Factors influencing the activity ranges of feral pigs (Sus scrofa) across four sites in eastern Australia
Cameron Wilson A B * , Matthew Gentle B C and Darren Marshall D
A B * , Matthew Gentle B C and Darren Marshall D
A Animal Biosecurity and Welfare, Biosecurity Queensland, Department of Agriculture and Fisheries, Bundaberg, Qld 4670, Australia.
B Pest Animal Research Centre, Biosecurity Queensland, Department of Agriculture and Fisheries, Toowoomba, Qld 4350, Australia.
C School of Sciences, University of Southern Queensland, Toowoomba, Qld 4350, Australia.
D Southern Queensland Landscapes, Toowoomba, Qld 4350, Australia.
Wildlife Research 50(11) 876-889 https://doi.org/10.1071/WR22095
Submitted: 31 May 2022 Accepted: 2 December 2022 Published: 16 January 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Understanding the home-range size and the ecological drivers that influence the spatial distribution of feral pigs is of paramount importance for exotic-disease modelling and the improvement of pest management programs.
Aims: To investigate various factors affecting home- and core-range size and test selection of habitat, to better inform disease modelling and pest management programs.
Methods: In this study, 59 GPS-collared feral pigs were tracked over four sites in eastern Australia between 2017 and 2021. Using minimum convex polygon (MCP) and the nearest-neighbour–local convex hull (k-LoCoH) as home-range estimators and foliage projective cover (FPC) as an estimator of landscape-scale shelter, we investigated the influence of sex, site, season, year and body weight on range size and tested selection of habitat by using chi-squared and Jacob’s index tests.
Key results: Home-range sizes were highly variable, with k-LoCoH90 (home) ranges between 0.08 and 54.97 km2 and k-LoCoH50 (core) ranges between 0.01 and 7.02 km2. MCP90 ranged between 0.15 and 242.30 km2, with MCP50 being between 0.07 and 60.61 km2. Sex and site both significantly (P < 0.001) influenced home-range size, but season and year did not. Home-range size was shown to increase with body mass for both sexes (P = 0.001). Importantly, the data indicated that feral pigs prefer habitat within 20–40% FPC (woodland), whereas open forests (51–80% FPC) and closed forests (>80% FPC) were actively avoided. Typically, use of open vegetation (1–10% FPC) was also avoided, but this behaviour varied and was dependent on site.
Conclusion: Feral pig ranges are influenced by sex, site and body mass but not by season and year. Broad-scale selection for shelter indicated that feral pigs prefer habitat between 20% and 40% FPC.
Implications: Targeting or avoiding such areas respectively for control or monitoring tool placement may result in improved, efficient outcomes to monitor or manage feral pig populations. Feral pig distribution modelling may also find benefit in the consideration and further study of the above factors and the influence of food and water sources on the activity ranges and behaviour of feral pigs.
Keywords: activity range, African swine fever, core range, disease modelling, feral pig, foliage projective cover, habitat selection, home range, k-LoCoH, MCP, pest management.
Introduction
Feral pigs (Sus scrofa) are a significant vertebrate pest, both in Australia and around the world. Despite control efforts, the distribution of feral pigs in Australia continues to expand through either natural dispersal (Saunders and McLeod 1999; Hone 2002; Cowled et al. 2009) or through anthropogenic means (Spencer and Hampton 2005). Their habits and distribution translate to wide-ranging impacts to the environment, agricultural economy and to human health. Feral pigs can damage important ecosystems through the dispersal of invasive plants (Lynes and Campbell 2000; Setter et al. 2002), the destruction of wetland habitats and water quality (Mitchell 2010), the predation on and/or competition with native animals (Fordham et al. 2006) and through the disruption of native plant establishment and dispersal (Hone 2002; Mitchell et al. 2007; Webber et al. 2010; Taylor et al. 2011). Feral pigs have been demonstrated to predate on young lambs (Pavlov et al. 1981; Choquenot et al. 1997) and selectively feed on crop species (Gentle et al. 2015), potentially resulting in significant economic impacts (Bradhurst et al. 2015). Feral pigs are also hosts for a variety of diseases, both zoonotic (Eales et al. 2010) and non-zoonotic (Ward et al. 2007; Chenais et al. 2019), which may result in human health concerns (Massey et al. 2011).
The recent outbreak of African swine fever (ASF) throughout Eurasia has resulted in severe economic losses (World Organisation for Animal Health 2021). An incursion of the disease in Australia could cost upwards of AUD$2 billion (ACIL Allen Consulting 2019). Despite strong biosecurity legislation and stakeholder awareness, there remains a considerable risk of wild pig populations complicating disease eradications by acting as viral reservoirs and spreading disease through both direct and indirect contact with domestic populations (Guinat et al. 2016; Miller et al. 2017; Podgórski et al. 2018; VanderWaal and Deen 2018; VerCauteren et al. 2018; Bradhurst et al. 2021; Animal Health Australia 2022). As a result, it is imperative that government and industry gain an improved understanding of the feral pig-related aspects of ASF disease ecology in Australia to support ASF preparedness and response strategies.
Epidemiological modelling is a useful tool for understanding the influence of different factors on the dynamics of a pathogen, and the potential effect of various prevention or management interventions on transmission, infection patterns and persistence in animal populations (Garner and Beckett 2005; Garner et al. 2007; Harvey et al. 2007). This approach is particularly useful for formulating preparation and policy guidelines for rare or exotic diseases where field data are limited (Bradhurst et al. 2021). The Australian animal disease spread (AADIS) modelling framework simulates the spread and control of emergency animal diseases (EADs) such as ASF (Bradhurst et al. 2021) and incorporates aspects of and interactions between domestic and feral pig populations. The AADIS–ASF model has been adapted to local conditions considering key disease parameters from international ASF outbreaks and a range of ecological data from Australian and international wild pig populations. The current iteration uses measures based on the movement of female pigs (sounders), but separate parameters for modelling males may be required where substantially different. Understanding the geospatial and ecological drivers that influence feral pig populations is a critical factor in modelling disease spread (Cowled and Garner 2008) and model refinement using field-based data will improve the accuracy and precision of model output. These drivers incorporate the distribution, density, movement, social and age structure of feral pig populations as well as the productivity of suitable habitat, climatic effects and the presence of alternate hosts (Pech and McIlroy 1990; Caley 1993; Choquenot et al. 1996; Kern et al. 1999; McCallum et al. 2001; Morgan et al. 2006; Cowled and Garner 2008).
However, suitable distribution and movement data from Australian feral pig populations is currently limited. While there have been studies assessing movement ecology in Australian feral pig populations (Saunders and Kay 1991; Saunders and Kay 1996; Caley 1997; Dexter 1999; Mitchell et al. 2009), the more recent availability and improvements in GPS technology provide a far greater quantity of high-quality data. This technology provides the means to more accurately quantify ranging behaviour (e.g. daily and/or seasonal home range and movements across a range of environments) and examine the factors that may influence such movements. There is also very little data examining the fine-scale habitat use of feral pig distributions across Australia and the geospatial relationships between key habitat types and/or focal points (e.g. water sources) to assist modellers to better predict connectivity of pig populations (Cowled and Garner 2008).
In addition to informing disease preparedness, spatial modelling data support wider feral pig management decision-making. Effective control of vertebrate pests to mitigate their impacts requires a strategic, integrated approach that considers the ecology of the targeted species. The refinement of pest management strategies through the application of ecological intelligence could significantly improve existing feral pig control methods (Nogueira et al. 2007), resulting in more efficient and effective mitigation of impacts. Maximising control tool encounter rates, by identifying and targeting focal sites with a high probability of pig use, may increase effectiveness but with concomitant reduction in effort and cost (Recio et al. 2017). Understanding how these focal sites are affected by various biological or environmental factors could enable even greater refinement of control strategies.
It is currently understood that the home range of female feral pigs is negatively correlated with landscape productivity (Dexter 1999; Clontz et al. 2022), whereas male pigs maintain ranges that maximise access to breeding females (Dexter 1999). As such, male ranges are typically larger than female ranges (Saunders and Kay 1991; Saunders and Kay 1996; Caley 1997; Dexter 1999; Mitchell 2002), although there are exceptions (e.g. Mitchell et al. (2009). Home range of pigs may also be influenced by body weight (Saunders and McLeod 1999) and climatic conditions (Baber and Coblentz 1986; Hone 1987; Mitchell et al. 2009), with various environmental factors influencing behaviour. Garza et al. (2018) found that mammal species richness was positively correlated with feral pig home-range size, although this, in turn, may be linked to other environmental factors such as latitude and elevation (Garza et al. 2018) and may be more relevant to overseas environs.
