Avian diversity and abundance across years: consistent patterns in forests but not grasslands on Viti Levu, Fiji
Alivereti N. Naikatini A , Gunnar Keppel B , Gilianne Brodie A and Sonia Kleindorfer
B , Gilianne Brodie A and Sonia Kleindorfer  C D *
C D *
A Institute of Applied Sciences, University of the South Pacific, Suva, Fiji.
B UniSA STEM and Future Industries Institute, University of South Australia, Mawson Lakes Campus, Adelaide, SA 5001, Australia.
C College of Science and Engineering, Flinders University, Adelaide, SA 5001, Australia.
D Konrad Lorenz Research Center for Behavior and Cognition and Department of Behavioral and Cognitive Biology, University of Vienna, Vienna, 1030, Austria.
Pacific Conservation Biology 29(3) 223-237 https://doi.org/10.1071/PC21039
Submitted: 30 June 2021 Accepted: 6 March 2022 Published: 1 April 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Habitat loss is a global problem and in Fiji >50% of the land area once covered by forests has been converted to grasslands and agricultural land. About 99% of Fiji’s endemic biodiversity and 80% of the land bird species have been identified as forest species.
Aims: In this study, we compare forest and grassland sites and test for consistency in avian diversity, abundance, foraging guild, and distribution status (endemic, native, introduced to Fiji) over a 5-year period (2016–2020).
Methods: We surveyed bird communities using the point count method with a 100 m radius and 7-min observation period per site.
Key results: A one-way analysis of similarities (ANOSIM) analysis showed significant differences in species composition and bird abundance between the forested habitats and grassland habitats. A general linear model test showed significant differences in foraging guild composition and distribution status between forested and grassland habitats. There were no significant differences between the three forested sites (primary montane forest, secondary old-growth forest, old-growth mahogany plantations with regenerating native species), while grassland sites had stronger annual change in species composition.
Implications: Forest cover, irrespective of whether these forests are of primary or secondary nature, therefore plays an important role in maintaining the native and endemic land bird species and other biodiversity in oceanic island ecosystems such as Viti Levu Island, Fiji.
Keywords: avian biodiversity, aves, community assemblage, foraging guild, forests, grasslands, introduced species, Pacific Islands, primary forest, secondary forest, species richness, vegetation structure, woodlands.
Introduction
Globally, biodiversity is declining at a rapid rate and two of the main drivers behind this decline are introduced (exotic alien) species and habitat loss, both of which are a result of human related activities (Birdlife International 2018; Bongaarts 2019; Soares et al. 2020). Birds comprise more than 11 000 species globally, with 40% being threatened due to declining populations and 183 species confirmed to have gone extinct in the past 500 years (Birdlife International 2018). Tropical forests are highly biodiverse ecosystems, containing 15 of the 25 global hotspots for biodiversity (Brooks et al. 2002; Birdlife International 2018; Şekercioğlu et al. 2019). Tropical oceanic island ecosystems are particularly important biodiversity hotspots, as they support a high number of endemic species with often small population sizes over relatively small geographic areas (Kier et al. 2009). Concurrently, birds and other taxa on oceanic islands have extremely high extinction risk with record-level local and species extinction and population decline (Steadman 1995; Brooks et al. 2002; Xu et al. 2017). Added to this biodiversity crisis is the relative lack of research into functional ecology in tropical systems compared with temperate areas (Clarke et al. 2017), and a paucity of long-term monitoring for species diversity and the distribution of functional foraging guilds (Birdlife International 2018; Domisch et al. 2019).
Long-term data sets on avian abundance and diversity can provide clues about potential threats or threatening processes, which is useful information for conservation managers. Several studies have found greater avian diversity and abundance associated with seasonal peaks in resource abundance and breeding onset (Clunie 1984; Watling 2001; Naikatini 2009; Almazán-Núñez et al. 2018; Kopij 2019). Avian surveys have increasingly documented patterns of avian decline, especially in island ecosystems that must contend with a suite of factors such as habitat loss, introduced predators, and introduced pathogens (Steadman 1993; Innes et al. 2009; Dvorak et al. 2012; Koop et al. 2016). Habitat loss is a key threatening process (Xu et al. 2017), as is climate change (Şekercioğlu et al. 2012), which is predicted to impact high-elevation species in particular (Birrell et al. 2020). Therefore, habitat cover types such as forest or grassland and elevation are additional valuable sources of information to interpret patterns of decline or increase from avian surveys (McCain 2009; Mueller-Dombois et al. 2012; Ferger et al. 2014; Sam et al. 2019). Understanding differences in the annual consistency of species or foraging guild composition in different habitat types and/or at different elevation ranges provides essential baseline information about potential changes that may guide management decisions and helps to frame testable hypotheses for the occurrence of such patterns.
The relative composition of different foraging guilds in different habitat cover types is a window through which to understand community structure (Verner 1984; Nally 1994; Korňan and Kropil 2014). There is a breadth of knowledge about the different composition of avian foraging guilds in different ecosystems including coastal systems, arid/grassland systems, and woodland systems (Vale et al. 1982; Nally 1994). For example, foliage gleaners (insectivores) may be most dominant and show higher diversity in forested habitat cover types, generalist feeders are common in desert-shrub habitat cover types, and ground-seed feeders (granivores) are dominant in grassland habitat cover types (Vale et al. 1982; Nally 1994; Somasundaram and Vijayan 2008; Bett et al. 2016; Howland et al. 2016; Ehlers Smith et al. 2018; Kopij 2019). Seasonal shifts in the prevalence of foraging guilds may also be observed such as, in an arid grassland habitat cover type in India, insectivores were dominant during the winter season and omnivores were dominant during summer (Varun and Dutta 2020). The number of avian foraging guilds varies but is often between five and nine per ecosystem (Taylor et al. 2017; Chatterjee et al. 2020). The guild structure and composition in an ecosystem is a product of the physical structure of the system, geographic location, resource availability and the ability of birds to exploit the available resources (Vale et al. 1982; Nally 1994; Chatterjee et al. 2020). The high bird species richness and guild diversity in forested habitats has been shown to be related to the heterogeneity of the vegetation structure and the plant guilds present (Cubley et al. 2020).
The Fijian archipelago has approximately 500 named islands and islets of which about 100 are inhabited, and a range of terrestrial habitat types such as grasslands and forests spanning elevational gradients from 0 to 1323 m a.s.l. (Derrick et al. 1965; Smith 1979). There are nine principal vegetation types recognised in Fiji: lowland rainforest, upland rainforest, cloud forest, dry forest, Talasiga vegetation, freshwater-wetland vegetation, mangrove forest and scrub, coastal strand vegetation and small island vegetation (Mueller-Dombois and Fosberg 1998). Fiji harbours 108 native bird species, of which 61% are land birds, 28% seabirds and 11% migrant shorebirds (Birdlife Datazone 2017). Of the 66 native land bird species, 52% are endemic to Fiji, 11% are threatened with extinction and one species has been confirmed to have gone extinct from Fiji (Watling 2001; Birdlife Datazone 2017).
Viti Levu island is the largest island in the Fijian archipelago with an area of 10 338 km2 (Smith 1979), which is approximately 57% of the total landmass for Fiji, with eight of nine principal vegetation types described for Fiji found on Viti Levu. Therefore, it is a good system to begin research into how habitat cover type affects avian diversity and foraging guild composition on Fiji. Furthermore, 49 (74%) of the 66 native land bird species are extant on Viti Levu island. The habitat associations for some of these native and endemic species have briefly been discussed by Gorman (1975) and Reid et al. (2019) including some observations and records (Clunie 1984; Watling 2001; Tabudravu 2009) but patterns of occurrence in different habitat types across years has never been quantitatively analysed. To better manage the extant avifauna in forest and anthropogenically modified grasslands in Fiji, we urgently need comparative data on patterns of avian diversity and abundance between habitat types.
