Citizen science and community action provide insights on a threatened species: nest box use by the brush-tailed phascogale (Phascogale tapoatafa)
Jessica A. Lawton A B * , Greg J. Holland A B C , Chris Timewell D , Asha Bannon D , Elizabeth Mellick E and Andrew F. Bennett A B
A B * , Greg J. Holland A B C , Chris Timewell D , Asha Bannon D , Elizabeth Mellick E and Andrew F. Bennett A B
A Department of Ecology, Environment and Evolution, La Trobe University, Melbourne, Vic. 3086, Australia.
B Research Centre for Future Landscapes, La Trobe University, Melbourne, Vic. 3086, Australia.
C Australian Wildlife Conservancy, GPO Box 464, Narrabri, NSW 2390, Australia.
D Connecting Country, PO Box 437, Castlemaine, Vic. 3450, Australia.
E Wettenhall Environment Trust, PO Box 669, Castlemaine, Vic. 3450, Australia.
Wildlife Research 49(6) 513-528 https://doi.org/10.1071/WR21102
Submitted: 8 July 2021 Accepted: 30 November 2021 Published: 16 March 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC)
Abstract
Context: Landscape management and restoration in rural environments is frequently driven by community groups, who often use ‘flagship’ species to generate broader engagement. In south-eastern Australia, installation of nest boxes for hollow-dependent fauna is undertaken by many groups. Monitoring the outcomes of such projects offers opportunities for citizen science.
Aims: The aim of the present study was to report on a community-led project to install and monitor nest boxes to enhance the conservation of a threatened species, the brush-tailed phascogale (Phascogale tapoatafa), and to
Methods: A community group installed 450 nest boxes across 150 sites to monitor and provide habitat for the brush-tailed phascogale. Of these, 102 sites were stratified in relation to: (1) geographic sub-region; (2) forest patch size; and (3) topographic position. Nest boxes were inspected five times over 8 years. We modelled factors influencing nest box use at the tree, site, and landscape level. We compared nest box data with data from camera traps at 50 sites to assess their value as a monitoring tool.
Key results: In any given survey, up to 6% of nest boxes had individuals present and up to 22% had evidence of use by the brush-tailed phascogale. There was greater use of nest boxes when installed on ‘stringybark’ type trees than ‘box’ and ‘gum-barked’ species. Nest box use was greater for sites on forest slopes than in gullies, and use varied between years. Surveys using remote cameras were more effective at detecting phascogales than monitoring nest boxes.
Conclusions: Nest box monitoring can provide insights into the distribution and habitat requirements of hollow-dependent species, and engage the community in citizen science. Elements that enhance community-led monitoring include scientific input to project design, collecting data in a consistent manner, allocating sufficient time for data curation, engaging people invested in project outcomes, maintaining good relationships with stakeholders, and sharing data for analysis.
Implications: Collaboration between scientists and community groups can be of benefit to both parties. However, to maximise scientific and conservation outcomes there must be effective engagement and adequate resourcing for project coordination.
Keywords: agricultural landscape, agri-environment schemes, Australia, brush-tailed phascogale, citizen science, community action, conservation, landscape restoration, nest box use, woodland.
Introduction
In rural regions worldwide, a major challenge is to protect, manage and restore natural habitats such that landscapes can provide for both people and biodiversity (Donald and Evans 2006; Kremen and Merenlender 2018). Community groups increasingly contribute to conservation and restoration actions in such landscapes, alongside government agencies and reserve managers (Saunders 1995; Berkes 2004; Pannell et al. 2006). Community groups typically carry out practical actions at a local scale, such as protecting remnant vegetation, restoring waterways, planting native vegetation, controlling pests and weeds, and promoting sustainable land management (Campbell 1994; Curtis 1998; Norton and Reid 2013). Key strengths of such groups are their capacity to build social capital and attract in-kind support (e.g. time, labour, land) among the community for nature conservation.
Community groups often identify a charismatic ‘flagship’ species to encourage conservation action (Heywood and Watson 1995). Examples in Australia include the southern cassowary (Casuarius casuarius) (Crome and Bentrupperbaumer 1993), malleefowl (Leipoa ocellata) (Williams 1995) and regent honeyeater (Xanthomyza phrygia) (Thomas 2009). Across a 14-year period, the Regent Honeyeater Project in north-east Victoria attracted the support of over 17 000 volunteers to restore 1060 ha of habitat on 95% of farms in the local area, to the benefit of many species (Thomas 2009). ‘Citizen science’ projects, where scientific research is conducted wholly or partly by community members, is growing in popularity amongst such groups (Steven et al. 2019). Community groups are encouraged, and may have an ethical obligation, to monitor the outcomes of their conservation work.
Installation of nest boxes for wildlife is a common activity undertaken by community groups in south-eastern Australia (Macak 2020) as a practical measure to address the loss of natural tree hollows for hollow-dependent fauna (Goldingay et al. 2018). Many species of birds and mammals in Australia depend on tree hollows for den and breeding sites (Goldingay 2009, 2011). Where land clearing or timber harvesting has occurred, hollow-bearing trees may be scarce, and replacement is slow; trees can take 100 years or more to grow and develop hollows (Bennett et al. 1994; Koch et al. 2008; Rayner et al. 2013).
A popular focal species for nest box projects in south-eastern Australia is the brush-tailed phascogale (Phascogale tapoatafa) (Macak 2020). This medium-sized (140–220 g) marsupial, a distinctive and charismatic species, uses multiple tree hollows as den and breeding sites (Rhind 2004; van der Ree et al. 2006) throughout its large home range (females ~20–50 ha, males ~100 ha; Soderquist 1995). This species has a short, unusual life history: all males die each year after a frenzied mating season in autumn (Soderquist 1993). It has undergone a marked contraction in geographic range nationally (Woinarski et al. 2014a), and a recent decline in Victoria (Holland et al. 2012) where it is listed as threatened (Department of Sustainability and Environment 2013). The development and trial of efficient survey and monitoring techniques is a priority action for this species (Woinarski et al. 2014b).
The brush-tailed phascogale was selected as a flagship species for conservation by ‘Connecting Country’, a community organisation in central Victoria that coordinates landscape-scale restoration (Mellick et al. 2009). Since inception in 2007, the group has conducted restoration work across more than 9500 ha of land. In 2010, Connecting Country implemented a project to install nest boxes for the brush-tailed phascogale to improve habitat for this species, to monitor its occurrence and distribution, and to provide opportunities for education and community engagement. Connecting Country sought advice from scientists on project design, installed nest boxes for phascogales at sites stratified systematically across the region, and has monitored a subset of nest boxes regularly.
Here, we use data collected by Connecting Country from its monitoring program to: (1) assess the extent of nest box use by the brush-tailed phascogale; (2) determine tree-, site-, and landscape-level factors that influence the use of nest boxes by this species; (3) examine whether nest box use has changed through time; and (4) evaluate the potential of nest boxes as a monitoring tool by comparing it with observations from camera surveys.
Methods
Study area
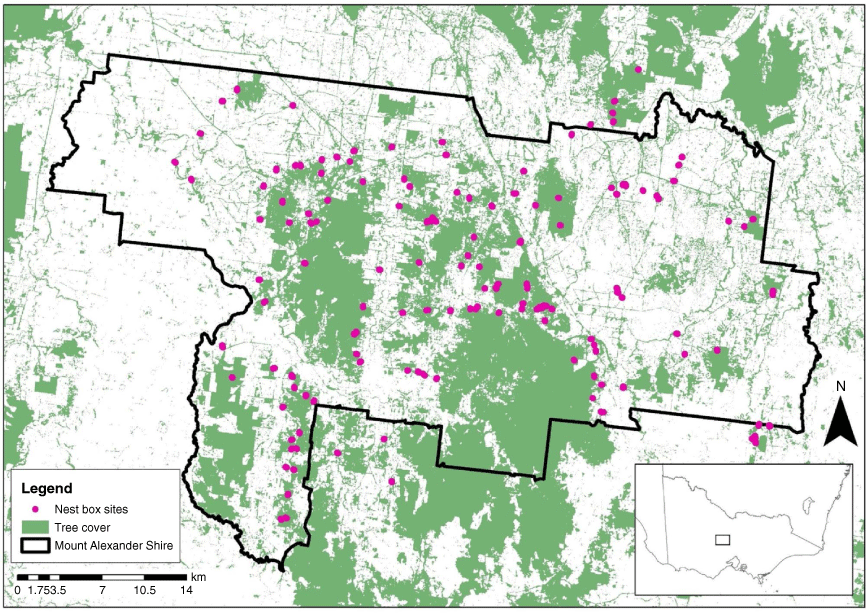
The Mount Alexander region (Fig. 1), an area of ~1530 km2, is primarily part of the Goldfields bioregion in central Victoria, Australia. It has gentle hills of 170–740 m elevation on Palaeozoic sediments with infertile soils (Department of Environment, Land, Water and Planning 2018). The climate is temperate, with a mean maximum temperature of 28.3°C in February (summer) and 11.7°C in July (winter), and mean annual rainfall of 590 mm (measured at Castlemaine Prison; Australian Bureau of Meteorology 2018).
