Inducible heat shock protein A1A (HSPA1A) is markedly expressed in rat myometrium by labour and secreted via myometrial cell-derived extracellular vesicles
M. F. Russell A B , G. C. Bailey B C , E. I. Miskiewicz B C and D. J. MacPhee B C D
B C D
A Department of Anatomy, Physiology and Pharmacology, College of Medicine, University of Saskatchewan, Saskatoon, SK, S7N 5E5, Canada.
B One Reproductive Health Research Group, University of Saskatchewan, Saskatoon, SK, S7N 5B4, Canada.
C Department of Veterinary Biomedical Sciences, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, SK, S7N 5B4, Canada.
D Corresponding author. Email: d.macphee@usask.ca
Reproduction, Fertility and Development 33(4) 279-290 https://doi.org/10.1071/RD20242
Submitted: 17 September 2020 Accepted: 24 November 2020 Published: 12 February 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
The myometrium goes through physiological, cellular and molecular alterations during gestation that necessitate effective cellular proteostasis. Inducible heat shock protein A1A (HSPA1A) is a member of the 70-kDa heat shock protein A (HSPA) family, which acts as a chaperone to regulate proteostasis; however, HSPA1A also participates as a cytokine in inflammatory regulation, leading to its designation as a chaperokine. This study examined the spatiotemporal expression of HSPA1A protein in the rat myometrium throughout gestation and assessed whether it is secreted as cargo of myometrial cell-derived extracellular vesicles (EVs). Immunoblot analysis demonstrated that HSPA1A expression was markedly elevated during late pregnancy and labour and increased by uterine distension. Myometrial HSPA1A expression in situ increased in myocytes of longitudinal and circular muscle layers from Day 19 through to postpartum, specifically in the cytoplasm and nuclei of myocytes from both muscle layers, but frequently detectable just outside myocyte membranes. Scanning electron microscopy examination of samples isolated from hTERT-HM cell-conditioned culture medium, using EV isolation spin columns, confirmed the presence of EVs. EV lysates contained HSPA8, HSPA1A and the EV markers apoptosis-linked gene 2-interacting protein X (Alix), the tetraspanin cluster of differentiation 63 (CD63), tumour susceptibility gene 101 (TSG101) and HSP90, but not the endoplasmic reticulum protein calnexin. These results indicate that HSPA1A may act as a chaperokine in the myometrium during pregnancy.

Keywords: HSPA1A, HSP70, chaperokine, pregnancy, myometrium, extracellular vesicles.
Introduction
During pregnancy, the uterine musculature, or myometrium, is altered structurally, physiologically and biochemically under the influence of endocrine, biophysical and inflammatory signals to produce a powerful contractile tissue capable of delivering a term fetus(es) for extrauterine survival (MacPhee and Miskiewicz 2017). In the rat, where this process has been characterised in detail, the myometrium undergoes growth by proliferation and hypertrophy interspersed by activation of an intrinsic apoptotic pathway in the myometrium, yet with no indication of significant apoptosis (Shynlova et al. 2009, 2010, 2013). The myometrial hypertrophy as well as increased production of interstitial extracellular matrix proteins, subsequent basement membrane proteins and their integrin receptors are driven, in part, by the ever-increasing stress of uterine distension induced by growing fetuses (Shynlova et al. 2004, 2010; Williams et al. 2005, 2010). During labour, contraction-associated proteins such as gap junction alpha-1 (GJA1) are expressed and physiological uterine inflammation occurs to enable myometrial activation and parturition (Tabb et al. 1992; Shynlova et al. 2020). Finally, in the postpartum period the uterus undergoes an involution process similar to wound healing that is marked by reorganisation of the myometrium and even cell death (Shynlova et al. 2007, 2008). Such a breadth of processes occurring in the myometrium during gestation necessitates the tissue to use efficient and effective cellular proteostasis.
The 70-kDa heat shock protein (HSP) family (HSPA; also known as HSP70) comprises 13 proteins encoded by 17 genes and 30 pseudogenes (Brocchieri et al. 2008; Kampinga et al. 2009). These chaperones work with other HSPs, such as the HSP90 family, and a large group of cochaperones including HSP40 (DNAJB1), stress-induced phosphoprotein-1 (STIP1; also known as Hsp70–Hsp90 organising protein (HOP)) and suppressor of tumorigenicity 13 (ST13; also known as HSP70-interacting protein (HIP); Radons 2016). HSPA and HSP90 proteins, along with their host of cochaperones, can comprise 3% of total protein mass in unstressed cells (Finka et al. 2015a). Canonically, HSPA and HSP90 family members are ATP-hydrolysing chaperones that, in partnership with their cochaperones, regulate cellular proteostasis (Finka et al. 2015b). In this capacity, they can regulate processes such as the stress-induced unfolded protein response, the breakdown of misfolded or aggregated proteins, and modulate the complexing of macromolecules as well as protein–protein interactions, including steroid hormone receptor maturation (Echeverria and Picard 2010; Yamamoto et al. 2014; Sisti et al. 2015; Lackie et al. 2017). HSPA1A is the major cytosolic stress-inducible HSPA member, whereas HSPA8 (heat shock cognate 70-kDa protein (HSC70)) is the constitutively expressed essential housekeeping protein of the family (Hartl et al. 2011; Radons 2016).
Beyond an intracellular role, reports have now clearly demonstrated that HSPs can also be secreted and have extracellular functions (De Maio 2011). A primary mode of secretion of HSPs is via extracellular vesicles (EVs), which can be released by many different cell types in response to stress (De Maio and Vasquez 2013). EVs are comprised of lipid bilayers containing transmembrane proteins and an inner cytosol of proteins and RNA. There is a considerable EV heterogeneity in terms of biogenesis, size, cargo and function (Kalluri and LeBleu 2020). EVs can be produced by cells from cell membrane budding (microvesicles) or from multivesicular bodies of endosomal origin that release the EVs (exosomes) and their contents upon fusion with the plasma membrane (Colombo et al. 2014; Tkach and Théry 2016). Small EVs <200 nm in diameter are represented by exosomes as well as small microvesicles, and these small EVs are marked by the presence of proteins, including apoptosis-linked gene 2-interacting protein X (Alix), the tetraspanin cluster of differentiation 63 (CD63) and tumour susceptibility gene 101 (TSG101) (Deng and Miller 2019; Stahl and Raposo 2019; Kalluri and LeBleu 2020). HSPA1A has also been identified as cargo in EVs and implicated in promoting inflammation (De Maio 2011). Recently, minimal information required for publishing functional studies of EVs have been recommended (Théry et al. 2018).
There is a growing body of knowledge regarding the role of stress proteins in pregnancy and labour (MacPhee and Miskiewicz 2017). Recently, it was reported that total HSP90, including HSP90AA1 and HSP90AB1 isoforms, was detected in the myometrium throughout pregnancy (Bhatti et al. 2019); however, because HSPA1A has roles in proteostasis and inflammatory events, it is imperative to investigate the expression and role of HSPA1A in the myometrium. Therefore, the objectives of the present study were to examine the spatiotemporal expression of HSPA1A in the rat myometrium throughout gestation and to assess whether HSPA1A could be cargo in myometrial cell-derived EVs.
Materials and methods
Animals
Sprague-Dawley rats were individually housed by the University Animal Care Unit under standard environmental conditions (12-h light–dark cycle). Rats had access to water ad libitum and were maintained on LabDiet Prolab (RMH 3000; PMI Nutrition International). For all experiments, virgin female rats were mated with stud males and the observation of a vaginal plug the morning after mating was designated Day 1 of the gestation period. Under these standard conditions, the time of delivery was Day 23. All experiments adhered to institutional and national standards for the care and welfare of animals and were granted ethics approval by the University Animal Care Committee under Protocols 08-02-DM to 11-02-DM.
