Who’s been lost from the landscape? Identifying missing terrestrial fauna to inform urban rewilding
Mareshell Wauchope A * , Patrick B. Finnerty
A * , Patrick B. Finnerty  A , Jennifer C. Pierson B , Peter B. Banks A , Alexandra J. R. Carthey C and Thomas M. Newsome A
A , Jennifer C. Pierson B , Peter B. Banks A , Alexandra J. R. Carthey C and Thomas M. Newsome A
A
B
C
Abstract
Urbanisation has driven native species declines and local extinctions, eroding ecological processes. However, urban areas with remnant native vegetation patches may offer opportunities for native wildlife rewilding.
We sought to identify potential urban rewilding candidates across a target landscape. We then examined their ecological traits to understand if those traits were shared by rewilding candidate species.
We developed and applied a decision framework to occurrence records of terrestrial, non-volant mammals and reptiles to identify two status groups: (1) extant; and (2) rewilding candidates. Data on four ecological traits (diet, size, habit, and habitat) were then analysed using multivariate statistics.
We identified 39 mammal and 47 reptile species historically present, with up to 62% of mammals and 93% of reptiles persisting since 2000. Eighteen species were categorised as locally missing, 11 of which are threatened. Two families (Canidae and Potoridae) were found to be locally extinct. Foraging habit (P-value = 0.047) and diet breadth (P-value = 0.024) were significantly different between our status groups.
Locally missing and/or declined species represent potential urban rewilding candidates with broadest geographic applicability in patchy urban contexts, and align with a rewilding goal to restore pre-disturbance assemblages. In an urban context, where the list of species lost might be high, additional factors require consideration to aid rewilding candidate prioritisation in resource constrained environments.
The decision framework efficiently pinpointed an initial suite of urban rewilding candidates. This framework can be applied by urban conservation managers. Trait analyses highlighted vulnerabilities critical to informing development of successful urban rewilding strategies.
Keywords: Atlas occurrence data, biodiversity loss, decision framework, mammals, reptiles, urbanisation, urban rewilding.
Introduction
Since the Late Pleistocene, there has been a period of unprecedented, rapid species loss, proclaimed the sixth mass extinction (Wake and Vredenburg 2008). Both mammals and reptiles are impacted by this extinction crises, with the former particularly hard hit (Davis et al. 2018). Since 1900, at least 53 squamates are estimated to have been extirpated globally, and 350 mammal species are estimated to have been extirpated since the late Pleistocene (Alroy 2015; Davis et al. 2018; Andermann et al. 2020). For mammals, this equates to the loss of 2.5 billion years of evolutionary history (Davis et al. 2018). Biodiversity loss has left many ecosystems profoundly altered (Soulé and Noss 1998) because species diversity regulates many ecosystem-level processes. For example, reductions in biodiversity have been linked to lowered ecosystem productivity (e.g. through trophic cascades), altered habitats (e.g. through the loss of key ecosystem engineers, such as beavers, resulting in changes to habitat formation), and lowered efficiency by which communities capture resources (e.g. nutrients, water, light, prey) (Cardinale et al. 2012). Ongoing biodiversity declines are predicted with 25% of mammals and 21% of reptiles categorised as threatened (Cox et al. 2022). Practitioners are responding to this emergency with a multitude of approaches spanning conservation biology and reintroduction biology, to restoration ecology. As biodiversity continues a downward spiral, exploring new conservation approaches and frontiers remains critical (Murphy and van Leeuwen 2021).
Rewilding is a potential conservation response to the biodiversity crisis (Jepson 2019). Initially characterised by the three Cs (i.e. cores, corridors, and carnivores), rewilding was positioned as a response to the emergence of the principles of island biogeography and the observation that the current extinction crisis had left ecosystems significantly altered and lacking in large keystone species (Soulé and Noss 1998). Since then, the term rewilding has quickly evolved. It has branched into use across a diversity of globally implemented conservation projects and experienced rapid growth in use, as evidenced by rising numbers of publications citing ‘rewilding’ (Pettorelli et al. 2019). Broadly, rewilding aims to restore ecological processes and functions, either passively or actively via human-initiated fauna translocations (Carver et al. 2021). Rewilding projects have predominantly occurred in large landscapes, in settings that remain relatively ‘wild’, or in human-altered areas that have been abandoned, left to undergo what has been termed ‘passive rewilding’ (Pettorelli et al. 2019; Sweeney et al. 2019). More recently, however, there has been growing interest in pursuing rewilding projects in people-dominated landscapes including cities (Van Heezik and Seddon 2018; Maller et al. 2019).
Although urbanisation has significantly disrupted the natural environment (McKinney 2002), rewilding urban areas via faunal reintroduction potentially presents unique opportunities for both biodiversity and humans. Arguably, cities could be good places for species protection as they have been found to support a diversity of both endemic and nonnative species (Lepczyk et al. 2017). Moreover, rewilding species into formerly occupied remnant patches of urban native vegetation can infill both local- and landscape-scale distribution gaps that may otherwise remain empty. This is particularly relevant for dispersal limited species due to challenges presented by a hostile urban landscape that may prevent them from naturally recolonising (Watson and Watson 2015; Leo et al. 2022). Restoring species to urban patches can also restore declining ecological processes such as pollination, pest control, and fire suppression (Seddon et al. 2014; Van Heezik and Seddon 2018; O’Rourke et al. 2020; Ryan et al. 2020). The more active rewilding approach of reintroducing historically present species likely presents a safer starting point to restore ecological function than other rewilding practices, such as reintroduction of surrogates, with lower risk of undesirable outcomes and mismatches with the local environment (IUCN/SSC 2013). Furthermore, exposure to nature has been linked to improvements in human well-being, both physical and psychological, and can also shift people towards pro-environmental behaviours (Berto 2014; Mackay and Schmitt 2019). Rewilding projects in urban areas thus offer opportunities to engage and educate local communities, and the possibility of nature exposure through hands-on conservation experiences (Parker 2008). Yet, only 38 animal translocations have been documented in urban areas worldwide (Brown et al. 2024), and within the IUCN Reintroduction Perspectives series (2010–2016), only 6 of 181 animal reintroductions occurred in urban areas (Van Heezik and Seddon 2018), highlighting a gap in rewilding efforts globally.
Rewilding projects in Australia echo these global trends. This continent has not been immune to the extinction crisis. At least 61 endemic species have gone extinct since European colonisation of Australia (10 invertebrates, 1 fish, 4 frogs, 3 reptiles, 9 birds, and 34 mammals) (Woinarski et al. 2019). Several traits have been identified and associated with these declines and extinctions, including body size (e.g. critical weight range (35–5500 g) mammals), ground-dwelling habits, and diet (e.g. carnivores) (Lunney et al. 1997). Mammals have been hardest hit, with their losses attributed to habitat loss and predation by introduced feral cats (Felis catus) and red foxes (Vulpes vulpes) (Woinarski et al. 2015). As such, rewilding initiatives in Australia have prioritised the conservation translocation of rare or threatened mammals into predator-free safe havens, including offshore islands and remote fenced areas (Sweeney et al. 2019). Only a handful of rewilding projects have been initiated in, or near to, urban settlements (Ryan et al. 2020; Dickman and La Marca 2021; Leo et al. 2022). For example, in south-eastern Australia, a fenced feral predator-free area has been established in urban western Sydney to reintroduce locally extirpated species, including eastern quoll (Dasyurus viverrinus) and eastern bettong (Bettongia gaimardi gaimardi) (Dickman and La Marca 2021). Three small mammal species, (bush rat Rattus fuscipes, eastern pygmy possum Cercartetus nanus and brown antechinus Antichinus stuartii) have also been reintroduced to a national park on the edge of Sydney, the most populous city in Australia (Leo et al. 2022). With 76% of people inhabiting major cities in Australia (Hill et al. 2021), urban rewilding presents a unique opportunity to restore biodiversity and repair human-nature connections (Maller et al. 2019).
To initiate further rewilding efforts in Australia, including outside of predator-free areas and within peopled landscapes, a key first step is to determine which historically present species are now locally missing (Morris et al. 2017). Species occurrence data is growing, particularly in urban areas. When species records are pooled in databases, they can provide a sizable dataset that can be used to evaluate species’ historical likelihood and persistence (Van Der Ree 2004; Major and Parsons 2010). Indeed, within in a patchy urban context, species or functional groups that may be missing across the entire landscape may present an initial suite of rewilding candidates with the broadest geographical scope for rewilding opportunities. Furthermore, this approach can identify candidates that are not limited by threat status. In Oceania, most conservation efforts have focused on rare and threatened species, yet common native species are fundamental to the structure of ecosystems and have also undergone substantial declines (Gaston and Fuller 2008). In a heavily altered urban landscape, working to ‘keep common species common’, may represent the best mantra for restoring ‘wildness’ (Watson and Watson 2015).
With a paucity of urban rewilding studies, our goal was to identify species that were historically likely to occur but are currently missing across an urban landscape in northern Sydney – generating an initial list of potential urban rewilding candidates – and consider how ecological traits influence their response to urbanisation. This is a critical step in the development of impactful and successful rewilding strategies in an urban setting (Hahs et al. 2023). To address this goal, we sought to answer the following questions: (1) which non-volant mammal and reptile species were likely to be present historically (i.e. pre-2000 and/or pre-European colonisation) in the landscape-scale study area of northern Sydney?; (2) which of these historically present species might be considered missing or declined from the study area today?; and (3) do missing and declined species share size, diet, habit (i.e. dominant foraging strata) and/or habitat traits? Our target landscape has remnant native vegetation within the urban matrix that has undergone vegetation restoration and feral predator (red fox) management over the past two decades, increasing the potential suitability for reintroductions of missing common native fauna species. To our knowledge, there has been no recent singular study spanning this urban landscape that systematically evaluates which historically present native fauna species are persisting and which may now be considered locally missing. We use the results to explore options for restoring species assemblages and ecological processes that may enhance or support local native biodiversity in northern Sydney and urban areas more broadly.