|
The region has a history of extensive land clearing for agriculture. Remaining native vegetation (~36% cover) primarily occurs on public land (state forests, conservation reserves) and has been subject to disturbance since ~1850, including gold mining, timber harvesting, stock grazing, weed invasion and altered fire regimes. Smaller patches of forest and stands of scattered trees occur on private land, along roadsides and creek lines. Many community members and landholders are actively interested in landscape restoration for conservation, particularly where larger farms have been subdivided into smaller lifestyle properties.
Native vegetation consists of dry eucalypt forests and woodlands, with relatively open mid and ground strata. Box Ironbark Forest, one of two common vegetation types, has a canopy of grey box (Eucalyptus microcarpa) and red box (E. polyanthemos), with red ironbark (E. tricarpa) on dryer slopes and yellow box (E. melliodora) in moister gullies and flats. The mid-storey and understorey include small trees and shrubs, herbs and grasses. Heathy Dry Forest has a canopy of red stringybark (E. macrorhyncha), red box and long-leaf box (E. goniocalyx and E. nortonii), with red ironbark in dryer parts, over a shrubby understorey. Other vegetation types, such as Alluvial Terraces Herb-rich Woodland, Valley Grassy Forest and Creekline Grassy Woodland, form along valleys, drainage lines and creek lines, and are typically dominated by grey box, yellow box and yellow gum (E. leucoxylon), with river red gum (E. camaldulensis) along drainage lines.
Study design
Connecting Country installed 450 nest boxes across 150 sites in the Mount Alexander region to enhance habitat for the brush-tailed phascogale, serve as a tool for monitoring the species, and provide opportunities for education. Nest boxes were installed primarily in 2010 and 2011. Of these 150 sites, 102 were set out in a stratified design, with sites located to: (1) represent each of five geographic sub-regions in the study area; (2) sample both larger (>50 ha) and smaller (<50 ha) patches of native vegetation; and (3) occur on slopes and in gullies. These 102 sites were surveyed each monitoring season. The additional 48 sites were established primarily on private land, based on landholder interest, to provide habitat and to promote engagement and education. These sites were surveyed opportunistically during monitoring seasons when resources permitted. Control of pests (European honey bee Apis mellifera) was undertaken opportunistically, by removing hives or installing deterrents (carpet, Perspex, or charring the lid of the nest box).
Nest boxes were designed specifically for the brush-tailed phascogale. They were constructed from 19 mm timber (lid 45 mm) with external dimensions of 200 × 240 × 350 mm, a hinged sloping lid, a 37 mm hole in the back (adjacent to the tree) and were painted dark green or beige. At each site, nest boxes were established in groups of three, typically in a line spaced 50 m apart. Each nest box was installed at ~3–3.5 m height, facing south east (~110°) and, where possible, on ‘rough-barked’ trees of at least 30 cm diameter at breast height and without obvious existing hollows.
Data collection
Nest box surveys
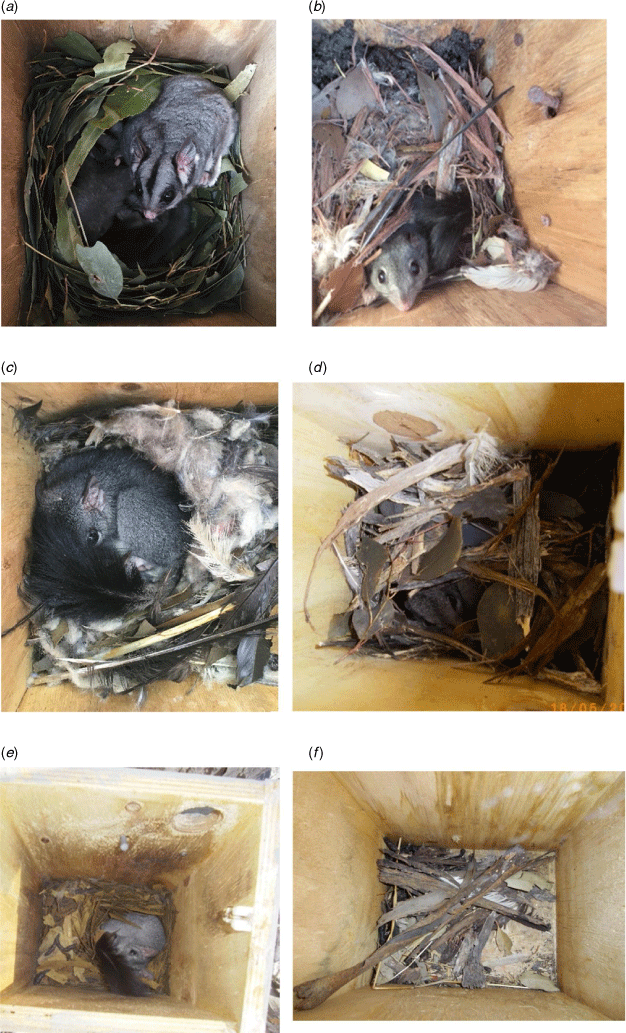
Nest boxes were inspected in 2011, 2012, 2014 and 2016 by Connecting Country staff accompanied by volunteers and landholders, and in 2018 by AB, EM and JL (in voluntary capacity), accompanied by volunteers and landholders. Inspections were conducted between April and early June (austral autumn), as recommended to avoid disturbing breeding females in winter (Soderquist et al. 1996). Nest box contents were visually inspected during daylight hours. Notes were taken on the type of nest, including nesting material (eucalypt leaves, feathers, shredded bark etc.) and the presence of scats. A photograph of the contents was taken. Animals were not handled and nesting material was not disturbed. The two species that commonly use these boxes in the study area, brush-tailed phascogale and Krefft’s glider (Petaurus notatus; previously Petaurus breviceps; Cremona et al. 2020), build nests that are easily distinguished (Appendix Fig. A1).
The timing of nest box inspections corresponds with the mating season when males travel widely, potentially through sub-optimal habitat, to search for mates (Soderquist 1993). Thus, there is a likelihood that a nest box record may represent sub-optimal habitat. To account for this, we used records and photographs from nest box inspections to collate three response variables: (1) the presence or absence of individual phascogales in a nest box; (2) the presence or absence of evidence of use by phascogales (i.e. large or small nests, scats, or individual animals present); and (3) the presence of an ‘established’ nest. An ‘established’ nest was typically large, with characteristic nesting materials such as shredded bark, dried leaves, feathers, or fur (see images in Fig. A1). The presence of an established nest indicates that the site represents suitable habitat for this species. A male moving rapidly and widely (through potentially sub-optimal habitat) in search of mates is unlikely to spend time building an elaborate nest.
Camera surveys
We surveyed 50 nest box sites in 2016 using camera traps as part of a separate study (Lawton et al. 2021). By conducting camera surveys concurrently with nest box inspections, we can compare the effectiveness of these approaches as monitoring tools. Camera sites were selected without prior knowledge of nest box use at the site. A Reconyx HC 600 and a Scoutguard 550 were set at two nest-box trees (of three nest boxes at each site) that were furthest apart (typically 100 m). Reconyx cameras were set to high sensitivity, to take the maximum number of photos at each trigger (i.e. 10 photos), with a 1-s interval between photos and a 3-min rest period between each trigger. Scoutguard cameras were also set to high sensitivity to take the maximum number of photos at each trigger (i.e. three photos), with a 3-min rest period between triggers. Brown packing tape was placed over the Scoutguard cameras LED to reduce over-exposure of close-range images (De Bondi et al. 2010).