In the Fijian archipelago, there is still a gap in knowledge about the number of guilds, guild structure, species diversity and community assemblage in the different terrestrial habitats, which this study aimed to address. We used bird point count data collected from forest and grassland habitat cover types on Viti Levu, Fiji and asked if avian species diversity and community assemblage differ across years and habitat cover types. Reid et al. (2019) carried out a short-term study during July in 2016 and found consistency of point count data across survey days. We compared (1) avian species diversity and community composition, (2) the proportion of different foraging guilds (insectivore, nectarivore, granivore, frugivore, omnivore), and (3) the distribution status (endemic, native, introduced) at the species level and the individual level across habitats. We predicted that the diversity of avian species would be greatest in the least-disturbed primary high-elevation forest, followed by the secondary mid-elevation forest and, mahogany plantation low-elevation forest, and be lowest in grasslands. As the grasslands and forests are composed of different vegetation communities and exhibit different plant phenology, we expected that the percentage of species per foraging guild would differ across different habitat cover types. Finally, we predicted that the primary high-elevation forest would sustain the most endemic species and we predicted comparable proportions of endemic and native species in the secondary mid-elevation and mahogany plantation low-elevation forest (see also Reid et al. 2019). Grasslands, which have been heavily modified by human activity, were predicted to support more introduced species (Reid et al. 2019). One overarching aim of this study was to assess whether avian forest communities and grassland communities show consistent patterns across several years.
Materials and methods
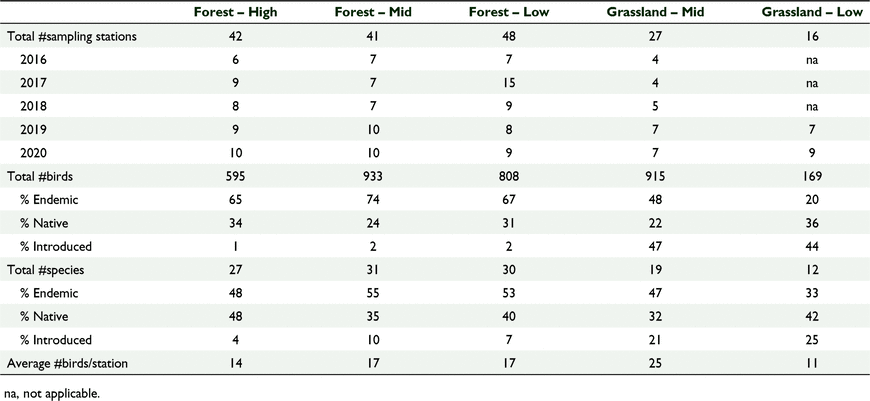
Our study was carried out on the island of Viti Levu, Fiji from 2016 to 2020 during the month of July, coinciding with the breeding season from June to November (Watling 2001; Naikatini 2009). A total of 174 point count stations were surveyed in three forest habitat cover types and two grassland habitat cover types: high-elevation forest (−17°40′55.9″S, 177°32′30.4″E), mid-elevation forest (−17°40′86.0″S, 177°32′38.2″E), low-elevation forest (−18°3′16.8″S, 178°27′41.1″E), mid-elevation grassland (−17°40′21.4″S, 177°32′40.0″E), and low-elevation grassland (–18°4′38.9″S, 177°24′12.9″E) (Fig. 1). Table 1 shows the number of point surveys per study site. The number of sampling stations in each habitat cover type was based on a previous study in Fiji whereby a minimum of five stations was sufficient to record 95% of the bird diversity in a forest habitat (Naikatini 2009). In this study, we noted all birds seen or heard at every survey point (Naikatini 2009; Reid et al. 2019).
Description of the study sites
Mt Koroyanitu national heritage park
Three study sites are located within the general area of this national park including high-elevation forest, mid-elevation forest and mid-elevation grassland. The Mt Koroyanitu National Heritage Park, unlike reserves managed by the Ministry of Forestry, uses a community-based management approach which was initiated in 1990 by the iTaukei Land Trust Board, Fiji Pine Limited and Ministry of Forestry (Thaman 1996; Waqaisavou 1997; Malani 2002). It covers an area of 25 000 ha with 700 documented plant species, 11 of which are endemic to the park (Thaman 1996). The park was set up with the main purposes being conservation and eco-tourism (Thaman 1996; Waqaisavou 1997; Malani 2002).
The high-elevation forest at 1000 m a.s.l. was located along the track to Mt. Batilamu. The vegetation type is montane rain forest which is still intact or in primary state with Agathis macrophylla (Araucariaceae) being the dominant tree species (Anderson et al. 2018).
The mid-elevation forest at 500 m a.s.l. was located along the Savuione waterfall track. The forest is secondary regrowth due to shifting subsistence agriculture and traditional tree removal practices (Reid et al. 2019). The dominant tree species include native trees such as Pterocimbium oceanicum (Sterculiaceae), Bischofia javanica (Phyllanthaceae), Syzygium malaccense (Myrtaceae), Vitex vitilevuense (Verbenaceae) and Dendrocnide harveyi (Urticaceae) (Keppel et al. 2022).
The mid-elevation grassland habitat at 500 m a.s.l. was located between the Abaca Nase lodge and Mt. Batilamu. It is a degraded lowland vegetation type, that developed as a result of constant burning and human activities like subsistence agriculture over a long period of time (Latham 1983). The grassland habitat is dominated by introduced grass (Poaceae) species such as Pennisetum polystachyon, Sporobolus elongatus and Panicum maximum, with patches of the native reed Miscanthus floridulus that had once dominated this vegetation type (Ash 1992; Mueller-Dombois and Fosberg 1998).
Colo-i-Suva forest park
The low-elevation forest sampled at this site was at 100 m a.s.l. The park was established in 1963, with an area of 92 ha and it is part of the Colo-i-Suva Forest Reserve which was established in 1953 covering an area of 369.5 ha (Waqaisavou 1997; Tuiwawa et al. 2018). Initially established as a mahogany plantation which has never been logged, it is now a conservation area and used for recreational and educational purposes and is open to the public (Malani 2002; Tuiwawa and Keppel 2012). About 50 000 people visit the park annually which includes the local public, school field trips and tourists (Tuiwawa et al. 2018). The introduced Mahogany (Swietenia macrophylla) is the dominant tree species but regenerating populations of native plant species in the Colo-i-Suva Forest Reserve have been observed and documented (Tuiwawa and Keppel 2012; Tuiwawa et al. 2018; Reid et al. 2019).
Tuva grassland
This low-elevation grassland occurs at 100 m a.s.l., in the Tuva river watershed. This is a degraded catchment with 65% of the area being non-forested, 26% being covered with Pine (Pinus caribaea) plantation and only 9% being still covered with secondary forest (Government of Fiji and United Nations Development Programme 2015; Institute of Applied Sciences 2019). It is one of the six designated sites in Fiji for the United Nations Development Programme (UNDP) Ridge to Reef Project for rehabilitation and reforestation activities (Government of Fiji and United Nations Development Programme 2015). The non-forested areas of the catchment are covered with grassland habitats similar to those in Abaca and are dominated by introduced grass species such as Pennisetum polystachyon.
