Behaviour of a large ungulate reflects temporal patterns of predation risk
Kevyn H. Wiskirchen A B * , Todd C. Jacobsen A C , Stephen S. Ditchkoff A , Steve Demarais D and Robert A. Gitzen A
A B * , Todd C. Jacobsen A C , Stephen S. Ditchkoff A , Steve Demarais D and Robert A. Gitzen A
A School of Forestry and Wildlife Sciences, Auburn University, 602 Duncan Drive, Auburn, AL 36849, USA.
B Present address: Missouri Department of Conservation, 3500 E Gans Road, Columbia, MO 65201, USA.
C Present address: Washington Department of Fish and Wildlife, 5525 S 11th Street, Ridgefield, WA 98642, USA.
D Department of Wildlife, Fisheries, and Aquaculture, Mississippi State University, Mississippi State, MS 39762, USA.
Wildlife Research 49(6) 500-512 https://doi.org/10.1071/WR21047
Submitted: 5 March 2021 Accepted: 19 November 2021 Published: 1 March 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Many prey species exhibit antipredator behaviours when threatened, yet prey response to temporal variation in predation risk is not well-understood, despite being foundational to predator–prey dynamics and an important consideration among game population managers and recreationalists.
Aims: To examine white-tailed deer (Odocoileus virginianus) behaviour in response to temporal variation in predation risk imposed by recreational hunters and to assess the effect of deer sex and age on antipredator response.
Methods: Global positioning system (GPS) collars were used to monitor behaviour of female and male deer in response to diel and weekly patterns of recreational hunting in Alabama, USA.
Key results: Deer behaviour on weekends (i.e. Friday–Sunday), corresponding to periods of elevated risk, was similar to behaviour on weekdays (i.e. Monday–Thursday). However, when behaviour was examined by individual day of the week, movement rate decreased by 17%, net displacement decreased by 31%, and the probability of activity decreased by 24% during daylight hours on Sundays compared with Fridays. Behavioural changes among days were not detected at night. Daytime behavioural shifts persisted until Wednesdays, despite lower weekday hunting activity. Behavioural variation by deer sex and age was also observed.
Conclusions: Deer perceive temporal variation in predation risk and modify their behaviour to reduce the likelihood of predation. Variation in response across sex and age classes may be driven by previous experience with hunters and/or survival- and fitness-related trade-offs that affect prey decisions at the individual level. Antipredator response was not initially detectable when examined at a broad temporal scale (i.e. weekend vs weekday); however, a behavioural response was shown with a finer-scale analysis (i.e. individual day of the week), which more closely reflected the pattern of risk fluctuation.
Implications: Our findings demonstrated the acute awareness of a large ungulate to temporal changes in predation risk and provided insight into ways in which these prey behaviourally respond to reduce the likelihood of predatory encounters. Future studies should consider the temporal scale of risk fluctuation when examining antipredator response to avoid false conclusions. Ungulate hunters and managers can use this information to more efficiently achieve their goals.
Keywords: Alabama, antipredator behaviour, hunting pressure, movement, Odocoileus virginianus, predation risk, predator–prey dynamics, white-tailed deer.
Introduction
Prey animals are faced with the challenge of avoiding predators to stay alive. However, prey have additional survival- and fitness-related considerations, such as foraging to meet nutritional requirements, thermoregulation, acquiring mates, providing care for offspring, and defending territories or other resources. Thus, prey animals must balance their time across multiple competing needs to maximise survival and lifetime fitness (Lima and Dill 1990; Sih et al. 2000).
A wealth of evidence demonstrates that prey across numerous animal taxa alter behaviour to avoid predators (Kats and Dill 1998). Further, researchers have offered predictions as to how prey should behave under different predation-risk scenarios to optimally balance predator avoidance and other necessary activities (Lima and Bednekoff 1999). These predictions are based, in part, on the assumption that prey can accurately assess predation risk and detect changes in risk through time. Tests of these predictions have yielded varied results, with some providing support and others providing opposing evidence (Gude 2004; Creel et al. 2008; Ferrari et al. 2009), creating uncertainty about the acuity with which prey can detect and respond to temporal variation in predation risk. Additional investigation into prey response to temporal variation in risk can provide important insights into predator-detection capabilities and prey decision-making that has shaped evolutionary history.
One important consideration when examining prey response to predation risk is the temporal scale (i.e. seconds, minutes, hours, etc.) at which risk fluctuates (Picardi et al. 2019). Prey behaviour should fluctuate at a temporal scale that corresponds to the scale of risk fluctuation (Basille et al. 2015). Without a priori knowledge of this temporal scale, it becomes difficult, or even impossible, to accurately detect a behavioural change as a result of the data being collected or examined at a different scale than the behaviour of interest (Hebblewhite and Haydon 2010). Some previous studies have failed to detect a behavioural response to predation risk, and suggested that their outcome could be related to the temporal scale at which the data were collected or examined (Neumann et al. 2009; Karns et al. 2012).
White-tailed deer, Odocoileus virginianus (hereafter ‘deer’), provide a useful model for investigation into prey response to temporal patterns of risk because humans present significant predation risk within many deer populations in the form of recreational hunting (Frid and Dill 2002). Furthermore, human hunters have predictable, and often quantifiable, temporal patterns of activity, which researchers can use to determine the scale of risk fluctuation (Proffitt et al. 2009; Ciuti et al. 2012). The goal of this study was to examine deer response to identified temporal patterns of predation risk imposed by recreational hunters. Additionally, we examined the effects of deer sex and age on antipredator response. We hypothesised that there would be a difference in response by deer age because of amassed experience with hunters through time. We also hypothesised that antipredator response would vary by sex because of different fitness-related considerations between females and males. We tested our predictions during a period prior to the onset of deer breeding, because breeding may have hindered previous investigations by masking deer response to recreational hunting (Sargent and Labisky 1995; Tomberlin 2007; Karns et al. 2012).
Study area
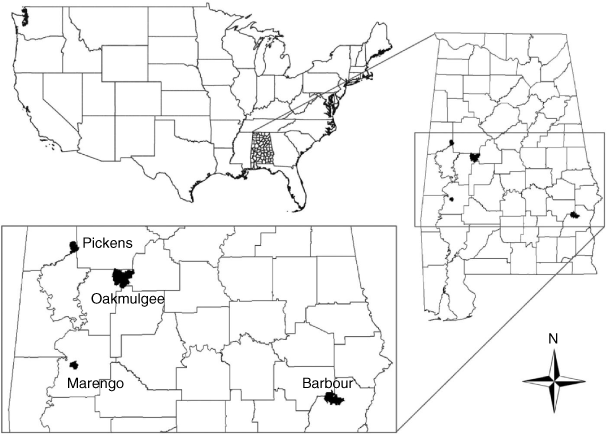
Our research was conducted across four areas in Alabama, USA (Fig. 1). Two areas were privately owned (i.e. Marengo and Pickens) and two were public-use Wildlife Management Areas (i.e. Barbour and Oakmulgee) managed by the Alabama Department of Conservation and Natural Resources (ADCNR). Combined land area across all locations was 374 km2 (Barbour = 114 km2, Marengo = 31 km2, Oakmulgee = 180 km2, Pickens = 49 km2). Barbour and Marengo were situated in Alabama’s lower coastal plain (Gray et al. 2002) and consisted of gently rolling terrain, while Oakmulgee and Pickens were situated in the southern foothills of the Appalachian Mountains and consisted of more rugged terrain with hills of steep to moderate slope. Habitat on public-use areas was predominantly mixed pine–hardwood stands consisting of loblolly (Pinus taeda) and shortleaf pine (P. echinata), oaks (Quercus spp.), maples (Acer spp.), sweetgum (Liquidambar styraciflua), sycamore (Platanus occidentalis), and yellow poplar (Liriodendron tulipifera) managed on a 3–5-year prescribed burn rotation. Portions of upland areas were mature or regenerating stands of longleaf pine (P. palustris) that were more frequently burned. Private-land areas were primarily managed for timber production and existed in various regenerative stages of loblolly and shortleaf pine. Winters in Alabama were mild relative to more northern portions of the white-tailed deer distribution, with mean diel temperatures of 11.5°C (min. = 5.1°C, max. = 17.9°C) and monthly precipitation of 16.0 cm during the study period (www.prism.oregonstate.edu). Portions of Alabama infrequently receive snow during the winter, but accumulation is short-lived and snow depth is not great enough to restrict deer movement or limit food resources (Cook and Gray 2003).
|
In the south-eastern United States, coyotes (Canis latrans) are known to be significant predators of deer neonates (Saalfeld and Ditchkoff 2007; Kilgo et al. 2012; Jackson and Ditchkoff 2013; Nelson et al. 2015), but with the exception of one study (Chitwood et al. 2014), coyotes have not been demonstrated as being important predators of adult deer in the region. Coyotes were present across our study areas; however, a concurrent study of adult deer survival on the same areas did not document any predation events (Wiskirchen 2017), suggesting predation risk from non-human predators was minimal compared with that presented by recreational hunters.