Cameras were set facing downwards, attached to a tree ~1.5 m above ground. This deployment reduces false triggers from moving vegetation and has previously been used successfully (De Bondi et al. 2010). Polyester fibre was soaked in a mixture of tuna oil, peanut butter, vanilla essence and linseed oil, enclosed in a PVC pipe with a ventilation cowl cap, and secured to the ground with a tent peg. This lure was placed directly below the camera and the camera aligned so that both lure and tree trunk were within the camera’s detection zone. Lures were refreshed after 20 days, and cameras were left to operate for 39 days in total.
Tree-level habitat measures
The tree on which the nest box was installed was identified to species and grouped into one of four broad categories (‘tree type’), based on bark structure: (1) ‘Gum’ (river red gum, candlebark E. rubida, manna gum E. viminalis, and yellow gum) which have smooth bark; (2) ‘Box’ (grey box, long-leaf box, red box), which have rough, tessellated bark; (3) ‘Stringybark’ (red stringybark and messmate stringybark E. obliqua), which have fibrous, stringy bark; and (4) ‘Yellow box’, allocated to a unique category because its bark varies from smooth, gum-type bark to rough, tessellated bark.
Site-level habitat measures
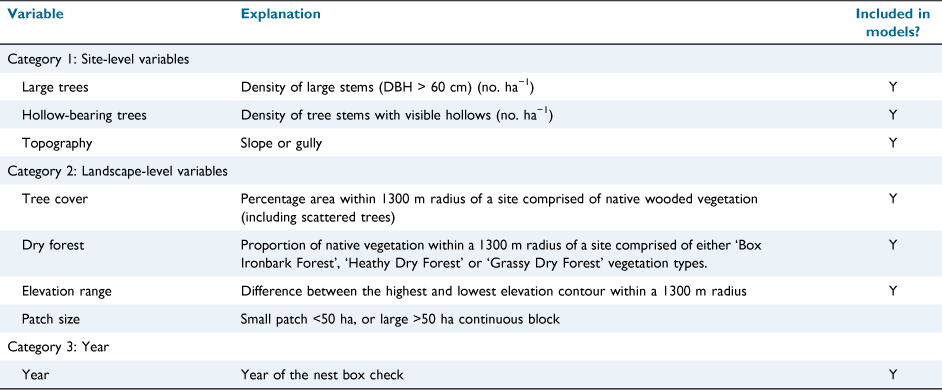
Data were available for the trees present in a 100 m × 10 m (0.1 ha) quadrat at 92 of the stratified sites (Monagle 2012), including the species and size class of each stem and whether trees had obvious hollows. We calculated the density (no. per ha) of stems ≥60 cm diameter at breast height as a measure of the abundance of large trees at each site, and the density of stems with visible hollows as a measure of the availability of natural hollows. Topographic position (slope or gully) was also relevant.
Landscape-level habitat measures
We measured ‘tree cover’, the cover of native wooded vegetation including scattered trees, in a buffer area surrounding each site, at each of four radii – 600 m (113 ha), 1000 m (314 ha), 1300 m (530 ha), and 2000 m (1256 ha) – using GIS layers of land use from the year 2015 from the Victorian Government. These measures were highly correlated, so only that from a 1300-m radius was used in further analysis, as a balance between an area large in relation to the home range of a phascogale, while avoiding extensive overlap between site buffer areas.
Vegetation in the study area ranges from dry, less productive box-ironbark woodlands on slopes and hilltops, to moister, more productive forests. Forest productivity influences the distribution of this species (Holland et al. 2012). We divided vegetation types surrounding sites into two categories: Dry Forests (the least productive vegetation types: Box Ironbark Forest; Heathy Dry Forests; Grassy Dry Forests), and more productive forests (i.e. all other vegetation types). We then calculated the proportion of the 1300-m buffer area comprised of ‘dry forest’.
To measure variation in elevation, we calculated the difference between the highest and lowest topographic contour within the 1300-m buffer. A greater range in elevation is an indicator of greater environmental heterogeneity. Finally, the size of the forest patch in which the site was located was assigned to one of two categories: (1) small (<50 ha); or (2) larger (>50 ha).
Data analysis
Simple summaries of data on nest box use were collated by Connecting Country volunteers and staff and reported to the community following each year of monitoring. These data were shared with scientists for further collation and analysis.
Does the type of tree on which a nest box is installed affect nest box use?
To examine the influence of ‘tree type’ on the likelihood of detecting the brush-tailed phascogale in a nest box, we used generalised linear mixed models (GLMM) with a random effect ‘nest box’ fitted to account for repeated visits to nest boxes through time. GLMMs were fitted with a binomial distribution and a logit function. The number of iterations was set to 100 000 using the BOBYQA optimisation to resolve convergence issues (Powell 2009). Models were fitted for two response variables: (1) the presence or absence of any evidence of use of a nest box by the brush-tailed phascogale (i.e. presence of a nest, feathers, scats, or an individual phascogale); and (2) the presence or absence of an established phascogale nest. Observations of individual animals in a nest box were too few for reliable modelling. The predictor variable was the tree type on which the nest box was installed. Tree species with a small sample size (<5 trees) and not assigned to a ‘tree type’ category (e.g. peppermints, ironbarks and non-indigenous trees), or trees otherwise not identified, were excluded from analysis. After these exclusions, there was a sample size of 1396 inspections across 306 nest box trees and 102 sites. The reference category for tree type was ‘box’, the most widespread group in the study area.
Do site-level and landscape-level variables, or year, influence nest box use by the brush-tailed phascogale?
To examine the influence of site- and landscape-level variables on the likelihood of detecting the brush-tailed phascogale at a site, we used a GLMM with a binomial distribution and a logit function. Again, the two response variables were: (1) the presence or absence of any evidence of the brush-tailed phascogale; and (2) the presence of an established nest, in any of the three nest boxes at a site (i.e. a single data point per site). The number of iterations was set to 100 000 using the BOBYQA optimisation (Powell 2009).
Modelling was limited to sites for which data were available on the occurrence of large trees and trees with obvious hollows (n = 92). Where sites were less than 1 km apart (i.e. less likely to be independent), we selected one site at random to include in the analysis. After these exclusions, 75 sites remained for analysis.
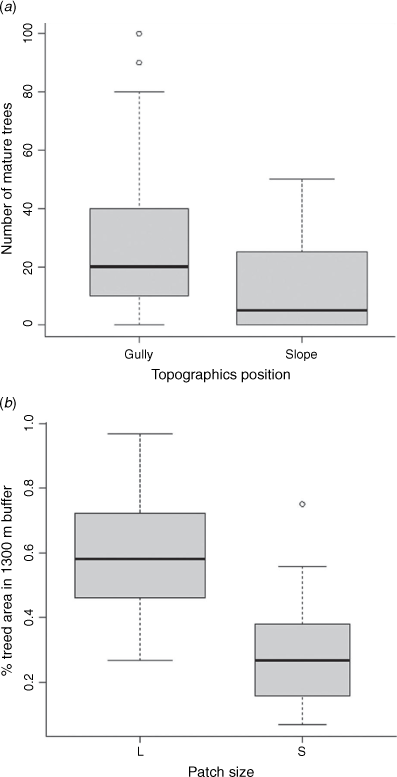
The variable ‘large trees’ was strongly associated with ‘topography’, with more large trees occurring in gullies than on slopes (Fig. A2a). We included only ‘topography’ in the analysis. The variable ‘patch size’ was strongly associated with ‘tree cover’ (i.e. small patches had a lower proportion of surrounding tree cover; Fig. A2b), so we included only ‘tree cover’ in analyses. After these were excluded, continuous predictors were checked for pair-wise collinearity and all had Pearson and Spearman coefficients of <0.6.
