Are sutures a pathway to infection? A multidisciplinary assessment of wound healing in sharks following internal acoustic tagging
Brittany Heath A , Charlie Huveneers
A , Charlie Huveneers  A , Ryan D. Hesse B , Lewis Vaughan C , Ondi L. Crino C , Chloe N. Roberts A , Xanthe Venn
A , Ryan D. Hesse B , Lewis Vaughan C , Ondi L. Crino C , Chloe N. Roberts A , Xanthe Venn  D and Jordan K. Matley A *
D and Jordan K. Matley A *
A
B
C
D
Abstract
Acoustic telemetry often involves a surgical method of internal tagging, wherein an animal is incised, a transmitter (hereafter referred to as a tag) internally inserted into the coelomic cavity, and the incision closed with sutures to aid wound closure, healing and tag retention. However, the act of tagging leads to additional handling and exposure to foreign materials (e.g. sutures), potentially increasing stress and the possibility of infection or fatality.
We assessed whether the absence of sutures would result in similar or different healing responses compared to incisions closed with sutures.
A 42-day captive study measured physical (i.e. macroscopic assessment of incision healing progress), bacterial colonisation (i.e. colony forming units) and blood chemistry (i.e. glucose and lactate concentrations) responses to the two internal acoustic tagging procedures (i.e. with and without sutures) in twelve Port Jackson sharks (Heterodontus portusjacksoni).
Macroscopic measurements (i.e. incision length and width) healed at different rates but overall healing was comparable between tagging procedures across the 42-day study. There was, however, a higher presence of inflammation and incisional swelling at sutured incisions and one case of visceral protrusion from a non-sutured incision. Sutured incisions had significantly more bacteria present than non-sutured incisions, despite the use of autoclaved surgical tools and surgeries performed in a controlled environment. Tag retention was 100% in both treatments.
Combined, these findings highlight that healing was broadly similar regardless of suturing and not suturing; however, sutures may offer a pathway to bacterial infection. Nevertheless, the potential lethal impact of visceral protrusion from non-sutured incisions is a critical concern; future work should examine whether smaller incisions and tags, or altering the position of tagging sites (e.g. further off the ventral midline) could limit protrusions, rendering non-suturing approaches as preferred under certain scenarios.
This research on wound healing contributes to our limited understanding of elasmobranch healing and tagging effects, which is an important consideration when minimising welfare impacts on animals used in research.
Keywords: acoustic tracking, animal welfare, bacteria, elasmobranchs, fish tracking, Heterodontus portusjacksoni, Port Jackson shark, suturing, tagging effects.
Introduction
Aquatic movement ecology is a rapidly developing field of study (Matley et al. 2022), and often incorporates electronic tagging to track the movements of fish and other marine and freshwater animals (Matley et al. 2024a). Implanting aquatic animals with transmitters (hereafter referred to as a tag), although largely considered a safe and responsible research practice, still lacks fundamental knowledge of how the tagging process (e.g. handling, holding, surgery, recovery; Clemens et al. 2023) affects the behaviour, physiology, fitness and survival of animals (Matley et al. 2024a; Shorgan et al. 2025). The healing process is a fundamental consideration when tagging, both in terms of animal welfare and operability of findings, and consists of a variety of responses at cellular and whole-organism levels (Bereiter-Hahn 1986) to promote recovery. Most studies that evaluate healing and recovery from tagging explore short-term impacts on tag retention, tag burden (i.e. relative weight of tag), growth and survival (Matley et al. 2024a; Shorgan et al. 2025). Although increasingly more studies are incorporating fine-scale techniques to evaluate physiology (e.g. stress hormones and swim performance, see Fuller et al. 2020 and Wosnick et al. 2023) and healing at a cellular level (e.g. inflammation and immune responses, see Borucinska et al. 2020), few studies have incorporated a multidisciplinary approach to investigate the diverse ways that elasmobranchs respond to tagging. See Jepsen et al. (2008a, 2008b) for information about tagging effects on bony fish. These lines of investigation are necessary because healing (and associated positive or deleterious effects) is usually not presented or effectively measured by a singular metric (Wagner et al. 2000; Panther et al. 2011; Sveen et al. 2020). Additionally, researchers working with animals (e.g. biologists, ecologists, veterinarians) have an ethical responsibility to ensure data are collected using methods that minimise impact and maximise animal welfare (Russell et al. 1959).
Intracoelomic (or internal) electronic tagging is an invasive process whereby an animal is incised, a tag is internally inserted into the coelomic cavity, and the incision is often closed with sutures to aid wound closure, healing and tag retention (Wagner et al. 2000; Jepsen et al. 2002). This method was developed as an alternative to external attachment of tags to avoid shedding and biofouling (Heupel et al. 1998; Dicken et al. 2011; Hammerschlag et al. 2011), and is commonplace in acoustic telemetry – one of the main methods of aquatic animal tracking (Matley et al. 2022). Nevertheless, the presence of sutures contribute to healing complications post-surgery such as inflammation, dehiscence (opening of wound edges) and ulceration (formation of a break on the skin) (Jepsen et al. 2002; Clemens et al. 2023), with reasonably little known of the long-term consequences to animal health and survival (Matley et al. 2024a). Suturing adds another level of risk during the tagging process in relation to puncturing vital organs. Suturing is also time consuming and can be challenging to perform in the field during suboptimal conditions (e.g. strong wind or large swell while on a boat), further increasing processing times. Increased duration between capture and release can lead to additional stress and may cause pathological changes which disrupt the healing process (Clemens et al. 2023). Alternative methods to suturing (e.g. staples, adhesives, bandages, glue) are typically discouraged because of the high incidence of dehiscence and mortality, difficulty of placement, retention duration, and other logistical challenges, such as the maintenance of aseptic technique during the procedure (Haeseker et al. 1996; Swanberg et al. 1999; Murray 2002; Jepsen et al. 2017). Another option is to leave the incision undisturbed following tag insertion. This approach is commonly used for passive integrated transponder (PIT) tags, relatively small (11–23 mm) electronic tags that use radio frequency identification (RFID) technology (Castro-Santos and Vono 2013; Larsen et al. 2013). Whether this approach can be reliably used for other tracking technologies, specifically acoustic telemetry, is not known. Only a limited number of studies have explored the viability of not suturing incisions following acoustic tagging, but all have shown promising results. For example, tag retention, levels of inflammation and wound closure rates of several different ‘non-sutured’ fish were similar or improved compared to sutured individuals (Huysman et al. 2020; Tsitrin et al. 2020; Kelican et al. 2021). These preliminary studies, in addition to minimising associated traumas from suturing, highlight the potential value of leaving incisions non-sutured following internal tag surgeries.
Elasmobranchs (e.g. sharks, skates, rays) are one of the most common animal groups studied using acoustic telemetry (Matley et al. 2022), yet relatively little is known regarding their healing following tag implantation. Elasmobranchs are considered resilient to natural and anthropogenic injuries because of their unique adaptive immune responses (Marra et al. 2017), making them ideal candidates to evaluate healing from internal tagging and test refinements of current practices (e.g. sutures v. no sutures). For example, rapid healing of wounds has been described across a range of elasmobranchs including blacktip reef sharks (Carcharhinus melanopterus), reef manta ray (Mobula alfredi), leopard shark (Triakis semifasciata), nurse shark (Ginglymostoma cirratum), whale shark (Rhincodon typus), whitespotted eagle ray (Aetobatus narinari) and white shark (Carcharhinus carcharias) (Reif 1978; Marshall and Bennett 2010; Domeier 2012; Towner et al. 2012; Chin et al. 2015; McGregor et al. 2019; Borucinska et al. 2020; Nasby-Lucas and Domeier 2020; Womersley et al. 2021; Rangel et al. 2022). Despite the documented resilience to injury, elasmobranchs have received limited attention regarding studies that specifically investigate behavioural, physiological and immunological responses to acoustic tagging. Therefore, useful information can be gained by investigating the characteristics of healing and affiliated responses that occur during healing and recovery following internal tagging.
The overall aim of this study was to use a multidisciplinary approach to evaluate different aspects of shark healing to investigate whether the absence of sutures negatively affects healing and potential for infection in a robust shark species. To do so, we assessed macroscopic (i.e. physical assessment of incision healing), bacterial (i.e. bacterial colony forming units) and blood chemistry (i.e. glucose and lactate concentrations) responses to the surgical procedures. Our specific questions were as follows:
Will wound closure at non-sutured incision sites be comparable to sutured sites?
Will the presence of sutures increase infection risk through inflammation and bacterial colonisation?
Will healing post-surgery or complications from tagging affect stress responses?
Ultimately, the presence of sutures (independent of type) acting as a foreign body that may prevent or delay the healing of tissue and muscle layers has received limited ethical or applied considerations in aquatic animal tracking. The outcomes of this study will address knowledge gaps in elasmobranch wound healing by examining a variety of responses that will create a better understanding of the immune response to acute injuries. Our results will contribute to understanding the effects of tagging procedures, and investigate whether the current practice of suturing could be replaced by a faster and less invasive method without negatively affecting healing.