Bird surveys
We conducted bird surveys at 174 point count stations and followed similar methods as in Reid et al. (2019). The point count method was used to determine the presence of birds in the area; this method has been recommended for forested habitats and used globally including the Pacific and Fiji in the past (Fancy 1997; Fancy et al. 1999; Jackson and Jit 2007; Naikatini 2009; O’Connor et al. 2010; Reid et al. 2019). The sampling stations were separated by 200 m to prevent any double counting of birds. At each site, all the birds detected visually and audibly were recorded. The location (GPS coordinates) of each station, time of survey and the estimated distance of each bird detected from the point count station, within 100 m radius, were also recorded. Seven minute counts were conducted in the early morning hours between 0630 and 0930 hours, following a 3-min rest and preparation period after arriving at each sampling station. The bird surveys were led by two experienced field ornithologists during 2016, 2017, 2018 (AN, SK) and only AN during 2019 and 2020 with assistance during all years from four undergraduate students noting calling directions (each student monitored a 90° field of view). The large team size monitoring a 360° field of view, reduced the possibility of double counting. All species were identified by AN and SK using the bird field guide book by Watling (2001) as reference.
The foraging guilds, distribution and conservation status categories
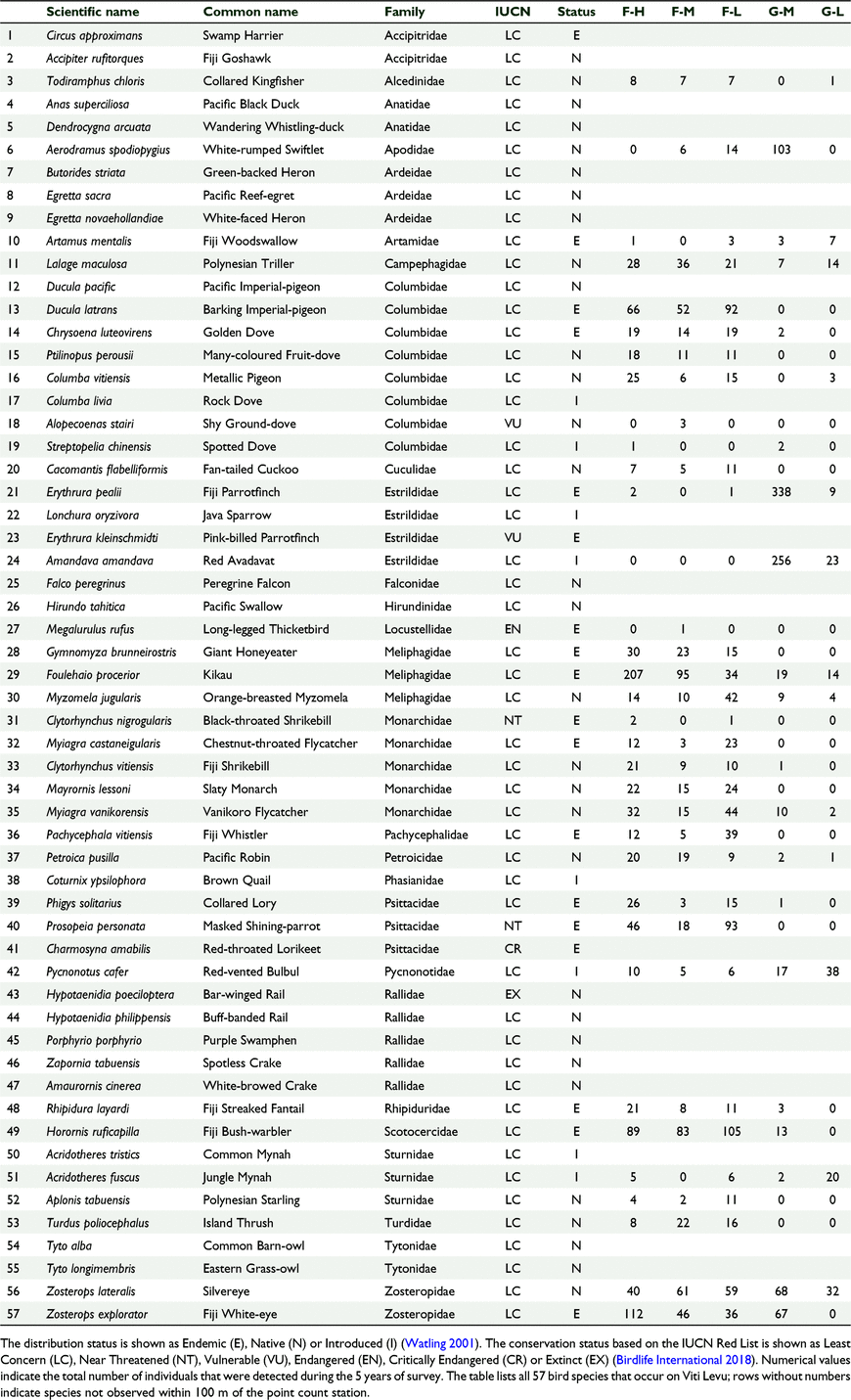
Each species was categorised according to its feeding guild, distribution status and conservation status. We used Watling (2001) to determine the foraging guilds and distribution status categories and the Birdlife Datazone (2017) to assign conservation categories to each species. For foraging guild, each species was categorised as either nectarivore, insectivore, frugivore, omnivore, granivore or carnivore. For the distribution status each species was categorised as either native, endemic or introduced species. Of the 57 land birds of Viti Levu Island, there are 16 insectivore, 13 omnivore, 10 carnivore, eight frugivore, five nectarivore, and five granivore species (Watling 2001). For distribution status of the 57 land bird species, there are 29 native, 20 endemic, and eight introduced species (Watling 2001; Birdlife Datazone 2017). For conservation status of the 57 land bird species, Birdlife Datazone (2017) lists one Extinct (Hypotaenidia poeciloptera), one Critically Endangered (Charmosyna amabilis), one Endangered (Megalurulus rufus), two Vulnerable (Alopecoenas stairi, Erythrura kleinschmidti), two Near Threatened (Clytorhynchus nigrogularis, Prosopeia personata), and the remaining 50 species as Least Concern (LC) (Table 2).
Statistical analysis
Prior to the statistical analysis, we transformed our data and expressed them as percentage of foraging guilds (insectivore, nectarivore, frugivore, granivore and omnivore) and distribution or geographic range status (endemic, native and introduced) at each site for each year. This was done both at the species (species richness) and individual (total individuals) level. To test for similarities in species composition among the five different survey sites over the 5 years, we used multivariate analysis of similarities (ANOSIM) for statistical analysis and non-metric multidimensional scaling (nMDS) for graphing purposes using the PAST 4.01 software (Hammer et al. 2001). The ANOSIM test is a non-parametric test looking at similarities between two or more tested samples (Clarke 1993). We assessed similarity in species composition between the different habitat cover types using the Jaccard similarity index (accounts for species presence versus absence only) and the Bray–Curtis similarity index (accounts for the quantity of different species) to create a dissimilarity matrix between sites. We then used nMDS to present the data in a scatter plot. To test the effects of the different habitat cover types on the foraging guilds (insectivore, nectarivore, frugivore, granivore and omnivore) and distribution/geographic range status (endemic, native and introduced) over the different years, we used General Linear Model (GLM) Multivariate Analysis in the IBM SPSS Statistics software (version 22, IBM Corp 2013). In the multivariate analysis we used habitat as the fixed variable, year was the covariate, and foraging guild and distribution status were the dependent variables.