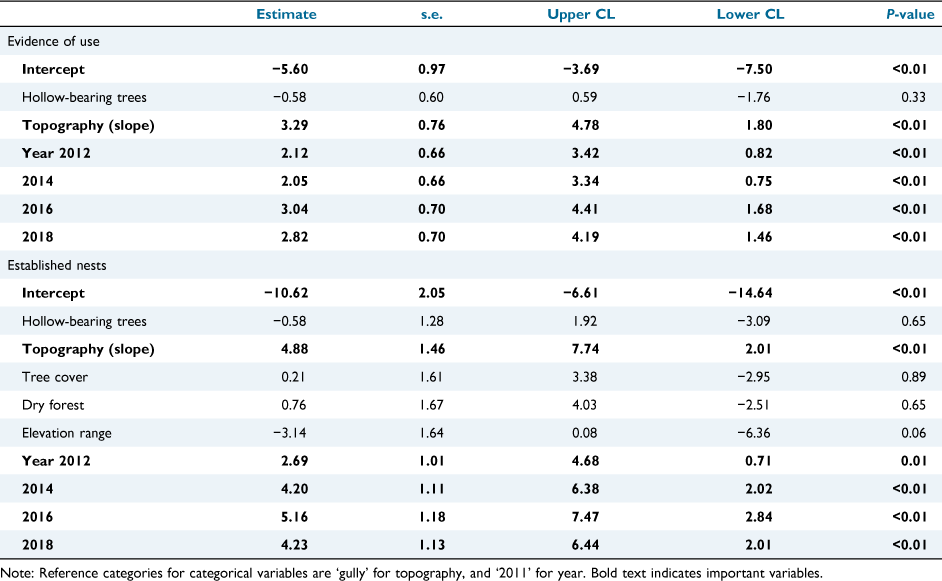
The final set of six predictors represented three categories: site-level variables, landscape-level variables and, to assess changes in detections of phascogales through time, the year of nest box inspection (Table 1). The variable ‘hollow-bearing trees’ was log-transformed; and continuous variables were standardised (Gelman 2008). We fitted ‘site’ as a random term to account for repeated visits to sites.
|
To determine the most parsimonious model, we ran a series of models based on each combination of the three categories of variables hypothesised to influence use of nest boxes by the brush-tailed phascogale (Table 1). Including a null model, this resulted in a set of eight candidate models. We ranked candidate models using Akaike’s information criterion corrected for small sample sizes (AICC). The difference (Δi) between the AICC value of the ‘best model’ (smallest AICC value) and that of other candidate models was calculated: models where Δi ≤ 2 are considered to have substantial support (Burnham and Anderson 2002). We also calculated Akaike weights (wi) for each model: those with higher weights are more likely to be the best approximating model (given the candidate set of models). We measured goodness-of-fit by calculating the marginal R2 (variance explained by fixed factors) and conditional R2 (variance explained by both fixed and random factors). We then generated a confidence set of models with ΔAICC < 2 and built a final model using all predictor variables in this set. Variables for which the 95% confidence limits of the coefficient did not overlap zero were considered an important influence on the likelihood of detecting evidence of the brush-tailed phascogale. We then ran this model again, systematically specifying each year of monitoring as the reference category to identify differences in detections between years.
Modelling was conducted using the packages ‘lme4’ version 1.1-21 (Bates et al. 2015), ‘arm’ version 1.10-1 (Gelman and Su 2018), ‘AICcmodavg’ version 2.2-2 (Mazerolle 2019) and ‘MuMIn’ version 1.43.6 (Bartoń 2019) in R version 3.6.0 (R Core Team 2017).
Results
Summary results
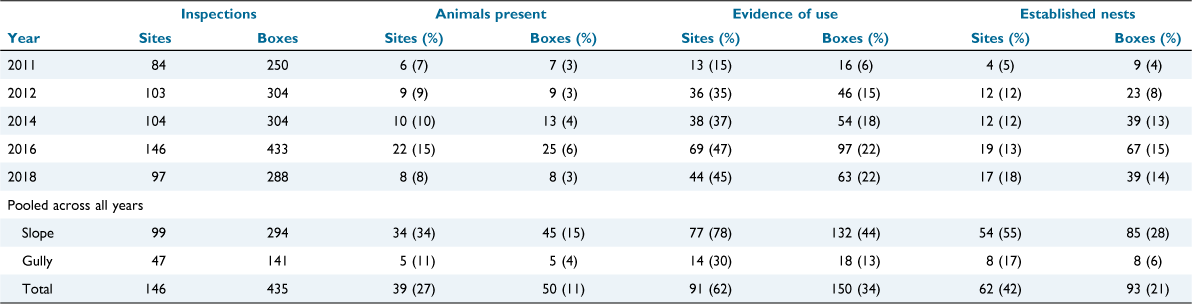
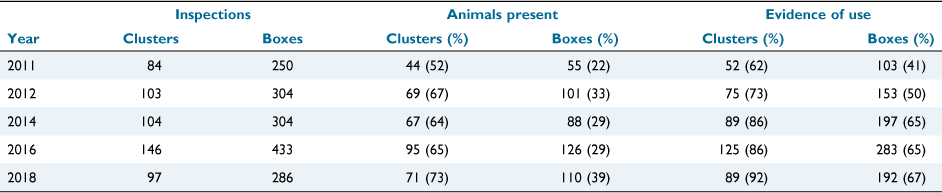
After 1579 nest box inspections across 146 sites over five monitoring years, evidence of use of nest boxes by the brush-tailed phascogale (nests, scats, or an individual animal) was detected on 276 occasions. On 62 occasions, a brush-tailed phascogale was present at the time of inspection. Established nests were detected on 177 occasions (Table 2). Across the 5 years of surveys, a total of 150 (34%) nest boxes had evidence of use by phascogales, and 93 (21%) nest boxes had established nests.
|
Non-target species were also detected. Krefft’s glider was detected (any evidence of use) at up to 92% of sites and 67% of nest boxes in a single year (2018), and 89% of sites and 78% of nest boxes across the study period (Table A1). Other mammals detected included the common brushtail possum (Trichosurus vulpecula), detected on four occasions, and the common ringtail possum (Pseudocheirus peregrinus) on one occasion. Active hives of European honey bees were detected in 3–18% of nest boxes in annual checks (2011, 18%; 2012, 9%; 2014, 3%; 2016, 4%; and 2018, 9%). Across all years of monitoring, ants were detected on 52 occasions, spiders on 47 occasions, wasps on three occasions and borer grubs on two occasions.
Does the type of tree on which a nest box is installed influence nest box use?
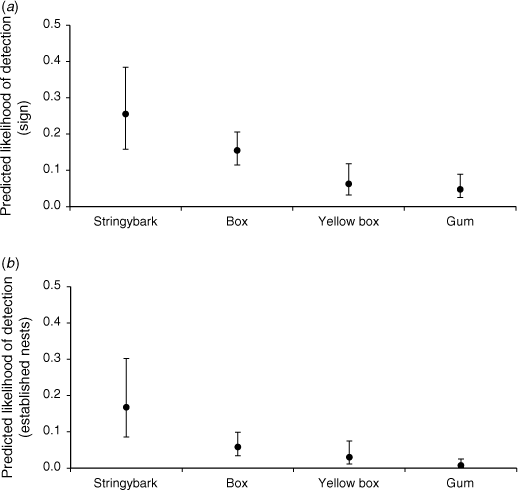
For both response variables, use of nest boxes by the brush-tailed phascogale differed in relation to tree type (Fig. 2, Table A2). For ‘any evidence of use’, nest boxes on ‘gum’ and ‘yellow box’ tree types had a lower likelihood (0.05; 95% CI 0.02–0.09 and 0.06, 95% CI 0.03–0.12, respectively), and ‘stringybark’ a higher likelihood (0.26, 95% CI 0.15–0.38), than those on trees of the reference category ‘box’ (0.15, 95% CI 0.11–0.21; Fig. 2). For the response variable ‘established nests’, there was a lower likelihood of detecting use of nest boxes on ‘gum’ tree types (<0.01, 95% CI < 0.01–0.03), and a higher likelihood on ‘stringybark’ tree types (0.16, 95% CI 0.08–0.30), compared with the reference category ‘box’ (0.06, 95% CI 0.03–0.10). There was no difference between the reference category and tree type ‘yellow box’ (0.03, 95% CI 0.01–0.08; Fig. 2).
|
Do site- and landscape-level variables, or year, influence nest box use by the brush-tailed phascogale?