Methods
Study species, capture and husbandry
The Port Jackson shark (Heterodontus portusjacksoni) was selected due to its resilience to capture, handling, captivity and repeat sampling (Frick et al. 2010). Heterodontus portusjacksoni is a medium-sized (up to 165-cm total length) shark endemic to Australian waters, and listed as Least Concern on the IUCN Red List (Huveneers and Simpfendorfer 2015). Twelve H. portusjacksoni (total length range: 57–90 cm; median: 70 cm) were collected across two locations (Long Spit and Grange Beach, SA, Australia) using 500-m bottom-set longlines. Each longline consisted of up to 50 circle hooks (size 13/0–16/0) spaced ~10 m apart. Upon capture, sharks were transported to Flinders University in temporary tanks with aerators to ensure water oxygen saturation levels remained above 80%. Sharks were housed in 1800-L round tanks (1.35-m diameter × 1.2-m height) with a maximum of two sharks per tank to reduce overcrowding. The flow-through tank system recirculated up to 10,000 L h−1, turning over the water ~3 times per hour. Additionally, protein skimmers and daily manual cleaning ensured tank cleanliness. We monitored water quality parameters (pH 7.9–8.4, dissolved oxygen 80–100%, nitrite <0.25 ppm, nitrate <5 ppm, ammonia <0.25 ppm, temperature 15–23°C, and salinity 26–36 ppt of NaCl in solution) weekly throughout the captivity period. Sharks were fed ~3–5% of their body mass every second day (Jones 2023). Diet consisted of sardines (Sardinops sagax), king prawns (Melicertus latisulcatus), blue mussels (Mytilus edulis) and southern calamari (Sepioteuthis australis). Weight was recorded on the day of capture, the day of surgery (day 0) and weekly over the observational period (day 7, 14, 21, 28, 35, 42, 49, 56 and 62) post-surgery.
Biopsy tissue samples were collected under approval of Flinders University Animal Ethics Committee (AEC BIOL5590-11) and animals approved to be caught under AEB4822. The housing and feeding of all sharks were monitored by the Flinders University Animal House technicians.
Experimental design
Sharks were acclimatised to captive conditions for 2 weeks prior to beginning internal surgery and tagging trials. For sampling, each shark was caught by hand from the tank surface, grabbing in front of the first dorsal fin and directly behind the second dorsal fin, and put into tonic immobility by turning the animal upside down (Kessel and Hussey 2015). A damp towel was placed over the sharks’ eyes to reduce stress and water was pumped into the mouth to ensure oxygenation. Surgeries were performed by two surgeons, one expert who has internally tagged over 300 animals ranging from small teleosts to large sharks, and one novice. The novice surgeon was trained by a veterinarian and supported during surgeries by a team of experts. During surgeries on day 0, two anterior–posterior incisions (~2 cm) were made, using a sterile disposable scalpel (Livingstone, blade size 10), on the ventral side lateral to the midline (i.e. left and right side of midline) of the shark into the peritoneal cavity. Two incisions were made per shark following ethical considerations to limit the number of sharks removed from the wild and held in captivity. A dummy tag (replicate of acoustic tags in size (30.5 × 13 mm) and weight (9.2 g in air; 5.1 g in water) but without electric components), which had been soaked in Betadine (povidone-iodine 10%) as per common field practices (Holmes et al. 2022) to ensure tag sterilisation, was inserted through one of the incisions. A sham insertion was applied to the other incision; this consisted of partially inserting the dummy tag through the incision (i.e. two-thirds of its length) and then removing it so that both incisions underwent the same tag-insertion process, but only one dummy tag was implanted in the shark. One incision was closed using two interrupted cruciate sutures (SilverGlide Polyglycolic Acid 50-mm USP 0/EP 3.5, 45-cm Violet Braided, absorbable), whereas the second incision was left non-sutured. The interrupted cruciate suture method is commonly used in fish and shark tagging because it ensures close apposition without tissue compression and reduces the time stitching takes compared to single stitches (Orrell et al. 2025). The number of sutures varied for two sharks due to incision size and animal welfare requirements; one shark had four single interrupted sutures and another shark only had one interrupted cruciate suture. Our closure plan was to space sutures 5–7.5 mm apart ~5 mm to the incision. Sharks were monitored for 30 min post-surgery to ensure there were no immediate complications from the surgeries.
Each shark was assigned to one of four treatment groups (A, B, C, D) that differed based on the sutured side (left or right) and dummy tag insertion side (left or right; Supplementary Fig. S1). Healing was monitored for 42 days post-surgery using three assessment types: (1) macroscopic assessment of the incision and healing process, (2) bacterial communities, and (3) blood glucose and lactate levels. Tag retention was monitored by checking tanks and filters daily. Sharks were also observed daily to monitor feeding events and any behavioural changes.
Macroscopic assessment
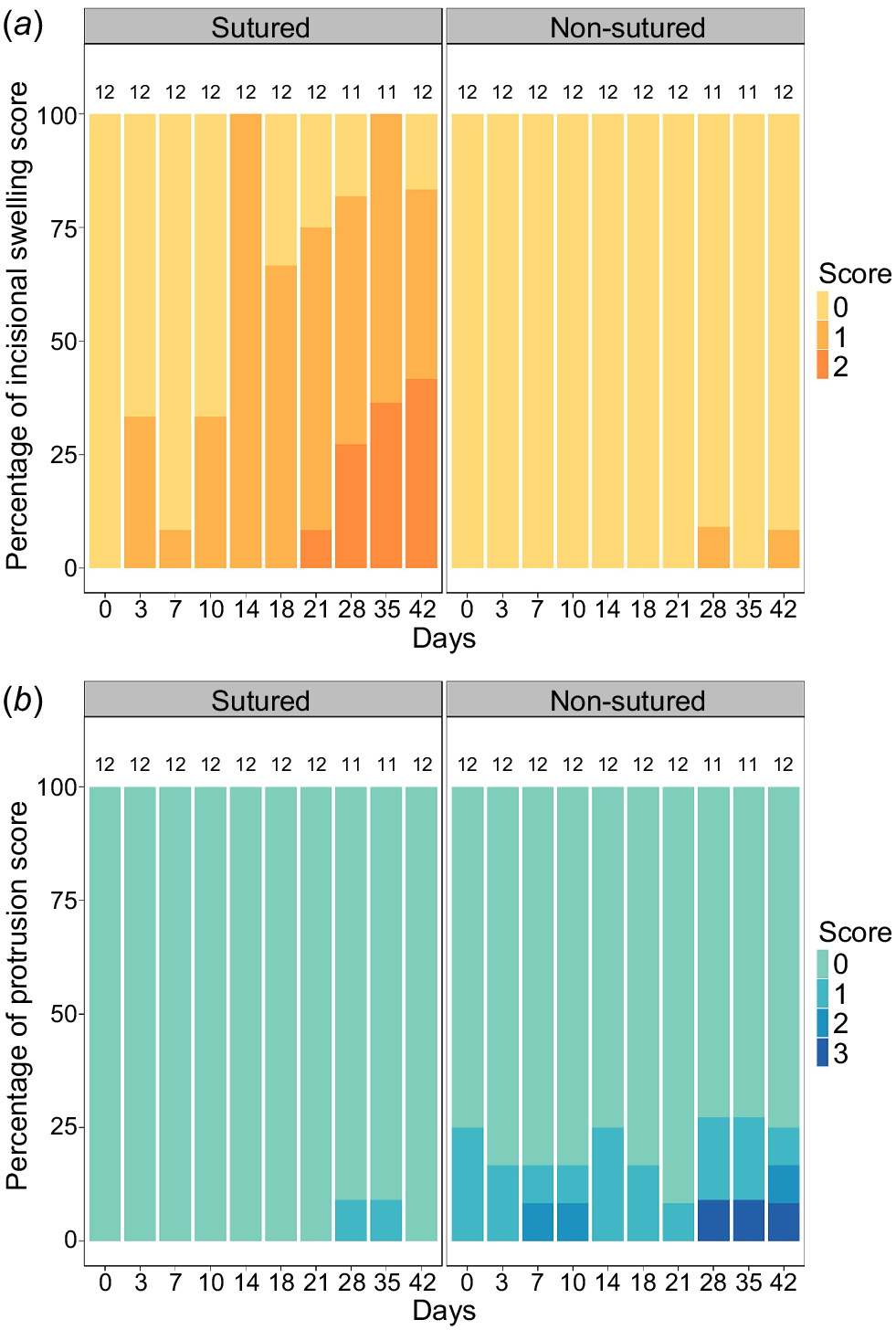
Standardised measurements and categorisations of wound healing at a macroscopic level were determined to monitor healing (Schoonyan et al. 2017). Photographs were taken immediately after surgery (day 0) and every 2–3 days (days 3, 7, 10, 14, 18, 21, 28, 35 and 42) to assess healing progress macroscopically (Supplementary Table S1). Photos were taken with a ruler parallel to the incisions for later measurement in ImageJ (ver. 1.54a, W. S. Rasband, US National Institutes of Health, Bethesda, MD, USA, see https://imagej.net/ij/; Table S1). The following measurements were recorded: inflamed area, incision length and incision width (Fig. 1). Inflamed area was measured based on skin discolouration around the incision (Fig. 1a; Wagner et al. 2000; Schoonyan et al. 2017). Incision length measured the length between distal ends of the incision (Fig. 1b). Incision width measured the widest part of the open wound, where applicable (Fig. 1c). Also, the extent of incisional swelling (i.e. growth of hardening or scarring tissue) and protrusion (i.e. dehiscence with visceral protrusion) were scored using criteria adapted from Panther et al. (2011), Schoonyan et al. (2017) and Wagner et al. (2000) (Table 1). Protrusion events were scored (0–3) by the severity of mass visible inside or outside the wound area (Fig. 1d). Incisional swelling was scored (0–2) based on the amount of macroscopically visible incisional swelling present (Fig. 1e). Three assessors blindly and independently measured and scored all incision photos (n = 142) to reduce bias from a single observer. Measurement and scoring values were averaged from the two assessors with the most similar measurements.
Diagrams illustrating the three measurements taken using ImageJ. Blue crosses represent the sutures. The black outlines and arrows indicate the measurement taken: (a) inflamed area, (b) incision length, and (c) incision width. Photos in (d) illustrate categories of protrusion (i.e. dehiscence with visceral protrusion) and numbers in bottom left corners correspond to the protrusion nature or score (see Table 1 for category descriptions). Photos in (e) illustrate categories of incisional swelling (see Table 1 for category descriptions).
