The response of reptiles and mammals to fire-driven vegetation succession in semi-arid Triodia-mallee woodlands
Jules E. Farquhar A B * , Wyn Russell B , Jesse B. Farquhar B , Simon Cook B § and Ashley R. Olson
A B * , Wyn Russell B , Jesse B. Farquhar B , Simon Cook B § and Ashley R. Olson  B
B
A
B
Abstract
Predicting faunal responses to fire is complex due to regional differences in fire-vegetation dynamics, necessitating locally calibrated studies.
This study examined the effects of fire-mediated vegetation succession on (1) the richness, diversity and composition of small vertebrate communities and (2) individual species abundance in Triodia-mallee landscapes.
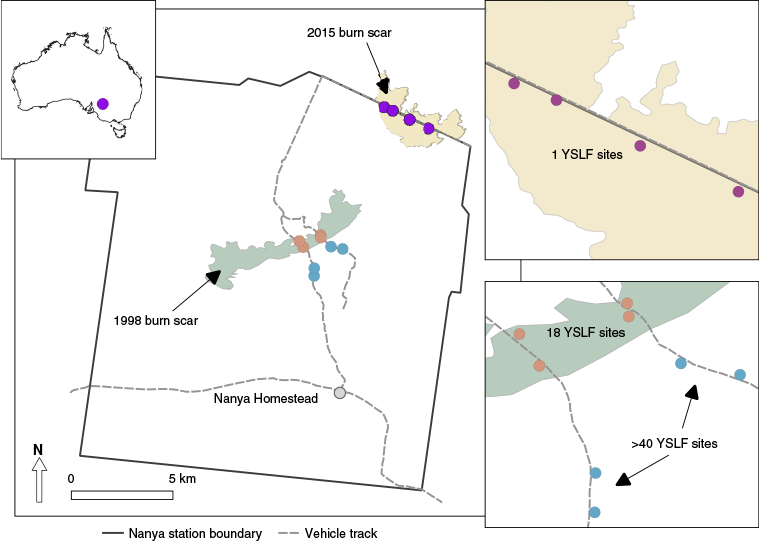
We sampled small vertebrates across 12 sites at Nanya Station, NSW, Australia, with fire histories spanning 1, 18 and >40 years since the last fire (YSLF).
531 vertebrates from 39 species were recorded. There was strong evidence that fire history influenced vegetation structure, which shaped reptile community composition and species abundance. Early successional sites (1 YSLF) supported higher abundances of lizards such as Ctenotus regius, Lerista labialis and Lucasium damaeum. These species peaked 1-year post-fire before declining by 18 YSLF. In contrast, the dasyurid Ningaui yvonneae was most abundant 20–40 YSLF, correlating with dense spinifex, and was nearly absent at recently burned sites.
Fire history drives varied successional responses among species, with some benefiting from early post-fire conditions and others requiring mature spinifex habitat.
Region-specific studies are essential for effective conservation and land management. Long-term research is needed to understand successional dynamics and guide fire management strategies.
Keywords: Community composition, fire management, habitat structure, lizards, mammals, post-fire recovery dynamics, successional response, Triodia-mallee.
Introduction
Fire is an integral form of landscape-scale disturbance that influences the distribution, richness and abundance of fauna globally (Andersen et al. 2005; Bond and Keeley 2005; Bowman et al. 2009; York et al. 2012; He et al. 2019; González et al. 2022; Moyo 2022), and is widely used as a management tool for biodiversity conservation (Andersen et al. 2005; Edwards et al. 2008; Driscoll et al. 2010). However, human activities have led to changes in fire regimes, and this presents a risk of extinction to species globally (Bradstock et al. 1998; Pausas and Keeley 2009; Griffiths et al. 2015; Woinarski et al. 2015; Nimmo et al. 2022). It is therefore imperative that management decisions are underpinned by robust knowledge of species responses to fire regimes (Driscoll et al. 2010).
Many faunal species depend on habitat structural features (Tews et al. 2004; Garden et al. 2007; Bunce et al. 2013; Farquhar et al. 2023) and populations often track the dynamic changes in vegetation structure following fire (Friend 1993; Monamy and Fox 2000; Loyn and Kennedy 2009; Clarke et al. 2021). Fox’s (1982) habitat accommodation model (HAM) has widely been used as a conceptual framework for predicting faunal responses to fire-driven secondary succession. The model posits that species will enter the succession when changes in vegetation structure result in a habitat that meets their specific ecological needs (Fox 1982). As community succession continues and conditions become unfavourable for a species, it may reduce in abundance or become eliminated from the succession (Fox 1982). The model suggests that species responses to fire follow predictable patterns of change related to the post-fire regeneration of vegetation, not time-since-disturbance per se (Monamy and Fox 2000; Fox et al. 2003; Letnic et al. 2004).
In the fire-prone mallee Eucalyptus woodlands of southern Australia, where vegetation structure is strongly influenced by time-since-fire (Haslem et al. 2011), Caughley (1985) developed a habitat accommodation model specific to the post-fire changes in abundance of desert reptiles. The model recognises three discreet responses to time-since-fire. First, burrowing species are most abundant in early seral stages, second, spinifex grass (Triodia spp.) specialists are most abundant beyond 6 years once sufficient Triodia has recovered and third, litter dwelling species are most abundant after 25 years once sufficient leaf litter has accumulated (Caughley 1985). Additionally, Driscoll and Henderson (2008) propose that logs are an important resource for reptiles and should be included into Caughley’s (1985) model. The temporal availability of these microhabitat resources is expected to have implications for the persistence of fauna in these ecosystems (Haslem et al. 2011).
Species responses to fire-mediated habitat changes will depend on their functional traits and how those traits influence interactions with the environment (Sousa 1984). As such, a common approach to fauna-fire studies is to use functional traits related to habitat-use to predict biotic responses to fire (Driscoll and Henderson 2008; Lindenmayer et al. 2008; Keith 2012; Nimmo et al. 2012). Species that depend on a particular vegetation resource are expected to peak in abundance when that resource becomes widely available after fire (Keith 2012). With this knowledge, it may be possible to extrapolate responses to fire for species with similar ecological traits (Watson et al. 2012). These generalisable models could potentially be used to identify seral stages important for species persistence (Watson et al. 2012; Clarke et al. 2021). However, such generalised response curves have had variable success in predicting species responses to fire (Driscoll and Henderson 2008; Lindenmayer et al. 2008; Nimmo et al. 2012; Smith et al. 2013). Geographical variation in variables unrelated to fire, such as rainfall and grazing history, also drive vegetation changes (Letnic et al. 2004), causing fauna-fire relationships to vary regionally (Nimmo et al. 2012; Smith et al. 2013; Nimmo et al. 2014). Thus, to achieve region specific conservation and land management goals, we need to know whether fire management actions are best guided by generalised species response models (Watson et al. 2012) or by knowledge of local fauna-fire relationships that may be idiosyncratic to the site being managed.
In this study, we explored the response of small terrestrial vertebrates (reptiles and mammals) to fire-driven vegetation succession in semi-arid Australia, where lizards comprise a remarkably abundant and diverse taxonomic group among faunal communities (Morton and James 1988). Because reptiles respond to fire over decadal and centennial time scales (Nimmo et al. 2012; Valentine et al. 2012), we examined fire histories up to 40 years using a space-for-time substitution approach where community data are collected from areas known to have burnt at different times (Pianka and Goodyear 2012; Smith et al. 2013). First, we investigated the effect of fire history on the richness, diversity and composition of vertebrate communities and the abundance of common species. We then investigated whether changes in the abundance of a species could be explained by changes in habitat structure associated with fire-driven vegetation succession.
Materials and methods
Study area
The study was conducted at Nanya Station, a 40,000 ha ex-pastoral property in the Scotia mallee region of far western NSW (33°7′48.70″S, 141°17′42.04″E) managed for conservation and teaching by Federation University Australia. The climate is semi-arid with a mean annual rainfall of 250 mm but can be highly variable between years. Summer temperatures are high (February mean daily max = 32°C, min = 16°C) while winters are mild (July mean daily max = 15°C, min = 5°C) (Westbrooke 2010). The area comprises relatively intact vegetation communities with no recent livestock grazing in the last 40 years (Westbrooke 2012). The most widespread vegetation community is Eucalyptus open-shrubland with Triodia scariosa understorey (Triodia-mallee) that occurs on low, undulating, sand dunes (Westbrooke et al. 1998). The fire history of Nanya has resulted in a mosaic of age classes spanning a century-long timeframe. Extensive wildfire occurred throughout much of the region in 1917 (Withers 1989) and large areas were burnt during 1976 (Rodda 1978), followed by a 5000 ha fire in December 1997 (Westbrooke 2010). A more recent 390 ha wildfire occurred on the northern boundary of Nanya Station in November 2015.
