Habitat use and survival of the endangered northern bettong (Bettongia tropica) after prescribed fire
Christopher A. Pocknee A D * , Sarah M. Legge
A D * , Sarah M. Legge  B , Jane McDonald C and Diana Fisher A
B , Jane McDonald C and Diana Fisher A
A
B
C
D Present address:
Abstract
Changed fire regimes play a role in the decline of many species, and prescribed fire is now commonly used as a management tool to restore or maintain habitat. The northern bettong (Bettongia tropica) is an endangered Australian mammal that has been lost from most of its previous range. Northern bettongs remain in only two locations, a core population of ~1000 at our study site and <20 individuals at their only other known location. In some locations, fire suppression over the past 200 years, typically for asset protection, appears to have reduced the suitability of the bettong’s preferred grassy woodland habitat. Prescribed fire is now being used as a tool to manage northern bettong habitat, to maintain open structure and promote grass growth; however, there is limited evidence about how prescribed fire affects bettongs in the short term. Food and shelter sources that northern bettongs rely on may be affected by fire. They are also in the critical weight range of Australian mammals that are prone to cat predation, which can be exacerbated by fire.
We aimed to test how northern bettongs respond to low-to-medium severity fires intended to improve habitat structure for the species.
We deployed 20 GPS collars on northern bettongs across two field seasons to obtain data on home range, active area, and nesting areas before and after fire. We performed mark–recapture surveys before and after fire to compare population density, fitness measures, and demography.
Bettongs shifted their nesting areas following fire to incorporate more unburned habitat, and there was no change to their active area. Bettongs’ overall home ranges do not shift following a low-to-medium severity fire, consistent with their food sources’ resistance to fire.
Northern bettongs can shift their nesting areas to unburned grassy patches. This supports the belief that northern bettongs are well-adapted to low-severity fires, but high-severity fire may result in a lack of appropriate nesting areas.
In sites where the northern bettong is in critically low numbers, the short-term risk from low-to-medium severity prescribed fire aimed at longer-term habitat improvement appears low.
Keywords: conservation, critical weight range, fire ecology, land management, mammal, potoroidae, prescribed fire, spatial ecology, threatened species, wet tropics.
Introduction
Fire is the dominant natural disturbance in many ecosystems worldwide (Bond et al. 2005; Bowman et al. 2009). Many ecological communities have evolved with, and are adapted to, particular fire regimes. The rapid environmental and climatic changes that are characteristic of the Anthropocene have influenced fire frequency and severity in many areas, with detrimental effects on biodiversity (Russell-Smith and Yates 2007; Jolly et al. 2015; Lindenmayer et al. 2020).
In the past 230 years, at least 33 Australian mammal species have become extinct, accounting for more than one-third of modern mammal extinctions (Woinarski et al. 2019; Legge et al. 2023). Extensive or severe fire is a current or potential threat to 80% of Australian mammal species and subspecies listed as Critically Endangered, Endangered or Vulnerable, for which information on threats are available through approved national threatened species listings (Environment Protection and Biodiversity Conservation Act 1999). Additionally, species not considered threatened by severe fires may become so as their frequency increases (Legge et al. 2022). The most reported impact of fire regimes on mammals is changed habitat suitability, rather than direct mortality (Letnic 2003; Legge et al. 2008; Jolly et al. 2022).
Species responses to fire can be unpredictable, owing to the interaction of factors such as vegetation structure, fire extent and severity, species biology and interspecific interactions (Leahy et al. 2015; Swan et al. 2016; Legge et al. 2019). Synergistic interactions with other threats can occur, including feral cats exploiting recent fire scars to hunt more effectively, with species falling within the critical weight range (CWR; 35–5500 g) being particularly susceptible (Burbidge and Mckenzie 1989; McGregor et al. 2014, 2016). Nevertheless, fire management is an increasingly common tool for conservation in Australia. Land managers are responding to evidence that appropriate prescribed fire regimes can improve habitat quality for threatened mammals, reduce predation by introduced predators, and increase both density and abundance of native species (Legge et al. 2011; McDonald et al. 2016; Flanagan-Moodie et al. 2018). In northern Australia, fires at the end of the wet season and early in the dry season typically burn with low severity, almost exclusively burn the ground layer or lower mid-storey and leave unburned patches of habitat (Wysong et al. 2021). Early season fire can be applied at small spatial scales to form habitat mosaics across a landscape, where patches of different fire ages provide habitat for a suite of mammals (Radford et al. 2015, 2020; McDonald et al. 2016).
For individual animals, the characteristics of a fire at the scale of a home range may determine their response (Pocknee et al. 2023). An animal’s home range size is predominantly driven by its energy requirements (Kelt and Van Vuren 2001). If resources are scarce or an animal’s energy requirements increase, it will need a larger area to obtain the resources to meet these demands (Prestrud 1992; Edwards et al. 2013; Bengsen et al. 2016; Stobo-Wilson et al. 2021). Immediately and shortly after fire, an individual’s home range may increase in size or shift in response to changed food availability or habitat structure (Murphy et al. 2010; Herzog et al. 2014; Cheyne et al. 2019). Recent fire will make nesting areas less secure for species that rely on flammable ground-layer vegetation or fallen timber for shelter, so nesting portions of the home range may change (Vernes and Pope 2001; Körtner et al. 2007).
The short-to-medium-term response (immediately to 6 months post-fire) of mammal populations to fire will also vary with the species’ ecology (Pocknee et al. 2023). Even species that use a common resource (e.g. grass) in different ways (e.g. food v. shelter) will show different responses to a fire (Doherty et al. 2022; Pocknee et al. 2023). Many grasses are advantaged by, and respond quickly to, fire (Linder et al. 2018). Rodents that shelter in grass have been recorded to decline in abundance following fire, predominantly through increased predation owing to the decreased grass cover (Leahy et al. 2015; Manyonyi et al. 2020). Conversely, species that rely on grass as a food source, often show increased abundance at a site following fire, owing to availability of fresh growth soon after fire (Eby et al. 2014; Chard et al. 2022; Pocknee et al. 2023). For example, Sharman’s rock-wallabies (Petrogale sharmani) tracked across an experimental fire did not shift their shelter ranges, because they shelter in rocks, but they did shift their foraging ranges into burned areas following fire to exploit new grass growth (Hayes 2019).
Species with food sources that are slower to recover after fire may show negative effects of fire relating to body condition or breeding success, rather than short-term abundance. The agile gracile opossum (Gracilinanus agilis) eats a range of fruits and showed a decrease in female body condition and breeding success following fire (Rossi and Leiner 2023). The Australian bush rat (Rattus fuscipes) has also demonstrated a decline in body condition after fire, which is likely to be due to a loss of food and shelter (Fordyce et al. 2016). Animals may also shift their diet in response to a change in availability of food. Radford (2012) found that northern quolls (Dasyurus hallucatus) and golden bandicoots (Isoodon auratus) consumed more large prey following fire, presumably owing to easier hunting conditions in a post-fire landscape. Where fire is expected to lead to medium-term gains in habitat quality, land managers must weigh up the risks associated with any deleterious short-term impacts of fire on species of interest.
In north-eastern Australia, the Australian Wet Tropics contain a diverse range of habitats supporting unique biodiversity. The rainforests and sclerophyll forests of this region each support different communities of flora and fauna, and the ecotone between these two forest types is historically dynamic (Harrington and Sanderson 1994; Wilf and Kooyman 2025). A range of threatened and endemic species are found in each forest type, and each have suffered significant losses since European colonisation of the region. Fire is used as a management tool to maintain the current boundary and prevent rainforest species establishing in areas that currently contain sclerophyll forest, although the widespread use of fire for this purpose is somewhat controversial (Wilf and Kooyman 2025). Rainforest extent in this region has declined drastically in modern times, despite significant rainforest establishment in areas that previously contained sclerophyll forests throughout the 20th century (Harrington and Sanderson 1994). Whereas the conservation of Australia’s remaining tropical rainforests should be a high priority, several studies have made it clear that strategic fire management is required at this interface to maintain the habitat required by threatened, endemic fauna such as the northern bettong (Bettongia tropica) (Vernes et al. 2001; Whitehead 2018).