Tissue collection
Individual female rats were euthanised using carbon dioxide gas asphyxiation. Tissue samples were collected from animals at 10 time points: non-pregnant (NP), throughout gestation (Days 6, 12, 15, 17, 19, 21, 22 and 23 (labour)) and 1 day postpartum (PP). Four independent sets of rat uterine tissue samples were used for experiments (n = 4 per time point). The labour samples collected on Day 23 were taken during active labour after the dam had delivered two to three pups.
For immunoblot analysis, the uterine horns were dissected and opened longitudinally in cold phosphate-buffered saline (PBS) solution (pH 7.4). Myometrial tissue was then isolated as described previously (Nicoletti et al. 2016). All samples collected were flash frozen in liquid nitrogen and stored at –80°C. For immunofluorescence detection, a portion of the intact rat uterine horn was fixed in 4% paraformaldehyde in PBS overnight at room temperature. Tissues were processed, paraffin embedded, sectioned and mounted on microscope slides as described previously (Bhatti et al. 2019).
To assess the effect of uterine distension on HSPA1A expression, a unilaterally pregnant rat model was used. This model has been used previously to study the effect of uterine distension or stretch on numerous genes and proteins, including the gap junction protein GJA1, the heat shock protein αB-crystallin (CRYAB) and signalling kinases such as mitogen-activated protein kinase (MAPK) 14 (Ou et al. 1997; Oldenhof et al. 2002; Nicoletti et al. 2016). Virgin female rats were anaesthetised and underwent a unilateral tubal ligation procedure as described previously in detail by White and MacPhee (2011). Animals were monitored postoperatively and allowed to recover for at least 5 days before matings were attempted. This model results in pregnant rats each having a gravid (distended) and non-gravid (empty, non-distended control) uterine horn for analyses. Samples of gravid and non-gravid horns were collected during gestation on Days 19 and 23 (n = 4 for each time point), and tissues were processed as described above.
Immunoblot analysis
Tissue samples were homogenised using a Precellys bead mill in RIPA lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS)) containing protease and phosphatase inhibitors, as described previously (Nicoletti et al. 2016). Sample protein concentrations were then determined using the Bio-Rad Protein Assay Kit II (500-0002). Protein samples (20 μg per lane) were mixed with 2× gel loading dye (100 mM Tris-HCl, pH 6.8, 10% β-mercaptoethanol, 4% SDS, 0.2% bromophenol blue, 20% glycerol) and electrophoretically separated on 10% SDS–polyacrylamide gels, followed by electroblotting to 0.2 μm nitrocellulose membranes (162-0097; Bio-Rad). Membranes were then stained with a Reversible Protein Stain kit (PI24580; ThermoFisher Scientific) according to the manufacturer’s instructions to verify protein transfer.
Nitrocellulose membranes were rinsed in Tris-buffered saline containing Tween 20 (TBST; 20 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.1% Tween 20) and then incubated in a blocking solution composed of 5% non-fat dry milk (w/v) in TBST. All antisera used for analyses (Table 1) were diluted in blocking solution. Blots were incubated with primary antisera overnight at 4°C with constant shaking, rinsed with TBST and probed with an appropriate horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Protein detection was accomplished using a SuperSignal West Pico chemiluminescence substrate detection system (PI34080; ThermoFisher) and multiple exposures were acquired using a Bio-Rad ChemiDoc MP digital imaging system. All membranes were subsequently stripped with Restore Western Blot Stripping Buffer (PI21059; ThermoFisher) according to the manufacturer’s instructions and re-probed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein expression, which served as a loading control, using a rabbit polyclonal GAPDH-specific antibody (Table 1).
|
Immunofluorescence analysis
Tissue sections were deparaffinised, rehydrated and underwent epitope retrieval as described previously (Nicoletti et al. 2016). Sections were incubated for 1 h in a blocking solution consisting of 5% normal goat serum, 1% normal horse serum and 1% fetal bovine serum (FBS) in PBS. Sections were then incubated overnight at 4°C with primary antiserum or non-specific rabbit IgG (Table 1) used at the same concentration as the primary antiserum to serve as a negative control. Sections were washed with PBS, then incubated with appropriate secondary antiserum (Table 1). Following washes in ice-cold PBS containing 0.02% Tween 20, sections were mounted in ProLong Diamond Anti-Fade Reagent with 4′,6′-diamidino-2-phenylindole (P36931; ThermoFisher). An Olympus IX83 microscope equipped with an Andor Zyla 4.2 sCMOS camera (2048-pixel × 2048-pixel array; Andor USA) and CellSens software (Olympus) were used for widefield epifluorescence image capture.
EV isolation and biochemical evaluation
The hTERT-HM human myometrium-derived cell line was established via stable transfection of human myometrial cells with expression vectors containing the human telomerase reverse transcriptase (hTERT), which maintains telomere length and immortalises cells (Condon et al. 2002). These cells retain myometrial cell characteristics, such as expression of calponin (CNN1) and oxytocin receptor (OXTR) proteins (Condon et al. 2002). hTERT-HM cells were regularly cultivated in 75-cm2 culture flasks at 37°C under 5% CO2 in air using DMEM/F12 medium with l-glutamine and 15 mM HEPES (11330-032; ThermoFisher) plus 10% FBS (12483-020; ThermoFisher) and 1% penicillin–streptomycin (P/S; 15140-122; ThermoFisher). The medium was refreshed every 24 h and cells were passaged when they reached approximately 90% confluence using trypsin (0.05% (v/v) trypsin–EDTA; 15400-054; ThermoFisher). Twenty-four hours before EV collection, cells were washed three times in Opti-MEM serum-free medium (31985-070; ThermoFisher) containing 1% P/S to remove residual FBS and then cultured in 8 mL fresh Opti-MEM/1% P/S per flask. The cell culture supernatant from four flasks was pooled and centrifuged at 500g for 5 min at room temperature to remove any residual detached cells. Subsequently, the supernatant was passed through a Millex-AA 0.8-μm syringe filter (SLAA033SS; Sigma-Aldrich) into two 50-mL tubes to remove any remaining cellular debris. The EVs were then isolated using an ExoEasy Maxi EV isolation kit (76064; Qiagen) according to the manufacturer’s instructions. Briefly, the supernatant was mixed by inversion with an equal volume of Buffer XBP and loaded onto ExoEasy affinity spin columns followed by centrifugation at 500g for 1 min. The spin columns were rinsed twice with 10 mL of Buffer XWP using centrifugation at 3000g for 5 min. The spin columns were then transferred to fresh collection tubes and EVs were eluted with 400 μL Buffer XE. The EV eluate was concentrated to 100 μL using a Vivaspin 500 centrifugal concentrator with a 100-kDa molecular mass cut-off (14-558-404; ThermoFisher) and then sonicated twice for 10 s each time on ice. Protein assays were conducted as described above. A 5-µg sample of the EV preparation per lane was used for SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblot analyses to assess the detection of Alix, CD63, HSPA8, HSPA1A, HSP90 and TSG101 and to confirm the absence of the endoplasmic reticulum protein calnexin (Asea et al. 2008) using specific antisera (Table 1).