Sampling
We used a space-for-time substitution to examine changes in reptile community structure along a 40-year post-fire chronosequence. We sampled from three discreet fire histories; 1-year since the last fire (burned in 2015), 18 years since the last fire (burned in 1998) and greater than 40 years since the last fire (burned in 1976 or earlier) (Fig. 1). Four survey sites were established within each treatment, equating to a total of 12 survey sites. All sites were placed approximately mid-dune, on sandy soils in Triodia-mallee vegetation between 50 and 89 m elevation and spaced at least 250 m apart to prevent pseudoreplication. Sampling was conducted over four 12-day fieldtrips across two Austral spring-summer periods (December 2016, February 2017, September 2017 and January 2018) using pit-fall and funnel trapping with a total sampling effort of 4144 trap nights. Importantly, every site was sampled the same number of times and on the same days, thereby removing the effects of survey time period, weather conditions and sampling effort on inter-site comparisons. This enabled us to use the sum total of captures at each site as the sampling unit. A 25 m trapping line was constructed at each site, consisting of five 20 L buckets spaced at 4 m intervals and connected by a 30 cm high flywire fence. Two funnel traps (Professional Trapping Supplies, Australia. Trap dimensions: 18 cm × 18 cm × 79 cm) were placed in the centre of the trapping line, one either side of the drift fence. Traps were checked daily. After taking basic morphological measurements, animals were marked on the ventral surface with non-toxic permanent marker to distinguish recaptures. Animals were released at the site of capture within 24 h, at least 20 m from the trapping line to minimise short-term recaptures.
Vegetation assessment
To quantify structural differences in habitat among fire ages, ground cover and understorey structure were assessed at each site using a structure ‘touch-pole’ over a 100-point grid (1 m intervals between points). Ground cover attributes contacting the base of the pole were recorded as either Triodia, litter/debris or bare ground. Vertical vegetation density was assessed by recording the number of understorey structural contacts with the pole up to 2 m high. Vertical vegetation structure contacts were grouped into two categories: Triodia and non-Triodia vegetation density (shrubs, mallee, herbs and tussock grasses). Canopy cover was quantified for each site by one observer placing a circular cardboard tube over one eye and looking directly upward to visually estimate the percentage of canopy foliage encroaching on the circular view. All vegetation above the 2 m touch-pole was considered to be canopy foliage.
A principal component analysis (PCA) was performed on vegetation variables to avoid collinearity and simplify analytical models. All variables were centred and scaled prior to conducting the PCA and structural density variables (Triodia, other vegetation <1 m and other vegetation 1–2 m) were square-root transformed. The resulting principal component (PC) dimensions used for subsequent analysis were selected by confirming that the variation captured in the dimension was significantly different from random variation in the data using the PCtest package.
Data analysis
We investigated the relationship between the number of years since the last fire (YSLF) and species richness, Shannon’s diversity index (H′), community composition (beta diversity), vegetation structure (PCA dimensions) and the abundance of the most common species (species that were observed >20 times in total). To do so, we fitted generalised linear models (GLMs) in a Bayesian framework using the brm function from the brms package in R (Bürkner 2017; R Development Core Team 2024) with YSLF as a categorical predictor variable with a level for each YSLF category (1, 18 and >40). We also investigated the effect of vegetation structure on the abundance of the most common species using Poisson GLMs using PCA dimensions of structure as predictor variables. All models were fitted using default priors and three Markov chain Monte Carlo (MCMC) simulations with 4000 iterations, after the first 1000 were discarded as burn-in, for a total of 12,000 iterations. Poisson GLMs (PGLM) or negative binomial GLMs (NBGLM) were used to determine support for relationships between response variables that were count data (richness and species abundance) and YSLF, depending on whether the data were overdispersed. For continuous response variables (H′ and PCA dimensions), we fitted Gaussian GLMs. For each model, we plotted parameter estimates and provided highest posterior density (HPD) intervals as an indication of uncertainty. Model diagnostics were performed by checking that Rhat values were less than 1.01, MCMC effective sample size was greater than 2000, and by performing graphical posterior predictive checks using the pp_check function. Finally, pairwise comparisons between YSLF categories were performed using the pairs function from the emmeans package which calculates the difference in estimated means and associated HPD intervals (Lenth 2025).
Community composition was compared among burn treatments using a PerMANOVA via the adonis function in the vegan package in R (Oksanen 2024). PerMANOVA is a distance-based nonparametric multivariate analysis that provides a pseudo F-statistic value and derives a P-value from permutation tests. PerMANOVA was performed using square-root transformed abundance data and a Bray–Curtis dissimilarity matrix. We used 10,000 random permutations to test for significant variation in community composition among different YSLF categories. A groupwise comparison among YSLF treatments was initially performed, followed by pair-wise comparisons to detect differences between each pair of treatments (Anderson 2001). The Bonferroni correction was applied to P-values calculated from pairwise comparisons to avoid false discoveries. Dissimilarities were visualised to illustrate reptile assemblage trends using non-metric multidimensional scaling (nMDS) among treatments using the metaMDS function in the vegan package (Oksanen 2024).
Results
Pit-fall trapping at the 12 sites resulted in a total of 531 captures comprising 34 reptile species, four mammals and one amphibian (Appendix 1). The most captured species were the dasyurid Ningaui yvonnae (n = 129), the agamid lizard Ctenophorus spinodomus (n = 113), the skinks Ctenotus atlas (n = 34), Ctenotus regius (n = 31) and Lerista labialis (n = 29), and the gecko Lucasium damaeum (n = 20). Fourteen species were captured less than three times; of those, 12 were reptile species (six snakes, three geckos, two skinks and one pygopod), one was a mammal, Cercartetus concinnus, and one was the amphibian, N. sudellae. Four reptile species and three mammal species are considered threatened in NSW (Appendix 1).
Response of community richness, diversity and composition to YSLF
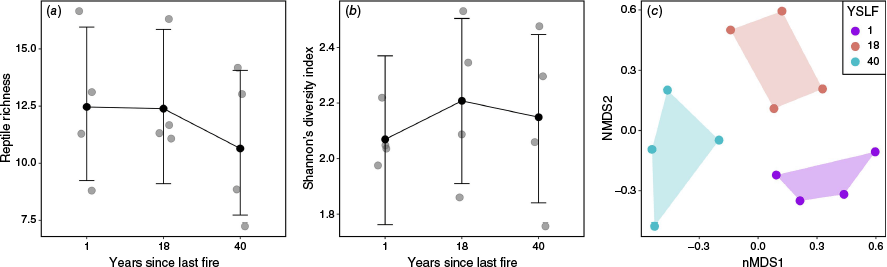
The overall mammal assemblage was too depauperate to investigate patterns of richness, diversity and composition (total richness = 4). Thus, we restricted our investigation of the ecological community to the reptile assemblage (Fig. 2). Pairwise comparisons revealed no support for differences between YSLF categories in species richness or Shannon’s diversity index (95% HPD included zero in all cases; Table 1). However, YSLF significantly influenced the composition of the reptile community (three-way PerMANOVA: F = 3.512, P < 0.001) (Fig. 2). Pairwise PerMANOVA comparisons indicated that the composition of species was significantly different between sites that were 1-year post-fire and 18-years post-fire (F = 3.561, P = 0.029), 1-year and > 40-years post-fire (F = 4.619, P = 0.029), and 18-years and >40 years post-fire (F = 2.46, P = 0.031).