Habitat selection has long been recognised as a useful tool in wildlife science (Neu et al. 1974). Feral pig habitat selection (preference/avoidance) in Australia has been examined using empirical field data (Saunders and Kay 1991; Caley 1997; Dexter 1999) and, more recently, Bayesian network modelling of expert knowledge has been applied to identify habitat suitability for feral pigs (Froese et al. 2017). However, these approaches could be refined given their reliance on site-specific assessments or expert knowledge, which may not be transferable to other areas. Foliage projective cover (FPC) is a quantitative measure of canopy cover density that is correlated with protection from heat and possibly disturbance. Because pigs lack sweat glands, they are restricted to shadier habitats during hot weather (Dexter 1999); thus, habitats with higher FPC values may be preferred. FPC can be quantified in all habitat types, allowing for comparison across sites, reducing potential errors from variable classification and nomenclature of landscape types. The application of FPC as a proxy for habitat type and as a measure of landscape-based shelter may allow for a higher accuracy in determining habitat preference and may indicate areas of higher use within the landscape.
Quantifying key parameters for feral pig spatial ecology is critical for applications of spatial modelling, disease modelling or management, and to inform future research on both broad-scale and fine-scale habitat preference. A deeper understanding of the factors that influence feral pig ranging behaviour and activity levels may also allow for modellers or practitioners to tailor pig management strategies that consider temporal, environmental or biological factors. Using GPS location data, this research quantifies the site, and seasonal and annual home range of feral pigs across four study areas in eastern Australia. Key factors influencing home-range size, specifically sex, site, season, year, bodyweight and habitat selection, are examined and the implications for management and future research are discussed.
Methods
Data collection
Data were collected from 59 feral pigs collared at four sites in eastern Australia, namely, Arcadia Valley, Downfall Creek, Gebar Island (Queensland) and Palerang (New South Wales), between 2017 and 2021. Details of pigs across all sites is displayed in Supplementary material Table S1. Feral pigs were captured through a combination of box, panel or corral traps, with a pre-feeding period of approximately 7–10 days or until an asymptote of feed consumption was achieved. Wheat (dry or fermented) was used as a lure, with the occasional additive (molasses or carasweet). Captured pigs were sedated with an intramuscular injection of Zoletil (Virbac Australia Pty Ltd, Milperra, NSW, Australia) at a dosage rate of 1 mg kg−1. Pigs were collared with Lotek Iridiumtrack Heavy Duty 3D collars (Lotek, Ontario, Canada), programmed to take a fix every 30 min and all data were remotely downloaded via their online portal. Animal ethics permits were approved by the University of New England (AEC 16-115 and AEC 20-023).
Study sites
Arcadia Valley is an ~15 km wide valley stretching between Carnarvon and Expedition National Parks, in the Central Highlands region of Queensland (Fig. 1). Land use is predominately free-range cattle grazing with scattered small-scale feedlots. Our study site extended over 960 km2 of open grassland with small pockets of eucalyptus woodland. It has a subtropical climate (Bureau of Meteorology 2022a), with mean annual rainfall of 635.8 mm (Bureau of Meteorology 2022e).
Downfall Creek was our study site in the Western Downs region of Queensland, covering an area of ~1380 km2 from Wandoan to Miles, bordering the Barakula State Forest. This study site is used for natural gas extraction and cattle production. Downfall Creek consists of mostly eucalypt medium woodland or open grassland, experiences a subtropical climate (Bureau of Meteorology 2022a) and a mean annual rainfall of 643.4 mm (Bureau of Meteorology 2022d).
Gebar Island is a small (~4.2 km2) uninhabited island in the Torres Strait, Queensland, that is owned by the Gebaralgal (Torres Strait Islanders) Corporation (National Native Title Tribunal 2004). It is a tropical island with an equatorial climate (Bureau of Meteorology 2022a), with the nearby Coconut Island receiving a mean annual rainfall of 1441.7 mm (Bureau of Meteorology 2022b).
Palerang is a locality in southern New South Wales, east of Canberra. Our study site (~777 km2) consisted mostly of open grassland interspersed with open eucalypt forests and the site is mostly used for livestock production. This area of Australia experiences a temperate climate (Bureau of Meteorology 2022a) and a mean annual rainfall of 624.4 mm (Canberra) (Bureau of Meteorology 2022c).
Collar-accuracy test
To determine a protocol for identifying and removing inaccurate points, 13 locations of deceased pigs were investigated. These locations were typified by large clusters of points in close proximity to one another because of variations in recorded locations prior to the collar being retrieved. The mean location across all points at each site was considered as the ‘true’ location of each deceased pig. We defined all points less than or equal to 20 m away from this location as being accurate, because this distance is unlikely to change the habitat type where the pig is present at, at that time. All points greater than 20 m away were investigated for their potential cause of error. A comparison of filtering on direct quality indicators [i.e. Bjørneraas non-movement model; Bjørneraas et al. (2010)] and indirect quality indicators [i.e. dilution of precision (DOP) values following Fancourt et al. (2021)] was investigated. Where the Bjørneraas model identifies and removes unrealistic ‘spikes’ in the data (Bjørneraas et al. 2010), the DOP value is the measure of satellite configuration relative to the GPS collar at the time of location fix (D’eon and Delparte 2005) and is often used as an indicator of accuracy (owing to the ease of obtaining a value) with varying success (Ironside et al. 2017). As supporting parameters of the Bjørneraas model (i.e. speed and turning angles) were not available for feral pigs, we used the 90th percentile threshold as a substitute, as recommended by Gupte et al. (2022). By comparing the Bjørneraas model and various DOP values to the points generated from the stationary pigs in this project, we determined a procedure for cleansing data of inaccurate points (>20 m), beyond which datapoint accuracy was unreliable for the purposes of our research questions.
Data cleaning
To ensure acclimatisation of animals to the collars, only locations recorded after 2 days following collar attachment were examined further. To clean the dataset of inaccurate points, all failed points (those with a zero in latitude or longitude) were deleted. All points considered ‘inaccurate’ through the collar-accuracy test were also deleted. Finally, a visual inspection of the remaining points on ArcMap® was conducted to identify and remove points that were erroneous or obvious outliers (e.g. 3 km out to sea), or not biologically plausible (e.g. 28 km movement outside their range between successive points in 30 min).
Home/core range
To represent the home range, or area utilised by each animal for normal foraging activities (Burt 1943), two methods were chosen, the minimum convex polygon (MCP) and the nearest-neighbour–local convex hull (k-LoCoH). The MCP method is a simple and practical method but has poor resolution to the true home range of the targeted species (Burgman and Fox 2003; Getz and Wilmers 2004; Wilson 2020). However, its wide usage allows for uniformity in comparisons with other studies. The nearest-neighbour local convex hull (k-LoCoH) is a more refined estimator than are other methods (Getz and Wilmers 2004) and has been shown to demonstrate low error rates with high conformity to utilised landscape features (Wilson 2020). The 90% isopleth was chosen to represent the home range, because of an increase in bias in higher isopleths (Börger et al. 2006). Similarly, because accuracy declines below the 50% isopleth (Börger et al. 2006), this value was chosen to represent the core range. The core range represents the highest density of points within the smallest area, thereby highlighting key areas of high activity (Edwards et al. 2001; Wilson 2020). Pigs were excluded from the analysis if they had less than 160 observations (four fixes/day over 40 days), so as to ensure sufficient sampling for convergence with the true extent of the home range (Leo et al. 2016). For seasonal range changes, only animals that had more than 28 observations (four fixes/day over 7 days) were used to present an indication of range size over that period.
Minimum convex polygon
MCP areas for each pig were estimated for the 50% and 90% isopleths by using the adehabitatHR package (Calenge 2006) in R (v. 4.0.5; see https://cran.r-project.org/). Four fixes per day (0200; 0800; 1400; 2000) were used in home range and core ranges analyses to avoid the influence of spatial auto-correlation. This temporal range of fixes allows a regular array of locations spanning night, morning, afternoon and evening. Home ranges were estimated for the entire monitored period (overall), each year (annual) and each season (autumn, winter, spring or summer). Calculated MCP values are displayed with pig details in Table S1.