The aims of this study were to understand the mechanisms by which low-severity, patchy experimental fire affects an endangered, range-restricted mammal, the northern bettong, in terms of both resistance (immediate impacts of the fire) and short-to-medium-term resilience. This timeframe is considered particularly relevant because we consider the most likely negative effects on bettongs following fire to be an immediate increase in predation risk owing to a lack of shelter and a decrease in body condition or reproductive rates if food availability declines post-fire. We hypothesise that a fine-scale, patchy fire regime benefits northern bettongs in the medium-to-long term, by maintaining the grassy ground layer and open mid-storey they require, without disadvantaging them in the short term, because their key food sources will be largely unaffected and unburned patches will provide nesting opportunities. We used mark–recapture surveys and GPS tracking to test the following predictions: (1) the population density, body condition and breeding activity of northern bettongs will not be affected by a low-severity, patchy fire because direct deleterious effects from these fires are highly unlikely and the food sources required by bettongs will be unaffected, (2) the population density of two co-occurring CWR mammals, namely, the endangered northern quoll and brown bandicoots (Isoodon spp.), will not be affected by this fire regime, because of the persistence of shelter and food sources required by these species; (3) northern bettongs do not shift their overall home range immediately after a low-severity, patchy fire, because the resources they require are still present; and (4) in the days and weeks following fire, bettongs concentrate their nesting in unburned patches of ground cover within their original home range, and foraging and other activity will not shift because their food sources will not be affected.
Materials and methods
Study species
The northern bettong is a small, endangered marsupial, restricted to a tiny section of open sclerophyll woodlands at the edge of the northern Queensland Wet Tropics, north-eastern Australia. In the past two decades, at least two known populations of northern bettong have been lost, resulting in an estimated 70% decline in the species’ area of occupancy (Cronin et al. 2018). Today, only two populations of northern bettongs remain, a relative stronghold in the Lamb Range, near Cairns, with 700–1000 individuals, and a population at Mount Spurgeon and Mount Lewis National Parks, estimated at 5–20 individuals. Northern bettongs are thought to be threatened by changed fire regimes, particularly fire suppression that promotes rainforest encroachment into the bettong’s preferred open grassy woodland habitat. Bettongs require an open mid-storey for ease of movement, and fire promotes grasses that they require for nesting and as a food source, particularly cockatoo grass (Alloteropsis semialata) (Abell et al. 2006; Bateman and Johnson 2011). Vernes and Pope (2001) radio-tracked 23 northern bettongs before and after a low-severity fire, and found that these individuals favoured unburned vegetation around boulder piles in the months following fire. In the absence of fire, mid-storey density increases, causing the loss of ground vegetation by shading. Vernes (2000) found no evidence of direct mortality from fire in two low–medium-severity experimental fires. However, the behavioural mechanisms through which bettongs and similar species survive a fire are poorly understood, and we do not know the impact of fire on northern bettongs and their habitat in the short to medium term.
Study site
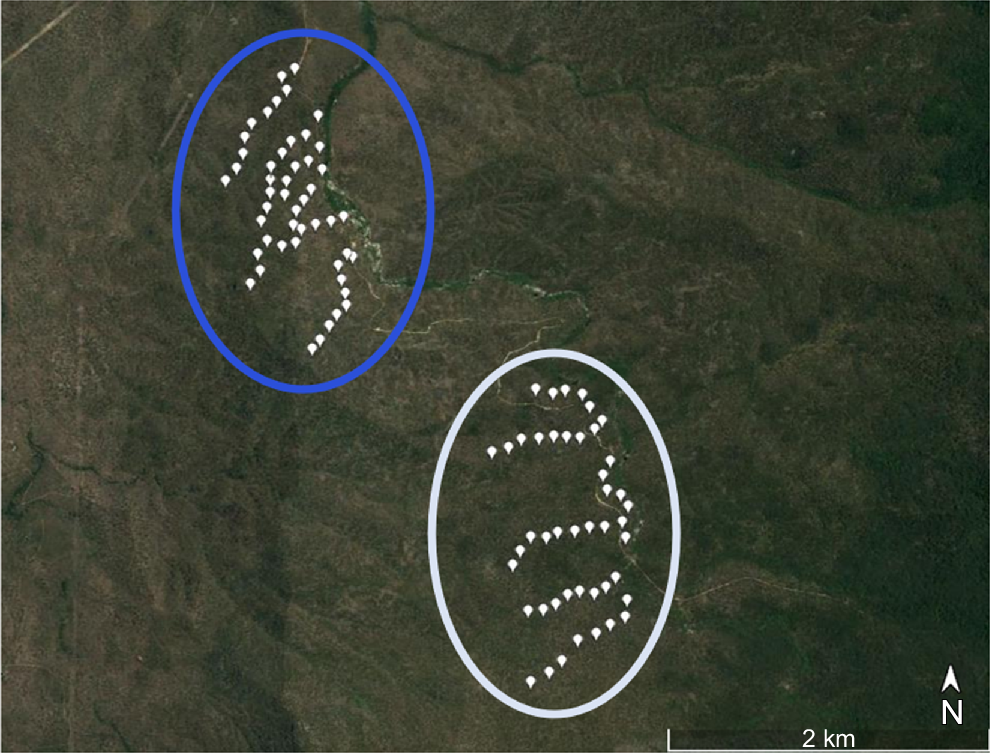
This study was conducted at Dinden and Davies Creek National Parks in far-northern Queensland, Australia, at Upper Davies Creek in the south-east and Lower Davies Creek in the north-west (Fig. 1). There is ~1.3 km of steep terrain between the eastern-most Lower Davies site and the western-most Upper Davies site. Lower Davies falls in a partial rain shadow, creating drier, more open forest at the lower sites than the wet sclerophyll forest at Upper Davies. The elevation at Lower Davies ranges from 456 to 694 m, and from 615 to 712 m at Upper Davies. Managers burn the drier Lower Davies early in the dry season and the wetter Upper Davies slightly later. The sites at Upper Davies have been the subject of northern bettong studies between 1993 and 2016, whereas the species has not been previously studied at Lower Davies. The Davies Creek subpopulation is part of the Lamb Range population, where over 90% of all remaining northern bettongs are now found.
Map of Davies Creek study sites. Cage trapping sites are white points. Lower Davies Creek sites are surrounded by a blue line, and Upper Davies Creek sites by a white line. Map (Queensland, Australia, 17°0′48.20″S, 145°34′33.04″E) (CNES/Airbus 2019) generated using Google Earth Pro (ver. 7.3, see http://www.earth.google.com).
The Queensland Department of Environment, Science and Innovation (DESI) is responsible for the management of most bettong habitat in the Lamb Range. Fire is the main tool used to maintain this habitat. Northern bettong conservation is a key focus of fire planning in the Lamb Range; target outcomes include providing refuges by leaving at least 30% of habitat unburned at the scale of a northern bettong home range, promoting truffle and grass diversity, and maintaining cockatoo grass (Department of Environment and Heritage Protection 2017). Management fires generally occur at short intervals (every 2–3 years) in northern bettong habitat at both the Lower Davies and Upper Davies study areas to achieve these goals; a management regime that has been in place for ~20 years at the time of this study.
Trapping surveys and GPS collaring
We collared bettongs during pre-fire mark–recapture trapping in both 2019 and 2021 (Table 1). We conducted the surveys for four consecutive nights at each site and set traps in lines of four to nine traps spaced 100 m apart. We baited and set traps in the late afternoon then checked and cleared from 23:00 hours. Bait was a mixture of peanut butter, rolled oats, vanilla essence and truffle-infused olive oil. In total, we performed 690 trap-nights pre-fire, and 588 post-fire. We microchipped all bettongs and northern quolls, except pouch young, for individual identification in both years, whereas we microchipped only bandicoots in 2019. We grouped captured bandicoots as Isoodon spp. for analysis, as both northern (I. macrourus) and Cape York (I. peninsulae) brown bandicoots have been recorded at the study site and are difficult to reliably tell apart. We recorded sex, weight, hind-foot length and reproductive status for all microchipped animals (65 individual northern bettongs, 17 northern quolls and 45 bandicoots), along with the approximate size of any pouch young. We measured body condition for bettongs on a scale of 1 (poor condition) to 3 (very healthy), on the basis of the amount of fat felt at the base of the tail. We used the following criteria to decide the suitability of captured bettongs for collaring: (1) adult bettongs only, (2) greater than 1 kg in weight, (3) average or better body condition, and (4) not carrying pouch young greater than ~50-mm head–body length.
| Activity | Date | ||||
|---|---|---|---|---|---|
| 2019 | 2021 | ||||
| Lower Davies | Upper Davies | Lower Davies | Upper Davies | ||
| Pre-fire trapping | 27–31 May | 3–7 June | 7–11 June | 14–18 June | |
| Fire | 21–25 June | 8–16 July (29 July A) | 5–6 July | 19–22 July | |
| Post-fire trapping | 30 July−2 August | 5–9 August | 20–24 July | 27–31 July | |
We fitted five bettongs with collars at each site in each field season, for a total of 20 deployments. We aimed to obtain an even sex ratio in collared bettongs, and nearly achieved this (11 males and 9 females). Following trapping, managers burned the sites, and trapping was repeated between 1 and 5 weeks post-fire, depending on the site and year. All post-fire trapping was completed before grass had regrown to a stage that would provide any shelter for bettongs, so consideration of difference in post-fire timing between trapping sessions was not considered necessary. When radio-tracking the collars prior to trapping, some were discovered to have been dropped before the burns, whereas faults or dead batteries were found in others upon recapturing the collared bettongs. Overall, we obtained useable pre- and post-fire home-range data for four collared bettongs in 2019 and six bettongs in 2021. We obtained sufficient data to estimate pre-fire home range from a further four bettongs in 2019 and three in 2021.