The effect of fire history (years since last fire (YSLF)) on (a) the richness, (b) diversity and (c) composition of reptile communities. For richness and diversity, points indicate predicted values of Poisson and Gaussian models, respectively, and error bars indicate 95% highest posterior density (HPD) intervals. Changes in community composition are demonstrated by a non-metric multidimensional scaling (nMDS) biplot showing dissimilarity of reptile community composition at sample sites 1, 18 and >40 years after being burned. Bray–Curtis dissimilarity was used to quantify the differences in community structure between each sample site.
| Response | Contrast | Δ estimate | 95% HPD intervals | |
|---|---|---|---|---|
| Richness | 1–18 | 0.00 | −0.37, 0.41 | |
| 1–40 | 0.16 | −0.25, 0.56 | ||
| 18–40 | 0.15 | −0.27, 0.55 | ||
| Diversity | 1–18 | −0.14 | −0.56, 0.28 | |
| 1–40 | −0.08 | −0.51, 0.34 | ||
| 18–40 | 0.06 | −0.36, 0.49 | ||
| C. spinodomus | 1–18 | 0.70 | −0.26, 1.65 | |
| 1–40 | 1.16 | 0.16, 2.09* | ||
| 18–40 | 0.46 | −0.54, 1.47 | ||
| C. atlas | 1–18 | −0.65 | −1.56, 0.19 | |
| 1–40 | −0.33 | −1.26, 0.61 | ||
| 18–40 | 0.32 | −0.49, 1.11 | ||
| C. regius | 1–18 | 3.66 | 1.73, 6.30* | |
| 1–40 | 11.94 | 2.51, 31.20* | ||
| 18–40 | 8.22 | −2.19, 28.10 | ||
| L. labialis | 1–18 | 1.39 | 0.49, 2.28* | |
| 1–40 | 9.34 | 1.81, 26.99* | ||
| 18–40 | 7.94 | 0.35, 25.76* | ||
| L. damaeum | 1–18 | 1.38 | 0.31, 2.61* | |
| 1–40 | 3.00 | 0.89, 5.51* | ||
| 18–40 | 1.62 | −0.59, 4.51 | ||
| N. yvonneae | 1–18 | −3.55 | −5.29, −2.26* | |
| 1–40 | −3.57 | −5.29, −2.25* | ||
| 18–40 | −0.02 | −0.37, 0.34 | ||
| Vegetation (PC1) | 1–18 | 3.44 | 2.15, 4.73* | |
| 1–40 | 3.54 | 2.25, 4.82* | ||
| 18–40 | 0.11 | −1.18, 1.40 |
Poisson generalised linear models were used for all analyses, except for C. spinodomus, where a negative binomial generalised linear model was used. Estimates and highest posterior density (HPD) intervals are reported on a log scale meaning strong evidence for a difference between groups is indicated by HPD intervals excluding zero and is denoted by an asterisk. PC1 refers to principal component 1.
Influence of YSLF on abundance
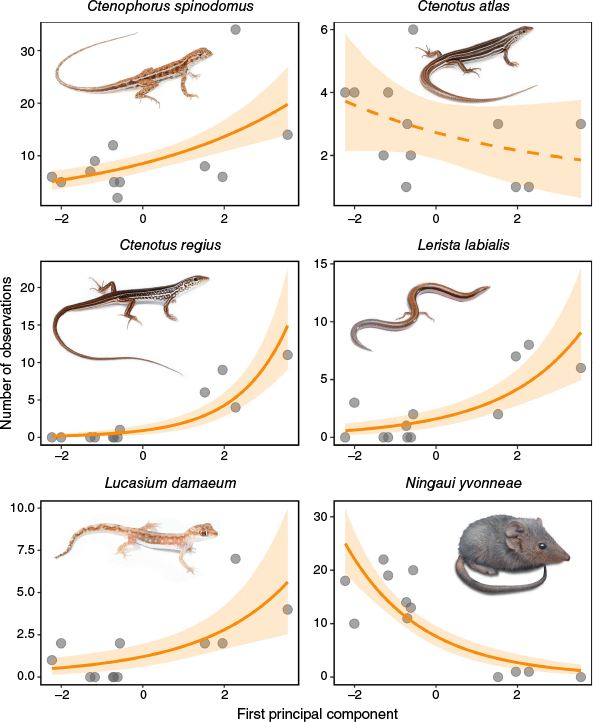
The six most commonly trapped vertebrate species varied in their response to fire, from ‘irruptive’ (high early succession abundance) to ‘plateau’ (abundance increases to an asymptote in the intermediate-late succession) and ‘null’ (Clarke et al. 2021; Fig. 3). The abundance of the agamid species C. spinodomus (the most frequently trapped reptile) showed a decline in abundance with YSLF, with strong evidence the species was more abundant 1-year post-fire than at >40-years post-fire (Table 1). The skinks C. regius and L. labialis, and the gecko L. damaeum, all showed a similar preference for recently burned sites; these species showed strong evidence that they were most abundant at the 1-year post-fire sites then displayed a substantial decline (i.e. irruptive response), more attenuated than that of C. spinodomus, between 1-year and 18-years post-fire (Table 1). There was no evidence that the abundance of C. atlas differed according to the fire history of a site (Table 1).
The effect of fire on the abundance of the six most common species at the 12 sample sites. Black points represent predicted values and error bars indicate 95% highest posterior density intervals based on Poisson generalised linear models. Grey points (observed values) are slightly transparent to show overlapping data more easily.
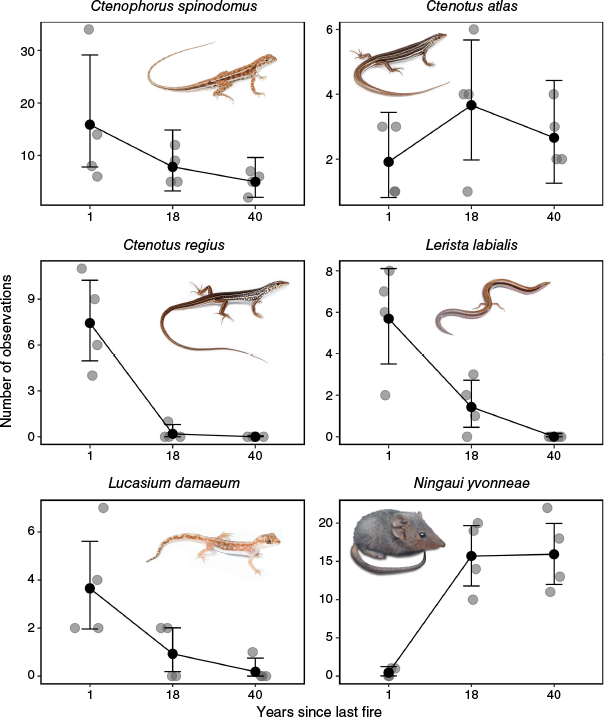
The most common mammal species, N. yvonneae showed for a positive relationship with YSLF (Fig. 3). This species was rarely observed at sites that had burned 1-year prior to our survey (total observations = 2) and there was strong evidence that it was substantially more abundant at sites that were 20–40 years post-fire (Table 1). In total, N. yvonneae was observed 63 times at sites that had burned 18 years prior to our study and 64 times at sites that had burned 40 years or more before our study. This indicates that the abundance of N. yvonneae increases to a plateau over a 40-year chronosequence.
Effect of vegetation on abundance
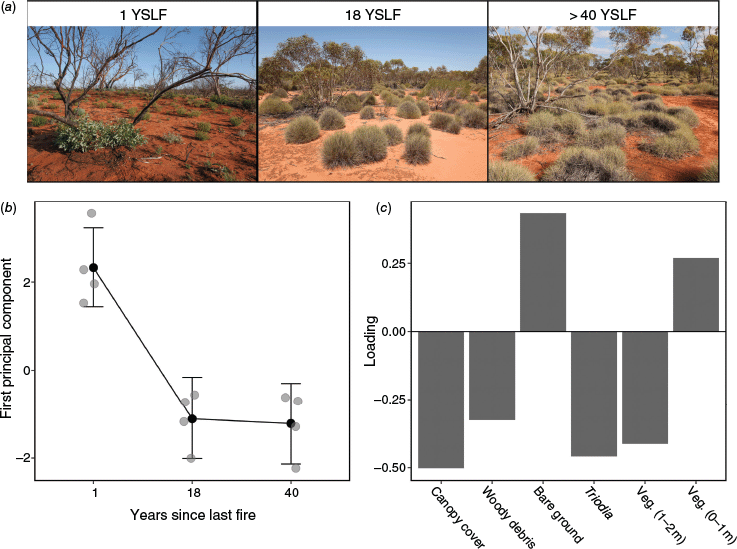
The first dimension (PC1) of the principal component analysis explained 57.3% of the variation in the habitat structure data and was the only dimension that was significantly different from random variation (P < 0.05). PC1 revealed a clear separation among fire age classes in vegetation structure (Fig. 4) as indicated by strong evidence for differences between the 1 YSLF treatment and both the 18 YSLF treatment and >40 YSLF treatment (Table 1). However, there was no evidence for a difference in PC1 between the 18 YSLF and the >40 YSLF treatments (Table 1). The differences in PC1 values between seral stages indicates that sites 1-year post-fire had greater coverage of bare ground and greater density of low (0–1 m high) non-Triodia vegetation (e.g. small herbs regenerating after the fire). By comparison, sites that were 18 or >40 years post-fire showed greater canopy cover, more woody debris on the soil surface and greater Triodia and mid-storey vegetation density.