On Barbour and Marengo, archery hunting for deer extended from 25 October to 10 February annually, with firearm portions (i.e. youth, primitive weapons, and rifle) occurring intermittently from 14 November to 10 February during the 2014–2015 season and from 13 November to 10 February during the 2015–2016 season. On Oakmulgee and Pickens, archery hunting for deer extended from 15 October to 31 January annually, with firearm portions occurring intermittently from 14 November to 31 January during the 2014–2015 season and from 13 November to 31 January during the 2015–2016 season. Hunting and trapping seasons for various other game species were concurrent with portions of deer season in Alabama; however, such activities were not allowed on public-use areas on days of gun hunting for deer, or during preceding nights. The primary activity on privately owned study areas during deer season was deer hunting.
Materials and methods
Capture and handling
During summers of 2014 and 2015, adult (≥1 year old) male and female deer were immobilised via intramuscular injection of Telazol (Fort Dodge Animal Health, Fort Dodge, IA, USA; 100 mg mL−1 at an approximate rate of 4.0 mg kg−1) and xylazine-hydrochloride (Lloyd Laboratories, Shenandoah, IA; 100 mg mL−1 at an approximate rate of 2.0 mg kg−1) by using a transmitter dart delivery system (Pneu-Dart, Inc., Williamsport, PA, USA), and sedatives were reversed via intramuscular hand-injection of tolazoline (Lloyd Laboratories; 100 mg mL−1 at an approximate rate of 2.0 mg kg−1) when animal handling was complete. Deer were equipped with a global positioning system (GPS) collar (Advanced Telemetry Systems, Isanti, MN, USA) and yellow, cattle ear tags (Y-Tex Corporation, Cody, WY, USA). GPS collars were fluorescent orange and, paired with yellow ear tags, were intended to be visible to hunters who were asked not to harvest GPS-collared deer to avoid sample size reduction (Wiskirchen et al. 2017). Deer age was estimated using a combination of tooth replacement and wear (Severinghaus 1949) and body characteristics (Demarais et al. 1999) to maximise aging accuracy (Bowman et al. 2007). Capture and handling methods were approved by the Auburn University Institutional Animal Care and Use Committee (PRN 2013–2323).
Data collection and censoring
GPS collars were programmed to acquire a location once per hour during the 2014–2015 and 2015–2016 deer hunting seasons. Mean error of GPS locations was reduced by removing all three-dimensional fixes with position dilution of precision (PDOP) values >10 or horizontal dilution of precision (HDOP) values >6, as well as two-dimensional fixes with HDOP values >5 (Moen et al. 1997; Dussault et al. 2001; D’Eon and Delparte 2005). Fixes <7 days post-capture were also removed to exclude movements that might be altered by capture (Karns et al. 2012), and fixes <7 days pre-mortem were removed except in the case of known hunter harvest.
Temporal patterns of risk and exposure period
Two temporal patterns of predation risk were identified that reflected activity patterns of recreational hunters across our study areas. The first was a diel temporal pattern, also observed within other hunted populations of deer (Proffitt et al. 2009; Ciuti et al. 2012), where predation risk was elevated during hours of legal hunting and reduced at night when hunters were absent. Two periods were defined to reflect diel temporal variation in predation risk; DIURNAL represented the period of elevated risk and was defined by the period of legal hunting (i.e. 30 min before sunrise to 30 min after sunset), and NOCTURNAL was the period outside of these bounds. Sunrise and sunset data were obtained from the US Naval Observatory (www.usno.navy.mil/USNO) for the municipality nearest to each study area.
A second, weekly temporal pattern of predation risk reflected variation in hunting activity by day of the week (i.e. Sunday, Monday, Tuesday, etc.). Daily hunting activity on privately owned areas was quantified using hunter records documenting the date and number of hours hunters spent afield, and are reported as hunter h ha−1 day−1. On public-use areas, hunters were required to obtain an area-use permit each day of gun hunting. Time spent afield was unknown on public areas, so daily hunting activity was represented as hunters km−2 day−1. Hunter h ha−1 day−1 and hunters km−2 day−1 are common indices of hunting pressure, allowing for direct comparison with previous studies (Murphy 1962; Root et al. 1988; Diefenbach et al. 2005; Little et al. 2016). Predation risk was greater on weekends (i.e. Fridays, Saturdays, and Sundays) than throughout the rest of the week, with additional risk variation within weekend and weekday periods. Two independent variables were created to reflect weekly variation in predation risk; DAY_TYPE represented coarse differences between weekends and weekdays, and DOW reflected finer-scale temporal-risk variation by individual day of the week. Although knowledge of daily hunting activity across study areas was likely imperfect, we had no reason to believe that missing records were more likely from one day of the week than from any other. Thus, our estimates represent minimum hunting pressure and are assumed to reflect relative risk throughout the week.
Breeding and hunting seasons frequently coincide within hunted populations of deer (Tomberlin 2007). Consequently, breeding activity has confounded previous investigations of deer response to human predators (Sargent and Labisky 1995; Karns et al. 2012). We selected a study period during hunting season, but prior to breeding season, to reduce this potentially confounding factor. This was possible given a 108-day deer hunting season within Alabama, most of which occurred prior to the onset of breeding. We determined area-specific breeding periods by using a 10-year conception dataset collected by the ADCNR (C. Cook, unpubl. data) at each study area. We selected a period for each area that began the opening day of youth season (14 November in 2014 and 13 November in 2015), when hunting activity became an appreciable source of predation risk, and ended on a date such that <5% of annual conceptions would be contained within the period of risk exposure. Owing to variation in breeding dates across study areas (Cook and Gray 2003), the selected period varied from 23 to 58 days (X̄ = 42.75 days).
Behavioural parameters
We evaluated deer response to temporal patterns of predation risk through the following four behavioural metrics: movement rate, net displacement, probability of activity, and temporal selection. These parameters were chosen or developed to monitor for spatial and temporal behavioural response. Movement rate was the Euclidean distance between successive hourly fixes (m h−1) and was classified as DIURNAL or NOCTURNAL on the basis of the period in which the movement ended. Movements that began in one period and ended in another were excluded from the analysis, as were movement windows exceeding 1 h because of failed fix attempts to avoid estimating total movement over variable-length periods (Little 2011).
Net displacement can be used to characterise animal movement behaviour (Crist et al. 1992), where large net displacement values correspond to extensive spatial exploration and small net displacement values represent localised movement or stationary behaviour. Net displacement was calculated as the Euclidean distance (m) from a pre-defined starting point to each subsequent point along the movement path throughout the period of interest. Net displacement values were classified as DIURNAL or NOCTURNAL on the basis of the period in which the movement ended, with DIURNAL net displacement being calculated using the first diurnal location of each day as the starting point and NOCTURNAL net displacement by using the first nocturnal location of each day (i.e. the first location following the DIURNAL period) as the starting point.
