Deletion of the PDZ-binding kinase (Pbk) gene does not affect male fertility in mice
Yuka Miki A , Lalitha Devi B C , Yuji Imai B , Naojiro Minami A , Tsuyoshi Koide B and Sandeep Goel A C D
A C D
A Laboratory of Reproductive Biology, Graduate School of Agriculture, Kyoto University, Kyoto 606-8502, Japan.
B Mouse Genomics Resource Laboratory, National Institute of Genetics, 1111 Yata, Mishima, Shizuoka-ken 411-8540, Japan.
C Laboratory for the Conservation of Endangered Species, Centre for Cellular and Molecular Biology, Council for Scientific and Industrial Research, Uppal Road, Hyderabad 500 007, India.
D Corresponding author. Email: sandeep@ccmb.res.in
Reproduction, Fertility and Development 32(10) 893-902 https://doi.org/10.1071/RD19445
Submitted: 5 December 2019 Accepted: 23 April 2020 Published: 9 June 2020
Abstract
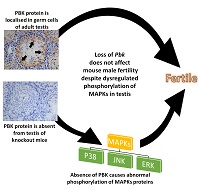
The PDZ-binding kinase (PBK) protein is localised exclusively in spermatogenic cells, such as spermatogonia, spermatocytes and round spermatids, of the adult testis. However, its role in male fertility remains unknown. Analysis of adult Pbk-knockout (KO) male mice showed no significant difference in the weight of the testes, epididymis and seminal vesicle compared with adult wild-type (WT) mice. There were no significant differences in testis morphology, tubule diameter and the number of offspring born to females mated with KO or WT male mice. Sperm number, motility and morphology did not differ significantly between KO and WT mice. The oocyte fertilisation rate and embryo development following IVF were comparable between groups fertilised using spermatozoa from KO versus WT mice (P > 0.05). Further analysis revealed that the phosphorylation of the mitogen-activated protein kinases (MAPKs) p38 kinase, c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinases was dysregulated in the testis of KO mice. In conclusion, Pbk-KO male mice are fertile and their spermatozoa and testis do not show any morphological and functional abnormalities despite the dysregulated phosphorylation of MAPKs. It is likely that functional redundancy of PBK and overlapping substrate specificities of the MAPK superfamily compensated for the loss of PBK from the testis.
Additional keywords: gametogenesis, IVF, testis.
References
Abe, Y., Matsumoto, S., Kito, K., and Ueda, N. (2000). Cloning and expression of a novel MAPKK-like protein kinase, lymphokine-activated killer T-cell-originated protein kinase, specifically expressed in the testis and activated lymphoid cells. J. Biol. Chem. 275, 21525–21531.| Cloning and expression of a novel MAPKK-like protein kinase, lymphokine-activated killer T-cell-originated protein kinase, specifically expressed in the testis and activated lymphoid cells.Crossref | GoogleScholarGoogle Scholar | 10781613PubMed |
Almog, T., and Naor, Z. (2008). Mitogen activated protein kinases (MAPKs) as regulators of spermatogenesis and spermatozoa functions. Mol. Cell. Endocrinol. 282, 39–44.
| Mitogen activated protein kinases (MAPKs) as regulators of spermatogenesis and spermatozoa functions.Crossref | GoogleScholarGoogle Scholar | 18177996PubMed |
Avilés, M., Castells, M. T., Martínez-Menárguez, J. A., Abascal, I., and Ballesta, J. (1997). Localization of penultimate carbohydrate residues in zona pellucida and acrosomes by means of lectin cytochemistry and enzymatic treatments. Histochem. J. 29, 583–592.
| Localization of penultimate carbohydrate residues in zona pellucida and acrosomes by means of lectin cytochemistry and enzymatic treatments.Crossref | GoogleScholarGoogle Scholar | 9347355PubMed |
Brown-Clay, J. D., Shenoy, D. N., Timofeeva, O., Kallakury, B. V., Nandi, A. K., and Banerjee, P. P. (2015). PBK/TOPK enhances aggressive phenotype in prostate cancer via beta-catenin-TCF/LEF-mediated matrix metalloproteinases production and invasion. Oncotarget 6, 15594–15609.