Materials and methods
Study area
The study area, situated north of the central business district (CBD) of Sydney, Australia, comprises seven Local Government Areas (LGAs) (Fig. 1). Covering ~110,000 ha, it stretches from Sydney Harbour in the south to the Hawkesbury River in the north, west to Lane Cove River and an arbitrary boundary along the Great Northern Road. The area is characterised by a strong urbanisation gradient moving from the south, with highest population densities around coastal areas, to peri-urban outskirts fringed by National Parks in the north (see Supplementary material Fig. S1). The study area contains 11 National Parks protecting ~44,000 ha of native vegetation, with a further ~500 council managed vegetation patches, ranging in size from <1 ha to ~300 ha, dispersed throughout the urban matrix. The National Parks in the study area were retained for this study as they are considered ‘urban impacted’ due to the relative ease of human access and high visitation compared with other protected areas (four of the parks were in the ‘top eight most visited parks in NSW’ in 2022 (DPE 2023)). The area is predominantly situated on Hawkesbury Sandstone, with some areas overlayed by shales, such as Narrabeen shales (Conroy et al. 2022). This geology contributes to the diversity of vegetation communities dominated by Sydney Coastal Dry Sclerophyll Forests, Sydney Coastal Heaths and North Coast Wet Sclerophyll Forests (DCCEEW 2016).
Fauna data
Occurrence records from within the study area for reptiles and non-volant mammals, taxa both dispersal-limited and greatly impacted by the extinction crises, were extracted from the Atlas of Living Australia (ALA) in October 2023 (ALA 2023). ALA incorporates data from several collecting institutions, environmental consultants and community groups, incorporating various occurrence record databases for the area, for example, the NSW BioNet, FrogID, Australian Museum, and iNaturalist. ALA records have various levels of reliability and there are variable levels of data associated with a record. Therefore, data were filtered to exclude spatially suspect records, pre-1700 records, duplicate records, and records where the year (date) was not provided, or species’ scientific name was missing or tentative (e.g. identified only to broad taxa – Marsupial, Macropod – or genus). Subspecies were grouped as a single species and marine species were deleted.
Locally missing species decision framework
A decision-making framework was developed to categorise native species’ records obtained from ALA into four categories: (1) extant; (2) extant, data deficient; (3) extant, declined; and (4) locally missing (Fig. 2). These categories were then combined into two status groups: (1) extant (extant and extant, data deficient); and (2) rewilding candidates (extant, declined and locally missing). Species in the latter status group were considered potential rewilding candidate species as they represent historically present species of relatively higher conservation value where they are currently either likely to be absent or declined. Prior to evaluating a species’ category, each species’ ALA records were organised by time period. ALA records dated prior to 2000 (i.e. 1788–1999) were assigned to be ‘historically present’ and those dated post 2000 (i.e. 2000–2023) were categorised as ‘currently present’ (Van Der Ree 2004).
Historically present
Reconstructing assemblages for Sydney from the time of European settlement through to the early 20th century has been acknowledged as being ‘almost completely speculative’, with historical assessments of status at the time of European settlement made using ‘educated guesses’ (Recher et al. 1993). As such, additional lines of evidence were used to aid inferences about historical status: (1) species’ known ecosystem was historically present in northern Sydney; e.g. excluded montane or arid species; and (2) published accounts on historical vertebrate fauna suggest the species was historically present in the study area or the broader Sydney Basin Bioregion e.g. (Recher et al. 1993; White and Burgin 2004; Burbidge et al. 2008; Cogger 2010; Recher 2010; Shea 2010; Dickman and La Marca 2021; Conroy et al. 2022). The latter inclusion of possible presence in the bioregion enabled a broader area to be considered from which to evaluate potential for historical presence (Ward et al. 2022; Smith et al. 2024). Where available, the type of record (e.g. human observation, preserved specimen) was also considered, such that if a record was a preserved specimen its veracity was given more weight in establishing a species’ historical presence. Using this approach, if a species had nil occurrence records pre-2000 (omitted from being ‘historically present’), yet had ALA records since 2000, and additional literature indicated likelihood of historical presence, then it was re-assigned to the ‘historically present’ classification (for a summary of additional considerations and deviations from ALA data driven decisions, see Supplementary material Table S1).
Currently present
Like the approach taken when establishing historical presence, additional lines of evidence were used to support the ‘currently present’ designation and to identify possible errors in the occurrence records. Consideration was given to: (1) current species distribution maps using field guides or literature; and (2) the source of the occurrence record (i.e. collection institute, name of observer) (where available), with greater confidence in species identification given to scientific datasets (e.g. NPWS) over community/citizen science databases (WIRES, iNaturalist). For instance, if a species had nil ‘historically present’ occurrence records and some ALA records since 2000 and if this species’ current range did not overlap with the study area, then it was not considered ‘currently present’ and was instead assigned to be ‘never present, likely error’, and excluded from further analysis (Supplementary material Table S1). For a list of species, provided along with the citation/rationale underpinning a status category decision, see Supplementary material Tables S2 and S3.
Status categories
In endeavouring to determine which species are extant, several factors influence the level of confidence we might have in making such an assertion. Using data associated with ALA records, extant species (i.e. ‘historically present’ and ‘currently present’) were reviewed giving consideration to: (1) total number of records; (2) year of most recent record; and (3) geographic spread (Fig. 3; Supplementary material Table S1). With large variability in the number of occurrence records within and between our taxonomic groups, we used each taxonomic group’s lower inter-quartile range (IQR1) value of the number of records to represent how many records was considered ‘low’, as IQR better accounts for the outliers in record values within each taxonomic group (Wauchope-Drumm et al. 2020). Species were considered to have a low numbers of records since 2000 if they had fewer than the lower inter-quartile range value number of all records: mammals: IQR1 = 9; reptiles: IQR1 = 44. To assess geographic spread, the occurrence records were mapped in QGIS and visually examined. Species with records spread out across the study area were generally considered ‘well dispersed’. If a species’ records were clumped or missing from multiple LGAs, National Parks and larger Council managed reserves (i.e. >100 ha such as, Manly Dam, St. Ives Wildflower Garden) these were considered to have geographic gaps. Species that had some, or all, of these characteristics remained classified as ‘extant’ but noted as ‘extant, data deficient’ (see Supplementary material Table S1). For instance, we would have less confidence in the extant status of a species that has only a few, old records, that are spatially close together (i.e. clumped) (Shea 2010). In these cases, additional survey work/investigations may be worthwhile to validate their status.
The combinations of relationships between the factors (i.e. number of records, age of record, geographic distribution) can influence the confidence we have in assigning a species to be ‘extant’. For example, if a species had a low number of records, that were also old and geographically clumped we would have low confidence in their being extant, and thus we categorised them as ‘extant, data deficient’.

Species additions were those species that had some records in the period since 2000, but nil records historically, and were assessed as not being historically present using published accounts per above. However, if the species’ current known or predicted range per field guides or literature did not coincide with the study area, it was categorised as ‘never present, probable error’ and removed from further analysis (Menkhorst and Knight 2011; Wilson and Swan 2017).
Species categorised as extant (per above) that had fewer occurrence records in the current time period since 2000 than in the historical time period were classified as ‘extant, declined’.
Species classified as ‘historically present’ but had no records since 2000 and distributions that overlapped the study area were categorised as ‘Locally missing’. Species with low numbers of records since 2000 (i.e. possibility of being extant), were further scrutinised in the context of two factors: (1) individual records examined; and (2) published accounts evaluating the status of fauna in the study area and/or greater Sydney region (see literature listed above within ‘Historically present’ methods). Where records were dubious and/or literature identified a species as locally (or regionally) extinct, it was reclassified from ‘extant’ to ‘locally missing’. In these instances, the rationale and citation underpinning such a decision is in Supplementary material Tables S2 and S3.
Ecological traits
Four broad ecological traits were examined to enable comparisons across the two taxonomic groups: (1) body size; (2) diet; (3) habit (i.e. dominant foraging stratum); and (4) habitat (Lunney et al. 1997; Hu et al. 2020). Selection of trait categories followed Dickman et al. (2001), with changes relevant to reflect the species’ pool under analyses; e.g. addition of a >5 kg size category due to species in our study area being in this size class (see Supplementary material Table S4 for ecological trait categories and their data sources). These traits were selected as they have been empirically linked to species’ responses to disturbances, provision of ecosystem services and used to assess drivers underlying a species’ conservation status (Lunney et al. 1997; Hu et al. 2020).
To evaluate associations between the status categories and ecological traits, the status categories were pooled into two groups: (1) rewilding candidates (locally missing/extant, declined); and (2) extant (extant/extant, data deficient). The analytical approach undertaken in Dickman et al. (2001) was then followed. First, associations between status and traits were explored using chi-squared contingency analyses; however, due to our small number of observations, the Fisher’s Exact Test was used (Clinton and Le Beouf 1993). Second, associations between status categories and traits were explored using non-metric multidimensional scaling (nMDS, (Clarke 1993)) with significance testing carried out using PERMANOVA on 5000 random permutations of the distance matrix data. As trait data were mixed (i.e. categorical, binary and continuous), the Gower distance was applied to trait data and a ‘gower distance matrix’ generated in R software using the ‘daisy’ function from the cluster package Maechler et al. (2023). Initial nMDS analysis on all traits found stress values to be within acceptable range <0.3.