Probability of activity was calculated for each DIURNAL and NOCTURNAL hourly period as the Euclidean distance between successive fixes and treated as a binary variable, with ‘active’ being assigned to step lengths of ≥49.05 m and ‘inactive’ to step lengths of <49.05 m. This activity threshold was based on Jerde and Visscher (2005) who recommended using ≥5 s.d. of the mean GPS locational error for assigning activity or inactivity states. Sullivan et al. (2016) field tested the locational accuracy of the same collar used in our study and found a mean locational error of 12.95 m (s.d. = 9.81 m). Movements that began in one period and ended in another, as well as movement windows >1 h, were excluded when assigning activity states.
Deer may decrease diurnal activity and increase nocturnal activity in response to human hunters (Kilgo et al. 1998). We used temporal selection to monitor for disproportionate movement during, or ‘selection’ for, either the DIURNAL or NOCTURNAL period, as well as shifts in selection in response to patterns of predation risk. Temporal selection was calculated as:
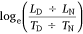
where LD was diurnal step length, LN was nocturnal step length, and TD and TN were the number of diurnal and nocturnal hours during the corresponding day respectively. Diurnal and nocturnal step lengths were the sum of hourly movements over the corresponding period. Hourly movements that began in one period and ended in another were excluded from the analysis, as were days that contained fewer than 23 hourly fixes. A natural log-transformation was used to normalise the distribution of fitted values (Bartlett 1947) after which values >0 indicated disproportionate movement during, or ‘selection’ for, the DIURNAL period and values <0 indicated selection for the NOCTURNAL period.
Data analysis
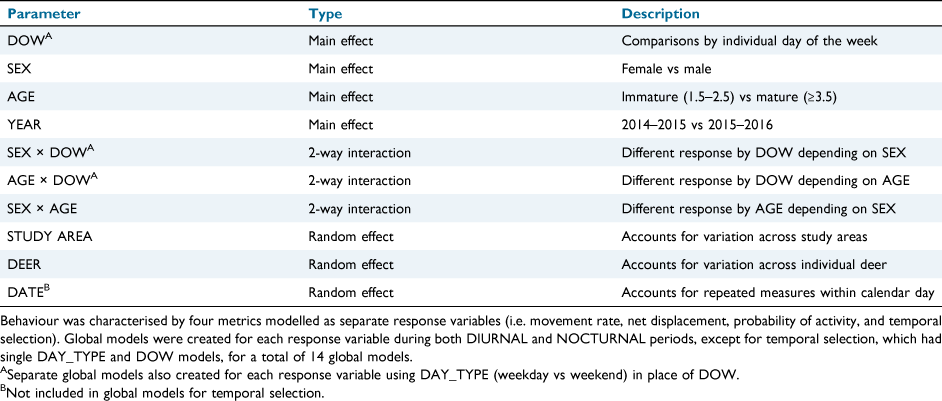
Movement rate and net displacement values were natural log-transformed to correct for a right-skewed distribution of residuals across fitted values (Bartlett 1947). Each of the four behavioural parameters were included as response variables within either linear mixed-effects models (i.e. movement rate, net displacement, temporal selection) or generalised linear mixed-effects models with a binomial distribution (i.e. probability of activity) in Program R (ver. 4.1.1, www.r-project.org). To examine differences in movement between DIURNAL and NOCTURNAL periods, data from three of the behavioural parameters (i.e. movement rate, net displacement, probability of activity) were subdivided into two groups on the basis of the corresponding diel period. Temporal selection was examined without subdividing the data because both diurnal and nocturnal movements were used to calculate the parameter values. DIURNAL and NOCTURNAL datasets, as well as the dataset for temporal selection, were each included in two global models (i.e. 14 total global models) to examine the effect of weekly temporal patterns of risk (i.e. DAY_TYPE and DOW) on deer antipredator behaviour. Course temporal differences between weekend and weekday behaviour were examined through DAY_TYPE models, and finer-scale temporal differences in behaviour by individual day of the week were examined through DOW models (Table 1). Global models included SEX, AGE, and YEAR as main effects, with AGE being modelled as a two-factor, categorical variable, as follows: 1.5–2.5 years old (hereafter ‘immature’) and ≥3.5 years old (hereafter ‘mature’). Global models also contained interaction terms with SEX and AGE to examine the effect of sex and age class on deer response to temporal patterns of risk. STUDY_AREA and DEER were included as nested random effects within each global model, reflecting the spatially nested study design and accounting for unmeasured variation between geographic locations and individuals. DATE was also included as a nested random effect in global models, except those with temporal selection as the response variable, to account for greater correlation among movements within, rather than between, days. Temporal selection was not modeled with DATE as a random effect because there was only a single parameter estimate per day.
A limited step-down approach was used to eliminate uninformative interaction terms and improve parsimony of global models (Harrell 2001). Global models were compared to reduced models with a two-way interaction excluded, using a likelihood ratio test (LRT). Interactions were removed if the LRT resulted in a P-value > 0.10. A liberal P-value was used to achieve an appropriate balance between parsimony and model accuracy (Harrell 2001). The model-reduction process was complete when all uninformative interactions were removed, as main effects were not candidates for removal, and inferences were based on final models.
Results
We deployed GPS collars on 38 adult deer across study areas, six of which were not included in the analysis owing to collar program malfunction (n = 2), mortality prior to the start of the risk-exposure period (n = 1), and failure by collars to detach (n = 3). Of the remaining 32 animals (16 males, 16 females), 20 contributed movement data from both the 2014–2015 and 2015–2016 deer hunting seasons. Movement rate and probability of activity were analysed from 36 718 locations, net displacement from 38 673 locations, and temporal selection from 24 196 locations within the risk-exposure period.
Hunting effort
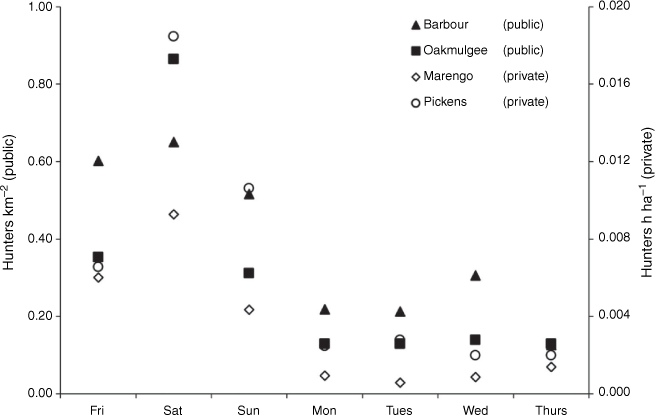
Hunting effort on public-use areas averaged 0.56 (s.e. = 0.10) hunters km−2 day−1 on weekends and declined by 66%, to an average of 0.19 (s.e = 0.04) hunters km−2 day−1 on weekdays (Fig. 2). On privately owned areas, hunting effort averaged 0.009 (s.e. = 0.001) hunter h ha−1 day−1 on weekends and declined by 83% to an average of 0.001 (s.e. < 0.001) hunter h ha−1 day−1 on weekdays. On both public and private areas, hunting effort was greatest on Saturdays, averaging 0.72 (s.e. = 0.19) hunters km−2 day−1 and 0.013 (s.e. = 0.002) hunter h ha−1 day−1 respectively.