| PBK/TOPK enhances aggressive phenotype in prostate cancer via beta-catenin-TCF/LEF-mediated matrix metalloproteinases production and invasion.Crossref | GoogleScholarGoogle Scholar | 25909225PubMed |
Devi, L., Pothana, L., and Goel, S. (2017). Dysregulation of angiogenesis-specific signalling in adult testis results in xenograft degeneration. Sci. Rep. 7, 2605.
| Dysregulation of angiogenesis-specific signalling in adult testis results in xenograft degeneration.Crossref | GoogleScholarGoogle Scholar | 28572601PubMed |
Dougherty, J. D., Garcia, A. D., Nakano, I., Livingstone, M., Norris, B., Polakiewicz, R., Wexler, E. M., Sofroniew, M. V., Kornblum, H. I., and Geschwind, D. H. (2005). PBK/TOPK, a proliferating neural progenitor-specific mitogen-activated protein kinase kinase. J. Neurosci. 25, 10773–10785.
| PBK/TOPK, a proliferating neural progenitor-specific mitogen-activated protein kinase kinase.Crossref | GoogleScholarGoogle Scholar | 16291951PubMed |
Ewen, K., Jackson, A., Wilhelm, D., and Koopman, P. (2010). A male-specific role for p38 mitogen-activated protein kinase in germ cell sex differentiation in mice. Biol. Reprod. 83, 1005–1014.
| A male-specific role for p38 mitogen-activated protein kinase in germ cell sex differentiation in mice.Crossref | GoogleScholarGoogle Scholar | 20739663PubMed |
Fujibuchi, T., Abe, Y., Takeuchi, T., Ueda, N., Shigemoto, K., Yamamoto, H., and Kito, K. (2005). Expression and phosphorylation of TOPK during spermatogenesis. Dev. Growth Differ. 47, 637–644.
| Expression and phosphorylation of TOPK during spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 16316408PubMed |
Haeussler, M., Schonig, K., Eckert, H., Eschstruth, A., Mianne, J., Renaud, J. B., Schneider-Maunoury, S., Shkumatava, A., Teboul, L., Kent, J., Joly, J. S., and Concordet, J. P. (2016). Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 17, 148.
| Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR.Crossref | GoogleScholarGoogle Scholar | 27380939PubMed |
Ho, Y., Wigglesworth, K., Eppig, J. J., and Schultz, R. M. (1995). Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol. Reprod. Dev. 41, 232–238.
| Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression.Crossref | GoogleScholarGoogle Scholar | 7654376PubMed |
Jackson, A. C., Tenniswood, M., Bird, C. E., and Clark, A. F. (1977). Effects of androgen and estradiol administration on the weight of the ventral prostate, seminal vesicles, and testes of immature rats. Invest. Urol. 14, 351–355.
| 844996PubMed |
Johnson, C., Jia, Y., Wang, C., Lue, Y. H., Swerdloff, R. S., Zhang, X. S., Hu, Z. Y., Li, Y. C., Liu, Y. X., and Hikim, A. P. (2008). Role of caspase 2 in apoptotic signaling in primate and murine germ cells. Biol. Reprod. 79, 806–814.
| Role of caspase 2 in apoptotic signaling in primate and murine germ cells.Crossref | GoogleScholarGoogle Scholar | 18614702PubMed |
Li, M. W., Mruk, D. D., and Cheng, C. Y. (2009a). Mitogen-activated protein kinases in male reproductive function. Trends Mol. Med. 15, 159–168.
| Mitogen-activated protein kinases in male reproductive function.Crossref | GoogleScholarGoogle Scholar | 19303360PubMed |
Li, M. W. M., Mruk, D. D., and Cheng, C. Y. (2009b). Mitogen-activated protein kinases in male reproductive function. Trends Mol. Med. 15, 159–168.