Changes in habitat structure following fire. Photos (a) show examples of the vegetation 1-year, 18 years and >40 years since last fire (YSLF). (b) The relationship between first principal component and fire history (YSLF), where black points represent predicted values and the error bars indicate 95% highest posterior density intervals based on a Poisson generated linear model. Grey points (observed values) are slightly transparent to show overlapping data more easily. (c) Principal component analysis loadings for each habitat variable.
Small vertebrates showed variable responses to the vegetational changes driven by fire (Fig. 5). Strong evidence for a positive correlation with PC1 was observed for four species of lizard; C. spinodomus (PGLM: β = 0.23, 95% HPD: [0.14, 0.33]), C. regius (PGLM: β = 0.80, 95% HPD: [0.55, 1.08]), Lerista labialis (PGLM: β = 0.49, 95% HPD: [0.29, 0.70]), and Lucasium damaeum (PGLM: β = 0.43, 95% HPD: [0.19, 0.68]). Positive correlations with PC1 indicate that the aforementioned four species preferred sites with relatively more bare ground and understorey vegetation that did not include Triodia. In comparison, there was no evidence for a relationship between PC1 and the abundance of C. atlas (PGLM: β = −0.13, 95% HPD: [−0.35, 0.07]). As the abundance of C. atlas was not correlated with YSLF either, it appears that the abundance of this species is not influenced by fire-driven vegetation succession at the fire intervals and locations we sampled. The mammal, N. yvonneae, showed strong evidence for a negative correlation with PC1 (PGLM: β = −0.53, 95% HPD: [−0.70, −0.38]), indicating a preference for sites with greater canopy cover and woody debris, as well as a higher density of Triodia and mid-storey vegetation.
The response of five lizards and one mammal (N. yvonneae) to habitat structure at the 12 sample sites. Structure was quantified by six habitat variables that were reduced to a single dimension (the first principal component (PC1)) via a principal component analysis (see Fig. 4 for variables and principal component loadings). For all plots, solid lines indicate strong evidence for a relationship between abundance and PC1 (slope highest posterior density (HPD) intervals do not include zero on a log scale) and ribbons represent HPD intervals. Lizard photos by Jules Farquhar; mammal photo by Kris Bell.
Discussion
Regional variability in fire regimes, biota, climate and landscape factors can make generalisations in fire ecology problematic (Keith 2012; Nimmo et al. 2013; Connell et al. 2022). Instead, fire management practices might best be guided by region-specific knowledge of vegetation communities to optimise habitat for fauna; however, such information is not available for most regions, and obtaining detailed region-specific data are impractical for board-scale fire management (Driscoll et al. 2010). Nonetheless, such arduous approaches may be necessary to meet the precision required for threatened species management, because fire regimes guided by inaccurate generalisations could have dire consequences for biodiversity. Although our study was small in scale, it can benefit local management and serves as a reference point for comparison with other regions and future assemblages that may experience different climates and fire-vegetation dynamics.
Vegetation-fire relations at Nanya Station
Clear successional changes in habitat structure were observed after fire. The most conspicuous change is the recovery of Triodia hummocks and the consequent decline in bare ground cover over time. Recently burnt (1 YSLF) areas have extensive bare ground coverage, and the only available form of above-ground vegetation cover is small regenerating herbaceous plants. At 18 YSLF, Triodia scariosa tussocks become large and dense, while mallee Eucalyptus spp. had regrown to >2 m tall. At 40 years, the cover of Triodia climaxes, forming large and connected hummocks (Rice and Westoby 1999). Similar patterns of vegetation recovery have been noted in other studies of Triodia-mallee communities (Caughley 1985; Haslem et al. 2011; Clarke et al. 2021).
Fauna-fire relationships at Nanya station
Despite strong evidence for successional changes in habitat structure, reptile richness and diversity did not vary according to fire history at our sites. This observation is consistent with studies in other ecosystems on reptile richness in south-western Australia, including in Banksia woodlands (Davis and Craig 2024) and temperate jarrah forests (Doherty et al. 2024a), and in a range of vegetation types in eastern Australia (Lindenmayer et al. 2008; Hu et al. 2013; Archer et al. 2024). Conversely, in a sclerophyll forest of southeastern Australia, Dixon et al. (2018) showed that reptile richness and abundance are significantly higher in sites >96 years post-fire than younger fire ages (0.5–12 years). Inconsistency between reptile richness and fire history was noted in a recent meta-analysis by González et al. (2022). Further, a global meta-analysis of organismal responses to fire (Moyo 2022) showed that fire had no effect on abundance and diversity of reptiles but had a small positive effect on richness of reptiles.
Reptiles can display strong turnover in community composition following fire, which likely masks variation in reptile species richness because late successional specialists are quickly replaced by early successional species (Doherty et al. 2024b). We found that reptile community composition differed in response to fire, with each of the three fire age classes supporting a substantially different assemblage of species. This supports an extensive base of literature demonstrating that fire-driven vegetation succession is a key driver of variation in reptile community assembly (Caughley 1985; Greenberg et al. 1994; Masters 1996; Letnic et al. 2004; Valentine et al. 2012; Clarke et al. 2021; González et al. 2022; Partridge et al. 2023).
Although PC1 suggests that broad vegetation structure is similar in 18 and >40 YSLF sites, reptile communities remain compositionally distinct. We attribute this to successional changes in ecological dynamics not measured in this study, but which may continue to diverge beyond 18 years post‐fire. Older sites (e.g. >40 YSLF) may continue to shift in finer-scale microhabitat features and invertebrate prey communities that are not captured by PC1, but underlie the nMDS-identified turnover between mid- and late-successional sites. Future studies should integrate metrics of prey availability, debris, litter depth, soil properties and moisture to fully explain the associations between fauna and fire history in these ecosystems.
As our models of individual species capture rates (Fig. 3) are limited to the six most frequently captured species, they cannot account for the rarer taxa that contribute substantially to the community‐level differences shown in the nMDS (Fig. 2c). While our individual‐species models clearly distinguish 1 YSLF from both 18 YSLF and >40 YSLF, they do not reveal any contrasts between the two older age classes. The nMDS, by including all species – even those too infrequent to model – demonstrates that 18 YSLF and >40 YSLF plots are themselves distinct. We therefore infer that low‐abundance species underlie the divergence between these older treatments. Targeted surveys and modelling of these rare taxa will be essential to fully understand how fire‐history shapes the entire reptile assemblage.
Individual species responses
A range of animal functional traits influence a species’ fire sensitivity and therefore response to fire (Lazzari et al. 2022; Batista et al. 2023). We explored responses by comparing relative abundance between sites, under the assumption that higher abundance in a given burn age is indicative of a species’ preference for that age class. Here, we discuss the fire-response of the six most commonly trapped species and compare them to studies from other regions to understand the degree of inter-regional variation.