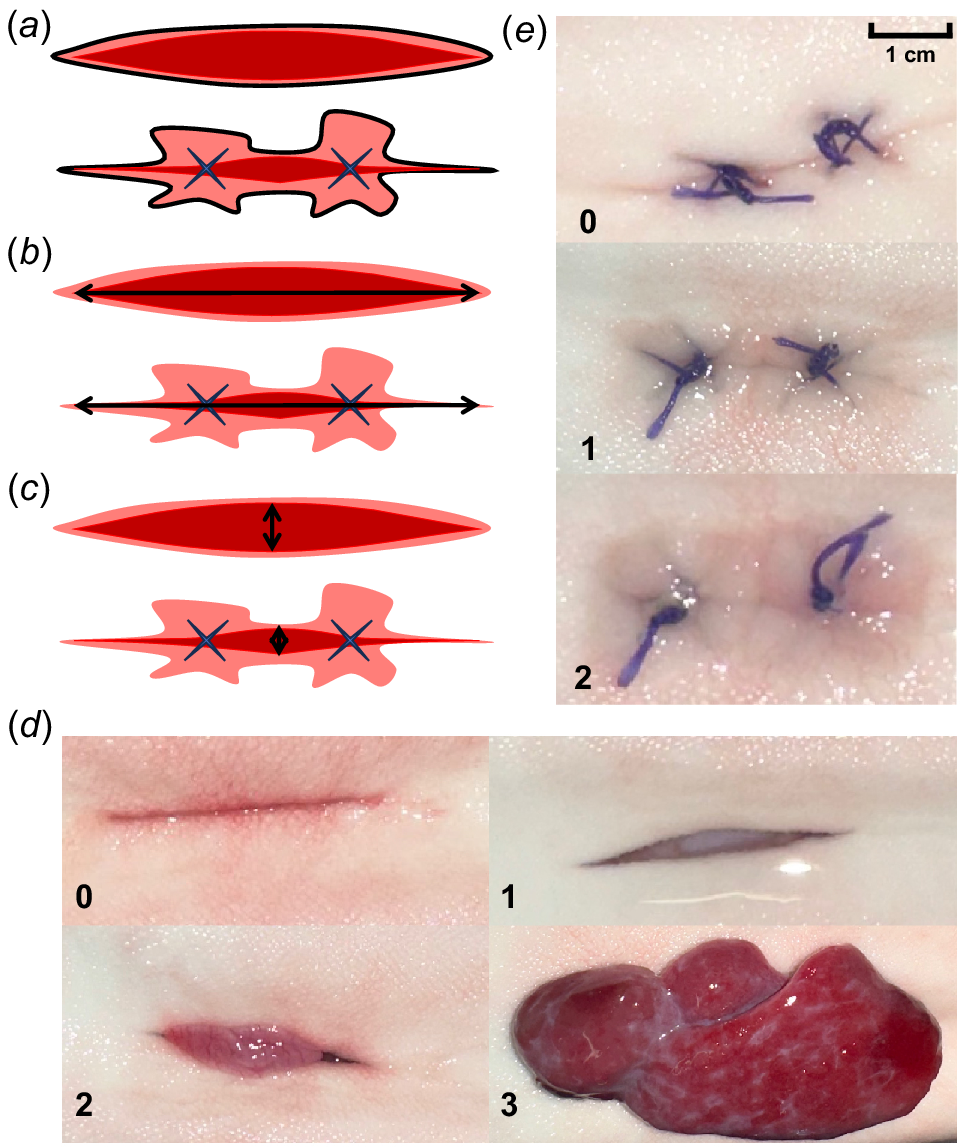
| Categories | Score | Description | |
|---|---|---|---|
| Incisional swelling | 0 | No incisional swelling present | |
| 1 | Moderate degree of incisional swelling present – raised, hardening or scarred skin area (less than 50%) present along the incisions and sutures (where applicable) | ||
| 2 | Advanced degree of incisional swelling present – raised, hardening or scarred skin area (more than 50%) around the entirety of the incision and sutures (where applicable) | ||
| Protrusion | 0 | No dehiscence or visceral protrusion | |
| 1 | Dehiscence without visceral protrusion, mass visible but remains inside the incision, incision visible and measurable | ||
| 2 | Dehiscence with a moderate degree of visceral protrusion, mass expelled and overhanging the incision, incision partly visible and incision length measurable | ||
| 3 | Dehiscence with severe degree of visceral protrusion, mass larger than the incision, incision line not visible or measurable |
Bacterial dynamics
Assessments of bacterial communities on the skin and wounds were conducted to determine how tagging may disrupt bacterial associates of elasmobranchs and if this affects the healing process (Luer et al. 2014; Caballero et al. 2020). Bacterial sampling was conducted to measure bacterial colonisation and growth following surgery. A control swab (FLOQSwabs) was first collected from the skin near the surgery area prior to any incisions. During the surgery process, an internal incision swab and wound swab were collected after initial (i.e. incision) and final (i.e. suture closure) surgical steps. Swabs of the incision site (i.e. sutured and non-sutured), skin control and water samples were taken weekly (on days 7, 14 and 21) post-surgery. Swab samples were placed into a 2-mL Eppendorf tube with 1 mL of sterilised seawater. Seawater was sterilised with tangential flow filtration (Dinsdale et al. 2008). Swabs were vortexed to mix at 300 rpm for 10 s and centrifuged at 2348g at room temperature for 60 s to dislodge bacteria from the swab into a working solution. Samples were then vortexed to homogenise the solutions and serially diluted in sterilised seawater. In total, 100 μL of the 1:1000 dilution was plated on blood agar (HBA Columbia Plates) and Marine Agar 2216 (Bacto). Cultures were grown for 48 h at 18°C after which colony forming units (CFUs) were counted at the dilution of 1:1000 and estimated as CFUs per millilitre:
Blood chemistry
Physiological responses to stress, determined through blood chemistry parameters (i.e. glucose and lactate), were measured to assess how the tagging and healing process influences stress response (Hoffmayer and Parsons 2001). Blood samples were taken on the day of surgery (day 0) and weekly post-surgery (days 7, 14, 21 and 42). Blood samples were drawn immediately after weighing the shark within the first 5 min of handling as the acid base balance of elasmobranchs blood can be severely disrupted by capture and handling (Cliff and Thurman 1984). Blood sampling was performed by a caudal vein puncture and ~4 mL of whole blood was collected using a sterile 21-gauge hypodermic needle (21G × 1.5ʺ Livingstone) and a 10-mL plastic disposable syringe (Luer Slip Livingstone). Whole blood was separated into 2 mL of plasma and 2 mL of red blood cells by centrifuging for 3 min at 845g at room temperature. Blood was stored in a −80°C freezer until blood assay analyses were conducted. Glucose and lactate levels were determined using enzyme-linked immunosorbent (ELISA) assays (Cayman Chemical Glucose Colorimetric Assay, Item Number 10009582). All plates were read on a CLARIOstar Plus microplate reader at 510 nm for glucose and 535 nm for lactate. Optimal sample dilution was determined from a pooled plasma sample that was serially diluted and assayed at five dilutions: 1:1, 1:2, 1:5, 1:10 and 1:20. As all dilutions except the 1:1 and 1:2 dilutions resulted in undetectable values, plasma samples were assayed at 1:1 dilution (undiluted) to ensure that both glucose and lactate levels were detectable by the assay. Glucose levels were calculated from a four parameter five-point standard curve ranging from 2.5 to 15 mg dL−1. Lactate levels were calculated from a linear regression fit standard curve ranging from 50 to 1000 μM. Lactate values from the assay are given in micromoles per litre and converted to millimoles per litre by multiplying values in micromoles per litre by 0.001. An external standard of 7.5 mg dL−1 for glucose and 200 μM for lactate was included on every plate and used to calculate inter-plate variation. All samples and standards were run in triplicate except for three samples that were run in duplicate (due to small sample volume). For all samples, the intra-sample coefficient of variation (CV) from the triplicate measurements was calculated. If the intra-sample CV was over 5%, the value that was most different was excluded and glucose levels and intra-sample CV were calculated from the two other remaining samples. The average intra-sample variation was calculated for glucose (2.71%) and lactate (6.56%). Inter-plate variation was determined from the external standard and was calculated for glucose (38.18%) and lactate (18.16%). Plate ID was included in the models to account for the inter-assay variation.
Statistical analysis
For all variables assessed, our main goal was to test whether leaving a wound non-sutured resulted in different relative responses across the study period compared to standard suturing practices. We constructed linear mixed-effects models (LMMs) for each response variable (except macroscopic scoring) using the lmer function from the lme4 package (ver. 1.1-35.3, D. Bates, see https://CRAN.R-project.org/package=lme4; Bates et al. 2015) in RStudio (ver. 4.4.0, Posit Software, PBC, Boston, MA, USA, see https://posit.co/products/open-source/rstudio/). Models included specific combinations of fixed interactions (e.g. days:treatment, sex:days:treatment) to assess if the rates of change differed across treatments. The following random effects were incorporated into the models depending on the specific response variables: crossed effects of shark identity, tag, side, swab and assay plate, and tank nested in water system; Table 2). Protrusion score was included as a fixed effect for the analysis of glucose and lactate to test whether the potentially compromised state of healing affected stress. For the macroscopic measurements (i.e. inflamed area, incision length and incision width), each value was converted to a percentage relative to day 0 to assess healing compared to the initial incision. All measurements and bacterial counts were log-transformed to normalise the variance and distribution of the response variables. The potential effect of the time taken to collect blood, i.e. duration between first handling and collection of blood sample, was explored to test whether it affected glucose and lactate levels. There was no correlation between time to take blood and biochemical parameters (Pearson correlation: P = 0.485 and 0.885; Fig. S2) and was therefore excluded from models. Correlations among the response variables and with predictor variables were tested using variance inflation factors (VIFs), with any VIFs >3 leading to the removal of correlated variables. Assumptions of heterogeneity of variance and normality was tested using residual and Q-Q plots respectively. Fixed effects within the model were considered significant (i.e. slope different from 0) when P-values were <0.05. The conditional (Rc) and marginal (Rm) R2 values (Nakagawa and Schielzeth 2013) of each model were calculated to estimate the proportion of variance explained by the fixed and random effects combined and fixed effects alone respectively. For macroscopic scoring (i.e. incisional swelling and protrusion), a non-parametric paired Wilcoxon rank sum test was used to compare the treatment types (i.e. sutured and non-sutured) within individuals, since the data was highly skewed (i.e. low occurrence of incisional swelling and protrusions). We also used a Wilcoxon rank sum test to compare surgeon expertise with wound healing metrics (inflamed area, incision length, incision width) using the relative healing rate at day 42 of each animal as the response variable. For blood chemistry results, glucose and lactate values were standardised (i.e. z-scored) relative to the plate it was processed on.
| Response variable | Global model | |
|---|---|---|
| Inflamed area, incision length, incision width, and bacterial CFUs | ~ days:treatment + sex:days:treatment + (1|shark identity) + (1|treatment) + (1|tag) + (1|side) + (1|system/tank) | |
| Glucose and lactate | ~ protrusion + days + sex:days + (1|assay plate) + (1|shark identity) |
CFU, colony forming unit.