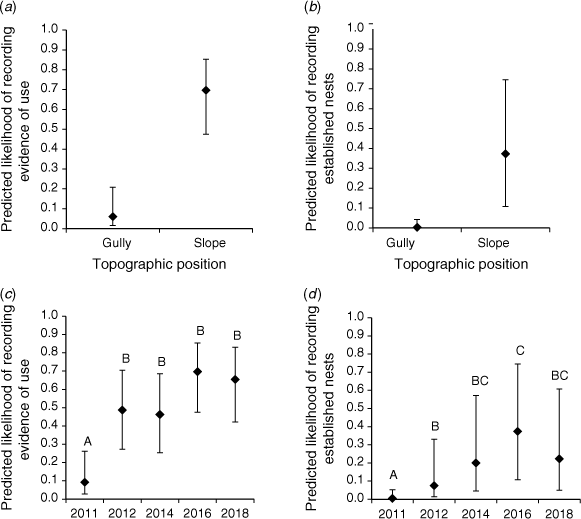
For ‘any evidence of use’, only one model had substantial support (i.e. Δi < 2): the model that included site-level variables and year (Table 3). The marginal R2 value (0.34) suggests that fixed effect variables explain a modest amount of variance in the data. Two variables, topography and year, were important influences (i.e. 95% confidence intervals of the coefficient did not overlap zero) (Table 4). There was a higher likelihood of nest box use at sites on slopes than on gullies (Fig. 3a), and in all other years compared to 2011 (Fig. 3c). The other site-level variable, density of trees with obvious hollows, was not influential (Table 4).
|
For ‘established nests’, two models had support (i.e. Δi < 2): the model including site-level variables and year, and the global model. Marginal R2 values, 0.34 and 0.43 respectively, again suggest fixed effect variables explain a modest amount of variance in the data (Table 3). In the final model, the variables topography and year were important (Table 4). There was a higher likelihood of nest boxes with established nests at sites on slopes than on gullies (Fig. 3b), and a lower likelihood in 2011 than all other years (Fig. 3d). The year 2016 had a higher likelihood of recording established nests than in 2012 (Fig. 3d). Other variables (hollow-bearing trees, tree cover, dry forest, elevation range) were not influential (Table 4).
Are nest boxes an appropriate monitoring tool?
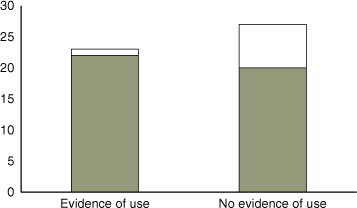
In 2016, baited cameras were deployed at 50 nest box sites. Overall, cameras were more effective at detecting phascogales (at 42/50 sites) than nest boxes (any evidence of use, 23/50 sites). Phascogales were detected by cameras at 95% (22/23) of sites at which there was evidence of use in nest boxes. However, cameras also detected phascogales at many additional sites (20/27) where there was no evidence of use of nest boxes (Fig. 4).
|
Discussion
The community group ‘Connecting Country’ established a nest box program for the brush-tailed phascogale, a flagship species for promoting nature conservation to landholders and other community members in a rural region. Nest box monitoring over an 8-year period provides evidence that the brush-tailed phascogale, a threatened species in Victoria, is widespread in the study area, with evidence of use of nest boxes at up to 47% of sites and 22% of boxes in a single year (2016), and 62% of sites and 34% of nest boxes over the study period.
Nest box use and the distribution of the brush-tailed phascogale
Nest box use differed according to the type of tree on which the nest box was installed. Phascogales were more likely to use nest boxes installed on trees with rough bark (here, box or stringybark trees), than smooth-barked gum-type trees or yellow box, consistent with other work on this species (Goldingay et al. 2020a). The brush-tailed phascogale was also more likely to use nest boxes at sites on forested slopes than in gullies, as also found for camera trap surveys (Lawton et al. 2021). A preference for dry slopes is consistent with habitats favoured by this species in Victoria, being strongly associated with drier forests of box, ironbark and stringybark vegetation on slopes (Cuttle 1982; Traill and Coates 1993; Menkhorst 1995).
In the study area, forests on slopes typically have a greater number of rough-barked box- and stringybark-type trees, whereas gullies have a greater number of trees with gum-type bark. Rough-barked trees provide a larger and more diverse surface area for foraging, and harbour a greater abundance of invertebrate food (Majer et al. 2003). They also are more amenable to climbing; phascogales are reported to have difficulty climbing smooth, gum-barked trees (Soderquist et al. 1996). The higher likelihood of detecting this species at sites on forested slopes may be associated with the greater occurrence of rough-barked trees on slopes. Phascogales were detected in nest boxes across a wide environmental range, in both small and large forest patches. There was no evidence that nest-box use was influenced by the broad vegetation type at the site, patch size, or landscape attributes such as the extent of surrounding tree cover. These results were consistent for both response variables modelled. This species has a large home range (Traill and Coates 1993; Soderquist 1995), and individuals are highly mobile; the structural connectivity provided by a mosaic of forest patches and networks of remnant vegetation along roadsides and creek lines likely contribute to a high level of functional connectivity.
The level of use of nest boxes differed across years, especially between the first year (2011) and later years. It can be expected that individuals will take time to find, or become familiar with, nest boxes (e.g. Lindenmayer et al. 2016). The highest number of detections of signs and established nests was in 2016, consistent with individuals having gained greater familiarity with nest boxes over several years. However, nesting material and scats were not cleared each survey and can remain in nest boxes for several years. It is not possible to draw firm conclusions regarding local population size, or changes in populations through time from these nest box data. Detections of individual animals in boxes were too few to analyse.
Survey methods and the value of nest box data
Camera traps were more effective than nest boxes for detecting phascogales, consistent with other studies (Scida and Gration 2017; Geyle et al. 2020). Cameras almost always detected phascogales at sites where there was evidence of occurrence in nest boxes, but also detected them at many additional sites where they were not recorded using nest boxes. Brush-tailed phascogales use multiple den sites within a large home range (Traill and Coates 1993; van der Ree et al. 2006), so it is not surprising there were few records of individuals present in nest boxes on the day of inspection. Nest material and other signs provide general evidence of occurrence, but not of the time or duration of occurrence. Nest boxes can be a useful survey method for community groups because nest boxes have additional benefits – they supplement habitat resources, community members can connect with wildlife by seeing animals ‘in the flesh’, and they often are used to sample over long timeframes. However, camera traps are recommended when confidence in the presence or absence of a species at a site, and the time of occurrence, is required, or when habitat augmentation is not a goal. Cameras are typically deployed for shorter durations (e.g. <8 weeks; De Bondi et al. 2010; Smith and Coulson 2012), and often longer when the target species are predators, are rare, or have a large home range (Geyle et al. 2020; Moore et al. 2020). Here, cameras were deployed for 39 nights. The detection probability of phascogales with camera traps decreased with the time since the lure was refreshed (Lawton et al. 2021).
It is often assumed that animals use nest boxes only where suitable natural hollows are unavailable; for example, because temperatures in nest boxes fluctuate more than in natural hollows (Rowland et al. 2017, but see Goldingay and Thomas 2021). Therefore, it can be argued that nest box use may not be a reliable indicator of habitat quality. However, outcomes from analysing these nest box data were similar to outcomes from camera surveys, with the same predictors being important (Lawton et al. 2021). This suggests that occurrence in nest boxes can be used to assess habitat attributes in the environment important for species.
Evidence from this study and others (Traill and Coates 1993; Dashper and Myers 2012; Goldingay et al. 2018, 2020a, 2020b) shows that nest boxes do provide a useful habitat resource for individual phascogales. More generally, nest box programs provide valuable distributional records of a species, access to individuals for study, and in some instances can be used to monitor population demography and change (Rhind and Bradley 2002; Beyer and Goldingay 2006). Few studies, however, particularly in an Australian context, provide a clear understanding of whether the installation and provision of nest boxes as artificial den or nest sites has benefits for the conservation status of local populations (but see Saunders et al. (2020) for an Australian example and Newton (1998) for a European example). To demonstrate an increase in population size or broader use of habitats following nest box installation requires an experimental approach that compares populations before and after installation of nest boxes, as well as in ‘control’ areas where nest boxes are not available. Given the investment of time and money by individuals, government and volunteer groups in the installation, monitoring and maintenance of nest boxes, this is an important issue to address.
