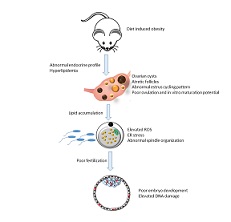
High-fat diet leads to elevated lipid accumulation and endoplasmic reticulum stress in oocytes, causing poor embryo development
Arpitha Rao A * , Aparna Satheesh A * , Guruprasad Nayak A , Pooja Suresh Poojary A , Sandhya Kumari A , Sneha Guruprasad Kalthur B , Srinivas Mutalik C , Satish Kumar Adiga A and Guruprasad Kalthur
A and Guruprasad Kalthur  A D
A D
A Department of Clinical Embryology, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal 576 104, Karnataka State, India.
B Department of Anatomy, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal 576 104, Karnataka State, India.
C Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal 576 104, Karnataka State, India.
D Corresponding author. Email: guru.kalthur@manipal.edu
Reproduction, Fertility and Development 32(14) 1169-1179 https://doi.org/10.1071/RD20112
Submitted: 30 April 2020 Accepted: 28 July 2020 Published: 1 October 2020
Abstract
The present study was designed to investigate the effect of diet-induced obesity on endoplasmic reticulum (ER) stress in oocytes. Swiss albino mice (3 weeks old) were fed with a high-fat diet (HFD) for 8 weeks. Oocytes were assessed for lipid droplet accumulation, oxidative stress, ER stress and their developmental potential in vitro. High lipid accumulation (P < 0.01) and elevated intracellular levels of reactive oxygen species were observed in both germinal vesicle and MII oocytes of HFD-fed mice (P < 0.05 and P < 0.01 respectively compared with control). Further, expression of the ER stress markers X-box binding protein 1 (XBP1), glucose-regulated protein 78 (GRP78), activating transcription factor 4 (ATF4) and activating transcription factor 6 (ATF6) was significantly (P < 0.001) higher in oocytes of the HFD than control group. Oocytes from HFD-fed mice exhibited poor fertilisation and blastocyst rates, a decrease in total cell number and high levels of DNA damage (P < 0.01) compared with controls. In conclusion, diet-induced obesity resulted in elevated lipid levels and higher oxidative and ER stress in oocytes, which contributed to the compromised developmental potential of embryos.
Additional keyword: reproduction.
References
Baldassano, S., Rappa, F., Amato, A., Cappello, F., and Mule, F. (2015). GLP-2 as beneficial factor in the glucose homeostasis in mice fed a high fat diet. J. Cell. Physiol. 230, 3029–3036.| GLP-2 as beneficial factor in the glucose homeostasis in mice fed a high fat diet.Crossref | GoogleScholarGoogle Scholar | 25967277PubMed |
Bansal, P., Paul, P., Mudgal, J., Nayak, P. G., Pannakal, S. T., Priyadarsini, K. I., and Unnikrishnan, M. K. (2012). Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp. Toxicol. Pathol. 64, 651–658.
| Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice.Crossref | GoogleScholarGoogle Scholar | 21208790PubMed |
Basar, M., Bozkurt, I., Guzeloglu-Kayisli, O., Sozen, B., Tekmen, I., Schatz, F., Arici, A., Lockwood, C. J., and Kayisli, U. A. (2014). Unfolded protein response prevents blastocyst formation during preimplantation embryo development in vitro. Fertil. Steril. 102, 1777–1784.
| Unfolded protein response prevents blastocyst formation during preimplantation embryo development in vitro.Crossref | GoogleScholarGoogle Scholar | 25305729PubMed |
Boudoures, A. L., Chi, M., Thompson, A., Zhang, W., and Moley, K. H. (2016). The effects of voluntary exercise on oocyte quality in a diet-induced obese murine model. Reproduction 151, 261–270.
| The effects of voluntary exercise on oocyte quality in a diet-induced obese murine model.Crossref | GoogleScholarGoogle Scholar | 26700938PubMed |
Broughton, D. E., and Moley, K. H. (2017). Obesity and female infertility: potential mediators of obesity’s impact. Fertil. Steril. 107, 840–847.
| Obesity and female infertility: potential mediators of obesity’s impact.Crossref | GoogleScholarGoogle Scholar | 28292619PubMed |
Chakraborty, T. R., Donthireddy, L., Adhikary, D., and Chakraborty, S. (2016). Long-term high fat diet has a profound effect on body weight, hormone levels, and estrous cycle in mice. Med. Sci. Monit. 22, 1601–1608.