Results
Insights from ALA occurrence records
There were 160,680 occurrence records of non-volant mammals (hereafter referred to as ‘mammals’) and 21,359 occurrence records of terrestrial reptiles (hereafter referred to as ‘reptiles’) across time (1788–2023) within the study area. The density of occurrence records was higher in areas with higher urban density (Supplementary material Fig S1).
Within the period 1788–2023, the ALA dataset identified 41 native mammal species in the study area. Six of these species were deemed unlikely to ever have been present in the study area: (1) long-tailed planigale (Planigale ingrami); (2) mountain pygmy possum (Burramys parvus); (3) northern brown bandicoot (Isoodon macrourus); (4) woylie (Bettongia pencillata); (5) red kangaroo (Osphranter rufus); and (6) mountain brushtail possum (Trichosurus cunninghami). These species either do not have suitable habitat (e.g. mountain pygmy possum occurs in alpine regions) and/or alternative sources indicated that their historical distributions (at the time of European settlement) were situated outside the study area (Recher et al. 1993; Burbidge et al. 2008; Yeatman and Groom 2012). Most remaining mammal species were ground-dwelling (70%) and critical weight range (i.e. 35–5500 g) (61%). The number of records for each species was highly variable, ranging from 71,493 records (swamp wallaby, Wallabia bicolor) to a single record (e.g. parma wallaby, Notamacropus parma), with an average of 4589 (IQR: 9–551) occurrence records (Supplementary material Table S2).
Within the same period, the ALA dataset included 92 native reptile species in the study area; however, 45 species were deemed unlikely to ever have been present in the study area. Alternative sources for these 45 species indicated that their historical distributions were situated outside the study area (Cogger 2010; Shea 2010) and current range maps did not overlap the study area (Wilson and Swan 2017). The remaining 47 species in this taxon had lower numbers of records per species, ranging from 3798 (eastern water dragon, Itellagama lesueruii) to a single record (mainland she-oak skink, Cyclodomorphus michaeli), with an average of 392 records (IQR: 44–273) (SM 4). Most were diurnal (n = 32) and in the Scincidae family (n = 18).
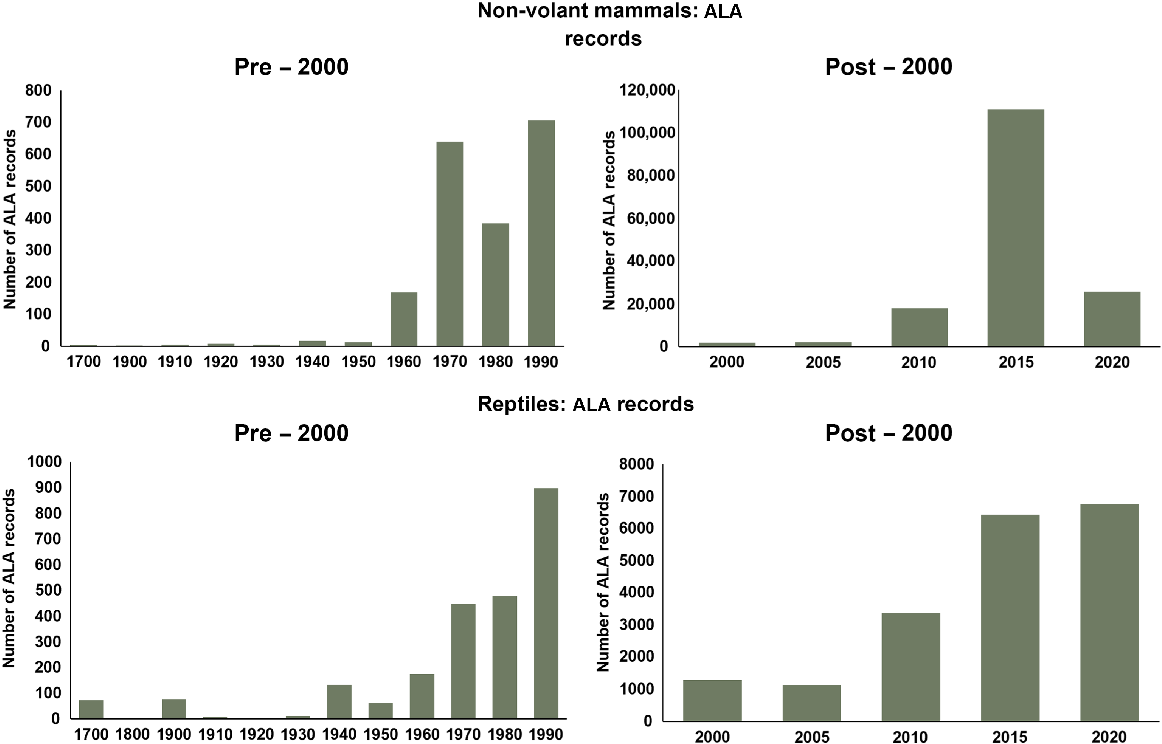
A temporal distribution of the records shows an increasing number of records for both taxa, highly biased towards the decades since 2000 (Fig. 4). There have been sizable increases in the volume of occurrence data, with 98% and 88% of mammal and reptile records respectively generated since 2000. In an urban landscape, this large growth in records does not necessarily imply changes to species’ abundances but is likely linked to increased sightings due to rising human population, environmental education, legislative obligations linked to development e.g. environmental impact statements and technological advances (i.e. smartphones, citizen science data collection applications).
Historical species
There were 39 mammal species, representing 15 families, and 47 reptile species, representing 11 families, assessed as being historically present within the study area. In nearly all instances, additional lines of inquiry supported the attribution of species to the historical category using ALA data. However, there were two exceptions: (1) yellow-footed antechinus (Antechinus flavipes); and (2) broad-headed snake (Hoplocephalus bungaroides).
The yellow-footed antechinus had historical ALA records; however, a secondary source had not identified it as being historically present in the Sydney Basin IBRA at the time of European settlement (Burbidge et al. 2008). The yellow-footed antechinus commonly occurs in coastal and sub-coastal areas in New South Wales and based on climatic criteria, are predicted to occur in the Greater Sydney region, as well as in the study area (Crowther 2002). There are reliable recent sightings in the Greater Sydney region (Mowat et al. 2015). The species has also been shown to occur in narrow sympatry with Antechinus stuartii in some areas in the Greater Sydney Region, including Yengo National Park in the north-west of the Sydney Basin (Mowat et al. 2015, Dickman and La Marca 2021). This information, in combination with the ALA records, four of which were listed as preserved specimens located in the Mosman LGA, supports the possibility of this species being historically present in the study area, as such, it was retained.
The broad-headed snake also had historical ALA records; however, secondary sources were conflicted with regards to evidence for the species’ historical status in the study area, with one source noting historical presence based upon interviews and another source considering these records to be dubious (White and Burgin 2004; Shea 2010). The broad-headed snake is endemic to the Greater Sydney region and strongly associated with sandstone habitats, which are present within the study area (Wilson and Swan 2017). The ALA records were preserved specimens located within the vicinity of Narrabeen, lending stronger support to the possibility this species was historically present in the study area.
Additional lines of inquiry identified a further four mammal species that may have been historically present within the study area despite there being nil ALA records across time for this locality. Two of these, black-striped wallaby (Notamacropus dorsalis) and red-necked pademelon (Thylogale thetis), have been reportedly identified in archaeological midden sites within the study area (Harris et al. 2010; Conroy et al. 2022). The other two species, the eastern quoll and eastern bettong, were both thought to be historically distributed along coastal areas in NSW (Burbidge et al. 2012). The eastern quoll can exploit a diversity of habitats and has been recorded in Sydney’s eastern suburbs (Dickman and La Marca 2021). The eastern bettong was found to have a distribution encompassing Sydney (Haouchar et al. 2016). Thus, they are included in the list of historically present species for completeness.