Three common, terrestrial burrowing species (L. labialis, C. regius and L. damaeum) displayed significant positive relationships with PC1, peaking in abundance at recently burned (1 YSLF) sites, characterised by high bare ground cover and lack of Triodia. This is consistent with the findings of similar studies (Fyfe 1980; Caughley 1985; Sass and Wilson 2006; Driscoll and Henderson 2008) and aligns with the ‘irruptive’ fire response type – species that burrow or favour open spaces for foraging display greatest frequency of occurrence in early post-fire habitat (Caughley 1985; Friend 1993; Clarke et al. 2021). Lerista labialis is a sand-swimming specialist, spending much of its time in the loose upper surface of the sand (Greenville and Dickman 2009), and hence may be relatively unaffected by the loss of biomass following fire. Greenville and Dickman (2009) experimentally show that site selection in L. labialis is largely driven by a preference for loose sandy soil, rather than vegetation, food availability or thermal regimes of a site. Such a preference may interact with fire history, given that fire destroys the soil crust and enables loose sand to redistribute (Greene et al. 1990), thereby creating more extensive burrowing opportunities for fossorial squamates like L. labialis (Greenville and Dickman 2009). Our evidence for higher abundance of L. labialis at recently burnt sites indicates that fire benefits this common species by removing soil-stabilising crusts and vegetation. Ctenotus regius is a generalist species found in a range of habitat types and regions that lack Triodia. Ctenotus regius shelters in burrows it constructs into soil, and is likely minimally affected by the loss of complex vegetation structures following fire. At Nanya Station, Ctenotus regius has been recorded eating the inflorescence of small herbaceous plants (Olson et al. 2018) which are markedly abundant at 1 YSLF sites. It is possible that occasional herbivory in Ctenotus regius is a trait that may enhance its capacity to persist in recently burnt sites compared to other more strictly insectivorous and Triodia dependent species. Lucasium damaeum forges widely in open spaces and shelters in disused burrows made by other animals (Henle 1990) – again, a strategy that likely enables this species to persist in the post-fire environment where vegetation is lacking. In mallee eucalypt woodlands, gecko body condition and growth rates are correlated with increased early successional invertebrate abundance (Smith 2018) suggesting that trophic interactions may influence successional changes in abundance of insectivorous geckos such as L. damaeum.
The response of C. spinodomus (a non-burrowing spinifex specialist) was consistent with the ‘decline’ fire response type (Clarke et al. 2021), showing a decline in occurrence with increasing time since fire, but in a more gradual manner than the irruptive response type. This suggests that, over successional time, the habitat becomes increasingly less suitable for this species in this ecosystem. Others have shown that C. spinodomus (formerly C. fordi in older studies in NSW) has highest capture rates in early successional vegetation (Cogger 1969, 1974; Caughley 1985; Avitabile 2014). Conversely, Nimmo et al. (2012) examined reptile responses to fire over a 100 year chronosequence, and found that C. spinodomus (=‘C. fordi northern subregion’ in that study) had a preference for sites 50–100 years post-fire. Spatial differences in rainfall and grazing can influence fire-vegetation dynamics, and this has been posited as an explanation for the varied responses of reptiles to fire (Nimmo et al. 2012, 2014). Ctenophorus spinodomus is widely regarded as a spinifex-obligate species (Cogger 1969, 1974; Caughley 1985; Schlesinger et al. 1997; Sadlier et al. 2019) but observations of the species occurring in highest abundance at recently burned sites lacking Triodia requires explanation. It is possible that, although closely associated with spinifex ecosystems, and frequently using Triodia hummocks, the species is not so strictly dependent on them. Indeed, Bell et al. (2021) experimentally showed that C. spinodomus displays considerable intra-specific variation in the usage of Triodia over other vegetation, from total avoidance in some individuals (1.3% use) to exclusive use (100%) in others. Trophic dynamics may also play a role in this species’ high abundance at recently burned sites. For instance, C. spinodomus feeds almost exclusively on ants (Sadlier et al. 2019), which can display a marked increase in their surface activity soon after fire (Andersen and Yen 1985). Given these observations, we suggest that C. spinodomus is an overall abundant species in part because of its ecological versatility, and this may contribute to explaining why past studies have found varying fire responses in this species. Further research on the post-fire resource use of C. spinodomus is needed before one confidently claims that it prefers a certain seral stage.
Although C. atlas counts peaked in the 18 YSLF plots (15 individuals versus 8 in 1 YSLF and 11 in >40 YSLF), neither years‐since‐fire nor the Triodia‐cover axis (PC1) predicted these differences statistically. This pattern is surprising given the species reliance on Triodia hummocks for foraging and shelter (Pianka 1969; Craig et al. 2006; Bell et al. 2021) and the strong positive relationships reported elsewhere (Caughley 1985; Driscoll and Henderson 2008; Driscoll et al. 2012; Smith et al. 2013; Verdon et al. 2020). Early post‐fire environments tend to lack the mature hummocks this skink prefers, yet we still detected them in such environments – perhaps reflecting transient persistence or the species’ overall abundance and resilience to fire. Targeted sampling across other successional classes (e.g. 5–10 YSLF) will be essential to resolve more precisely how C. atlas tracks hummock regeneration over time at Nanya Station.
Ningaui yvonneae showed a ‘plateau’ response to fire history that is typical of a spinifex specialist (Clarke et al. 2021), being virtually absent from 1 YSLF sites, and becoming very abundant between 18 and 40 YSLF sites. This finding is comparable with previous studies demonstrating the species’ preference for sites older than ~20 YSLF (Kelly et al. 2010, 2011; Clarke et al. 2021), suggesting that accurate inter-regional predictions of this species’ occurrence and abundance can be derived from fire history and vegetation data. For instance, Verdon et al. (2020) show that N. yvonneae returns to the succession when a site’s Triodia cover is extensive and >20 cm tall (a feature of older sites). Preserving patches of long-unburnt spinifex habitat would benefit the conservation of this species.
Management implications
Our findings suggest that some common burrowing reptiles are able to tolerate a range of seral stages, and the need to provide a particular vegetation age class may not be as urgent for such species. Others, such as Ningaui yvonneae (Vulnerable in NSW), clearly require old growth habitat with mature Triodia. Given the variability in fauna responses to fire, both inter-regionally and interspecifically, we urge that a careful management of fire is required at Nanya Station.
Specific fire management recommendations for Nanya are difficult to derive from the present study alone, for several reasons. First, we focused on small terrestrial vertebrates (primarily reptiles), hence separate and tailored fire management actions must be considered for birds, mammals and invertebrates. Second, although time-since-fire has a major effect on fauna in many studies (reviewed in González et al. 2022), these fauna-fire responses can be difficult to disentangle from the interacting effects of animal dispersal capabilities (Nimmo et al. 2019), habitat loss and fragmentation (Driscoll et al. 2021), the timing of fire in relation to prevailing climatic conditions (Connell et al. 2022), fire severity, burn area size and vegetation type (Miritis et al. 2024). Given this, we provisionally make the general recommendation that a fine-grained mosaic of fire age classes should be a management priority, as each fire age class examined in this study had significantly different community composition (Fig. 2). Recent fire simulations by French et al. (2024) in mallee eucalypt woodlands support this management action, showing that small fires form fine-grained mosaics with a stable habitat mixture and with habitat diversity occurring at fine scales.
Aside from maximising beta diversity in a landscape, another critical conservation aim is to tailor fire management around the most fire-sensitive and threatened species. Such species may be especially vulnerable to fire due to their low population size and restricted ranges (Connell et al. 2019). Many threatened species depend on critical habitat attributes that are often most available in long-unburnt habitats, such as coarse woody debris or large grass hummocks; hence, maintaining long-unburnt habitat is critical for conserving most threatened species (von Takach et al. 2022). This is likely the case for small vertebrates at Nanya Station also – in our study, four reptile species and three mammal species are considered threatened in NSW, most of which are spinifex-obligate species (i.e. Strophurus elderi, Delma australis, Cyclodomorphus melanops elongatus, Ningaui yvonneae). Unfortunately, some of these species can be difficult to detect by virtue of their rarity and cryptic habitat-use patterns (e.g. S. elderi and C. m. elongatus rarely leave spinifex), and hence their fire responses are difficult to quantify without a more targeted sampling strategy. Most studies, including ours, are dominated by a small number of common species, allowing only a small portion of the assemblage to be modelled (e.g. Schlesinger et al. 1997), leaving many species' fire responses unknown. We encourage more detailed and ongoing investigations into fauna-fire relations at Nanya Station in order to further refine management decisions. Until then, priority should be placed on maximising pyrodiversity (French et al. 2024), but with emphasis on maintaining the availability of key habitat features that are found only in long-unburnt habitats (Dixon et al. 2018; von Takach et al. 2022).
