Deep snow and human influence affect survival of an ungulate at the northern part of its range
Alexej P. K. Sirén A * , Nathan R. Bieber B and Kyle Ravana B
A * , Nathan R. Bieber B and Kyle Ravana B
A
B
Abstract
Winter is generally considered the limiting season for wildlife populations living in high-latitude/altitude regions. Logically, managers have used temperature and snow variables or indices to predict survival and adjust harvest quotas according to winter severity. A common approach is to use a winter severity index (WSI) to evaluate the effects of winter severity on game species (e.g. ungulates). However, a WSI requires both snow and temperature data, ideally collected at broad spatial scales and at high temporal resolution (e.g. weekly), and can take considerable effort to collect the data.
We evaluated the influence of several winter variables, including a WSI that has been used for predicting winter survival over the past several decades, on adult female white-tailed deer (Odocoileus virginianus) survival in northern New England. We also considered the influence of humans (anthropogenic influence), primarily in the form of artificial feeding, on deer survival.
We monitored 200 radio-collared deer in four different management units that spanned the gradient of winter climate and anthropogenic influence in the region over the course of seven winters (2015–2021). We used known-fate, staggered-entry, binomial models in a Bayesian framework to evaluate factors that influenced deer survival.
We recorded 64 mortalities that were primarily associated with predation in the northern regions but also from deer–vehicle collisions. Average (95% credible intervals [CI]) winter survival was 78% (66–89%), with the lowest and highest estimates occurring during the harshest (72%; CI = 54–85%) and mildest (82%; CI = 67–93%) winters respectively. The top survival model included snow depth under closed canopy and an additive effect of anthropogenic influence; snow depth had a negative effect, and anthropogenic influence had a positive effect on survival. However, WSI had low predictive power, and predicted higher survival in most winters and regions.
Our study indicated that winter survival rates of adult female deer were most influenced by snow and anthropogenic factors in northern New England and that WSI indices for evaluating the impacts of winter on deer populations were not robust for monitoring survival and determining harvest quotas.
Natural resource agencies should consider using alternative field methods (e.g. remote cameras) for monitoring annual snowpack and deer survival in high latitude regions. Further research is needed to determine which anthropogenic factors have a negative and positive impact on deer survival.
Keywords: abiotic stress, anthropogenic factors, artificial feeding, forest cover, Odocoileus virginianus, snow depth, survival, white-tailed deer, winter severity, WSI.
Introduction
Understanding the impact of climate and anthropogenic factors on wildlife populations is important for effective management. According to theory, harsh climate (e.g. deep snow and low temperatures) is considered a limiting factor for populations at high latitude range margins (Louthan et al. 2015; Sirén and Morelli 2020). However, biotic factors such as food or habitat availability can sometimes buffer the negative effects of harsh abiotic stress and allow populations to persist (or even expand ranges) at high latitudes (Sirén and Morelli 2020). Consequently, it is imperative to understand the relative contribution of abiotic and biotic factors influencing populations along range margins. This is especially important for game species in high-latitude regions where pre-harvest quotas need to be established on the basis of the severity of winter conditions during the previous year (Norton et al. 2021). As such, developing models that best predict sensitive demographic parameters (e.g. survival) can improve wildlife management and allow for a better understanding of the impacts of climate and habitat change on game populations (Weiskopf et al. 2019).
White-tailed deer (Odocoileus virginianus) are subject to severe winter conditions in the northern parts of their range (Fieberg et al. 2008; Kautz et al. 2020; Norton et al. 2021). Although deer are adapted to tolerate some harsh winter conditions (e.g. reduced movement during snowy periods; Moen 1976), severe winters can result in significant decreases in winter survival for northern populations (Fuller 1990; Whitlaw et al. 1998; Dumont et al. 2000; Patterson and Power 2002; Norton et al. 2021; Lasharr et al. 2023). Deep snow makes movement more energetically costly, and low temperatures make it more difficult for deer to maintain body temperature (Moen 1976). There may also be some threshold effects with regards to snow depth and temperature and a mediating effect from anthropogenic factors. For example, Norton et al. (2021) found that cumulative days above 30.5 cm snow depth and days above 0°C influenced survival in forested areas and cumulative days above 0°C influenced survival in farmlands, where there was more access to food. To account for winter survival, wildlife managers must have a solid understanding of how different variables affect winter survival of deer, including abiotic factors (e.g. snow depth, temperature) and biotic factors such as habitat availability and artificial feeding, or food provided by humans to improve survival or viewing opportunities (Norton et al. 2021; Lasharr et al. 2023).
Northern deer populations often migrate to deer wintering areas, or yards, to adjust to snow conditions and food availability (Nelson 1995; Van Deelen et al. 1998; Ditchkoff and Servello 2002; Sabine et al. 2002). The habitat within these natural deer wintering areas is often characterized by mature softwoods with high levels of canopy closure and interspersed or adjacent hardwood browse (Morrison et al. 2003; DelGiudice et al. 2013). These closed-canopy softwood areas intercept snow, resulting in lower snowpack at ground level, which facilitates establishment of trail networks, and serves as thermal and wind cover (Morrison et al. 2003; Lefort et al. 2007). In Maine, and in other northern regions, deer may have historically used natural winter cover (DelGiudice et al. 2013; Simons-Legaard et al. 2018) but now often frequent towns and developed areas where they have access to compacted snow, refuge from predation, and artificial feed (Dawe et al. 2014; Fortin et al. 2015). In particular, artificial feeding is widespread in some northern regions, but the extent to which it provides a survival advantage is context dependent (Tarr and Pekins 2002; Milner et al. 2014; Fortin et al. 2015).
Some studies have used a winter severity index (WSI) to describe multiple winter weather variables that affect winter survival (DelGiudice et al. 2002; Kautz et al. 2020). For example, DelGiudice et al. (2002) found that a WSI, which accounted for the number of days with snow depth of ≥38 cm and ambient temperature lower than −17.7°C during 1 November−31 May, best predicted winter survival, but snow depth alone was nearly as predictive. Kautz et al. (2020), by using the same WSI as DelGiudice et al. (2002), found the best fit model of winter survival to include WSI, cumulative snow-free days, and body mass at capture. The number of snow free days was the most impactful variable, followed by WSI and body mass. Contrastingly, Garroway and Broders (2005) found that snow depth rather than rainfall and temperature best predicted winter survival. These WSIs are often used by wildlife management agencies to estimate and account for winter survival rates and develop pre-harvest quotas (Norton et al. 2021).
Historically, Maine Department of Inland Fisheries and Wildlife (MDIFW) estimated winter mortality rates by using a WSI model developed in the 1990s from dead deer surveys in deer wintering areas and deer density estimates from pellet group counts (Maine Department of Inland Fisheries and Wildlife (MDIFW) 2007). This modeling work (hereafter ‘historical WSI model’) related snow and temperature conditions with observations of dead deer in deer wintering areas from mid-December through April. Specifically, the WSI was calculated using the following formula:
where SD = snow depth in open canopy forests, SK = deer sinking depths in open canopy forests, Tm = long-term mean temperature, and Ts = current season mean temperature are multiplied by 33.3. MDIFW would then predict winter mortality rates by exponentiating 2.29 by 0.222 × WSI for each region and winter (Maine Department of Inland Fisheries and Wildlife (MDIFW) 2007). In summary, the WSI formula combined with the predictions of winter mortality rates constituted the historical WSI model. The estimates of winter mortality were then used to adjust doe removal rates when developing recommendations for antlerless harvest. Because the estimates were not specific to adult females, it was not ideal to use them to adjust doe removal rates and estimates specific to adult females were needed. Further, the estimates relied on site visits, which were labor intensive and costly. For example, using the historical WSI model developed in the 1990s, Maine collected weekly snow depth, deer sinking depth, and temperature data at 26 sites across the state over a 20-week period to produce annual winter mortality estimates, and because some of these data required weekly site visits, they were very costly and time-consuming to maintain.