GPS collars were designed and manufactured by Telemetry Solutions (model Small GPS 4000ER, Concord, CA, USA). Each collar weighs ~35 g and contains a GPS transmitter and VHF transmitter for radio tracking. These collars store GPS location data on-board and data can be downloaded through a remote download base station when within the range of a collar.
We programmed collars in Telemetry Solutions software (see https://www.telemetrysolutions.com/) to record GPS location every 45 min overnight (between 18:00 and 05:15 hours) and at 12:00 hours each day. GPS battery life was 6–8 weeks. Before deployment we added a weak link in each collar by cutting halfway through the width of the cable tie that holds the collars together.
Data collection for this project was conducted under approval from The University of Queensland Animal Ethics Committee (AE47305), and under permit from the Queensland Department of Environment and Science (PTU19-001697, SPP19-001702).
Population density, demography and fitness
We analysed capture histories for all microchipped individuals in the program Mark (ver. 10.0, see http://www.phidot.org/software/mark/index.html; White and Burnham 1999), by using the robust mark–recapture method (Pollock 1982). This analysis returns an estimate of the abundance of the species within the study area for each trapping session. We converted abundance to density by dividing abundance estimates by the estimated effective area of our study sites, namely, 2.64 and 2.99 km2 for Lower Davies and Upper Davies respectively. These estimates for effective area were calculated using the same method as in Whitehead (2018), so as to allow comparison of density estimates between that study and the current study. We created a polygon by applying a buffer of 431 m (the mean home-range radius of a male northern bettong as defined by Whitehead 2018) around all trapping sites and including only the area to the south of Davies Creek, because this creek is assumed impassable for bettongs. We analysed the number of captures before and after fire using Chi-Square tests. We also compared northern bettong body condition, sex ratio and proportion of females showing evidence of breeding (either carrying pouch young or with an elongated teat indicating a current young-at-foot) before and after fire with Chi-Square tests, and body-mass index (BMI), which was calculated as a ratio of mass (g) to pes length (mm), with a linear model.
Home range and fire mapping
We categorised each GPS point as pre- or post-fire (based on date and time), and nesting or active. We assumed that the 12:00-hours GPS point, and any points at this location the previous morning and following evening, corresponded to that day’s nesting site. We considered that all GPS fixes after a bettong had left its starting nesting site and before it settled at its next nesting site as active. We used the package adehabitatHR (ver. 0.4.19, see https://cran.r-project.org/package=adehabitatHR; Calenge 2006) in R (ver. 4.1.1, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/) to estimate 95% kernel density estimates (KDEs) of overall home range, active range, and nesting areas pre- and post-fire for individuals with sufficient data points. The KDEs were exported as ESRI Shapefiles for further analysis in ArcGIS Pro (ver. 2.8.29751, Environmental Systems Research Institute, Redlands, CA, USA).
We mapped the differenced normalised burn ratio (dNBR) of the study area in the Google Earth Engine online code editor. This process compares infrared data from satellite images before and after a fire, to assess burn severity. First, we produced and upload an ESRI Shapefile with a buffer with radius 600 m (Lower Davies) or 700 m (Upper Davies) around study sites. These values ensured that the area analysed included the full extent of collared bettongs’ home-range estimates. The next step is to specify a date range for pre- and post-fire satellite images, in this case from the Sentinel-2 satellite, which takes 10-m resolution images every 5 days. We used date ranges spanning between 10 and 30 days for the pre- and post-fire image collection, depending on how many captures were required to avoid cloud cover masking the study sites. This process produces NBR maps for pre- and post-fire periods, with each pixel assigned a value calculated as:
where NIR represents the near-infrared reflectance and SWIR is the short-wave infrared reflectance. The post-fire value for each pixel is subtracted from the pre-fire value and scaled by 103 to produce a dNBR map. The resulting fire-severity maps were downloaded as ESRI Shapefiles. We plotted dNBR fire-severity maps for Upper Davies and Lower Davies in ArcGIS Pro, and the 95% KDE contours from the bettong GPS data were overlayed to visualise how burn severity affected northern bettong habitat use. Three collared bettongs from 2021 had home ranges that were almost entirely unburned, so they were treated as a control group for home range analysis.
Fire severity was defined in discrete classes by the scaled dNBR values as recommended by the United States Geological Survey (USGS), as follows: <100 unburned, 100–269 low, 270–439 low–moderate, 440–659 high–moderate, and >659 high. It is important to consider what this severity is representing in this open grassy woodland habitat; areas classified as high severity, for example, will have most of their ground cover vegetation consumed in the fire, but this does not necessarily represent a high intensity fire.
Within 5 days of each fire in 2021, seventeen 100-m line-intercept transects were used to ground truth the dNBR fire mapping, each orientated approximately east to west. From the starting point, whether the habitat was burned at each metre along the transect was recorded. These point recordings were mapped on the dNBR mapping to judge how well it detected fine-scale patterns. If the post-fire transects showed that the dNBR mapping was underestimating or overestimating burned areas, then the thresholds that define dNBR classes can be adjusted. These transects were generally consistent with the dNBR mapping, so no calibration was performed. As noted above, severity does not necessarily correlate with intensity, and areas mapped as high severity showed little to no indication of having burned with a high intensity (e.g. scorch heights, level of canopy tree damage).
Home-range analysis
We used a repeated measures two-way ANOVA to investigate the impact of treatment (seven experimental, three control) and sex on the difference between pre- and post-fire home ranges of the 10 bettongs for which sufficient GPS data were attained across both study sites and years. We accounted for individual variation by including a random effect of individual ID. We ran a similar ANOVA comparing the percentage of pre-fire nesting area that remained unburned following fire with the percentage of post-fire nesting area that was unburned, for a total of seven bettongs with sufficient data (five experimental, two control).