| Long-term high fat diet has a profound effect on body weight, hormone levels, and estrous cycle in mice.Crossref | GoogleScholarGoogle Scholar | 27171231PubMed |
Chambers, T. J. G., Morgan, M. D., Heger, A. H., Sharpe, R. M., and Drake, A. J. (2016). High-fat diet disrupts metabolism in two generations of rats in a parent-of-origin specific manner. Sci. Rep. 6, 31857.
| High-fat diet disrupts metabolism in two generations of rats in a parent-of-origin specific manner.Crossref | GoogleScholarGoogle Scholar |
Choi, H. G., Kim, J. K., Kwak, D. H., Cho, J. R., Kim, J. Y., Kim, B. J., Jung, K. Y., Choi, B. K., Shin, M. K., and Choo, Y. K. (2002). Effects of high molecular weight water-soluble chitosan on in vitro fertilization and ovulation in mice fed a high-fat diet. Arch. Pharm. Res. 25, 178–183.
| Effects of high molecular weight water-soluble chitosan on in vitro fertilization and ovulation in mice fed a high-fat diet.Crossref | GoogleScholarGoogle Scholar | 12009032PubMed |
Després, J. P., Lemieux, I., Bergeron, J., Pibarot, P., Mathieu, P., Larose, E., Rodes-Cabau, J., Bertrand, O. F., and Poirier, P. (2008). Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler. Thromb. Vasc. Biol. 28, 1039–1049.
| Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk.Crossref | GoogleScholarGoogle Scholar | 18356555PubMed |
Diakogiannaki, E., Welters, H. J., and Morgan, N. G. (2008). Differential regulation of the endoplasmic reticulum stress response in pancreatic beta-cells exposed to long-chain saturated and monounsaturated fatty acids. J. Endocrinol. 197, 553–563.
| Differential regulation of the endoplasmic reticulum stress response in pancreatic beta-cells exposed to long-chain saturated and monounsaturated fatty acids.Crossref | GoogleScholarGoogle Scholar | 18492819PubMed |
Dickey, R. P., Taylor, S. N., Curole, D. N., Rye, P. H., Lu, P. Y., and Pyrzak, R. (1997). Relationship of clomiphene dose and patient weight to successful treatment. Hum. Reprod. 12, 449–453.
| Relationship of clomiphene dose and patient weight to successful treatment.Crossref | GoogleScholarGoogle Scholar | 9130738PubMed |
Durmanova, A. K., Otarbayev, N. K., Kaiyrlykyzy, A., Zhangazieva, K. K., Ibrayeva, Z. N., Donenbayeva, G. B., Zhusupova, Z. T., Saidakhmetov, A. S., Temirgaliyeva, G. S., Salykbayeva, Z. K., Tazhigulova, Z. M., Yensebayeva, A. M., Seksembayeva, K. K., Vasilenko, O. K., Timanova, S., and Akkozhina, B. (2016). Ovarian reserve and adipokine levels in reproductive-aged obese women. Ter. Arkh. 88, 46–50.
| Ovarian reserve and adipokine levels in reproductive-aged obese women.Crossref | GoogleScholarGoogle Scholar | 27801419PubMed |
Dyaczyński, M., Scanes, C. G., Koziec, H., Koziec, H., and Pierzchała-Koziec, K. (2018). Endocrine implications of obesity and bariatric surgery. Endokrynol. Pol. 69, 574–597.
| 30379322PubMed |
Esfahlan, R. J., Zarghami, N., Esfahlan, A. J., Mollazadeh, M., Nejati, K., and Nasiri, M. (2011). The possible impact of obesity on androgen, progesterone and estrogen receptors (ERα and ERβ) gene expression in breast cancer patients. Breast Cancer (Auckl.) 5, 227–237.
| The possible impact of obesity on androgen, progesterone and estrogen receptors (ERα and ERβ) gene expression in breast cancer patients.Crossref | GoogleScholarGoogle Scholar | 22174584PubMed |
Evans, D. J., Hoffmann, R. G., Kalkhoff, R. K., and Kissebah, A. H. (1983). Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J. Clin. Endocrinol. Metab. 57, 304–310.
| Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women.Crossref | GoogleScholarGoogle Scholar | 6345569PubMed |
Finger, B. J., Harvey, A. J., Green, M. P., and Gardner, D. K. (2015). Combined parental obesity negatively impacts preimplantation mouse embryo development, kinetics, morphology and metabolism. Hum. Reprod. 30, 2084–2096.
| Combined parental obesity negatively impacts preimplantation mouse embryo development, kinetics, morphology and metabolism.Crossref | GoogleScholarGoogle Scholar | 26089300PubMed |
Fribley, A., Zhang, K., and Kaufman, R. J. (2009). Regulation of apoptosis by the unfolded protein response. Methods Mol. Biol. 559, 191–204.
| Regulation of apoptosis by the unfolded protein response.Crossref | GoogleScholarGoogle Scholar | 19609758PubMed |
Gambineri, A., Laudisio, D., Marocco, C., Radellini, S., Colao, A., and Savastano, S. (2019). Female infertility: which role for obesity? Int. J. Obes. Suppl. 9, 65–72.
| Female infertility: which role for obesity?Crossref | GoogleScholarGoogle Scholar | 31391925PubMed |
Ge, Z. J., Luo, S. M., Lin, F., Liang, Q., Huang, L., Wei, Y., Hou, Y., Han, Z., Schatten, H., and Sun, Q. (2014). DNA methylation in oocytes and liver of female mice and their offspring: effects of high-fat-diet-induced obesity. Environ. Health Perspect. 122, 159–164.
| DNA methylation in oocytes and liver of female mice and their offspring: effects of high-fat-diet-induced obesity.Crossref | GoogleScholarGoogle Scholar | 24316659PubMed |
Harding, H. P., Zhang, Y., Scheuner, D., Chen, J. J., Kaufman, R. J., and Ron, D. (2009). Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development. Proc. Natl Acad. Sci. USA 106, 1832–1837.
| Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development.Crossref | GoogleScholarGoogle Scholar | 19181853PubMed |
Harte, R. A., Kirk, E. A., Rosenfeld, M. E., and LeBoeuf, R. C. (1999). Initiation of hyperinsulinemia and hyperleptinemia is diet dependent in C57BL/6 mice. Horm. Metab. Res. 31, 570–575.
| Initiation of hyperinsulinemia and hyperleptinemia is diet dependent in C57BL/6 mice.Crossref | GoogleScholarGoogle Scholar | 10596967PubMed |
Hendershot, L. M. (2004). The ER function BiP is a master regulator of ER function. Mt Sinai J. Med. 71, 289–297.
| 15543429PubMed |
Hou, Y. J., Zhu, C. C., Duan, X., Liu, H. L., Wang, Q., and Sun, S. C. (2016). Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci. Rep. 6, 18858.
| Both diet and gene mutation induced obesity affect oocyte quality in mice.Crossref | GoogleScholarGoogle Scholar | 26732298PubMed |
Howell, G. E., Mulligan, C., Meek, E., and Chambers, J. E. (2015). Effect of chronic p,p′-dichlorodiphenyldichloroethylene (DDE) exposure on high fat diet-induced alterations in glucose and lipid metabolism in male C57BL/6H mice. Toxicology 328, 112–122.
| Effect of chronic p,p′-dichlorodiphenyldichloroethylene (DDE) exposure on high fat diet-induced alterations in glucose and lipid metabolism in male C57BL/6H mice.Crossref | GoogleScholarGoogle Scholar | 25541407PubMed |
Igosheva, N., Abramov, A. Y., Poston, L., Eckert, J. J., Fleming, T. P., Duchen, M. R., and McConnell, J. (2010). Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One 5, e10074.
| Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes.Crossref | GoogleScholarGoogle Scholar | 20404917PubMed |
Jia, Z., Feng, Z., Wang, L., Li, H., Wang, H., Xu, D., Zhao, X., Feng, D., and Feng, X. (2018). Resveratrol reverses the adverse effects of a diet-induced obese murine model on oocyte quality and zona pellucida softening. Food Funct. 9, 2623–2633.
