Is the Felixer cat control device safe for marsupial carnivores?
Holly Rickards A B * , John L. Read C , Chris N. Johnson A , Menna E. Jones A , Matthew D. Pauza B , Joss Bentley D , Andry Sculthorpe E , Morgan Humphrey A and Rowena Hamer
A B * , John L. Read C , Chris N. Johnson A , Menna E. Jones A , Matthew D. Pauza B , Joss Bentley D , Andry Sculthorpe E , Morgan Humphrey A and Rowena Hamer  A F
A F
A University of Tasmania, Hobart, Tas., Australia.
B Department of Natural Resource and Environment Tasmania, Hobart, Tas., Australia.
C University of Adelaide, Adelaide, SA, Australia.
D NSW Office of Environment and Heritage, Ecosystems and Threatened Species Queanbeyan, Queanbeyan, NSW, Australia.
E Tasmanian Aboriginal Centre Inc, Hobart, Tas., Australia.
F Tasmanian Land Conservancy, Hobart, Tas., Australia.
Wildlife Research 50(5) 356-365 https://doi.org/10.1071/WR21175
Submitted: 9 December 2021 Accepted: 21 May 2022 Published: 10 August 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: The Felixer grooming device (‘Felixer’) is a lethal method of feral cat control designed to be cost-effective and target specific.
Aims: This study aims to test the target specificity of the Felixer in Tasmania, with a particular focus on Tasmanian devil and quoll species due to the overlap in size, habitats and behaviour between these native carnivores and feral cats.
Methods: Our study deployed Felixer devices set in a non-lethal mode in nine field sites in Tasmania, one field site in New South Wales and two Tasmanian wildlife sanctuaries.
Key results: Our study recorded 4376 passes by identifiable vertebrate species including 528 Tasmanian devil passes, 507 spotted-tailed quoll passes and 154 eastern quoll passes. Our data showed that the Felixer can successfully differentiate quoll species from feral cats with spotted-tailed quolls and eastern quolls targeted in 0.19% and 0% of passes, respectively. However, Tasmanian devils and common wombats were targeted in 23.10% and 12% of passes, respectively, although sample size was low for common wombats (n = 25).
Conclusions: The Felixer could not reliably identify Tasmanian devils and possibly common wombats as non-target species. Further data is needed to confirm the potential for impacts on the common wombat and other potential non-target species in Tasmania, and the likelihood of the toxin being ingested by falsely targeted individuals.
Implications: Our study suggest that the Felixer device is safe for use in the presence of two species of conservation concern, the eastern and spotted-tailed quoll. It also supports evidence from previous studies that the Felixer is unlikely to impact bettongs and potoroos. Use of Felixer devices across much of Tasmania would have to balance the conservation or economic benefits of cat control against potential impacts on Tasmanian devils. We suggest that active Felixer deployments be preceded by surveys to establish the range of species present at the control site, and the season of control considered carefully to minimise potential impacts on more susceptible juvenile animals. In addition, modifications to the Felixer device such as the proposed incorporation of AI technology should be tested against the Tasmanian devil and other non-target species.
Keywords: feline control, Felis catus, feral cats, grooming trap, lethal control, management, Sarcophilus harrishii, target specificity, Tasmanian carnivores.
Introduction
The feral cat (Felis catus) is a versatile predator that has established across Australia and most of its offshore islands (Doherty et al. 2014, 2017; Legge et al. 2017). Feral cats have contributed to most of the 30 native mammal extinctions since European settlement of Australia and are directly contributing to the decline of numerous other populations of small vertebrates (Woinarski et al. 2015; Woinarski 2016; Doherty et al. 2017). Feral cats threaten wildlife, livestock, and humans by transmitting disease (Doherty et al. 2017; Woinarski et al. 2019; Legge et al. 2020a). Therefore, extensive management programs have been developed to limit their impacts via eradication or population reduction (Australia Co 2015; Doherty et al. 2017; Legge et al. 2020b).
Management of feral cats can use lethal or non-lethal approaches, depending on the desired outcome of the program. The specific methods used in a region depend on the feasibility, labour intensity, humaneness and target-specificity of the approach (Parkes et al. 2014). Non-lethal trapping and relocation/re-homing and trap-neuter-release (TNR) often attract community support although opinions on welfare outcomes for released cats differ widely (Robertson 2008; Wallach et al. 2018; Wolf and Schaffner 2019; Read et al. 2020). Non-lethal management methods entail high effort for limited benefit, and still leave non-target and prey animals vulnerable to predation, disease and competition (Andersen et al. 2004; Foley et al. 2005; Robertson 2008; Schmidt et al. 2009; Lohr et al. 2013; Moseby et al. 2019; Wolf and Schaffner 2019). Lethal methods are usually applied to either eradicate a population from a defined area where recolonisation is limited (e.g. an island or fenced exclusion area), or control the density of a treated population to limit impacts on native wildlife (Moseby et al. 2019). Such methods include baiting, trapping, shooting, or use of biocontrol agents (Short et al. 2002; Moseby and Read 2006; de Tores et al. 2011; Leo et al. 2018). Lethal methods are often the most efficient methods of eradication or control as they can reduce invasive populations in a shorter time frame with less effort (Short et al. 1997; Kinnear et al. 2010; Moseby and Hill 2011; Comer et al. 2018).
