| Distribution, habitat, and conservation status of the giant barred frog Mixophyes iteratus in the Bungawalbin catchment, northeastern New South Wales.Crossref | GoogleScholarGoogle Scholar |
MacKenzie, D. I., Nichols, J. D., Lachman, G. B., Droege, S., Royle, J. A., and Langtimm, C. A. (2002). Estimating site occupancy rates when detection probabilities are less than one. Ecology 83, 2248–2255.
| Estimating site occupancy rates when detection probabilities are less than one.Crossref | GoogleScholarGoogle Scholar |
McCarthy, M. A., and Masters, P. (2005). Profiting from prior information in Bayesian analyses of ecological data. Journal of Applied Ecology 42, 1012–1019.
| Profiting from prior information in Bayesian analyses of ecological data.Crossref | GoogleScholarGoogle Scholar |
Meyer, E., Hines, H., and Hero, J.-M. (2001). ‘Wet forest frogs of south-east Queensland.’ (Griffith University: Qld.)
Murray, K., Skerratt, L., Marantelli, G., Berger, L., Hunter, D., Mahony, M., and Hines, H. (2011). Hygiene protocols for the control of diseases in Australian frogs. A report for the Australian Government Department of Sustainability, Environment, Water, Population and Communities. (James Cook University: Brisbane, Qld., Australia.)
Pilgrim, S. E., Cullen, L. C., Smith, D. J., and Pretty, J. (2008). Ecological knowledge is lost in wealthier communities and countries. Environmental Science & Technology 42, 1004–1009.
| Ecological knowledge is lost in wealthier communities and countries.Crossref | GoogleScholarGoogle Scholar |
Rutherford, I., Anderson, B., and Ladson, A. (2007). Managing the effects of riparian vegetation on flooding. In ‘Principles for riparian lands management’. (Eds S. Lovett and P. Price.) pp. 63–84. (Land and Water Australia: Canberra, ACT.)
Scheele, B. C., Legge, S., Armstrong, D. P., Copley, P., Robinson, N., Southwell, D., Westgate, M. J., and Lindenmayer, D. B. (2018). How to improve threatened species management: an Australian perspective. Journal of Environmental Management 223, 668–675.
| How to improve threatened species management: an Australian perspective.Crossref | GoogleScholarGoogle Scholar | 29975894PubMed |
Scheele, B. C., Legge, S., Blanchard, W., Garnett, S., Geyle, H., Gillespie, G., Harrison, P., Lindenmayer, D., Lintermans, M., Robinson, N., and Woinarski, J. (2019). Continental-scale assessment reveals inadequate monitoring for threatened vertebrates in a megadiverse country. Biological Conservation 235, 273–278.
| Continental-scale assessment reveals inadequate monitoring for threatened vertebrates in a megadiverse country.Crossref | GoogleScholarGoogle Scholar |
Specht, H. M., Reich, H. T., Iannarilli, F., Edwards, M. R., Stapleton, S. P., Weegman, M. D., Johnson, M. K., Yohannes, B. J., and Arnold, T. W. (2017). Occupancy surveys with conditional replicates: an alternative sampling design for rare species. Methods in Ecology and Evolution 8, 1725–1734.
| Occupancy surveys with conditional replicates: an alternative sampling design for rare species.Crossref | GoogleScholarGoogle Scholar |
Streatfield, C. (1999). Spatial movements of Mixophyes iteratus and Mixophyes fasciolatus in southeast Queensland. Honours thesis. Griffith University, Bundall, Qld.
Toledo, L. F., and Haddad, C. (2009). Colors and some morphological traits as defensive mechanisms in Anurans. International Journal of Zoology 1–4, 1–12.
| Colors and some morphological traits as defensive mechanisms in Anurans.Crossref | GoogleScholarGoogle Scholar |
Vermeiren, P., Reichert, P., and Schuwirth, N. (2020). Integrating uncertain prior knowledge regarding ecological preferences into multi-species distribution models: effects of model complexity on predictive performance. Ecological Modelling 420, 1–15.
| Integrating uncertain prior knowledge regarding ecological preferences into multi-species distribution models: effects of model complexity on predictive performance.Crossref | GoogleScholarGoogle Scholar |