Scanning electron microscopy examination of EVs
For examination of EVs by scanning electron microscopy, samples were fixed for 15 min with 2.5% glutaraldehyde in Buffer XE while on 0.1% poly-L-lysine-coated glass coverslips. Samples were then washed with 0.1 M sodium cacodylate buffer (pH 7.2), followed by dehydration with an ethanol series (30%, 50%, 70%, 80%, 90%, 95% and 3 × 100% ethanol; 5 min at each concentration). Following critical point drying, samples were coated with 5 nm chromium and then imaged with a Hitachi SU8010 electron microscope.
Data analysis
Densitometric analysis of immunoblot data was conducted using Image Lab software (Bio-Rad) and densitometric values for protein expression were normalised to the GAPDH loading control. Statistical analysis and data plotting were performed using GraphPad Prism version 8.0 for Mac (GraphPad Software; www.graphpad.com). Datasets were subjected to one-way analysis of variance (ANOVA) and post hoc Tukey–Kramer multiple comparisons tests. Statistical differences were considered significant at P < 0.05.
Results
Myometrial HSPA1A expression during pregnancy
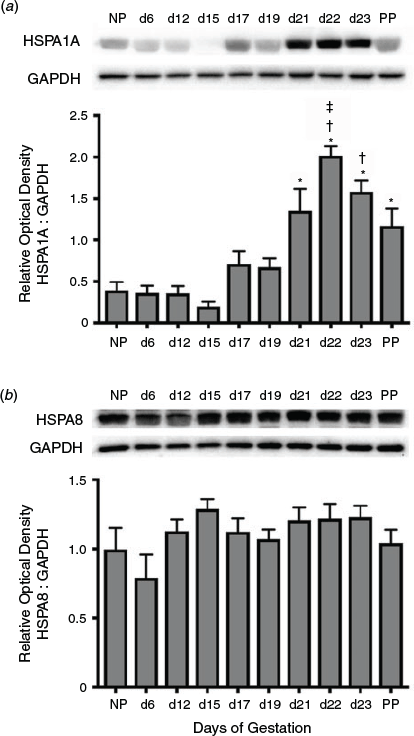
Immunoblot analysis was used to determine the temporal expression of HSPA1A during gestation (Fig. 1a). Total HSPA1A protein rapidly increased towards the end of pregnancy through to parturition. Expression of HSPA1A increased significantly from Day 21 of gestation to PP compared with NP, and Days 6, 12 and 15 of gestation (P < 0.05), and was significantly elevated on Days 22 and 23 of gestation compared with Days 17 and 19 (P < 0.05). HSPA1A expression on Day 22 was markedly elevated compared with PP (P < 0.05). In contrast, levels of the essential housekeeping protein HSPA8 were not significantly altered in the myometrium during gestation (Fig. 1b).
|
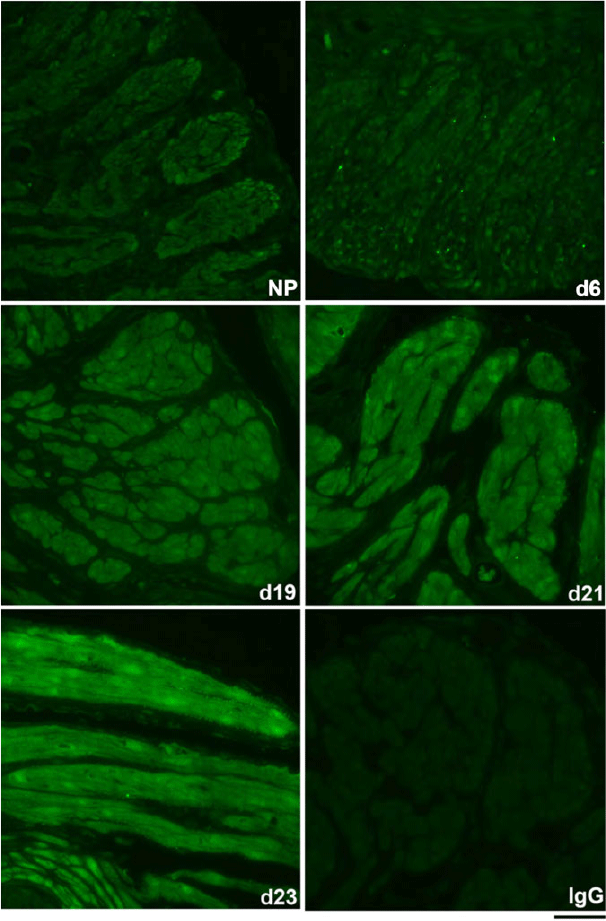
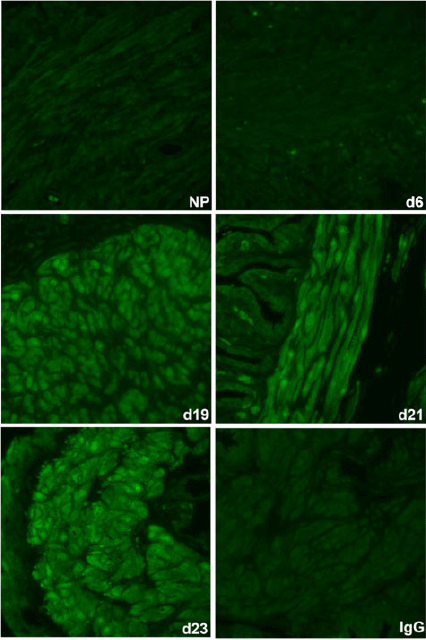
The spatiotemporal expression of HSPA1A was examined by immunofluorescence analysis. HSPA1A was nearly undetectable in myometrium from NP to Day 6 of gestation, but expression gradually increased in myocytes of longitudinal and circular muscle layers from Day 19 of gestation through to PP (Figs 2, 3). HSPA1A expression appeared greater in the longitudinal than circular layer. During this period, HSPA1A was found throughout the cytoplasm and in the nuclei of myocytes from both muscle layers (Fig. S1) and was frequently detectable in apparent extracellular areas just outside myocyte plasma membranes (Fig. 4).
|
|
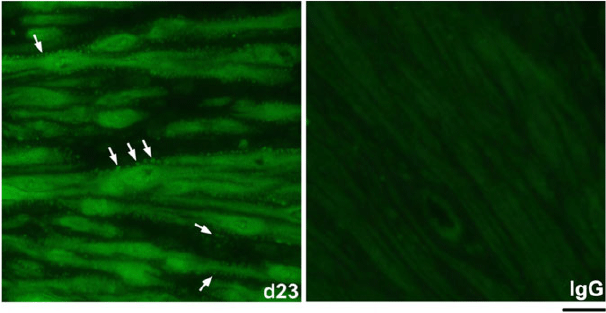
|
Effect of uterine distension on HSPA1A expression
Since uterine distension resulting from fetal growth is a significant source of mechanical stress during late pregnancy, myometrial protein extracts and uterine tissue were collected from unilaterally pregnant rats at Days 19 and 23 of gestation for both immunoblot and immunofluorescence analysis. Immunoblot analyses demonstrated that HSPA1A protein levels at Day 19 were not significantly altered by uterine distension in gravid compared with non-gravid horns (data not shown). In contrast, HSPA1A expression was significantly increased in gravid horns on Day 23 compared with non-gravid contralateral horns (P < 0.05; Fig. 5a). Similarly, immunofluorescence analysis revealed that HSPA1A detection at Day 23 was increased in the myometrium from gravid compared with corresponding non-gravid horns (Fig. 5b).