Nearest-neighbour–local convex hull
The nearest-neighbour–local convex hull (k-LoCoH) areas for each pig were estimated for the 50% and 90% isopleths by using the adehabitatHR package (Calenge 2006) in R (v. 4.0.5). As with the MCP, only four fixes per day were used and ranges were estimated overall, annually and seasonally to test for changes. The default parameter of the square root of the number of points was used, but where polygon errors (e.g. orphaned holes, etc.) caused the script to fail, a value of at least five was added to the parameter value (k). This parameter influences the number of neighbouring locations each point is associated with for the creation of hulls (Getz and Wilmers 2004). Adjusting the k-value is akin to the ‘minimum spurious hole covering’ rule in Getz and Wilmers (2004). The utilised k-value was recorded with the estimated area and is displayed with pig details in Table S1.
Home- and core-range statistics
All range estimations were log-transformed (log10) to conform to normality prior to analysis. A Student’s unpaired t-test was conducted in R (v, 4.0.5) to compare overall range sizes between male and female pigs (Zar 2014). An ANOVA was conducted to determine whether range size varied by study site, season and year (Zar 2014). A linear regression model was used to determine the significance of body mass in influencing the activity range of feral pigs (Zar 2014). Both the dependent (activity range) and independent variables (mass) were log-transformed to conform to normality. All statistical tests were calculated through the ‘stats’ package (R Core Team 2021) or ‘car’ package (Fox and Weisberg 2019) in R (R Core Team 2021).
Habitat preference
Foliage projective cover data were chosen to represent landscape-scale shelter for feral pigs. For the three Queensland sites, FPC data (raster with 30 m resolution) was downloaded from the Long Paddock website (The State of Queensland 2021). For Palerang (New South Wales), FPC data (raster with 25 m resolution) was accessed through the Department of Planning, Industry and Environment (NSW Government 2021). To calculate the habitat available to each pig, the MCP100 was calculated for each pig to encompass the entire landscape used. The original FPC raster layer was cropped to the MCP100 range for each pig and converted to an ESRI shapefile by using R packages ‘raster’ (Hijmans 2021), ‘rgdal’ (Bivand et al. 2021), ‘sp’ (Pebesma and Bivand 2005) and ‘sf’ (Pebesma 2018). According to their percentage foliage cover, individual attributes were grouped into FPC ranges with increments of 10% and the total area within each FPC group was calculated to provide a proportion of each FPC group within the pig’s home range. These proportions were then used to estimate the expected proportion of GPS points per FPC group. Observed points per group were calculated through an intersection tool (‘sp::over’) in R (Pebesma and Bivand 2005). Points that fell within an area of ‘no data’ (i.e. in an area of no recorded data or a waterbody) were removed from the analysis. Following McDonald (2014), FPC ranges with an expected value of less than five were also removed from the analysis.
To investigate preference, proportional use or avoidance of habitat, the observed and expected proportions of points within each FPC range were compared through the chi-squared goodness-of-fit (GOF) test (following Saunders and Kay (1991) and Fernanda Cuevas et al. (2013) and the Jacob’s index (Jacobs 1974; as per Moseby et al. 2021). Using a Dunn–Sidak corrected α-value of 0.00087, a chi-squared test was estimated using the ‘stats::chisq.test’ function in R (R Core Team 2021). The median residual per FPC range was used to avoid the skewing of averages by extreme values and each residual was inspected for preferential use. Any residual greater than two was considered as ‘preference’, less than negative two as ‘avoidance’ and between two and negative two as ‘proportional’. All pigs that demonstrated an insignificance in the chi-squared test were also removed from the Jacob’s index test and for further uniformity, the median Jacob’s index (JI) per FPC range was also calculated. To distinguish among strong, normal and weak selection, the JI output was classified as being ‘strong preference’ (≥0.75), ‘preference’ (≥0.45 and <0.75), ‘weak preference’ (≥0.15 and <0.45), ‘used in proportion’ (>−0.15 and <0.15), ‘weak avoidance’ (≤−0.15 and >−0.45), ‘avoidance’ (≤−0.45 and >−0.75) and ‘strong avoidance’ (≤−0.75). Pigs may demonstrate preferential selection or avoidance for more than one FPC range.
Results
Collar-accuracy test
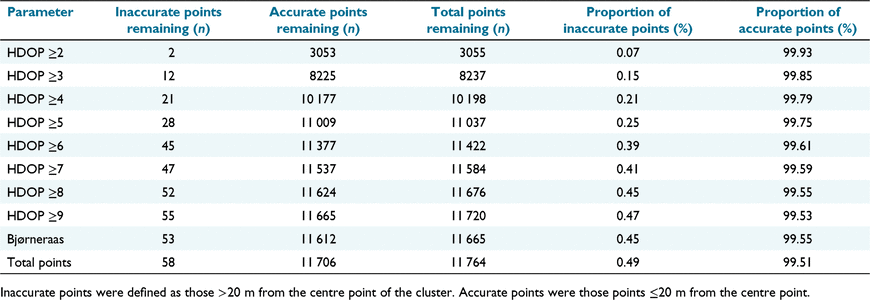
The Bjørneraas non-movement model (Bjørneraas et al. 2010) and DOP values (D’eon and Delparte 2005) were investigated as potential means of cleaning data of inaccurate points. Using the 90th percentile threshold for speed and turning angle (Gupte et al. 2022), the Bjørneraas model removed fewer accurate points than did DOP ≥5, but removed just 8.6% of inaccurate points (compared to 51.7% of inaccurate points by DOP ≥5), resulting in a dataset with a lower proportion of accurate points (see Table 1). Higher removal of inaccurate points was possible with a lower DOP value but with the corresponding sacrifice of too many accurate points (DOP ≥5 retained 94% of accurate points). We thus used the DOP ≥5 as an acceptable proxy of locational error, resulting in a final dataset that contained 99.75% accurate points.
|
Home range size
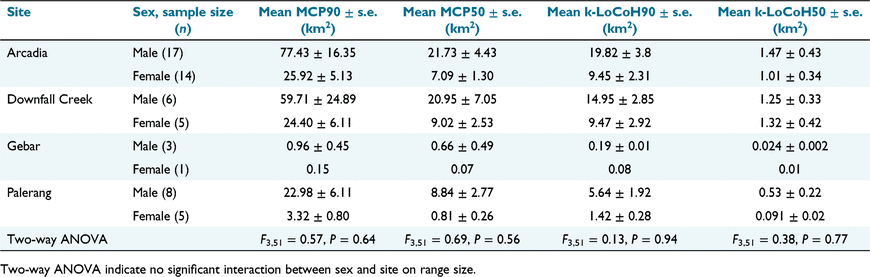
The mean male and female home- and core ranges are displayed in Table 2.
|
The mean Arcadia home ranges for both sexes (pooled) were >17% larger than in any other site, while their core ranges were marginally smaller than those at Downfall Creek. Feral pigs at the Palerang site had considerably smaller home and core ranges than did pigs at located at both Arcadia or Downfall Creek. Pigs on Gebar Island had by far the smallest home and core ranges. Study site was the most important factor affecting home- and core-range sizes (all methods: P = <0.001). We also found a statistically significant difference in range size between sexes (MCP90, MCP50, k-LoCoH90: P = <0.001), with the exception of k-LoCoH50 (P = 0.07), although the interaction between the study site and sex was not significant for either method or isopleth (P = >0.05). The large standard errors also indicate a large variation in the size of the ranges across the sample.
Home ranges by body mass (kg)
Linear regressions of log-transformed mass to log-transformed activity range (Fig. 2) for both 90% and 50% range isopleths showed a positive association. The most significant relationship was demonstrated by the MCP90 (n = 25, r2 = 0.36, P = 0.001) for female pigs and (n = 34, r2 = 0.25, P = 0.001) for male pigs. The least significant relationship was demonstrated by k-LoCoH50, which demonstrated gentler slopes and weaker r2-values. The k-LoCoH50 demonstrated y = −6.06 + 3.17x (n = 25, r2 = 0.18, P = 0.02) for female pigs and y = −3.11 + 1.52x (n = 34, r2 = 0.10, P = 0.04) for male pigs. Both methods and isopleths indicated a critical point where regression lines of male and female pigs intersect. Inverse logarithms of these points indicated a body mass of between 69 and 82 kg for home ranges and 62 and 87 kg for core ranges where female pig ranges will surpass that of similarly sized males. Multiple linear regressions examining interactions between body mass and site found no significant interactions (P = >0.05) across either method, isopleth or sex. When split into weight categories of 25 kg increments, male pigs >100 kg demonstrated significantly larger k-LoCoH90 ranges (F3,30 = 3.07, P = 0.04) than did all smaller pigs. However, there was no significant difference between the sizes of the core range (k-LoCoH50) (F3,30 = 1.17, P = 0.34).