Our primary objectives were to determine what factors most influenced winter survival in adult female white-tailed deer in Maine and to develop a predictive equation allowing for the estimation of winter survival. We also examined differences in winter survival rates between deer living in traditional deer wintering areas and those exposed to anthropogenic influences, primarily in the form of artificial feeding. Our primary hypotheses were that winter temperature and snowpack variables would influence winter survival (Table 1). We predicted that colder and snowier winters would be negatively correlated with survival and that deer exposed to anthropogenic influences would have higher survival rates (Table 1). We also evaluated the WSI that Maine has used for predicting winter survival as a predictor variable on deer survival and compared it with other winter variables. Last, we compared predictions of overwinter deer survival across the entire state of Maine for a seven-winter period (2015–2021) between the top model and the historical WSI model that MDIFW has used to predict winter mortality rates since the 1990s.
| Variable name | Description | Hypothesis | Prediction | |
|---|---|---|---|---|
| odep | Snow depth measured under open canopy | Snow influences the mobility of deer, primarily via access to food and ability to escape predators, thereby influencing survival | Negative | |
| cdep | Snow depth measured under closed canopy | |||
| osnk | Sinking depth of snow measured under open canopy | |||
| csnk | Sinking depth of snow measured under closed canopy | |||
| cm25.4 | Number of snowstorms >25.4 cm recorded within a 2-week period (cumulative over entire winter) | |||
| d45.7 | Number of days with snow depth of ≥45.7 cm (cumulative over entire winter) | |||
| atmp | Average temperature (°C) during a 2-week period | Temperature affects thermoregulation of deer, especially in the winter, and can affect their metabolism, which will influence survival rates | Negative | |
| d0C | Number of days with temperature below 0°C during a 2-week period (cumulative over entire winter) | |||
| dneg17.8C | Number of days with temperature below −17.8°C during a 2-week period (cumulative over entire winter) | |||
| wsi | Winter severity index (WSI) was calculated using snow and sinking depths in open canopy cover and long-term and current season mean temperatures | The severity of winter can affect both their mobility and ability to thermoregulate, and the combined effects will influence survival | Negative | |
| ANTHRO | Anthropogenic status included artificial feed and human development determined using knowledge of capture and study sites and aerial imagery where status was uncertain | Artificial feeding from humans can offset the negative impacts of deep snow and cold winters, providing energy to replenish depleted fat reserves and affecting survival. Human development may provide refuge from predation and easier travel in plowed walkways and on sled trails | Positive | |
| wmd | Wildlife management district (WMD) was included as a random effect | Deer within different WMDs will be affected by similar (and unique) environmental conditions to those of other WMDs and therefore have similar survival rates | – | |
| winter | Winter of study was included as a random effect. | Annual variability in climate and food availability influence deer survival | – |
Materials and methods
Study area
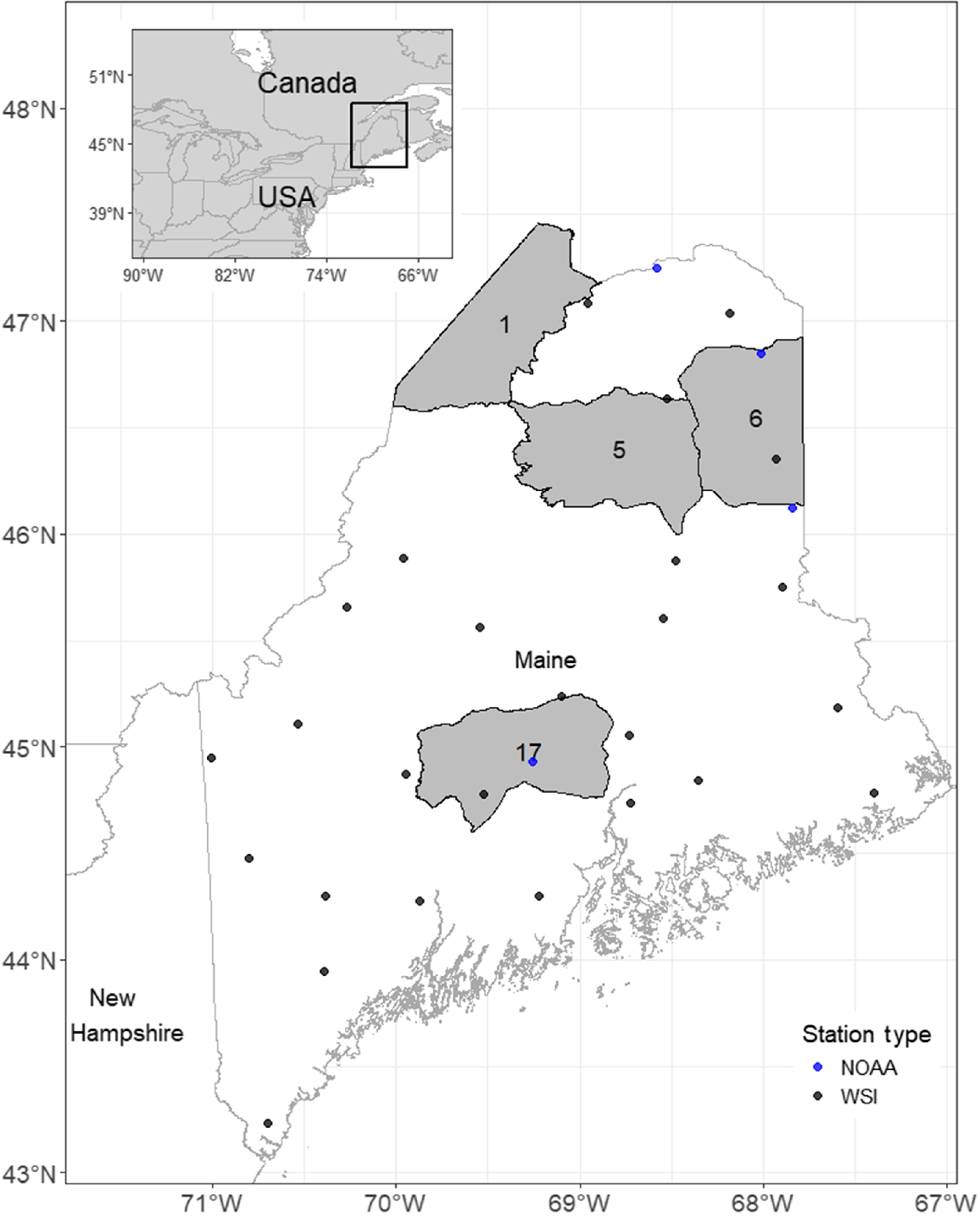
We captured and radio-collared deer in four Wildlife Management Districts (WMDs) in central and northern Maine (WMDs 1, 5, 6, 17; Fig. 1). Our WMD 1 study site extended from the town of Allagash, ME (47°05′N, 69°02′W), south along the Allagash River to the Ben Glazier Brook deer wintering area in T14 R12 WELS, Maine (46°55′N, 69°14′W). Landcover in WMD 1 was primarily Laurentian–Acadian Northern Hardwood Forest and Acadian Low Elevation Spruce-Fir-Hardwood Forest (Ferree and Anderson 2013). Deer in WMD 1 were collared in the Ben Glazier Brook deer wintering area as well as around the town of Allagash. Deer in the town of Allagash had access to artificial winter feed, primarily in the form of lucerne hay bales placed by a regional conservation group. Winter snow depth in open cover averaged 53.6 cm, and winter temperature averaged −7.9°C. Our WMD 5 study site was centered around the Scraggly Lake Maine Public Reserved Land in T7 R8 WELS, Maine (46°14′N, 68°45′W). Deer in WMD 5 were collared around Scraggly, Millimagassett, and Millinocket lakes. Landcover was primarily Laurentian–Acadian Northern Hardwood Forest and Acadian Low Elevation Spruce–Fir–Hardwood Forest (Ferree and Anderson 2013). These deer did not have access to artificial feed in the winter. Winter snow depth in open cover averaged 50.0 cm, and winter temperature averaged −6.1°C. Our WMD 6 study site was geographically broad, ranging from Caribou, ME (46°51′N, 68°00′W), south to Houlton, ME (46°07′N, 67°50′W). Deer were collared widespread throughout WMD 6, and landcover in the study site was primarily Laurentian–Acadian Northern Hardwood Forest, Acadian Low Elevation Spruce-Fir-Hardwood Forest, and mixed agriculture (Ferree and Anderson 2013). Winter snow depth in open cover averaged 50.0 cm, and winter temperature averaged −6.1°C. Our WMD 17 study site was geographically broad ranging from Parkman, ME (45°08′N, 69°25′W), south to Canaan, ME (44°45′N, 69°33′W). Deer were collared throughout WMD 17, and landcover was primarily Laurentian-Acadian Pine-Hemlock-Hardwood Forest, Laurentian-Acadian Northern Hardwood Forest, Acadian Low Elevation Spruce-Fir-Hardwood Forest, and mixed agriculture (Ferree and Anderson 2013). Winter snow depth in open cover averaged 24.1 cm, and winter temperature averaged −3.6°C. The WMD 6 and WMD 17 study sites were in more developed parts of the state with higher road densities, abundant edge habitat, and widespread artificial feeding of deer in winter. There was no allowable antlerless harvest in WMDs 1 and 5 during the study. Annual antlerless harvest was 0.01–0.03 adult females/km2 in WMD 6 and 0.14–0.43 adult females/km2 in WMD 17 during the study. Predators on adult deer in Maine are primarily coyotes and bobcat or lynx, depending on location (N. Bieber, unpubl. data, Major and Sherburne 1987; Fuller 2004).