DIURNAL period
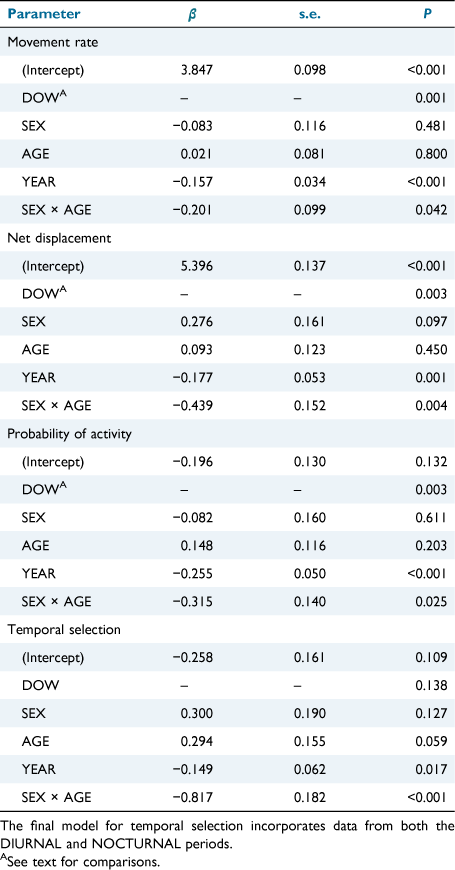
Within the final DAY_TYPE, DIURNAL-period models, and the final DAY_TYPE temporal selection model, no interactions between DAY_TYPE and other main effects were retained. Furthermore, the main effect of DAY_TYPE did not explain deer behaviour within any of the final models (P ≥ 0.216), suggesting similar behaviour between weekends and weekdays regardless of sex or age class of deer. However, DOW explained daytime variation in movement rate (P = 0.001), net displacement (P = 0.003), and probability of activity (P = 0.003), indicating that diurnal behaviour fluctuated by individual day of the week. DOW was not an informative parameter in the temporal selection model (P = 0.138), indicating that deer did not respond temporally by shifting movement from one period to another throughout the week (Table 2).
|
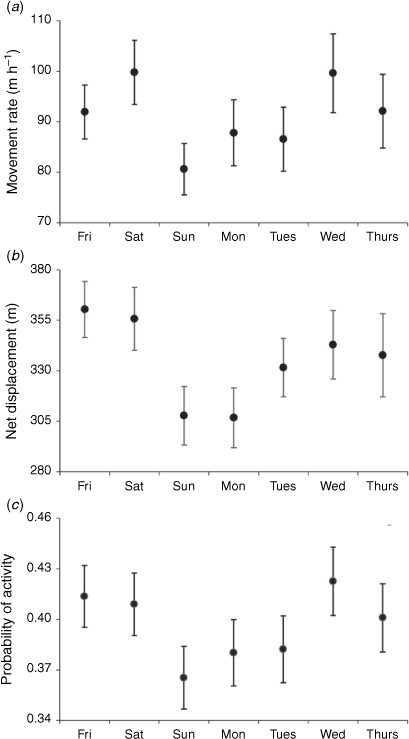
We observed no change in diurnal movement rate, net displacement, or probability of activity from Thursdays to Fridays (P ≥ 0.179) or from Fridays to Saturdays (P ≥ 0.761; Fig. 3). However, from Saturdays to Sundays, we observed an 18% (95% CI = 7–29%, P = 0.001) decrease in movement rate, 28% (95% CI = 10–49%, P = 0.002) decrease in net displacement, and 22% (95% CI = 6–40%, P = 0.005) decrease in probability of activity. Each of these behavioural metrics then gradually increased, and, by Wednesdays, they were once again greater than on Sundays (P ≤ 0.006).
|
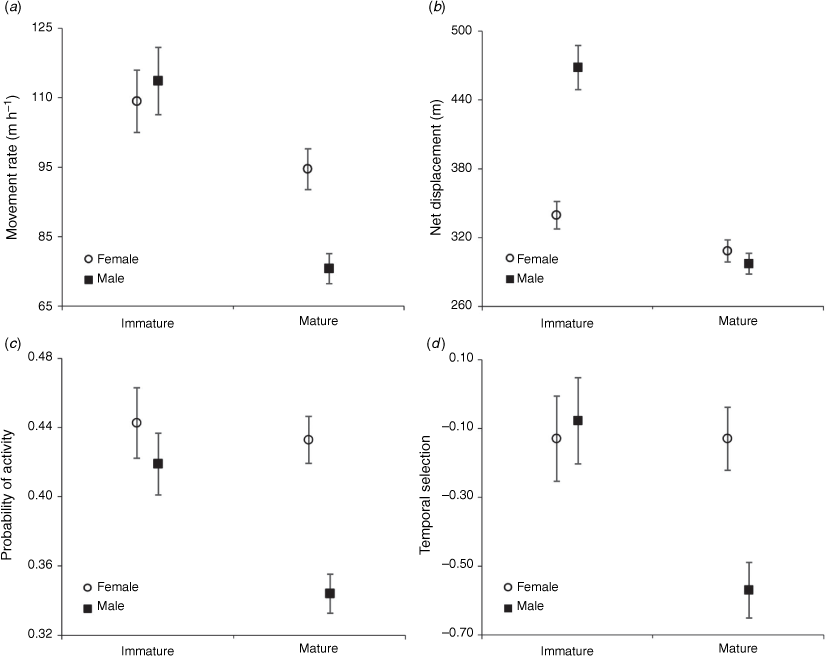
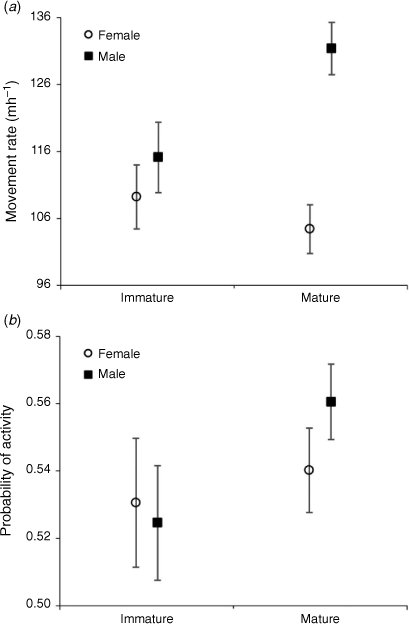
All final DAY_TYPE and DOW, DIURNAL-period and temporal selection models retained a SEX × AGE interaction (P ≤ 0.042), indicating differences in daytime behaviours and temporal selection between immature and mature deer varied by sex, or vice versa (Fig. 4). Diurnal movement rate of mature males was 15% (95% CI = 0–33%; P = 0.047) less than that of immature males and net displacement of mature males was 31% (95% CI = 3–67%, P = 0.025) less than that of immature males. However, there was no difference in diurnal movement rate or net displacement between female age classes (P ≥ 0.689). Mature males were also 50% (95% CI = 5–113%; P = 0.025) less likely to be active during the day than were mature females and expressed 60% (95% CI = 9–133%; P = 0.027) greater selection for the NOCTURNAL period than did mature females. However, probability of activity and temporal selection did not differ between immature males and females (P ≥ 0.514).
Final DIURNAL models indicated greater (P ≤ 0.001) movement rate, net displacement, and probability of activity during the 2014–2015 hunting season compared to the 2015–2016 season.
NOCTURNAL period
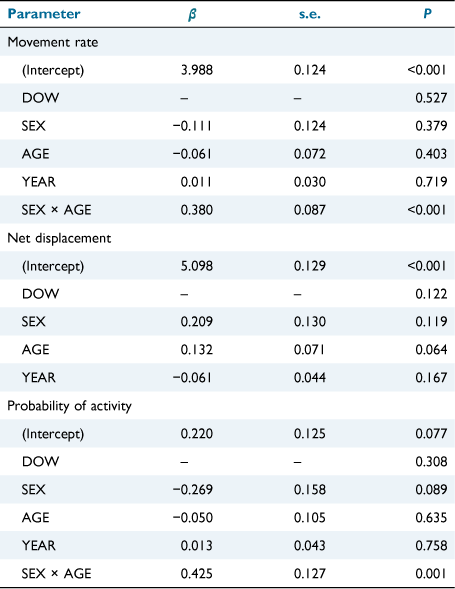
As with DIURNAL models, the final DAY_TYPE, NOCTURNAL-period models retained no interactions between DAY_TYPE and other main effects, and the main effect of DAY_TYPE did not explain deer behaviour within any of the NOCTURNAL models (P > 0.169). Similarly, final DOW models for the NOCTURNAL period retained no interactions between DOW and other main effects, and the main effect of DOW was not an explanatory variable for any of the behavioural parameters (P > 0.122; Table 3). The absence of interactions containing DAY_TYPE or DOW, and the lack of support for both DAY_TYPE and DOW as main effects, indicate consistent behaviour between weekends and weekdays, and across days of the week, during the NOCTURNAL period.