| Mitogen-activated protein kinases in male reproductive function.Crossref | GoogleScholarGoogle Scholar |
Li, S., Zhu, F., Zykova, T., Kim, M. O., Cho, Y. Y., Bode, A. M., Peng, C., Ma, W., Carper, A., Langfald, A., and Dong, Z. (2011). T-LAK cell-originated protein kinase (TOPK) phosphorylation of MKP1 protein prevents solar ultraviolet light-induced inflammation through inhibition of the p38 protein signaling pathway. J. Biol. Chem. 286, 29601–29609.
| T-LAK cell-originated protein kinase (TOPK) phosphorylation of MKP1 protein prevents solar ultraviolet light-induced inflammation through inhibition of the p38 protein signaling pathway.Crossref | GoogleScholarGoogle Scholar | 21715333PubMed |
Naito, Y., Hino, K., Bono, H., and Ui-Tei, K. (2015). CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123.
| CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites.Crossref | GoogleScholarGoogle Scholar | 25414360PubMed |
Nandi, A., Tidwell, M., Karp, J., and Rapoport, A. P. (2004). Protein expression of PDZ-binding kinase is up-regulated in hematologic malignancies and strongly down-regulated during terminal differentiation of HL-60 leukemic cells. Blood Cells Mol. Dis. 32, 240–245.
| Protein expression of PDZ-binding kinase is up-regulated in hematologic malignancies and strongly down-regulated during terminal differentiation of HL-60 leukemic cells.Crossref | GoogleScholarGoogle Scholar | 14757441PubMed |
Ni, F. D., Hao, S. L., and Yang, W. X. (2019). Multiple signaling pathways in Sertoli cells: recent findings in spermatogenesis. Cell Death Dis. 10, 541.
| Multiple signaling pathways in Sertoli cells: recent findings in spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 31316051PubMed |
Ohashi, T., Komatsu, S., Ichikawa, D., Miyamae, M., Okajima, W., Imamura, T., Kiuchi, J., Nishibeppu, K., Kosuga, T., Konishi, H., Shiozaki, A., Fujiwara, H., Okamoto, K., Tsuda, H., and Otsuji, E. (2016). Overexpression of PBK/TOPK contributes to tumor development and poor outcome of esophageal squamous cell carcinoma. Anticancer Res. 36, 6457–6466.
| Overexpression of PBK/TOPK contributes to tumor development and poor outcome of esophageal squamous cell carcinoma.Crossref | GoogleScholarGoogle Scholar | 27919968PubMed |
Ohashi, T., Komatsu, S., Ichikawa, D., Miyamae, M., Okajima, W., Imamura, T., Kiuchi, J., Kosuga, T., Konishi, H., Shiozaki, A., Fujiwara, H., Okamoto, K., Tsuda, H., and Otsuji, E. (2017). Overexpression of PBK/TOPK relates to tumour malignant potential and poor outcome of gastric carcinoma. Br. J. Cancer 116, 218–226.
| Overexpression of PBK/TOPK relates to tumour malignant potential and poor outcome of gastric carcinoma.Crossref | GoogleScholarGoogle Scholar | 27898655PubMed |
Ran, F. A., Hsu, P. D., Wright, J., Agarwala, V., Scott, D. A., and Zhang, F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308.
| Genome engineering using the CRISPR-Cas9 system.Crossref | GoogleScholarGoogle Scholar | 24157548PubMed |
Sacher, F., Moller, C., Bone, W., Gottwald, U., and Fritsch, M. (2007). The expression of the testis-specific Dyrk4 kinase is highly restricted to step 8 spermatids but is not required for male fertility in mice. Mol. Cell. Endocrinol. 267, 80–88.
| The expression of the testis-specific Dyrk4 kinase is highly restricted to step 8 spermatids but is not required for male fertility in mice.Crossref | GoogleScholarGoogle Scholar | 17292540PubMed |
Scanlan, M. J., Simpson, A. J., and Old, L. J. (2004). The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 4, .