Home ranges by season and year
There was considerable variation in the seasonal home-range size among different sites and sexes. Using the k-LoCoH90 home-range size, mean feral pig ranges appear to be marginally larger in autumn for male pigs at Arcadia and Downfall Creek and larger in summer at Gebar and winter at Palerang. For female pigs, autumn displayed larger ranges at all sites except Palerang (winter). Summer at Arcadia and Palerang represented considerably smaller ranges for male pigs, whereas for the other two sites, spring represented the smallest ranges. Winter (Downfall Creek and Gebar), spring (Arcadia) and summer (Palerang) displayed the smallest ranges for female pigs. Despite these seasonal changes, k-LoCoH90 did not demonstrate a significance in home range-size change through interactions between season and site (F9,149 = 0.45, P = 0.91), nor between season and sex (F3,157 = 0.37, P = 0.77). No method or isopleth indicated a significant change in range size through these interactions. There was also no significant difference indicated among years, nor any interaction among sex, site and year on activity-range size (MCP90; F4,68 = 1.42, P = 0.23).
Habitat preference
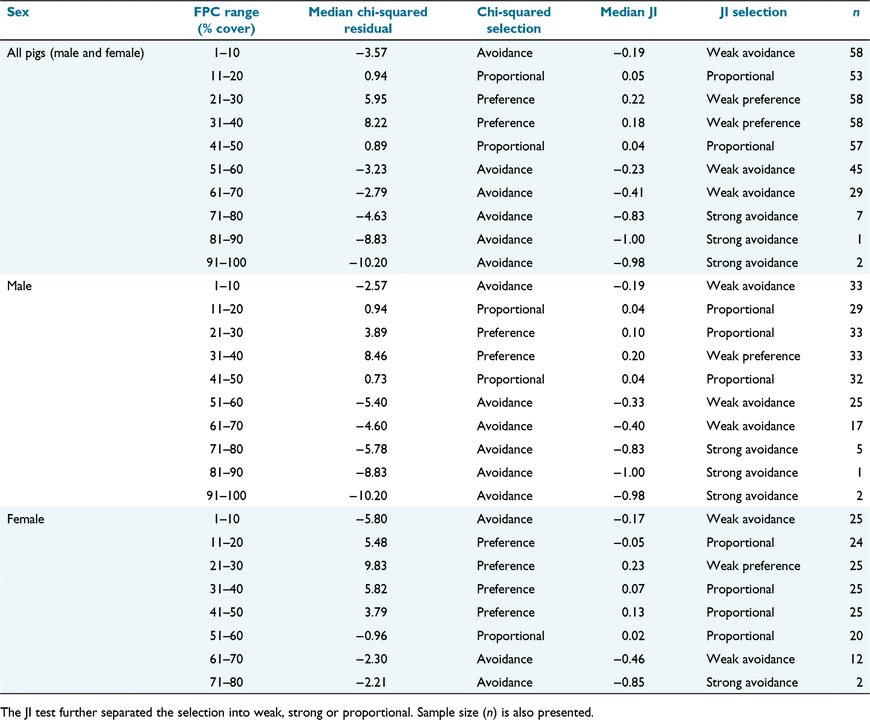
Through the chi-squared test, 58 of the 59 pigs in this study demonstrated a significance in habitat selection. Residual medians of both the chi-squared test and Jacob’s index for all pigs, male and female, are presented in Table 3.
|
Across all pigs, both methods indicated a preferential selection of habitat between 21% and 40% FPC, whereas the ranges immediately below (11–20% FPC) and above (41–50% FPC) were used in proportion to their availability. All other FPC ranges indicated avoidance, with the JI demonstrating a strong avoidance for habitat, with an FPC value of >70%. Overall, 91% (chi-squared) and 81% (JI) of pigs demonstrated a preference for one or more FPC groups within the range of 11–50% FPC. Both methods indicated that female pigs have a marginally wider range of proportional or preferential use (11–60% FPC) than do males. Although males potentially have a wider range of habitat available because of their greater home-range size, they are slightly more selective, either preferring or using habitat in proportion to its availability between 11% and 50% FPC. There were some slight differences among sites, with the vast majority of pigs at Arcadia (97%), Palerang (92%), Downfall Creek (82%) and Gebar (75%) demonstrating a preference for habitat within 11–50% FPC. Across all pigs, 57% (chi-squared) and 47% (JI) demonstrated an avoidance of very open habitat (1–10% FPC), with no statistical difference between the sexes (P = 0.72 (chi-squared), P = 0.75 (JI)]. However, there was a greater disparity among sites for the use of open habitat (F5,55 = 4.25, P = 0.002), with 68% pigs at Arcadia and 50% at Palerang demonstrating avoidance, whereas at Downfall and Gebar, 55% and 75% (respectively) demonstrated a preference for this habitat type. Almost half (43%, chi-squared, and 46%, JI) of all pigs actively avoided habitat containing the highest FPC density that is available to them. In all circumstances, the resultant JI was more conservative in determining preference than was the chi-squared test, but where the JI indicated the strongest preference (in this case, weak), the corresponding chi-squared residual median was the highest or second-highest presented (Table 3). ANOVA tests indicated that there was no significant difference (P = >0.05) between the proportions of retained and deleted points (removed during our cleaning process) for all FPC values, except for 51–60% FPC (P = 0.016), where a slightly higher proportion was deleted than retained.
Discussion
Feral pig range sizes were highly variable, with both home and core ranges being significantly affected by study site and bodyweight, but not by season or year. Sex significantly influenced MCP90, k-LoCoH90 and MCP50 ranges but did not significantly influence k-LoCoH50 ranges. Across all sites, feral pigs preferentially used habitat with 20–40% FPC (woodland), proportionally used habitats of 11–20% FPC and 41–50% FPC and tended to avoid habitats with all other FPC ranges. Identifying the critical factors affecting feral pig home-range size and habitat preference are informative for monitoring, management, and modelling of feral pig populations, and to guide future research.
The findings indicated study site as the most significant factor in determining feral pig home- and core-range sizes. These results support the hypothesis that the considerable variation demonstrated both herein and across previous Australian studies (Saunders and Kay 1991; Saunders and Kay 1996; Caley 1997; Dexter 1999; Mitchell et al. 2009) reflects differences in landscape productivity (Singer et al. 1981; Dexter 1999; Bengsen et al. 2016; Clontz et al. 2022). Female range size is believed to be negatively scaled to landscape productivity (Singer et al. 1981; Bengsen et al. 2016), whereas male ranges are scaled positively to the range size of coexisting females (Dexter 1999). The limitation of island size and the more compact resources in tropical island biomes such as Gebar Island (this study) and Hawaiian Islands (Salbosa and Lepczyk 2009) are likely to influence more compact range sizes. In contrast, the likely lower productive areas of Downfall Creek and Arcadia coincide with considerably larger ranges in the feral pigs studied. Paradoxically, previous home-range estimates from pigs in semi-arid regions of New South Wales (NSW; Dexter 1999) have shown considerably smaller ranges than our study sites (excluding Gebar), although this difference is likely to be due to differences in data-collection quantity and quality (i.e. radio-telemetry vs GPS technology). Similarly, there are disparities between our Palerang site and other studies in similar sites in southern NSW, with both sexes in Saunders and Kay (1996) and female pigs in Saunders and Kay (1991) demonstrating considerably larger home ranges (MCP) than in our study. Even though both site and sex have significant effects on home-range size, the lack of significant interaction between site and sex supports the theory that relationships between the sexes is not different per site. Because home-range size is highly site-specific, generalising or using mean values generated across vastly different areas may not be appropriate where more accurate estimates are critical, such as in disease-spread modelling. Alternatively, feral pig distribution modelling may benefit from factoring in landscape productivity, through the use of a metric of vegetative vigour such as in Campbell et al. (2021) or through more complicated Bayesian networks (Froese et al. 2017).