Study area map of the four wildlife management districts (1, 5, 6, 17) where we evaluated overwinter survival of adult female white-tailed deer (Odocoileus virginianus) from winter 2015–2016 to winter 2021–2022. The points are locations of the weather stations where we collected environmental data to evaluate relationships between winter variables and survival.
Capture and survival monitoring
We captured and radio-collared adult (>1.5 years) female deer from winter 2015–2016 through winter 2021–2022. Since we captured most deer in clover traps and handled them by using only physical restraint, it was not possible to examine teeth and so we distinguished only between fawn and adult deer. We baited capture sites primarily with a wildlife grain mix containing corn, oats, barley, and molasses. We processed and released deer captured in Clover traps (Clover 1954) by using physical restraint and no chemical immobilization. In cases where physical restraint was not possible and with deer captured with drop nets (Ramsey 1968), rocket nets (Hawkins et al. 1968), darting (Hawkins and Klimstra 1970), and helicopter net gunning (Barrett et al. 1982), we chemically immobilized deer with butorphanol–azaperone–medetomidine (BAM; Miller et al. 2009). We administered naltrexone and atipamezole to reverse the effects of BAM. We applied an ear tag to each ear and a global positioning system (GPS) collar to select deer. All trapping, handling, and radio-collaring of deer were approved by the University of Maine Animal Care and Use Committee (IACUC Protocols A2016-12-03 and A2019-08-04). GPS collars were manufactured by VECTRONIC Aerospace and programmed to transmit two GPS locations per day on a 12-h interval. Collars transmitted mortality signals when motionless for 4 h. We monitored locations using VECTRONIC software, GPS Plus X (VECTRONIC Aerospace GmbH, Berlin, Germany). When a collar transmitted a mortality signal, we attempted to find and investigate the site of death. We used a standardized protocol looking for evidence to describe the cause of death including location, blood spatter, signs of chase, and animal scats and tracks (McLellan et al. 2018). Date of mortality was determined on the basis of the date of mortality signal transmission in addition to location.
Predictor variables
We measured snow depth in open canopy and closed canopy cover and deer sinking depth in open and closed canopy cover bi-weekly from mid-December through April at 26 WSI monitoring stations throughout Maine (Fig. 1). We obtained deer sinking depths by searching near the WSI monitoring station for recent deer tracks and measuring their depth. In the absence of recent deer tracks, we searched for tracks in surrounding areas with similar cover. If there were no deer tracks in the area, we used human tracks as an index to estimate sinking depths, which is highly correlated with deer sinking depths (Bunnell et al. 1990). This occurred infrequently (of the data that were available, 27 of 414 (7%) of the surveys used human tracks as an index for estimating sinking depths) and primarily at one site during a period of shallow snow. We averaged snow and sinking depths from the nearest WSI monitoring stations for each study site. Daily temperature and snowpack (depth and snowstorm totals) data were obtained from National Oceanic and Atmospheric Administration (NOAA) weather stations in Corinna, Houlton, Caribou, and Fort Kent, Maine (NOAA Online Weather Data: https://www.weather.gov/wrh/climate, accessed 18 June 2024). We averaged temperatures (bi-weekly) from the nearest NOAA weather stations for each study site. Other weather variables considered included the number of cumulative days below 0°C and −17.8°C, cumulative days with ≥45.7 cm of snow depth, and number of storms producing ≥25.4 cm of snow; these were calculated using data from the previously mentioned NOAA weather monitoring stations and are based on similar thresholds that affect deer survival (Sabine et al. 2002; Morrison et al. 2003; Kautz et al. 2020; Norton et al. 2021). We also calculated WSIs from the aforementioned weather variables by using the formula described in the Introduction that was developed in Maine (Maine Department of Inland Fisheries and Wildlife (MDIFW) 2007) and used these as predictors of bi-weekly survival. Given the difficulty in separating survival effects from artificial feeding and other anthropogenic influences, such as possible refuge from predation and snow-cleared trails, we decided to look only generally at anthropogenic influences rather than artificial feeding specifically. We considered deer to be influenced by anthropogenic factors (hereafter ANTHRO) if we captured them at a site where artificial feeding was occurring or if their winter range overlapped with towns, yards, or parks. Artificial feeding patterns and deer movements varied during the study and within WMDs, so anthropogenic influence could change annually for radio-collared deer. We recorded this information annually for each deer (Supplementary Table S1).
Survival analyses
To evaluate factors that influenced deer survival, we constructed a series of known-fate, staggered-entry, binomial models in a Bayesian framework. We used bi-weekly survey occasions from 200 deer (monitored >1 occasion) over seven winters (2015–2021) to match the temporal scale of our environmental variables. We censored deer that died within 2 weeks of capture. For each winter, there were nine bi-weekly survey occasions between 16 December and 30 April. We included several weather variables (snow and temperature), WSI outputs, and ANTHRO status (see variable descriptions in the Predictor Variables section) in models that we hypothesized would most influence annual overwinter (mid-December–April) deer survival (Table 1). To account for the spatial and temporal hierarchy of the data, we fit WMD and winter as a random effect (Table 1). Because of high correlations among environmental predictor variables (most had Pearson’s correlation coefficients of >0.5) and our limited sample size of radio-collared deer, we did not include multiple environmental predictors in the same model. Instead, we evaluated single environmental predictors with ANTHRO status as an additive effect. Also, we did not consider interactive terms between weather variables and ANTHRO status because there was no evidence to support their inclusion.