The 10 most recorded mammal species historically were dominated by small (<35 g, n = 1) and critical weight range mammals (35 g−5.5 kg, n = 7), the exceptions to this were the swamp wallaby and koala (Phascolarctos cinereus). All these species, except the koala, remained within the top 10 most recorded species since 2000 (Table 1). Historically for reptiles, the 10 most recorded species were predominantly small skinks and snakes. Only half remained the most recorded since 2000, a period that has seen a greater number of sightings of larger reptiles, such as elapids and lace monitors.
| Species | # ALA records pre-2000 | Species | # ALA records post-2000 | |
|---|---|---|---|---|
| Mammals | ||||
| Common brushtail possum Trichosurus vulpecula | 322 | Swamp wallaby Wallabia bicolor | 71,292 | |
| Common ringtail possum Pseudocheirus peregrinus | 282 | Common brushtail possum Trichosurus vulpecula | 22,555 | |
| Swamp wallaby Wallabia bicolor | 201 | Bush rat Rattus fuscipes | 22,398 | |
| Long-nosed bandicoot Perameles nasuta | 199 | Common ringtail possum Pseudocheirus peregrinus | 16,569 | |
| Brown antechinus Antechinus stuartii | 178 | Long-nosed bandicoot Perameles nasuta | 16,327 | |
| Koala Phascolarctos cinereus | 150 | Brown antechinus Antechinus stuartii | 4346 | |
| Bush rat Rattus fuscipes | 135 | Short-beaked echidna Tachyglossus aculeatus | 1851 | |
| Sugar glider Petaurus breviceps | 106 | Eastern pygmy possum Cercartetus nanus | 1674 | |
| Short-beaked echidna Tachyglossus aculeatus | 64 | Sugar glider Petaurus breviceps | 509 | |
| Southern brown bandicoot Isoodon obesulus | 58 | Southern brown bandicoot Isoodon obesulus | 430 | |
| Reptiles | ||||
| Dark-flecked garden sunskink Lampropholis delicata | 231 | Eastern water dragon Intellagama lesueurii | 3704 | |
| Eastern blue-tongue Tiliqua scincoides | 168 | Eastern blue-tongue Tiliqua scincoides | 3192 | |
| Copper-tailed skink Ctenotus taeniolatus | 130 | Red-bellied black snake Pseudechis porphyriacus | 1747 | |
| Weasel skink Saproscincus mustelinus | 130 | Diamond python Morelia spilota | 1449 | |
| Golden-crowned snake Cacophis squamulosus | 128 | Lace monitor Varanus varius | 1299 | |
| Three-toed skink Saiphos equalis | 127 | Green tree snake Dendrelaphis punctulatus | 1100 | |
| Blackish blind snake Anilios nigrescens | 108 | Eastern water skink Eulamprus quoyii | 884 | |
| Southern leaf-tailed gecko Phyllurus platurus | 105 | Golden-crowned snake Cacophis squamulosus | 744 | |
| Eastern water dragon Intellagama lesueurii | 94 | Dark-flecked garden sunskink Lampropholis delicata | 730 | |
| Pale-flecked garden skink Lampropholis guichenoti | 81 | Southern leaf-tailed gecko Phyllurus platurus | 432 | |
Species in bold represent the temporal changes within the groupings.
Species status
Most mammals and reptiles historically thought to be present within the study area remain extant (62% and 93% respectively). Nevertheless, both groups appear to have experienced declines in richness. Of those likely to be persisting, nearly 40% were either data deficient or potentially declining. Mammals were found have a higher number of species that could be confirmed as locally missing, likely due to additional lines of evidence confirming their local extirpation. In contrast, reptiles had higher numbers of species ‘extant, declined’. For both groups, when these categories were combined, they represented 51% and 32% of mammal and reptile species respectively (Table 2). There were no native species additions identified.
| Status | Mammals | Reptiles | |
|---|---|---|---|
| Extant | 35.9% | 57.5% | |
| Extant, data deficient | 12.8% | 10.6% | |
| Extant, declined | 12.8% | 25.5% | |
| Locally missing | 38.4% | 6.4% |
Within the study area, mammals have potentially lost representation of two entire families: (1) Canidae; and (2) Potoridae, with species missing or in decline within an additional six families. The Macropodidae family was found to have the highest numbers of locally missing species (Fig. 5), with the study area losing 75% of historically present species. For reptiles, all families continue to have representation. Nevertheless, seven families have species missing or in likely decline (Fig. 5).
Rewilding candidates
Fifteen mammal species and three reptiles were assigned to be ‘locally missing’. Ten of these mammals and one reptile were listed under either or both the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) (Commonwealth)/Biodiversity Conservation Act 2016 (NSW) (BC Act) (Table 3). All three reptiles had ALA evidence of historical presence yet nil records since 2000, however, this ALA data scenario only applied to two mammals: (1) short-eared possum (Trichosurus caninus); and (2) long-nosed potoroo (Potorous tridactylus). In all other instances, this status was assigned based on external evidence from the literature, combined with a general paucity of data and/or lower reliability of some data sources, e.g. community databases.
| Scientific name | Common name | Current status | EPBC Act | BC Act | |
|---|---|---|---|---|---|
| Phascogale tapoatafa | Brush-tailed phascogale | Locally missing | V | ||
| Dasyurus viverrinus | Eastern quoll | Locally missing | E | Ex | |
| Petauroides volans | Greater glider | Locally missing | E | E | |
| Trichosurus caninus | Short-eared possum | Locally missing | |||
| Aepyprymnus rufescens | Rufous bettong | Locally missing | V | ||
| Bettongia gaimardi gaimardi | Eastern bettong | Locally missing | EX | ||
| Potorous tridactylus | Long-nosed potoroo | Locally missing | V | V | |
| Notamacropus parma | Parma wallaby | Locally missing | V | V | |
| Notamacropus rufogriseus | Red-necked wallaby | Locally missing | |||
| Osphranter robustus | Common wallaroo | Locally missing | |||
| Petrogale penicillata | Brush-tailed rock-wallaby | Locally missing | V | E | |
| Notamacropus dorsalis | Black-striped wallaby | Locally missing | E | ||
| Thylogale thetis | Red-necked pademelon | Locally missing | |||
| Pseudomys gracilicaudatus | Eastern chestnut mouse | Locally missing | V | ||
| Canis lupus | Dingo | Locally missing | |||
| Antechinus flavipes | Yellow-footed antechinus | Extant, declined | |||
| Dasyurus maculatus | Spotted-tailed quoll | Extant, declined | E | V | |
| Planigale maculata | Common planigale | Extant, declined | V | ||
| Sminthopsis murina | Common dunnart | Extant, declined | |||
| Phascolarctos cinereus | Koala | Extant, declined | E | E | |
| Reptiles | |||||
| Anomalopus swansoni | Punctate worm-skink | Locally missing | |||
| Cyclodomorphus michaeli | Mainland she-oak skink | Locally missing | |||
| Hoplocephalus bungaroides | Broad-headed snake | Locally missing | E | E | |
| Rankinia diemensis | Mountain dragon | Extant, declined | |||
| Underwoodisaurus milii | Thick-tailed gecko | Extant, declined | |||
| Amalosia lesueurii | Lesueur’s velvet gecko | Extant, declined | |||
| Diplodactylus vittatus | Wood gecko | Extant, declined | |||
| Furina diadema | Red-naped snake | Extant, declined | |||
| Notechis scutatus | Tiger snake | Extant, declined | |||
| Lialis burtonis | Burton’s snake-lizard | Extant, declined | |||
| Acritoscincus platynotus | Red-throated skink | Extant, declined | |||
| Ctenotus taeniolatus | Copper-tailed skink | Extant, declined | |||
| Lygisaurus foliorum | Tree-base litter-skink | Extant, declined | |||
| Saproscincus mustelinus | Weasel skink | Extant, declined | |||
| Anilios nigrescens | Blackish blind snake | Extant, declined | |||
EPBC Act/BC Act status: V, vulnerable; E, endangered; EX, extinct in the wild.
For instance, four mammal species were identified as ‘locally missing’ that had nil ALA records historically (pre-2000) but did have current ALA records: (1) rufous bettong (Aepyprymnus rufescent); (2) parma wallaby; (3) brush-tailed rock wallaby (Petrogale penicillata); and (4) brush-tailed phascogale (Phascogale tapoatafa). Additional lines of evidence suggested these species occurred in the broader Sydney Basin IBRA at the time of European Settlement (Schlager 1992; Recher et al. 1993; Burbidge et al. 2008), giving rise to the possibility of historical presence. Hence, their status as an addition to the study area seemed unlikely. Each species had very few post-2000 records (n < 6), prompting external literature searches and examination of each record. These reviews suggested that these species are considered locally extinct within the broader Sydney region (DECC 2007; Dickman and La Marca 2021; Conroy et al. 2022) (Supplementary material Table S2). For instance, the brush-tailed rock wallaby had two records post-2000: one was sourced from Questagame (a mobile game that encourages people to get outdoors, which was considered relatively unreliable identification); and the other from Department of Planning Industry Environment, however was listed as ‘Invalid, in quarantine’. Three brush-tailed phascogale records were associated with a single dead male, potentially indicative of a nearby population; nevertheless with remaining records from a community survey (lower identification reliability), the other record labelled ‘Invalid, in quarantine’, along with additional lines of evidence noting that the species has ‘disappeared from the Sydney region’ (Dickman and La Marca 2021) (Supplementary material Table S2), its persistence was deemed unlikely. Further each of these species has experienced disruptions and declines in their distributions (Short and Milkovits 1990; OEH 2003, 2020; Aussie Ark 2021). These lines of evidence casted doubt on an extant status in the study area and thus they were categorised as ‘locally missing’.
A further 5 mammals and 12 reptiles were categorised as ‘extant, declined’ (Table 3). These species’ changes in ALA records over time were contrary to the general trend of increasing records, declining during a period from which most occurrence data have been generated. This trend may be a sampling artefact; however, for the purposes of this analysis it is considered a possible early signal of decline.
Ecological traits
Of the 86 species in this study, 51 were grouped as extant and 35 as rewilding candidates (locally missing/extant, declined). Associations between individual ecological traits and status groups (i.e. extant and rewilding candidates) were significant for two traits: habit (Fisher’s Exact Test: P-value = 0.047) and diet breadth (Fisher’s Exact Test: P-value = 0.024) (Fig. 6). Most rewilding candidate species had ground dwelling habits (29 of 35 species), with only 8 species in other habit categories as compared to 30 extant species (arboreal, 16; aquatic, 7; saxicolous, 4; fossorial, 3). Rewilding candidates were dominated by species with 1 diet category (22 of 35 species; predominant categories were invertebrates (n = 10) or other plants (n = 8)), with none having more than 3 diet categories.