Results
Population density, demography and fitness
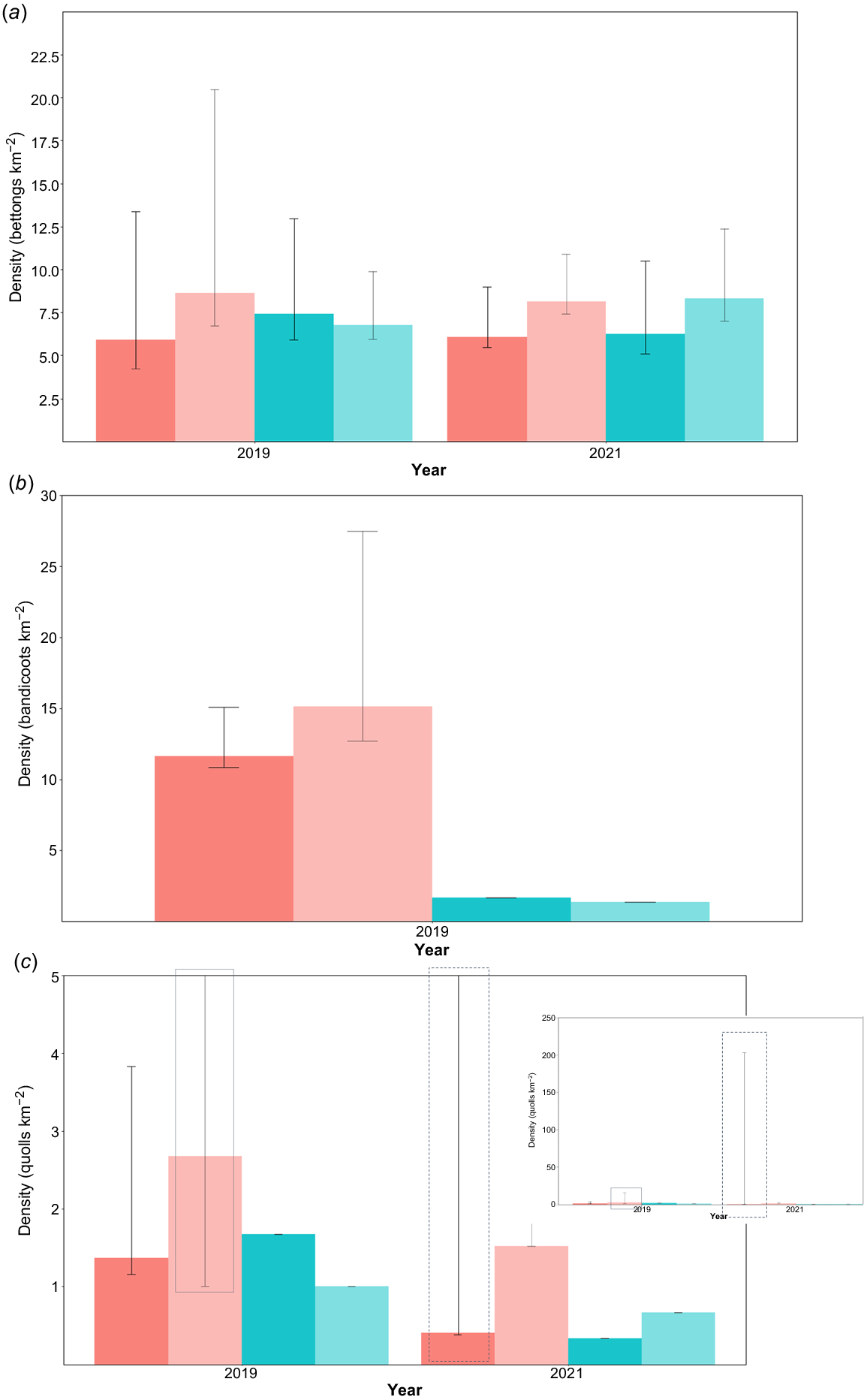
We found no evidence of an effect of fire on northern bettong, northern quoll or bandicoot density (Fig. 2). There were more bettong captures at Upper Davies after fire than pre-fire (χ2 = 6.01, P = 0.01) and more bandicoot captures after fire at both study sites (Lower Davies: χ2 = 4.86, P = 0.03; Upper Davies: χ2 = 8.33, P < 0.01). The number of quoll captures was not significantly different between sites or trapping sessions (Lower Davies: χ2 = 0.24, P = 0.62; Upper Davies: χ2 = 0.33, P = 0.56), nor were the numbers of bettongs caught at Lower Davies (χ2 = 1.62, P = 0.20) (Table 2). The increased capture rates of bandicoots (28.60% and 75.67% increase in captures per 100 trap nights at Lower Davies and Upper Davies respectively) and bettongs (58.82% at Upper Davies) post-fire did not represent increased densities. Instead, some individuals entered traps more often post-fire. The average number of captures per individual per session were slightly higher post-fire despite fewer trap nights than in pre-fire trapping (Fig. 2).
Estimated density and 95% confidence interval of of (a) northern bettongs, (b) bandicoots, and (c) northern quolls. Red bars represent Lower Davies surveys, and blue bars Upper Davies surveys. Darker colouring represents pre-fire surveys, and pale colouring post-fire. Error bars represent 95% confidence intervals; the inset plot in c shows the full extent of 95% confidence intervals.
| Species | Lower Davies | Upper Davies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-fire | Post-fire | Pre-fire | Post-fire | ||||||
| Captures (expected) | Captures per 100 trap nights | Captures (expected) | Captures per 100 trap nights | Captures (expected) | Captures per 100 trap nights | Captures (expected) | Captures per 100 trap nights | ||