Results
Avian diversity, abundance and conservation status
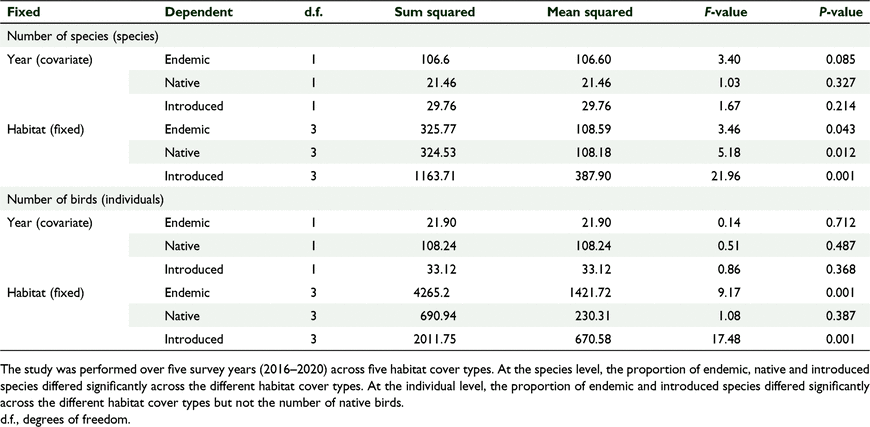
We counted 3421 birds including 34 species of birds and 22 bird families across 174 sampling stations (Tables 1, 2). Sixty-one percent (n = 2097) of all birds counted were endemic, 28% (n = 943) were native and 11% (n = 374) were introduced. At the species level 50% (n = 17) of the recorded species were endemic, 13% (n = 13) were native and 12% (n = 4) were introduced (Table 2). The mid-elevation forest supported the most species and the lowland grassland recorded the least number of species (Table 1, Fig. 2). The mid-elevation grassland had the most birds per sampling station and the lowland grassland recorded the least number of birds per sampling station (Table 1, Fig. 3).
Community composition (species and abundance) across the sites
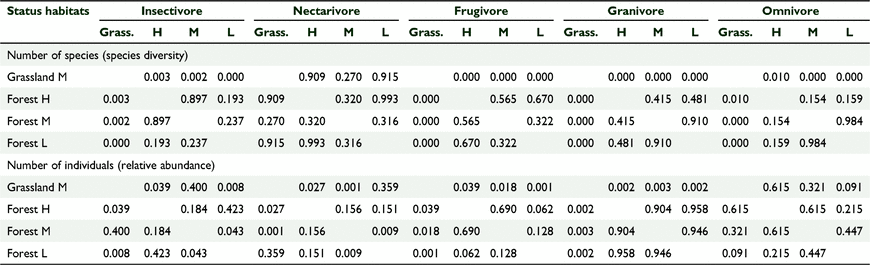
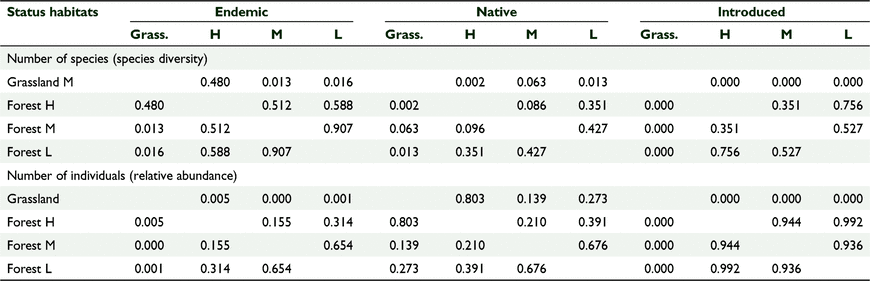
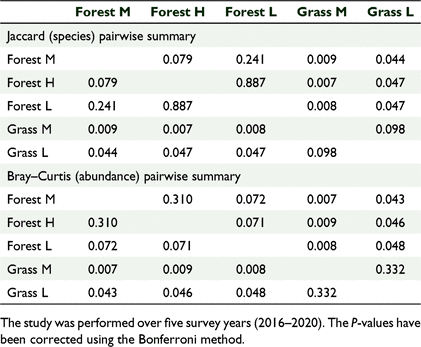
There was a significant difference in the bird species composition between the five sites based on a one-way ANOSIM, using presence/absence (R = 0.5678, P = 0.0001) and the Jaccard similarity index. There was also a significant difference in the bird abundance data between different habitat types (R = 0.4716, P = 0.0001) using the Bray–Curtis similarity index. The pairwise comparison tests showed significant differences between the forested versus the grassland sites (Table 3). The scatter plots based on nMDS showed that the three forest sites clustered together, suggesting similar distribution patterns for species composition when compared to the two grassland sites (Fig. 4). The number of birds across habitat cover types also followed a similar distribution pattern (Fig. 5).
|
Avian foraging guild composition (insectivore, nectarivore, granivore, frugivore, omnivore) across sites
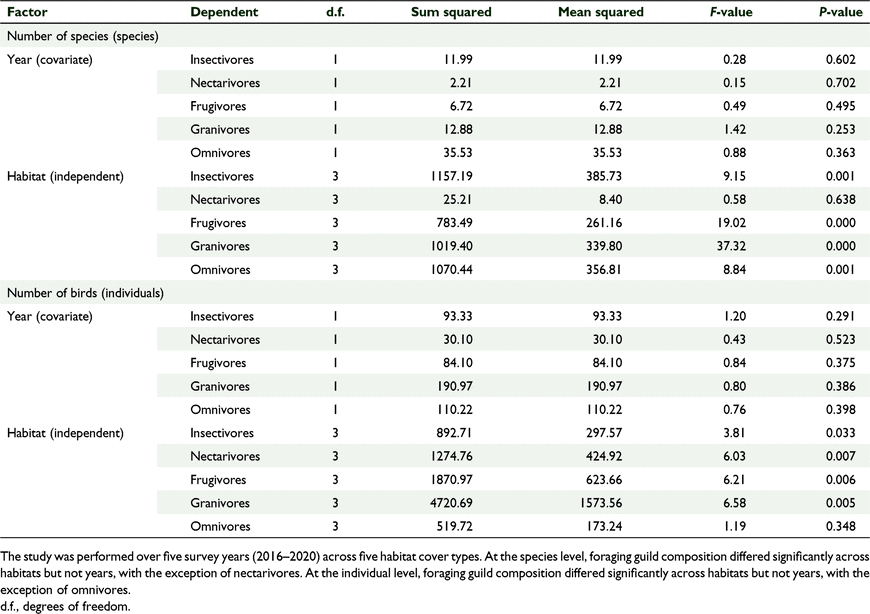
There was a significant effect of habitat cover type on the relative proportion of birds per foraging guild, and no significant effect of year, which we tested using GLM one-way MANCOVA (F(15, 30.768) = 4.781, P = 0.001, Wilks’ Λ = 0.036, partial η2 = 0.000) (Fig. 5, Tables 4, 5). Similarly, there was a significant effect of habitat cover type on the number of birds per foraging guild and no significant effect of year (F(15, 30.768) = 3.065, P = 0.004, Wilks’ Λ = 0.080, partial η2 = 0.004) (Fig. 5, Tables 4, 5). In general, forests supported more insectivores and grasslands supported more granivores, and this pattern was stable across years (Fig. 6).