|
Final NOCTURNAL models for movement rate and probability of activity contained a SEX × AGE interaction (P ≤ 0.001), indicating that night-time movement rate and activity levels were different between mature and immature deer, but the magnitude of difference was dependent on sex (Fig. 5). Namely, mature males were 56% (95% CI = 27–92%; P < 0.001) more likely to be active during nocturnal hours than were immature males, and movement rate was 42% (95% CI = 24–64%; P < 0.001) greater among mature males than immature males. Conversely, there was no difference in movement rate (P = 0.221) or probability of activity (P = 0.362) between female age classes. Last, YEAR was not a significant parameter in any of the final NOCTURNAL models (P ≥ 0.167), indicating consistent nocturnal movement, space use, and activity between years.
|
Discussion
Differences in patterns of behaviour between diurnal and nocturnal periods suggest that deer are able to distinguish between the presence and absence of hunters on the landscape and alter antipredator behaviour accordingly. Furthermore, our results demonstrated that even low levels of hunting pressure can elicit antipredator response within ungulate populations. Previously examined ungulate populations have received various hunting pressure intensities, ranging from 4 hunters km−2 (Diefenbach et al. 2005) to 77 hunters km−2 (Murphy 1962), and from 0.05 hunter h ha−1 (Little et al. 2016) to 1.31 hunter h ha−1 (Root et al. 1988). In comparison, estimated hunting pressure on our study areas was less, even less than a previously reported level below which behavioural response was not detected (0.45 hunter h ha−1 day−1, Root et al. 1988). However, most previous studies have been conducted during the breeding season, because periods of hunting and breeding typically coincide within ungulate populations (Tomberlin 2007). This study took place prior to breeding, which not only limited a nearly ubiquitous confounding factor, but also allowed for examination of the effect of hunting during a time when deer may be more responsive to predatory threats. Intense competition for mates among male conspecifics (Mysterud et al. 2004), and, to a lesser degree, among females (Sullivan et al. 2017), during the rut may cause breeding activity to dominate behaviour until an environmental stimulus surpasses the desire to breed (Neumann et al. 2009). On the basis of our findings, compared with previous studies, outside of the breeding season deer may be more willing to employ behavioural trade-offs to avoid predators, even when the risk of predation is low.
We further attribute detection of behavioural changes within low-risk environments to having conducted our analysis at a temporal scale that corresponded to the scale of risk fluctuation throughout the week. Our DIURNAL, DAY_TYPE models suggested no change in behaviour between weekends and weekdays, despite a substantial difference in hunting activity. Yet, we found a population-level response when a greater degree of temporal variation was incorporated (i.e. DOW models), more accurately reflecting temporal variation in hunting pressure by day of the week. Surprisingly, we did not observe an immediate response to increased hunting pressure on weekends. Rather, we detected a ‘delayed’ response to weekend hunting where a population-level change in deer behaviour became evident on Sundays. The ‘delayed’ population-level response could suggest that individual deer did not immediately respond to temporal changes in predation risk. However, this possibility seems unlikely, because detection of, or direct encounters with, predators should elicit a rapid antipredator response (Lima and Bednekoff 1999). More plausible is that individual deer responded to encounters with hunters as they occurred throughout the weekend, but in ways that were not detectable by our selected behavioural metrics. Karns et al. (2012) found no population-level response to hunting among adult male deer in Maryland, USA. However, they observed individuals displaying temporary and short-distance flight responses to encounters with hunters, and attributed their ability to detect these fine-scale movements to high-resolution GPS data collected at 20-min intervals. Likewise, Neumann et al. (2009) found no behavioural response by moose (Alces alces) to hunting when using a GPS fix rate of 30–60 min. However, the researchers postulated that behavioural changes may have been detectable on an individual basis rather than at the population level. Our 1-h GPS fix intervals are likely to have precluded our ability to detect fine-scale or individually based behavioural changes at the beginning of the weekend. Our selected behavioural metrics also do not reflect the full array of possible antipredator behaviours that have been previously observed among hunted ungulates, such as changes in habitat utilisation, that may not otherwise affect activity levels or movements (Swenson 1982; Kufeld et al. 1988; Kilgo et al. 1998). Despite failing to detect an initial response, three of our metrics indicated a population-level change in behaviour by Sundays, which may have been the result of multiple encounters with hunters by the third day of the weekend. Laurila et al. (2004) observed a graded behavioural response by common frog (Rana temporaria) tadpoles to a predatory dragonfly (Aeshna sp.), where tadpole activity decreased with each additional predatory encounter throughout phases of development. Our results may indicate a similar graded response among ungulates, where antipredator behaviour becomes more pronounced after multiple encounters with hunters.
Following the diurnal behavioural depression detected on Sundays, daytime behaviours did not return to pre-weekend levels until Wednesdays, despite less hunting pressure on Mondays and Tuesdays. Other studies have observed a similar display of prolonged antipredator behaviour following periods of risk, and have postulated that this may be the result of uncertainty as to whether or not predators have vacated the field (Sih 1992; Little 2011). However, we observed a difference in patterns of behaviour between diurnal and nocturnal periods, suggesting that deer were able to accurately distinguish between the presence and absence of hunters. The ‘delayed’ return to pre-weekend behaviours could support previous findings where prey learned and responded in a predictive manner to temporal patterns of predation risk (Ferrari et al. 2008). Deer avoid recently hunted stand locations, resulting from a learned spatial pattern of risk (Sullivan et al. 2018). Deer may possess a similar ability to learn temporal patterns of risk and respond accordingly. Our results suggested that 3 days of exposure to a temporal pattern of risk throughout the weekend may have caused deer to associate diurnal periods with a greater risk of predation than duringnocturnal periods, resulting in heightened antipredator behaviour despite a decrease in hunting activity on Mondays and Tuesdays. Likewise, previous studies have found that 3 days of exposure to predators has been sufficient to allow other prey species to make an adequate assessment of temporal patterns of risk (Sih and McCarthy 2002; Laurila et al. 2004; Foam et al. 2005; Brown et al. 2006; Ferrari et al. 2009).
Differences in life-history events between male and female ungulates result in dissimilar energy demands throughout the year (Edwards 1983; Ruckstuhl and Kokko 2002). Consequently, males and females are differentially susceptible to predation as they forage to meet energetic requirements (Labisky and Fritzen 1998) and we should expect antipredator responses to vary by sex as males and females balance survival- and fitness-related costs within risky environments (Wolfe et al. 2000; White and Berger 2001; Neumann et al. 2009). We found that mature females were 50% more likely to be active than were mature males during the day, which could be explained by additional fitness-related considerations of females compared with males. In our study areas, parturition typically occurs during late summer and early fall (Leuth 1955; Gray et al. 2002), with some young being born as late as October (Leuth 1967). Given that natural forage is often limited in quality and quantity at that time of year (Cook and Gray 2003), females must maintain heightened levels of activity to support lactation (Beier and McCullough 1990; Rhind et al. 2002). In Alabama, weaning may not occur until 6 months of age (Cook and Gray 2003), causing nutrient intake to remain a priority well into the hunting season. Thus, mature females may be less willing to reduce activity than are mature males because of differences in the impact on long-term fitness (Clark 1994).
Increased exposure to hunters through time may cause ungulates to become increasingly wary (Kilpatrick and Lima 1999) and may yield different antipredator responses between age classes or from 1 year to the next within individuals. We observed behavioural differences between mature and immature deer, with differences dependent on sex and diel period of risk (i.e. DIURNAL/NOCTURNAL). During the day when hunters were present, mature bucks displayed lower movement rates, occupied smaller areas, and were less likely to be active than were immature males. However, at night, mature bucks displayed greater movement and were more likely to be active than were immature males. In fact, mature males were more mobile at night than was any other segment of the deer population, likely out of a need to meet nutritional demands or recuperate other resources that were forfeited during the day. We observed fewer behavioural differences between mature and immature females, which may reflect the strong spatial association of matriarchal groupings that consist of a dam and her offspring (Hawkins and Klimstra 1970).