| Resveratrol reverses the adverse effects of a diet-induced obese murine model on oocyte quality and zona pellucida softening.Crossref | GoogleScholarGoogle Scholar | 29682638PubMed |
Kalthur, G., Salian, S. R., Nair, R., Mathew, J., Adiga, S. K., Kalthur, S. G., Zeegers, D., and Hande, M. P. (2016). Distribution pattern of cytoplasmic organelles, spindle integrity, oxidative stress, octamer-binding transcription factor 4 (Oct4) expression and developmental potential of oocytes following multiple superovulation. Reprod. Fertil. Dev. 28, 2027–2038.
| Distribution pattern of cytoplasmic organelles, spindle integrity, oxidative stress, octamer-binding transcription factor 4 (Oct4) expression and developmental potential of oocytes following multiple superovulation.Crossref | GoogleScholarGoogle Scholar | 26173898PubMed |
Kawada, T., Andou, T., and Fukumitsu, M. (2016). Waist circumference, visceral abdominal fat thickness and three components of metabolic syndrome. Diabetes Metab. Syndr. 10, 4–6.
| Waist circumference, visceral abdominal fat thickness and three components of metabolic syndrome.Crossref | GoogleScholarGoogle Scholar | 26376586PubMed |
Lashen, H., Fear, K., and Sturdee, D. W. (2004). Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum. Reprod. 19, 1644–1646.
| Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study.Crossref | GoogleScholarGoogle Scholar | 15142995PubMed |
Leenen, R., van der Kooy, K., Seidell, J. C., Deurenberg, P., and Koppeschaar, H. P. (1994). Visceral fat accumulation in relation to sex hormones in obese men and women undergoing weight loss therapy. J. Clin. Endocrinol. Metab. 78, 1515–1520.
| 8200956PubMed |
Li, J., Wang, S., Wang, B., Wei, H., Liu, X., Hao, J., Duan, Y., Hua, J., Zheng, X., Feng, X., and Yan, X. (2018). High-fat-diet impaired mitochondrial function of cumulus cells but improved the efficiency of parthenogenetic embryonic quality in mice. Anim. Cells Syst. (Seoul) 22, 243–252.
| High-fat-diet impaired mitochondrial function of cumulus cells but improved the efficiency of parthenogenetic embryonic quality in mice.Crossref | GoogleScholarGoogle Scholar | 30460104PubMed |
Li, X., Wang, H., Wang, T., Zheng, F., Wang, H., and Wang, C. (2019). Dietary wood pulp-derived sterols modulation of cholesterol metabolism and gut microbiota in high-fat-diet-fed hamsters. Food Funct. 10, 775–785.
| Dietary wood pulp-derived sterols modulation of cholesterol metabolism and gut microbiota in high-fat-diet-fed hamsters.Crossref | GoogleScholarGoogle Scholar | 30667436PubMed |
Luo, S., Mao, C., Lee, B., and Lee, A. S. (2006). GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol. Cell. Biol. 26, 5688–5697.
| GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development.Crossref | GoogleScholarGoogle Scholar | 16847323PubMed |
Luzzo, K. M., Wang, Q., Purcell, S. H., Chi, M., Jimenez, P. T., Grindler, N., Schedl, T., and Moley, K. H. (2012). High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One 7, e49217.
| High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects.Crossref | GoogleScholarGoogle Scholar | 23152876PubMed |
Malhi, H., and Gores, G. J. (2008). Molecular mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Semin. Liver Dis. 28, 360–369.
| Molecular mechanisms of lipotoxicity in non-alcoholic fatty liver disease.Crossref | GoogleScholarGoogle Scholar | 18956292PubMed |
Malhotra, J. D., and Kaufman, R. J. (2007). Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 9, 2277–2293.
| Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword?Crossref | GoogleScholarGoogle Scholar | 17979528PubMed |
Malhotra, N., Bahadur, A., Singh, N., Kalaivani, M., and Mittal, S. (2013). Does obesity compromise ovarian reserve markers? A clinician’s perspective. Arch. Gynecol. Obstet. 287, 161–166.
| Does obesity compromise ovarian reserve markers? A clinician’s perspective.Crossref | GoogleScholarGoogle Scholar | 22930149PubMed |
Nair, R., Singh, V. J., Salian, S. R., Kalthur, S. G., D’Souza, A. S., Shetty, P. K., Mutalik, S., Kalthur, G., and Adiga, S. K. (2014). Methyl parathion inhibits the nuclear maturation, decreases the cytoplasmic quality in oocytes and alters the developmental potential of embryos of Swiss albino mice. Toxicol. Appl. Pharmacol. 279, 338–350.