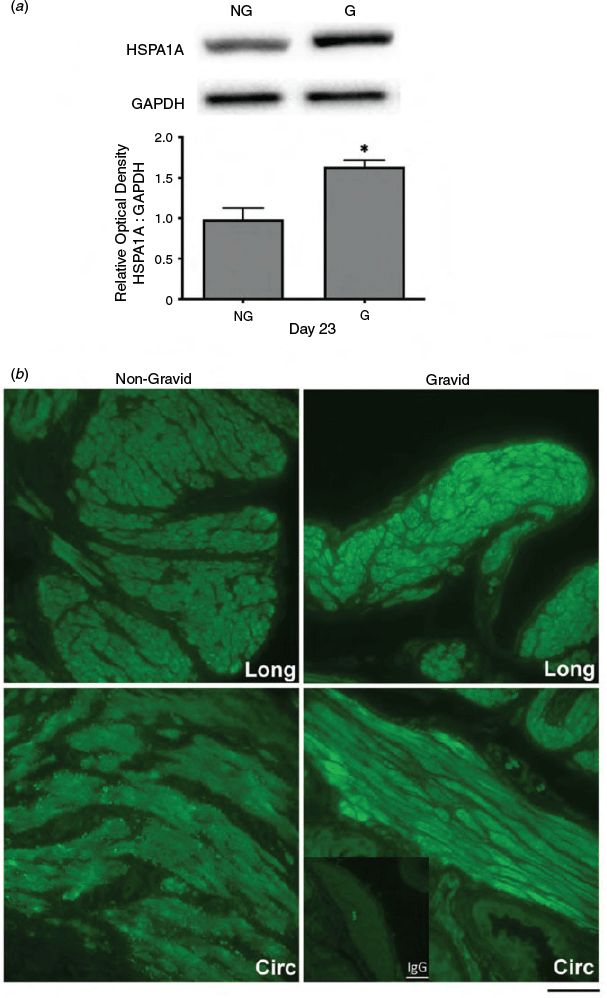
|
Expression of HSPA1A-associated chaperones and cochaperones
ST13 was dynamically expressed in the myometrium during pregnancy, with high levels in the early proliferative and late contractile as well as labour phases of myometrial programming (Fig. 6a). Specifically, expression on Day 6 of gestation remained similar to NP expression and levels at both time points, as well as on Days 22, 23 and PP, were significantly higher than on Days 12, 15, 17 and 19 of gestation (P < 0.05). In contrast to ST13, STIP-1 was constitutively expressed at high levels in the myometrium throughout pregnancy (Fig. 6b), whereas DNAJB1 expression was slightly higher at Day 12 of gestation compared with Days 19, 21, 22, 23 and PP (P < 0.05; Fig. 6c).
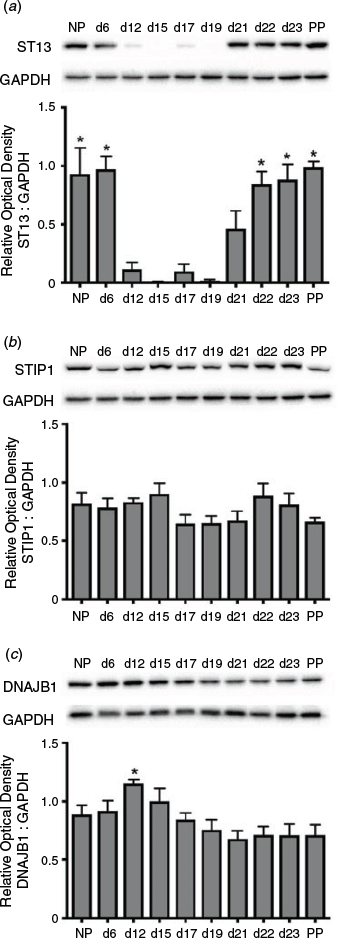
|
Assessment of HSPA1A as cargo of myometrial cell-derived EVs
Scanning electron microscopy examination of samples isolated from hTERT-HM cell-conditioned culture medium confirmed the presence of EVs ranging from 20 to 200 nm in diameter, representative of exosomes and small microvesicles (Fig. 7a). Samples were also examined for the presence of key marker proteins in EV preparations by SDS-PAGE and immunoblot analysis. EV preparations demonstrated an enrichment of mid to high molecular weight proteins (Fig. 7b). Furthermore, EV lysates contained Alix, CD63, TSG101, HSP90, HSPA8 and HSPA1A, but not the endoplasmic reticular protein calnexin (Fig. 7c).
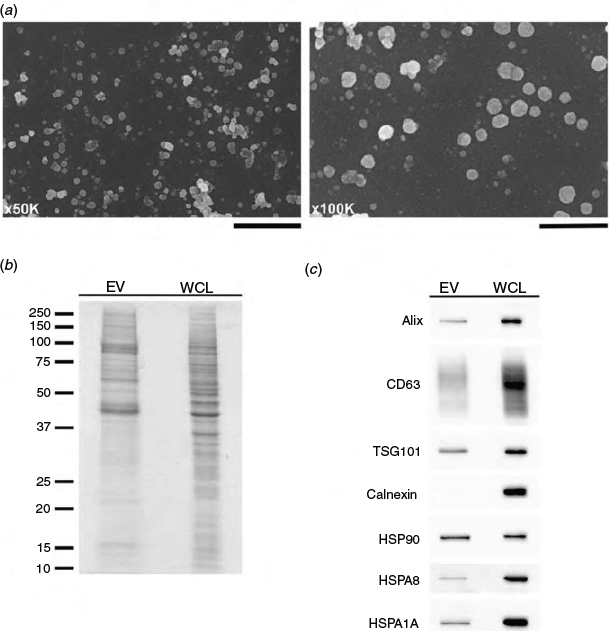
|
Discussion
HSPA1A, the major stress-inducible member of the HSPA family, is an ATP-dependent protein with important intracellular and extracellular roles, such as regulating the breakdown of misfolded or aggregated proteins and inducing inflammation respectively (De Maio and Vasquez 2013; Finka et al. 2015b). Nevertheless, the expression of this stress protein in the myometrium is poorly characterised. Thus, the objectives of the present study were to examine where and when HSPA1A was expressed in the rat myometrium during pregnancy and to assess whether HSPA1A could be secreted in myometrial cell-derived EVs.
Intracellular HSPA1A expression
In contrast with constitutive HSPA8 protein expression, HSPA1A protein expression in the myometrium rapidly increased towards the end of pregnancy through to parturition. Immunofluorescence detection of HSPA1A during this period was prominent throughout the cytoplasm and in the nuclei of myocytes from both longitudinal and circular muscle layers. We recently reported that the stress-induced HSP90AA1 and constitutively expressed HSP90AB1, as well as associated cochaperones p23/prostaglandin E synthase 3 (PTGES3) and activator of heat shock 90 kDa protein ATPase homologue 1 (AHSA1), were readily expressed in rat myometrium during pregnancy (Bhatti et al. 2019). The HSPA and HSP90 family members and their cochaperones DNAJB1, STIP1, ST13, PTGES3 and AHSA1 are important regulators of steroid hormone receptor family maturation and activation (Echeverria and Picard 2010). ST13 can also exhibit chaperone activity, aid in the interaction of HSPA1A with target proteins (Radons 2016) and was highly expressed at late pregnancy and labour in the present study, in parallel with HSPA1A expression. Using quantitative proteomics, Dhamad et al. (2016) showed that HSPA1A and HSPA8 were the two most abundant HSPs binding to the oestrogen receptor ERα, followed by HSP90AA1 and HSP90AB1. ERα mRNA and protein levels in the rat myometrium increase significantly during late pregnancy and labour, whereas progesterone receptor levels are readily detectable but unchanged during this period (Fang et al. 1996; Murata et al. 2003). Furthermore, HSPA1A can interact with ERα and associate with transcriptionally active chromatin (Dhamad et al. 2016). Thus, it is possible that through a HSP90–HSPA1A chaperone machine, increased myometrial HSPA1A expression during late pregnancy and labour may produce a shift to transcriptionally active, ligand-bound ERα–HSPA1A complexes, because HSPA1A was detected in both the cytoplasm and nuclei of myometrial cells during this period. This could facilitate ligand–ERα signalling as circulating concentrations of 17β-oestradiol rise for the initiation of parturition (Lye et al. 1993).