Results
All animals survived the 42-day experimental trial except shark 239, which was euthanased during welfare monitoring after the study (i.e. 89 days post-surgery) due to a severe visceral protrusion from the non-sutured incision that initially occurred on day 28. The expert surgeon averaged ~13 min and the novice ~18 min for surgery time, from the time the animal was caught to being put back in the water. There were no significant differences in healing between expert and novice surgeons for inflamed area (W = 64, P = 0.82), incision length (W = 72, P = 0.46) and incision width (W = 75, P = 0.35). Tag retention was 100% in all individuals. Suture retention was 100% on day 42 following internal tagging. Animal weights (range: 1.74–6.35 kg; median: 2.4 kg) remained stable over the experimental trial.
Macroscopic assessment results
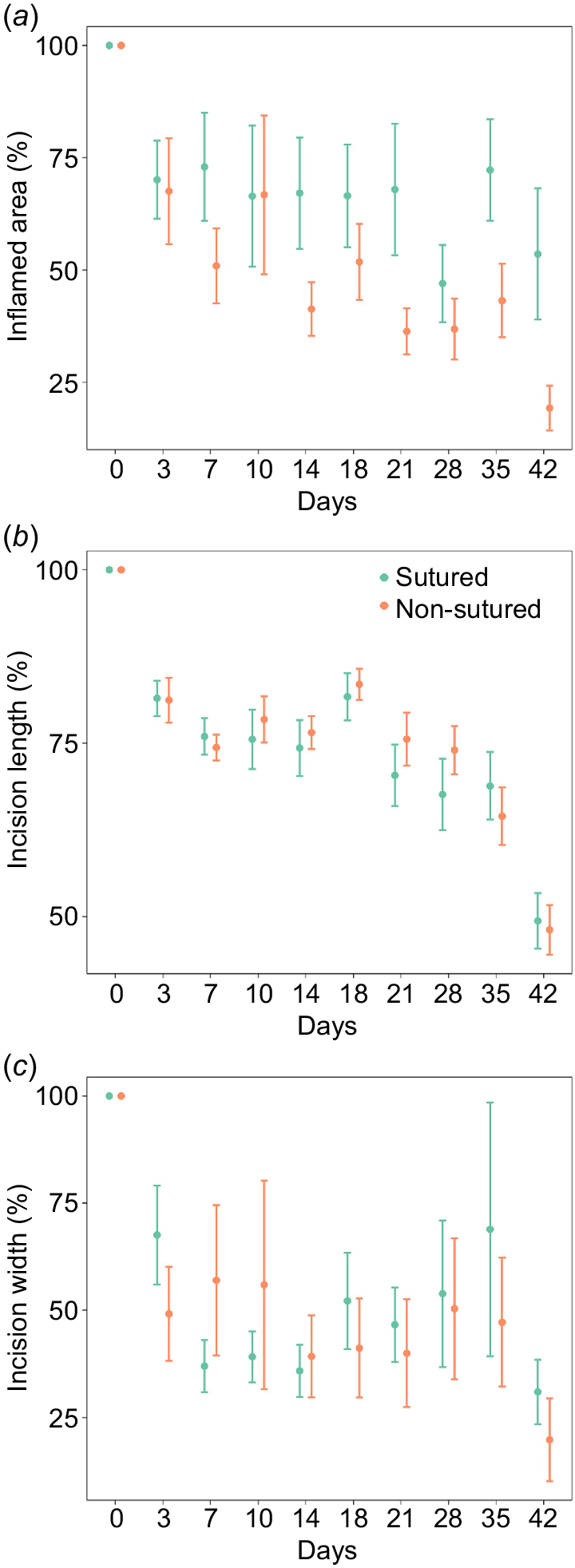
A total of 142 incision photos were measured and scored by three assessors across the 42-day study period. Macroscopic measurements (inflamed area, incision length and incision width) for shark 239 were excluded due to a protrusion hiding the incision. Scores for incisional swelling and protrusion could, however, still be recorded. Tag (i.e. sham and tag) and side (i.e. left and right) was included in the model and did not significantly affect the results of inflamed area, incision length and incision width. The interaction between treatment (i.e. sutured and non-sutured) and time (i.e. days) significantly affected the inflamed area, incision length and incision width (Fig. 2), and this effect was consistent across sex (Table 3). The fixed effects accounted for 29, 34 and 19% of variation, whereas the fixed and random effects accounted for 65, 71 and 60% of the variation respectively. Inflammation was higher in sutured incisions over the 42-day observational period, with non-sutured incisions steadily declining over time (Fig. 2a). Incision length was similar for sutured and non-sutured incisions over the 42-day observational period, healing quickly from day 35–42 (Fig. 2b). Differences in incision width were observed in the earlier stages of the study (days 0–10) – non-sutured incisions were initially healing at a slower rate with higher rates of variation – but after day 14 non-sutured incision width remained stable whereas sutured incision width increased until day 35 before quickly declining at day 42 (Fig. 2c).
Macroscopic changes of sutured and non-sutured incisions over the study period: (a) inflamed area percentage (%); (b) incision length (%); and (c) incision width (%). Error bars represent standard error, n = 11. The slight increase in incision length (%) in (b) from day 14 to 18 may reflect minor inconsistencies in photo quality.
| Days:treatment | Days:treatment:sex | ||||||
|---|---|---|---|---|---|---|---|
| d.f. | F-value | P-value | d.f. | F-value | P-value | ||
| Macroscopic assessment | |||||||
| Inflamed area | 17 | 7.532 | <0.001 | 18 | 0.764 | 0.734 | |
| Incision length | 19 | 20.179 | <0.001 | 20 | 1.264 | 0.245 | |
| Incision width | 17 | 3.290 | <0.001 | 18 | 0.519 | 0.940 | |
| Bacterial dynamics | |||||||
| Morphotype 1 | 15 | 23.496 | 0.042 | 16 | 0.735 | 0.748 | |
| Morphotype 2 | 15 | 6.218 | 0.147 | 16 | 0.366 | 0.988 | |
| Blood chemistry | Protrusion | Days | Days:sex | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | F-value | P-value | d.f. | F-value | P-value | d.f. | F-value | P-value | ||
| Glucose | 1 | 0.215 | 0.645 | 4 | 0.732 | 0.575 | 5 | 0.254 | 0.935 | |
| Lactate | 1 | 1.584 | 0.215 | 4 | 0.305 | 0.872 | 5 | 0.858 | 0.534 | |
P-values were considered significantly different (from the null hypothesis) when <0.05.
There was a significant difference in the extent of incisional swelling between treatments (Wilcoxon paired test: P < 0.001). Incisional swelling gradually increased throughout the study period in sutured incisions whereas non-sutured incisions had little to no incisional swelling present (Fig. 3a). Protrusion was also significantly different between treatments (Wilcoxon paired test: P < 0.001) and were irrespective of a tag or sham insertion. Protrusion events occurred predominantly in non-sutured incisions, though most cases were of least concern (i.e. score = 1) (Fig. 3b). Overall, 7 out of the 12 sharks (58.3%) experienced a protrusion event (n = 6 non-sutured incisions, n = 1 sutured incisions) across the 42-day observation period.