| Methyl parathion inhibits the nuclear maturation, decreases the cytoplasmic quality in oocytes and alters the developmental potential of embryos of Swiss albino mice.Crossref | GoogleScholarGoogle Scholar | 25038315PubMed |
Nteeba, J., Ganesan, S., and Keating, A. F. (2014). Progressive obesity alters ovarian folliculogenesis with impacts on pro-inflammatory and steroidogenic signaling in female mice. Biol. Reprod. 91, 86.
| Progressive obesity alters ovarian folliculogenesis with impacts on pro-inflammatory and steroidogenic signaling in female mice.Crossref | GoogleScholarGoogle Scholar | 25143355PubMed |
Ozcan Dag, Z., Oguzturk, O., Isik, Y., Turkel, Y., and Bulcun, E. (2015). Personality profile in patients with polycystic ovary syndrome. Gynecol. Endocrinol. 31, 540–542.
| Personality profile in patients with polycystic ovary syndrome.Crossref | GoogleScholarGoogle Scholar | 25884894PubMed |
Park, H. J., Park, J. Y., Kim, J. W., Yang, S. G., Jung, J. M., Kim, M. J., Kang, M. J., Cho, Y. H., Wee, G., Yang, H. Y., Song, B. S., Kim, S. U., and Koo, D. B. (2018). Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J. Pineal Res. 64, e12458.
| Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation.Crossref | GoogleScholarGoogle Scholar | 29149522PubMed |
Pasquali, R., Pelusi, C., Genghini, S., Cacciari, M., and Gambineri, A. (2003). Obesity and reproductive disorders in women. Hum. Reprod. Update 9, 359–372.
| Obesity and reproductive disorders in women.Crossref | GoogleScholarGoogle Scholar | 12926529PubMed |
Podrini, C., Cambridge, E. L., Lelliott, C. J., Carragher, D. M., Estabel, J., Gerdin, A. K., Karp, N. A., and Scudamore, C. L. (2013). High-fat feeding rapidly induces obesity and lipid derangements in C57BL/6N mice. Mamm. Genome 24, 240–251.
| High-fat feeding rapidly induces obesity and lipid derangements in C57BL/6N mice.Crossref | GoogleScholarGoogle Scholar | 23712496PubMed |
Pohlmeier, W. E., Xie, F., Kurz, S. G., Lu, N., and Wood, J. R. (2014). Progressive obesity alters the steroidogenic response to ovulatory stimulation and increases the abundance of mRNAs stored in the ovulated oocyte. Mol. Reprod. Dev. 81, 735–747.
| Progressive obesity alters the steroidogenic response to ovulatory stimulation and increases the abundance of mRNAs stored in the ovulated oocyte.Crossref | GoogleScholarGoogle Scholar | 24824196PubMed |
Reddy, R. K., Mao, C., Baumeister, P., Austin, R. C., Kaufman, R. J., and Lee, A. S. (2003). Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J. Biol. Chem. 278, 20915–20924.
| Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation.Crossref | GoogleScholarGoogle Scholar | 12665508PubMed |
Reynolds, K. A., Boudoures, A. L., Chi, M. M., Wang, Q., and Moley, K. H. (2015). Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod. Fertil. Dev. 27, 716–724.
| Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice.Crossref | GoogleScholarGoogle Scholar | 25775080PubMed |
Ron, D., and Walter, P. (2007). Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529.
| Signal integration in the endoplasmic reticulum unfolded protein response.Crossref | GoogleScholarGoogle Scholar | 17565364PubMed |
Segula, D. (2014). Complications of obesity in adults: a short review of the literature. Malawi Med. J. 26, 20–24.
| 24959321PubMed |
Shang, C. G., Liu, Z. H., Wang, X. H., Feng, Z. H., and Zhang, Y. (2017). Effect of high-fat diet-induced disorders on rat with endometrial hyperplasia and adiponectin system in circulation and uterus. Chin. Med. J. (Engl.) 130, 1831–1837.
| Effect of high-fat diet-induced disorders on rat with endometrial hyperplasia and adiponectin system in circulation and uterus.Crossref | GoogleScholarGoogle Scholar | 28748857PubMed |
Skaznik-Wikiel, M. E., Swindle, D. C., Allshouse, A. A., Polotsky, A. J., and McManaman, J. L. (2016). High-fat diet causes subfertility and compromised ovarian function independent of obesity in mice. Biol. Reprod. 94, 108.