Ecological traits for two species’ status categories: extant (light green) vs rewilding candidates (i.e. locally missing/extant, declined) (dark green). Shaded charts are those where status category is influenced by ecological attribute (Fisher’s Exact Test: P-value <0.05).

There was an overall difference in ecological traits between the two status groups (Global PERMANOVA; R2 = 0.02961, P = 0.012). Visual inspection of the nMDS plot indicated mild separation of extant and rewilding candidate species, particularly evident in a group of nMDS plot points of macropods and arboreal mammal rewilding candidate species situated close together, yet further apart from extant species points (Fig. 7) (stress value = 0.23).
Discussion
Using occurrence records combined with a decision framework, 15 terrestrial mammals and 3 reptile species were identified as historically present but now locally missing in northern Sydney, representing a likely loss of 38% of mammals and 6% of reptiles. Seventeen additional species were found to be potentially in decline. Together, these 35 species are a first cut at potential urban rewilding candidates. Missing species included multiple families; notably, two entire mammal families (Potoridae and Canidae). Other impacted taxa included Macropodidae, elapids, skinks, and geckoes. These findings align with broader patterns of loss and decline in these taxonomic groups across Greater Sydney (DECC 2007; Shea 2010). Landscape-scale drivers of species’ declines are varied and often multi-faceted (Cox et al. 2022). At a species scale, however, a potential driver of decline is ecological traits. Here, we found broad ecological traits influence a species’ status (locally missing/declined (i.e. rewilding candidates) versus extant species) (Fig. 7). Specifically, status was found to be significantly influenced by diet breadth and habit. Most locally missing or declined species are ground-dwelling species and dietary specialists, reliant upon one diet category (Fig. 6). These findings are consistent with studies evaluating drivers of species’ extinction risk (Lunney et al. 1997; McKenzie et al. 2007; Böhm et al. 2016). These notable traits highlight vulnerabilities that may be faced by species rewilded into urban contexts and if exhibited by a potential rewilding candidate, may be used to inform development of targeted conservation initiatives, which we discuss further below along with other factors that may influence rewilding success.
As well as the importance for a suitable urban rewilding candidate to have been historically present, rewilding literature emphasises a requirement to restore degraded ecosystem functions and processes (Carver et al. 2021), thus a rewilding candidate should, by preference, restore a key ecological function. Our results found that the study area has potentially lost species from taxonomic groups providing important functions. For example, potoroids, such as the long-nosed potoroo and rufous bettong, are bioturbators – crucial ecological engineers (Davies et al. 2019) whose diggings trap organic matter, supporting higher soil fertility and moisture content (Davies et al. 2019). Further, they are mycophgagous mammals, contributing to fungi spore dispersal linked to ecosystem health (Standaloft and Kirkpatrick 2022). Elapids, such as tiger snakes (Notechis scutatus), are also key predators that may help with pest rodent suppression (Shine et al. 2024). Strategically rewilding such species could therefore restore lost or depleted ecosystem processes in urban landscapes.
Persistence of restored ecosystem function requires that species rewilded are capable of becoming self-sustaining, viable populations (IUCN/SSC 2013; Carver et al. 2021). In urbanised places, fragmented remnant vegetation means landscape factors such as patch size and connectivity significantly influence species’ population viability. Thus, species movement traits (e.g. home range (HR) and dispersal needs) will form key considerations for urban rewilding candidate selection. Indeed, within the study area’s urbanised core (<25 km from CBD) patch sizes range from <5–350 ha, with some connected to large national parks on the urban fringe. Thus, the ability to occupy smaller areas of suitable habitat may improve rewilding feasibility for certain species. For instance, the dingo, a locally missing apex predator, requires large areas with a HR of 1700 ha (urban) up to 41,400 ha (arid monsoonal), moving over 6 km/day in urban settings (McNeill et al. 2016). In contrast, the brush-tailed phascogale, a smaller-sized, locally missing species, is a marsupial carnivore typically associated with dry sclerophyll forests. Formerly widespread in eastern and south-western Australia it is now present in less than 50% of its former range, including on the urban fringes of Perth, Western Australia (DBCA 2024) and Melbourne, Victoria (Nillumbik Shire Council 2025). This species has recorded HRs of between 2.3–8 ha and 20–70 ha, foraging over a range of 4–5 ha (Strahan 1991; Van Der Ree et al. 2001) potentially making them suited to smaller urban patch sizes.
Other locally missing species identified with small ‘HR to patch size’ ratios and/or lower dispersal requirements include the common dunnart (movement 440–1100 m, mean distance 154 m between activity locations (Paull 2013)), red-necked pademelon (HR: 5–30 ha; (Strahan 1991)), parma wallaby (HR: 5 ha (Lentle et al. 2004)), Burton’s snake-lizard (Lialis burtonis (average movement 5 m per day; (Wall and Shine 2013)), and the copper-tailed skink (HR: unknown, however, though other Ctenotus sp. move 40–60 m (Read 1998)). Several successful rewilding projects in small urban patches targeted species with relatively small HRs (Vieira et al. 2015; Miskelly 2018; Saumure et al. 2021). For example, Eurasian red squirrels (Sciurus vulgaris) rewilded into small urban parks (13 ha, 35 ha) (Vieira et al. 2015) (HR: 2.7–7.5 ha; Wauters et al. 2001); the relict leopard frogs (Rana fisheri) into an urban wetland (73 ha); and north island Kaka (Nestor meridionalis) into a fenced sanctuary (225 ha) (HR: 2–21 ha (Recio et al. 2016)). Globally, rewilding projects have typically been large scale, yet these examples highlight that cities can be suitable for rewilding, particularly where they comprise multiple types of green spaces, such as native vegetation, gardens and road verges that may aid connectivity by reducing the hostility of the urban matrix (Lepczyk et al. 2017). Hence, landscape factors need not constrain rewilding projects. Nevertheless, these factors are likely to have relatively greater influence on rewilding suitability of a species in an urban context than in other landscape settings. As such, they form a fundamental consideration for decision-makers progressing species that will survive when rewilded into an urban context.
Other traits that were examined individually found status (extant/declined/locally missing) to be significantly influenced by diet breadth and habit (Fig. 6). Most locally missing or declined species are ground-dwelling species (n = 29) and dietary specialists (n = 22), reliant upon one diet category. In Australia, ground-dwelling habits have been associated with declines and extinctions of birds and mammals (Lunney et al. 1997). Ecological specialisation is another factor often correlated with vulnerability to decline and extinction (McKenzie et al. 2007). Ecological specialists are thought to be more vulnerable to changes in their environment and availability of resources compared to generalists (Harcourt et al. 2002). Urban-adapted species are often associated with having dietary flexibility (e.g. bush rats, long-nosed bandicoots, eastern blue tongues (Fardell and Dickman 2023)), and thus species with broader diets may have greater urban rewilding success. Rewilding candidates with the highest dietary breadth (n = 3) included eastern quoll, short-eared possum, and brush-tailed phascogale.
Body size is another trait associated with species persistence (Lunney et al. 1997). In Australia, the majority of small mammals (11–5000 g) are threatened with extinction, and there are more threatened reptiles in larger size classes (1–5 kg) than smaller classes (1–10 g) (Lunney et al. 1997). In our urban context, species have been lost or declined across all size categories, albeit fewer than those persisting in all but the largest size category (>5 kg), where more species (n = 6) have been lost/declined than remain extant. Several small mammals (n = 13, Fig. 6) were identified as potential urban rewilding candidates, including the eastern chestnut mouse (Pseudomys gracilicaudatus) and long-nosed potoroo. The eastern chestnut mouse is associated with four broad habitat types. However, these mainly occur in dense, wet heathland, experiencing peak densities in recently burnt heath (Pereoglou et al. 2011). The long-nosed potoroo is associated with three broad habitat types, however, vegetation communities dominated by species known to host hypogeous ectomycorrhizal associations (e.g. eucalypts, acacias, allocasuarinas, casuarinas, Leptospermum) are potentially favoured due to the species’ dietary intake of hypogeal fungi (Claridge et al. 1993).
The key threatening processes of predation by red foxes and cats and habitat loss/fragmentation threaten the persistence of small vertebrate species (McKenzie et al. 2007; Doherty et al. 2016). These feral predators, omnipresent in urban areas (Campbell et al. 2020), may pose a threat to establishment and persistence of both small and ground-dwelling (n = 29) rewilding candidates. In Australia, these feral predators are managed via exclusion fencing, trapping, poison baiting and shooting, not all of which are viable in urban settings. Thus, urban rewilding offers an opportunity to explore alternative predation risk mitigation techniques. Vegetation structure has been found to mediate predation risk in a variety of ecosystems (Braun et al. 2024), and there is interest in the use of artificial structures to provide refuge from predation (Cowan et al. 2021; Carthey 2025). Moreover, olfactory misinformation has also been posited as a potential tool to aid species reintroduction efforts in the face of invasive (or native) predators (Price and Banks 2012; Finnerty et al. 2022). Reptile rewilding candidates, including broad-headed snake, red-throated skink, wood gecko, and Lesueur’s velvet gecko, may be declining due to habitat fragmentation and loss, such as sandstone outcrops, crevices, or historical removal of bush rock for gardens (Shea 2010). However, sandstone habitats persist in the study area, and targeted habitat suitability surveys could assess the viability of this habitat for rewilding, or confirm localised distributions. Habitat restoration e.g. rock piles, is also relevant here to support these species’ restoration (Herbert et al. 2023). Ongoing threats, particularly where mitigation is unavailable, challenge rewilding feasibility, especially for at-risk species (IUCN/SSC 2013). Thus, decision makers might prioritise urban rewilding candidates that are more common and whose threats can be mitigated.