Bacterial dynamic results
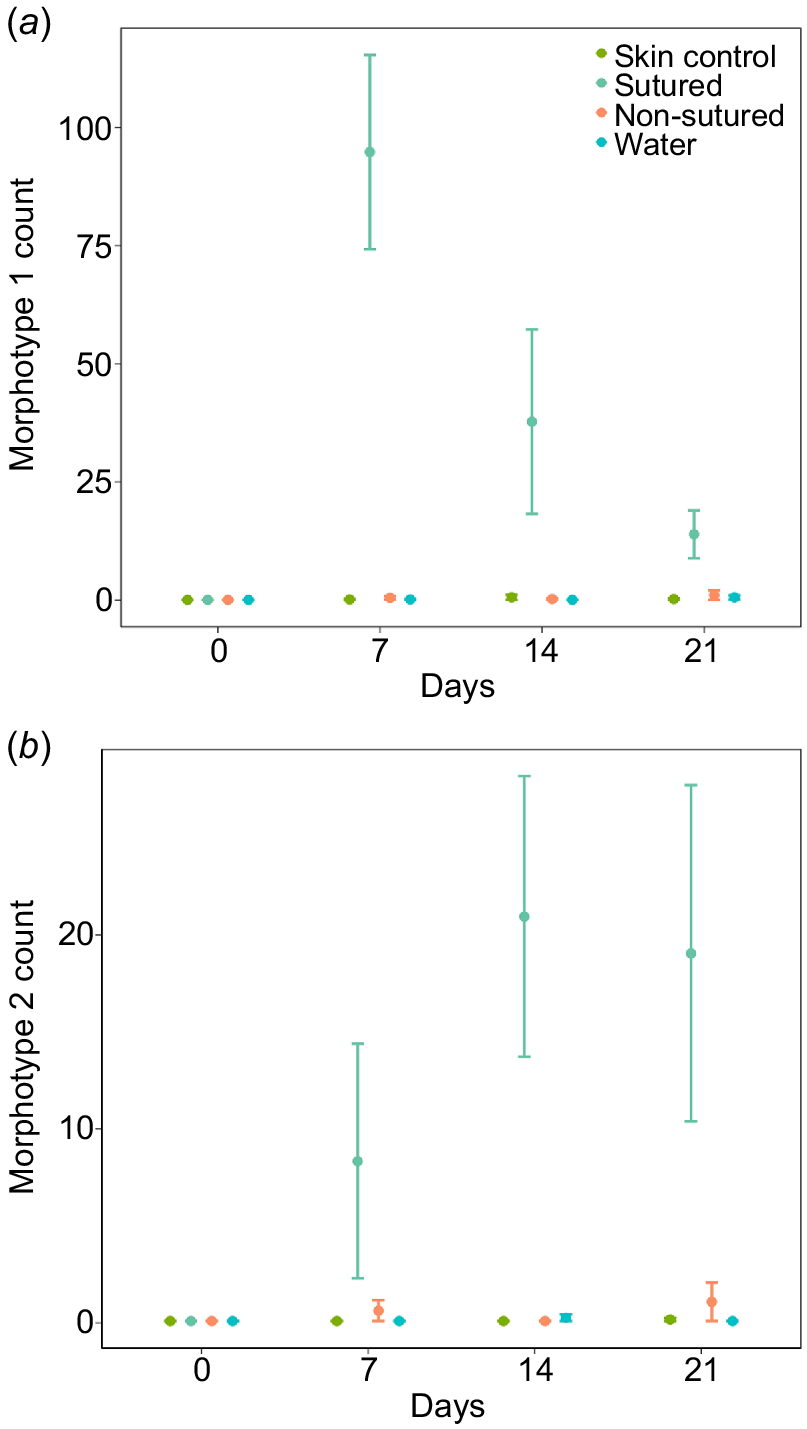
Two distinct bacterial colony types were observed, distinguished by colour, size and motility. Morphotype 1 was opaque, circular and rounded, and did not readily spread. Morphotype 2 was white, blotchy and asymmetrically round, and was often clumping with other bacterial colonies. The interaction between treatment (i.e. sutured and non-sutured) and time (i.e. days) significantly affected the number of morphotype 1 colonies but not morphotype 2 (Table 3). The fixed effects accounted for 68 and 37% of variation whereas the fixed and random effects accounted for 69 and 47% of the variation for morphotype 1 and 2 respectively. Independent of the interaction between treatment and time (which was aligned to our main question of whether rates of healing differed between treatments), it was evident that both morphotypes had higher counts at sutured sites compared to all other sample sites (Fig. 4a, b). Morphotype 1 had the highest counts, which peaked on day 7 before decreasing consistently afterwards (Fig. 4a). Counts of morphotype 2 peaked at day 14 with no evident decrease by day 21 (Fig. 4b).
Blood chemistry results
None of the tested variables or interactions affected the concentration of glucose or lactate in H. portusjacksoni (Table 3), which remained between 0.08 and 0.64 mmol L−1 for glucose and 0.32–8.36 mmol L−1 for lactate throughout the study period for both components (Fig. S3a, b).
Discussion
Our study assessed wound healing responses of H. portusjacksoni to two types of wound closure procedures following internal tagging. Specifically, we tested whether leaving an incision to heal without sutures to keep the incision closed would result in similar or different macroscopic, physiological and bacterial responses compared to sutured wounds. We found that tag retention (100%) did not differ between treatments. Similarly, although different rates of healing occurred, as measured by incision length and width, both methods were largely comparable across the 42-day study. Results were consistent irrespective of the tag or sham insertion. Blood biochemistry (i.e. glucose and lactate levels) was also similar regardless of the wound healing status. By contrast, inflammation and incisional swelling were higher in sutured incisions compared to non-sutured incisions. Bacterial dynamics (i.e. morphotype counts) between treatments were also different with more colonies forming on sutured sites than water samples, skin controls and non-sutured sites. Visceral protrusion events occurred from one sutured incision compared to six in non-sutured incisions, but most of these were minor and healed completely. Overall, these findings show considerable benefits to leaving wounds non-sutured but must be weighed with the risk of tissue protrusions. Ongoing evaluation of alternative tagging approaches contributes practical knowledge to animal care protocols, helping ensure research is conducted ethically and study findings are not biased from undue tagging effects.
Most of the healing metrics that we investigated supported the practice of leaving the wound non-sutured either through reduction of potentially debilitating factors or lack of difference between methods. Non-sutured incisions had reduced inflammation, incisional swelling and bacterial counts. Sutured incisions had consistently high (e.g. ~70% of initial values) relative areas of inflammation with no evident decrease by day 42. The occurrence and severity of incisional swelling from sutured incisions also increased during the study. By contrast, inflammation area in non-sutured incisions consistently decreased to <25% and occurrence of incisional swelling was rare. Inflammation and incisional swelling are concomitant processes associated with healing (Gurevich et al. 2020). Inflammation is an immune response to a foreign body, whereas incisional swelling restores tissue through excess deposition of extracellular matrix components (Schmidt et al. 2016; Gurevich et al. 2020). The presence of sutures commonly leads to a foreign body reaction in animals (i.e. when materials fail to integrate into the skin with a negative immune response), resulting in inflammation and eliciting repair mechanisms of fibrous tissue (Sveen et al. 2020). Inflammation was also higher in sutured juvenile white sturgeon (Acipenser transmontanus; Liss et al. 2018) and alewife (Alosa pseudoharengus; Tsitrin et al. 2020) compared to non-sutured individuals tagged with acoustic tags. For example, Liss et al. (2018) observed higher infection rates in sutured white sturgeon than non-sutured individuals. Furthermore, although incisional swelling can provide support and structure to a wound, potentially reducing the likelihood of a sutured incision reopening (Panther et al. 2011), the only sutured incision that resulted in a protrusion (on day 28) in our study also had severe and moderate incisional swelling on days 28 and 35 respectively. Thus, incisional swelling may still be detrimental in the context of wound closure and infection risk. The steady decrease in area of inflammation with non-sutured incisions highlights a lack of foreign body reaction, likely due to the lack of irritation from sutures.
Although all surgical equipment was sterilised before use, significant bacterial growth only at sutured sites highlights a biological response with the presence of sutures, and associated tissue inflammation, likely facilitating the growth and colonisation of bacteria. Bacterial colonisation from sutures has readily been hypothesised in literature on the tagging effects (Wagner et al. 2000; Schoonyan et al. 2017); however, few studies have measured bacterial dynamics in this context. Knights and Lasee (1996) identified bacterial isolates microscopically and concluded that tagged (and sutured) bluegill (Lepomis macrochirus) were not more susceptible to infection than reference fish. Similarly, in free-ranging black-tip reef sharks (Carcharhinus melanopterus), there were no differences in bacterial community compositions between healthy and injured skin (Pogoreutz et al. 2019). By contrast, Liss et al. (2018) observed higher fungal infection rates in sutured white sturgeon than non-sutured individuals. Recirculated water systems of limited volumes could have contributed to a different bacterial load than in the wild. For example, in the leopard shark (Triakis semifasciata), epidermal microbiomes were consistent between wild populations and populations reared in captivity in terms of alpha diversity; however, the relative abundance of certain key microbes differed in captive individuals (Goodman et al. 2022). Furthermore, bacteria adhesion can differ among suture materials and can be higher in multifilament suture compared to monofilament (Fowler et al. 2013), such that using a different material, e.g. monofilament, could have led to lower bacterial load. Although inflammation and incisional swelling are part of the healing process, chronic inflammation caused by closure method (i.e. cruciate, simple interrupted, simple continuous) or suture placement (i.e. restrictive or loose) may also increase the risk of infection. Colony forming units for morphotype 1 peaked at day 7 before declining substantially by day 21, which may be indicative of wound healing, immune response and inability to colonise the wound site. Antimicrobial properties present in the mucus layer of fishes contribute to host defences, microbial community regulation and wound healing (Luer et al. 2014; Ritchie et al. 2017), whereas the unique cutaneous structure of shark skin is also suspected to hinder bacterial infection (Pogoreutz et al. 2019). Although a clear decrease in CFUs was not observed in Morphotype 2 by the end of the sampling period, peak counts did not occur until day 14, suggesting there may have been a delay in growth (and defence) for this morphotype. Species identification through genetic sequencing was outside of the scope of this study limiting our ability to comment further on the origin and effect of the morphotypes identified.
Despite the role of elasmobranch microbiomes in host health not being well understood (Black et al. 2021), bacterial communities are known to provide explicit benefits to their marine hosts (Apprill 2017). For example, elasmobranchs often demonstrate remarkable ability to heal, and wounds are rarely observed infected (Doane et al. 2017), whereas the bacterial community composition remains highly conserved (Pogoreutz et al. 2019). Further work is needed to evaluate whether H. portusjacksoni microbiomes differ between experimental and wild conditions, as well as the effect of introduced synthetic materials (i.e. sutures) on the composition of the microbiome. Wild shark microbiomes fluctuate in taxonomy over time within a population, but the functional genes and ecological niches in the microbiome remain consistent and different from those of the environment (Doane et al. 2023). The consistency suggests that shark microbiomes are generally resistant to microbial colonisation from the environment, even in captivity and with some human intervention, but the introduction of a foreign body with different physical properties than shark epidermis may increase the potential for colonisation by foreign bacteria.