We fit models in JAGS (Plummer 2003) by using the jagsUI package (ver. 1.5.2, https://CRAN.R-project.org/package=jagsUI; Kellner 2021) in R (R Core Team 2024). We ran three Markov-chain Monte Carlo (MCMC) chains of 12,000 iterations with an adaptive phase of 2000 iterations that was followed by an initial burn-in phase of 2000 iterations. We drew inference from the posterior distribution by saving every 10th iteration, resulting in 1000 samples per MCMC chain. We inspected each model and parameter for convergence by using trace plots and the Gelman–Rubin statistic (Gelman and Rubin 1992), assuming convergence when MCMC chains mixed adequately (assessed via visual inspection) and when Rhat values were <1.1 (Link and Barker 2010). We evaluated goodness-of-fit (GOF) and model performance by using leave-one-out cross-validation (LOO-CV) and information criterion (LOO-IC) respectively, by using the ‘loo’ function in the loo R package (ver. 2.8.0, https://mc-stan.org/loo/; Vehtari et al. 2024). GOF was assessed using Pareto-k diagnostics from LOO-CV (Vehtari et al. 2017) and, similar to other IC approaches (e.g. AIC; Burnham and Anderson 2002), the model with the lowest LOO-IC was considered to be the top model.
Model comparison
We also compared winter survival estimates produced using the WSI model developed in Maine during the 1990s (Maine Department of Inland Fisheries and Wildlife (MDIFW) 2007), i.e. the historical WSI model described above, with predictions (based on the linear equation) from the top survival model that used radio-collared animals. First, we compared estimates of survival for each winter (n = 7; 2015–2016 through 2021–2022) and WMD (1, 5, 6, 17) that corresponded with the winters and WMDs of our study. We then compared estimates of survival for each winter across the entire state in those WMDs that we did not sample (i.e. WMDs 2–4, 7–16, 18–28). For the latter approach, we did not include the betas for the winter and WMD from the top survival model. Note that we converted winter mortality rates from the historical WSI model to survival rates by subtracting the rates by 1. Also, the historical WSI model does not contain estimates of uncertainty (Maine Department of Inland Fisheries and Wildlife (MDIFW) 2007).
Results
Capture and survival monitoring
We captured 428 unique deer, and we radio-collared 268 of these, including 61 in WMD 1, 39 in WMD 5, 99 in WMD 6, and 69 in WMD 17. We captured 345 deer with Clover traps, 39 with drop nets, 35 with rocket nets, five with dart gun, and four via helicopter net gunning. We monitored 200 radio-collared female deer during a seven-winter period (2015–2021) in central and northern Maine. The mean (±s.e.; range) number of winters that deer were monitored was 2.26 (0.07; 1–5). We recorded 64 mortalities (32%), most of which were in the northern regions (WMD 1 and 6; Table 2). The primary cause of mortality was predation (n = 30), primarily from canids (Table 2). Other sources included deer–vehicle collisions and natural mortality (Table 2). However, there were many cases where the cause of the mortalities was unknown (Table 2).
| Cause of mortality | WMD 1 | WMD 5 | WMD 6 | WMD 17 | Total | |
|---|---|---|---|---|---|---|
| Predation: canine | 8 | 3 | 9 | 1 | 21 | |
| Predation: feline | 0 | 2 | 0 | 1 | 3 | |
| Predation: unknown predator | 4 | 2 | 0 | 0 | 6 | |
| Deer–vehicle collision | 0 | 0 | 6 | 3 | 9 | |
| Other natural causes | 4 | 2 | 1 | 0 | 7 | |
| Poaching | 0 | 0 | 0 | 1 | 1 | |
| Rumen acidosis | 0 | 0 | 1 | 0 | 1 | |
| Unknown | 7 | 0 | 7 | 2 | 16 | |
| Total | 23 | 9 | 24 | 8 | 64 |
Survival analyses
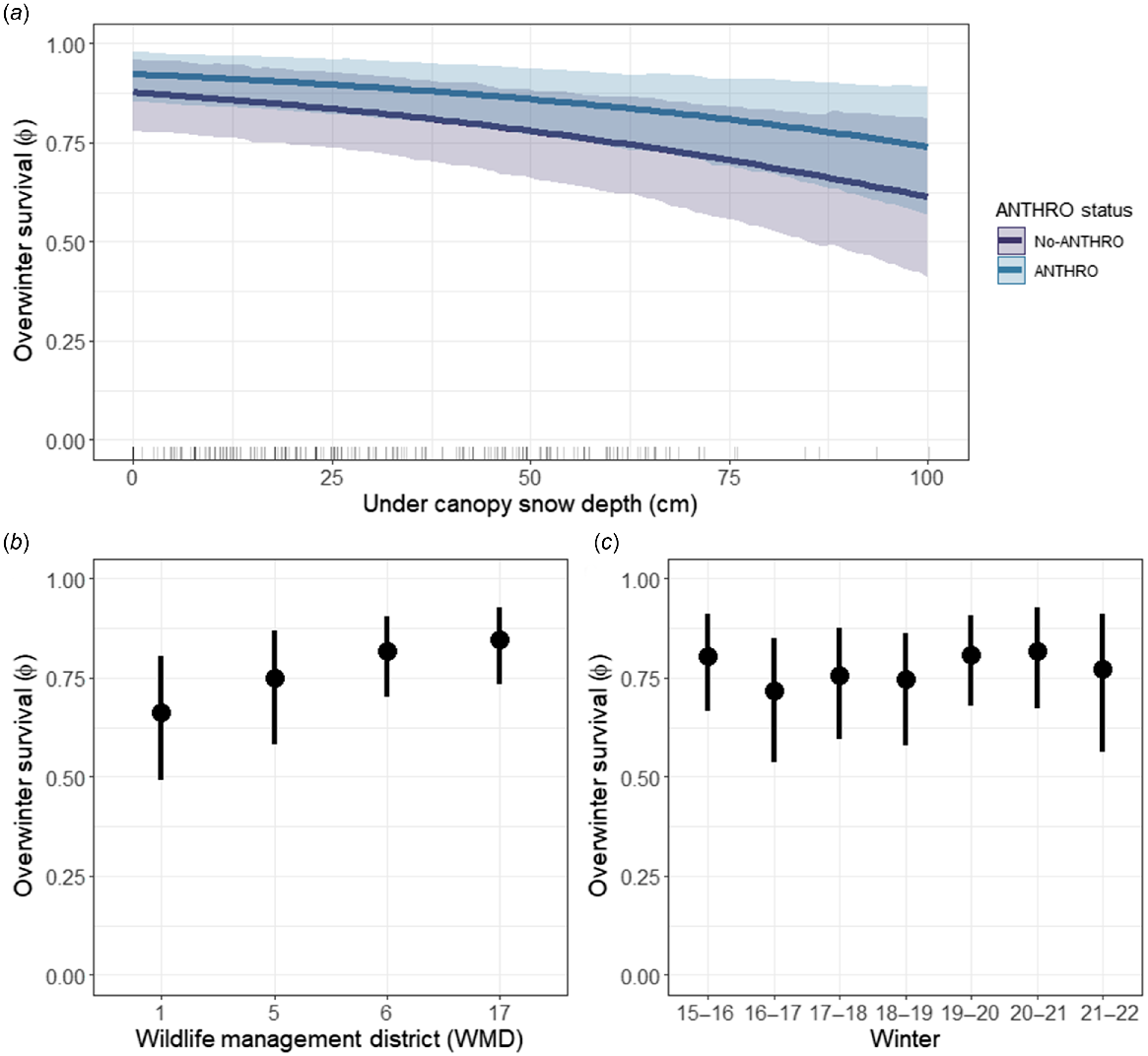
The top survival model, as determined by the lowest LOO-IC score, included closed-canopy snow depth and ANTHRO status (cdep + ANTHRO); all other models, which included weather variables and ANTHRO status, had low predictive power and credible intervals of variables overlapped zero (Table 3). The GOF test indicated that the top model fit the data well, with 100% of the Pareto-k estimates being <0.7. Further, Rhat values were <1.1 and the MCMC chains mixed well, indicating convergence. Snow depth under closed-canopy forests had a negative effect on survival (Table 3, Fig. 2a). Also, deer living close to artificial feeding and developed areas (i.e. ANTHRO) had higher survival rates, yet there was some uncertainty in this relationship as credible intervals slightly overlapped zero (Table 3, Fig. 2a). Survival varied by WMD, with the lowest and highest overwinter survival rates in the northernmost (WMD 1: ϕ = 0.66, CI = 0.49–0.81) and southernmost (WMD 17: ϕ = 0.85, CI = 0.73–0.93) regions respectively (Fig. 2b, Table S2). Overwinter survival varied annually, with the lowest estimate during the 2016–2017 winter (ϕ = 0.72, CI = 0.54–0.85) and the highest estimate during the 2020–2021 winter (ϕ = 0.82, CI = 0.67–0.93) (Fig. 2c, Table S2).