Avian distribution status (endemic, native, introduced) across sites
There was a significant effect of habitat cover type on the number of species per ‘distribution status’ and no significant effect of year, which we tested using GLM one-way MANCOVA (F(9, 2.054) = 4.309, P = 0.002, Wilks’ Λ = 0.085, partial η2 = 0.561) (Tables 6, 7). Similarly, there was a significant effect of habitat type on the number of birds per ‘distribution status’ and no significant effect of year (F(9,22.054 3) = 5.121, P = 0.01, Wilks’ Λ = 0.064, partial η2 = 0.600) (Tables 6, 7). The forests supported comparable diversity and number of endemic and native birds, but grasslands supported more introduced species, and this pattern was consistent across years.
Discusssion
There were similarities in avian species diversity, number of birds, and proportion of foraging guilds across the 5 years in all three forest habitats irrespective of elevation (range 100–1100 m a.s.l.), and different species diversity, number of birds, and foraging guilds in the two grassland sites (range 100–500 m a.s.l.). The forest habitat cover types had 27–31 species on average and the grassland sites 12–19 species. In general, the number of birds per year was more stable and ranged from 11 to 17 birds per sampling station in forest habitat cover types and from 10 to 27 birds per sampling station in grassland habitat cover types. Both forests and grasslands had comparable diversity and abundance of endemic and native birds, but grasslands had more introduced species, and this pattern was consistent across the 5 years.
Our initial objective tested for diversity and community composition for which we predicted that species diversity and avian abundance would be highest in high-elevation primary forest, followed by mid-elevation secondary forest and lowland plantation forest, respectively, and lowest in the modified grassland habitats. This assumption was based on studies that have shown plant and invertebrate diversity to be highest in primary forest systems and lowest in modified or degraded systems (Barlow et al. 2007; Kormos et al. 2017; Neoh et al. 2017; Sayer et al. 2017). In contrast to this prediction, we found comparable species diversity across the three forest habitats (Fig. 2). We also found lower species diversity in the grasslands compared with the forest habitats. Our study therefore corroborated the prediction that forests have higher species diversity than grasslands but did not find differences among forest types related to elevation. One factor that has been widely described as being significant for avian diversity globally is forest cover, with greater diversity in areas with greater forest cover (Jayapal et al. 2009; Newmark et al. 2010). Tropical tree diversity and other terrestrial biodiversity generally tends to decrease with increasing elevation (Diamond 1988; Givnish 1999; Lomolino 2001). Species diversity tends be the highest around mid-elevation along an elevation gradient, a phenomenon known as the mid-domain effect (Colwell and Lees 2000), which has also been observed for Coleoptera in Fiji where diversity was highest at mid-elevations on Viti Levu Island (Waqa-Sakiti et al. 2018). Similarly, high avian diversity in mid-elevation forest was observed in the Pacific island of Mauna Loa, Hawaii, where it was interpreted to be the result of vegetation structure (Mueller-Dombois et al. 2012), and at Mt. Wilhelm, Papua New Guinea (Sam et al. 2020). Although vegetation studies carried out in Fiji show that tree species diversity is highest in primary tropical rainforest systems, studies on plant diversity in Fiji have failed to find significant differences among different elevations (Ash 1992). Our finding that species diversity was comparable in the forest habitats could indicate strong niche specialisation of the forest birds. Further research is needed, however, to identify the association between plant diversity and forest structure and cover, which is not known for Viti Levu.
For our second objective, we tested for the proportion of different foraging guilds (insectivore, nectarivore, granivore, frugivore, omnivore) in the four different habitat cover types. We predicted that the percentage of species per foraging guild will differ across cover types due to the different vegetation structure and plant community. Insectivores recorded the highest species diversity in all the habitats, and it was the most common guild recorded in all the habitats except for grassland, where granivores were more abundant (Fig. 6). Grassland sites often contain many seed heads and can sustain large groups of birds in a small area, which is congruent with our observation of more granivore species in the grasslands. Granivores often form large flocks while foraging in open grassland areas and this increases feeding efficiency and safety (Perea et al. 2014). Forest birds were mostly insectivores and frugivores, which tend to defend territories for invertebrate consumption, flowers and fruits (Stamps 1994). Such differences in search behaviour for food resources, foraging guild and territory defence behaviour could explain our observation of varying abundances of birds in the two habitat types. The high diversity and abundance of insectivores in forest habitats has been linked to forest structure and has been observed, for example, in Papua New Guinea (Sam et al. 2019; Sam et al. 2020), in Tanzania (Ferger et al. 2014) and India (Jayapal et al. 2009). The diversity and number of the phytophagous guilds (frugivores, nectarivores) have been observed to be influenced by food availability and not forest structure alone (Jayapal et al. 2009). This could have affected nectarivore and frugivore diversity observed in the five study sites but remains to be investigated in the future.
Lastly, we tested for distribution status (native, endemic, introduced) focusing on species diversity and abundance of birds across the different habitat cover types. We found similar results compared with two earlier studies in Fiji by Gorman (1975) and Reid et al. (2019) who also found evidence that endemic bird species tend to prefer forested habitats; and grasslands contained the greatest number of introduced birds and introduced species. Apart from the grassland habitat, all the three forested habitats (primary, secondary and plantation forest) showed no significant difference in the diversity and abundance of endemic, native and introduced species. These results agree with the ‘vegetation structure theory’ (Hurlbert 2004; Ferger et al. 2014), which posits that diversity and abundance are primarily determined by vegetation structure. However, this should be explored in more detail in future studies as we did not specifically compare food availability, climatic conditions, or vegetation structures in this study. The fact that 80% of breeding land birds in Fiji are forest species (Gorman 1975; Birdlife International 2006) suggests that the ancestors of Fijian birds would have arrived and evolved in a forest dominated ecosystem or perhaps chosen forested habitats. Furthermore, the fact that there was no significant difference between the three forest cover types despite differences in disturbance status, elevation, tree species composition and climatic conditions would also suggest that most of the forest species in Fiji are generalists occupying a wide range of forest habitats, which has been observed in other island ecosystems (MacArthur and Wilson 1967; Lack 1973).
Conclusion
The main findings of this study show stability and consistency across 5 years in Fiji’s forest bird communities, irrespective of differences in forest type, forest age or elevation. However, such consistency was lacking in grasslands. Given that most of Fiji’s forests have been degraded, the finding that the age of the forest was not a strong predictor for avian species diversity or abundance is a positive sign for bird conservation in Fiji. It suggests that secondary forests have great value for native Fijian birds and that reforestation and improved habitat connectivity should be effective tools to sustain Fiji’s forest birds. We conclude that, for Fiji, there is evidence for stable avian community structure in forests that is maintained across years and is not strongly influenced by elevation, while grasslands have a more variable avian community composition.
Data availability
Data are available on dryad https://doi.org/10.5061/dryad.37pvmcvmh.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Funding was provided by the Australian Government New Colombo Plan from 2016 to 2019 and from the University of South Pacific in 2020.
Acknowledgements
We acknowledge and thank all the students from Flinders University and University of South Australia that were funded by the Australian Government New Colombo Plan who participated in the bird surveys from 2016 to 2019. We also thank the volunteers from Nature Fiji Mareqeti Viti and University of the South Pacific who took part in the surveys. We are grateful to the Ministry of Forestry and Abaca community for giving us permission to access and conduct surveys in the Colo-i-Suva Mahogany Reserve and the Koroyanitu National Heritage Park. We thank the Abaca Eco-Lodge project management, Mr Pauliasi Kubu, Ms. Kalesi Bose, and all the local guides for accommodating the survey team and providing research assistance.