With the exception of Mitchell et al. (2009), all other published Australian feral pig-range studies have demonstrated a significant range-size difference between sexes (Saunders and Kay 1991, 1996; Caley 1997; Dexter 1999). The larger ranges demonstrated by males in this study (P = <0.001) and others are likely to be related to their biological desire to actively seek out breeding females. Regardless, this suggests that focussing distribution models exclusively on sounder behaviour may lead to the model underestimating true feral pig movements and home-range sizes. Although females are more gregarious (Choquenot et al. 1996), the typically larger ranges of males may mean greater distribution and, therefore, greater inter-sounder connectivity than for females. Dexter (1996) also suggested that exotic-disease spread may result from a combination of simple diffusion and through long-distance animal movements. Our analysis, across both range methods and isopleths, indicated that range size increases as a function of body mass. This may be related to the greater energetic requirement of larger animals (Harestad and Bunnel 1979) and supports the findings of Saunders and McLeod (1999) and Mitchell et al. (2009). However, the high standard errors and a less robust r2-value observed in this study than in that of Saunders and McLeod (1999) suggests that there may be other, more influential, factors affecting range size at the four study sites. Differences in range size as a function of the interaction between study site and body mass yielded no significant interaction across either sex, method or isopleth. This suggests that while body mass and study site individually influence range size, the effect of body mass on range size is not different per site. Differences in range size per site are likely to be influenced by extrinsic factors such as landscape composition and productivity rather than body mass. The demonstration that large male pigs (>100 kg) have significantly larger home (k-LoCoH90) ranges while having core (k-LoCoH50) ranges similar-sized to those of smaller males, suggests that resources within the core range support basic needs (food, water, shelter), whereas an extended home range is influenced by other factors such as higher sexual motivation (Singer et al. 1981; Caley 1997; Dexter 1999). Linear regressions across both methods and isopleths indicated that both the home- and core-range sizes of female feral pigs with a body mass of greater than 62 kg will surpass that of similarly sized males, suggesting that larger females may require larger range sizes, and hence, resources to meet their needs. However, this result may have been affected by the higher number of males (n = 30) than females (n = 17) greater than 62 kg in this dataset rather than a biological feature; therefore, further investigation to confirm the findings is recommended for future study.
Seasonal range fluctuations across all pigs were evident, yet statistically insignificant in this study (Fig. 3). Variations in home-range size among seasons appear to be site-dependent. Larger winter ranges were evident at Palerang and in Saunders and Kay (1991) and Dexter (1999), which is indicative of greater spatial distribution in cooler seasons, owing to lower requirements for water and protection from heat (Dexter 1999). Saunders and Kay (1991) suggested that management programs are ‘best implemented in the winter when movement within the population is greatest’. However, this also means that they are more widely distributed, which may influence control tool encounter rates, if control tool volume and spatial distribution are not increased in proportion to the relative home-range size. In our study, autumn typically produced larger ranges, corresponding with the results of Caley (1997), Massei et al. (1997) and Dexter (1999). There was no uniform season where ranges were condensed, neither in our study nor others (Saunders and Kay 1991; Caley 1997; Massei et al. 1997; Dexter 1999; Mitchell et al. 2009), suggesting that variations in seasonal ranges across different sites and studies is influenced by other factors. Varying climatic conditions (e.g. unpredictable rainfall, ambient air temperatures, abundance of and distance to food, water and shelter) are more likely to influence range size than is a particular month or season (Heitman and Hughes 1949; Dexter 1999; Kay et al. 2017). In periods of unseasonal weather, using averaged seasonal predictors may affect the outcome. In this situation, lagged meteorological conditions may be a more appropriate variable than is month or season (Kay et al. 2017). However, the application of site-specific abiotic factors (e.g. rainfall, food abundance) may prove challenging for broad-scale modelling. Similarly, the changes seen among years appear to be highly individualistic and not a factor of sex or site, and are probably a result of factors not investigated here (e.g. breeding, sexual competition, food availability, hunting or other disturbances).
|
|
Habitat selection based on FPC should be a considered as a covariate in future modelling of habitat assessments, and to assist in determining strategies for feral pig control. We found a significant preference for habitat with 21–40% FPC and a proportional usage of habitat with 11–20% and 41–50% FPC. In Australia, closed forests are classified as those where tree canopies (FPC) cover more than 80% of the land area, open forest as 51–80%, woodland forest as 20–50% and non-forest carrying other woody vegetation <20% (ABARES 2013). Feral pigs in this study indicated a preferential use of woodland forest, whereas actively avoiding open forests and closed forests. This finding contradicts other Australian studies on habitat use, which found that pigs avoid open woodland and woodland areas but show a preference for open and closed forests (Saunders and Kay 1991; Caley 1997). This discrepancy may result from the small sample size and high error rate associated with radio-tracking in previous studies compared with the significantly larger dataset and highly accurate GPS locations in this study. Caley (1997) noted that the radio-tracking error rate is close to the width of some habitats and therefore use may be underestimated. However, more recent modelling studies have also predicted woodlands to be unsuitable habitat for breeding (in dry seasons) for feral pigs (Froese et al. 2017). However, this is likely to be site-dependent and, with fewer parameters examined, this study was limited to shelter only. Saunders and Kay (1991) also suggested that concentration of effort at the interface between heavy vegetation and open pasture may improve control/monitoring tool encounter rates. Further research into ecotone use and finer-scale habitat use, using large, fine-resolution datasets across sites, is required. It is unlikely that the disproportionate removal of high DOP-value points from specific FPC categories during our cleaning process affected the habitat preference results, because all but one FPC range (51–60%) indicated an insignificant (P = >0.05) difference between the proportion of retained and deleted points within each FPC range.
Site-specific differences in habitat use may be related to the distribution of resources at each site, which was not accounted for in this study. For example, at Gebar, pigs appear to forage for food in inter-tidal zones, hence demonstrating a higher preference for open habitat, owing to this site-specific food availability. This study clearly indicated that feral pigs avoid open forest and avoid (chi-squared) or strongly avoid (JI) closed forests. It is possible that habitats with higher density canopies do not provide significantly greater shelter than do lower FPC-range habitats, while also yielding lower food and water availability. This may not be the case in some habitats (e.g. wet tropical rainforests) and future work may be required to understand this better. The use of FPC is appropriate for examining landscape-based shelter for feral pigs but does not account for other components of the landscape that may affect how an animal uses the landscape. Future work to identify and determine the influence of detailed food- and water-source availability on habitat preference will enable refinement of feral pig landscape-use data to better inform modelling and control programs.
This research has quantified feral pig home ranges across four sites in eastern Australia, and assessed the influence of site, sex, season and bodyweight on home-range size. The preference for habitat with 20–40% FPC indicated that targeting such areas may result in improved feral pig control or monitoring at significantly reduced cost. Both home-range and habitat-use analyses provide insights into factors that influence feral pig activity ranges for further consideration in distribution modelling and refining control strategies. It is also evident from this study and others that feral pig home range is highly variable and likely to be dependent on a multitude of factors, limiting the appropriateness of a ‘one-size-fits-all’ approach to home-range analyses. Understanding the ecological drivers and using this intelligence will allow us to tailor our approaches according to biotic and abiotic predictors. Further studies to understand these factors and how they influence feral pig behaviour is critical to both appropriately model disease spread (Cowled and Garner 2008) and to inform feral pig control programs.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study were obtained from Southern Queensland Landscapes by permission. Data will be shared upon reasonable request to the corresponding author with permission from Southern Queensland Landscapes.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Fieldwork was supported by funding provided by the Queensland Murray Darling Committee (QMDC), Santos GNLG, Arrow Energy, Southern Queensland Landscapes, South East Local Land Services and the NSW Environmental Trust. Spatial modelling analysis was undertaken as part of the Queensland state government funded African swine fever prevention and preparedness project.
Acknowledgements
All field work and data collected was completed by DM; CW analysed the data and led the writing of the manuscript; MG assisted in data analysis, project design and manuscript writing. Thanks to Peter Elsworth, Robyn Grob and our anonymous reviewer for comments that improved the paper.