Data availability
Data and R code used in this study are available on the Monash University Bridges data repository: 10.26180/29532416
Conflicts of interest
The authors have no conflicts of interests to declare. Simon Cook (author deceased): no conflicts of interest available.
Acknowledgements
We thank Federation University for managing Nanya Station and facilitating our research on the property.
References
Anderson MJ (2001) A new method for non‐parametric multivariate analysis of variance. Austral Ecology 26, 32-46.
| Crossref | Google Scholar |
Andersen AN, Yen AL (1985) Immediate effects of fire on ants in the semi‐arid mallee region of north‐western Victoria. Australian Journal of Ecology 10(1), 25-30.
| Crossref | Google Scholar |
Andersen AN, Cook GD, Corbett LK, Douglas MM, Eager RW, Russell-Smith J, Setterfield SA, Williams RJ, Woinarski JC (2005) Fire frequency and biodiversity conservation in Australian tropical savannas: implications from the Kapalga fire experiment. Austral Ecology 30, 155-167.
| Crossref | Google Scholar |
Archer ML, Letnic M, Murray BR, Webb JK (2024) After the megafires: effects of fire severity on reptile species richness and occupancy in South-Eastern Australia. Fire 7, 349.
| Crossref | Google Scholar |
ASH (2023) Australian Society of Herpetologists Official List of Australian Species. Available at https://www.australiansocietyofherpetologists.org/ash-official-list-of-australian-species [verified 1 December 2024].
Batista EK, Figueira JE, Solar RR, de Azevedo CS, Beirão MV, Berlinck CN, Brandão RA, de Castro FS, Costa HC, Costa LM, Feitosa RM, et al. (2023) In case of fire, escape or die: a trait-based approach for identifying animal species threatened by fire. Fire 6(6), 242.
| Crossref | Google Scholar |
Bell KJ, Doherty TS, Driscoll DA (2021) Predators, prey or temperature? Mechanisms driving niche use of a foundation plant species by specialist lizards. Proceedings of the Royal Society B. Biological Sciences 288, 20202633.
| Crossref | Google Scholar | PubMed |
Bond WJ, Keeley JE (2005) Fire as a global “herbivore”: the ecology and evolution of flammable ecosystems. Trends in Ecology & Evolution 20, 387-394.
| Crossref | Google Scholar | PubMed |
Bowman DM, Balch JK, Artaxo P, Bond WJ, Carlson JM, Cochrane MA, D’Antonio CM, DeFries RS, Doyle JC, Harrison SP, Johnston FH (2009) Fire in the earth system. Science 324, 481-484.
| Crossref | Google Scholar | PubMed |
Bradstock RA, Bedward M, Kenny BJ, Scott J (1998) Spatially-explicit simulation of the effect of prescribed burning on fire regimes and plant extinctions in shrublands typical of south-eastern Australia. Biological Conservation 86, 83-95.
| Crossref | Google Scholar |
Bunce RGH, Bogers MM, Evans D, Halada L, Jongman RHG, Mucher CA, Bauch B, de Blust G, Parr TW, Olsvig-Whittaker L (2013) The significance of habitats as indicators of biodiversity and their links to species. Ecological Indicators 33, 19-25.
| Crossref | Google Scholar |
Bürkner P-C (2017) brms: An R Package for Bayesian Multilevel Models using Stan. Journal of Statistical Software 80, 1-28.
| Crossref | Google Scholar |
Clarke MF, Kelly LT, Avitabile SC, Benshemesh J, Callister KE, Driscoll DA, Ewin P, Giljohann K, Haslem A, Kenny SA, Leonard S (2021) Fire and its interactions with other drivers shape a distinctive, semi-arid ‘Mallee’ ecosystem. Frontiers in Ecology and Evolution 9, 647557.
| Crossref | Google Scholar |
Cogger HG (1974) Thermal relations of the mallee dragon Amphibolurus fordi (Lacertilia: Agamidae). Australian Journal of Zoology 22, 319-339.
| Crossref | Google Scholar |
Connell J, Watson SJ, Taylor RS, Avitabile SC, Schedvin N, Schneider K, Clarke MF (2019) Future fire scenarios: predicting the effect of fire management strategies on the trajectory of high-quality habitat for threatened species. Biological Conservation 232, 131-141.
| Crossref | Google Scholar |
Connell J, Hall MA, Nimmo DG, Watson SJ, Clarke MF (2022) Fire, drought and flooding rains: the effect of climatic extremes on bird species’ responses to time since fire. Diversity and Distributions 28(3), 417-438.
| Crossref | Google Scholar |
Craig MD, Withers PC, Bradshaw SD (2006) Patterns of diet and microhabitat use by four species of sympatric Ctenotus lizards: do they reveal foraging specialization?. Journal of the Royal Society of Western Australia 89, 1-5.
| Google Scholar |
Davis RA, Craig MD (2024) Long-term post-fire succession of reptiles in an urban remnant in south-western Australia. International Journal of Wildland Fire 33(6), WF24033.
| Crossref | Google Scholar |
Dixon KM, Cary GJ, Worboys GL, Gibbons P (2018) The disproportionate importance of long‐unburned forests and woodlands for reptiles. Ecology and Evolution 8(22), 10952-10963.
| Crossref | Google Scholar | PubMed |
Doherty TS, Johnson B, Friend GR, Wayne AF (2024a) Multi‐year responses of reptiles to prescribed burning in a eucalypt forest ecosystem. Austral Ecology 49(8), e13572.
| Crossref | Google Scholar |
Doherty TS, Bohórquez Fandiño DF, Watchorn DJ, Legge SM, Dickman CR (2024b) Experimentally testing animal responses to prescribed fire size and severity. Conservation Biology 38(3), e14231.
| Crossref | Google Scholar |
Driscoll DA, Henderson MK (2008) How many common reptile species are fire specialists? A replicated natural experiment highlights the predictive weakness of a fire succession model. Biological Conservation 141, 460-471.
| Crossref | Google Scholar |
Driscoll DA, Lindenmayer DB, Bennett AF, Bode M, Bradstock RA, Cary GJ, Clarke MF, Dexter N, Fensham R, Friend G, Gill M (2010) Fire management for biodiversity conservation: key research questions and our capacity to answer them. Biological Conservation 143, 1928-1939.
| Crossref | Google Scholar |
Driscoll DA, Smith AL, Blight S, Maindonald J (2012) Reptile responses to fire and the risk of post-disturbance sampling bias. Biodiversity and Conservation 21, 1607-1625.
| Crossref | Google Scholar |
Driscoll DA, Armenteras D, Bennett AF, Brotons L, Clarke MF, Doherty TS, et al. (2021) How fire interacts with habitat loss and fragmentation. Biological Reviews 96, 976-998.
| Crossref | Google Scholar | PubMed |
Edwards GP, Allan GE, Brock C, Duguid A, Gabrys K, Vaarzon-Morel P (2008) Fire and its management in central Australia. The Rangeland Journal 30, 109-121.
| Crossref | Google Scholar |
Farquhar JE, Russell W, Chapple DG (2023) Identifying the abiotic factors that determine the inland range limits of a mesic-adapted lizard species. Integrative and Comparative Biology 64, 55-66.
| Crossref | Google Scholar |
Fox BJ (1982) Fire and mammalian secondary succession in an Australian coastal heath. Ecology 63, 1332-1341.
| Crossref | Google Scholar |
Fox BJ, Taylor JE, Thompson PT (2003) Experimental manipulation of habitat structure: a retrogression of the small mammal succession. Journal of Animal Ecology 72, 927-940.
| Crossref | Google Scholar |
French BJ, Murphy BP, Bowman DM (2024) Promoting optimal habitat availability by maintaining fine-grained burn mosaics: a modelling study in an Australian semi-arid temperate woodland. Fire 7(6), 172.
| Crossref | Google Scholar |
Friend GR (1993) Impact of fire on small vertebrates in mallee woodlands and heathlands of temperate Australia: a review. Biological Conservation 65, 99-114.
| Crossref | Google Scholar |
Fyfe G (1980) The effect of fire on lizard communities in central Australia. Herpetofauna 12, 1-9.