Common lethal methods of managing feral cats, such as shooting and trapping, require a high effort and can be ineffective, particularly when feral cat densities are low (Short et al. 2002; Fancourt et al. 2021). Baiting is commonly used, and while relatively cheap to implement, it can risk uptake by non-target species including native carnivores. Bait avoidance is known to occur in feral cats if prey are abundant or if they develop avoidance behaviour (Algar et al. 2007a).
The Felixer grooming ‘trap’ (hereafter ‘Felixer’) has been designed as a cost-effective, targeted technique to control feral cats and foxes while minimising impacts on non-target native animals. The device distinguishes target species (i.e. feral cats and foxes) from non-target species based on the size, shape and speed of the animals recorded by a detection system linked to the firing mechanism (Read et al. 2014). When the Felixer is set to active mode, it fires the contents of a cartridge containing sodium fluoroacetate (1080) dissolved in a sticky gel onto the flank of an animal that is identified as a target, that will then be ingested when the animal grooms the gel off its fur. Importantly, the Felixer has an inbuilt photographic monitoring system, which allows users to determine which (if any) animals triggered the Felixer during its deployment.
One of the key advantages of the Felixer is its target specificity. The accuracy and precision of species identification by the Felixer has been tested on mainland Australia (Read et al. 2019; Moseby et al. 2020) but not yet in Tasmania, where native carnivores similar in body size and gait to cats are common. The purpose of this study is to test the ability of the Felixer to discriminate between feral cats and native Tasmanian wildlife in different regions across Tasmania. The study focuses mainly on native Tasmanian carnivores, specifically the spotted-tailed quoll (Dasyurus maculatus), eastern quoll (Dasyurus viverrinus) and Tasmanian devil (Sarcophilus harrisii), due to their similarities in size, shape and habitat use to the feral cat. For any native species that are regularly (>5% passes) misidentified as a target by the Felixer, we also assess the number of target events required to deliver a lethal 1080 dose to assess the risk posed to these species. The data collected from these trials will provide important information on the efficacy and suitability of the Felixer for controlling feral cats and will form part of the application for national registration for use in Australia.
Methods
Field trials
Felixer
The Felixer’s detection system comprises a camera linked to an array of four LIDAR (range-finding laser) sensors in a diamond shaped configuration (Fig. 1). The camera is triggered whenever an object breaks any of the sensors. For each trigger event, the Felixer stores a photo along with information on the timing to millisecond accuracy and distance at which each sensor beam was intercepted, enabling the number and speed of animals to be calculated. To be identified as a target, animals must simultaneously intercept two activation sensor beams at approximately equal distance from the Felixer, without intercepting the top blocking sensor or blocking the bottom blocking sensor any longer than the typical passage of walking cat’s legs. Moreover, the classification algorithm matches the speed of movement between sensors to the average speed of target (feral cat and red fox) species to further discriminate between target and non-target species. When the Felixer is set in ‘active’ mode, recognition of a target results in the firing of the contents of a gel cartridge.
Trial locations
Felixers were deployed during January 2020, June–September 2020 and January 2021 (Table 1). Felixers were set up within both captive enclosures and at field sites. The captive trials were undertaken at two Tasmanian wildlife parks: Bonorong Wildlife Sanctuary and Trowunna Wildlife Sanctuary and targeted the three native carnivore species. Use of these sanctuaries enabled the recording of large numbers of detections over short periods of time. Also, captive trials allowed us to select individuals for the trial in a way that ensured a large variation in age, size and sex.

|
Field deployments of the Felixers were used to collect data on target specificity in realistic management contexts, recognising that body size, age and behaviour may differ from captive individuals. Nine field sites throughout Tasmania were selected for field trials, which were run between June and September 2020 (Fig. 2, Table 1). We chose trial locations using two criteria. First, some were chosen because of known high densities of focal species. We selected the Arthur Pieman Conservation Area, because it has high densities of Tasmanian devils and spotted-tailed quolls (Andersen et al. 2016, 2017, 2020), and Bruny Island which has high densities of eastern quolls (Fancourt 2016). Second, we conducted trials in places where use of Felixers for cat control is being planned or considered by land managers. These sites were Bruny Island, lungtalanana/Clarke Island, Three Hummock Island, and the Tasmanian Midlands. To increase sample sizes, additional data on spotted-tailed quoll targeting rates were incorporated from a study in Barren Grounds Nature Reserve, NSW.
|
|
Deployment details
Felixers can be set with either aggressive or conservative settings, which apply different algorithms to the sensor data to discriminate between target and non-target detections (Table 1). The conservative mode is designed to reduce triggers by non-target animals, but, as a consequence, reduces the target rate for feral cats and foxes. It is typically used where non-target animals are abundant. During Tasmanian Felixer trials, conservative mode was used for 1 week, where a very small number of passes were recorded. Therefore, these results were pooled with aggressive mode results for analysis.
Felixers were set in non-lethal, photo-only mode for all deployments. The Felixer detection range (i.e. the distance from the unit at which the camera is triggered and the mechanism would fire if set to lethal mode) was adjusted on a site-specific basis to avoid triggering by vegetation or animals beyond the range of the firing mechanism. For captive trials, the maximum detection range was typically set at 1 m due to proximity of enclosure features and defined paths, though in an eastern quoll trial at Trowunna a range of 3 m was used due to the large enclosure size. In wild deployments, detection range varied from 2.5 m to 4 m.