Several study metrics showed no difference between methods (i.e. sutured v. non-sutured), including macroscopic incision length and width, physiological parameters and tag retention. The lack of difference provides further support for leaving wounds non-sutured since handling times are reduced and there may be fewer pathways to infection (Liss et al. 2018). Medical grade absorbable sutures were selected for this study because of their demonstrated effectiveness in tagging studies and are expected to be absorbed after 3–6 months (Cooke et al. 2003; Deters et al. 2010; Holmes et al. 2022). Studies on the effects of different suture material (i.e. braided and monofilament) have not found substantial differences in wound healing or incision inflammation (Cooke et al. 2003; Wagner et al. 2000). We used braided suture material because, in our view, it holds knots better in the field. Although there were fluctuations in healing rates, incision sizes were similar between treatment type by day 42. Few studies have assessed wound healing in elasmobranchs, i.e. only 5 of 154 studies on acoustic telemetry tagging effects have been conducted on elasmobranchs (Matley et al. 2024b). The healing of H. portusjacksoni in our study was slower than C. melanopterus (n = 4), which showed complete wound closure from internal tagging within 29 days (Chin et al. 2015). We found ~45% of sutured and ~82% of non-sutured incisions were considered closed by day 42. Nevertheless, reasonably rapid healing from internal tagging is not surprising because elasmobranchs are capable of healing from severe wounds in the wild without intervention (Towner et al. 2012; Chin et al. 2015; Womersley et al. 2021). The few studies that have investigated the healing of non-sutured incisions following tagging were also supportive of the method. For example, healing in Oncorhynchus mykiss following acoustic tag insertion was similar, if not quicker, in non-sutured incisions (Huysman et al. 2020; Kelican et al. 2021). Complete wound closure in O. mykiss typically took up to 7 weeks in both sutured and non-sutured incisions (Kelican et al. 2021), which aligns with healing rate in this study. Similarly, Liss et al. (2018) found that non-sutured incisions in juvenile white sturgeon had greater incision gape than sutured incisions, but only for the first 14 days post-surgery – a trend that we also observed for incision width.
Blood chemistry assessment of glucose and lactate was used to evaluate secondary responses to stress. In elasmobranchs, stressful events trigger the secretion of 1α-hydrocorticosterone (a glucocorticoid ‘stress’ hormone). Glucocorticoid hormones mobilise energy reserves (e.g. by increasing glucose production) to provide vertebrates with energy to cope with stressors (Sapolsky et al. 2000; Skomal and Bernal 2010; Skomal and Mandelman 2012). Lactate is produced as a byproduct of anaerobic metabolism, increasing when an animal is physically exerting itself (Skomal and Bernal 2010; Skomal and Mandelman 2012). The low and consistent levels of glucose and lactate throughout the study suggest minimal physiological stress regardless of the healing status. Others have also noted the resilience of H. portusjacksoni to capture and handling stress, with the species showing 100% survival in all studies assessing post-release mortality (Frick et al. 2009; Braccini et al. 2012; Dapp et al. 2016). Physiological stress has been observed in H. portusjacksoni during captive stress trials (i.e. 30-min gill-net entanglement and serial blood sampling over 3 h), with glucose increasing from ~1.88 ± 0.08 mmol L−1 (baseline) to ~2.09 ± 0.14 mmol L−1 (Frick et al. 2009). Yet, our glucose levels were within or lower than the baseline levels in Frick et al. (2009), further supporting the lack of physiological stress in H. portusjacksoni throughout our study. Although glucose and lactate are standard stress markers in elasmobranchs (Cooper and Morris 1998; Hoffmayer and Parsons 2001; Fuller et al. 2020), the lack of detectable changes in the shark with severe protrusion questions whether other markers might be more appropriate to assess stress related to healing and should be investigated in future studies. We also could not specifically test whether the presence of sutures affected glucose and lactate levels due to each shark having both sutured and non-sutured incisions.
A final metric that supported the method of leaving surgical incisions non-sutured was the 100% tag retention. Although this metric needs to be evaluated in conjunction with visceral protrusion events (see below), the loss of tags is a significant technical concern in animal tracking studies. Tag loss often occurs independent of suture presence as part of an animal’s foreign body reaction, where tags are expelled through the body wall (often away from the incision site) following deposition of fibrous protein and encapsulation (Marty and Summerfelt 1986). An open wound may facilitate tag loss independent of a foreign body response; for example, if movement or friction with substrate in the environment pushes the tag through the open incision or if the sutures have not been adequately closed (Jepsen et al. 2013). We expected high tag retention, despite the presence of two incisions, because the ventral skin of H. portusjacksoni is inflexible and firm, and by minimising the size of the incision, the tag could only be inserted utilising a twisting motion (i.e. not reproducible from inside–out). Tag retention at non-sutured sites was also 100% in juvenile white sturgeon (Liss et al. 2018) and alewife (Tsitrin et al. 2020), and higher in non-sutured rainbow trout (compared to sutured individuals).
The occurrence of internal organ visceral protrusions was the most detrimental aspect of this study, particularly for non-sutured incisions, which made up six (of the seven) protrusion events. The protrusion of one individual was so severe that we had to intervene and euthanase the animal. All other individuals that experienced a protrusion event (scores 1 or 2) recovered without intervention, suggesting that not all protrusion events are detrimental to healing. Not surprisingly, a visceral protrusion can increase the risk of infection or predation. For example, a captive blacktip reef shark (C. melanopterus) with a prolapsed intestine suffered attacks from conspecifics, eventually resulting in infection and death (Morales and Dunker 1999). The benthic nature of H. portusjacksoni may also interfere with the healing of wounds because incisions on the ventral side are more likely to come into contact with substrate (Wagner et al. 2011). An alternate solution would be to make incisions more laterally (e.g. Huysman et al. 2020) to limit friction and reduce the direct impact of gravity pushing internal organs down through the wound opening. Although the relative tag:body size ratio was smaller than in other studies that left incisions non-sutured following tagging with an acoustic tag (e.g. ~24:335 mm in Kelican et al. 2021 and ~29:445 mm in Miller et al. 2014 v. ~30.5:800 mm), the tags were larger in our study. As a result, the wound opening in H. portusjacksoni was larger, presumably resulting in a higher likelihood of protrusion. Our sample size was too low to investigate additional variables such as incision placement and tag size; thus, future work could evaluate practical alternatives (e.g. lateral incisions and smaller tag to enable smaller incision) to avoid protrusions for H. portusjacksoni and similar species.
Conclusion
Our study provided a positive initial assessment of not suturing following internal tagging as shown by 11 out of 12 sharks (91.67%) healing consistently across treatments. However, the potential lethal impacts of protrusion events need to be accounted for when determining whether to suture or keep the incision non-sutured. For resilient species like H. portusjacksoni that can withstand capture and long handling times, the possibility of severe protrusion and possible lethal effects likely outweighs the benefits of saving handling time by not suturing. However, in species like hammerheads (Sphyrna spp.) sensitive to capture, handling and tagging (Gallagher et al. 2014), a reasonably small incision without the use of sutures may be viable to reduce handling stress and improve chances of survival, especially under difficult field conditions (e.g. time until retrieval, sea state).
Comprehensive assessments of wounds that incorporate macroscopic, bacterial and physiological components are rare in research on tagging effects, but are needed to establish safe and sustainable practices. Infections are one of the main concerns in fish tagging that may affect the behaviour and survival of animals and how researchers interpret movement data (Matley et al. 2024b), but bacterial investigations are almost non-existent. This study is one of the few that have investigated bacterial growth, empirically demonstrating that suture presence is the main route for colonisation, although the implications of this finding in connection to infection risk is not known. Future research on wound healing in elasmobranchs should also focus on identifying the types of bacteria that are established on wounds to better understand specific defence and healing mechanisms under varying conditions. Future trials could include a systemic assessment of bacteria load (e.g. from cultures of blood, coelomic fluid or kidneys), concurrent with a general post-mortem and histological assessment of the local incision to provide a more detailed health appraisal. Overall, this project provides novel information about healing using a spectrum of methods, and contributes to practical knowledge to help design future studies with animal welfare considerations in mind.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
Charlie Huveneers is an editor of Wildlife Research. Despite this relationship, they took no part in the review and acceptance of this manuscript, in line with the publishing policy. The authors declare that they have no further conflicts of interest.
Author contributions
This study was designed by Charlie Huveneers, Jordan Matley and Lewis Vaughan in conjunction with Brittany Heath, Xanthe Strudwick, Ryan Hesse and Ondi Crino. Internal tagging procedures were performed by C. Huveneers and B. Heath with the assistance of J. Matley, L. Vaughan, R. Hesse, C. Roberts and volunteers. Weekly sampling procedures (i.e. photos, blood samples, swabs and weights) were taken by B. Heath, R. Hesse, C. Roberts and volunteers. Laboratory work was conducted by B. Heath with assistance from R. Hesse and O. Crino. Data analysis was carried out by B. Heath, with assistance and guidance from C. Huveneers, J. Matley, X. Strudwick and O. Crino. B. Heath wrote the manuscript with suggestions provided by C. Huveneers, J. Matley, L. Vaughan, X. Strudwick, R. Hesse, O. Crino and C. Roberts.
Acknowledgements
We thank Bradley Hayman, Chloe Paterson, Esha Sarai, James Whitelaw, Laura Holmes, Lewis Hurley, Kiarna Taylor and Tessa Herewane who aided during sample collection, surgical procedures and animal care. We also thank James Harris for advice on tissue processing and The University of Adelaide microscopy unit of The Faculty of Health and Medical Sciences for sample processing.
