Producer-initiated field research leads to a new diagnostic test for footrot
C. A. Gaden A E , B. F. Cheetham B , E. Hall C , G. Green D and M. E. Katz BA ‘Beaumont’, Invergowrie, NSW 2350, Australia.
B Molecular and Cellular Biology, University of New England, Armidale, NSW 2351, Australia.
C Margaret Street, Launceston, Tas. 7250, Australia.
D Livestock Health and Pest Authority, Armidale, NSW 2350, Australia.
E Corresponding author. Email: cagaden@iprimus.com.au
Animal Production Science 53(8) 610-617 https://doi.org/10.1071/AN11175
Submitted: 13 August 2011 Accepted: 2 March 2012 Published: 10 July 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
The Cicerone Project was formed in 1998 to address problems faced by wool producers. In the New England area, the issue of suspected false positive diagnoses of virulent footrot, which can be a significant cause of economic loss to individual producers, was investigated. In New South Wales, footrot diagnosis is primarily a field diagnosis supported by the gelatin gel laboratory test. The principal causative agent of footrot is Dichelobacter nodosus. If the gelatin gel test finds strains of D. nodosus to be thermostable (gel stable), a finding of virulent footrot is likely and quarantine of the affected property follows. However, livestock producers and inspectors reported that there were a considerable number of cases where laboratory tests found strains to be stable but these strains did not cause virulent footrot in the field. Preliminary results using DNA markers associated with virulent footrot showed that one of these markers, intA, was absent in gel stable, field benign strains but present in all strains tested which caused field virulent footrot. A trial conducted at Uralla, New South Wales, demonstrated conclusively that there were strains of D. nodosus which were stable in the gelatin gel test but did not cause virulent footrot in the field. All of these strains were negative in the intA DNA test. These results were confirmed in a second field trial at Molong, New South Wales. These trials were instrumental in establishing that the gelatin gel test at times gave results inconsistent with the clinical expression of footrot, potentially leading to a false positive diagnosis of virulent footrot. Subsequent research led to confirmation of the intA test, which is now available as an additional tool for footrot diagnosis.
Introduction
Footrot is a mixed bacterial infection of the hooves of sheep, caused by the anaerobic bacterium Dichelobacter nodosus. Different strains of D. nodosus cause disease of different severity, ranging from mild (benign) to severe (virulent) footrot. Virulent footrot results in lameness, which impairs the ability of the sheep to graze, resulting in loss of condition and reduced wool production. As virulent footrot results in considerable economic loss, it is subject to regulatory action, including quarantine. Benign footrot, however, is not subject to the same control procedures, so distinguishing between the different forms of the disease is crucial to the success of footrot eradication programs.
In the state of New South Wales (NSW), a footrot control program was introduced in 1988 with the aim of eradicating virulent footrot. Under this program, diagnosis was based on field observations of the severity of the disease, taking into account climatic conditions and backed up by laboratory tests. Properties found to have flocks with virulent footrot are subject to quarantine, which can result in economic loss to farmers due to their restricted ability to sell sheep.
The severity of the damage to the hoof by the footrot infection can be measured using a 1–5 scoring system (Whittington and Nicholls 1995) and virulent footrot is usually diagnosed if the lesions are scores 4 or 5 or where a significant proportion of the flock shows underrunning of the hoof (score 3B or above). Warm, moist conditions and lush pasture favour the expression of the disease (Stewart et al. 1984). However, in the initial stages of infection or under poor (or drier) climatic conditions, it is often difficult to distinguish benign from virulent footrot.
A variety of laboratory tests have been developed to aid in footrot diagnosis. The two most common tests measure differences in the properties of the proteases secreted by the bacterium to degrade the hoof. In general, virulent strains secrete proteases that are more thermostable, which is the basis for the gelatin gel test (Palmer 1993). In addition, proteases secreted by virulent strains have higher elastase activity, which is the basis of the elastase test (Stewart 1979).
In March 1985, the NSW Footrot Steering Committee endorsed the use of the gelatin gel test to assist in footrot diagnosis. This test classifies D. nodosus strains as unstable or stable. Strains classified as unstable could substantiate a diagnosis of benign footrot, but could not overturn a diagnosis of virulent footrot as there were known isolates which gave false negative results. If the gelatin gel test result was stable but the field diagnosis did not suggest virulent footrot, further investigations were carried out. These included revision of the flock history, waiting for further periods of suitable climatic conditions, examining more sheep and, in some cases, using the elastase test.