The Felixers have pre-installed audio lures of birds, cats, foxes and small mammals which can be played on a customised or standard schedule. Pre-installed audio lures of birds and small mammals were used during trials at lungutalanana/Clarke Island and in the Midlands on a standard schedule. An olfactory lure (tuna oil) was used in deployments on Bruny Island and in the Arthur Pieman Conservation Area. Approximately 90 mL of oil was poured onto the ground approximately 2 m either side of the Felixer, to attract animals without affecting their gait or behaviour when passing the Felixer sensors.
Data analysis
Photos and sensor logs from field and captive trials were uploaded to the Felixer Management System (FMS), a cloud-based data storage and processing system, and classified to species level wherever possible. Once images were tagged, metadata were exported as csv files. Subsequent data analyses were conducted in the R statistical environment using packages ‘dplyr’, ‘tidyr’, ‘ggplot2’, ‘AICcmodavg’ and ‘boot’. This included calculating percent targeted by expressing the number of times a species was targeted as a percentage of the total number of detections of that species.
Target rate
We tested the influence of species on the Felixer targeting rate (the likelihood that a pass was classified as a target object) using a binomial generalised linear model (GLM). We excluded any species with less than 10 passes recorded across all sites. For species never classified as a target, we added a single target event to the dataset to avoid boundary fit errors. We attempted to include study site as a random factor, but models failed to converge due to insufficient replicates of each species at each site
For species with a relatively high risk (>5%) of being falsely targeted by the Felixer, we undertook additional analyses as discussed below.
Likelihood of lethal dose
To understand the potential consequences of non-target species ingesting a lethal dose of toxin, we plotted body weights of non-target species with a relatively high risk (>5%) of being targeted by the Felixer, against the number of cartridges required for a lethal dose, based on published estimates of 1080 lethal dose. The range of body sizes plotted for each species was based on UTAS and NRE trapping datasets (M. Jones, unpubl. data, and S. Huxtable, pers. comm., respectively). The minimum body size for each species represents that of the smallest individual likely to encounter a Felixer. For Tasmanian devils and common wombats this is the approximate weight when they are weaned and become independent. For eastern grey kangaroos this is the approximate weight they leave the pouch. Maximum body size was limited to 40 kg for common wombats and eastern grey kangaroos.
Influence of body size on target rate
Given that smaller (and therefore younger) animals are more likely to receive a lethal dose, we reran the binomial GLM analyses of target rate for these species including age as an additional covariate. We classified animals in each detection as either subadult (<1 year old) or adult, using either the known age (captive individuals) or estimated age from photos (wild individuals) based on size, fur condition and structural definition of facial features. This analysis was undertaken only for species where we could confidently assign age.
Approvals and permits
This project operated under Animal Ethics Approval 20223 and AEC7/2019–20, scientific permit TFA20105 and APVMA permit 80926.
Results
During deployment at wildlife parks and field sites, the Felixer sensors were activated 6650 times. Activations where the cause of the trigger could not be identified, or which were caused by humans, vehicles, litter, unidentifiable objects, or movements of vegetation, were removed from the dataset. This left 4376 activations by identifiable vertebrate species.
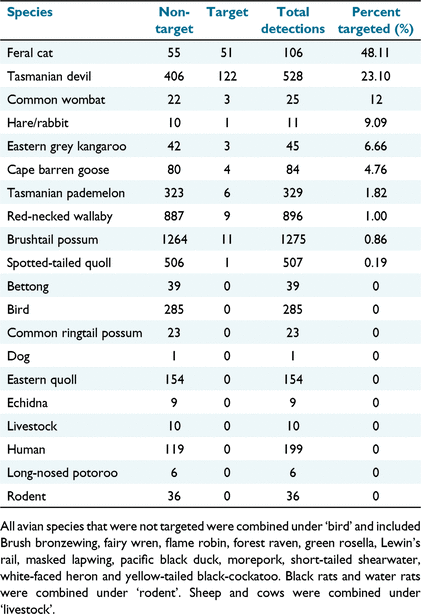
Species that were identified by the Felixer as targets included the feral cat, Tasmanian devil, common wombat, hare/rabbit, eastern grey kangaroo, Cape Barren goose, Tasmanian pademelon, Bennett’s wallaby, brushtail possum, and spotted-tailed quoll (Table 2). The highest target rate was for the feral cat (48.11% of passes classified as a target), followed by the Tasmanian devil (23.10%) and common wombat (12%).
|
Targeting rate
The binomial GLM indicated that targeting rate differed among species. Model predictions of targeting rate for each species are shown in Fig. 3 with associated confidence intervals.
Of the native species analysed, Tasmanian devils, common wombats and eastern grey kangaroos were targeted in more than 5% of passes. Additional analyses were therefore undertaken for these species.
Lethal dose
The average lethal dose (LD50: the amount of toxin needed to kill 50% of the population) is 4.2 mg of 1080 kg−1 for Tasmanian devils, 1.5 mg kg−1 for common wombats and 0.29 mg kg−1 for Eastern Grey Kangaroos (Department of Primary Industries, Parks, Water and Environment 2011; Department of Primary Industries and Regional Development 2018). The relationship between the minimum number of Felixer capsules and body weight required to deliver a lethal dose is shown in Fig. 4, based on 8 mg of 1080 per Felixer cartridge.