| Model | LOO-IC | Parameter | Mean | s.d. | Lower CI | Upper CI | |
|---|---|---|---|---|---|---|---|
| cdep + ANTHRO | 553.08 | cdep | −0.41 | 0.15 | −0.72 | −0.11 | |
| ANTHRO | 0.52 | 0.29 | −0.04 | 1.08 | |||
| osnk + ANTHRO | 554.60 | osnk | 0.09 | 0.14 | −0.16 | 0.37 | |
| ANTHRO | 0.51 | 0.29 | −0.07 | 1.08 | |||
| csnk + ANTHRO | 555.14 | csnk | 0.04 | 0.13 | −0.21 | 0.30 | |
| ANTHRO | 0.51 | 0.29 | −0.05 | 1.10 | |||
| odep + ANTHRO | 555.30 | odep | 0.03 | 0.14 | −0.24 | 0.32 | |
| ANTHRO | 0.49 | 0.30 | −0.09 | 1.07 | |||
| cm25.4 + ANTHRO | 555.50 | cm25.4 | 0.05 | 0.13 | −0.21 | 0.31 | |
| ANTHRO | 0.50 | 0.29 | −0.07 | 1.08 | |||
| d0C + ANTHRO | 555.67 | d0C | −0.05 | 0.13 | −0.30 | 0.19 | |
| ANTHRO | 0.51 | 0.29 | −0.06 | 1.07 | |||
| atmp + ANTHRO | 555.73 | atmp | 0.04 | 0.13 | −0.21 | 0.29 | |
| ANTHRO | 0.51 | 0.29 | −0.07 | 1.07 | |||
| wsi + ANTHRO | 555.79 | wsi | −0.14 | 0.15 | −0.43 | 0.14 | |
| ANTHRO | 0.51 | 0.29 | −0.07 | 1.07 | |||
| d45.7 + ANTHRO | 555.91 | d45.7 | −0.08 | 0.15 | −0.38 | 0.22 | |
| ANTHRO | 0.53 | 0.29 | −0.04 | 1.10 | |||
| dneg17.8C + ANTHRO | 555.92 | dneg17.8C | −0.19 | 0.15 | −0.49 | 0.10 | |
| ANTHRO | 0.52 | 0.28 | −0.05 | 1.08 |
Note: variables highlighted in bold had CIs that did not overlap zero.
Predictive plots from the top model (cdep + ANTHRO), indicating (a) the relationship between under-canopy snow depth and ANTHRO status on overwinter (mid-December–April) survival of adult female white-tailed deer (Odocoileus virginianus), (b) estimates of overwinter survival for each wildlife management district, and (c) estimates of overwinter survival for each winter of the study. Points and lines are posterior estimates (means), and error bars and colored bands are 95% credible intervals respectively.
Model comparison
The predictive equation from our top model (cdep + ANTHRO) that we compared with the historical WSI model described above was
where overwinter survival (ϕ) within a WMD (w) during a winter (t) is the inverse logit of the sum of the intercept (α) and slopes (β; here cdep and ANTHRO), given the unique cover depth and ANTHRO status values within a WMD (w) and winter (t). Overwinter survival, as a function of snow cover depth and ANTHRO status, was predicted at the population level by exponentiating the number of bi-weekly survey occasions in a winter (n = 9). Note, that the random effects (WMD and winter) were retained for making comparisons between approaches for the WMDs and winters that we sampled but were marginalized from the likelihood to make comparisons across the entire state (i.e. WMDs other than 1, 5, 6, 17) for each winter.
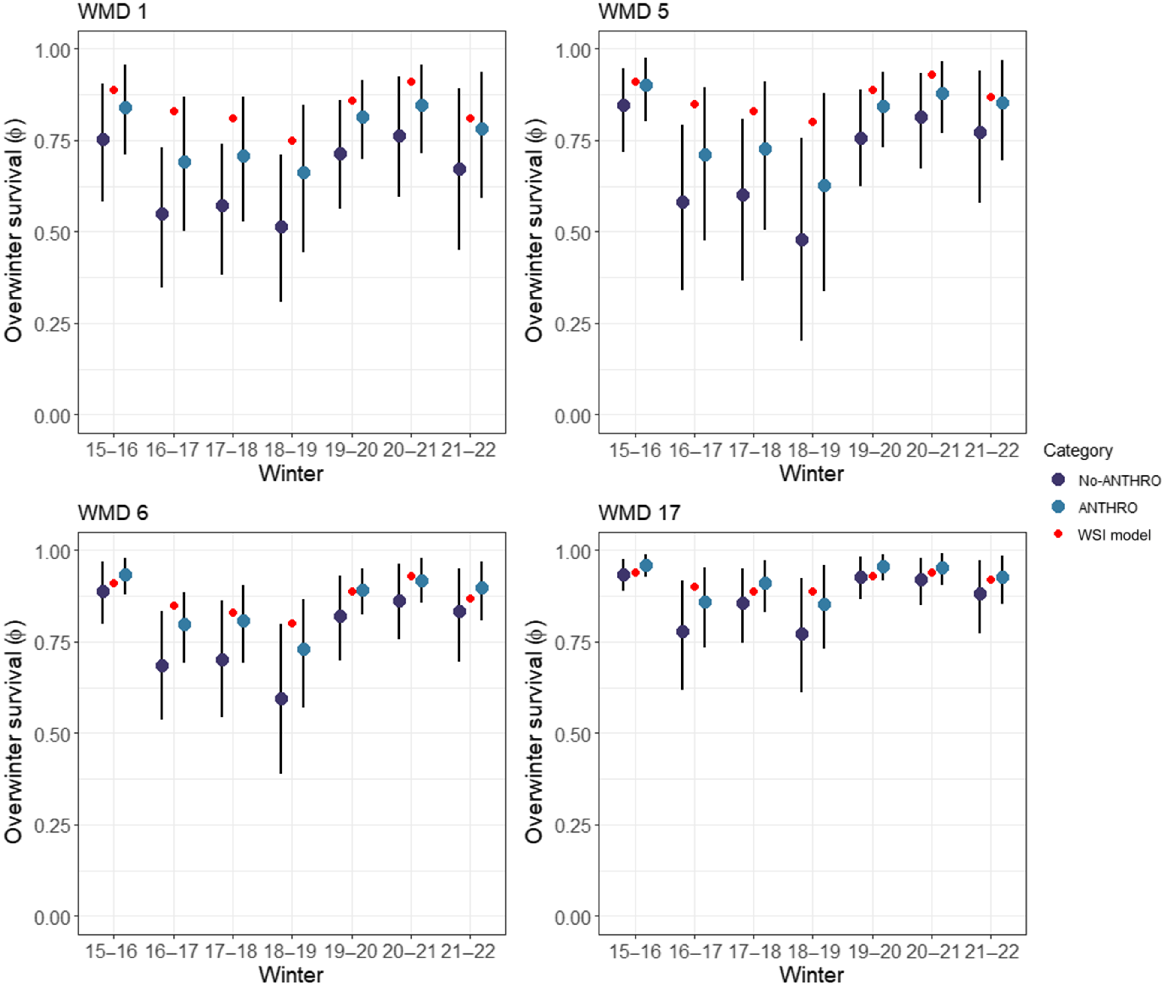
The historical WSI model predicted higher overwinter survival for the majority of WMD and winter combinations that coincided with our study (Fig. 3), than were predictions of overwinter survival from the top model using radio-collared deer (cdep + ANTHRO). We also compared predictions in other WMDs across the state and found a similar pattern; the historical WSI model predicted higher survival rates with less differences among annual mean survival rates than did the top model (Supplementary Figs S1–S6). One common trend was that the lowest survival rates were predicted for the winter of 2018–2019, yet there was high uncertainty associated with predictions from the top model (cdep + ANTHRO) (Fig. 3).