Vulnerability to extinction based upon threat status is often used as a criterion to prioritise species for conservation (Mace et al. 2007). Yet, urban rewilding candidates here spanned from common (unlisted) to highly threatened species. Of the 35 potential rewilding candidates, the majority (60%) were common species not listed as threatened under Australian legislation. Common species have not been immune to human impacts of urbanisation, with many populations in decline (Gaston and Fuller 2008; Finn et al. 2023). However, the importance of common species in ecosystems is often under-represented, as many provide crucial ecosystem structure and function roles, such as food web stabilisation, seed dispersal and pollination (Gaston and Fuller 2008; Lindenmayer et al. 2011). Notable common species identified include the common dunnart, yellow-footed antechinus and three gecko species (thick-tailed gecko, Lesueur’s velvet gecko, and wood gecko; noted above). These species represent taxonomic groups broadly lost from our study area and with them a potential erosion of ecosystem functions. For example, gecko species are viewed by some communities as playing an important ecosystem role because their diet includes mosquitoes and invertebrates (Ceríaco et al. 2011). Common dunnarts and yellow-footed antechinus are carnivorous marsupials with a wide range of habitat associations that include dry sclerophyll forests that still dominate the study area. These marsupials have small HRs (e.g. yellow-footed antechinus: 0.7–1.2 ha; Kelly 2006), which may improve the species’ viability in relatively small urban patches. They were also recently found to display dietary diversity for fungi, important for maintaining natural variation in fungal community composition (Nest et al. 2023). Furthermore, these small, relatively cryptic species pose no known serious threat to humans, making them potentially more appealing to communities with whom they will be sharing the broader ‘urban backyard’.
When rewilding in an urban context, social licence is another essential consideration to ensure a rewilding project’s success. Undertaking rewilding in human-dominated landscapes presents opportunities to engage communities and address the ‘nature deficit disorder’ associated with people’s increasing disconnection from nature (Maller et al. 2019). Yet social benefits may be eroded if social acceptability and engagement factors were ignored. For example, rewilding candidates that present human–wildlife conflict, perceived or otherwise, may spark community opposition and lost community engagement opportunities. This study identified several predators and rodents as locally missing from the landscape (e.g. dingoes, eastern, and spotted-tailed quolls). Concerns generated by these taxa include zoonotic disease transmission, predation on domestic pets/poultry, loss of amenity or fouling of recreational areas, and risks to human safety and psychological wellbeing, including elevated anxiety/fear of attacks (McNeill et al. 2016). As such, these species may not represent ideal candidates to prioritise in an urban rewilding context. Nevertheless, if such species were considered essential to meeting an urban rewilding project’s goal, then carefully constructed community engagement and educational programs that bring the community along the urban rewilding journey, an important aspect of any such project, will be even more critical in this scenario.
Our desktop approach efficiently used an existing database to identify potential rewilding candidates. Yet databases of occurrence records present opportunities and challenges for evaluating species presence and temporal distribution changes. With over 180,000 records for the study area, the ALA provided a sizeable dataset from which to begin evaluations of a species’ ongoing presence. The dataset combines diverse sources of occurrence sightings providing a breadth of data unattainable from a single survey, yet may include inherent biases, such as observer identification error. Examination of records and incorporation of additional evidence can mitigate biases. For instance, reviewing species distribution maps revealed species unlikely to have been present, particularly reptiles (n = 45). We also pursued a landscape approach for our analysis, yet the scale where a similar analysis is undertaken will be project dependent. For example, if only a single patch was available, then an analysis of species conducted at the patch level may be more suitable and would likely generate a different number and suite of missing species. This scale dependency was evident when analysing missing species from our largest urban reserve (Manly Dam), where an additional seven mammals and four reptiles had no ALA records in this reserve, presenting potential rewilding candidates worthy of consideration for this patch (Supplementary material Table S5).
Rewilding urban landscapes is an emerging area for conservation projects that diverges from current rewilding practices taking place in rural or remote areas, predominantly because of a higher degree of landscape fragmentation and activities of people (Maller et al. 2019). With the majority of people globally living in urban contexts, it presents an approach that may respond to the dual rising concerns of declining biodiversity and health and wellbeing of people living in cities (Maller et al. 2019). A critical first step of rewilding is identifying historically present, locally extinct species. Our approach leveraged occurrence records to pinpoint a suite of rewilding candidate species that were historically present, but currently missing or declined in a landscape, and assessed their ecological traits. This study provided greater awareness of trait generated vulnerabilities, current taxonomic gaps, and associated ecosystem functions that may be missing from our urban study area, irrespective of a species’ threat status. In an urban context, where the list of species lost might be high, there are several additional factors that require consideration to aid rewilding candidate prioritisation in a resource constrained environment, along with highlighting needs for follow-up investigations on the ground. Species that best suit these factors, such as the examples highlighted above, are likely to have the greatest chance of urban rewilding success. Urban ecosystems, often viewed as degraded and beyond repair, continue to support high concentrations of biodiversity, even despite species losses (Ives et al. 2016). Several rewilding paradigms seek re-integration of nature back into human places (Pettorelli et al. 2019), positioning it as an ideal response to biodiversity losses in urban landscapes, viewing them instead as conservation openings. Through rebuilding populations of locally missing species, urban rewilding can fortify resilience of native species’ and begin shifting our urban ecosystems along the degradation gradient towards a more biodiverse and functional state.
Data availability
The data that support this study can be shared upon reasonable request to the corresponding author.
Acknowledgements
We thank Dr Chris Jolly for feedback on the reptile species list generated by our approach.
References
ALA (2023) Atlas of living Australia occurrence download. Available at https://doi.org/10.26197/ala.4c337c23-6028-4ba9-9cbf-0658a79989bb [accessed 14 November 2023]
Alroy J (2015) Current extinction rates of reptiles and amphibians. Proceedings of the National Academy of Sciences 112(42), 13003-13008.
| Crossref | Google Scholar |
Andermann T, Faurby S, Turvey ST, Antonelli A, Silvestro D (2020) The past and future human impact on mammalian diversity. Science Advances 6(36), eabb2313.
| Crossref | Google Scholar |
Aussie Ark (2021) Parma wallaby. Available at https://www.aussieark.org.au/parma-wallaby/ [accessed 4 April 2024]
Berto R (2014) The role of nature in coping with psycho-physiological stress: a literature review on restorativeness. Behavioral Sciences 4(4), 394-409.
| Crossref | Google Scholar | PubMed |
Böhm M, Williams R, Bramhall HR, McMillan KM, Davidson AD, Garcia A, Bland LM, Bielby J, Collen B (2016) Correlates of extinction risk in squamate reptiles: the relative importance of biology, geography, threat and range size. Global Ecology and Biogeography 25(4), 391-405.
| Crossref | Google Scholar |
Braun S, Ritchie EG, Doherty TS, Nimmo DG (2024) The red fox (Vulpes vulpes) is the dominant predator of lizard models in a semi-arid landscape, and predation risk is reduced by vegetation cover. Austral Ecology 49(5), e13530.
| Crossref | Google Scholar |
Brown J, Williams NSG, Soanes K (2024) Conservation translocations in urban environments: state of the knowledge and future directions. Basic and Applied Ecology 81, 85-95.
| Crossref | Google Scholar |
Burbidge AA, McKenzie NL, Brennan KEC, Woinarski JCZ, Dickman CR, Baynes A, Gordon G, Menkhorst PW, Robinson AC (2008) Conservation status and biogeography of Australia’s terrestrial mammals. Australian Journal of Zoology 56(6), 411-422.
| Crossref | Google Scholar |
Campbell SJ, Ashley W, Gil-Fernandez M, Newsome TM, Di Giallonardo F, Ortiz-Baez AS, Mahar JE, Towerton AL, Gillings M, Holmes EC, Carthey AJR, Geoghegan JL (2020) Red fox viromes in urban and rural landscapes. Virus Evolution 6(2), veaa065.
| Crossref | Google Scholar |
Cardinale B, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Larigauderie A, Srivastava D, Naeem S (2012) Biodiversity loss and its impact on humanity. Nature 486, 59-67.
| Crossref | Google Scholar | PubMed |
Carthey AJR (2025) Space, structure, and refuge from predation: artificial habitat for ground-dwelling wildlife. Australian Zoologist 44(2), 343-355.
| Crossref | Google Scholar |
Carver S, Convery I, Hawkins S, Beyers R, Eagle A, Kun Z, Van Maanen E, Cao Y, Fisher M, Edwards SR, Nelson C, Gann GD, Shurter S, Aguilar K, Andrade A, Ripple WJ, Davis J, Sinclair A, Bekoff M, Noss R, Foreman D, Pettersson H, Root-Bernstein M, Svenning J-C, Taylor P, Wynne-Jones S, Featherstone AW, Fløjgaard C, Stanley-Price M, Navarro LM, Aykroyd T, Parfitt A, Soulé M (2021) Guiding principles for rewilding. Conservation Biology 35(6), 1882-1893.
| Crossref | Google Scholar | PubMed |
Ceríaco LMP, Marques MP, Madeira NC, Vila-Viçosa CM, Mendes P (2011) Folklore and traditional ecological knowledge of geckos in Southern Portugal: implications for conservation and science. Journal of Ethnobiology and Ethnomedicine 7, 26.