Body size
Model selection for devil data supported the hypothesis that target rate is influenced by age. Devils identified as subadults through size, fur condition and facial structure were targeted 2.6% more often than adult devils. However, the model predictions suggest that the differences in target rate between adults and subadults are small and probably not biologically significant. The influence of body size on Felixer targeting rate could not be shown for wombats or eastern grey kangaroos during our trial, given the difficulty of distinguishing subadult from adult individuals from the images, and the limited sample size (25 passes) for wombats.
Discussion
The extent of incorporation of novel methods of feral cat control into cat management programs will be determined by demonstrated limits on non-target impacts, in line with the requirements of the feral cat Threat Abatement Plan (Australia Co 2015). Our data indicate that the Felixer can reliably differentiate eastern and spotted-tailed quolls from feral cats, as well as other native non-target animals including Bennett’s wallabies and Tasmanian pademelons. However, the Felixer mis-identified Tasmanian devils as a target animal in 23.10% of detections, common wombats in 12% of detections and eastern grey kangaroos in 6.66% of detections (although our sample sizes were small for the latter two species). Mitigation measures, including judicious placement or timing of deployment or improvements in nontarget distinction, would be required before the device could be safely used in areas with Tasmanian devil populations.
Quoll species, particularly the spotted-tailed quoll, were selected as focal species for this study as their similar body size and shape to feral cats suggested they were likely to be at high risk of misidentification by Felixers. However, these species had some of the lowest target rates, with only one of 507 spotted-tailed quoll passes (0.2%) identified as a target, and none of the 154 eastern quoll passes. These results are supported by another large trial, which reported no target events from over 4000 spotted-tailed quoll passes in captivity and the wild in NSW (A. Claridge, unpubl. data). Inspection of the Felixer photos indicates that this differentiation is most likely due to differences in size, leg length, speed and gait. Eastern quolls are quite small and would likely trigger the lower blocking sensor and only one of the activation sensors. Spotted-tailed quolls are similar in body weight to a cat but have relatively shorter legs and their torso would likely trigger the lower blocking sensor. Also, most quolls were recorded running with both sets of legs either close together or far apart and off the ground, characteristic of their typical gait (Jones and Stoddart 1998; Jones 2003), whereas cats were typically photographed walking past the Felixer.
Data from most macropod and potoroid species recorded were also encouraging. The Bennett’s wallaby, Tasmanian pademelon and eastern grey kangaroo were the only species to be identified as targets, in approximately 1% of 896 Bennett’s wallaby, 1.82% of 329 Tasmanian pademelon passes and 6.66% of eastern grey kangaroos. Of the two other species recorded (eastern bettong and long-nosed potoroo) none were identified as targets, however sample sizes were low (39 and six detections respectively), and these results should be treated with caution. However, previous studies which recorded related species including burrowing bettongs (0% target rate, Read et al. 2019; Moseby et al. 2020), and 1.6% of 8419 kangaroo records and also 1.6% of 11 535 wallaby records Australia wide were targeted by Felixers between January 2000 and March 2022 (Felixer Management System, unpubl. data) suggest that target rates for these species are unlikely to be high.
Tasmanian devils are listed as Endangered under state and federal legislation due to the impacts of devil facial tumour disease (DFTD, Hawkins et al. 2006). The high target rate of Tasmanian devils (23.10%, n = 528), caused by their similarities in body shape, size and gait to cats, is therefore particularly concerning, and has potential implications for the use of these devices across most of mainland Tasmania. Also of concern was the 12% targeting rate recorded for common wombats, however given the low sample size (n = 25) we recommend that additional data be collected to confirm these results for this species. The estimated targeting rate of eastern grey kangaroos (6.66%, n = 45) was high but should also be treated with caution due to the low sample size. Kangaroo results show similarities to other studies such as 4.23% kangaroo false targets (Read et al. 2019)
The relatively high targeting rate for hare/rabbits (9%) should also be treated with caution given the very low sample size (n = 11), especially given that 0.0004% of 4895 hare/rabbits have been targeted by Felixers from January 2020 to March 2022 (FMS, unpubl. data). Non-target impacts on these introduced pest species are unlikely to affect approvals for use of this device, but could have implications for the cost, efficacy, and safety of control efforts. Traditional methods of cat control such as trapping and baiting have reduced efficacy when preferred prey such as rabbits are abundant. Passive devices such as Felixers would therefore be potentially preferred. If there is a high target rate for rabbits and hares, however, the large numbers of non-target activations and the associated need to replace Felixer cartridges more frequently, as the Felixer can only hold 20 cartridges at one time, may outweigh any benefits for cat control in areas or during periods of very high rabbit abundance. In addition, whereas sublethal doses of 1080 are readily metabolised and excreted, it does not readily degrade in carcasses (Eason et al. 2013), meaning there is some risk of secondary poisoning to native carnivores if large numbers of rabbits ingest a lethal dose.
Suggested mitigation
Although our study recorded high pass numbers from focal species; devils and quolls, lack of data for other native wildlife limits the strength of inference of overall target-specificity in Tasmania. Increased sample sizes for common wombats, Bennett’s wallaby, Tasmanian pademelons and eastern grey kangaroos may be required. Another factor in determining risks associated with Felixer deployments is whether non-targets are likely to groom, ingest and receive a lethal dose from a Felixer cartridge. Further research on grooming behaviour and the number of full cartridges required for a lethal dose will help quantify such risks.