References
Almazán-Núñez, RC, Alvarez-Alvarez, EA, Pineda-López, R, and Corcuera, P (2018). Seasonal variation in bird assemblage composition in a dry forest of Southwestern Mexico. Ornitología Neotropical 29, 215–224.Anderson, J, Keppel, G, Thomson, S-M, Randell, A, Raituva, J, Koroi, I, Anisi, R, Charlson, T, Boehmer, HJ, and Kleindorfer, S (2018). Changes in climate and vegetation with altitude on Mount Batilamu, Viti Levu, Fiji. Journal of Tropical Ecology 34, 316–325.
| Changes in climate and vegetation with altitude on Mount Batilamu, Viti Levu, Fiji.Crossref | GoogleScholarGoogle Scholar |
Ash, J (1992). Vegetation ecology of Fiji: past, present and future perspectives. Pacific Science 46, 111–127.
Barlow, J, Gardner, TA, Araujo, IS, Ávila-Pires, TC, Bonaldo, AB, Costa, JE, Esposito, MC, Ferreira, LV, Hawes, J, Hernandez, MIM, Hoogmoed, MS, Leite, RN, Lo-Man-Hung, NF, Malcolm, JR, Martins, MB, Mestre, LAM, Miranda-Santos, R, Nunes-Gutjahr, AL, Overal, WL, Parry, L, Peters, SL, Ribeiro-Junior, MA, da Silva, MNF, da Silva Motta, C, and Peres, CA (2007). Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proceedings of the National Academy of Sciences of the United States of America 104, 18555–18560.
| Quantifying the biodiversity value of tropical primary, secondary, and plantation forests.Crossref | GoogleScholarGoogle Scholar | 18003934PubMed |
Bett, MC, Muchai, M, and Waweru, C (2016). Avian species diversity in different habitat types in and around North Nandi Forest, Kenya. African Journal of Ecology 54, 342–348.
| Avian species diversity in different habitat types in and around North Nandi Forest, Kenya.Crossref | GoogleScholarGoogle Scholar |
Birdlife Datazone (2017) Species list: Fiji.. Available at http://datazone.birdlife.org/species/downloadcsv/C6C19152-AA6E-47F3-84BO-A12A4E4BDC7E. [Accessed 18 August 2017]
Birdlife International (2006) Important bird areas in Fiji – conserving Fiji’s natural heritage. Birdlife International Pacific Partnership Secretariat, Suva, Fiji.
Birdlife International (2018) State of the world’s birds: taking the pulse of the planet. BirdLife International, Cambridge, UK.
Birrell, JH, Shah, AA, Hotaling, S, Giersch, JJ, Williamson, CE, Jacobsen, D, and Woods, HA (2020). Insects in high-elevation streams: life in extreme environments imperiled by climate change. Global Change Biology 26, 6667–6684.
| Insects in high-elevation streams: life in extreme environments imperiled by climate change.Crossref | GoogleScholarGoogle Scholar | 32931053PubMed |
Bongaarts J (2019) IPBES. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Available at https://onlinelibrary.wiley.com/doi/full/10.1111/padr.12283. [Accessed 15 October 2020]
Brooks, TM, Mittermeier, RA, Mittermeier, CG, Da Fonseca, GAB, Rylands, AB, Konstant, WR, Flick, P, Pilgrim, J, Oldfield, S, Magin, G, and Hilton-Taylor, C (2002). Habitat loss and extinction in the hotspots of biodiversity. Conservation Biology 16, 909–923.
| Habitat loss and extinction in the hotspots of biodiversity.Crossref | GoogleScholarGoogle Scholar |
Chatterjee, A, Adhikari, S, Pal, S, and Mukhopadhyay, SK (2020). Foraging guild structure and niche characteristics of waterbirds wintering in selected sub-Himalayan wetlands of India. Ecological Indicators 108, 105693.
| Foraging guild structure and niche characteristics of waterbirds wintering in selected sub-Himalayan wetlands of India.Crossref | GoogleScholarGoogle Scholar |
Clarke, DA, York, PH, Rasheed, MA, and Northfield, TD (2017). Does biodiversity–ecosystem function literature neglect tropical ecosystems? Trends in Ecology & Evolution 32, 320–323.
| Does biodiversity–ecosystem function literature neglect tropical ecosystems?Crossref | GoogleScholarGoogle Scholar |
Clarke, KR (1993). Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18, 117–143.
| Non-parametric multivariate analyses of changes in community structure.Crossref | GoogleScholarGoogle Scholar |
Clunie F (1984) ‘Birds of the Fiji bush’. (Fiji Museum: Suva, Fiji Islands)
Colwell, RK, and Lees, DC (2000). The mid-domain effect: geometric constraints on the geography of species richness. Trends in Ecology & Evolution 15, 70–76.
| The mid-domain effect: geometric constraints on the geography of species richness.Crossref | GoogleScholarGoogle Scholar |
Cubley, ES, Bateman, HL, Merritt, DM, and Cooper, DJ (2020). Using vegetation guilds to predict bird habitat characteristics in riparian areas. Wetlands 40, 1843–1862.
| Using vegetation guilds to predict bird habitat characteristics in riparian areas.Crossref | GoogleScholarGoogle Scholar |
Derrick RA, Hughes CAA, Riddell RB (1965) ‘The Fiji Islands: a geographic handbook.’ (Fiji Government Press: Suva, Fiji Islands)
Diamond, J (1988). Factors controlling species diversity: overview and synthesis. Annals of the Missouri Botanical Garden 75, 117–129.
| Factors controlling species diversity: overview and synthesis.Crossref | GoogleScholarGoogle Scholar |
Domisch, S, Friedrichs, M, Hein, T, Borgwardt, F, Wetzig, A, Jähnig, SC, and Langhans, SD (2019). Spatially explicit species distribution models: a missed opportunity in conservation planning? Diversity and Distributions 25, 758–769.
| Spatially explicit species distribution models: a missed opportunity in conservation planning?Crossref | GoogleScholarGoogle Scholar |
Dvorak, M, Fessl, B, Nemeth, E, Kleindorfer, S, and Tebbich, S (2012). Distribution and abundance of Darwin’s finches and other land birds on Santa Cruz Island, Galápagos: evidence for declining populations. Oryx 46, 78–86.
| Distribution and abundance of Darwin’s finches and other land birds on Santa Cruz Island, Galápagos: evidence for declining populations.Crossref | GoogleScholarGoogle Scholar |
Ehlers Smith, DAE, Si, X, Ehlers Smith, YCE, and Downs, CT (2018). Seasonal variation in avian diversity and tolerance by migratory forest specialists of the patch-isolation gradient across a fragmented forest system. Biodiversity and Conservation 27, 3707–3727.
| Seasonal variation in avian diversity and tolerance by migratory forest specialists of the patch-isolation gradient across a fragmented forest system.Crossref | GoogleScholarGoogle Scholar |
Fancy, SG (1997). A new approach for analyzing bird densities from variable circular-plot counts. Pacific Science 51, 107–114.
Fancy, SG, Lusk, MR, and Grout, D (1999). Status of the Mariana crow population on Rota, Mariana Islands. Micronesica 32, 3–10.
Ferger, SW, Schleuning, M, Hemp, A, Howell, KM, and Böhning-Gaese, K (2014). Food resources and vegetation structure mediate climatic effects on species richness of birds. Global Ecology and Biogeography 23, 541–549.
| Food resources and vegetation structure mediate climatic effects on species richness of birds.Crossref | GoogleScholarGoogle Scholar |
Givnish, TJ (1999). On the causes of gradients in tropical tree diversity. Journal of Ecology 87, 193–210.
| On the causes of gradients in tropical tree diversity.Crossref | GoogleScholarGoogle Scholar |
Gorman, ML (1975). Habitats of the land-birds of Viti Levu, Fiji Islands. Ibis 117, 152–161.
| Habitats of the land-birds of Viti Levu, Fiji Islands.Crossref | GoogleScholarGoogle Scholar |
Government of Fiji and United Nations Development Programme (2015) Project document: implementing a ridge to reef approach to preserve ecosystem services, sequester carbon, improve climate resilience and sustain livelihoods in Fiji. Available at https://open.undp.org/projects/00083111 [Accessed 20 April 2020]
Hammer, Ø, Harper, DA, and Ryan, PD (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4, 9.