However, the elastase test also measured protease activity so was not entirely independent of the gelatin gel test. Some farmers with flocks which were identified with stable isolates claimed that the disease was causing little distress to the sheep, wool production remained high and the hoof problems seemed to come and go without treatment. Thus, there was a strong feeling that some strains classified as stable by the gelatin gel test were only capable of causing benign footrot. Although there had been anecdotal reports of such strains before in the scientific literature (Depiazzi et al. 1991), it was not widely accepted that the gelatin gel test gave false positive results and Rural Lands Protection Boards (RLPB) inspectors were discouraged from making a benign diagnosis after stable isolates were found.
The areas covered by the RLPB (now Livestock Health and Pest Authorities) of NSW were classified as ‘protected’ if the prevalence of flocks with virulent footrot was less than 1%; ‘control’ if the prevalence was between 1 and 10%; or ‘residual’ if the prevalence was greater than 10%. Measures such as the mandatory notification to the RLPB of sheep introduced from outside the protected area, and the use of Footrot Vendor Declarations were introduced to prevent the re-introduction of virulent footrot into protected areas and to reduce the incidence of virulent footrot in all areas. Infected flocks in the protected and control areas were quarantined, with sale of sheep restricted to slaughter only, leading to economic loss. In residual areas, trade in infected sheep was still allowed and the formation of local footrot control groups encouraged.
The Armidale Pastures Protection Board, (which became the RLPB and thence the New England Livestock Health and Pest Authority) was declared a footrot ‘protected’ area in 1969 after an extensive local campaign to eradicate virulent footrot. This protected area was extended to cover the majority of the New England area with the introduction of the NSW Footrot Strategic Plan in 1988, and was assumed to have a prevalence of virulent footrot of less than 1% of flocks. However, auditing of the New England protected area in 1997, which relied heavily on gelatin gel testing, revealed that 11.9% of flocks in the Armidale RLPB had virulent footrot while, in the neighbouring Glen Innes RLPB, the incidence was as high as 22.2%. Thus, it appeared that footrot had re-emerged as a major problem in the New England area and there was frustration on the part of both sheep producers and advisors as much of this ‘disease’ was not causing significant symptoms in infected sheep.
After considerable consultation among livestock producers and the research and extension community, the Cicerone Project was formed in 1998 as a result of livestock producers expressing a desire for more input into how their levy money was spent on research and on adoption. The specific aim of the Cicerone Project was ‘to co-learn, through a partnership between livestock producers, research, extension and other specialists, about ways to improve the profitability and sustainability of grazing enterprises in the summer-rainfall, Northern Tablelands region of NSW’ (Sutherland et al. 2013).
The Cicerone Project organised a series of meetings about footrot with wool producers in Armidale and the neighbouring areas of Glen Innes, Guyra and Walcha in 1998 and 1999, which were attended by over 120 people. It was evident at these meetings that there was anger and frustration with the footrot control program. In particular, farmers and RLPB staff were unhappy with the high degree of reliance on the gelatin gel test and claimed that there were a substantial number of isolates of D. nodosus that were stable in the gelatin gel test but did not cause virulent footrot in the field. This was causing resentment towards the footrot control program and the issue urgently needed to be resolved. In particular, it was suggested that, while the control strategies of the NSW Footrot Steering Committee may work well in southern NSW, with its cold, wet winters and hot, dry summers, it was obvious to local producers that the different conditions on the Northern Tablelands relating to its specific strains and the summer-rainfall environment warranted investigation.
Following these meetings, vigorous applications for Producer Initiated Research and Development (PIRD) funding from Meat and Livestock Australia and the then Woolmark Co., resulted in sufficient funds to conduct a field trial on 14 ha of the CSIRO research property ‘Chiswick’ near Uralla, NSW with the collaboration of the Cicerone Project, the Armidale RLPB, NSW Agriculture, the University of New England and CSIRO.
The aim of the first field trial, conducted at Uralla in 1999, was to compare the field expression of various gel stable and unstable isolates of footrot under similar conditions during spring when footrot expression was likely to occur in this region.