References
ABARES (2013) Australia’s state of the forests report. ABARES, Canberra, ACT, Australia.ACIL Allen Consulting (2019) ‘Economic analysis of African swine fever incursion into Australia.’ (Prepared for Australian Pork Limited, ACIL Allen Consulting: Melbourne, Vic., Australia)
Animal Health Australia (2022) ‘Response strategy: African swine fever (version 5.1).’ 5th edn. (Australian Veterinary Emergency Plan (AUSVETPLAN): Canberra, ACT, Australia)
Baber, DW, and Coblentz, BE (1986). Density, home range, habitat use, and reproduction in feral pigs on Santa Catalina Island. Journal of Mammalogy 67, 512–525.
| Density, home range, habitat use, and reproduction in feral pigs on Santa Catalina Island.Crossref | GoogleScholarGoogle Scholar |
Bengsen, AJ, Algar, D, Ballard, G, Buckmaster, T, Comer, S, Fleming, PJS, Friend, JA, Johnston, M, McGregor, H, Moseby, K, and Zewe, F (2016). Feral cat home-range size varies predictably with landscape productivity and population density. Journal of Zoology 298, 112–120.
| Feral cat home-range size varies predictably with landscape productivity and population density.Crossref | GoogleScholarGoogle Scholar |
Bivand R, Keitt T, Rowlingson B (2021) rgdal: bindings for the ‘geospatial’ data abstraction library. Version 1.5-23. Available at https://CRAN.R-project.org/package=rgdal
Bjørneraas, K, Van Moorter, B, Rolandsen, CM, and Herfindal, I (2010). Screening global positioning system location data for errors using animal movement characteristics. The Journal of Wildlife Management 74, 1361–1366.
| Screening global positioning system location data for errors using animal movement characteristics.Crossref | GoogleScholarGoogle Scholar |
Börger, L, Franconi, N, De Michele, G, Gantz, A, Meschi, F, Manica, A, Lovari, S, and Coulson, T (2006). Effects of sampling regime on the mean and variance of home range size estimates. Journal of Animal Ecology 75, 1393–1405.
| Effects of sampling regime on the mean and variance of home range size estimates.Crossref | GoogleScholarGoogle Scholar |
Bradhurst, RA, Roche, SE, East, IJ, Kwan, P, and Garner, MG (2015). A hybrid modeling approach to simulating foot-and-mouth disease outbreaks in Australian livestock. Frontiers in Environmental Science 3, 17.
| A hybrid modeling approach to simulating foot-and-mouth disease outbreaks in Australian livestock.Crossref | GoogleScholarGoogle Scholar |
Bradhurst RA, Garner G, Roche SE, Iglesias R, Kung N, Robinson B, Willis S, Cozens M, Richards K, Cowled BD, Oberin M, Tharle C, Fireston S, Stevenson M (2021) Modelling the spread and control of African swine fever in domestic and feral pigs. Technical report for CEBRA project 20121501. University of Melbourne, Melbourne, Vic., Australia.
Bureau of Meteorology (2022a) Climate classification maps. Available at http://www.bom.gov.au/jsp/ncc/climate_averages/climate-classifications/index.jsp?maptype=kpngrp#maps [Accessed 8 April 2022]
Bureau of Meteorology (2022b) Summary statisitcs Coconut Island. Available at http://www.bom.gov.au/climate/averages/tables/cw_027054.shtml [Accessed 8 April 2022]
Bureau of Meteorology (2022c) Summary statistics Canberra airport. Available at http://www.bom.gov.au/climate/averages/tables/cw_070351.shtml [Accessed 8 April 2022]
Bureau of Meteorology (2022d) Summary statistics Miles Post Office. Available at http://www.bom.gov.au/climate/averages/tables/cw_042023.shtml [Accessed 8 April 2022]
Bureau of Meteorology (2022e) Summary statistics Rolleston. Available at http://www.bom.gov.au/climate/averages/tables/cw_035059.shtml [Accessed 8 April 2022]
Burgman, MA, and Fox, JC (2003). Bias in species range estimates from minimum convex polygons: implications for conservation and options for improved planning. Animal Conservation 6, 19–28.
| Bias in species range estimates from minimum convex polygons: implications for conservation and options for improved planning.Crossref | GoogleScholarGoogle Scholar |
Burt, WH (1943). Territoriality and home range concepts as applied to mammals. Journal of Mammalogy 24, 346–352.
| Territoriality and home range concepts as applied to mammals.Crossref | GoogleScholarGoogle Scholar |
Calenge, C (2006). The package ‘adehabitat’ for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling 197, 516–519.
| The package ‘adehabitat’ for the R software: a tool for the analysis of space and habitat use by animals.Crossref | GoogleScholarGoogle Scholar |
Caley, P (1993). Population dynamics of feral pigs (Sus scrofa) in a tropical riverine habitat complex. Wildlife Research 20, 625–636.
| Population dynamics of feral pigs (Sus scrofa) in a tropical riverine habitat complex.Crossref | GoogleScholarGoogle Scholar |
Caley, P (1997). Movements, activity patterns and habitat use of feral pigs (Sus scrofa) in a tropical habitat. Wildlife Research 24, 77–87.
| Movements, activity patterns and habitat use of feral pigs (Sus scrofa) in a tropical habitat.Crossref | GoogleScholarGoogle Scholar |
Campbell, HA, Loewensteiner, DA, Murphy, BP, Pittard, S, and McMahon, CR (2021). Seasonal movements and site utilisation by Asian water buffalo (Bubalus bubalis) in tropical savannas and floodplains of northern Australia. Wildlife Research 48, 230–239.
| Seasonal movements and site utilisation by Asian water buffalo (Bubalus bubalis) in tropical savannas and floodplains of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Chenais, E, Depner, K, Guberti, V, Dietze, K, Viltrop, A, and Ståhl, K (2019). Epidemiological considerations on African swine fever in Europe 2014–2018. Porcine Health Management 5, 6.
| Epidemiological considerations on African swine fever in Europe 2014–2018.Crossref | GoogleScholarGoogle Scholar |
Choquenot D, McIlroy J, Korn T (1996) ‘Managing vertebrate pests: feral pigs.’ (Bureau of Resource Sciences: Canberra, ACT, Australia)
Choquenot, D, Lukins, B, and Curran, G (1997). Assessing lamb predation by feral pigs in Australia’s semi-arid rangelands. Journal of Applied Ecology 34, 1445–1454.
| Assessing lamb predation by feral pigs in Australia’s semi-arid rangelands.Crossref | GoogleScholarGoogle Scholar |
Clontz, LM, Pepin, KM, VerCauteren, KC, and Beasley, JC (2022). Influence of biotic and abiotic factors on home range size and shape of invasive wild pigs (Sus scrofa). Pest Management Science 78, 914–928.
| Influence of biotic and abiotic factors on home range size and shape of invasive wild pigs (Sus scrofa).Crossref | GoogleScholarGoogle Scholar |
Cowled, B, and Garner, G (2008). A review of geospatial and ecological factors affecting disease spread in wild pigs: considerations for models of foot-and-mouth disease spread. Preventive Veterinary Medicine 87, 197–212.
| A review of geospatial and ecological factors affecting disease spread in wild pigs: considerations for models of foot-and-mouth disease spread.Crossref | GoogleScholarGoogle Scholar |
Cowled, BD, Giannini, F, Beckett, SD, Woolnough, A, Barry, S, Randall, L, and Garner, G (2009). Feral pigs: predicting future distributions. Wildlife Research 36, 242–251.
| Feral pigs: predicting future distributions.Crossref | GoogleScholarGoogle Scholar |
D’eon, RG, and Delparte, D (2005). Effects of radio-collar position and orientation on GPS radio-collar performance, and the implications of PDOP in data screening. Journal of Applied Ecology 42, 383–388.
| Effects of radio-collar position and orientation on GPS radio-collar performance, and the implications of PDOP in data screening.Crossref | GoogleScholarGoogle Scholar |
Dexter, N (1996). The effect of an intensive shooting exercise from a helicopter on the behaviour of surviving feral pigs. Wildlife Research 23, 435–441.
| The effect of an intensive shooting exercise from a helicopter on the behaviour of surviving feral pigs.Crossref | GoogleScholarGoogle Scholar |
Dexter, N (1999). The influence of pasture distribution, temperature and sex on home-range size of feral pigs in a semi-arid environment. Wildlife Research 26, 755–762.
| The influence of pasture distribution, temperature and sex on home-range size of feral pigs in a semi-arid environment.Crossref | GoogleScholarGoogle Scholar |
Eales, KM, Norton, RE, and Ketheesan, N (2010). Brucellosis in northern Australia. The American Journal of Tropical Medicine and Hygiene 83, 876–878.
| Brucellosis in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Edwards, GP, De Preu, N, Shakeshaft, BJ, Crealy, IV, and Paltridge, RM (2001). Home range and movements of male feral cats (Felis catus) in a semiarid woodland environment in central Australia. Austral Ecology 26, 93–101.
| Home range and movements of male feral cats (Felis catus) in a semiarid woodland environment in central Australia.Crossref | GoogleScholarGoogle Scholar |
Fancourt, BA, Augusteyn, J, Cremasco, P, Nolan, B, Richards, S, Speed, J, Wilson, C, and Gentle, MN (2021). Measuring, evaluating and improving the effectiveness of invasive predator control programs: feral cat baiting as a case study. Journal of Environmental Management 280, 111691.
| Measuring, evaluating and improving the effectiveness of invasive predator control programs: feral cat baiting as a case study.Crossref | GoogleScholarGoogle Scholar |
Fernanda Cuevas, M, Ojeda, RA, and Jaksic, FM (2013). Multi-scale patterns of habitat use by wild boar in the Monte Desert of Argentina. Basic and Applied Ecology 14, 320–328.