Howland, BWA, Stojanovic, D, Gordon, IJ, Radford, JQ, Manning, AD, and Lindenmayer, DB (2016). Birds of a feather flock together: using trait-groups to understand the effect of macropod grazing on birds in grassy habitats. Biological Conservation 194, 89–99.
| Birds of a feather flock together: using trait-groups to understand the effect of macropod grazing on birds in grassy habitats.Crossref | GoogleScholarGoogle Scholar |
Hurlbert, AH (2004). Species-energy relationships and habitat complexity in bird communities. Ecology Letters 7, 714–720.
| Species-energy relationships and habitat complexity in bird communities.Crossref | GoogleScholarGoogle Scholar |
IBM Corp. (2013) ‘IBM SPSS Statistics for Windows, Version 22.0’ (IBM Corp: Armonk, NY)
Innes, J, Kelly, D, Overton, JM, and Gillies, C (2009). Predation and other factors currently limiting New Zealand forest birds. New Zealand Journal of Ecology 34, 86–114.
Institute of Applied Sciences (2019) Tuva BioRAP report. Activity 1.1.2.11. Fiji Ridge to Reef Project. Institute of Applied Sciences, University of the South Pacific, Suva, Fiji.
Jackson, DB, and Jit, R (2007). Population densities and detectability of 3 species of Fijian forest birds. Notornis 54, 99–111.
Jayapal, R, Qureshi, Q, and Chellam, R (2009). Importance of forest structure versus floristics to composition of avian assemblages in tropical deciduous forests of Central Highlands, India. Forest Ecology and Management 257, 2287–2295.
| Importance of forest structure versus floristics to composition of avian assemblages in tropical deciduous forests of Central Highlands, India.Crossref | GoogleScholarGoogle Scholar |
Keppel, G, Peters, S, Taoi, J, Raituku, N, and Thomas-Moko, N (2022). The threat by the invasive African tulip tree, Spathodea campanulata P.Beauv., for the critically endangered Fijian tree, Pterocymbium oceanicum A.C.Sm.; revisiting an assessment based on expert knowledge after extensive field surveys. Pacific Conservation Biology 28, 164–173.
| The threat by the invasive African tulip tree, Spathodea campanulata P.Beauv., for the critically endangered Fijian tree, Pterocymbium oceanicum A.C.Sm.; revisiting an assessment based on expert knowledge after extensive field surveys.Crossref | GoogleScholarGoogle Scholar |
Kier, G, Kreft, H, Lee, TM, Jetz, W, Ibisch, PL, Nowicki, C, Mutke, J, and Barthlott, W (2009). A global assessment of endemism and species richness across island and mainland regions. Proceedings of the National Academy of Sciences of the United States of America 106, 9322–9327.
| A global assessment of endemism and species richness across island and mainland regions.Crossref | GoogleScholarGoogle Scholar | 19470638PubMed |
Koop, JAH, Kim, PS, Knutie, SA, Adler, F, and Clayton, DH (2016). An introduced parasitic fly may lead to local extinction of Darwin’s finch populations. Journal of Applied Ecology 53, 511–518.
| An introduced parasitic fly may lead to local extinction of Darwin’s finch populations.Crossref | GoogleScholarGoogle Scholar |
Kopij, G (2019). Population densities and community structure of birds breeding in a suburban wooded grassland in the Highveld of Lesotho. Vestnik Zoologii 53, 155–164.
| Population densities and community structure of birds breeding in a suburban wooded grassland in the Highveld of Lesotho.Crossref | GoogleScholarGoogle Scholar |
Kormos CF, Mackey B, DellaSala DA, Kumpe N, Jaeger T, Mittermeier RA, Filardi C (2017) Primary forests: definition, status and future prospects for global conservation. In ‘The encyclopedia of the anthropocene’. (Eds DA DellaSala, MI Goldstein) vol. 2, pp. 31–41. (Elsevier: Oxford)
Korňan, M, and Kropil, R (2014). What are ecological guilds? Dilemma of guild concepts. Russian Journal of Ecology 45, 445–447.
| What are ecological guilds? Dilemma of guild concepts.Crossref | GoogleScholarGoogle Scholar |
Lack D (1973) The numbers of species of hummingbirds in the West Indies. Evolution, 326–337.
| Crossref |
Latham M (1983). Origin of talasiga formation. In ‘The Eastern Islands of Fiji’. (Eds M Latham, HC Brookfield) pp. 129–141. (UNESCO/UNFPA: Paris)
Lomolino, MV (2001). Elevation gradients of species-density: historical and prospective views. Global Ecology and Biogeography 10, 3–13.
| Elevation gradients of species-density: historical and prospective views.Crossref | GoogleScholarGoogle Scholar |
MacArthur RH, Wilson EO (1967) ‘The theory of island biogeography.’ (Princeton University Press: New Jersey)
Malani M (2002) Ecotourism in Fiji. In ‘Linking green productivity to ecotourism: experiences in the Asia-Pacific Region’. (Ed. T. Hundloe) pp. 45–55. (Asian Productivity Organization)
McCain, CM (2009). Global analysis of bird elevational diversity. Global Ecology and Biogeography 18, 346–360.
| Global analysis of bird elevational diversity.Crossref | GoogleScholarGoogle Scholar |
Mueller-Dombois D, Bridges KW, Carson H (Eds) (2012) ‘Island ecosystems: biological organization in selected Hawaiian communities.’ US/IBP synthesis series 15. (Blackburn Press: Caldwell, New Jersey)
Mueller-Dombois D, Fosberg FR (1998) ‘Vegetation of the tropical Pacific Islands.’ (Springer: New York)
Naikatini A (2009) Monitoring comparative and temporal variation in the landbirds of Vago-Savura Forest Reserve, a native lowland rainforest in South East Viti Levu, Fiji. MSc thesis, University of the South Pacific, Suva, Fiji.
Nally, RM (1994). Habitat-specific guild structure of forest birds in south-eastern Australia: a regional scale perspective. Journal of Animal Ecology 63, 988–1001.
| Habitat-specific guild structure of forest birds in south-eastern Australia: a regional scale perspective.Crossref | GoogleScholarGoogle Scholar |
Neoh, KB, Bong, LJ, Muhammad, A, Itoh, M, Kozan, O, Takematsu, Y, and Yoshimura, T (2017). The effect of remnant forest on insect successional response in tropical fire-impacted peatland: a bi-taxa comparison. PLoS ONE 12, e0174388.
| The effect of remnant forest on insect successional response in tropical fire-impacted peatland: a bi-taxa comparison.Crossref | GoogleScholarGoogle Scholar | 28334021PubMed |
Newmark, WD, Mkongewa, VJ, and Sobek, AD (2010). Ranging behavior and habitat selection of terrestrial insectivorous birds in north-east Tanzania: implications for corridor design in the Eastern Arc Mountains. Animal Conservation 13, 474–482.