A second field trial, conducted at Molong, NSW in 2000, aimed to ascertain if these New England strains behaved in a similar manner when taken out of the region into a supposedly more ‘expressive’ environment (where moist, clover-dominant pastures are common in spring). This would test the hypothesis that the gelatin gel test result is a predictor of virulence and that the field benign gel stable isolates from the New England area would express as field virulent in another environment, more conducive to the disease.
The same strains were also compared using DNA fingerprinting. Strains which were classified as stable by the gelatin gel test but were not associated with clinical virulent footrot (gel stable, field benign), were obtained from farms in the Armidale RLPB in 1998. All the gel stable, field benign samples tested were found to lack the intA gene. By contrast, intA was present in all virulent strains tested so far. These preliminary results suggested that intA could be used to determine whether gelatin gel stable isolates were associated with virulent or benign footrot.
For the past 10 years, we have analysed genes associated with the virulence of the footrot pathogen D. nodosus. Several genes have been identified and characterised, some of which may have a role in virulence (Bloomfield et al. 1997). Southern blot analysis of DNA prepared from isolates of D. nodosus from the Cicerone footrot trial was carried out. Using one of the potential virulence-associated genes, intA, we found that DNA from the field benign gel stable strains did not hybridise, so there were no bands on the Southern blot. By contrast, DNA from all virulent strains tested hybridised to the intA probe.
This paper reports the results of those two field trials and summarises the evolution of a reliable DNA test for virulent footrot.
Materials and methods
With the assistance of two PIRD grants, the newly established Cicerone Project and collaborators conducted two trials in different locations in NSW, Uralla and Molong, to compare the field expression of various strains of footrot with laboratory tests of the disease.
Uralla field trial (spring 1999)
Sheep and strains
There were seven flocks, each comprised of 10 sheep distributed evenly across 14 paddocks (two replicates), each of 1 ha. The sheep infected with different strains of D. nodosus came from six different properties located within the New England region while the clean (control) sheep came from a 7th property. These strains included sheep carrying typical gel stable virulent footrot (HVS), gel stable isolates classified as low virulent (LVS), typical gel unstable benign footrot (BUS), three flocks with gel stable, field benign footrot (BS1, BS2 and BS3) and control sheep found to be free of footrot (CON) (Table 1). All of the CON sheep were from the same flock. Five of the 10 ‘clean’ sheep in the control paddocks were randomly assigned to ‘clean’ and ‘infected’ groups, respectively, to create a balanced design to assist in statistical analysis.
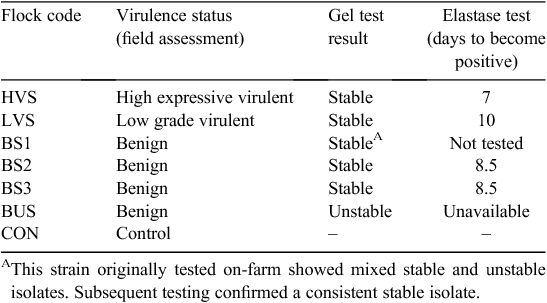
|
Quarantine precautions
During all interactions between investigators, the sheep and the paddocks, care was taken to ensure that there was no cross-contamination between paddocks. Each person going into the paddocks wore rubber over-boots and entered and exited via a footbath containing Hibitane or Stericide. Dogs also were subject to going through the footbaths. Inspectors washed hands and foot clippers between each plot.
Pastures
In August 1999, all 14 paddocks were slashed and fertilised with Starter 15 fertiliser (15% N; 13% P; 11% S) at 100 kg/ha. Half of the paddocks were oversown with lime pelleted and inoculated white clover (Trifolium repens cv. Huia) seed (2 kg/ha), which was surface spread together with the fertiliser to encourage legume growth in half the paddocks by late spring. Paddocks were ungrazed for ~12 weeks before the sheep were introduced.
The pastures were assessed for legume content by the NSW DPI District Agronomist for Armidale (Ms Clare Edwards) on 28 October 1999 who allocated ‘high’ and ‘low’ clover paddocks for the two replicates allocated to each treatment. As the legume did not become established uniformly across all paddocks, there was considerable variability in the clover content of many of the paddocks.
The final allocation of the paddocks to each treatment was confirmed with the RLPB veterinarian (Mr John McFarlane) on 29 October 1999 and the sheep were delivered to the trial site on 1 November 1999.