In unilaterally pregnant rats, HSPA1A protein expression in gravid horns on Day 23 of gestation was significantly increased and more immunodetectable in situ compared with non-gravid horns. In support of this finding, mechanical distension has also been shown to upregulate HSPA1A expression in the smooth muscle of the urinary bladder and vascular smooth muscle cells (Galvin et al. 2002; Metzler et al. 2003). Although there were no significant differences in HSPA1A expression or immunostaining on Day 19 of gestation between non-gravid and gravid horns (data not shown), this may just reflect a stress in the gravid horns below the threshold required to induce HSPA1A expression, because it became significantly elevated during gestation after Day 19. The distension stress would peak at Day 23 due to the maximum size of the conceptus. The effect of such a stress on HSPA1A expression is likely complex. Studies using rat and human myometrial cells have demonstrated that distension stress induces inflammation (Shynlova et al. 2013; Keelan 2018). Inflammation and oxidative stress are closely interrelated processes that can mutually amplify one another (Hajjar and Gotto 2013). In skeletal and cardiac muscles, as well as in organs such as the liver, oxidative stress was complemented by high levels of HSPA1A (Salo et al. 1991). When HSPA1A or HSPA8 were overexpressed in human amnion epithelial cells, interleukin (IL)-8 and Toll-like receptor (TLR) 4 mRNA expression increased, and these effects were abrogated upon respective short interfering RNA targeting (Geng et al. 2017). Thus, the expression of HSPA members in the myometrium may be influenced by the integrated effects of myometrial distension, inflammation and oxidative stress, and may play a role in regulating immune activation within the tissue.
Extracellular HSPA1A
From Day 19 of gestation onwards in this study, HSPA1A was frequently detectable in apparent extracellular areas just outside myocyte plasma membranes of both the longitudinal and circular layers of the myometrium. HSPA1A is considered an intracellular protein, but under stressful conditions it is now known to be released into the surrounding environment and circulation (De Maio and Vasquez 2013). HSPs, including HSPA1A, have been found to be released from cells as cargo in EVs (Bausero et al. 2005; Clayton et al. 2005; Conde-Vancells et al. 2008; Vega et al. 2008). In fact, HSPA and HSP90 family members are now considered markers of such EVs (Kalluri and LeBleu 2020). EVs produced by pregnancy-associated tissues, including the conceptus, uterus and trophoblast, as well as within the amniotic fluid and serum of pregnant women, have been reported to contain cargo such as small RNAs, mRNA and proteins (Fukushima et al. 2005; Asea et al. 2008; Stenqvist et al. 2013; Burns et al. 2016; Bidarimath et al. 2017). The roles of extracellular HSPA1A (eHSPA1A) revolve, in particular, around regulation of inflammation, including stimulating proinflammatory cytokine activity (Bausero et al. 2005).
In this study, the novel detection of EVs from hTERT-HM cell-conditioned medium that were free of contaminating endoplasmic reticular calnexin, confirmation of HSPA1A as EV cargo from these cells and the immunodetection of eHSPA1A in rat myometrium in situ suggest that myometrial cells in vivo could release HSPA1A during pregnancy as a cytokine in EVs. HSPA8 and HSPA1A in particular can become inserted into membranes and exist on the cell surface, dependent on membrane fluidity and phospholipid-specific association, enabling interaction with cell receptors (Ferrarini et al. 1992; Multhoff et al. 1995; Asea et al. 2000; McCallister et al. 2016). eHSPA1A is now considered a damage-associated molecular pattern (DAMP) that induces cytokine production and activation of nuclear factor-κB (Asea 2003). Asea et al. (2002) reported that eHSPA1A from human embryonic kidney cells bound in a CD14-dependent manner to TLR2 and TLR4 to stimulate the upregulation of tumour necrosis factor-α, IL-1β and IL-6.
The initiation and process of labour have been described as sterile inflammatory events (Shynlova et al. 2013). It has been observed that there is a marked increase in immune cell infiltration in pregnancy-associated tissues during labour (Hamilton et al. 2012). Leucocytes enhance the effects of proinflammatory cytokines, which, in turn, recruit additional leucocytes. Immune cells, such as macrophages and neutrophils, infiltrate the myometrium and cervix during labour and are implicated in triggering myometrial contractions and inducing cervical ripening via the local release of inflammatory mediators (Osman et al. 2003). The release of eHSPA1A from myometrial cells via EVs could result in uptake by additional myometrial cells, the interstitial telocytes of the myometrium that also produce EVs (Cretoiu et al. 2013), capillaries and immune cells in the region as part of an intercellular communication network to potentiate immune activation within the myometrium leading to parturition.
Evidence for the detection of eHSPA1A in pregnancy-related tissues or in sera from pregnant women has been reported previously. Molvarec et al. (2007) demonstrated a significant positive correlation between gestational age and serum HSPA1A concentrations. Furthermore, it has been reported that HSPA1A serum concentrations were significantly elevated in pregnant women at higher risk of preterm delivery or diagnosed with pre-eclampsia compared with healthy pregnant women (Fukushima et al. 2005). Term parturition has also been associated with elevated amniotic fluid concentrations of HSPA1A (Chaiworapongsa et al. 2008).
Conclusion
The present study is the first to demonstrate that HSPA1A expression in the myometrium is significantly upregulated in late pregnancy and labour, localises to both the cytoplasm and nuclei of myocytes and is regulated, in part, by uterine distension. Intracellular HSPA1A could act as part of a chaperone machine to regulate the function of steroid receptors, but novel detection of HSPA1A-laden EVs from human-derived myometrial cells suggests eHSPA1A could be packaged out of myometrial cells as a cytokine to act as a significant mediator of the inflammatory processes and intercellular communication cascades required for parturition. Such dual roles in other cells have led to the designation of HSPA1A as a chaperokine (Asea 2003). Investigations at the molecular level are required to understand the mechanism(s) of HSPA1A action intracellularly and extracellularly within the myometrium. Furthermore, specific assessment of the range of vesicle sizes produced by myometrial-derived hTERT-HM cells and potential size-related functions are warranted.
Conflicts of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgements
This research was supported by grants awarded to D. J. MacPhee from the Natural Sciences and Engineering Research Council of Canada (Discovery Grant no. RGPIN-2017-04951) and the Canada Foundation for Innovation John R. Evans Leaders Fund (Project no. 32512). The authors thank Eiko Kawamura and Larhonda Sobchishin of the Western College of Veterinary Medicine Imaging Centre (University of Saskatchewan) for assistance with electron microscopy, EV sample preparation and imaging. The authors also gratefully acknowledge the Departments of Physiology and Pharmacology and Veterinary Biomedical Sciences for stipend support for Mckenzie F. Russell and Georgia C. Bailey respectively.