| Google Scholar |
Garden JG, McAlpine CA, Possingham HP, Jones DN (2007) Habitat structure is more important than vegetation composition for local‐level management of native terrestrial reptile and small mammal species living in urban remnants: a case study from Brisbane, Australia. Austral Ecology 32, 669-685.
| Crossref | Google Scholar | PubMed |
González TM, González-Trujillo JD, Muñoz A, Armenteras D (2022) Effects of fire history on animal communities: a systematic review. Ecological Processes 11(1), 11.
| Crossref | Google Scholar |
Greenberg CH, Neary DG, Harris LD (1994) Effect of high-intensity wildfire and silvicultural treatments on reptile communities in sand-pine scrub. Conservation Biology 8, 1047-1057.
| Crossref | Google Scholar |
Greene RSB, Chartres CJ, Hodgkinson KC (1990) The effects of fire on the soil in a degraded semiarid woodland. I. Cryptogam cover and physical and micromorphological properties. Soil Research 28(5), 755-777.
| Crossref | Google Scholar |
Greenville AC, Dickman CR (2009) Factors affecting habitat selection in a specialist fossorial skink. Biological Journal of the Linnean Society 97, 531-544.
| Crossref | Google Scholar |
Griffiths AD, Garnett ST, Brook BW (2015) Fire frequency matters more than fire size: testing the pyrodiversity-biodiversity paradigm for at-risk small mammals in an Australian tropical savanna. Biological Conservation 186, 337-346.
| Crossref | Google Scholar |
Haslem A, Kelly LT, Nimmo DG, Watson SJ, Kenny SA, Taylor RS, Avitabile SC, Callister KE, Spence-Bailey LM, Clarke MF, Bennett AF (2011) Habitat or fuel? Implications of long-term, post-fire dynamics for the development of key resources for fauna and fire. Journal of Applied Ecology 48, 247-256.
| Crossref | Google Scholar |
He T, Lamont BB, Pausas JG (2019) Fire as a key driver of Earth’s biodiversity. Biological Reviews 94, 1983-2010.
| Crossref | Google Scholar | PubMed |
Henle K (1990) Population ecology and life history of three terrestrial geckos in arid Australia. Copeia 1990, 759.
| Crossref | Google Scholar |
Hu Y, Urlus J, Gillespie G, Letnic M, Jessop TS (2013) Evaluating the role of fire disturbance in structuring small reptile communities in temperate forests. Biodiversity and Conservation 22, 1949-1963.
| Crossref | Google Scholar |
Keith DA (2012) Functional traits: their roles in understanding and predicting biotic responses to fire regimes from individuals to landscapes. In ‘Flammable Australia: Fire Regimes, Biodiversity and Ecosystems in a Changing World’. (Eds RA Bradstock, MA Gill, JE Williams) pp. 97–125. (CSIRO Publishing: Melbourne, Vic, Australia)
Kelly LT, Nimmo DG, Spence-Bailey LM, Clarke MF, Bennett AF (2010) The short-term responses of small mammals to wildfire in semiarid mallee shrubland, Australia. Wildlife Research 37(4), 293-300.
| Crossref | Google Scholar |
Kelly LT, Nimmo DG, Spence-Bailey LM, Haslem A, Watson SJ, Clarke MF, Bennett AF (2011) Influence of fire history on small mammal distributions: insights from a 100‐year post‐fire chronosequence. Diversity and Distributions 17(3), 462-473.
| Crossref | Google Scholar |
Lazzari J, Sato CF, Driscoll DA (2022) Traits influence reptile responses to fire in a fragmented agricultural landscape. Landscape Ecology 37(9), 2363-2382.
| Crossref | Google Scholar |
Lenth R (2025) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.11.1-00001. Available at https://rvlenth.github.io/emmeans/
Letnic M, Dickman CR, Tischler MK, Tamayo B, Beh CL (2004) The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. Journal of Arid Environments 59, 85-114.
| Crossref | Google Scholar |
Lindenmayer DB, Wood JT, MacGregor C, Michael DR, Cunningham RB, Crane M, Montague-Drake R, Brown D, Muntz R, Driscoll DA (2008) How predictable are reptile responses to wildfire? Oikos 117, 1086-1097.
| Crossref | Google Scholar |
Loyn RH, Kennedy SJ (2009) Designing old forest for the future: old trees as habitat for birds in forests of Mountain Ash Eucalyptus regnans. Forest Ecology and Management 258, 504-515.
| Crossref | Google Scholar |
Masters P (1996) The effects of fire-driven succession on reptiles in spinifex grasslands at Uluru National Park, Northern Territory. Wildlife Research 23, 39-48.
| Crossref | Google Scholar |
Miritis V, Dickman CR, Nimmo DG, Doherty TS (2024) After the ‘Black Summer’ fires: faunal responses to megafire depend on fire severity, proportional area burnt and vegetation type. Journal of Applied Ecology 61(1), 63-75.
| Crossref | Google Scholar |
Monamy V, Fox BJ (2000) Small mammal succession is determined by vegetation density rather than time elapsed since disturbance. Austral Ecology 25, 580-587.
| Crossref | Google Scholar |
Morton SR, James CD (1988) The diversity and abundance of lizards in arid Australia: a new hypothesis. American Naturalist 132, 237-256.
| Crossref | Google Scholar |
Moyo S (2022) Community responses to fire: a global meta-analysis unravels the contrasting responses of fauna to fire. Earth 3, 1087-1111.
| Crossref | Google Scholar |
Nimmo DG, Kelly LT, Spence-Bailey LM, Watson SJ, Haslem A, White JG, Clarke MF, Bennett AF (2012) Predicting the century-long post-fire responses of reptiles. Global Ecology and Biogeography 21, 1062-1073.
| Crossref | Google Scholar |
Nimmo DG, Kelly LT, Spence‐Bailey LM, Watson SJ, Taylor RS, Clarke MF, Bennett AF (2013) Fire mosaics and reptile conservation in a fire‐prone region. Conservation Biology 27, 345-353.
| Crossref | Google Scholar | PubMed |
Nimmo DG, Kelly LT, Farnsworth LM, Watson SJ, Bennett AF (2014) Why do some species have geographically varying responses to fire history? Ecography 37, 805-813.
| Crossref | Google Scholar |
Nimmo DG, Avitabile S, Banks SC, Bliege Bird R, Callister K, Clarke MF, Dickman CR, Doherty TS, Driscoll DA, Greenville AC, Haslem A (2019) Animal movements in fire‐prone landscapes. Biological Reviews 94(3), 981-998.
| Crossref | Google Scholar | PubMed |
Nimmo DG, Andersen AN, Archibald S, Boer MM, Brotons L, Parr CL, Tingley MW (2022) Fire ecology for the 21st century. Diversity and Distributions 28(3), 350-356.
| Crossref | Google Scholar |
Oksanen J (2024) Vegan: an introduction to ordination. Available at https://cran.r-project.org/web/packages/vegan/vignettes/intro-vegan.pdf [verified 1 December 2024].
Olson AR, Farquhar JE, Bettio MK (2018) Ctenotus regius (Regal Striped Skink). Diet. Herpetological Review 49(2), 328-329.
| Google Scholar |
Partridge DA, Lewis T, Tran CT, Castley JG (2023) Fire and habitat variables explain reptile community abundance and richness in subtropical open eucalypt forests. International Journal of Wildland Fire 32, 1089-1108.
| Crossref | Google Scholar |
Pausas JG, Keeley JE (2009) A burning story: the role of fire in the history of life. BioScience 59, 593-601.
| Crossref | Google Scholar |
Pianka ER (1969) Sympatry of desert lizards (Ctenotus) in Western Australia. Ecology 50, 1012-1030.
| Crossref | Google Scholar |
Pianka ER, Goodyear SE (2012) Lizard responses to wildfire in arid interior Australia: Long-term experimental data and commonalities with other studies. Austral Ecology 37, 1-11.
| Crossref | Google Scholar |
R Development Core Team (2024) R: A language and environment for statistical computing. Version 3.3.3. (R Foundation for Statistical Computing: Vienna) Available at https://www.r-project.org/ [verified 1 December 2024].