Strengths and limitations of community monitoring
There were important benefits that arose because this project was community led. First, it provided significant opportunities for education and engagement, with up to 20 volunteers and >100 property owners and Landcare members involved in a single year of monitoring. Second, Connecting Country leveraged community networks to gain access to sites on private land, an important requirement for studies in agricultural landscapes. Third, the project has been maintained over the long term and has remained resilient to funding fluctuations. Key project participants continued monitoring even after the program was unfunded (in 2018).
We identified four main elements in the implementation of this project that contributed to positive outcomes. First, in planning the project, Connecting Country defined the aims, sought scientific advice on study design before establishing nest boxes, and installed an appropriate number of boxes to allow for analysis. Second, there was a commitment to consistency in approach including: (1) installing nest boxes in a consistent manner; (2) co-ordinating regular nest box inspections at the same time of year; (3) allocating time to collate, manage and check the data; and (4) maintaining the program over a number of years. Third, Connecting Country engaged, and provided training for, staff and volunteers who were committed to the project, prioritised good relationships with landholders, and communicated frequently with volunteers and landholders. This helped to ensure data were collected carefully, and that volunteers and landholders were retained through time. Finally, Connecting Country were willing to share data to enable analysis of the outcomes.
Citizen science monitoring projects also have limitations. They can be perceived as being more cost effective to operate than those run by professional scientists. This may be true for the data collection phase when protocols for data collection are clear and straightforward and projects span a large geographic area (Sullivan et al. 2009; Parrish et al. 2018). Experience in this project reveals that much time is required for recruitment, support and training of community-group staff and volunteers. Likewise, although there was a commitment to the study design and methods, continued effort was required by a coordinator to maintain consistency of data collection, check the data and ensure quality control (Parrish et al. 2018). Others have reported that it can be difficult to produce publishable science from community-led projects (Gadermaier et al. 2018). Here, not all data collected could be used for analysis. Community-led projects are most successful when they receive ongoing support for a project coordinator (Curtis 1998), yet funding for such ongoing roles is difficult to obtain.
Our experience is that collaboration between scientists and community groups can be to the benefit of both parties, while recognising differences in culture and expectations. Contributions by scientists to study design and analysis of data can help overcome limitations in such skills among community groups. Prior consultation and design can help reduce the likelihood of citizen science data being wasted because of inconsistencies, lack of independence or other ‘noise’ in the data. A key requirement is that projects accommodate priorities for both parties. Community groups typically value practical outputs, simple summaries and clear messages to communicate to members and stakeholders, and scientists are driven by the necessity to publish and the associated requirement for novelty and rigour.
Citizen science projects, such as this one, can produce positive outcomes not readily quantified or measured by scientific outcomes. These include the benefits of citizens learning about and connecting with nature and place, interacting with scientific methods and processes, feeling a sense of purpose, and building social connections and community (Trumbull et al. 2000; Brossard et al. 2005; Haywood et al. 2016). Scientists also benefit from engagement with communities, access to local knowledge, access to private lands, achievement from contributing to practical conservation outcomes, and a sense of hope for conservation (Garnett and Crowley 1997; Swaisgood and Sheppard 2010; Frigerio et al. 2018).
Projects such as the provision of nest boxes for wildlife are widely undertaken by community groups (Thomas 2009; Goldingay et al. 2018; Michael et al. 2021), and often supported by governmental and philanthropic funding. Unfortunately, in many such projects monitoring is not undertaken systematically, collation and curation of data are incomplete, and few projects are analysed and reported in a manner that allows for learning and new insight (Macak 2020). Given the large investment of time and resources by individuals and community groups, significant gains could be achieved for participants and for conservation outcomes by greater collaboration in project design and analysis, and by adequate resourcing for effective co-ordination and consistency in data collection by citizen scientists.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
JL is employed part-time by Connecting Country; CT and AB are former employees. EM is employed by the Wettenhall Environment Trust, which provided funding and in-kind support for nest box surveys.
Declaration of funding
We thank the Wettenhall Environment Trust, the Holsworth Wildlife Research Endowment and the Arthur Rylah Institute for Environmental Research for funding support. JL was supported by a La Trobe University postgraduate research scholarship.
Acknowledgements
The staff of Connecting Country, many landholders and volunteers generously contributed time and knowledge. In particular, Cara Byrt, Bryan McMullan, Max Schlachter and Amy Monagule contributed substantially to the nest box project. Angie Haslem provided advice on analysis. Helpful comments from two reviewers improved the manuscript. Camera trapping was conducted in accordance with Department of Environment, Land, Water and Planning Permit number 10007687 and La Trobe University Animal Ethics Committee AEC15-59.
References
Australian Bureau of Meteorology (2018) Castlemaine prison, climate data online, Weather Station # 088110. Bureau of Meteorology, Melbourne, Vic., Australia. Available at http://www.bom.gov.au/climate/data [Accessed 8 Feburary 2018]Bartoń K (2019) MuMIn: Multi-Model Inference. R package version 1.43.6. Available at https://CRAN.R-project.org/package=MuMIn
Bates, D, Maechler, M, Bolker, BW, and Walker, S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bennett, AF, Lumsden, LF, and Nicholls, AO (1994). Tree hollows as a resource for wildlife in remnant woodlands: spatial and temporal patterns across the northern plains of Victoria, Australia. Pacific Conservation Biology 1, 222–235.
| Tree hollows as a resource for wildlife in remnant woodlands: spatial and temporal patterns across the northern plains of Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Berkes, F (2004). Rethinking community-based conservation. Conservation Biology 18, 621–630.
| Rethinking community-based conservation.Crossref | GoogleScholarGoogle Scholar |
Beyer, GL, and Goldingay, RL (2006). The value of nest boxes in the research and management of Australian hollow-using arboreal marsupials. Wildlife Research 33, 161–174.
| The value of nest boxes in the research and management of Australian hollow-using arboreal marsupials.Crossref | GoogleScholarGoogle Scholar |
Brossard, D, Lewenstein, B, and Bonney, R (2005). Scientific knowledge and attitude change: the impact of a citizen science project. International Journal of Science Education 27, 1099–1121.
Burnham KP, Anderson DR (2002) ‘Model Selection and Multimodel Inference: a Practical Information–theoretic Approach.’ 2nd edn. (Springer: New York, NY)
Campbell A (1994) ‘Landcare. Communities Shaping the Land and the Future.’ (Allen & Unwin: Sydney, NSW)
Cremona, T, Baker, AM, Cooper, SJB, Montague-Drake, R, Stobo-Wilson, AM, and Carthew, SM (2020). Integrative taxonomic investigation of Petaurus breviceps (Marsupialia: Petauridae) reveals three distinct species. Zoological Journal of the Linnean Society 191, 503–527.
| Integrative taxonomic investigation of Petaurus breviceps (Marsupialia: Petauridae) reveals three distinct species.Crossref | GoogleScholarGoogle Scholar |
Crome FHJ, Bentrupperbaumer J (1993) Special people, a special animal and a special vision: the first steps to restoring a fragmented tropical landscape. In ‘Nature Conservation 3: the Reconstruction of Fragmented Ecosystems’. (Eds DA Saunders, RJ Hobbs, PR Ehrlich) pp. 267–279. (Surrey Beatty & Sons: Sydney, NSW, Australia)
Curtis, A (1998). Agency–community partnership in Landcare: lessons for state-sponsored citizen resource management. Environmental Management 22, 563–574.
| Agency–community partnership in Landcare: lessons for state-sponsored citizen resource management.Crossref | GoogleScholarGoogle Scholar | 9582392PubMed |
Cuttle P (1982) Life history strategy of the dasyurid marsupial Phascogale tapoatafa. In ‘Carnivorous Marsupials’. (Ed. M Archer) pp. 13–22. (Royal Zoological Society of New South Wales: Sydney, NSW)
Dashper, S, and Myers, S (2012). The use of artificial nestboxes by brush-tailed phascogales Phascogale tapotafa in Rushworth Forest. The Victorian Naturalist 120, 40–48.