Our behavioural metrics indicated that deer decreased diurnal movement, activity, and space use in response to predation risk, yet we did not detect variation during the nocturnal period that would suggest that movement had been shifted from days to nights. Rather, we observed a consistently greater selection for night than for day, and nocturnal selection remained constant throughout the week. These findings could suggest that diel nutritional and other resource demands were being met primarily during the nocturnal period, regardless of the level of predation risk during the day. Therefore, when daytime risk was perceived to be elevated, deer could afford to decrease their activity and movement without being forced to increase nocturnal movement to recuperate lost resources. Alternatively, deer may have already been moving at, or near, a maximum sustainable level at night and were incapable, or unwilling, to further elevate nocturnal movement to compensate for lost opportunities to acquire resources during the day. In that case, deer may have been operating under a nutritional deficiency by Wednesdays, after 3 days of suppressed diurnal movement, which may further explain why daytime changes in behaviour were not observed in response to elevated weekend hunting activity until Sundays. This is supported by Lima and Bednekoff (1999) who theorised that antipredator behaviour will be reduced during high-risk periods when such periods are frequent or lengthy, or when periods of lesser risk are insufficient for meeting nutritional demands.
We observed greater diurnal movement rate, net displacement, and probability of activity during the first hunting season than during the second. A number of unmeasured biotic and abiotic factors could have contributed to the observed annual variation, including differences in mast production (Ryan et al. 2004), average temperature (Webb et al. 2010), and precipitation (Bello et al. 2004). Variation in seasonal precipitation, for example, greatly affects the quantity and quality of forage vegetation available to deer and has been shown to affect deer body weights as much as 2 years later (Campbell and Wood 2013). If environmental factors had driven annual variation in movement, we would have expected greater movement during Year 1 across temporal periods, and especially during the low-risk nocturnal period when resource acquisition would be a priority (Lima and Bednekoff 1999). However, annual variation was limited to the high-risk, diurnal period. Available data suggested that effort on days of hunting was 1.06 times greater in Year 2 than in Year 1 of the study, and average deer age within our sample increased from 3.1 to 3.6 years between the first and second years of the study. Therefore, decreased diurnal movement the second year may have been the result of heightened antipredator behaviour driven by a modest increase in hunting pressure, a greater average age among the cohort of marked deer, or a combination of both factors.
Conclusions
Our findings demonstrated the ability of a large ungulate to detect and respond to predatory threats, and suggested that even low levels of disturbance outside of peak breeding season are sufficient to elicit behavioural shifts within ungulate populations. However, the degree of antipredator response varies within and among ungulate populations because of numerous factors, including habitat type and availability of escape cover (Marshall and Whittington 1968; Kammermeyer and Marchinton 1976), as well as demographic parameters such as sex ratio and age structure. Thus, the concept of a disturbance-level threshold that must be exceeded to elicit a behavioural response within ungulate populations (Root et al. 1988; Karns et al. 2012) may be an oversimplification of reality, as predation risk influences prey in a non-uniform manner. The complexity of predator–prey relationships presents a challenge to researchers when the goal is to generalise the behavioural response to certain types or magnitudes of disturbance. However, at times, such generalisations are desired to help understand the effect of predator communities among prey at the population level. Our results demonstrated the importance of taking into account the temporal variation in risk, inherent within all predator–prey relationships, so as to accurately reflect population-level antipredator response. We recommend that future studies on the topic take a similar approach to avoid underestimating the impact that predator-mediated disturbances have on prey survival and lifetime fitness. Our results may also be useful to managers of ungulate populations for understanding the impacts that human hunters have on ungulate behaviour and the implications this may have for the success of management programs in which removal by hunters is the goal. In light of findings that deer are able to perceive temporal patterns of risk fluctuation and respond accordingly, we recommend short-duration (e.g. <3 days) hunts separated by periods (e.g. ≥2 days) of minimal disturbance to avoid establishing a discernable pattern of risk and to maximise deer vulnerability to harvest. Under the scenario of lengthy and continuous hunting seasons, understanding typical patterns of hunting activity, and response by deer thereto, may provide insight into periods when deer are most vulnerable to harvest.
Data availability
The data that support this study cannot be publicly shared because of ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Funding was provided by the Alabama Department of Conservation and Natural Resources – Division of Wildlife and Freshwater Fisheries, The Westervelt Company, B. Bishop, T. Couvillion, and J. Gaddy.
Acknowledgements
We thank B. Baker, R. Basinger, W. Bentley, N. Deig, J. Makemson, J. Meares, R. Morgan, A. Pritchett, S. Rankins, A. Riddle, H. Smith, T. Teel, H. Wood, and many other ADCNR and Westervelt staff for field assistance. We also thank C. Newbolt for his insight during the development of this project, as well as the Associate Editor and two anonymous reviewers for comments that improved this paper.
References
Bartlett, MS (1947). The use of transformations. Biometrics 3, 39–52.| The use of transformations.Crossref | GoogleScholarGoogle Scholar | 20240416PubMed |
Basille, M, Fortin, D, Dussault, C, Bastille-Rousseau, G, Ouellet, J-P, and Courtois, R (2015). Plastic response of fearful prey to the spatiotemporal dynamics of predator distribution. Ecology 96, 2622–2631.
| Plastic response of fearful prey to the spatiotemporal dynamics of predator distribution.Crossref | GoogleScholarGoogle Scholar | 26649384PubMed |
Beier, P, and McCullough, DR (1990). Factors influencing white-tailed deer activity patterns and habitat use. Wildlife Monographs 109, 3–51.
Bello, J, Gallina, S, and Equihua, M (2004). Movements of the white-tailed deer and their relationship with precipitation in northeastern Mexico. Interciencia 29, 357–361.
Bowman, JL, Jacobson, HA, Coggin, DS, Heffelfinger, JR, and Leopold, BD (2007). Survival and cause-specific mortality of adult male white-tailed deer managed under the quality deer management paradigm. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 61, 76–81.
Brown, GE, Rive, AC, Ferrari, MCO, and Chivers, DP (2006). The dynamic nature of antipredator behavior: prey fish integrate threat-sensitive antipredator responses within background levels of predation risk. Behavioral Ecology and Sociobiology 61, 9–16.
| The dynamic nature of antipredator behavior: prey fish integrate threat-sensitive antipredator responses within background levels of predation risk.Crossref | GoogleScholarGoogle Scholar |
Campbell, SAB, and Wood, TC (2013). Influences of precipitation, temperature, and acorn mast on white-tailed deer body weight in the northern Piedmont of Virginia. Northeastern Naturalist 20, 469–477.
| Influences of precipitation, temperature, and acorn mast on white-tailed deer body weight in the northern Piedmont of Virginia.Crossref | GoogleScholarGoogle Scholar |
Chitwood, MC, Lashley, MA, Moorman, CE, and DePerno, CS (2014). Confirmation of coyote predation on adult female white-tailed deer in the southeastern United States. Southeastern Naturalist 13, N30–N32.
| Confirmation of coyote predation on adult female white-tailed deer in the southeastern United States.Crossref | GoogleScholarGoogle Scholar |
Ciuti, S, Northrup, JM, Muhly, TB, Simi, S, Musiani, M, Pitt, JA, and Boyce, MS (2012). Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. PLoS One 7, e50611.
| Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear.Crossref | GoogleScholarGoogle Scholar | 23226330PubMed |
Clark, CW (1994). Antipredator behavior and the asset-protection principle. Behavioral Ecology 5, 159–170.
| Antipredator behavior and the asset-protection principle.Crossref | GoogleScholarGoogle Scholar |
Cook C, Gray B (2003) Biology and management of white-tailed Deer in Alabama. Division of Wildlife and Freshwater Fisheries, Alabama Department of Conservation and Natural Resources, AL, USA.