To limit impacts on Tasmanian devils and other non-target species in Tasmania, we suggest two main avenues for further investigation: reducing false positive target identifications; and deactivating Felixers when and where false-positive non-targets are most likely. Reducing the targeting rate by adjusting the Felixer sensing technology, settings or the firing algorithm would be the safest method of reducing impacts. Our results did not include enough passes to determine whether the conservative setting would be less likely to target Tasmanian devils, and this should be included in future trials. The targeting rate for feral cats in this study (46.8%) is already substantially lower than that recorded in previous studies (82%, Read et al. 2019; 77%, Moseby et al. 2020), and targeting rate will significantly impact both the cost and ability of control programs to achieve a long-term reduction in cat population numbers. Continuous improvement in targeting algorithms achievable through larger datasets and planned inclusion of Artificial Intelligence cameras to augment target identification in Felixers are anticipated to improve targeting rates for feral cats and foxes and reduce false-positive targeting of non-target species.
Adjusting the timing and/or placement of Felixer deployments may help to limit lethal impacts on species such as Tasmanian devils. Body size had a significant, although small effect on targeting rate. It is worth noting that our study did not include any dependent juveniles (those smaller than ~2.5 kg). Moreover, the shorter leg length of such juveniles, as well as their limited ranging behaviour away from the maternal den, may make them less likely to be targeted. Avoiding periods around weaning age when juveniles are present and active may therefore reduce the risk of lethal impacts. However, the Tasmanian devil breeding season has become more variable since the onset of disease (Jones et al. 2008). A decline in the age of sexual maturity means some female Tasmanian devils are breeding in their first season, but typically commencing weeks or months after older females in the population (Jones et al. 2008; Lachish et al. 2009). The resulting extended breeding season means that the period in which vulnerable-sized animals are active is a much larger proportion of the year.
Adult Tasmanian devils (and other species such as wombats) may still incur a lethal dose if they are repeatedly targeted by Felixers. In our trial, only 2 of 38 individual devils (identified using markings) observed in wild trials were observed more than once. The likelihood of repeat visits would increase with higher numbers or density of devices deployed, although it may also be reduced if the animal is fired upon by a Felixer, as the high speed impact of the gel is likely to encourage future avoidance, if recognition of the device and learning occurs. However, even if adult devils do not ingest a lethal dose, sublethal impacts of 1080 toxicity may still be incurred. Sublethal doses of 1080 are readily metabolised and excreted relative to other toxins, but this does not eliminate the risk of toxic effects (Eason et al. 2011). Sublethal impacts may include sickness (lack of coordination, seizures, lethargy, retching and spasm), cessation of grooming, feeding and activity, vulnerability to disease, impacts on pouched and dependent young, behavioural changes leading to increased risk of roadkill, dog attacks, and inability to defend themselves against other devils (Mcilroy 1984; Littin et al. 2009). However, sublethal symptoms are short-term, as 1080 has likely passed through the system within 8–24 h in mammals (Mead et al. 1979; Twigg et al. 2003). We therefore suggest that adjusting the timing of Felixer deployments is unlikely to eliminate the risk of impact on species such as the Tasmanian devil. However, use of Felixers should be considered when eradicating or controlling cats in Tasmanian devil free locations such as offshore islands.
Conclusion
Our study demonstrated that Felixers can target, and therefore be used to control, feral cats in Tasmania. Trials throughout the nine Tasmanian field sites, two Tasmanian wildlife parks and one NSW nature reserve generated data on a wide range of native and invasive species and revealed that the two quolls species are consistently identified as nontargets correctly. However, devils are being targeted at a concerning rate, and future use of the devices would need to balance conservation or economic benefits of cat control with potential impacts on non-target species.
Our study focused on native carnivores, in contexts where the Felixer is most likely to be deployed, such as agricultural and semi-urban areas with high cat densities. We therefore did not detect all species of Tasmanian wildlife that have the potential to trigger the Felixer. Additional test deployments across a broader range of contexts would build the knowledge base of non-target risks for wildlife in Tasmania.
Data availability
All raw data used in this study are maintained on the Felixer Management System (https://felixerlogs.thylation.com/) and can be made available on request.
Conflicts of interest
John Read declares that he is the CEO of Thylation R&D, that is developing and commercialising Felixers. The remaining authors declare no conflicts of interest.
Declaration of funding
Funding was secured from Greening Australia’s Smart Farming small grants program and Rowena Hamers crowd-funding campaign.
Acknowledgements
We thank the landholders who provided sites for our research to take place, Julian and Annabel von Bibra, Rae and Lindsay Young, John and Isabelle Atkinson and Paul Davis. We extend thanks to Elisa Raulings and Greening Australia for their generous grants and interest in our research. We thank Hugh McGregor for initial crowdfunding and project design. To the kind and helpful staff at Trowunna Wildlife Sanctuary and Bonorong Wildlife Sancturary, we are grateful for the opportunity to work with your animals, within your facilities. Thanks extends to other stakeholders and members of the Bruny Island Feral Cat Eradication Team, Cindy Hull from NRM South, Kaylene Allan from Kingborough Council and Conrad Daniels from Bruny Island farming. Thank you to Iona Flett from the Cradle Coast Authority Tasmania, for her dedication, support and professionalism in progressing a cat-free Three Hummock Island.