De Bondi, N, White, JG, Stevens, M, and Cooke, R (2010). A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities. Wildlife Research 37, 456–465.
| A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities.Crossref | GoogleScholarGoogle Scholar |
Department of Environment, Land, Water and Planning (2018) Bioregions and EVC benchmarks. Available at https://www.environment.vic.gov.au/biodiversity/bioregions-and-evc-benchmarks
Department of Sustainability and Environment (2013) Advisory list of threatened vertebrate fauna in Victoria – 2013. Department of Sustainability and Environment, Melbourne, Vic., Australia.
Donald, PF, and Evans, AD (2006). Habitat connectivity and matrix restoration: the wider implications of agri-environment schemes. Journal of Applied Ecology 43, 209–218.
| Habitat connectivity and matrix restoration: the wider implications of agri-environment schemes.Crossref | GoogleScholarGoogle Scholar |
Frigerio, D, Pipek, P, Kimmig, S, Winter, S, Melzheimer, J, Diblíková, L, Wachter, B, and Richter, A (2018). Citizen science and wildlife biology: synergies and challenges. Ethology 124, 365–377.
| Citizen science and wildlife biology: synergies and challenges.Crossref | GoogleScholarGoogle Scholar |
Gadermaier, G, Dörler, D, Heigl, F, Mayr, S, Rüdisser, J, Brodschneider, R, and Marizzi, C (2018). Peer-reviewed publishing of results from Citizen Science projects. Journal of Science Communication 17, 3.
Garnett ST, Crowley GM (1997) The golden-shouldered parrot of Cape York Peninsula: the importance of cups of tea to effective conservation. In ‘Conservation outside Nature Reserves’. (Eds P Hale, D Lamb) pp. 201–205. (Centre for Conservation Biology, University of Queensland: Brisbane, Qld)
Gelman, A (2008). Scaling regression inputs by dividing by two standard deviations. Statistics in Medicine 27, 2865–2873.
| Scaling regression inputs by dividing by two standard deviations.Crossref | GoogleScholarGoogle Scholar | 17960576PubMed |
Gelman A, Su Y-S (2018). arm: data analysis using regression and multilevel/hierarchical models. R package version 1.11-2. Available at https://CRAN.R-project.org/package=arm
Geyle, HM, Woolley, L-A, Davies, HF, Woinarski, JCZ, and Murphy, BP (2020). Targeted sampling successfully detects the cryptic and declining arboreal marsupial (Phascogale pirata) in northern Australia. Pacific Conservation Biology 26, 395–403.
Goldingay, RL (2009). Characteristics of tree hollows used by Australian birds and bats. Wildlife Research 36, 394–409.
| Characteristics of tree hollows used by Australian birds and bats.Crossref | GoogleScholarGoogle Scholar |
Goldingay, RL (2011). Characteristics of tree hollows used by Australian arboreal and scansorial mammals. Australian Journal of Zoology 59, 277–294.
| Characteristics of tree hollows used by Australian arboreal and scansorial mammals.Crossref | GoogleScholarGoogle Scholar |
Goldingay, RL, Thomas, KJ, and Shanty, D (2018). Outcomes of decades long installation of nest boxes for arboreal mammals in southern Australia. Ecological Management & Restoration 19, 204–211.
| Outcomes of decades long installation of nest boxes for arboreal mammals in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Goldingay, RL, Quin, DG, Talamo, O, and Mentiplay-Smith, J (2020a). Nest box revealed habitat preferences of arboreal mammals in box-ironbark forest. Ecological Management & Restoration 21, 131–142.
| Nest box revealed habitat preferences of arboreal mammals in box-ironbark forest.Crossref | GoogleScholarGoogle Scholar |
Goldingay, RL, Rohweder, D, and Taylor, BD (2020b). Nest box contentions: are nest boxes used by the species they target? Ecological Management & Restoration 21, 115–122.
| Nest box contentions: are nest boxes used by the species they target?Crossref | GoogleScholarGoogle Scholar |
Goldingay, RL, and Thomas, KJ (2021). Tolerance to high temperature by arboreal mammals using nest boxes in southern Australia. Journal of Thermal Biology 98, 102899.
| Tolerance to high temperature by arboreal mammals using nest boxes in southern Australia.Crossref | GoogleScholarGoogle Scholar | 34016330PubMed |
Haywood, BK, Parrish, JK, and Dolliver, J (2016). Place-based and data-rich citizen science as a precursor for conservation action. Conservation Biology 30, 476–486.
| Place-based and data-rich citizen science as a precursor for conservation action.Crossref | GoogleScholarGoogle Scholar | 27110934PubMed |
Heywood VH, Watson RT (1995) ‘Global Biodiversity Assessment.’ (Cambridge University Press: Cambridge, UK)
Holland, GJ, Alexander, JSA, Johnson, P, Arnold, AH, Halley, M, and Bennett, AF (2012). Conservation cornerstones: capitalising on the endeavours of long-term monitoring projects. Biological Conservation 145, 95–101.
| Conservation cornerstones: capitalising on the endeavours of long-term monitoring projects.Crossref | GoogleScholarGoogle Scholar |
Koch, A, Munks, S, and Driscoll, D (2008). The use of hollow-bearing trees by vertebrate fauna in wet and dry Eucalyptus obliqua forest, Tasmania. Wildlife Research 35, 727–746.
| The use of hollow-bearing trees by vertebrate fauna in wet and dry Eucalyptus obliqua forest, Tasmania.Crossref | GoogleScholarGoogle Scholar |
Kremen, C, and Merenlender, AM (2018). Landscapes that work for biodiversity and people. Science 362, 6412.
| Landscapes that work for biodiversity and people.Crossref | GoogleScholarGoogle Scholar |
Lawton, JA, Holland, GJ, and Bennett, AF (2021). What determines the distribution of a threatened species, the brush-tailed phascogale Phascogale tapoatafa (Marsupialia: Dasyuridae), in a highly modified region? Austral Ecology 48, 1404–1417.
| What determines the distribution of a threatened species, the brush-tailed phascogale Phascogale tapoatafa (Marsupialia: Dasyuridae), in a highly modified region?Crossref | GoogleScholarGoogle Scholar |
Lindenmayer, D, Crane, M, Blanchard, W, Okada, S, and Montague-Drake, R (2016). Do nest boxes in restored woodlands promote the conservation of hollow-dependent fauna? Restoration Ecology 24, 244–251.
Macak, PV (2020). Nest boxes for wildlife in Victoria: an overview of nest box distribution and use. The Victorian Naturalist 137, 4–14.
Majer, JD, Recher, HF, Graham, R, and Gupta, R (2003). Trunk invertebrate faunas of Western Australian forests and woodlands: influence of tree species and season. Austral Ecology 28, 629–641.
| Trunk invertebrate faunas of Western Australian forests and woodlands: influence of tree species and season.Crossref | GoogleScholarGoogle Scholar |
Mazerolle MJ (2019) AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 2.3-1. Available at https://cran.r-project.org/package=AICcmodavg
Mellick, E, Brown, J, and Jones, M (2009). A ‘bottom up’ approach to landscape restoration. Australasian Plant Conservation: Journal of the Australian Network for Plant Conservation 18, 21–22.
Menkhorst PW (1995) Brush-tailed phascogale. In ‘Mammals of Victoria: Distribution, Ecology and Conservation’. (Ed. PW Menkhorst) pp. 58–60. (Oxford University Press: Melbourne, Vic., Australia)
Michael, DR, Niedra, S, and McWhinney, D (2021). The conservation of arboreal marsupials in the Albury–Wodonga region of south-eastern Australia. Ecological Management & Restoration 22, 45–52.
| The conservation of arboreal marsupials in the Albury–Wodonga region of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Monagle A (2012). The occurrence of arboreal mammals in a modified forest landscape. BSc(Hons) Thesis, Deakin University, Vic., Australia.