Creel, S, Winnie Jr, JA, Christianson, D, and Liley, S (2008). Time and space in general models of antipredator response: tests with wolves and elk. Animal Behaviour 76, 1139–1146.
| Time and space in general models of antipredator response: tests with wolves and elk.Crossref | GoogleScholarGoogle Scholar |
Crist, TO, Guertin, DS, Wiens, JA, and Milne, BT (1992). Animal movement in heterogeneous landscapes: an experiment with Eleodes beetles in shortgrass prairie. Functional Ecology 6, 536–544.
| Animal movement in heterogeneous landscapes: an experiment with Eleodes beetles in shortgrass prairie.Crossref | GoogleScholarGoogle Scholar |
Demarais S, Stewart D, Griffin RN (1999) A hunter’s guide to aging and judging live white-tailed deer in the Southeast. Mississippi State University Extension Service Report NSU-ES Publication 2206.
D’Eon, RG, and Delparte, D (2005). Effects of ratio-collar position and orientation on GPS radio-collar performance, and the implications of PDOP in data screening. Journal of Applied Ecology 42, 383–388.
| Effects of ratio-collar position and orientation on GPS radio-collar performance, and the implications of PDOP in data screening.Crossref | GoogleScholarGoogle Scholar |
Diefenbach, DR, Finley, JC, Luloff, AE, Stedman, R, Swope, CB, Zinn, HC, and Julian, GJ (2005). Bear and deer hunter density and distribution on public land in Pennsylvania. Human Dimensions of Wildlife 10, 201–212.
| Bear and deer hunter density and distribution on public land in Pennsylvania.Crossref | GoogleScholarGoogle Scholar |
Dussault, C, Courtois, R, Ouellet, JP, and Huot, J (2001). Influence of satellite geometry and differential correction on GPS locational accuracy. Wildlife Society Bulletin 29, 171–179.
Edwards, J (1983). Diet shifts in moose due to predator avoidance. Oecologia 60, 185–189.
| Diet shifts in moose due to predator avoidance.Crossref | GoogleScholarGoogle Scholar | 28310485PubMed |
Ferrari, MCO, Messier, F, and Chivers, DP (2008). Larval amphibians learn to match antipredator response intensity to temporal patterns of risk. Behavioral Ecology 19, 980–983.
| Larval amphibians learn to match antipredator response intensity to temporal patterns of risk.Crossref | GoogleScholarGoogle Scholar |
Ferrari, MCO, Sih, A, and Chivers, DP (2009). The paradox of risk allocation: a review and prospectus. Animal Behaviour 78, 579–585.
| The paradox of risk allocation: a review and prospectus.Crossref | GoogleScholarGoogle Scholar |
Foam, PE, Mirza, RS, Chivers, DP, and Brown, GE (2005). Juvenile convict cichlids (Archocentrus nigrofasciatus) allocate foraging and antipredator behaviour in response to temporal variation in predation risk. Behaviour 142, 129–144.
| Juvenile convict cichlids (Archocentrus nigrofasciatus) allocate foraging and antipredator behaviour in response to temporal variation in predation risk.Crossref | GoogleScholarGoogle Scholar |
Frid, A, and Dill, LM (2002). Human-caused disturbance stimuli as a form of predation risk. Conservation Ecology 6, 11.
| Human-caused disturbance stimuli as a form of predation risk.Crossref | GoogleScholarGoogle Scholar |
Gray II, WN, Ditchkoff, SS, Causey, MK, and Cook, CW (2002). The yearling disadvantage in Alabama deer: effect of birth date on development. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 56, 255–264.
Gude JA (2004) Applying risk allocation theory in a large mammal predator–prey system: elk–wolf behavioral interactions. MSc thesis, Montana State University, Bozeman, MT, USA.
Harrell Jr FE (2001) Multivariate modeling strategies. In ‘Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis’. (Ed. FE Harrell) pp. 53–85. (Springer-Verlag: New York, NY, USA)
Hawkins, RE, and Klimstra, WD (1970). A preliminary study of the social organization of white-tailed deer. Journal of Wildlife Management 34, 407–419.
| A preliminary study of the social organization of white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Hebblewhite, M, and Haydon, DT (2010). Distinguishing technology from biology: a critical review of the use of GPS telemetry data in ecology. Philosophical Transactions of the Royal Society 365, 2303–2312.
| Distinguishing technology from biology: a critical review of the use of GPS telemetry data in ecology.Crossref | GoogleScholarGoogle Scholar |
Jackson, AM, and Ditchkoff, SS (2013). Survival estimates of white-tailed deer fawns at Fort Rucker, Alabama. American Midland Naturalist 170, 184–190.
| Survival estimates of white-tailed deer fawns at Fort Rucker, Alabama.Crossref | GoogleScholarGoogle Scholar |
Jerde, CL, and Visscher, DR (2005). GPS measurement error influences on movement model parameterization. Ecological Applications 15, 806–810.
| GPS measurement error influences on movement model parameterization.Crossref | GoogleScholarGoogle Scholar |
Kammermeyer, KE, and Marchinton, RL (1976). The dynamic aspects of deer populations utilizing a refuge. Proceedings of the Annual Conference of the Southeastern Association of Game and Fish Commissioners 29, 466–475.
Karns, GR, Lancia, RA, DePerno, CS, and Conner, MC (2012). Impact of hunting pressure on adult male white-tailed deer behavior. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 66, 120–125.
Kats, LB, and Dill, LM (1998). The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5, 361–394.
| The scent of death: chemosensory assessment of predation risk by prey animals.Crossref | GoogleScholarGoogle Scholar |
Kilgo, JC, Labisky, RF, and Fritzen, DE (1998). Influences of hunting on the behavior of white-tailed deer: implications for conservation of the Florida panther. Conservation Biology 12, 1359–1364.
| Influences of hunting on the behavior of white-tailed deer: implications for conservation of the Florida panther.Crossref | GoogleScholarGoogle Scholar |
Kilgo, JC, Ray, HS, Vukovich, M, Goode, MJ, and Ruth, C (2012). Predation by coyotes on white-tailed deer neonates in South Carolina. Journal of Wildlife Management 76, 1420–1430.
| Predation by coyotes on white-tailed deer neonates in South Carolina.Crossref | GoogleScholarGoogle Scholar |
Kilpatrick, HJ, and Lima, KK (1999). Effects of archery hunting on movement and activity of female white-tailed deer in an urban landscape. Wildlife Society Bulletin 27, 433–440.
Kufeld, RC, Bowden, DC, and Schrupp, DL (1988). Influence of hunting on movements of female mule deer. Journal of Range Management 41, 70–72.
| Influence of hunting on movements of female mule deer.Crossref | GoogleScholarGoogle Scholar |
Labisky, RF, and Fritzen, DE (1998). Spatial mobility of breeding female white-tailed deer in low-density populations. Journal of Wildlife Management 62, 1329–1334.
| Spatial mobility of breeding female white-tailed deer in low-density populations.Crossref | GoogleScholarGoogle Scholar |
Laurila, A, Järvi-Laturi, M, Pakkasmaa, S, and Merilä, J (2004). Temporal variation in predation risk: stage-dependency, graded responses and fitness costs in tadpole antipredator defenses. Oikos 107, 90–99.
| Temporal variation in predation risk: stage-dependency, graded responses and fitness costs in tadpole antipredator defenses.Crossref | GoogleScholarGoogle Scholar |
Leuth, FX (1955). The birth dates of Alabama deer. Proceedings of the Annual Conference of the Southeastern Association of Game and Fish Commissioners 10, 129–131.
Leuth, FX (1967). Reproductive studies of some Alabama deer herds. Proceedings of the Annual Conference of the Southeastern Association of Game and Fish Commissioners 21, 62–68.
Lima, SL, and Dill, LM (1990). Behavioral decisions made under the risk of predations: a review and prospectus. Canadian Journal of Zoology 68, 619–640.
| Behavioral decisions made under the risk of predations: a review and prospectus.Crossref | GoogleScholarGoogle Scholar |
Lima, SL, and Bednekoff, PA (1999). Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. American Naturalist 153, 649–659.
| Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis.Crossref | GoogleScholarGoogle Scholar |
Little AR (2011) Human predation risk effects on adult, male white-tailed deer antipredator behavior. MSc thesis, Mississippi State University, Mississippi State, MS, USA.