References
Algar, D, Angus, GJ, Williams, MR, and Mellican, AE (2007a). Influence of bait type, weather and prey abundance on bait uptake by feral cats (Felis catus) on Peron Peninsula, Western Australia. Conservation Science Western Australia 6, 109–149.Andersen, MC, Martin, BJ, and Roemer, GW (2004). Use of matrix population models to estimate the efficacy of euthanasia versus trap-neuter-return for management of free-roaming cats. Journal of the American Veterinary Medical Association 225, 1871–1876.
| Use of matrix population models to estimate the efficacy of euthanasia versus trap-neuter-return for management of free-roaming cats.Crossref | GoogleScholarGoogle Scholar |
Andersen, GE, Johnson, CN, and Jones, ME (2016). Sympatric predator odour reveals a competitive relationship in size-structured mammalian carnivores. Behavioral Ecology and Sociobiology 70, 1831–1841.
| Sympatric predator odour reveals a competitive relationship in size-structured mammalian carnivores.Crossref | GoogleScholarGoogle Scholar |
Andersen, GE, Johnson, CN, Barmuta, LA, and Jones, ME (2017). Use of anthropogenic linear features by two medium-sized carnivores in reserved and agricultural landscapes. Scientific Reports 7, 11624.
| Use of anthropogenic linear features by two medium-sized carnivores in reserved and agricultural landscapes.Crossref | GoogleScholarGoogle Scholar |
Andersen, GE, Johnson, CN, and Jones, ME (2020). Space use and temporal partitioning of sympatric Tasmanian devils and spotted-tailed quolls. Austral Ecology 45, 355–365.
| Space use and temporal partitioning of sympatric Tasmanian devils and spotted-tailed quolls.Crossref | GoogleScholarGoogle Scholar |
Australia Co (2015) ‘Threat abatement plan for predation by feral cats.’ (Department of Environment)
Comer, S, Speldewinde, P, Tiller, C, Clausen, L, Pinder, J, Cowen, S, and Algar, D (2018). Evaluating the efficacy of a landscape scale feral cat control program using camera traps and occupancy models. Scientific Reports 8, 5335.
| Evaluating the efficacy of a landscape scale feral cat control program using camera traps and occupancy models.Crossref | GoogleScholarGoogle Scholar |
de Tores, PJ, Sutherland, DR, Clarke, JR, Hill, RF, Garretson, SW, Bloomfield, L, Strümpher, L, Glen, AS, and Cruz, J (2011). Assessment of risks to non-target species from an encapsulated toxin in a bait proposed for control of feral cats. Wildlife Research 38, 39–50.
| Assessment of risks to non-target species from an encapsulated toxin in a bait proposed for control of feral cats.Crossref | GoogleScholarGoogle Scholar |
Department of Primary Industries, Parks, Water and Environment (2011) ‘1080 poison.’ (Department of Natural Resources and Environment Tasmania: Hobart, Tas., Australia)
Department of Primary Industries and Regional Development (2018) ‘1080 characteristics and use.’ (Department of Primary Industries and Regional Development: Perth, WA, Australia)
Doherty, TS, Bengsen, AJ, and Davis, RA (2014). A critical review of habitat use by feral cats and key directions for future research and management. Wildlife Research 41, 435–446.
| A critical review of habitat use by feral cats and key directions for future research and management.Crossref | GoogleScholarGoogle Scholar |
Doherty, TS, Dickman, CR, Johnson, CN, Legge, SM, Ritchie, EG, and Woinarski, JCZ (2017). Impacts and management of feral cats Felis catus in Australia. Mammal Review 47, 83–97.
| Impacts and management of feral cats Felis catus in Australia.Crossref | GoogleScholarGoogle Scholar |
Eason, C, Miller, A, Ogilvie, S, and Fairweather, A (2011). An updated review of the toxicology and ecotoxicology of sodium fluoroacetate (1080) in relation to its use as a pest control tool in New Zealand. New Zealand Journal of Ecology 35, 1–20.
Eason, CT, Ross, J, and Miller, A (2013). Secondary poisoning risks from 1080-poisoned carcasses and risk of trophic transfer—a review. New Zealand Journal of Zoology 40, 217–225.
| Secondary poisoning risks from 1080-poisoned carcasses and risk of trophic transfer—a review.Crossref | GoogleScholarGoogle Scholar |
Fancourt, BA (2016). Diagnosing species decline: a contextual review of threats, causes and future directions for management and conservation of the eastern quoll. Wildlife Research 43, 197–211.
| Diagnosing species decline: a contextual review of threats, causes and future directions for management and conservation of the eastern quoll.Crossref | GoogleScholarGoogle Scholar |
Fancourt, BA, Augusteyn, J, Cremasco, P, Nolan, B, Richards, S, Speed, J, Wilson, C, and Gentle, MN (2021). Measuring, evaluating and improving the effectiveness of invasive predator control programs: feral cat baiting as a case study. Journal of Environmental Management 280, 111691.
| Measuring, evaluating and improving the effectiveness of invasive predator control programs: feral cat baiting as a case study.Crossref | GoogleScholarGoogle Scholar |
Foley, P, Foley, JE, Levy, JK, and Paik, T (2005). Analysis of the impact of trap-neuter-return programs on populations of feral cats. Journal of the American Veterinary Medical Association 227, 1775–1781.
| Analysis of the impact of trap-neuter-return programs on populations of feral cats.Crossref | GoogleScholarGoogle Scholar |
Hawkins, CE, Baars, C, Hesterman, H, Hocking, GJ, Jones, ME, Lazenby, B, Mann, D, Mooney, N, Pemberton, D, Pyecroft, S, Restani, M, and Wiersma, J (2006). Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biological Conservation 131, 307–324.
| Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii.Crossref | GoogleScholarGoogle Scholar |
Jones ME (2003) Convergence in ecomorphology and guild structure among marsupial and placental carnivores. In ‘Predators with pouches: the biology of carnivorous marsupials’. (Eds M Jones, C Dickman, M Archer) pp. 285–296. (CSIRO Publishing: Melbourne)
Jones, ME, and Stoddart, DM (1998). Reconstruction of the predatory behaviour of the extinct marsupial thylacine (Thylacinus cynocephalus). Journal of Zoology 246, 239–246.
| Reconstruction of the predatory behaviour of the extinct marsupial thylacine (Thylacinus cynocephalus).Crossref | GoogleScholarGoogle Scholar |
Jones, ME, Cockburn, A, Hamede, R, Hawkins, C, Hesterman, H, Lachish, S, Mann, D, McCallum, H, and Pemberton, D (2008). Life-history change in disease-ravaged Tasmanian devil populations. Proceedings of the National Academy of Sciences of the United States of America 105, 10023–10027.
| Life-history change in disease-ravaged Tasmanian devil populations.Crossref | GoogleScholarGoogle Scholar |
Kinnear, JE, Krebs, CJ, Pentland, C, Orell, P, Holme, C, and Karvinen, R (2010). Predator-baiting experiments for the conservation of rock-wallabies in Western Australia: a 25-year review with recent advances. Wildlife Research 37, 57–67.
| Predator-baiting experiments for the conservation of rock-wallabies in Western Australia: a 25-year review with recent advances.Crossref | GoogleScholarGoogle Scholar |
Lachish, S, McCallum, H, and Jones, M (2009). Demography, disease and the devil: life-history changes in a disease-affected population of Tasmanian devils (Sarcophilus harrisii). Journal of Animal Ecology 78, 427–436.
| Demography, disease and the devil: life-history changes in a disease-affected population of Tasmanian devils (Sarcophilus harrisii).Crossref | GoogleScholarGoogle Scholar |
Legge, S, Murphy, BP, McGregor, H, Woinarski, JCZ, Augusteyn, J, Ballard, G, Baseler, M, Buckmaster, T, Dickman, CR, Doherty, T, Edwards, G, Eyre, T, Fancourt, BA, Ferguson, D, Forsyth, DM, Geary, WL, Gentle, M, Gillespie, G, Greenwood, L, Hohnen, R, Hume, S, Johnson, CN, Maxwell, M, McDonald, PJ, Morris, K, Moseby, K, Newsome, T, Nimmo, D, Paltridge, R, Ramsey, D, Read, J, Rendall, A, Rich, M, Ritchie, E, Rowland, J, Short, J, Stokeld, D, Sutherland, DR, Wayne, AF, Woodford, L, and Zewe, F (2017). Enumerating a continental-scale threat: how many feral cats are in Australia? Biological Conservation 206, 293–303.
| Enumerating a continental-scale threat: how many feral cats are in Australia?Crossref | GoogleScholarGoogle Scholar |
Legge, S, Taggart, PL, Dickman, CR, Read, JL, and Woinarski, JCZ (2020a). Cat-dependent diseases cost Australia AU$6 billion per year through impacts on human health and livestock production. Wildlife Research 47, 731–746.
| Cat-dependent diseases cost Australia AU$6 billion per year through impacts on human health and livestock production.Crossref | GoogleScholarGoogle Scholar |
Legge, S, Woinarski, JCZ, Dickman, CR, Doherty, TS, McGregor, H, and Murphy, BP (2020b). Cat ecology, impacts and management in Australia. Wildlife Research 47, i–vi.
| Cat ecology, impacts and management in Australia.Crossref | GoogleScholarGoogle Scholar |
Leo, BT, Anderson, JJ, Ha, J, Phillips, RB, and Ha, RR (2018). Modeling impacts of hunting on control of an insular feral cat population. Pacific Science 72, 57–67.
| Modeling impacts of hunting on control of an insular feral cat population.Crossref | GoogleScholarGoogle Scholar |
Littin, KE, Gregory, NG, Airey, AT, Eason, CT, and Mellor, DJ (2009). Behaviour and time to unconsciousness of brushtail possums (Trichosurus vulpecula) after a lethal or sublethal dose of 1080. Wildlife Research 36, 709–720.
| Behaviour and time to unconsciousness of brushtail possums (Trichosurus vulpecula) after a lethal or sublethal dose of 1080.Crossref | GoogleScholarGoogle Scholar |
Lohr, CA, Cox, LJ, and Lepczyk, CA (2013). Costs and benefits of trap-neuter-release and euthanasia for removal of urban cats in Oahu, Hawaii. Conservation Biology 27, 64–73.
| Costs and benefits of trap-neuter-release and euthanasia for removal of urban cats in Oahu, Hawaii.Crossref | GoogleScholarGoogle Scholar |
Mcilroy, JC (1984). The sensitivity of Australian animals to 1080 poison Vii. Native and introduced birds. Wildlife Research 11, 373–385.
| The sensitivity of Australian animals to 1080 poison Vii. Native and introduced birds.Crossref | GoogleScholarGoogle Scholar |
Mead, RJ, Oliver, AJ, and King, DR (1979). Metabolism and defluorination of fluoroacetate in the brush-tailed possum (Trichosurus vulpecula). Australian Journal of Biological Sciences 32, 15–26.