References
Asea, A. (2003). Chaperokine-induced signal transduction pathways. Exerc. Immunol. Rev. 9, 25–33.| 14686091PubMed |
Asea, A., Kraeft, S.-K., Kurt-Jones, E. A., Stevenson, M. A., Chen, L. B., Finberg, R. W., Koo, G. C., and Calderwood, S. K. (2000). HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 6, 435–442.
| HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine.Crossref | GoogleScholarGoogle Scholar | 10742151PubMed |
Asea, A., Rehli, M., Kabingu, E., Boch, J. A., Bare, O., Auron, P. E., Stevenson, M. A., and Calderwood, S. K. (2002). Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277, 15028–15034.
| Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4.Crossref | GoogleScholarGoogle Scholar | 11836257PubMed |
Asea, A., Jean-Pierre, C., Kaur, P., Rao, P., Linhares, I. M., Skupski, D., and Witkin, S. S. (2008). Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J. Reprod. Immunol. 79, 12–17.
| Heat shock protein-containing exosomes in mid-trimester amniotic fluids.Crossref | GoogleScholarGoogle Scholar | 18715652PubMed |
Bausero, M. A., Gastpar, R., Multhoff, G., and Asea, A. (2005). Alternative mechanism by which IFN-gamma enhances tumor recognition: active release of heat shock protein 72. J. Immunol. 175, 2900–2912.
| Alternative mechanism by which IFN-gamma enhances tumor recognition: active release of heat shock protein 72.Crossref | GoogleScholarGoogle Scholar | 16116176PubMed |
Bhatti, M., Dinn, S., Miskiewicz, E. I., and MacPhee, D. J. (2019). Expression of heat shock factor 1, heat shock protein 90 and associated signaling proteins in pregnant rat myometrium: implications for myometrial proliferation. Reprod. Biol. 19, 374–385.
| Expression of heat shock factor 1, heat shock protein 90 and associated signaling proteins in pregnant rat myometrium: implications for myometrial proliferation.Crossref | GoogleScholarGoogle Scholar | 31522994PubMed |
Bidarimath, M., Khalaj, K., Kridli, R. T., Kan, F. W. K., Koti, M., and Tayade, C. (2017). Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: a new paradigm for conceptus endometrial cross-talk. Sci. Rep. 7, 40476.
| Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: a new paradigm for conceptus endometrial cross-talk.Crossref | GoogleScholarGoogle Scholar | 28079186PubMed |
Brocchieri, L., de Conway, M. E., and Macario, A. J. (2008). hsp70 genes in the human genome: conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol. Biol. 8, 19.
| hsp70 genes in the human genome: conservation and differentiation patterns predict a wide array of overlapping and specialized functions.Crossref | GoogleScholarGoogle Scholar | 18215318PubMed |
Burns, G. W., Brooks, K. E., and Spencer, T. E. (2016). Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol. Reprod. 94, 56.
| Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep.Crossref | GoogleScholarGoogle Scholar | 26819476PubMed |
Chaiworapongsa, T., Erez, O., Kusanovic, J. P., Vaisbuch, E., Mazaki-Tovi, S., Gotsch, F., Than, N. G., Mittal, P., Kim, Y. M., Camacho, N., Edwin, S., Gomez, R., Hassan, S. S., and Romero, R. (2008). Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J. Matern. Fetal Neonatal Med. 21, 449–461.
| Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition.Crossref | GoogleScholarGoogle Scholar | 18570125PubMed |
Clayton, A., Turkes, A., Navabi, H., Mason, M. D., and Tabi, Z. (2005). Induction of heat shock proteins in B-cell exosomes. J. Cell Sci. 118, 3631–3638.
| Induction of heat shock proteins in B-cell exosomes.Crossref | GoogleScholarGoogle Scholar | 16046478PubMed |
Colombo, M., Raposo, G., and Théry, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289.
| Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles.Crossref | GoogleScholarGoogle Scholar | 25288114PubMed |
Conde-Vancells, J., Rodriguez-Suarez, E., Embade, N., Gill, D., Matthiesen, R., Valle, M., Elortza, F., Lu, S. C., Mato, J. M., and Falcon-Perez, J. M. (2008). Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J. Proteome Res. 7, 5157–5166.
| Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes.Crossref | GoogleScholarGoogle Scholar | 19367702PubMed |
Condon, J., Yin, S., Mayhew, B., Word, R. A., Wright, W. E., Shay, J. W., and Rainey, W. E. (2002). Telomerase immortalization of human myometrial cells. Biol. Reprod. 67, 506–514.
| Telomerase immortalization of human myometrial cells.Crossref | GoogleScholarGoogle Scholar | 12135889PubMed |
Cretoiu, S. M., Cretoiu, D., Marin, A., Radu, B. M., and Popescu, L. M. (2013). Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium. Reproduction 145, 357–370.
| Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium.Crossref | GoogleScholarGoogle Scholar | 23404846PubMed |
De Maio, A. (2011). Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. Cell Stress Chaperones 16, 235–249.
| Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage.Crossref | GoogleScholarGoogle Scholar | 20963644PubMed |
De Maio, A., and Vasquez, D. (2013). Extracellular heat shock proteins: a new location, a new function. Shock 40, 239–246.
| Extracellular heat shock proteins: a new location, a new function.Crossref | GoogleScholarGoogle Scholar | 23807250PubMed |
Deng, F., and Miller, J. (2019). A review on protein markers of exosome from different bio-resources and the antibodies used for characterization. J. Histotechnol. 42, 226–239.
| A review on protein markers of exosome from different bio-resources and the antibodies used for characterization.Crossref | GoogleScholarGoogle Scholar | 31432761PubMed |
Dhamad, A. E., Zhou, Z., Zhou, J., and Du, Y. (2016). Systematic proteomic identification of the heat shock proteins (Hsp) that interact with estrogen receptor alpha (ERα) and biochemical characterization of the ERα–Hsp70 interaction. PLoS One 11, e0160312.
| Systematic proteomic identification of the heat shock proteins (Hsp) that interact with estrogen receptor alpha (ERα) and biochemical characterization of the ERα–Hsp70 interaction.Crossref | GoogleScholarGoogle Scholar | 27483141PubMed |
Echeverria, P. C., and Picard, D. (2010). Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim. Biophys. Acta 1803, 641–649.
| Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility.Crossref | GoogleScholarGoogle Scholar | 20006655PubMed |
Fang, X., Wong, S., and Mitchell, B. F. (1996). Relationships among sex steroids, oxytocin and their receptors in the rat uterus during late gestation and at parturition. Endocrinology 137, 3213–3219.
| Relationships among sex steroids, oxytocin and their receptors in the rat uterus during late gestation and at parturition.Crossref | GoogleScholarGoogle Scholar | 8754742PubMed |
Ferrarini, M., Heltai, S., Zocchi, M. R., and Rugarli, C. (1992). Unusual expression and localization of heat-shock proteins in human tumor cells. Int. J. Cancer 51, 613–619.
| Unusual expression and localization of heat-shock proteins in human tumor cells.Crossref | GoogleScholarGoogle Scholar | 1601523PubMed |
Finka, A., Sood, V., Quadroni, M., Rios, P. L., and Goloubinoff, P. (2015a). Quantitative proteomics of heat-treated human cells show an across-the-board mild depletion of housekeeping proteins to massively accumulate few HSPs. Cell Stress Chaperones 20, 605–620.
| Quantitative proteomics of heat-treated human cells show an across-the-board mild depletion of housekeeping proteins to massively accumulate few HSPs.Crossref | GoogleScholarGoogle Scholar | 25847399PubMed |
Finka, A., Sharma, S. K., and Goloubinoff, P. (2015b). Multi-layered molecular mechanisms of polypeptide holding, unfolding, and disaggregation by HSP70/HSP110 chaperones. Front. Mol. Biosci. 2, 29.