| Northern bettong | 39 (45.0) | 11.3 | 46 (40.0) | 14.9 | 45 (56.8) | 13.0 | 58 (46.2) | 20.7 | |
| Isoodon sp. | 99 (113) | 28.8 | 114 (100) | 37.0 | 40 (53.5) | 11.6 | 57 (43.5) | 20.4 | |
| Northern quoll | 6 (7.41) | 1.74 | 8 (6.59) | 2.60 | 18 (16.0) | 5.22 | 11 (13.00) | 3.93 | |
Values that are significantly different from expected are in bold.
There was no significant difference in BMI, body condition or the proportion of breeding females before or after fire. Mean BMI ratios were 11.8 pre-fire and 11.6 post-fire (t = 0.74, P = 0.46). The proportion of captured bettongs in the three body condition categories were 0.17 v. 0.18 (1, poor condition), 0.67 v. 0.77 (2, average) and 0.17 v. 0.05 (3, very healthy) pre-fire v. post-fire (χ2 = 4.38, P = 0.11). The proportion of adult females with evidence of breeding was 0.96 pre-fire and 0.93 post-fire (χ2 = 1.56 × 10−32, P = 1.00).
Home-range analysis
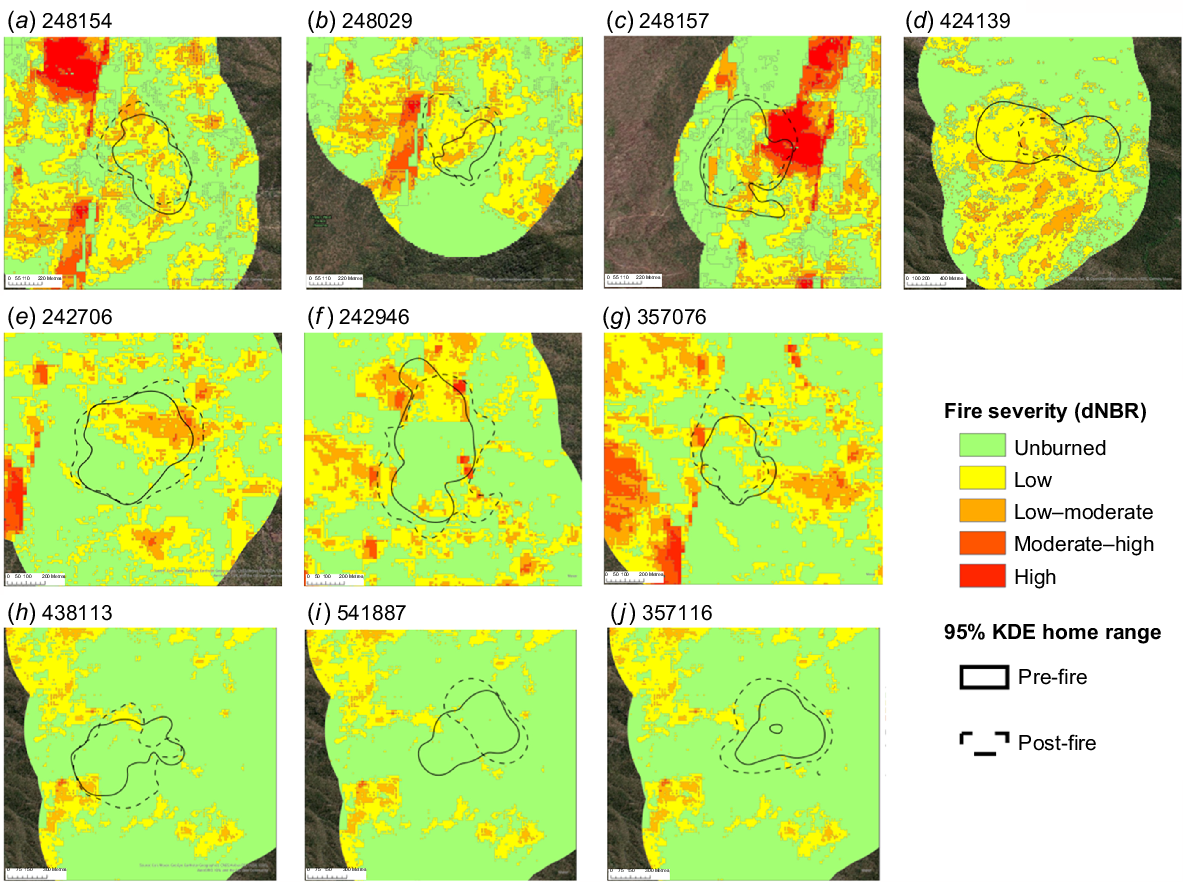
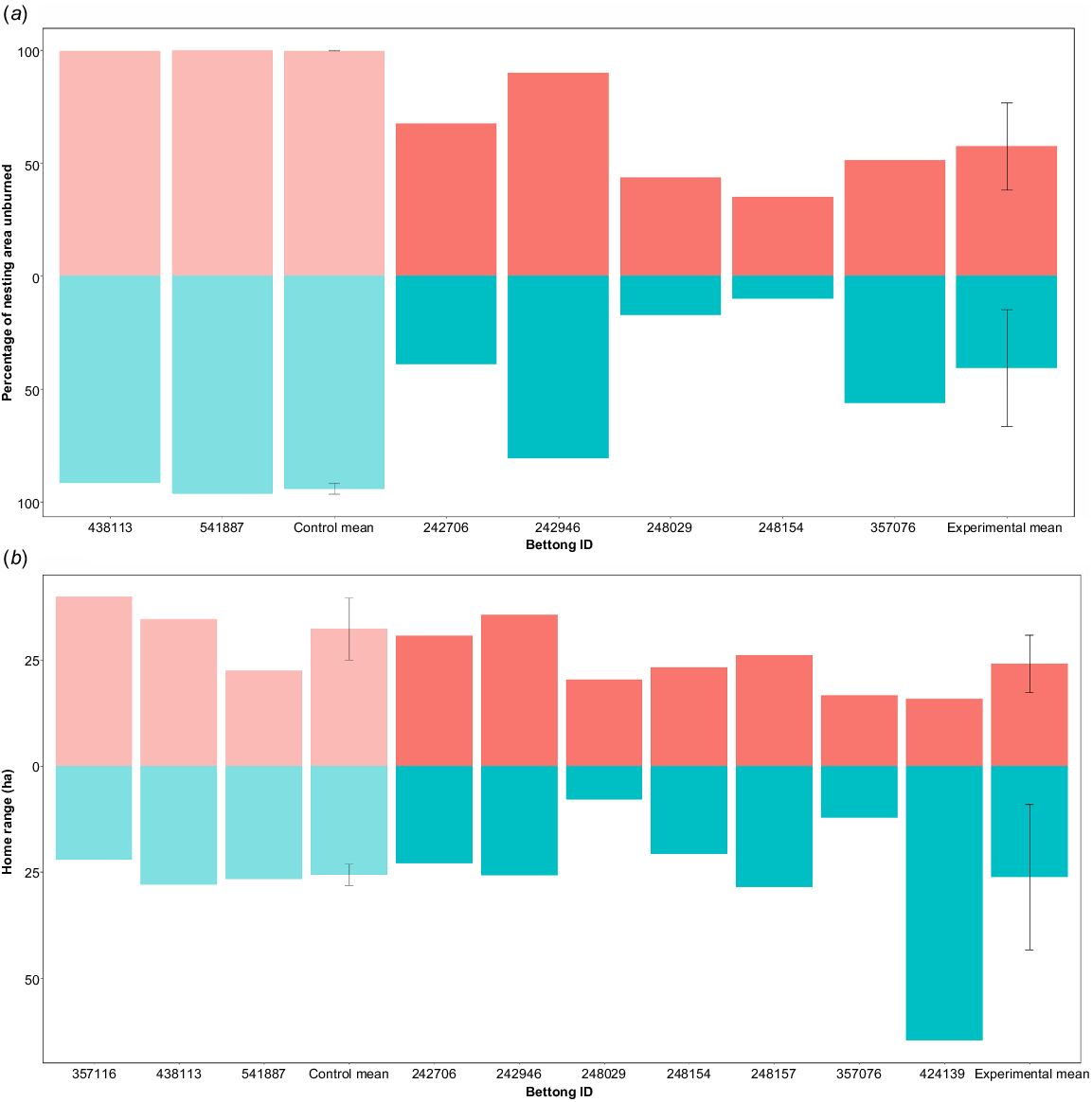
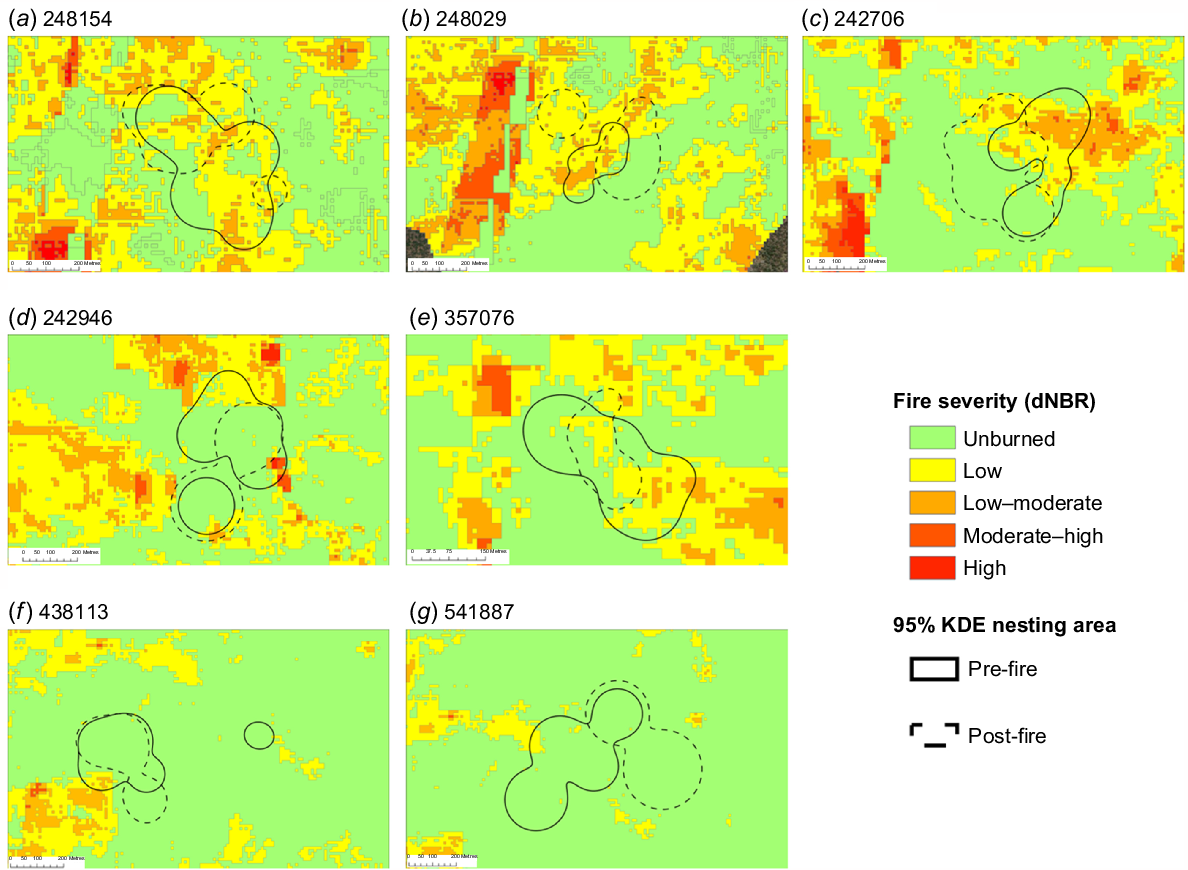
Following fire, bettongs shifted their nesting areas; the percentage of unburned habitat in the new nesting areas was significantly higher than in the nesting areas they used before the fire that were now partially or wholly burned (F = 7.74, P = 0.04; Fig. 3a, 4).
Pre-fire and post-fire (a) percentage of unburned habitat in northern bettong nesting areas and (b) northern bettong home-range sizes. Blue bars represent pre-fire data, and red bars post-fire data. Pale bars indicate individuals and mean values from control bettongs. Error bars indicate ±1 standard deviation from the mean.
Pre-fire and post-fire nesting area estimates of individual bettongs collared in (a–b) 2019 and (c–g) 2021. Bettongs in (a–e) the experimental area and (f, g) the control area.