Predictions and 95% credible intervals (error bars) of overwinter (mid-December–April) survival from the top model (cdep + ANTHRO status), which used known fates of radio-collared, adult female white-tailed deer (Odocoileus virginianus), for a seven-winter period in four wildlife management districts (WMD) compared with predictions from the historical WSI model developed during the 1990s that used a winter severity index (WSI) to estimate overwinter survival. Note that the WSI model does not include estimates of uncertainty.
Discussion
Our study has provided a long-term evaluation of factors influencing adult female white-tailed deer survival at the northern end of their range. We showed that winter weather has a strong impact on survival of deer, yet the availability of anthropogenic resources (e.g. food availability) can ameliorate mortality rates, supporting theory on factors that influence populations along high latitude range margins (Sirén and Morelli 2020). These results are similar to other studies of northern ungulates (DelGiudice et al. 2002; Kautz et al. 2020; Norton et al. 2021) and indicate that it is critical to monitor relevant winter weather variables to adjust harvest quotas for future seasons and use radio-collared animals for developing such metrics.
Snow depth under closed canopy cover had a strong negative effect on deer survival compared with other snow and temperature variables. The negative effect of deep snow on deer is well-documented (Robinette et al. 1952; Fuller 1990; DelGiudice et al. 2002; Lasharr et al. 2023). However, we were surprised that other snow and temperature variables, especially those that represent thresholds (e.g. number of days with >45.2 cm of snow), were not impactful, especially because our study included several long and severe winters. Deep snow, under closed-canopy forests, restricts the ability of deer to access food (Dumont et al. 1998, 2005) and avoid predators (Witt et al. 2012; Moratz et al. 2018; Kautz et al. 2020). Indeed, predation was the primary cause of mortalities, especially by canids (Table 2). Further, the amount and quality of deeryards have declined in northern Maine since the 1970s as a result of the logging practices of the past half-century that were primarily in response to a large spruce-budworm (Choristoneura fumiferana) outbreak (Simons-Legaard et al. 2018). Consequently, exceptionally deep snow in closed-canopy forests, including deer wintering areas, can result in higher overall mortality and potentially explains this finding.
In contrast, anthropogenic influences provided a survival advantage for deer. Previous work has indicated that artificial feeding (in the form of deer winter feed stations) and byproducts from agriculture can have a positive impact on survival during harsh winters (Baker and Hobbs 1985; Norton et al. 2021) and our results align with theoretical expectations. For instance, positive biotic factors such as food and habitat availability can ameliorate harsh climate for populations along high latitude range margins and allow for population persistence (Sirén and Morelli 2020). However, there are numerous unintended consequences of feeding deer, including increased risk of disease transmission (Dunkley and Cattet 2003; Murray et al. 2016), habitat degradation around feeding sites (Inslerman et al. 2006; Milner et al. 2014), incidental deer–vehicle collisions (Dunkley and Cattet 2003), increased competition among deer (Dunkley and Cattet 2003; Milner et al. 2014), and sickness or death from rumenitis and/or rumen acidosis (Wobeser and Runge 1975) or enterotoxemia (Rideout 2003). There are preferred alternatives to artificial feeding, and timing timber harvests to benefit deer in nearby wintering areas (e.g. Maine Department of Inland Fisheries and Wildlife (MDIFW) 2012) is one such approach.
While we found a positive effect from anthropogenic influence on deer survival, it is difficult to determine whether artificial feeding was the primary contributor. Artificial feeding tends to occur near human developed areas, and survival may be affected by human development in other ways. For example, predator success and hunting methods may differ from more natural environments (Fleming and Bateman 2018), deer may have access to trails that have compacted snow (e.g. snowmobile trails; Richens and Lavigne 1978), and deer may encounter vehicles more frequently (Fudge et al. 2007). Indeed, we did record some road-kill mortalities in the developed areas, so the influence of humans is not always positive for deer and could have been the source of high variance associated with this variable (see Table 3). Clearly, more study is warranted to better understand which anthropogenic factors have a negative and positive impact on deer survival.
One of the motivations of this study was to understand the limitations of the historical WSI model as a monitoring tool. Prior work in our region has relied on a WSI to monitor deer survival; however, compared with the top model, the WSI performed poorly and explained little variation in deer survival. Additionally, our comparison of the predictive equation from the top model and the historical WSI model (that did not use radio-collared deer), indicated that the latter overpredicted survival for all WMDs and that there was little variation among winters. Our findings of WSIs performing poorly are in line with a recent study in Wisconsin, which used a large sample size of radio-collared deer (Norton et al. 2021), yet in contrast to previous work that found WSIs to have high predictive power (DelGiudice et al. 2002; Kautz et al. 2020). WSIs can be valuable because they capture the cumulative impacts of snow and temperature that deer experience over an entire winter (Dawe and Boutin 2012). Other variables that capture winter length (e.g. number of days above freezing), instead of the weather within a shorter time frame, can also have a strong impact on survival (Kautz et al. 2020; Norton et al. 2021). Although our deepest snowpack primarily occurred later in the winter, we did not find support for variables associated with winter length. It is important to note that our applications are specific to adult female deer, so additional research is needed to assess factors, including WSIs, that influence juvenile and adult male survival.
Our study has several management implications and caveats worth noting. First, estimating winter survival rates in areas with low deer densities and limited antlerless harvest (e.g. northern Maine) may be less important for controlling deer populations if the range of possible management actions is very limited, such as in areas with restricted or no antlerless harvest opportunities. However, monitoring survival in these areas may be beneficial because mild winters will likely increase population sizes and provide hunting opportunities and a greater range of possible management actions. Further, keeping densities somewhat lower in areas where deer overlap with moose (Alces alces) could be an important management strategy to reduce the transmission of diseases that negatively affect moose (e.g. Parelaphostrongylus tenuis). Our study also indicated that it will be important to continue monitoring snow depth in closed-canopy forests. Although MDIFW historically collected weekly snow depth data, the number and distribution of the weather stations were sparsely distributed (see Fig. 1). Future monitoring could benefit from increased sampling to better capture the variation among regions, particularly in regions of greater deer densities and a greater range of possible management actions but where deer are still subject to occasional severe winters; however, adding more snow stations in each WMD would be prohibitively costly because snow depth data are collected manually on a weekly basis (Maine Department of Inland Fisheries and Wildlife (MDIFW) 2007). One solution would be to use remote cameras to record snow depths (Sirén et al. 2018) because these can be deployed in a variety of conditions to evaluate forest-snow dynamics (Sirén et al. 2018; Breen et al. 2023). Further, recent developments in cellular and satellite cameras and automated snow depth extraction from snow stakes (Breen et al. 2024) could greatly increase the efficiency of monitoring snowpack. Additionally, both monitoring of snow and demographic parameters (abundance, survival, and recruitment) can be achieved using cameras (Sirén et al. 2024) and allow for efficient large-scale monitoring of climate and deer populations. We suggest exploring these options for developing cost-effective and robust approaches for monitoring deer populations in northern regions.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
Dr Alexej Sirén is an Associate Editor of Wildlife Research but was not involved in the peer review or any decision-making process for this paper. The authors have no further conflicts of interest to declare.