| Multi-scale patterns of habitat use by wild boar in the Monte Desert of Argentina.Crossref | GoogleScholarGoogle Scholar |
Fordham, D, Georges, A, Corey, B, and Brook, BW (2006). Feral pig predation threatens the indigenous harvest and local persistence of snake-necked turtles in northern Australia. Biological Conservation 133, 379–388.
| Feral pig predation threatens the indigenous harvest and local persistence of snake-necked turtles in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Fox J, Weisberg S (2019) ‘An R companion to applied regression.’ 3rd edn. (Sage)
Froese, JG, Smith, CS, Durr, PA, McAlpine, CA, and van Klinken, RD (2017). Modelling seasonal habitat suitability for wide-ranging species: invasive wild pigs in northern Australia. PLoS ONE 12, e0177018.
| Modelling seasonal habitat suitability for wide-ranging species: invasive wild pigs in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Garner, MG, and Beckett, SD (2005). Modelling the spread of foot-and-mouth disease in Australia. Australian Veterinary Journal 83, 758–766.
| Modelling the spread of foot-and-mouth disease in Australia.Crossref | GoogleScholarGoogle Scholar |
Garner, MG, Dubé, C, Stevenson, MA, Sanson, RL, Estrada, C, and Griffin, J (2007). Evaluating alternative approaches to managing animal disease outbreaks: the role of modelling in policy formulation. Veterinaria Italiana 43, 285–298.
Garza, SJ, Tabak, MA, Miller, RS, Farnsworth, ML, and Burdett, CL (2018). Abiotic and biotic influences on home-range size of wild pigs (Sus scrofa). Journal of Mammalogy 99, 97–107.
| Abiotic and biotic influences on home-range size of wild pigs (Sus scrofa).Crossref | GoogleScholarGoogle Scholar |
Gentle, M, Speed, J, and Marshall, D (2015). Consumption of crops by feral pigs (Sus scrofa) in a fragmented agricultural landscape. Australian Mammalogy 37, 194–200.
| Consumption of crops by feral pigs (Sus scrofa) in a fragmented agricultural landscape.Crossref | GoogleScholarGoogle Scholar |
Getz, WM, and Wilmers, CC (2004). A local nearest-neighbor convex-hull construction of home ranges and utilization distributions. Ecography 27, 489–505.
| A local nearest-neighbor convex-hull construction of home ranges and utilization distributions.Crossref | GoogleScholarGoogle Scholar |
Guinat, C, Gogin, A, Blome, S, Keil, G, Pollin, R, Pfeiffer, DU, and Dixon, L (2016). Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Veterinary Record 178, 262–267.
| Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions.Crossref | GoogleScholarGoogle Scholar |
Gupte, PR, Beardsworth, CE, Spiegel, O, Lourie, E, Toledo, S, Nathan, R, and Bijleveld, AI (2022). A guide to pre-processing high-throughput animal tracking data. Journal of Animal Ecology 91, 287–307.
| A guide to pre-processing high-throughput animal tracking data.Crossref | GoogleScholarGoogle Scholar |
Harestad, AS, and Bunnel, FL (1979). Home range and body weight: a reevaluation. Ecology 60, 389–402.
| Home range and body weight: a reevaluation.Crossref | GoogleScholarGoogle Scholar |
Harvey, N, Reeves, A, Schoenbaum, MA, Zagmutt-Vergara, FJ, Dubé, C, Hill, AE, Corso, BA, McNab, WB, Cartwright, CI, and Salman, MD (2007). The North American Animal Disease Spread Model: a simulation model to assist decision making in evaluating animal disease incursions. Preventive Veterinary Medicine 82, 176–197.
| The North American Animal Disease Spread Model: a simulation model to assist decision making in evaluating animal disease incursions.Crossref | GoogleScholarGoogle Scholar |
Heitman, H, and Hughes, EH (1949). The effects of air temperature and relative humidity on the physiological well being of swine. Journal of Animal Science 8, 171–181.
| The effects of air temperature and relative humidity on the physiological well being of swine.Crossref | GoogleScholarGoogle Scholar |
Hijmans RJ (2021) raster: geographic data analysis and modeling. R package version 3.4-13. Available at https://CRAN.R-project.org/package=raster
Hone J (1987) Theoretical and practical aspects of feral pig control. PhD. thesis, Australian National University.
Hone, J (2002). Feral pigs in Namadgi National Park, Australia: dynamics, impacts and management. Biological Conservation 105, 231–242.
| Feral pigs in Namadgi National Park, Australia: dynamics, impacts and management.Crossref | GoogleScholarGoogle Scholar |
Ironside, KE, Mattson, DJ, Arundel, TR, and Hansen, JR (2017). Is GPS telemetry location error screening beneficial? Wildlife Biology 2017, 1–7.
| Is GPS telemetry location error screening beneficial?Crossref | GoogleScholarGoogle Scholar |
Jacobs, J (1974). Quantitative measurement of food selection. Oecologia 14, 413–417.
| Quantitative measurement of food selection.Crossref | GoogleScholarGoogle Scholar |
Kay, SL, Fischer, JW, Monaghan, AJ, Beasley, JC, Boughton, R, Campbell, TA, Cooper, SM, Ditchkoff, SS, Hartley, SB, Kilgo, JC, Wisely, SM, Wyckoff, AC, VerCauteren, KC, and Pepin, KM (2017). Quantifying drivers of wild pig movement across multiple spatial and temporal scales. Movement Ecology 5, 14.
| Quantifying drivers of wild pig movement across multiple spatial and temporal scales.Crossref | GoogleScholarGoogle Scholar |
Kern, B, Depner, KR, Letz, W, Rott, M, Thalheim, S, Nitschke, B, Plagemann, R, and Liess, B (1999). Incidence of classical swine fever (CSF) in wild boar in a densely populated area indicating CSF. Virus persistence as a mechanism for virus perpetuation. Journal of Veterinary Medicine, Series B 46, 63–68.
| Incidence of classical swine fever (CSF) in wild boar in a densely populated area indicating CSF. Virus persistence as a mechanism for virus perpetuation.Crossref | GoogleScholarGoogle Scholar |
Leo, BT, Anderson, JJ, Phillips, RB, and Ha, RR (2016). Home range estimates of feral cats (Felis catus) on Rota Island and determining asymptotic convergence. Pacific Science 70, 323–331.
| Home range estimates of feral cats (Felis catus) on Rota Island and determining asymptotic convergence.Crossref | GoogleScholarGoogle Scholar |
Lynes, BC, and Campbell, SD (2000). Germination and viability of mesquite (Prosopis pallida) seed following ingestion and excretion by feral pigs (Sus scrofa). Tropical Grasslands 34, 125–128.
Massei, G, Genov, PV, Staines, BW, and Gorman, ML (1997). Factors influencing home range and activity of wild boar (Sus scrofa) in a Mediterranean coastal area. Journal of Zoology 242, 411–423.
| Factors influencing home range and activity of wild boar (Sus scrofa) in a Mediterranean coastal area.Crossref | GoogleScholarGoogle Scholar |
Massey, PD, Polkinghorne, BG, Durrheim, DN, Lower, T, and Speare, R (2011). Blood, guts and knife cuts: reducing the risk of swine brucellosis in feral pig hunters in north-west New South Wales, Australia. Rural and Remote Health 11, 1793.
| Blood, guts and knife cuts: reducing the risk of swine brucellosis in feral pig hunters in north-west New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
McCallum, H, Barlow, N, and Hone, J (2001). How should pathogen transmission be modelled? Trends in Ecology & Evolution 16, 295–300.
| How should pathogen transmission be modelled?Crossref | GoogleScholarGoogle Scholar |
McDonald JH (2014) ‘Handbook of biological statistics.’ 3rd edn. (Sparky House Publishing: Baltimore, MD, USA)
Miller, RS, Sweeney, SJ, Slootmaker, C, Grear, DA, Di Salvo, PA, Kiser, D, and Shwiff, SA (2017). Cross-species transmission potential between wild pigs, livestock, poultry, wildlife, and humans: implications for disease risk management in North America. Scientific Reports 7, 7821.
| Cross-species transmission potential between wild pigs, livestock, poultry, wildlife, and humans: implications for disease risk management in North America.Crossref | GoogleScholarGoogle Scholar |
Mitchell JL (2002) Ecology and management of feral pigs (Sus scrofa) in rainforests. PhD thesis, James Cook University, Townsville, Qld, Australia.