References
Apprill A (2017) Marine animal microbiomes: toward understanding host–microbiome interactions in a changing ocean. Frontiers in Marine Science 4, 222.
| Crossref | Google Scholar |
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1), 1-48.
| Crossref | Google Scholar |
Bereiter-Hahn J (1986) Epidermal cell migration and wound repair. In ‘Biology of the integument: 2 vertebrates’. (Eds J Bereiter-Hahn, AG Matoltsy, KS Richards) pp. 443–471. (Springer) doi:10.1007/978-3-662-00989-5_23
Black C, Merly L, Hammerschlag N (2021) Bacterial communities in multiple tissues across the body surface of three coastal shark species. Zoological Studies 60, e69.
| Crossref | Google Scholar | PubMed |
Borucinska J, Adams DH, Frazier BS (2020) Histologic observations of dermal wound healing in a free-ranging blacktip shark from the Southeastern US atlantic coast: a case report. Journal of Aquatic Animal Health 32(4), 141-148.
| Crossref | Google Scholar | PubMed |
Braccini M, Van Rijn J, Frick L (2012) High post-capture survival for sharks, rays and chimaeras discarded in the main shark fishery of Australia? PLoS ONE 7(2), e32547.
| Crossref | Google Scholar | PubMed |
Caballero S, Maria Galeano A, Diego Lozano J, Vives M (2020) Description of the microbiota in epidermal mucus and skin of sharks (Ginglymostoma cirratum and Negaprion brevirostris) and one stingray (Hypanus americanus). PeerJ 8, e10240.
| Crossref | Google Scholar |
Castro-Santos T, Vono V (2013) Posthandling survival and PIT tag retention by alewives – a comparison of gastric and surgical implants. North American Journal of Fisheries Management 33(4), 790-794.
| Crossref | Google Scholar |
Chin A, Mourier J, Rummer JL (2015) Blacktip reef sharks (Carcharhinus melanopterus) show high capacity for wound healing and recovery following injury. Conservation Physiology 3(1), cov062.
| Crossref | Google Scholar |
Clemens BJ, Matley JK, Klinard NV, Lennox RJ, Sortland LK, Cooke SJ (2023) The need for reporting rationale and detailed methods in studies that surgically implant fish with electronic tracking devices. Fisheries Magazine 48(9), 388-394.
| Crossref | Google Scholar |
Cliff G, Thurman G (1984) Pathological and physiological effects of stress during capture and transport in the juvenile dusky shark, Carcharhinus obscurus. Comparative Biochemistry and Physiology – A. Physiology 78(1), 167-173.
| Crossref | Google Scholar |
Cooke SJ, Graeb BDS, Suski CD, Ostrand KG (2003) Effects of suture material on incision healing, growth and survival of juvenile largemouth bass implanted with miniature radio transmitters: case study of a novice and experienced fish surgeon. Journal of Fish Biology 62(6), 1366-1380.
| Crossref | Google Scholar |
Cooper AR, Morris S (1998) The blood respiratory, haematological, acid–base and ionic status of the Port Jackson shark, Heterodontus portusjacksoni, during recovery from anaesthesia and surgery: a comparison with sampling by direct caudal puncture. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 119(4), 895-903.
| Crossref | Google Scholar |
Dapp DR, Walker TI, Huveneers C, Reina RD (2016) Respiratory mode and gear type are important determinants of elasmobranch immediate and post-release mortality. Fish and Fisheries 17(2), 507-524.
| Crossref | Google Scholar |
Deters KA, Brown RS, Carter KM, Boyd JW, Eppard MB, Seaburg AC (2010) Performance assessment of suture type, water temperature, and surgeon skill in juvenile Chinook salmon surgically implanted with acoustic transmitters. Transactions of the American Fisheries Society 139(3), 888-899.
| Crossref | Google Scholar |
Dicken M, Nance SP, Smale M (2011) Sessile biofouling on tags from recaptured raggedtooth sharks (Carcharias taurus) and their effects on tagging studies. Marine and Freshwater Research 62(4), 359-364.
| Crossref | Google Scholar |
Dinsdale EA, Edwards RA, Hall D, Angly F, Breitbart M, Brulc JM, Furlan M, Desnues C, Haynes M, Li L, McDaniel L, Moran MA, Nelson KE, Nilsson C, Olson R, Paul J, Brito BR, Ruan Y, Swan BK, Stevens R, Valentine DL, Thurber RV, Wegley L, White BA, Rohwer F (2008) Functional metagenomic profiling of nine biomes. Nature 452(7187), 629-632.
| Crossref | Google Scholar | PubMed |
Doane MP, Haggerty JM, Kacev D, Papudeshi B, Dinsdale EA (2017) The skin microbiome of the common thresher shark (Alopias vulpinus) has low taxonomic and gene function β-diversity. Environmental Microbiology Reports 9(4), 357-373.
| Crossref | Google Scholar | PubMed |
Doane MP, Johnson CJ, Johri S, Kerr EN, Morris MM, Desantiago R, et al. (2023) The epidermal microbiome within an aggregation of leopard sharks (Triakis semifasciata) has taxonomic flexibility with gene functional stability across three time-points. Microbial Ecology 85(2), 747-764.
| Crossref | Google Scholar |
Domeier ML (2012) Boat-strike wound healing in carcharodon carcharias. In ‘Global perspectives on the biology and life history of the white shark’. (Ed. ML Domeier) pp. 102–109. (CRC Press) doi:10.1201/b11532-11
Fowler JR, Perkins TA, Buttaro BA, Truant AL (2013) Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clinical Orthopaedics and Related Research 471(2), 665-671.
| Crossref | Google Scholar | PubMed |
Frick LH, Reina RD, Walker TI (2009) The physiological response of Port Jackson sharks and Australian swellsharks to sedation, gill-net capture, and repeated sampling in captivity. North American Journal of Fisheries Management 29(1), 127-139.
| Crossref | Google Scholar |
Frick LH, Reina RD, Walker TI (2010) Stress related physiological changes and post-release survival of Port Jackson sharks (Heterodontus portusjacksoni) and gummy sharks (Mustelus antarcticus) following gill-net and longline capture in captivity. Journal of Experimental Marine Biology and Ecology 385(1), 29-37.
| Crossref | Google Scholar |
Fuller L, Stell E, Leary C, Parsons G (2020) Circulating adrenocorticotropic hormone levels, lactate levels, hematocrit and osmolality in relation to capture stress in Atlantic sharpnose sharks, Rhizoprionodon terraenovae. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 243, 110655.
| Crossref | Google Scholar |
Gallagher AJ, Serafy JE, Cooke SJ, Hammerschlag N (2014) Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. Marine Ecology Progress Series 496, 207-218.
| Crossref | Google Scholar |
Goodman AZ, Papudeshi B, Doane MP, Mora M, Kerr E, Torres M, et al. (2022) Epidermal microbiomes of leopard sharks (Triakis semifasciata) are consistent across captive and wild environments. Microorganisms 10(10), 2081.
| Crossref | Google Scholar |
Gurevich DB, French KE, Collin JD, Cross SJ, Martin P (2020) Live imaging the foreign body response in zebrafish reveals how dampening inflammation reduces fibrosis. Journal of Cell Science 133(5), jcs236075.
| Crossref | Google Scholar |
Haeseker SL, Carmichael JT, Hightower JE (1996) Summer distribution and condition of striped bass within Albemarle Sound, North Carolina. Transactions of the American Fisheries Society 125(5), 690-704.
| Crossref | Google Scholar |
Hammerschlag N, Gallagher AJ, Lazarre DM (2011) A review of shark satellite tagging studies. Journal of Experimental Marine Biology and Ecology 398(1–2), 1-8.
| Crossref | Google Scholar |
Heupel M, Simpfendorfer CA, Bennett MB (1998) Analysis of tissue responses to fin tagging in Australian carcharhinids. Journal of Fish Biology 52(3), 610-620.
| Crossref | Google Scholar |
Hoffmayer ER, Parsons GR (2001) The physiological response to capture and handling stress in the Atlantic sharpnose shark, Rhizoprionodon terraenovae. Fish Physiology and Biochemistry 25, 277-285.
| Crossref | Google Scholar |
Holmes BJ, Williams SM, Barnett A, Awruch CA, Currey-Randall LM, Ferreira LC, Huveneers C, Jones RL, Nowland SJ, Taylor A, Tracey SR, Waltrick D (2022) 13 Research methods for marine and estuarine fishes. In ‘Wildlife research in Australia: practical and applied methods’. (Eds BP Smith, HP Waudby, C Alberthsen, JO Hampton) pp. 257–286. (CSIRO Publishing)
Huveneers C, Simpfendorfer C (2015) Port Jackson Shark Heterodontus portusjacksoni. In ‘The IUCN Red List of Threatened Species 2015’. e.T39334A68625721. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/39334/68625721 [Verified 30 July 2025]
Huysman N, White S, Kientz J, Voorhees JM, Barnes ME (2020) Suture-less implantation of acoustic transmitters in two salmonids. International Journal of Sciences 9(03), 60-64.
| Crossref | Google Scholar |
Jepsen N, Koed A, Thorstad EB, Baras E (2002) Surgical implantation of telemetry transmitters in fish: how much have we learned? In ‘Aquatic Telemetry: Proceedings of the Fourth Conference on Fish Telemetry in Europe’, 26–30 June 2001, Trondheim, Norway. (Eds EB Thorstad, IA Fleming, TF Næsje) Developments in Hydrobiology 165, 239–248. (Springer) doi:10.1007/978-94-017-0771-8_28
Jepsen N, Christoffersen M, Munksgaard T (2008a) The level of predation used as an indicator of tagging/handling effects. Fisheries Management and Ecology 15(5–6), 365-368.
| Crossref | Google Scholar |
Jepsen N, Mikkelsen JS, Koed A (2008b) Effects of tag and suture type on survival and growth of brown trout with surgically implanted telemetry tags in the wild. Journal of Fish Biology 72(3), 594-602.
| Crossref | Google Scholar |
Jepsen N, Boutrup TS, Midwood JD, Koed A (2013) Does the level of asepsis impact the success of surgically implanting tags in Atlantic salmon? Fisheries Research 147, 344-348.
| Crossref | Google Scholar |
Jepsen N, Larsen MH, Aarestrup K (2017) Performance of fast absorbable sutures and histo-glue for closing incisions in brown trout. Transactions of the American Fisheries Society 146(6), 1233-1237.