Declaration of funding
Funding for this study was provided by Maine Federal Aid in Wildlife Restoration Grant W-87-R, Maine Department of Inland Fisheries and Wildlife, and J.D. Irving, Limited. Alexej P. K. Sirén was supported in part by the Iola Hubbard Climate Change Endowment managed by the Earth Systems Research Center at the University of New Hampshire.
Acknowledgements
We thank J. Matijas, C. White, A. DeMusz, K. Marden, and our many interns and technicians for their assistance with fieldwork and thank J. Vashon and G. Franzoi for feedback on our final draft. We also thank H. Gaya and M. Hallworth for providing advice on the Bayesian modeling analysis. Thanks go to the numerous landowners that have worked with our team and allowed access to their lands. Finally, we thank the editors and two anonymous reviewers for their helpful comments on this paper.
References
Baker DL, Hobbs NT (1985) Emergency feeding of mule deer during winter: tests of a supplemental ration. The Journal of Wildlife Management 49(4), 934-942.
| Crossref | Google Scholar |
Barrett MW, Nolan JW, Roy LD (1982) Evaluation of a hand-held net-gun to capture large mammals. Wildlife Society Bulletin 10(2), 108-114.
| Google Scholar |
Breen C, Vuyovich C, Odden J, Hall D, Prugh L (2023) Evaluating MODIS snow products using an extensive wildlife camera network. Remote Sensing of Environment 295, 113648.
| Crossref | Google Scholar |
Breen CM, Currier WR, Vuyovich C, Miao Z, Prugh LR (2024) Snow depth extraction from time-lapse imagery using a keypoint deep learning model. Water Resources Research 60(7), e2023WR036682.
| Crossref | Google Scholar |
Bunnell FL, Parker KL, McNay RS, Hovey FW (1990) Sinking depths of black-tailed deer in snow, and their indices. Canadian Journal of Zoology 68(5), 917-922.
| Crossref | Google Scholar |
Clover MR (1954) Single-gate deer trap. California Fish and Game 42, 199-201.
| Google Scholar |
Dawe KL, Boutin S (2012) Winter severity index using widely available weather information. Wildlife Research 39(4), 321-328.
| Crossref | Google Scholar |
Dawe KL, Bayne EM, Boutin S (2014) Influence of climate and human land use on the distribution of white-tailed deer (Odocoileus virginianus) in the western boreal forest. Canadian Journal of Zoology 92(4), 353-363.
| Crossref | Google Scholar |
DelGiudice GD, Riggs MR, Joly P, Pan W (2002) Winter severity, survival, and cause-specific mortality of female white-tailed deer in north-central Minnesota. The Journal of Wildlife Management 66(3), 698-717.
| Crossref | Google Scholar |
DelGiudice GD, Fieberg JR, Sampson BA (2013) A long-term assessment of the variability in winter use of dense conifer cover by female white-tailed deer. PLoS ONE 8, e65368.
| Crossref | Google Scholar | PubMed |
Ditchkoff SS, Servello FA (2002) Patterns in winter nutritional status of white-tailed deer Odocoileus virginianus populations in Maine, USA. Wildlife Biology 8(2), 137-143.
| Crossref | Google Scholar |
Dumont A, Ouellet J-P, Crête M, Huot J (1998) Caractéristiques des peuplements forestiers recherchés par le cerf de Virginie en hiver à la limite nord de son aire de répartition. Canadian Journal of Zoology 76(6), 1024-1036.
| Crossref | Google Scholar |
Dumont A, Crête M, Ouellet J-P, Huot J, Lamoureux J (2000) Population dynamics of northern white-tailed deer during mild winters: evidence of regulation by food competition. Canadian Journal of Zoology 78(5), 764-776.
| Crossref | Google Scholar |
Dumont A, Ouellet J-P, Crête M, Huot J (2005) Winter foraging strategy of white-tailed deer at the northern limit of its range. Écoscience 12(4), 476-484.
| Crossref | Google Scholar |
Dunkley L, Cattet MRL (2003) A comprehensive review of the ecological and human social effects of artificial feeding and baiting of wildlife. Canadian Cooperative Wildlife Health Centre: Newsletters & Publications 21, 68.
| Google Scholar |
Ferree C, Anderson MG (2013) A map of terrestrial habitats of the Northeastern United States: Methods and approach. The Nature Conservancy, Eastern Conservation Science, Eastern Regional Office, Boston, MA. Available at https://www.sciencebase.gov/catalog/item/5a611943e4b06e28e9c25976
Fieberg J, Kuehn DW, Delgiudice GD (2008) Understanding variation in autumn migration of northern white-tailed deer by long-term study. Journal of Mammalogy 89(6), 1529-1539.
| Crossref | Google Scholar |
Fleming PA, Bateman PW (2018) Novel predation opportunities in anthropogenic landscapes. Animal Behaviour 138, 145-155.
| Crossref | Google Scholar |
Fortin NL, Pekins PJ, Gustafson KA (2015) Productivity measures of white-tailed deer in New Hampshire: assessing reduced recruitment. Wildlife Society Bulletin 39(1), 56-64.
| Crossref | Google Scholar |
Fudge D, Reedman B, Rowell M, Nette T, Power VA (2007) Road-kill of mammals in Nova Scotia. The Canadian Field-Naturalist 121(3), 265-273.
| Crossref | Google Scholar |
Fuller TK (1990) Dynamics of a declining white-tailed deer population in north-central Minnesota. Wildlife Monographs 110, 3-37.
| Google Scholar |
Fuller AK (2004) Canada lynx predation on white-tailed deer. Northeastern Naturalist 11(4), 395-398.
| Crossref | Google Scholar |
Garroway CJ, Broders HG (2005) The quantitative effects of population density and winter weather on the body condition of white-tailed deer (Odocoileus virginianus) in Nova Scotia, Canada. Canadian Journal of Zoology 83(9), 1246-1256.
| Crossref | Google Scholar |
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Statistical Science 7, 457-511.
| Crossref | Google Scholar |
Hawkins RE, Klimstra WD (1970) A preliminary study of the social organization of white-tailed deer. The Journal of Wildlife Management 34(2), 407-419.
| Crossref | Google Scholar |
Hawkins RE, Martoglio LD, Montgomery GG (1968) Cannon-netting deer. The Journal of Wildlife Management 32(1), 191-195.
| Crossref | Google Scholar |
Kautz TM, Belant JL, Beyer DE, Jr, Strickland BK, Duquette JF (2020) Influence of body mass and environmental conditions on winter mortality risk of a northern ungulate: evidence for a late-winter survival bottleneck. Ecology and Evolution 10(3), 1666-1677.
| Crossref | Google Scholar | PubMed |
Kellner K (2021) jagsUI: a wrapper around ‘rjags’ to streamline ‘JAGS’ analyses. version 1.5.2. Available at https://cran.r-project.org/package=jagsUI
LaSharr TN, Dwinnell SPH, Jakopak RP, Randall J, Kaiser RC, Thonhoff M, Scurlock B, Fieseler T, Hymas N, Hymas A, Roberts N, Hobbs J, Zornes M, Brimeyer DG, Fralick G, Monteith KL (2023) Behavior, nutrition, and environment drive survival of a large herbivore in the face of extreme winter conditions. Ecosphere 14(7), e4601.