Mitchell J (2010) Experimental research to quantify the environmental impact of feral pigs within tropical freshwater ecosystems. Final report to the Department of the Environment, Water, Heritage and the Arts, Canberra, ACT, Australia.
Mitchell, J, Dorney, W, Mayer, R, and McIlroy, J (2007). Ecological impacts of feral pig diggings in north Queensland rainforests. Wildlife Research 34, 603–608.
| Ecological impacts of feral pig diggings in north Queensland rainforests.Crossref | GoogleScholarGoogle Scholar |
Mitchell, J, Dorney, W, Mayer, R, and McIlroy, J (2009). Migration of feral pigs (Sus scrofa) in rainforests of north Queensland: fact or fiction? Wildlife Research 36, 110–116.
| Migration of feral pigs (Sus scrofa) in rainforests of north Queensland: fact or fiction?Crossref | GoogleScholarGoogle Scholar |
Morgan, ER, Lundervold, M, Medley, GF, Shaikenov, BS, Torgerson, PR, and Milner-Gulland, EJ (2006). Assessing risks of disease transmission between wildlife and livestock: the Saiga antelope as a case study. Biological Conservation 131, 244–254.
| Assessing risks of disease transmission between wildlife and livestock: the Saiga antelope as a case study.Crossref | GoogleScholarGoogle Scholar |
Moseby, KE, Read, JL, and Andersen, GE (2021). Goat movement patterns inform management of feral goat populations in semiarid rangelands. Wildlife Research 48, 44–54.
| Goat movement patterns inform management of feral goat populations in semiarid rangelands.Crossref | GoogleScholarGoogle Scholar |
National Native Title Tribunal (2004) Extract from the National Native Title Register. Federal Court Number(s): QUD6066/1998. (Federal Court of Australia) Available at http://www.nntt.gov.au/searchRegApps/NativeTitleRegisters/NNTR%20Extracts/QCD2004_008/NNTRExtract_QCD2004_008.pdf [Accessed 8 April 2022]
Neu, CW, Byers, CR, and Peek, JM (1974). A technique for analysis of utilization-availability data. The Journal of Wildlife Management 38, 541–545.
| A technique for analysis of utilization-availability data.Crossref | GoogleScholarGoogle Scholar |
Nogueira, SSdC, Nogueira-Filho, SLG, Bassford, M, Silvius, K, and Fragoso, JMV (2007). Feral pigs in Hawai‘i: using behavior and ecology to refine control techniques. Applied Animal Behaviour Science 108, 1–11.
| Feral pigs in Hawai‘i: using behavior and ecology to refine control techniques.Crossref | GoogleScholarGoogle Scholar |
NSW Government (2021) Landsat woody extent and foliage projective cover (FPC) Ver 2.1 (25m) 2008. Available at https://datasets.seed.nsw.gov.au/dataset/landsat-woody-extent-and-foliage-projective-cover-fpc-ver-2-1-25m-20087355d [Accessed 16 April 2021]
Pavlov, PM, Hone, J, Kilgour, RJ, and Pedersen, H (1981). Predation by feral pigs on Merino lambs at Nyngan, New South Wales. Australian Journal of Experimental Agriculture 21, 570–574.
| Predation by feral pigs on Merino lambs at Nyngan, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Pebesma, E (2018). Simple features for R: standardized support for spatial vector data. The R Journal 10, 439–446.
| Simple features for R: standardized support for spatial vector data.Crossref | GoogleScholarGoogle Scholar |
Pebesma, EJ, and Bivand, RS (2005). Classes and methods for spatial data in R. R News 5, 9–13.
Pech, RP, and McIlroy, JC (1990). A model of the velocity of advance of foot and mouth disease in feral pigs. Journal of Applied Ecology 27, 635–650.
| A model of the velocity of advance of foot and mouth disease in feral pigs.Crossref | GoogleScholarGoogle Scholar |
Podgórski, T, Apollonio, M, and Keuling, O (2018). Contact rates in wild boar populations: implications for disease transmission. The Journal of Wildlife Management 82, 1210–1218.
| Contact rates in wild boar populations: implications for disease transmission.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2021) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria)
Recio, MR, Maloney, RF, Mathieu, R, Virgós, E, Moore, AB, and Seddon, PJ (2017). Optimizing control programmes by integrating data from fine-scale space use by introduced predators. Biological Invasions 19, 209–221.
| Optimizing control programmes by integrating data from fine-scale space use by introduced predators.Crossref | GoogleScholarGoogle Scholar |
Salbosa L-L, Lepczyk C (2009) Analysis of feral pig (Sus scrofa) movement in a Hawaiian forest ecosystem using GPS satellite collars. Nature Precedings 2009. 10.1038/npre.2009.3903.1
Saunders, G, and Kay, B (1991). Movements of feral pigs (Sus scrofa) at Sunny Corner, New South Wales. Wildlife Research 18, 49–61.
| Movements of feral pigs (Sus scrofa) at Sunny Corner, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Saunders, G, and Kay, B (1996). Movements and home ranges of feral pigs (Sus scrofa) in Kosciusko National Park, New South Wales. Wildlife Research 23, 711–719.
| Movements and home ranges of feral pigs (Sus scrofa) in Kosciusko National Park, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Saunders, G, and McLeod, S (1999). Predicting home range size from the body mass or population densities of feral pigs, Sus scrofa (Artiodactyla: Suidae). Australian Journal of Ecology 24, 538–543.
| Predicting home range size from the body mass or population densities of feral pigs, Sus scrofa (Artiodactyla: Suidae).Crossref | GoogleScholarGoogle Scholar |
Setter M, Bradford M, Dorney B, Lynes B, Mitchell J, Setter S, Westcott D (2002) Pond apple: are the endangered cassowary and feral pig helping this weed to invade Queensland’s wet tropics. In ‘Proceedings of Australian Weeds Conference volume 13’. pp. 173–176.
Singer, FJ, Otto, DK, Tipton, AR, and Hable, CP (1981). Home ranges, movements, and habitat use of European wild boar in Tennessee. The Journal of Wildlife Management 45, 343–353.
| Home ranges, movements, and habitat use of European wild boar in Tennessee.Crossref | GoogleScholarGoogle Scholar |
Spencer, PBS, and Hampton, JO (2005). Illegal translocation and genetic structure of feral pigs in Western Australia. The Journal of Wildlife Management 69, 377–384.
| Illegal translocation and genetic structure of feral pigs in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Taylor, DL, Leung, LK-P, and Gordon, IJ (2011). The impact of feral pigs (Sus scrofa) on an Australian lowland tropical rainforest. Wildlife Research 38, 437–445.
| The impact of feral pigs (Sus scrofa) on an Australian lowland tropical rainforest.Crossref | GoogleScholarGoogle Scholar |
The State of Queensland (2021) The long paddock. Available at https://www.longpaddock.qld.gov.au/forage/ [Accessed 16 April 2021]
VanderWaal, K, and Deen, J (2018). Global trends in infectious diseases of swine. Proceedings of the National Academy of Sciences of the United States of America 115, 11495–11500.
| Global trends in infectious diseases of swine.Crossref | GoogleScholarGoogle Scholar |
VerCauteren, KC, Lavelle, MJ, and Campa, H (2018). Persistent spillback of bovine tuberculosis from white-tailed deer to cattle in Michigan, USA: status, strategies, and needs. Frontiers in Veterinary Science 5, 301.
| Persistent spillback of bovine tuberculosis from white-tailed deer to cattle in Michigan, USA: status, strategies, and needs.Crossref | GoogleScholarGoogle Scholar |
Ward, MP, Laffan, SW, and Highfield, LD (2007). The potential role of wild and feral animals as reservoirs of foot-and-mouth disease. Preventive Veterinary Medicine 80, 9–23.
| The potential role of wild and feral animals as reservoirs of foot-and-mouth disease.Crossref | GoogleScholarGoogle Scholar |
Webber, BL, Norton, BA, and Woodrow, IE (2010). Disturbance affects spatial patterning and stand structure of a tropical rainforest tree. Austral Ecology 35, 423–434.
| Disturbance affects spatial patterning and stand structure of a tropical rainforest tree.Crossref | GoogleScholarGoogle Scholar |
Wilson C (2020) Spatial ecology of feral cats (Felis catus) and the implications for effective management. Honours thesis, University of New England, Armidale, NSW, Australia.
World Organisation for Animal Health (2021) African swine fever. Available at https://www.oie.int/en/disease/african-swine-fever/ [Accessed 11 November 2021]
Zar JH (2014) ‘Biostatistical analysis.’ 5th edn. (Pearson Education Limited: Essex, UK)