Rice B, Westoby M (1999) Regeneration after fire in Triodia R. Br. Australian Journal of Ecology 24, 563-572.
| Crossref | Google Scholar |
Rodda G (1978) 1975 bushfires in Northern Scotia country and their aftermath. Range Management Newsletter 78, 4-5.
| Google Scholar |
Sadlier RA, Colgan D, Beatson CA, Cogger HG (2019) Ctenophorus spinodomus sp. nov., a new species of dragon lizard (Squamata: Agamidae) from Triodia mallee habitat of Southeast Australia. Records of the Australian Museum 71, 199-215.
| Crossref | Google Scholar |
Sass S, Wilson A (2006) Effects of fire on lizard communities in the mallee shrublands of western New South Wales. Herpetofauna 36, 106-108.
| Google Scholar |
Schlesinger CA, Noble JC, Weir T (1997) Fire studies in mallee (Eucalyptus spp.) communities of western New South Wales: reptile and beetle populations in sites of differing fire history. The Rangeland Journal 19(2), 190-205.
| Crossref | Google Scholar |
Smith AL (2018) Successional changes in trophic interactions support a mechanistic model of post-fire population dynamics. Oecologia 186(1), 129-139.
| Crossref | Google Scholar | PubMed |
Smith AL, Bull CM, Driscoll DA (2013) Successional specialization in a reptile community cautions against widespread planned burning and complete fire suppression. Journal of Applied Ecology 50, 1178-1186.
| Crossref | Google Scholar |
Sousa WP (1984) The role of disturbance in natural communities. Annual Review of Ecology and Systematics 15, 353-391.
| Crossref | Google Scholar |
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. Journal of Biogeography 31, 79-92.
| Crossref | Google Scholar |
Valentine LE, Reaveley A, Johnson B, Fisher R, Wilson BA (2012) Burning in banksia woodlands: how does the fire-free period influence reptile communities? PLoS One 7, e34448.
| Crossref | Google Scholar | PubMed |
Verdon SJ, Watson SJ, Nimmo DG, Clarke MF (2020) Are all fauna associated with the same structural features of the foundation species Triodia scariosa?. Austral Ecology 45(6), 773-787.
| Crossref | Google Scholar |
von Takach B, Jolly CJ, Dixon KM, Penton CE, Doherty TS, Banks SC (2022) Long-unburnt habitat is critical for the conservation of threatened vertebrates across Australia. Landscape Ecology 37(6), 1469-1482.
| Crossref | Google Scholar |
Watson SJ, Taylor RS, Nimmo DG, Kelly LT, Haslem A, Clarke MF, Bennett AF (2012) Effects of time since fire on birds: how informative are generalized fire response curves for conservation management? Ecological Applications 22, 685-696.
| Crossref | Google Scholar | PubMed |
Westbrooke ME (2012) The pastoral history, biological and cultural significance of the Scotia Country, far western New South Wales. Proceedings of the Linnean Society of New South Wales 134, A55-A68.
| Google Scholar |
Westbrooke ME, Miller JD, Kerr MKC (1998) Vegetation of the Scotia 1:100 000 Map Sheet, Western New South Wales. Cunninghamia 5, 685-684.
| Google Scholar |
Woinarski JC, Burbidge AA, Harrison PL (2015) Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences 112(15), 4531-4540.
| Crossref | Google Scholar | PubMed |
York A, Bell TL, Weston CJ (2012) Fire regimes and soil-based ecological processes: implications for biodiversity. In ‘Flammable Australia: Fire Regimes, Biodiversity and Ecosystems in a Changing World’. (Eds RA Bradstock, MA Gill, JE Williams) pp. 127–148. (CSIRO Publishing: Melbourne, Vic, Australia)
Appendix 1.Abundance data for all small terrestrial vertebrates trapped in each fire age (years since last fire (YSLF))
| Class | Family | Species | 1 YSLF | 18 YSLF | >40 YSLF | Total | |
|---|---|---|---|---|---|---|---|
| Reptilia | Carphodactylidae | Nephrurus levis | 3 | 5 | 3 | 11 | |
| Reptilia | Diplodactylidae | Diplodactylus vittatus | 0 | 0 | 1 | 1 | |
| Reptilia | Diplodactylidae | Lucasium damaeum | 15 | 4 | 1 | 20 | |
| Reptilia | Diplodactylidae | Rhynchoedura angusta | 11 | 3 | 0 | 14 | |
| Reptilia | Diplodactylidae | Strophurus elderiVU | 0 | 5 | 1 | 6 | |
| Reptilia | Diplodactylidae | Strophurus williamsi | 0 | 2 | 0 | 2 | |
| Reptilia | Gekkonidae | Gehyra versicolor | 0 | 0 | 1 | 1 | |
| Reptilia | Pygopodidae | Delma australisEN | 0 | 1 | 0 | 1 | |
| Reptilia | Pygopodidae | Lialis burtonis | 1 | 1 | 1 | 3 | |
| Reptilia | Scincidae | Ctenotus atlas | 8 | 15 | 11 | 34 | |
| Reptilia | Scincidae | Ctenotus brachyonyx | 7 | 0 | 7 | 14 | |
| Reptilia | Scincidae | Ctenotus regius | 30 | 1 | 0 | 31 | |
| Reptilia | Scincidae | Ctenotus schomburgkii | 3 | 2 | 7 | 12 | |
| Reptilia | Scincidae | Ctenotus taeniatus | 0 | 15 | 1 | 16 | |
| Reptilia | Scincidae | Cyclodomorphus melanops elongatusEN | 1 | 0 | 1 | 2 | |
| Reptilia | Scincidae | Lerista aericeps | 3 | 3 | 1 | 7 | |
| Reptilia | Scincidae | Lerista labialis | 23 | 6 | 0 | 29 | |
| Reptilia | Scincidae | Lerista punctatovittata | 1 | 1 | 6 | 8 | |
| Reptilia | Scincidae | Liopholis inornata | 0 | 2 | 4 | 6 | |
| Reptilia | Scincidae | Menetia greyii | 2 | 2 | 5 | 9 | |
| Reptilia | Scincidae | Morethia boulengeri | 0 | 0 | 1 | 1 | |
| Reptilia | Agamidae | Ctenophorus pictus | 3 | 0 | 0 | 3 | |
| Reptilia | Agamidae | Ctenophorus spinodomus | 62 | 31 | 20 | 113 | |
| Reptilia | Agamidae | Diporiphora nobbi | 6 | 1 | 4 | 11 | |
| Reptilia | Agamidae | Pogona vitticeps | 2 | 4 | 3 | 9 | |
| Reptilia | Varanidae | Varanus gouldii | 2 | 2 | 1 | 5 | |
| Reptilia | Typhlopidae | Anilios bicolor | 0 | 2 | 0 | 2 | |
| Reptilia | Typhlopidae | Anilios bituberculatus | 1 | 0 | 6 | 7 | |
| Reptilia | Elapidae | Brachyurophis australis | 0 | 1 | 1 | 2 | |
| Reptilia | Elapidae | Demansia psammophis | 1 | 0 | 0 | 1 | |
| Reptilia | Elapidae | Suta nigriceps | 0 | 2 | 0 | 2 | |
| Reptilia | Elapidae | Pseudonaja mengdeni | 1 | 0 | 1 | 2 | |
| Reptilia | Elapidae | Pseudonaja modestaEN | 4 | 2 | 0 | 6 | |
| Reptilia | Elapidae | Vermicella annulata | 0 | 2 | 0 | 2 | |
| Amphibia | Limnodynastidae | Neobatrachus sudellae | 1 | 0 | 0 | 1 | |
| Mammalia | Dasyuridae | Ningaui yvonneaeVU | 2 | 63 | 64 | 129 | |
| Mammalia | Burramyidae | Cercartetus concinnusEN | 0 | 0 | 1 | 1 | |
| Mammalia | Muridae | Pseudomys bolamiEN | 2 | 1 | 0 | 3 | |
| Mammalia | Muridae | Mus musculus | 1 | 3 | 0 | 4 | |
| Total abundance | 196 | 182 | 153 | 531 |
Each YSLF treatment had four replicate sites. Nomenclatures of reptiles follows the ASH (2023) Official List of Australian Species. The conservation status is presented as ‘VU’ = Vulnerable and ‘EN’ = Endangered next to names of species listed as threatened in the state of NSW.