The home-range size of northern bettongs across two fire seasons did not differ before and after fire (F = 0.01, P = 0.93) or between the control and experimental bettongs (F = 0.47, P = 0.51). There was no statistically significant difference between pre-fire home range size of male (mean = 31.9 ha, s.e. = 5.42) and female (mean = 20.2 ha, s.e. = 3.66) bettongs (t = 1.74, P = 0.10), nor in the mean percentage home-range change between sexes (males: mean = +16.22%, s.e. = 32.9; females: mean = +35.6%, s.e. = 25.7; F = 0.22, P = 0.66) or groups (control: mean = +28.9%, s.e. = 27.6; experimental: mean = +27.4%, s.e. = 26.3). However, there was large variation among individuals in their pre-fire home range, post-fire home range and direction and magnitude of the change in their home-range size following fire (Fig. 3b, 5).
Discussion
This study suggests that the northern bettong is well adapted to low–moderate-severity fire, because of their ability to survive fire events, shift nesting areas to unburned patches and maintain body condition and reproductive rates. Additionally, this fire regime does not appear to affect the abundance of co-occurring CWR mammals at this site. Setting explicit targets for prescribed fire and measuring success at the scale of an individual animal’s home range will likely produce very different outcomes to coarse, whole-of-landscape scale targets or less specific ‘patchy’ targets, which may still result in some individuals’ home ranges being largely or completely burned (Parr and Andersen 2006). We measured the response of northern bettongs to fires that resulted in a matrix of burned and unburned habitat at the bettong home-range scale. By setting management targets at the scale of a bettong home range, individual animals always have unburned shelter available following prescribed fire (Department of Environment and Heritage Protection 2017).
This study has added to growing evidence of the importance of strategic fire management that leaves unburned patches within fire scars. Shaw et al. (2021) found that the pale field rat (Rattus tunneyi) showed high home-range fidelity following fire, and that survival was strongly influenced by the amount of vegetation cover remaining post-fire. Leahy et al. (2015) found similar results for the pale field rat and the western chestnut mouse (Pseudomys nanus), with abundance of both species declining as the amount of remaining vegetation cover decreased following fire. Similar patterns have been observed in birds; red-backed fairy-wren (Malurus melanocephalus) individuals moved from burned to unburned areas following fire and showed a decrease in reproductive activity and success following a more extensive and severe fire than after a patchier fire (Murphy et al. 2010).
We also provide evidence of the importance of strategic fire management at the rainforest–sclerophyll forest interface in the Australian Wet Tropics. Whereas there are concerns regarding the impact of widespread fire in this ecotone on rainforest habitats (e.g. Wilf and Kooyman 2025), fire is the most effective tool land managers possess to maintain the current state of this transition zone and enable the northern bettong to persist in this region (Harrington and Sanderson 1994; Vernes et al. 2001; Whitehead 2018). Provided fire is applied in a strategic manner with clear objectives, then we strongly recommend the continuation, and potentially expansion, of this management approach in this part of the Australian Wet Tropics.
No mortality occurred among collared bettongs during this study, which is in agreement with low rates of direct animal mortality from fire observed in other studies across vertebrate taxa (Jolly et al. 2022). We believe that the short interval between fires under the current management regime helps ensure long-term resilience of this bettong population through several mechanisms. This fire regime decreases the likelihood of large, severe wildfires, and prevents dominant grasses from shading out cockatoo grass and mid-storey thickening that could shade out grasses altogether (Bateman and Johnson 2011). In this habitat, fire is the key driver maintaining the ecotone vegetation, preventing the transition to rainforest over time (Harrington and Sanderson 1994).
We observed an increase in average captures per individual bettong following fire, possibly owing to a decline in food availability between trapping sessions, making the baits more attractive. This is unlikely to be due to a fire-induced food deficit, because the northern bettong’s key food sources (underground truffles) are unlikely to be affected by these low-severity fires. Seasonal differences in food availability may play a role, because truffle abundance may decrease throughout the dry season (Abell et al. 2006).
The same pattern was observed in bandicoots, with a higher average capture rate per individual driving an increase in total captures post-fire. Bandicoots have a diverse, generalised diet and are also unlikely to be affected by a decrease in food availability following fire (McIlwee and Johnson 1998; Keiper and Johnson 2004). Both northern quolls and bandicoots appear to be unaffected by this fire regime at a population level, despite other studies reporting negative effects of fire on both species (Woinarski et al. 2010; Moore et al. 2021). This contrast is likely to be due to the low severity of the fires that occurred in this study, supporting the ongoing implementation of current fire management at this site.
The stability of the size and location of bettong home ranges pre- and post-fire suggests that the amount and dispersion of their food sources were little-changed by the fires and supports previous findings from radio-tracked northern bettongs (Vernes and Pope 2001). This stability may occur because bettongs feed on underground fungi and grass roots, which survive these fires.
By contrast, bettongs apparently shifted their nesting areas after fire, to incorporate unburned habitat in their home range. Northern bettongs generally require grass to build a nest, so we can expect that they will seek out areas with a grassy ground layer for nesting. This result contrasts with Vernes and Pope (2001) who, by using radio-tracking, found no significant changes to bettong nest area location following fire. It is likely that the difference in tracking methods contributed to this difference; whereas we obtained high accuracy GPS locations from collared bettongs over a short timeframe, Vernes and Pope (2001) relied on estimates of nest locations from manual radio-tracking over a longer study period. The difference highlights the value of using updated methods to enhance our understanding of behavioural mechanisms underpinning the responses of animals to fire.
The two collared bettongs in the control area, which did not have their pre-fire nesting areas burned, showed contrasting behaviours, with one seeming to largely shift its nesting area over the timeframe of our monitoring, whereas the other did not. These results suggest that the northern bettong inherently does possess behavioural flexibility rather than having high fidelity to nesting areas, and this could be an evolutionary adaptation to the dynamic, fire-shaped landscapes they occur within. Further work, with a larger sample size of control individuals, would clarify whether bettongs regularly shift their nesting areas over this timeframe.
Given unburned patches seem valuable for nesting, high-severity fires may be detrimental for bettongs. Severe fires are less likely to leave sufficient nesting areas and bettongs may be forced to either shift their home range, or choose suboptimal nesting sites, exposing themselves to potential predation. Tracking of feral cats across fire events has shown that they travel large distances to hunt in high-severity fire scars and have greater hunting success in these open habitats in Kimberley savannas (McGregor et al. 2015, 2016). In fire-prone forests in south-eastern Australia, the occurrence of invasive predators increased five-fold following fire, and predation on native mammals increased, and similar results have been recorded in the tropical savannas of north-western Australia (Leahy et al. 2015; Hradsky et al. 2017). The current fire management practices in the Lamb Range may be playing a significant role in the stability of this population.
Deploying GPS collars on bettongs before (and after) a high-severity fire would provide an interesting comparison with the data collected in this study. This may be possible at Mount Spurgeon because land managers seek to restore habitat that has not been burned as frequently as in the Lamb Range, by using high-severity fire. Predators, particularly feral cats, should be monitored and controlled following any high-severity fires in northern bettong habitat, because of their preference for hunting in high-severity burn scars (McGregor et al. 2016).
We have provided further evidence for the importance of low-severity, patchy fire to maintain habitat for a threatened species in the long term, without disadvantaging it or co-occurring species in the short term. We have shown that northern bettongs are resistant to the immediate impacts of these fires, which is likely to be due to their mobility to avoid direct mortality, and the retention of unburned vegetation patches, logs, and rock piles for nesting. We found no evidence of bettong, bandicoot, or quoll declines resulting from fires, and similar reproductive rates and BMI pre- and post-fire indicate that bettongs are resilient to this fire regime. The similarity of pre- and post-fire home ranges in bettongs suggests that individuals are still able to meet their energy requirements within their pre-fire home ranges following fire. This research supports the effectiveness of current management of the Lamb Range habitat and will provide guidance for habitat management in other parts of the northern bettong’s range.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
Sarah Legge is an editor for Wildlife Research. Despite this relationship, she took no part in the review and acceptance of this manuscript, in line with the publishing policy. The authors declare that they have no further conflicts of interest.
Declaration of funding
This research was supported by an Australian Government Research Training Program Scholarship awarded to Christopher Pocknee. The project was funded by the National Environmental Science Program through the Threatened Species Recovery Hub, the Ecological Society of Australia (Holsworth Wildlife Research Endowment), the Royal Zoological Society of New South Wales and Paddy Pallin Foundation (Paddy Pallin Research Grant), and the Wet Tropics Management Authority (Student Research Grant).