| Metabolism and defluorination of fluoroacetate in the brush-tailed possum (Trichosurus vulpecula).Crossref | GoogleScholarGoogle Scholar |
Moseby, KE, and Hill, BM (2011). The use of poison baits to control feral cats and red foxes in arid South Australia I. Aerial baiting trials. Wildlife Research 38, 338–349.
| The use of poison baits to control feral cats and red foxes in arid South Australia I. Aerial baiting trials.Crossref | GoogleScholarGoogle Scholar |
Moseby, KE, and Read, JL (2006). The efficacy of feral cat, fox and rabbit exclusion fence designs for threatened species protection. Biological Conservation 127, 429–437.
| The efficacy of feral cat, fox and rabbit exclusion fence designs for threatened species protection.Crossref | GoogleScholarGoogle Scholar |
Moseby, KE, Letnic, M, Blumstein, DT, and West, R (2019). Understanding predator densities for successful co-existence of alien predators and threatened prey. Austral Ecology 44, 409–419.
| Understanding predator densities for successful co-existence of alien predators and threatened prey.Crossref | GoogleScholarGoogle Scholar |
Moseby, KE, McGregor, H, and Read, JL (2020). Effectiveness of the Felixer grooming trap for the control of feral cats: a field trial in arid South Australia. Wildlife Research 47, 599–609.
| Effectiveness of the Felixer grooming trap for the control of feral cats: a field trial in arid South Australia.Crossref | GoogleScholarGoogle Scholar |
Parkes, J, Fisher, P, Robinson, S, and Aguirre-Muñoz, A (2014). Eradication of feral cats from large islands: an assessment of the effort required for success. New Zealand Journal of Ecology 38, 307–314.
Read, J, Gigliotti, F, Darby, S, and Lapidge, S (2014). Dying to be clean: pen trials of novel cat and fox control devices. International Journal of Pest Management 60, 166–172.
| Dying to be clean: pen trials of novel cat and fox control devices.Crossref | GoogleScholarGoogle Scholar |
Read, JL, Bowden, T, Hodgens, P, Hess, M, McGregor, H, and Moseby, K (2019). Target specificity of the felixer grooming “trap”. Wildlife Society Bulletin 43, 112–120.
| Target specificity of the felixer grooming “trap”.Crossref | GoogleScholarGoogle Scholar |
Read, JL, Dickman, CR, Boardman, WSJ, and Lepczyk, CA (2020). Reply to Wolf et al.: why trap-neuter-return (TNR) is not an ethical solution for stray cat management. Animals 10, 1525.
| Reply to Wolf et al.: why trap-neuter-return (TNR) is not an ethical solution for stray cat management.Crossref | GoogleScholarGoogle Scholar |
Robertson, SA (2008). A review of feral cat control. Journal of Feline Medicine and Surgery 10, 366–375.
| A review of feral cat control.Crossref | GoogleScholarGoogle Scholar |
Schmidt, PM, Swannack, TM, Lopez, RR, and Slater, MR (2009). Evaluation of euthanasia and trap-neuter-return (TNR) programs in managing free-roaming cat populations. Wildlife Research 36, 117–125.
| Evaluation of euthanasia and trap-neuter-return (TNR) programs in managing free-roaming cat populations.Crossref | GoogleScholarGoogle Scholar |
Short, J, Turner, B, Risbey, DA, and Carnamah, R (1997). Control of feral cats for nature conservation. II. Population reduction by poisoning. Wildlife Research 24, 703–714.
| Control of feral cats for nature conservation. II. Population reduction by poisoning.Crossref | GoogleScholarGoogle Scholar |
Short, J, Turner, B, and Risbey, D (2002). Control of feral cats for nature conservation. III. Trapping. Wildlife Research 29, 475–487.
| Control of feral cats for nature conservation. III. Trapping.Crossref | GoogleScholarGoogle Scholar |
Twigg, LE, Martin, GR, Eastman, AF, King, the late DR, and Kirkpatrick, WE (2003). Sensitivity of some Australian animals to sodium fluoroacetate (1080): additional species and populations, and some ecological considerations. Australian Journal of Zoology 51, 515–531.
| Sensitivity of some Australian animals to sodium fluoroacetate (1080): additional species and populations, and some ecological considerations.Crossref | GoogleScholarGoogle Scholar |
Wallach, AD, Bekoff, M, Batavia, C, Nelson, MP, and Ramp, D (2018). Summoning compassion to address the challenges of conservation. Conservation Biology 32, 1255–1265.
| Summoning compassion to address the challenges of conservation.Crossref | GoogleScholarGoogle Scholar |
Woinarski, JCZ (2016). National context for the conservation fate of Victoria’s mammal fauna. The Victorian Naturalist 133, 74–78.
Woinarski, JCZ, Burbidge, AA, and Harrison, PL (2015). Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences of the United States of America 112, 4531–4540.
| Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement.Crossref | GoogleScholarGoogle Scholar |
Woinarski JCZ, Legge SM, Dickman CR (2019) ‘Cats in Australia: companion and killer.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Wolf, PJ, and Schaffner, JE (2019). The road to TNR: examining trap-neuter-return through the lens of our evolving ethics. Frontiers in Veterinary Science 5, 341.
| The road to TNR: examining trap-neuter-return through the lens of our evolving ethics.Crossref | GoogleScholarGoogle Scholar |