| Crossref | Google Scholar |
Claridge AW, Tanton MT, Cunningham RB (1993) Hypogeal fungi in the diet of the long-nosed potoroo (Potorous tridactylus) in mixed-species and regrowth eucalypt forest stands in south-eastern Australia. Wildlife Research 20(3), 321-338.
| Crossref | Google Scholar |
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18(1), 117-143.
| Crossref | Google Scholar |
Clinton WL, Le Beouf BJ (1993) Sexual selection’s effects on male life history and the pattern of male mortality. Ecology 74(6), 1884-1892.
| Crossref | Google Scholar |
Conroy RJ, Bonzol UA, Illingsworth JJ, Martyn JE, Mitchell PB, Percival IG, Robinson AM, Robson DF, Walsh JB (2022) The natural and cultural history of the Ku-ring-gai GeoRegion, New South Wales. Proceedings of the Linnean Society of New South Wales 144, 129-226.
| Google Scholar |
Cowan MA, Callan MN, Watson MJ, Watson DM, Doherty TS, Michael DR, Dunlop JA, Turner JM, Moore HA, Watchorn DJ, Nimmo DG (2021) Artificial refuges for wildlife conservation: what is the state of the science? Biological Reviews 96(6), 2735-2754.
| Crossref | Google Scholar | PubMed |
Cox N, Young BE, Bowles P, Fernandez M, Marin J, Rapacciuolo G, Böhm M, Brooks TM, Hedges SB, Hilton-Taylor C, Hoffmann M, Jenkins RKB, Tognelli MF, Alexander GJ, Allison A, Ananjeva NB, Auliya M, Avila LJ, Chapple DG, Cisneros-Heredia DF, Cogger HG, Colli GR, de Silva A, Eisemberg CC, Els J, Fong GA, Grant TD, Hitchmough RA, Iskandar DT, Kidera N, Martins M, Meiri S, Mitchell NJ, Molur S, Nogueira CdC, Ortiz JC, Penner J, Rhodin AGJ, Rivas GA, Rödel M-O, Roll U, Sanders KL, Santos-Barrera G, Shea GM, Spawls S, Stuart BL, Tolley KA, Trape J-F, Vidal MA, Wagner P, Wallace BP, Xie Y (2022) A global reptile assessment highlights shared conservation needs of tetrapods. Nature 605, 285-290.
| Crossref | Google Scholar | PubMed |
Crowther MS (2002) Distributions of species of the Antechinus stuartii-A. flavipes complex as predicted by bioclimatic modelling. Australian Journal of Zoology 50(1), 77-91.
| Crossref | Google Scholar |
Davies GTO, Kirkpatrick JB, Cameron EZ, Carver S, Johnson CN (2019) Ecosystem engineering by digging mammals: effects on soil fertility and condition in Tasmanian temperate woodland. Royal Society Open Science 6, 180621.
| Crossref | Google Scholar | PubMed |
Davis M, Faurby S, Svenning J-C (2018) Mammal diversity will take millions of years to recover from the current biodiversity crisis. Proceedings of the National Academy of Sciences 115(44), 11262-11267.
| Crossref | Google Scholar |
DBCA (2024) Brush-tailed phascogale. Available at https://library.dbca.wa.gov.au/static/FullTextFiles/071549.pdf [accessed 4 April 2024]
DCCEEW (2016) The native vegetation of the sydney metropolitan area – version 3.1: VIS_ID 4489. Available at https://datasets.seed.nsw.gov.au/dataset/the-native-vegetation-of-the-sydney-metropolitan-area-oeh-2016-vis-id-4489 [accessed 1 October 2023]
Dickman CR, Lunney D, Matthews A (2001) Ecological attributes and conservation of dasyurid marsupials in New South Wales, Australia. Pacific Conservation Biology 7(2), 124-133.
| Crossref | Google Scholar |
Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR (2016) Invasive predators and global biodiversity loss. Proceedings of the National Academy of Sciences 113(40), 11261-11265.
| Crossref | Google Scholar |
DPE (2023) Park visitor survey. Available at https://www.environment.nsw.gov.au/topics/parks-reserves-and-protected-areas/park-management/park-visitor-survey [accessed 29 October 2024]
Finn C, Grattarola F, Pincheira-Donoso D (2023) More losers than winners: investigating Anthropocene defaunation through the diversity of population trends. Biological Reviews 98(5), 1732-1748.
| Crossref | Google Scholar | PubMed |
Finnerty PB, McArthur C, Banks P, Price C, Shrader AM (2022) The olfactory landscape concept: a key source of past, present, and future information driving animal movement and decision-making. BioScience 72(8), 745-752.
| Crossref | Google Scholar |
Gaston KJ, Fuller RA (2008) Commonness, population depletion and conservation biology. Trends in Ecology & Evolution 23(1), 14-19.
| Crossref | Google Scholar |
Hahs AK, Fournier B, Aronson MFJ, Nilon CH, Herrera-Montes A, Salisbury AB, Threlfall CG, Rega-Brodsky CC, Lepczyk CA, La Sorte FA, MacGregor-Fors I, Scott MacIvor J, Jung K, Piana MR, Williams NSG, Knapp S, Vergnes A, Acevedo AA, Gainsbury AM, Rainho A, Hamer AJ, Shwartz A, Voigt CC, Lewanzik D, Lowenstein DM, O’Brien D, Tommasi D, Pineda E, Carpenter ES, Belskaya E, Lövei GL, Makinson JC, Coleman JL, Sadler JP, Shroyer J, Shapiro JT, Baldock KCR, Ksiazek-Mikenas K, Matteson KC, Barrett K, Siles L, Aguirre LF, Armesto LO, Zalewski M, Herrera-Montes MI, Obrist MK, Tonietto RK, Gagné SA, Hinners SJ, Latty T, Surasinghe TD, Sattler T, Magura T, Ulrich W, Elek Z, Castañeda-Oviedo J, Torrado R, Kotze DJ, Moretti M (2023) Urbanisation generates multiple trait syndromes for terrestrial animal taxa worldwide. Nature Communications 14, 4751.
| Crossref | Google Scholar | PubMed |
Haouchar D, Pacioni C, Haile J, McDowell MC, Baynes A, Phillips MJ, Austin JJ, Pope LC, Bunce M (2016) Ancient DNA reveals complexity in the evolutionary history and taxonomy of the endangered Australian brush-tailed bettongs (Bettongia: Marsupialia: Macropodidae: Potoroinae). Biodiversity and Conservation 25, 2907-2927.
| Crossref | Google Scholar |
Harcourt AH, Parks SA, Coppeto SA (2002) Rarity, specialization and extinction in primates. Journal of Biogeography 29(4), 445-456.
| Crossref | Google Scholar |
Herbert S, Knox C, Clarke D, Bell T (2023) Use of constructed rock piles by lizards in a grassland habitat in Otago, New Zealand. New Zealand Journal of Ecology 47(1), 3543.
| Crossref | Google Scholar |
Hill S, Cumpston Z, Quintana Vigiola G (2021) Urban. In ‘Australia state of the environment 2021’. (Eds I Creswell, T Janke, E Johnston) (Department of Agriculture, Water and the Environment, Australian Government). Available at https://soe.dcceew.gov.au/urban/environment/population-and-buildings [accessed 1 October 2024]
Hu Y, Doherty TS, Jessop TS (2020) How influential are squamate reptile traits in explaining population responses to environmental disturbances? Wildlife Research 47(3), 249-259.
| Crossref | Google Scholar |
Ives CD, Lentini PE, Threlfall CG, Ikin K, Shanahan DF, Garrard GE, Bekessy SA, Fuller RA, Mumaw L, Rayner L, Rowe R, Valentine LE, Kendal D (2016) Cities are hotspots for threatened species. Global Ecology and Biogeography 25(1), 117-126.
| Crossref | Google Scholar |
Jepson P (2019) Recoverable earth: a twenty-first century environmental narrative. Ambio 48, 123-130.
| Crossref | Google Scholar | PubMed |
Kelly LT (2006) Distribution and habitat requirements of the Yellow-footed Antechinus Antechinus flavipes at multiple scales: a review. The Victorian Naturalist 123(2), 91-100.
| Google Scholar |
Lentle RG, Hume ID, Stafford KJ, Kennedy M, Springett BP, Browne R, Haslett S (2004) Temporal aspects of feeding events in tammar (Macropus eugenii) and parma (Macropus parma) wallabies. I. Food acquisition and oral processing. Australian Journal of Zoology 52(1), 81-95.
| Crossref | Google Scholar |
Lepczyk CA, Aronson MFJ, Evans KL, Goddard MA, Lerman SB, MacIvor JS (2017) Biodiversity in the city: fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. BioScience 67(9), 799-807.
| Crossref | Google Scholar |
Lindenmayer DB, Wood JT, McBurney L, MacGregor C, Youngentob K, Banks SC (2011) How to make a common species rare: a case against conservation complacency. Biological Conservation 144(5), 1663-1672.
| Crossref | Google Scholar |
Lunney D, Curtin AL, Fisher D, Ayers D, Dickman CR (1997) Ecological attributes of the threatened fauna of New South Wales. Pacific Conservation Biology 3(1), 13-26.
| Crossref | Google Scholar |
Mackay CML, Schmitt MT (2019) Do people who feel connected to nature do more to protect it? A meta-analysis. Journal of Environmental Psychology 65, 101323.