Moore, HA, Valentine, LE, Dunlop, JA, and Nimmo, DG (2020). The effect of camera orientation on the detectability of wildlife: a case study from north-western Australia. Remote Sensing in Ecology and Conservation 6, 546–556.
| The effect of camera orientation on the detectability of wildlife: a case study from north-western Australia.Crossref | GoogleScholarGoogle Scholar |
Newton I (1998) ‘Population Limitation in Birds.’ (Academic Press: London, UK)
Norton D, Reid N (2013) ‘Nature and Farming. Sustaining Native Biodiversity in Agricultural Landscapes.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Pannell, DJ, Marshall, GR, Barr, N, Curtis, A, Vanclay, F, and Wilkinson, R (2006). Understanding and promoting adoption of conservation practices by rural landholders. Australian Journal of Experimental Agriculture 46, 1407–1424.
| Understanding and promoting adoption of conservation practices by rural landholders.Crossref | GoogleScholarGoogle Scholar |
Parrish, JK, Burgess, H, Weltzin, JF, Fortson, L, Wiggins, A, and Simmons, B (2018). Exposing the science in citizen science: fitness to purpose and intentional design. Integrative and Comparative Biology 58, 150–160.
| Exposing the science in citizen science: fitness to purpose and intentional design.Crossref | GoogleScholarGoogle Scholar | 29790942PubMed |
Powell MJ (2009) The BOBYQA algorithm for bound constrained optimization without derivatives. In ‘Cambridge NA report NA2009/06’. pp. 26–46. (University of Cambridge: Cambridge, UK)
Rayner, L, Ellis, M, and Taylor, JE (2013). Hollow occurrence and abundance varies with tree characteristics and among species in temperate woodland Eucalyptus. Austral Ecology 39, 145–157.
| Hollow occurrence and abundance varies with tree characteristics and among species in temperate woodland Eucalyptus.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rhind SG (2004) Direct impacts of logging and forest management on the brush-tailed phascogale Phascogale tapoatafa and other arboreal marsupials in Jarrah forest of Western Australia. In ‘Conservation of Australia’s Forest Fauna’. (Ed. D Lunney) pp. 639–655. (Royal Zoological Society of New South Wales: Sydney, NSW)
Rhind, SG, and Bradley, JS (2002). The effect of drought on body size, growth and abundance of wild brush-tailed phascogales (Phascogale tapoatafa) in south-western Australia. Wildlife Research 29, 235–212.
| The effect of drought on body size, growth and abundance of wild brush-tailed phascogales (Phascogale tapoatafa) in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Rowland, JA, Briscoe, NJ, and Handasyde, KA (2017). Comparing the thermal suitability of nest-boxes and tree-hollows for the conservation-management of arboreal marsupials. Biological Conservation 209, 341–348.
| Comparing the thermal suitability of nest-boxes and tree-hollows for the conservation-management of arboreal marsupials.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA (1995). Does our lack of vision threaten the viability of the reconstruction of disturbed ecosystems? Pacific Conservation Biology 2, 321–326.
| Does our lack of vision threaten the viability of the reconstruction of disturbed ecosystems?Crossref | GoogleScholarGoogle Scholar |
Saunders, DA, Dawson, R, Mawson, PR, and Cunningham, RB (2020). Artificial hollows provide an effective short-term solution to the loss of natural nesting hollows for Carnaby’s Cockatoo Calyptorhynchus latirostris. Biological Conservation 245, 108556.
| Artificial hollows provide an effective short-term solution to the loss of natural nesting hollows for Carnaby’s Cockatoo Calyptorhynchus latirostris.Crossref | GoogleScholarGoogle Scholar |
Scida, M, and Gration, R (2017). Monitoring the threatened brush-tailed phascogale (Phascogale tapoatafa tapoatafa) at Sugarloaf Reservoir, Victoria. Australian Mammalogy 40, 307–311.
| Monitoring the threatened brush-tailed phascogale (Phascogale tapoatafa tapoatafa) at Sugarloaf Reservoir, Victoria.Crossref | GoogleScholarGoogle Scholar |
Smith, JK, and Coulson, G (2012). A comparison of vertical and horizontal camera trap orientations for detection of potoroos and bandicoots. Australian Mammalogy 34, 196–201.
| A comparison of vertical and horizontal camera trap orientations for detection of potoroos and bandicoots.Crossref | GoogleScholarGoogle Scholar |
Soderquist, TR (1993). Maternal strategies of Phascogale-tapoatafa (Marsupialia, Dasyuridae). 1. Breeding seasonality and maternal investment. Australian Journal of Zoology 41, 549–566.
| Maternal strategies of Phascogale-tapoatafa (Marsupialia, Dasyuridae). 1. Breeding seasonality and maternal investment.Crossref | GoogleScholarGoogle Scholar |
Soderquist, TR (1995). Spatial organisation of the arboreal carnivorous marsupial Phascogale tapoatafa. Journal of Zoology 237, 385–398.
| Spatial organisation of the arboreal carnivorous marsupial Phascogale tapoatafa.Crossref | GoogleScholarGoogle Scholar |
Soderquist, TR, Traill, BJ, Faris, F, and Beasley, K (1996). Using nest boxes to survey for the brush-tailed phascogale. The Victorian Naturalist 113, 256–261.
Steven, R, Barnes, M, Garnett, ST, Garrard, G, O’Connor, J, Oliver, JL, Robinson, C, Tulloch, A, and Fuller, RA (2019). Aligning citizen science with best practice: threatened species conservation in Australia. Conservation Science and Practice 1, e100.
| Aligning citizen science with best practice: threatened species conservation in Australia.Crossref | GoogleScholarGoogle Scholar |
Sullivan, BL, Wood, CL, Iliff, MJ, Bonney, RE, Fink, D, and Kelling, S (2009). eBird: a citizen-based bird observation network in the biological sciences. Biological Conservation 142, 2282–2292.
| eBird: a citizen-based bird observation network in the biological sciences.Crossref | GoogleScholarGoogle Scholar |
Swaisgood, RR, and Sheppard, JK (2010). The culture of conservation biologists: show me the hope! BioScience 60, 626–630.
| The culture of conservation biologists: show me the hope!Crossref | GoogleScholarGoogle Scholar |
Thomas, R (2009). Regent honeyeater habitat restoration project Lurg Hills, Victoria. Ecological Management & Restoration 10, 84–97.
| Regent honeyeater habitat restoration project Lurg Hills, Victoria.Crossref | GoogleScholarGoogle Scholar |
Traill, BJ, and Coates, TD (1993). Field observations on the brush-tailed phascogale Phascogale tapoatafa (Marsupialia: Dasyuridae). Australian Mammology 16, 61–65.
| Field observations on the brush-tailed phascogale Phascogale tapoatafa (Marsupialia: Dasyuridae).Crossref | GoogleScholarGoogle Scholar |
Trumbull, DJ, Bonney, R, Bascom, D, and Cabral, A (2000). Thinking scientifically during participation in a citizen-science project. Science Education 84, 265–275.
| Thinking scientifically during participation in a citizen-science project.Crossref | GoogleScholarGoogle Scholar |
van der Ree, R, Bennett, AF, and Soderquist, TR (2006). Nest-tree selection by the threatened brush-tailed phascogale (Phascogale tapoatafa) (Marsupialia: Dasyuridae) in a highly fragmented agricultural landscape. Wildlife Research 33, 113–117.
| Nest-tree selection by the threatened brush-tailed phascogale (Phascogale tapoatafa) (Marsupialia: Dasyuridae) in a highly fragmented agricultural landscape.Crossref | GoogleScholarGoogle Scholar |
Williams, SL (1995). Malleefowl as a flagship for conservation on farms in the Murray Mallee of South Australia. Nature Conservation 4, 316–320.
Woinarski JC, Burbidge AA, Harrison PL, (2014a) Species conservation summary: brush-tailed phascogale. In ‘The Action Plan for Australian Mammals 2012’. (Eds JC Woinarski, AA Burbidge, PL Harrison) pp. 127–130. (CSIRO Publishing: Melbourne, Vic., Australia)
Woinarski JC, Burbidge AA, and Harrison PL, (2014b) Subspecies conservation summary: brush-tailed phascogale (eastern). In ‘The Action Plan for Australian Mammals 2012’. (Eds JC Woinarski, AA Burbidge, PL Harrison) pp. 130–132. (CSIRO Publishing: Melbourne, Vic., Australia)
Appendix
|
|
|