| Multi-layered molecular mechanisms of polypeptide holding, unfolding, and disaggregation by HSP70/HSP110 chaperones.Crossref | GoogleScholarGoogle Scholar | 26097841PubMed |
Fukushima, A., Kawahara, H., Isurugi, C., Syoji, T., Oyama, R., Sugiyama, T., and Horiuchi, S. (2005). Changes in serum levels of heat shock protein 70 in preterm delivery and pre-eclampsia. J. Obstet. Gynaecol. Res. 31, 72–77.
| Changes in serum levels of heat shock protein 70 in preterm delivery and pre-eclampsia.Crossref | GoogleScholarGoogle Scholar | 15669997PubMed |
Galvin, D. J., Watson, R. W., Gillespie, J. I., Brady, H., and Fitzpatrick, J. M. (2002). Mechanical stretch regulates cell survival in human bladder smooth muscle cells in vitro. Am. J. Physiol. Renal Physiol. 283, F1192–F1199.
| Mechanical stretch regulates cell survival in human bladder smooth muscle cells in vitro.Crossref | GoogleScholarGoogle Scholar | 12388384PubMed |
Geng, J., Li, H., Huang, C., Chai, J., Zheng, R., Li, F., and Jiang, S. (2017). Functional analysis of HSPA1A and HSPA8 in parturition. Biochem. Biophys. Res. Commun. 483, 371–379.
| Functional analysis of HSPA1A and HSPA8 in parturition.Crossref | GoogleScholarGoogle Scholar | 28025138PubMed |
Hajjar, D. P., and Gotto, A. M. (2013). Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. Am. J. Pathol. 182, 1474–1481.
| Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases.Crossref | GoogleScholarGoogle Scholar | 23608224PubMed |
Hamilton, S., Oomomian, Y., Stephen, G., Shynlova, O., Tower, C. L., Garrod, A., Lye, S. J., and Jones, R. L. (2012). Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol. Reprod. 86, 39.
| Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor.Crossref | GoogleScholarGoogle Scholar | 22011391PubMed |
Hartl, F. U., Bracher, A., and Hayer-Hartl, M. (2011). Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332.
| Molecular chaperones in protein folding and proteostasis.Crossref | GoogleScholarGoogle Scholar | 21776078PubMed |
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367, eaau6977.
| The biology, function, and biomedical applications of exosomes.Crossref | GoogleScholarGoogle Scholar | 32029601PubMed |
Kampinga, H. H., Hageman, J., Vos, M. J., Kubota, H., Tanguay, R. M., Bruford, E. A., Cheetham, M. E., Chen, B., and Hightower, L. E. (2009). Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14, 105–111.
| Guidelines for the nomenclature of the human heat shock proteins.Crossref | GoogleScholarGoogle Scholar | 18663603PubMed |
Keelan, J. A. (2018). Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J. Reprod. Immunol. 125, 89–99.
| Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth.Crossref | GoogleScholarGoogle Scholar | 29329080PubMed |
Lackie, R. E., Maciejewski, A., Ostapchenko, V. G., Marques-Lopes, J., Choy, W.-Y., Duennwald, M. L., Prado, V. F., and Prado, M. A. M. (2017). The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases. Front. Neurosci. 11, 254.
| The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases.Crossref | GoogleScholarGoogle Scholar | 28559789PubMed |
Lye, S. J., Nicholson, B. J., Mascarenhas, M., MacKenzie, L., and Petrocelli, T. (1993). Increased expression of connexin-43 in the rat myometrium during labour is associated with an increase in the plasma estrogen:progesterone ratio. Endocrinology 132, 2380–2386.
| Increased expression of connexin-43 in the rat myometrium during labour is associated with an increase in the plasma estrogen:progesterone ratio.Crossref | GoogleScholarGoogle Scholar | 8389279PubMed |
MacPhee, D. J., and Miskiewicz, E. I. (2017). The potential functions of small heat shock proteins in the uterine musculature during pregnancy. Adv. Anat. Embryol. Cell Biol. 222, 95–116.
| The potential functions of small heat shock proteins in the uterine musculature during pregnancy.Crossref | GoogleScholarGoogle Scholar | 28389752PubMed |
McCallister, C., Kdeiss, B., and Nikolaidis, N. (2016). Biochemical characterization of the interaction between HspA1A and phospholipids. Cell Stress Chaperones 21, 41–53.
| Biochemical characterization of the interaction between HspA1A and phospholipids.Crossref | GoogleScholarGoogle Scholar | 26342809PubMed |
Metzler, B., Abia, R., Ahmad, M., Wernig, F., Pachinger, O., Hu, Y., and Xu, Q. (2003). Activation of heat shock transcription factor 1 in atherosclerosis. Am. J. Pathol. 162, 1669–1676.
| Activation of heat shock transcription factor 1 in atherosclerosis.Crossref | GoogleScholarGoogle Scholar | 12707051PubMed |
Molvarec, A., Rigo, J., Nagy, B., Walentin, S., Szalay, J., Fust, G., Karadi, I., and Prohaszka, Z. (2007). Serum heat shock protein 70 levels are decreased in normal human pregnancy. J. Reprod. Immunol. 74, 163–169.
| Serum heat shock protein 70 levels are decreased in normal human pregnancy.Crossref | GoogleScholarGoogle Scholar | 17296233PubMed |
Multhoff, G., Botzler, C., Wiesnet, M., Muller, E., Meier, T., and Wilmanns, W. (1995). A stress-inducible 72 kDa heat shock protein (Hsp72) is expressed on the surface of human tumor cells but not on normal cells. Int. J. Cancer 61, 272–279.
| A stress-inducible 72 kDa heat shock protein (Hsp72) is expressed on the surface of human tumor cells but not on normal cells.Crossref | GoogleScholarGoogle Scholar | 7705958PubMed |
Murata, T., Narita, K., Honda, K., Matsukawa, S., and Higuchi, T. (2003). Differential regulation of estrogen receptor α and β mRNAs in the rat uterus during pregnancy and labor: possible involvement of estrogen receptors in oxytocin receptor regulation. Endocr. J. 50, 579–587.
| Differential regulation of estrogen receptor α and β mRNAs in the rat uterus during pregnancy and labor: possible involvement of estrogen receptors in oxytocin receptor regulation.Crossref | GoogleScholarGoogle Scholar | 14614214PubMed |
Nicoletti, J. G., White, B. G., Miskiewicz, E. I., and MacPhee, D. J. (2016). Induction of expression and phosphorylation of heat shock protein B5 (CRYAB) in rat myometrium during pregnancy and labour. Reproduction 152, 69–79.
| Induction of expression and phosphorylation of heat shock protein B5 (CRYAB) in rat myometrium during pregnancy and labour.Crossref | GoogleScholarGoogle Scholar | 27107034PubMed |
Oldenhof, A. D., Shynlova, O. P., Liu, M., Langille, B. L., and Lye, S. J. (2002). Mitogen-activated protein kinases mediate stretch-induced c-fos mRNA expression in myometrial smooth muscle cells. Am. J. Physiol. Cell Physiol. 283, C1530–C1539.
| Mitogen-activated protein kinases mediate stretch-induced c-fos mRNA expression in myometrial smooth muscle cells.Crossref | GoogleScholarGoogle Scholar | 12372814PubMed |
Osman, I., Young, A., Ledingham, M. A., Thomson, A. J., Jordan, F., Greer, I. A., and Norman, J. E. (2003). Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 9, 41–45.
| Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term.Crossref | GoogleScholarGoogle Scholar | 12529419PubMed |
Ou, C.-W., Orsino, A., and Lye, S. J. (1997). Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology 138, 5398–5407.
| Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals.Crossref | GoogleScholarGoogle Scholar | 9389525PubMed |
Radons, J. (2016). The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones 21, 379–404.
| The human HSP70 family of chaperones: where do we stand?Crossref | GoogleScholarGoogle Scholar | 26865365PubMed |
Salo, D. C., Donovan, C. M., and Davies, K. J. A. (1991). HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic. Biol. Med. 11, 239–246.
| HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise.Crossref | GoogleScholarGoogle Scholar | 1937141PubMed |
Shynlova, O., Mitchell, J. A., Tsampalieros, A., Langille, B. L., and Lye, S. J. (2004). Progesterone and gravidity differentially regulate expression of extracellular matrix components in the pregnant rat myometrium. Biol. Reprod. 70, 986–992.
| Progesterone and gravidity differentially regulate expression of extracellular matrix components in the pregnant rat myometrium.Crossref | GoogleScholarGoogle Scholar | 14645109PubMed |
Shynlova, O., Tsui, P., Dorogin, A., Langille, B. L., and Lye, S. J. (2007). Insulin-like growth factors and their binding proteins define specific phases of myometrial differentiation during pregnancy in the rat. Biol. Reprod. 76, 571–578.
| Insulin-like growth factors and their binding proteins define specific phases of myometrial differentiation during pregnancy in the rat.Crossref | GoogleScholarGoogle Scholar | 17123939PubMed |
Shynlova, O., Tsui, P., Dorogin, A., and Lye, S. J. (2008). Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labour. J. Immunol. 181, 1470–1479.
| Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labour.Crossref | GoogleScholarGoogle Scholar | 18606702PubMed |
Shynlova, O., Tsui, P., Jaffer, S., and Lye, S. J. (2009). Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur. J. Obstet. Gynecol. Reprod. Biol. 144, S2–S10.
| Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour.Crossref | GoogleScholarGoogle Scholar | 19299064PubMed |
Shynlova, O., Kwong, R., and Lye, S. J. (2010). Mechanical stretch regulates hypertrophic phenotype of the myometrium during pregnancy. Reproduction 139, 247–253.
| Mechanical stretch regulates hypertrophic phenotype of the myometrium during pregnancy.Crossref | GoogleScholarGoogle Scholar | 19776098PubMed |
Shynlova, O., Lee, Y. H., Srikhajon, K., and Lye, S. J. (2013). Physiologic uterine inflammation and labour onset: integration of endocrine and mechanical signals. Reprod. Sci. 20, 154–167.
| Physiologic uterine inflammation and labour onset: integration of endocrine and mechanical signals.Crossref | GoogleScholarGoogle Scholar | 22614625PubMed |
Shynlova, O., Nadeem, L., Zhang, J., Dunk, C., and Lye, S. J. (2020). Myometrial activation: novel concepts underlying labor. Placenta 92, 28–36.
| Myometrial activation: novel concepts underlying labor.Crossref | GoogleScholarGoogle Scholar | 32056784PubMed |
Sisti, G., Kanninen, T. T., Ramer, I., and Witkin, S. S. (2015). Interaction between the inducible 70-kDa heat shock protein and autophagy: effects on fertility and pregnancy. Cell Stress Chaperones 20, 753–758.
| Interaction between the inducible 70-kDa heat shock protein and autophagy: effects on fertility and pregnancy.Crossref | GoogleScholarGoogle Scholar | 26081752PubMed |
Stahl, P. D., and Raposo, G. (2019). Extracellular vesicles: exosomes and microvesicles, integrators of homeostasis. Physiology (Bethesda) 34, 169–177.
| Extracellular vesicles: exosomes and microvesicles, integrators of homeostasis.Crossref | GoogleScholarGoogle Scholar | 30968753PubMed |
Stenqvist, A.-C., Nagaeva, O., Baranov, V., and Mincheva-Nilsson, L. (2013). Exosomes secreted by human placenta carry functional fas ligand and trail molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol. 191, 5515–5523.
| Exosomes secreted by human placenta carry functional fas ligand and trail molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus.Crossref | GoogleScholarGoogle Scholar | 24184557PubMed |
Tabb, T., Thilander, G., Grover, A., Hertzberg, E., and Garfield, R. (1992). An immunochemical and immunocytologic study of the increase in myometrial gap junctions (and connexin 43) in rats and humans during pregnancy. Am. J. Obstet. Gynecol. 167, 559–567.
| An immunochemical and immunocytologic study of the increase in myometrial gap junctions (and connexin 43) in rats and humans during pregnancy.Crossref | GoogleScholarGoogle Scholar | 1323215PubMed |
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., Antoniou, A., Arab, T., Archer, F., Atkin-Smith, G. K., Ayre, D. C., Bach, J.-M., Bachurski, D., Baharvand, H., Balaj, L., Baldacchino, S., Bauer, N. N., Baxter, A. A., Bebawy, M., Beckham, C., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750.
| Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines.Crossref | GoogleScholarGoogle Scholar | 30637094PubMed |
Tkach, M., and Théry, C. (2016). Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232.
| Communication by extracellular vesicles: where we are and where we need to go.Crossref | GoogleScholarGoogle Scholar | 26967288PubMed |
Vega, V. L., Rodriguez-Silva, M., Frey, T., Gehrmann, M., Diaz, J. C., Steinem, C., Multhoff, G., Arispe, N., and De Maio, A. (2008). Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J. Immunol. 180, 4299–4307.
| Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages.Crossref | GoogleScholarGoogle Scholar | 18322243PubMed |
White, B. G., and MacPhee, D. J. (2011). Distension of the uterus induces HspB1 expression in rat uterine smooth muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1418–R1426.
| Distension of the uterus induces HspB1 expression in rat uterine smooth muscle.Crossref | GoogleScholarGoogle Scholar | 21900647PubMed |
Williams, S. J., White, B. G., and MacPhee, D. J. (2005). Expression of α5 integrin (Itga5) is elevated in the rat myometrium during late pregnancy and labour: implications for development of a mechanical syncytium. Biol. Reprod. 72, 1114–1124.
| Expression of α5 integrin (Itga5) is elevated in the rat myometrium during late pregnancy and labour: implications for development of a mechanical syncytium.Crossref | GoogleScholarGoogle Scholar | 15635129PubMed |
Williams, S. J., Shynlova, O., Lye, S. J., and MacPhee, D. J. (2010). Spatiotemporal expression of α1, α3 and β1 integrin subunits is altered in rat myometrium during pregnancy and labour. Reprod. Fertil. Dev. 22, 718–732.
| Spatiotemporal expression of α1, α3 and β1 integrin subunits is altered in rat myometrium during pregnancy and labour.Crossref | GoogleScholarGoogle Scholar | 20353731PubMed |
Yamamoto, S., Subedi, G. P., Hanashima, S., Satoh, T., Otaka, M., Wakui, H., Sawada, K.-I., Yokota, S.-I., Yamaguchi, Y., Kubota, H., and Itoh, H. (2014). ATPase activity and ATP-dependent conformational change in the co-chaperone HSP70/HSP90-organizing protein (HOP). J. Biol. Chem. 289, 9880–9886.
| ATPase activity and ATP-dependent conformational change in the co-chaperone HSP70/HSP90-organizing protein (HOP).Crossref | GoogleScholarGoogle Scholar | 24535459PubMed |