| Crossref | Google Scholar |
Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K (2023) Cluster: cluster analysis basics and extensions. R package version 2.1.6 — For new features, see the ‘NEWS’ and the ‘Changelog’ file in the package source. Available at https://CRAN.R-project.org/package=cluster
Major RE, Parsons H (2010) What do museum specimens tell us about the impact of urbanisation? A comparison of the recent and historical bird communities of Sydney. Emu - Austral Ornithology 110, 92-103.
| Crossref | Google Scholar |
McKenzie NL, Burbidge AA, Baynes A, Brereton RN, Dickman CR, Gordon G, Gibson LA, Menkhorst PW, Robinson AC, Williams MR, Woinarski JCZ (2007) Analysis of factors implicated in the recent decline of Australia’s mammal fauna. Journal of Biogeography 34(4), 597-611.
| Crossref | Google Scholar |
McKinney ML (2002) Urbanization, biodiversity, and conservation: the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 52(10), 883-890.
| Crossref | Google Scholar |
McNeill AT, Leung LK-P, Goullet MS, Gentle MN, Allen BL (2016) Dingoes at the doorstep: home range sizes and activity patterns of dingoes and other wild dogs around urban areas of North-Eastern Australia. Animals 6(8), 48.
| Crossref | Google Scholar | PubMed |
Miskelly CM (2018) Changes in the forest bird community of an urban sanctuary in response to pest mammal eradications and endemic bird reintroductions. Notornis 65(3), 132-151.
| Crossref | Google Scholar |
Mowat EJ, Webb JK, Crowther MS (2015) Fire-mediated niche-separation between two sympatric small mammal species. Austral Ecology 40(1), 50-59.
| Crossref | Google Scholar |
Nest C, Elliott TF, Cooper T, Vernes K (2023) Seasonal consumption of mycorrhizal fungi by a marsupial-dominated mammal community. Fungal Ecology 64, 101247.
| Crossref | Google Scholar |
Nillumbik Shire Council (2025) Brush-tailed phascogale. Nillumbik Shire Council. Available at https://www.nillumbik.vic.gov.au/Explore/Environment/Natural-environment/Animals/Native-animals/Brush-tailed-Phascogale
OEH (2003) Brush-tailed rock-wallaby (Petrogale penicillata) – endangered species listing. Available at https://www.environment.nsw.gov.au/topics/animals-and-plants/threatened-species/nsw-threatened-species-scientific-committee/determinations/final-determinations/2000-2003/brush-tailed-rock-wallaby-petrogale-penicillata-endangered-species-listing [accessed 4 April 2024]
OEH (2020) Rufous bettong – profile. Available at https://threatenedspecies.bionet.nsw.gov.au/profile?id=10033 [accessed 4 April 2024]
O’Rourke RL, Anson JR, Saul AM, Banks PB (2020) Limits to alien black rats (Rattus rattus) acting as equivalent pollinators to extinct native small mammals: the influence of stem width on mammal activity at native Banksia ericifolia inflorescences. Biological Invasions 22, 329-338.
| Crossref | Google Scholar |
Parker KA (2008) Translocations: providing outcomes for wildlife, resource managers, scientists, and the human community. Restoration Ecology 16(2), 204-209.
| Crossref | Google Scholar |
Paull DC (2013) Refuge sites, activity and torpor in wild common dunnarts (Sminthopsis murina) in a temperate heathland. Australian Mammalogy 35(2), 153-159.
| Crossref | Google Scholar |
Pereoglou F, Macgregor C, Banks SC, Ford F, Wood J, Lindenmayer DB (2011) Refuge site selection by the eastern chestnut mouse in recently burnt heath. Wildlife Research 38(4), 290-298.
| Crossref | Google Scholar |
Price CJ, Banks PB (2012) Exploiting olfactory learning in alien rats to protect birds’ eggs. Proceedings of the National Academy of Sciences 109(47), 19304-19309.
| Crossref | Google Scholar |
Read JL (1998) The ecology of sympatric scincid lizards (Ctenotus) in arid South Australia. Australian Journal of Zoology 46(6), 617-629.
| Crossref | Google Scholar |
Recher HF, Hutchings PA, Rosen S (1993) The biota of the Hawkesbury-Nepean catchment: reconstruction and restoration. Australian Zoologist 29(1–2), 3-41.
| Crossref | Google Scholar |
Recio MR, Payne K, Seddon PJ (2016) Emblematic forest dwellers reintroduced into cities: resource selection by translocated juvenile kaka. Current Zoology 62(1), 15-22.
| Crossref | Google Scholar | PubMed |
Ryan CM, Hobbs RJ, Valentine LE (2020) Bioturbation by a reintroduced digging mammal reduces fuel loads in an urban reserve. Ecological Applications 30(2), e02018.
| Crossref | Google Scholar | PubMed |
Saumure R, Rivera R, Jaeger JR, O’Toole T, Ambros A, Guadelupe K, Bennett A, Marshall Z (2021) Leaping from extinction: rewilding the relict leopard frog in Las Vegas, Nevada, USA. In ‘Global conservation translocation perspectives: 2021. Case studies from around the globe.’ (Ed PS Soorae) pp. 76–81. (Gland, Switzerland)
Schlager F (1992) The distribution, status and ecology of the rufous rat-kangaroo, ‘aepyrymnus rufescens’, in Northern NSW. Available at https://rune.une.edu.au/server/api/core/bitstreams/4ec7b52b-2725-49d8-8847-c9ac7cc20173/content [accessed 31 January 2024]
Seddon PJ, Griffiths CJ, Soorae PS, Armstrong DP (2014) Reversing defaunation: restoring species in a changing world. Science 345(6195), 406-412.
| Crossref | Google Scholar | PubMed |
Shine R, Dunstan N, Abraham J, Mirtschin P (2024) Why Australian farmers should not kill venomous snakes. Animal Conservation 27(4), 415-425.
| Crossref | Google Scholar |
Short J, Milkovits G (1990) Distribution and status of the Brush-Tailed Rock-Wallaby in South-Eastern Australia. Wildlife Research 17(2), 169-179.
| Crossref | Google Scholar |
Smith KJ, Pierson JC, Evans MJ, Gordon IJ, Manning AD (2024) Continental-scale identification and prioritisation of potential refugee species; a case study for rodents in Australia. Ecography 2024(9), e07035.
| Crossref | Google Scholar |
Soulé M, Noss R (1998) Rewilding and biodiversity: complementary goals for continental conservation. Wild Earth 8, 19-28.
| Google Scholar |
Standaloft I, Kirkpatrick JB (2022) Identifying important environmental variables in the niche partitioning of two keystone ecosystem engineers (Bettongia gaimardi and Potorous tridactylus) in Tasmania. Wildlife Research 50(7), 507-516.
| Crossref | Google Scholar |
Sweeney OF, Turnbull J, Jones M, Letnic M, Newsome TM, Sharp A (2019) An Australian perspective on rewilding. Conservation Biology 33(4), 812-820.
| Crossref | Google Scholar | PubMed |
Van Der Ree R, Soderquist TR, Bennett AF (2001) Home-range use by the brush-tailed phascogale (Phascogale tapoatafa) (Marsupialia) in high-quality, spatially limited habitat. Wildlife Research 28(5), 517-525.
| Crossref | Google Scholar |
Van Heezik Y, Seddon PJ (2018) Animal reintroductions in peopled landscapes: moving towards urban-based species restorations in New Zealand. Pacific Conservation Biology 24(4), 349-359.
| Crossref | Google Scholar |
Vieira BP, Fonseca C, Rocha RG (2015) Critical steps to ensure the successful reintroduction of the Eurasian red squirrel. Animal Biodiversity and Conservation 38, 49-58.
| Crossref | Google Scholar |
Wake DB, Vredenburg VT (2008) Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proceedings of the National Academy of Sciences 105(Supp 1), 11466-11473.
| Crossref | Google Scholar |
Wall M, Shine R (2013) Ecology and behaviour of burton’s legless lizard (Lialis burtonis, Pygopodidae) in Tropical Australia. Asian Herpetological Research 4(1), 9-21.
| Crossref | Google Scholar |
Ward M, Watson JEM, Possingham HP, Garnett ST, Maron M, Rhodes JR, MacColl C, Seaton R, Jackett N, Reside AE, Webster P, Simmonds JS (2022) Creating past habitat maps to quantify local extirpation of Australian threatened birds. Environmental Research Letters 17(2), 024032.
| Crossref | Google Scholar |
Watson DM, Watson MJ (2015) Wildlife restoration: mainstreaming translocations to keep common species common. Biological Conservation 191, 830-838.
| Crossref | Google Scholar |
Wauchope-Drumm M, Bentley J, Beaumont LJ, Baumgartner JB, Nipperess DA (2020) Using a species distribution model to guide NSW surveys of the long-footed potoroo (Potorous longipes). Austral Ecology 45(1), 15-26.
| Crossref | Google Scholar |
Wauters LA, Gurnell J, Preatoni D, Tosi G (2001) Effects of spatial variation in food availability on spacing behaviour and demography of Eurasian Red Squirrels. Ecography 24, 525-538.
| Google Scholar |
Woinarski JCZ, Burbidge AA, Harrison PL (2015) Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences 112(15), 4531-4540.
| Crossref | Google Scholar |
Woinarski JCZ, Braby MF, Burbidge AA, Coates D, Garnett ST, Fensham RJ, Legge SM, McKenzie NL, Silcock JL, Murphy BP (2019) Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia. Biological Conservation 239, 108261.
| Crossref | Google Scholar |