| 15546177PubMed |
Shang, P., Baarends, W. M., Hoogerbrugge, J., Ooms, M. P., van Cappellen, W. A., de Jong, A. A., Dohle, G. R., van Eenennaam, H., Gossen, J. A., and Grootegoed, J. A. (2010). Functional transformation of the chromatoid body in mouse spermatids requires testis-specific serine/threonine kinases. J. Cell Sci. 123, 331–339.
| Functional transformation of the chromatoid body in mouse spermatids requires testis-specific serine/threonine kinases.Crossref | GoogleScholarGoogle Scholar | 20053632PubMed |
Simons-Evelyn, M., Bailey-Dell, K., Toretsky, J. A., Ross, D. D., Fenton, R., Kalvakolanu, D., and Rapoport, A. P. (2001). PBK/TOPK is a novel mitotic kinase which is upregulated in Burkitt’s lymphoma and other highly proliferative malignant cells. Blood Cells Mol. Dis. 27, 825–829.
| PBK/TOPK is a novel mitotic kinase which is upregulated in Burkitt’s lymphoma and other highly proliferative malignant cells.Crossref | GoogleScholarGoogle Scholar | 11783945PubMed |
Simpson, A. J., Caballero, O. L., Jungbluth, A., Chen, Y. T., and Old, L. J. (2005). Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer 5, 615–625.
| Cancer/testis antigens, gametogenesis and cancer.Crossref | GoogleScholarGoogle Scholar | 16034368PubMed |
Sosnik, J., Miranda, P. V., Spiridonov, N. A., Yoon, S. Y., Fissore, R. A., Johnson, G. R., and Visconti, P. E. (2009). Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J. Cell Sci. 122, 2741–2749.
| Tssk6 is required for Izumo relocalization and gamete fusion in the mouse.Crossref | GoogleScholarGoogle Scholar | 19596796PubMed |
Su, B., and Karin, M. (1996). Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 8, 402–411.
| Mitogen-activated protein kinase cascades and regulation of gene expression.Crossref | GoogleScholarGoogle Scholar | 8793994PubMed |
Suzuki, S., Nozawa, Y., Tsukamoto, S., Kaneko, T., Manabe, I., Imai, H., and Minami, N. (2015). CHD1 acts via the Hmgpi pathway to regulate mouse early embryogenesis. Development 142, 2375–2384.
| CHD1 acts via the Hmgpi pathway to regulate mouse early embryogenesis.Crossref | GoogleScholarGoogle Scholar | 26092847PubMed |
Wang, X., Wei, Y., Fu, G., Li, H., Saiyin, H., Lin, G., Wang, Z., Chen, S., and Yu, L. (2015). Tssk4 is essential for maintaining the structural integrity of sperm flagellum. Mol. Hum. Reprod. 21, 136–145.
| Tssk4 is essential for maintaining the structural integrity of sperm flagellum.Crossref | GoogleScholarGoogle Scholar | 25361759PubMed |
Wong, C. H., and Cheng, C. Y. (2005). Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: a review of recent data. Dev. Biol. 286, 1–15.
| Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: a review of recent data.Crossref | GoogleScholarGoogle Scholar | 16153630PubMed |
Xu, B., Hao, Z., Jha, K. N., Zhang, Z., Urekar, C., Digilio, L., Pulido, S., Strauss, J. F., Flickinger, C. J., and Herr, J. C. (2008). TSKS concentrates in spermatid centrioles during flagellogenesis. Dev. Biol. 319, 201–210.
| TSKS concentrates in spermatid centrioles during flagellogenesis.Crossref | GoogleScholarGoogle Scholar | 18495105PubMed |
Zhao, S., Dai, J., Zhao, W., Xia, F., Zhou, Z., Wang, W., Gu, S., Ying, K., Xie, Y., and Mao, Y. (2001). PDZ-binding kinase participates in spermatogenesis. Int. J. Biochem. Cell Biol. 33, 631–636.
| PDZ-binding kinase participates in spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 11378444PubMed |