Acknowledgements
We acknowledge the Buluwai people as the Traditional Owners of the land on which this project took place and pay our respects to their Elders past and present. We thank the Buluwai and Djabugay Bulmba rangers for their involvement in and support of this work. Rob Miller and Brittney Butler of Queensland Parks and Wildlife Service were instrumental in this project, as were Manuela Fischer, Felicity L’Hotellier, Christine Mauger and Andy Howe of the Australian Wildlife Conservancy, and Stephanie Todd. We also thank all the volunteers – too many to name here – who were vital in the data collection for this project.
References
Abell SE, Gadek PA, Pearce CA, Congdon BC (2006) Seasonal resource availability and use by an endangered tropical mycophagous marsupial. Biological Conservation 132(4), 533-540.
| Crossref | Google Scholar |
Bateman BL, Johnson CN (2011) The influences of climate, habitat and fire on the distribution of cockatoo grass (Alloteropsis semialata) (Poaceae) in the Wet Tropics of northern Australia. Australian Journal of Botany 59(4), 315-323.
| Crossref | Google Scholar |
Bengsen AJ, Algar D, Ballard G, Buckmaster T, Comer S, Fleming PJS, Friend JA, Johnston M, McGregor H, Moseby K, Zewe F (2016) Feral cat home-range size varies predictably with landscape productivity and population density. Journal of Zoology 298(2), 112-120.
| Crossref | Google Scholar |
Bond WJ, Woodward FI, Midgley GF (2005) The global distribution of ecosystems in a world without fire. New Phytologist 165(2), 525-538.
| Crossref | Google Scholar | PubMed |
Bowman DMJS, Balch JK, Artaxo P, Bond WJ, Carlson JM, Cochrane MA, D’Antonio CM, Defries RS, Doyle JC, Harrison SP, Johnston FH, Keeley JE, Krawchuk MA, Kull CA, Marston JB, Moritz MA, Prentice IC, Roos CI, Scott AC, Swetnam TW, Van der Werf GR, Pyne SJ (2009) Fire in the Earth system. Science 324(5926), 481-484.
| Crossref | Google Scholar | PubMed |
Burbidge AA, Mckenzie NL (1989) Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50(1–4), 143-198.
| Crossref | Google Scholar |
Calenge C (2006) The package ‘adehabitat’ for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling 197(3–4), 516-519.
| Crossref | Google Scholar |
Chard M, Foster CN, Lindenmayer DB, Cary GJ, MacGregor CI, Blanchard W (2022) Time since fire influences macropod occurrence in a fire-prone coastal ecosystem. Austral Ecology 47(3), 507-518.
| Crossref | Google Scholar |
Cheyne SM, Capilla BR, Abdulaziz K, Supiansyah A, Cahyaningrum E, Smith DE (2019) Home range variation and site fidelity of Bornean southern gibbons [Hylobates albibarbis] from 2010–2018. PLoS ONE 14(7), e0217784.
| Crossref | Google Scholar | PubMed |
Cronin T, Abell S, Nuske S, Whitehead T, Todd S (2018) Northern Bettong Project 2013–2018. Bettongia tropica population status, distribution, habitat use and impact of fire. Final report. (WWF-Australia: Sydney, NSW, Australia) Available at https://assets.wwf.org.au/image/upload/v1674690981/website-media/resources/WWF_Northern_Bettong_Project_FINAL_1106.pdf
Doherty TS, Geary WL, Jolly CJ, Macdonald KJ, Miritis V, Watchorn DJ, Cherry MJ, Conner LM, González TM, Legge SM, Ritchie EG, Stawski C, Dickman CR (2022) Fire as a driver and mediator of predator–prey interactions. Biological Reviews 97, 1539-1558.
| Crossref | Google Scholar | PubMed |
Eby SL, Anderson TM, Mayemba EP, Ritchie ME (2014) The effect of fire on habitat selection of mammalian herbivores: the role of body size and vegetation characteristics. Journal of Animal Ecology 83(5), 1196-1205.
| Crossref | Google Scholar | PubMed |
Edwards MA, Derocher AE, Nagy JA (2013) Home range size variation in female Arctic grizzly bears relative to reproductive status and resource availability. PLoS ONE 8(7), e68130.
| Crossref | Google Scholar | PubMed |
Flanagan-Moodie AK, Holland GJ, Clarke MF, Bennett AF (2018) Prescribed burning reduces the abundance of den sites for a hollow-using mammal in a dry forest ecosystem. Forest Ecology and Management 429, 233-243.
| Crossref | Google Scholar |
Fordyce A, Hradsky BA, Ritchie EG, Di Stefano J (2016) Fire affects microhabitat selection, movement patterns, and body condition of an Australian rodent (Rattus fuscipes). Journal of Mammalogy 97(1), 102-111.
| Crossref | Google Scholar |
Harrington GN, Sanderson KD (1994) Recent contraction of wet sclerophyll forest in the wet tropics of Queensland due to invasion by rainforest. Pacific Conservation Biology 1(4), 319-327.
| Crossref | Google Scholar |
Herzog NM, Parker CH, Keefe ER, Coxworth J, Barrett A, Hawkes K (2014) Fire and home range expansion: a behavioral response to burning among savanna dwelling vervet monkeys (Chlorocebus aethiops). American Journal of Physical Anthropology 154(4), 554-560.
| Crossref | Google Scholar | PubMed |
Hradsky BA, Mildwaters C, Ritchie EG, Christie F, Di Stefano J (2017) Responses of invasive predators and native prey to a prescribed forest fire. Journal of Mammalogy 98(3), 835-847.
| Crossref | Google Scholar |
Jolly WM, Cochrane MA, Freeborn PH, Holden ZA, Brown TJ, Williamson GJ, Bowman DMJS (2015) Climate-induced variations in global wildfire danger from 1979 to 2013. Nature Communications 6, 7537.
| Crossref | Google Scholar | PubMed |
Jolly CJ, Dickman CR, Doherty TS, Van Eeden LM, Geary WL, Legge SM, Woinarski JCZ, Nimmo DG (2022) Animal mortality during fire. Global Change Biology 28(6), 2053-2065.
| Crossref | Google Scholar | PubMed |
Keiper P, Johnson CN (2004) Diet and habitat preference of the Cape York short-nosed bandicoot (Isoodon obesulus peninsulae) in north-east Queensland. Wildlife Research 31(3), 259-265.
| Crossref | Google Scholar |
Kelt DA, Van Vuren DH (2001) The ecology and macroecology of mammalian home range area. American Naturalist 157(6), 637-645.
| Crossref | Google Scholar | PubMed |
Körtner G, Pavey CR, Geiser F (2007) Spatial ecology of the mulgara in arid Australia: impact of fire history on home range size and burrow use. Journal of Zoology 273(4), 350-357.
| Crossref | Google Scholar |
Leahy L, Legge SM, Tuft K, McGregor HW, Barmuta LA, Jones ME, Johnson CN (2015) Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildlife Research 42(8), 705-716.
| Crossref | Google Scholar |
Legge S, Murphy S, Heathcote J, Flaxman E, Augusteyn J, Crossman M (2008) The short-term effects of an extensive and high-intensity fire on vertebrates in the tropical savannas of the central Kimberley, northern Australia. Wildlife Research 35(1), 33-43.
| Crossref | Google Scholar |
Legge S, Murphy S, Kingswood R, Maher B, Swan D (2011) EcoFire: restoring the biodiversity values of the Kimberley region by managing fire. Ecological Management & Restoration 12(2), 84-92.
| Crossref | Google Scholar |
Legge S, Smith JG, James A, Tuft KD, Webb T, Woinarski JCZ (2019) Interactions among threats affect conservation management outcomes: livestock grazing removes the benefits of fire management for small mammals in Australian tropical savannas. Conservation Science and Practice 1(7), e52.