| High-fat diet causes subfertility and compromised ovarian function independent of obesity in mice.Crossref | GoogleScholarGoogle Scholar | 27030045PubMed |
Sohrabi, M., Roushandeh, A. M., Alizadeh, Z., Vahidinia, A., Vahabian, M., and Hosseini, M. (2015). Effect of a high fat diet on ovary morphology, in vitro development, in vitro fertilisation rate and oocyte quality in mice. Singapore Med. J. 56, 573–579.
| Effect of a high fat diet on ovary morphology, in vitro development, in vitro fertilisation rate and oocyte quality in mice.Crossref | GoogleScholarGoogle Scholar | 26512150PubMed |
Tang, L. L., Tang, X. H., Li, X., Yu, H. B., Xie, Z. G., Liu, X. Y., and Zhou, Z. G. (2014). Effect of high-fat or high-glucose diet on obesity and visceral adipose tissue in mice. Acta Academiae Medicinae Sinicae 36, 614–619.
| 25556734PubMed |
van Swieten, E. C., van der Leeuw-Harmsen, L., Badings, E. A., and van der Linden, P. J. (2005). Obesity and clomiphene challenge test as predictors of outcome of in vitro fertilization and intracytoplasmic sperm injection. Gynecol. Obstet. Invest. 59, 220–224.
| Obesity and clomiphene challenge test as predictors of outcome of in vitro fertilization and intracytoplasmic sperm injection.Crossref | GoogleScholarGoogle Scholar | 15753618PubMed |
Wang, N., Luo, L. L., Xu, J. J., Xu, M. Y., Zhang, X. M., Zhou, X. L., Liu, W. J., and Fu, Y. C. (2014). Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism 63, 94–103.
| Obesity accelerates ovarian follicle development and follicle loss in rats.Crossref | GoogleScholarGoogle Scholar | 24135502PubMed |
Wang, H., Cheng, Q., Li, H., Hu, F., Han, L., Zhang, H., Li, L., Ge, J., Ying, X., Guo, X., and Wang, Q. (2018). Loss of TIGAR induces oxidative stress and meiotic defects in oocytes from obese mice. Mol. Cell. Proteomics 17, 1354–1364.
| Loss of TIGAR induces oxidative stress and meiotic defects in oocytes from obese mice.Crossref | GoogleScholarGoogle Scholar | 29776966PubMed |
Wu, L. L., Dunning, K. R., Yang, X., Russell, D. L., Lane, M., Norman, R. J., and Robker, R. L. (2010). High-fat diet causes lipotoxicity responses in cumulus–oocyte complexes and decreased fertilization rates. Endocrinology 151, 5438–5445.
| High-fat diet causes lipotoxicity responses in cumulus–oocyte complexes and decreased fertilization rates.Crossref | GoogleScholarGoogle Scholar | 20861227PubMed |
Wu, L. L., Russell, D. L., Norman, R. J., and Robker, R. L. (2012). Endoplasmic reticulum (ER) stress in cumulus–oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (ΔΨm) and embryo development. Mol. Endocrinol. 26, 562–573.
| Endoplasmic reticulum (ER) stress in cumulus–oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (ΔΨm) and embryo development.Crossref | GoogleScholarGoogle Scholar | 22383462PubMed |
Yang, X., Wu, L. L., Chura, L. R., Liang, X., Lane, M., Norman, R. J., and Robker, R. L. (2012). Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil. Steril. 97, 1438–1443.
| Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes.Crossref | GoogleScholarGoogle Scholar | 22440252PubMed |
Zeeshan, H. M., Lee, G. H., Kim, H. R., and Chae, H. J. (2016). Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 17, 327.
| Endoplasmic reticulum stress and associated ROS.Crossref | GoogleScholarGoogle Scholar | 26950115PubMed |
Zhang, J. Y., Diao, Y. F., Kim, H. R., and Jin, D. I. (2012). Inhibition of endoplasmic reticulum stress improves mouse embryo development. PLoS One 7, e40433.
| Inhibition of endoplasmic reticulum stress improves mouse embryo development.Crossref | GoogleScholarGoogle Scholar | 23300774PubMed |