| Ranging behavior and habitat selection of terrestrial insectivorous birds in north-east Tanzania: implications for corridor design in the Eastern Arc Mountains.Crossref | GoogleScholarGoogle Scholar |
O’Connor, JA, Sulloway, FJ, and Kleindorfer, S (2010). Avian population survey in the Floreana highands: Is Darwin’s medium tree finch declining in remnant patches of Scalesia forest? Bird Conservation International 20, 343–353.
| Avian population survey in the Floreana highands: Is Darwin’s medium tree finch declining in remnant patches of Scalesia forest?Crossref | GoogleScholarGoogle Scholar |
Perea, R, Venturas, M, and Gil, L (2014). Seed predation on the ground or in the tree? Size-related differences in behavior and ecology of granivorous birds. Acta Ornithologica 49, 119–130.
| Seed predation on the ground or in the tree? Size-related differences in behavior and ecology of granivorous birds.Crossref | GoogleScholarGoogle Scholar |
Reid, E, Naikatani, A, Keppel, G, and Kleindorfer, S (2019). The conservation value of secondary vegetation for Fijian woodland birds. Emu 119, 286–295.
| The conservation value of secondary vegetation for Fijian woodland birds.Crossref | GoogleScholarGoogle Scholar |
Sam, K, Koane, B, Bardos, DC, Jeppy, S, and Novotny, V (2019). Species richness of birds along a complete rain forest elevational gradient in the tropics: Habitat complexity and food resources matter. Journal of Biogeography 46, 279–290.
| Species richness of birds along a complete rain forest elevational gradient in the tropics: Habitat complexity and food resources matter.Crossref | GoogleScholarGoogle Scholar |
Sam, K, Koane, B, Sam, L, Mrazova, A, Segar, S, Volf, M, Moos, M, Simek, P, Sisol, M, and Novotny, V (2020). Insect herbivory and herbivores of Ficus species along a rain forest elevational gradient in Papua New Guinea. Biotropica 52, 263–276.
| Insect herbivory and herbivores of Ficus species along a rain forest elevational gradient in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Sayer, CA, Bullock, JM, and Martin, PA (2017). Dynamics of avian species and functional diversity in secondary tropical forests. Biological Conservation 211, 1–9.
| Dynamics of avian species and functional diversity in secondary tropical forests.Crossref | GoogleScholarGoogle Scholar |
Şekercioğlu, ÇH, Mendenhall, CD, Oviedo-Brenes, F, Horns, JJ, Ehrlich, PR, and Daily, GC (2019). Long-term declines in bird populations in tropical agricultural countryside. Proceedings of the National Academy of Sciences of the United States of America 116, 9903–9912.
| Long-term declines in bird populations in tropical agricultural countryside.Crossref | GoogleScholarGoogle Scholar | 31036662PubMed |
Şekercioğlu, ÇH, Primack, RB, and Wormworth, J (2012). The effects of climate change on tropical birds. Biological Conservation 148, 1–18.
| The effects of climate change on tropical birds.Crossref | GoogleScholarGoogle Scholar |
Smith AC (1979) ‘Flora Vitiensis Nova.’ Vol. 1. (Pacific Tropical Botanical Garden: Lawai, Kauai, Hawaii)
Soares, FC, Panisi, M, Sampaio, H, Soares, E, Santana, A, Buchanan, GM, Leal, AI, Palmeirim, JM, and de Lima, RF (2020). Land-use intensification promotes non-native species in a tropical island bird assemblage. Animal Conservation 23, 573–584.
| Land-use intensification promotes non-native species in a tropical island bird assemblage.Crossref | GoogleScholarGoogle Scholar |
Somasundaram, S, and Vijayan, L (2008). Foraging behaviour and guild structure of birds in the montane wet temperate forest of the Palni Hills, South India. Podoces 3, 79–91.
Stamps, JA (1994). Territorial behavior: testing the assumptions. Advances in the Study of Behavior 23, 173–232.
| Territorial behavior: testing the assumptions.Crossref | GoogleScholarGoogle Scholar |
Steadman, DW (1993). Biogeography of Tongan birds before and after human impact. Proceedings of the National Academy of Sciences of the United States of America 90, 818–822.
| Biogeography of Tongan birds before and after human impact.Crossref | GoogleScholarGoogle Scholar | 11607357PubMed |
Steadman, DW (1995). Prehistoric extinctions of Pacific Island birds: biodiversity meets zooarchaeology. Science 267, 1123–1131.
| Prehistoric extinctions of Pacific Island birds: biodiversity meets zooarchaeology.Crossref | GoogleScholarGoogle Scholar | 17789194PubMed |
Tabudravu MSV (2009) Spatial relationships between forest birds and habitats in degraded and nondegraded forests in IBA FJ 10. MSc thesis, University of the South Pacific, Suva.
Taylor, MDRB, Salazar, JLR, Enríquez, P, León-Cortés, JL, and García-Estrada, C (2017). Variation in hierarchical guild structure between two bird assemblages of a wetland in the Mexican Pacific. Revista de Biología Tropical 65, 1540–1553.
| Variation in hierarchical guild structure between two bird assemblages of a wetland in the Mexican Pacific.Crossref | GoogleScholarGoogle Scholar |
Thaman, R (1996). The biodiversity of Koroyanitu National Park. Domodomo 10, 28–51.
Tuiwawa M, Sakiti-Waqa H, Tuiwawa S, Naikatini A, Copeland L, Rashni S (2018) ‘Colo-i-Suva Forest Park Wildlife.’ (The University of the South Pacific Press: Suva, Fiji)
Tuiwawa, SH, and Keppel, G (2012). Species diversity, composition and the regeneration potential of native plants at the Wainiveiota Mahoganu Plantation, Viti Levu, Fiji Islands. The South Pacific Journal of Natural and Applied Sciences 30, 51–57.
| Species diversity, composition and the regeneration potential of native plants at the Wainiveiota Mahoganu Plantation, Viti Levu, Fiji Islands.Crossref | GoogleScholarGoogle Scholar |
Vale, TR, Parker, AJ, and Parker, KC (1982). Bird communities and vegetation structure in the United States. Annals of the Association of American Geographers 72, 120–130.
| Bird communities and vegetation structure in the United States.Crossref | GoogleScholarGoogle Scholar |
Varun, K, and Dutta, S (2020). Understanding land-use responses of bird communities in an arid ecosystem of India. bioRxiv , .
| Understanding land-use responses of bird communities in an arid ecosystem of India.Crossref | GoogleScholarGoogle Scholar |
Verner, J (1984). The guild concept applied to management of bird populations. Environmental Management 8, 1–13.
| The guild concept applied to management of bird populations.Crossref | GoogleScholarGoogle Scholar |
Waqa-Sakiti, HVF, Hodge, S, and Winder, L (2018). Distribution of long-horn beetles (Cerambycidae: Coleoptera) within the Fijian archipelago. The South Pacific Journal of Natural and Applied Sciences 36, 1–8.
| Distribution of long-horn beetles (Cerambycidae: Coleoptera) within the Fijian archipelago.Crossref | GoogleScholarGoogle Scholar |
Waqaisavou T (1997) Parks, reserves and tourism in Fiji: native landowner attitude and involvement. PhD thesis, Victoria University of Technology, Melbourne.
Watling D (2001) ‘A guide to the birds of Fiji and Western Polynesia.’ (Environmental Consultants: Suva, Fiji)
Xu, Y, Lin, S, He, J, Xin, Y, Zhang, L, Jiang, H, and Li, Y (2017). Tropical birds are declining in the Hainan Island of China. Biological Conservation 210, 9–18.
| Tropical birds are declining in the Hainan Island of China.Crossref | GoogleScholarGoogle Scholar |