| Crossref | Google Scholar |
Legge S, Rumpff L, Woinarski JCZ, Whiterod NS, Ward M, Southwell DG, Scheele BC, Nimmo DG, Lintermans M, Geyle HM, Garnett ST, Hayward-Brown B, Ensbey M, Ehmke G, Ahyong ST, Blackmore CJ, Bower DS, Brizuela-Torres D, Burbidge AH, Burns PA, Butler G, Catullo R, Chapple DG, Dickman CR, Doyle KE, Ferris J, Fisher D, Gallagher R, Gillespie GR, Greenlees MJ, Hohnen R, Hoskin CJ, Hunter D, Jolly C, Kennard M, King A, Kuchinke D, Law B, Lawler I, Lawler S, Loyn R, Lunney D, Lyon J, MacHunter J, Mahony M, Mahony S, McCormack RB, Melville J, Menkhorst P, Michael D, Mitchell N, Mulder E, Newell D, Pearce L, Raadik TA, Rowley JL, Sitters H, Spencer R, Valavi R, West M, Wilkinson DP, Zukowski S (2022) The conservation impacts of ecological disturbance: time-bound estimates of population loss and recovery for fauna affected by the 2019–2020 Australian megafires. Global Ecology and Biogeography 31(10), 2085-2104.
| Crossref | Google Scholar |
Legge S, Rumpff L, Garnett ST, Woinarski JCZ (2023) Loss of terrestrial biodiversity in Australia: magnitude, causation, and response. Science 381(6658), 622-631.
| Crossref | Google Scholar | PubMed |
Letnic M (2003) The effects of experimental patch burning and rainfall on small mammals in the Simpson Desert, Queensland. Wildlife Research 30(6), 547-563.
| Crossref | Google Scholar |
Lindenmayer DB, Kooyman RM, Taylor C, Ward M, Watson JEM (2020) Recent Australian wildfires made worse by logging and associated forest management. Nature Ecology & Evolution 4, 898-900.
| Crossref | Google Scholar | PubMed |
Linder HP, Lehmann CER, Archibald S, Osborne CP, Richardson DM (2018) Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biological Reviews 93(2), 1125-1144.
| Crossref | Google Scholar | PubMed |
Manyonyi AM, Mariki SB, Mnyone LL, Belmain SR, Mulungu LS (2020) Effects of prescribed burning on rodent community ecology in Serengeti National Park. Journal of Vertebrate Biology 69(2), 20001.
| Crossref | Google Scholar |
McDonald PJ, Stewart A, Schubert AT, Nano CEM, Dickman CR, Luck GW (2016) Fire and grass cover influence occupancy patterns of rare rodents and feral cats in a mountain refuge: implications for management. Wildlife Research 43(2), 121-129.
| Crossref | Google Scholar |
McGregor HW, Legge SM, Jones ME, Johnson CN (2014) Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS ONE 9(10), e109097.
| Crossref | Google Scholar | PubMed |
McGregor HW, Legge SM, Jones ME, Johnson CN (2015) Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS ONE 10(8), e0133915.
| Crossref | Google Scholar | PubMed |
McGregor HW, Legge SM, Jones ME, Johnson CN (2016) Extraterritorial hunting expeditions to intense fire scars by feral cats. Scientific Reports 6, 22559.
| Crossref | Google Scholar | PubMed |
McIlwee AP, Johnson CN (1998) The contribution of fungus to the diets of three mycophagous marsupials in Eucalyptus forests, revealed by stable isotope analysis. Functional Ecology 12, 223-231.
| Crossref | Google Scholar |
Moore HA, Dunlop JA, Jolly CJ, Kelly E, Woinarski JCZ, Ritchie EG, Burnett S, van Leeuwen S, Valentine LE, Cowan MA, Nimmo DG (2021) A brief history of the northern quoll (Dasyurus hallucatus): a systematic review. Australian Mammalogy 44(2), 185-207.
| Crossref | Google Scholar |
Murphy SA, Legge SM, Heathcote J, Mulder E (2010) The effects of early and late-season fires on mortality, dispersal, physiology and breeding of red-backed fairy-wrens (Malurus melanocephalus). Wildlife Research 37(2), 145-155.
| Crossref | Google Scholar |
Parr CL, Andersen AN (2006) Patch mosaic burning for biodiversity conservation: a critique of the pyrodiversity paradigm. Conservation Biology 20(6), 1610-1619.
| Crossref | Google Scholar | PubMed |
Pocknee CA, Legge SM, McDonald J, Fisher DO (2023) Modeling mammal response to fire based on species’ traits. Conservation Biology 37(4), e14062.
| Crossref | Google Scholar | PubMed |
Pollock KH (1982) A capture-recapture design robust to unequal probability of capture. The Journal of Wildlife Management 46(3), 752-757.
| Crossref | Google Scholar |
Prestrud P (1992) Denning and home-range characteristics of breeding arctic foxes in Svalbard. Canadian Journal of Zoology 70(7), 1276-1283.
| Crossref | Google Scholar |
Radford IJ (2012) Threatened mammals become more predatory after small-scale prescribed fires in a high-rainfall rocky savanna. Austral Ecology 37(8), 926-935.
| Crossref | Google Scholar |
Radford IJ, Gibson LA, Corey B, Carnes K, Fairman R (2015) Influence of fire mosaics, habitat characteristics and cattle disturbance on mammals in fire-prone savanna landscapes of the northern Kimberley. PLoS ONE 10(6), e0130721.
| Crossref | Google Scholar | PubMed |
Radford IJ, Woolley L-A, Corey B, Vigilante T, Wunambal Gaambera Aboriginal Corporation, Hatherley E, Fairman R, Carnes K, Start AN (2020) Prescribed burning benefits threatened mammals in northern Australia. Biodiversity and Conservation 29, 2985-3007.
| Crossref | Google Scholar |
Rossi RC, Leiner NO (2023) Effects of severe fires on the survival and body condition of Gracilinanus agilis in a Cerrado remnant. Mammalian Biology 103, 205-214.
| Crossref | Google Scholar |
Russell-Smith J, Yates CP (2007) Australian savanna fire regimes: context, scales, patchiness. Fire Ecology 3, 48-63.
| Crossref | Google Scholar |
Shaw RE, James AI, Tuft K, Legge S, Cary GJ, Peakall R, Banks SC (2021) Unburnt habitat patches are critical for survival and in situ population recovery in a small mammal after fire. Journal of Applied Ecology 58(6), 1325-1335.
| Crossref | Google Scholar |
Stobo-Wilson AM, Cremona T, Murphy BP, Carthew SM (2021) Resource availability drives variation in a marsupial glider’s home-range size. Journal of Zoology 315(3), 199-212.
| Crossref | Google Scholar |
Swan M, Galindez-Silva C, Christie F, York A, Di Stefano J (2016) Contrasting responses of small mammals to fire and topographic refugia. Austral Ecology 41(4), 437-445.
| Crossref | Google Scholar |
Vernes K (2000) Immediate effects of fire on survivorship of the northern bettong (Bettongia tropica): an endangered Australian marsupial. Biological Conservation 96(3), 305-309.
| Crossref | Google Scholar |
Vernes K, Pope LC (2001) Stability of nest range, home range and movement of the northern bettong (Bettongia tropica) following moderate-intensity fire in a tropical woodland, north-eastern Queensland. Wildlife Research 28(2), 141-150.
| Crossref | Google Scholar |
Vernes K, Castellano M, Johnson CN (2001) Effects of season and fire on the diversity of hypogeous fungi consumed by a tropical mycophagous marsupial. Journal of Animal Ecology 70(6), 945-954.
| Crossref | Google Scholar |
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46(S1), S120-S138.
| Crossref | Google Scholar |
Wilf P, Kooyman RM (2025) Paleobotany reframes the fiery debate on Australia’s rainforest edges. New Phytologist 245(4), 1355-1365.
| Crossref | Google Scholar | PubMed |
Woinarski JCZ, Armstrong M, Brennon K, Fisher A, Griffiths AD, Hill B, Milne DJ, Palmer C, Ward S, Watson M, Winderlich S, Young S (2010) Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia. Wildlife Research 37(2), 116-126.
| Crossref | Google Scholar |
Woinarski J, Braby M, Burbidge A, Coates D, Garnett ST, Fensham RJ, Legge SM, McKenzie NL, Silcock JL, Murphy BP (2019) Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia. Biological Conservation 239, 108261.
| Crossref | Google Scholar |
Wysong M, Legge S, Clarke A, Maier S, Bardi Jawi Rangers, Nyul Nyul Rangers, Yawuru Country Managers, Cowell S, Mackay G (2021) The sum of small parts: changing landscape fire regimes across multiple small landholdings in north-western Australia with collaborative fire management. International Journal of Wildland Fire 31(2), 97-111.
| Crossref | Google Scholar |