Little, AR, Webb, SL, Demarais, S, Gee, KL, Riffell, SK, and Gaskamp, JA (2016). Hunting intensity alters movement behaviour of white-tailed deer. Basic and Applied Ecology 17, 360–369.
| Hunting intensity alters movement behaviour of white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Marshall, AD, and Whittington, RW (1968). A telemetric study of deer home ranges and behavior of deer during managed hunts. Proceedings of the Annual Conference of the Southeastern Association of Game and Fish Commissioners 22, 30–46.
Moen, R, Pastor, J, and Cohen, Y (1997). Accuracy of GPS telemetry collar locations with differential correction. Journal of Wildlife Management 61, 530–539.
| Accuracy of GPS telemetry collar locations with differential correction.Crossref | GoogleScholarGoogle Scholar |
Murphy, DA (1962). Deer harvests from refuge areas in Missouri. Proceedings of the Annual Conference of the Southeastern Association of Game and Fish Commissioners 16, 37–42.
Mysterud, A, Langvatn, R, and Stenseth, NC (2004). Patterns of reproductive effort in male ungulates. Journal of Zoology 264, 209–215.
| Patterns of reproductive effort in male ungulates.Crossref | GoogleScholarGoogle Scholar |
Nelson, MA, Cherry, MJ, Howze, MB, Warren, RJ, and Conner, LM (2015). Coyote and bobcat predation on white-tailed deer fawns in a longleaf pine ecosystem in southwestern Georgia. Journal of the Southeastern Association of Fish and Wildlife Agencies 2, 208–213.
Neumann, W, Ericsson, G, and Dettki, H (2009). The non-impact of hunting on moose Alces alces movement, diurnal activity, and activity range. European Journal of Wildlife Research 55, 255–265.
| The non-impact of hunting on moose Alces alces movement, diurnal activity, and activity range.Crossref | GoogleScholarGoogle Scholar |
Picardi, S, Basille, M, Peters, W, Ponciano, JM, Boitani, L, and Cagnacci, F (2019). Movement responses of roe deer to hunting risk. Journal of Wildlife Management 83, 43–51.
| Movement responses of roe deer to hunting risk.Crossref | GoogleScholarGoogle Scholar |
Proffitt, KM, Grigg, JL, Hamlin, KL, and Garrott, RA (2009). Contrasting effects of wolves and human hunters on elk behavioral responses to predation risk. Journal of Wildlife Management 73, 345–356.
| Contrasting effects of wolves and human hunters on elk behavioral responses to predation risk.Crossref | GoogleScholarGoogle Scholar |
Rhind, SM, Archer, ZA, and Adam, CL (2002). Seasonality of food intake in ruminants: recent developments in understanding. Nutrition Research Reviews 15, 43–65.
| Seasonality of food intake in ruminants: recent developments in understanding.Crossref | GoogleScholarGoogle Scholar | 19087398PubMed |
Root, BG, Fritzell, EK, and Giessman, NF (1988). Effects of intensive hunting on white-tailed deer movement. Wildlife Society Bulletin 16, 145–151.
Ruckstuhl, KE, and Kokko, H (2002). Modelling sexual segregation in ungulates: effects of group size, activity budgets and synchrony. Animal Behaviour 64, 909–914.
| Modelling sexual segregation in ungulates: effects of group size, activity budgets and synchrony.Crossref | GoogleScholarGoogle Scholar |
Ryan, CW, Pack, JC, Igo, WK, Rieffenberger, JC, and Billings, AB (2004). Relationships of mast production to big-game harvests in West Virginia. Wildlife Society Bulletin 32, 786–794.
| Relationships of mast production to big-game harvests in West Virginia.Crossref | GoogleScholarGoogle Scholar |
Saalfeld, ST, and Ditchkoff, SS (2007). Survival of neonatal white-tailed deer in an exurban population. Journal of Wildlife Management 71, 940–944.
| Survival of neonatal white-tailed deer in an exurban population.Crossref | GoogleScholarGoogle Scholar |
Sargent, RA, and Labisky, RF (1995). Home range of male white-tailed deer in hunted and non-hunted populations. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 49, 389–398.
Severinghaus, CW (1949). Tooth development and wear as criteria of age in white-tailed deer. Journal of Wildlife Management 13, 195–215.
| Tooth development and wear as criteria of age in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Sih, A (1992). Prey uncertainty and the balancing of antipredator and feeding needs. American Naturalist 139, 1052–1069.
| Prey uncertainty and the balancing of antipredator and feeding needs.Crossref | GoogleScholarGoogle Scholar |
Sih, A, and McCarthy, TM (2002). Prey response to pulses of risk and safety: testing the risk allocation hypothesis. Animal Behaviour 63, 437–443.
| Prey response to pulses of risk and safety: testing the risk allocation hypothesis.Crossref | GoogleScholarGoogle Scholar |
Sih, A, Ziemba, R, and Harding, KC (2000). New insights on how temporal variation in predation risk shapes prey behaviour. Trends in Ecology and Evolution 15, 3–4.
| New insights on how temporal variation in predation risk shapes prey behaviour.Crossref | GoogleScholarGoogle Scholar |
Sullivan, JD, Ditchkoff, SS, Collier, BA, Ruth, CR, and Raglin, JB (2016). Movement and the moon: white-tailed deer activity and solunar events. Journal of the Southeastern Association of Fish and Wildlife Agencies 3, 225–232.
Sullivan, JD, Ditchkoff, SS, Collier, BA, Ruth, CR, and Raglin, JB (2017). Breeding behavior of female white-tailed deer relative to conception: evidence for female mate choice. Ecology and Evolution 7, 2395–2402.
| Breeding behavior of female white-tailed deer relative to conception: evidence for female mate choice.Crossref | GoogleScholarGoogle Scholar | 28405302PubMed |
Sullivan, JD, Ditchkoff, SS, Collier, BA, Ruth, CR, and Raglin, JB (2018). Recognizing the danger zone: response of female white-tailed to discrete hunting events. Wildlife Biology 2018, 1–8.
| Recognizing the danger zone: response of female white-tailed to discrete hunting events.Crossref | GoogleScholarGoogle Scholar |
Swenson, JE (1982). Effects of hunting on habitat use by mule deer on mixed-grass prairie in Montana. Wildlife Society Bulletin 10, 115–120.
Tomberlin JW (2007) Movement, activity, and habitat use of adult male white-tailed deer at Chesapeake Farms, Maryland. MSc thesis, North Carolina State University, Raleigh, NC, USA.
Webb, SL, Gee, KL, Strickland, BK, Demarais, S, and DeYoung, RW (2010). Measuring fine-scale white-tailed deer movements and environmental influences using GPS collars. International Journal of Ecology 2010, 459610.
| Measuring fine-scale white-tailed deer movements and environmental influences using GPS collars.Crossref | GoogleScholarGoogle Scholar |
White, KS, and Berger, J (2001). Antipredator strategies of Alaskan moose: are maternal trade-offs influenced by offspring activity. Canadian Journal of Zoology 79, 2055–2062.
| Antipredator strategies of Alaskan moose: are maternal trade-offs influenced by offspring activity.Crossref | GoogleScholarGoogle Scholar |
Wiskirchen KH (2017) Survival of adult white-tailed deer and movement relative to temporal patterns of predation risk. MSc thesis, Auburn University, Auburn, AL, USA.
Wiskirchen, KH, Jacobsen, TC, Sullivan, JD, and Ditchkoff, SS (2017). Hunter cooperation with requests to avoid a visibly marked ungulate. Wildlife Society Bulletin 41, 301–308.
| Hunter cooperation with requests to avoid a visibly marked ungulate.Crossref | GoogleScholarGoogle Scholar |
Wolfe, SA, Griffith, B, and Wolfe, CAG (2000). Response of reindeer and caribou to human activities. Polar Research 19, 63–73.
| Response of reindeer and caribou to human activities.Crossref | GoogleScholarGoogle Scholar |