| Crossref | Google Scholar |
Lefort S, Tremblay J-P, Fournier F, Potvin F, Huot J (2007) Importance of balsam fir as winter forage for white-tailed deer at the northeastern limit of their distribution range. Ecoscience 14(1), 109-116.
| Crossref | Google Scholar |
Louthan AM, Doak DF, Angert AL (2015) Where and when do species interactions set range limits? Trends in Ecology & Evolution 30(12), 780-792.
| Crossref | Google Scholar | PubMed |
Maine Department of Inland Fisheries and Wildlife (MDIFW) (2012) Living on the edge – winter feeding of deer: what you should know. MDIFW. Available at https://www.maine.gov/ifw/docs/deer_winter_feeding.pdf
Major JT, Sherburne JA (1987) Interspecific relationships of coyotes, bobcats, and red foxes in western Maine. The Journal of Wildlife Management 51(3), 606-616.
| Crossref | Google Scholar |
McLellan SR, Vashon JH, Johnson EL, Crowley SM, Vashon AD (2018) Fisher predation on Canada lynx in the northeastern United States. The Journal of Wildlife Management 82(8), 1775-1783.
| Crossref | Google Scholar |
Miller BF, Osborn DA, Lance WR, Howze MB, Warren RJ, Miller KV (2009) Butorphanol-azaperone-medetomidine for immobilization of captive white-tailed deer. Journal of Wildlife Diseases 45(2), 457-467.
| Crossref | Google Scholar |
Milner JM, Van Beest FM, Schmidt KT, Brook RK, Storaas T (2014) To feed or not to feed? Evidence of the intended and unintended effects of feeding wild ungulates. The Journal of Wildlife Management 78(8), 1322-1334.
| Crossref | Google Scholar |
Moen AN (1976) Energy conservation by white-tailed deer in the winter. Ecology 57(1), 192-198.
| Crossref | Google Scholar |
Moratz KL, Gullikson BS, Michel ES, Jenks JA, Grove DM, Jensen WF (2018) Assessing factors affecting adult female white-tailed deer survival in the Northern Great Plains. Wildlife Research 45(8), 679-684.
| Crossref | Google Scholar |
Morrison SF, Forbes GJ, Young SJ, Lusk S (2003) Within-yard habitat use by white-tailed deer at varying winter severity. Forest Ecology and Management 172(2–3), 173-182.
| Crossref | Google Scholar |
Murray MH, Becker DJ, Hall RJ, Hernandez SM (2016) Wildlife health and supplemental feeding: a review and management recommendations. Biological Conservation 204, 163-174.
| Crossref | Google Scholar |
Nelson ME (1995) Winter range arrival and departure of white-tailed deer in northeastern Minnesota. Canadian Journal of Zoology 73(6), 1069-1076.
| Crossref | Google Scholar |
Norton AS, Storm DJ, Van Deelen TR (2021) White-tailed deer, weather and predation: a new understanding of winter severity for predicting deer mortality. The Journal of Wildlife Management 85(6), 1232-1242.
| Crossref | Google Scholar |
Patterson BR, Power VA (2002) Contributions of forage competition, harvest, and climate fluctuation to changes in population growth of northern white-tailed deer. Oecologia 130, 62-71.
| Crossref | Google Scholar | PubMed |
R Core Team (2024) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Available at https://www.r-project.org/
Ramsey CW (1968) A drop-net deer trap. The Journal of Wildlife Management 32(1), 187-190.
| Crossref | Google Scholar |
Richens VB, Lavigne GR (1978) Response of white-tailed deer to snowmobiles and snowmobile trails in Maine. The Canadian Field-Naturalist 92(4), 334-344.
| Crossref | Google Scholar |
Rideout BA (2003) Clostridial diseases in all taxa. In ‘Zoo and wild animel medicine’. (Eds ME Fowler, RE Miller) ch. 72, pp. 712–718. (Saunders: St Louis, MO, USA) 10.1016/B978-1-4160-4047-7.X5001-5
Robinette WL, Julander O, Gashwiler JS, Smith JG, Ashman C, Garrard R (1952) Winter mortality of mule deer in Utah in relation to range condition. The Journal of Wildlife Management 16(3), 289-299.
| Crossref | Google Scholar |
Sabine DL, Morrison SF, Whitlaw HA, Ballard WB, Forbes GJ, Bowman J (2002) Migration behavior of white-tailed deer under varying winter climate regimes in New Brunswick. The Journal of Wildlife Management 66(3), 718-728.
| Crossref | Google Scholar |
Simons-Legaard EM, Harrison DJ, Legaard KR (2018) Ineffectiveness of local zoning to reduce regional loss and fragmentation of wintering habitat for white-tailed deer. Forest Ecology and Management 427, 78-85.
| Crossref | Google Scholar |
Sirén APK, Morelli TL (2020) Interactive range-limit theory (iRLT): an extension for predicting range shifts. Journal of Animal Ecology 89(4), 940-954.
| Crossref | Google Scholar | PubMed |
Sirén APK, Somos-Valenzuela M, Callahan C, Kilborn JR, Duclos T, Tragert C, Morelli TL (2018) Looking beyond wildlife: using remote cameras to evaluate accuracy of gridded snow data. Remote Sensing in Ecology and Conservation 4(4), 375-386.
| Crossref | Google Scholar |
Sirén APK, Hallworth MT, Kilborn JR, Bernier CA, Fortin NL, Geider KD, Patry RK, Cliché RM, Prout LS, Gifford SJ, Wixsom S, Morelli TL, Wilson TL (2024) Monitoring animal populations with cameras using open, multistate, N-mixture models. Ecology and Evolution 14(2), e70583.
| Crossref | Google Scholar |
Tarr MD, Pekins PJ (2002) Influences of winter supplemental feeding on the energy balance of white-tailed deer fawns in New Hampshire, USA. Canadian Journal of Zoology 80(1), 6-15.
| Crossref | Google Scholar |
Van Deelen TR, Campa HI, Hamaday M, Haufler JB (1998) Migration and seasonal range dynamics of deer using adjacent deeryards in northern Michigan. The Journal of Wildlife Management 62(1), 205-213.
| Crossref | Google Scholar |
Vehtari A, Gelman A, Gabry J (2017) Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Statistics and Computing 27, 1413-1432.
| Crossref | Google Scholar |
Vehtari A, Gabry J, Magnusson M, Yao Y, Bürkner P, Paananen T, Gelman A (2024) loo: efficient approximate leave-one-out cross-validation for fitted Bayesian models. version 2.8.0. Available at https://mc-stan.org/loo/
Weiskopf SR, Ledee OE, Thompson LM (2019) Climate change effects on deer and moose in the Midwest. The Journal of Wildlife Management 83(4), 769-781.
| Crossref | Google Scholar |
Whitlaw HA, Ballard WB, Sabine DL, Young SJ, Jenkins RA, Forbes GJ (1998) Survival and cause-specific mortality rates of adult white-tailed deer in New Brunswick. The Journal of Wildlife Management 62(4), 1335-1341.
| Crossref | Google Scholar |
Witt JC, Webster CR, Froese RE, Drummer TD, Vucetich JA (2012) Scale-dependent drivers of ungulate patch use along a temporal and spatial gradient of snow depth. Canadian Journal of Zoology 90(8), 972-983.
| Crossref | Google Scholar |
Wobeser G, Runge W (1975) Rumen overload and rumenitis in white-tailed deer. The Journal of Wildlife Management 39(3), 596-600.
| Crossref | Google Scholar |