| Crossref | Google Scholar |
Jones R (2023) Vitamin/mineral supplements for sharks and rays. (The Aquarium Vet: Melbourne, Vic., Australia) Available at https://www.theaquariumvet.com/wp-content/uploads/2018/09/Discussion-Paper-Vitamin-Supplements-for-Sharks-and-Rays.pdf
Kelican AN, Huysman N, Van Rysselberge LA, Voorhees JM, Barnes ME (2021) Assessment of a novel surgical technique for acoustic transmitter insertion. Open Journal of Veterinary Medicine 11(7), 247-257.
| Crossref | Google Scholar |
Kessel ST, Hussey NE (2015) Tonic immobility as an anaesthetic for elasmobranchs during surgical implantation procedures. Canadian Journal of Fisheries and Aquatic Sciences 72(9), 1287-1291.
| Crossref | Google Scholar |
Knights BC, Lasee BA (1996) Effects of implanted transmitters on adult bluegills at two temperatures. Transactions of the American Fisheries Society 125(3), 440-449.
| Crossref | Google Scholar |
Larsen MH, Thorn AN, Skov C, Aarestrup K (2013) Effects of passive integrated transponder tags on survival and growth of juvenile Atlantic salmon Salmo salar. Animal Biotelemetry 1, 19.
| Crossref | Google Scholar |
Liss SA, Ashton NK, Brown RS, Walker RW, Bates P, Klassen C, Backhouse S (2018) Evaluation of four surgical implantation techniques for age-0 white sturgeon (Acipenser transmontanus Richardson, 1836) with a new acoustic transmitter. Journal of Applied Ichthyology 34(2), 382-389.
| Crossref | Google Scholar |
Luer CA, Walsh C, Ritchie K, Edsberg L, Wyffels J, Luna V, Bodine A (2014) Novel compounds from shark and stingray epidermal mucus with antimicrobial activity against wound infection pathogens. Annual report, 15 Feb 2013–14 Feb 2014. (Mote Marine Laboratory: Sarasota, FL, USA) Available at https://apps.dtic.mil/sti/html/tr/ADA600463/
Marra NJ, Richards VP, Early A, Bogdanowicz SM, Pavinski Bitar PD, Stanhope MJ, Shivji MS (2017) Comparative transcriptomics of elasmobranchs and teleosts highlight important processes in adaptive immunity and regional endothermy. BMC Genomics 18(1), 87.
| Crossref | Google Scholar |
Marshall AD, Bennett MB (2010) The frequency and effect of shark-inflicted bite injuries to the reef manta ray Manta alfredi. African Journal of Marine Science 32(3), 573-580.
| Crossref | Google Scholar |
Marty GD, Summerfelt RC (1986) Pathways and mechanisms for expulsion of surgically implanted dummy transmitters from channel catfish. Transactions of the American Fisheries Society 115(4), 577-589.
| Crossref | Google Scholar |
Matley JK, Klinard NV, Martins APB, Aarestrup K, Aspillaga E, Cooke SJ, Cowley PD, Heupel MR, Lowe CG, Lowerre-Barbieri SK, Mitamura H, Moore J-S, Simpfendorfer CA, Stokesbury MJW, Taylor MD, Thorstad EB, Vandergoot CS, Fisk AT (2022) Global trends in aquatic animal tracking with acoustic telemetry. Trends in Ecology & Evolution 37(1), 79-94.
| Crossref | Google Scholar | PubMed |
Matley JK, Klinard NV, Jaine FR, Lennox RJ, Koopman N, Reubens JT, Harcourt RG, Cooke SJ, Huveneers C (2024a) Long-term effects of tagging fishes with electronic tracking devices. Fish and Fisheries 25(6), 1009-1025.
| Crossref | Google Scholar |
Matley JK, Klinard NV, Martins AB, Oakley-Cogan A, Huveneers C, Vandergoot CS, Fisk AT (2024b) TrackdAT, an acoustic telemetry metadata dataset to support aquatic animal tracking research. Scientific Data 11(1), 143.
| Crossref | Google Scholar | PubMed |
McGregor F, Richardson AJ, Armstrong AJ, Armstrong AO, Dudgeon CL (2019) Rapid wound healing in a reef manta ray masks the extent of vessel strike. PLoS ONE 14(12), e0225681.
| Crossref | Google Scholar | PubMed |
Miller EA, Froehlich HE, Cocherell DE, Thomas MJ, Cech JJ Jr, Klimley AP, Fangue NA (2014) Effects of acoustic tagging on juvenile green sturgeon incision healing, swimming performance, and growth. Environmental Biology of Fishes 97, 647-658.
| Crossref | Google Scholar |
Morales P, Dunker F (1999) Suspected intestinal torsion in a blacktip reef shark (Carcharhinus melanopterus). Journal of Zoo and Wildlife Medicine 30(1), 170-172.
| Google Scholar |
Murray MJ (2002) Fish surgery. Seminars in Avian and Exotic Pet Medicine 11(4), 246-257.
| Crossref | Google Scholar |
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution 4(2), 133-142.
| Crossref | Google Scholar |
Nasby-Lucas N, Domeier ML (2020) Impact of satellite linked radio transmitting (SLRT) tags on the dorsal fin of subadult and adult white sharks (Carcharodon carcharias). Bulletin of Marine Science 96(1), 23-30.
| Crossref | Google Scholar |
Orrell DL, Andrzejaczek S, Armstrong AO, Barbosa Martins A, Branco I, Charvet P, Chin A, Elston C, Espinoza M, Greenway ESI, McCully Phillips SR, Mickle MF, Murray TS, Silva JF, Thorburn J, Wosnick N (2025) Current methods and best practice recommendations for skate and ray (Batoidea) research: capture, handling, anaesthesia, euthanasia, and tag attachment. Reviews in Fish Biology and Fisheries 35, 117-144.
| Crossref | Google Scholar |
Panther JL, Brown RS, Gaulke GL, Deters KA, Woodley CM, Eppard MB (2011) Influence of incision location on transmitter loss, healing, survival, growth, and suture retention of juvenile Chinook salmon. Transactions of the American Fisheries Society 140(6), 1492-1503.
| Crossref | Google Scholar |
Pogoreutz C, Gore MA, Perna G, Millar C, Nestler R, Ormond RF, Clarke CR, Voolstra CR (2019) Similar bacterial communities on healthy and injured skin of black tip reef sharks. Animal Microbiome 1(1), 9.
| Crossref | Google Scholar | PubMed |
Rangel BS, Viegas R, Bettcher VB, Garla RC (2022) Eye healing in a free-ranging whitespotted eagle ray (Aetobatus narinari) following shark-inflicted bite injuries. Journal of Fish Biology 100(2), 590-593.
| Crossref | Google Scholar | PubMed |
Reif W-E (1978) Wound healing in sharks: form and arrangement of repair scales. Zoomorphologie 90(2), 101-111.
| Crossref | Google Scholar |
Ritchie KB, Schwarz M, Mueller J, Lapacek VA, Merselis D, Walsh CJ, Luer CA (2017) Survey of antibiotic-producing bacteria associated with the epidermal mucus layers of rays and skates. Frontiers in Microbiology 8, 1050.
| Crossref | Google Scholar | PubMed |
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews 21(1), 55-89.
| Crossref | Google Scholar | PubMed |
Schmidt JG, Andersen EW, Ersbøll BK, Nielsen M (2016) Muscle wound healing in rainbow trout (Oncorhynchus mykiss). Fish & Shellfish Immunology 48, 273-284.
| Crossref | Google Scholar | PubMed |
Schoonyan A, Kraus RT, Faust MD, Vandergoot CS, Cooke SJ, Cook HA, Hayden TA, Krueger CC (2017) Estimating incision healing rate for surgically implanted acoustic transmitters from recaptured fish. Animal Biotelemetry 5(1), 15.
| Crossref | Google Scholar |
Shorgan MB, Raby GD, Fedus AL, Howell BE, Haniford LSE, Howitt LC, Klinard NV, Matley JK, Brownscombe JW, Cooke SJ, Fisk AT (2025) Effects of surgical implantation of electronic tags in fishes: a review and meta-analysis. ResearchSquare 2025, ver. 1 [Preprint, published 6 March 2025].
| Crossref | Google Scholar |
Skomal GB, Mandelman JW (2012) The physiological response to anthropogenic stressors in marine elasmobranch fishes: a review with a focus on the secondary response. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 162(2), 146-155.
| Crossref | Google Scholar |
Sveen L, Karlsen C, Ytteborg E (2020) Mechanical induced wounds in fish – a review on models and healing mechanisms. Reviews in Aquaculture 12(4), 2446-2465.
| Crossref | Google Scholar |
Swanberg TR, Schmetterling DA, McEvoy DH (1999) Comparison of surgical staples and silk sutures for closing incisions in rainbow trout. North American Journal of Fisheries Management 19(1), 215-218.
| Crossref | Google Scholar |
Tsitrin E, McLean MF, Gibson AJF, Hardie DC, Stokesbury MJW (2020) Feasibility of using surgical implantation methods for acoustically tagging alewife (Alosa pseudoharengus) with V5 acoustic transmitters. PLoS ONE 15(11), e0241118.
| Crossref | Google Scholar | PubMed |
Wagner GN, Stevens ED, Byrne P (2000) Effects of suture type and patterns on surgical wound healing in rainbow trout. Transactions of the American Fisheries Society 129(5), 1196-1205.
| Crossref | Google Scholar |
Wagner GN, Cooke SJ, Brown RS, Deters KA (2011) Surgical implantation techniques for electronic tags in fish. Reviews in Fish Biology and Fisheries 21, 71-81.
| Crossref | Google Scholar |
Womersley F, Hancock J, Perry CT, Rowat D (2021) Wound-healing capabilities of whale sharks (Rhincodon typus) and implications for conservation management. Conservation Physiology 9(1), coaa120.
| Crossref | Google Scholar |
Wosnick N, Leite RD, Balanin S, Chaves AP, de Senna Gastal ER, Hauser-Davis RA, Giareta EP (2023) Behavioral and visual stress-induced proxies in elasmobranchs. Reviews in Fish Biology and Fisheries 33(1), 175-199.
| Crossref | Google Scholar |