Foot scoring
All sheep were initially foot scored after being placed in their respective treatment paddocks on 1 November 1999. Foot scoring was again conducted on 12 November 1999 and thereafter weekly up to the 20th (final) scoring on 17 March 2000. A field day was held a week later on 24 March 2000 and swabs were collected from all infected sheep by RLPB staff on 28 March 2000.
Every sheep had each claw of each foot scored separately by RLPB inspectors every week for 20 weeks starting on 1 November 1999. Whenever hoof treatment was required for animal welfare reasons, the foot was trimmed only. However, on the few occasions when maggots were present, the foot was treated with diazanon to remove the maggots. No other chemicals were used but diazanon can be expected to at least suppress the growth of D. nodosus. Once any foot was treated, the data beyond that point were excluded from records of infection subjected to analysis. During the trial, two sheep died from wild dog attack or fly strike.
The foot scoring system used was that of Stewart et al. (1984), the standard system recommended by the NSW Footrot Strategic Plan, with the modification that scores were allocated to each claw rather than to each foot. The weekly foot score results were converted to a numerical score (Table 2) to assist in statistical analysis and were subsequently re-coded to the Stewart et al. (1984) score before creating figures.

|
Foot conformation
In the second week of the trial, the conformation of each hoof was examined and designated to be: open (where air could circulate as the two claws were apart when the animal was walking), touching (where the two claws remained touching when the animal walked) or closed (where the claws were so close together they crossed over and thus excluded air, even when the animal put weight on its foot).
Seasonal conditions
The trial was carried out at the beginning of November when it was warm and moist with relatively lush pasture growth, which created suitable conditions for the development of footrot, especially over the first 14 weeks of the trial (Fig. 1).
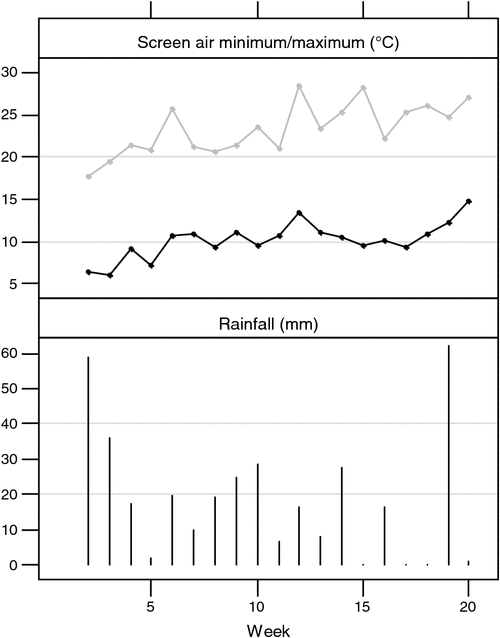
|
Stocking rate
From the beginning of November 1999, four or five wethers carrying each of the described field strains of footrot were run on either a higher or lower clover content paddock through the 20 weeks of the trial. Five initially ‘clean’, uninfected sheep were also run with each of the six strains to ascertain if spread was occurring. The clean sheep were all four-tooth wethers from the same bloodline. Thus, the stocking rate on each plot was a total of 9–10 wethers per ha. Each of the two replicate control paddocks contained 10 clean sheep throughout the trial.
After the trial
The sheep from the Uralla trial were swabbed before and at the end of the study to ensure that no transfer or change in the strains had occurred. There were no changes in gelatin gel results over the period of the trial. At the end of the 20 weeks, all sheep except the field virulent strains (LVS and HVS) were retained on their respective paddocks. Subsequently, these retained sheep were kept over the winter period, drenched and shorn in their paddocks and foot baths of disinfectant continued to be used by dogs and people whenever they entered and left a paddock. Over this period, it was decided that a second trial would be run by transferring these sheep to another, potentially more ‘expressive’ environment and conducting further weekly inspections to observe how the remaining strains reacted in a different environment (Molong).
Molong field trial – 2000
The second trial, which was carried out in the Molong RLPB, ~500 km south of Armidale, commenced in late August 2000. The trial was set up in a similar way to the previous trial including the sheep transported from the Uralla trial: the CON, BUS and BS1, BS2, BS3 groups were compared with one flock of local sheep identified (via laboratory and field tests) as HVS. A local uninfected flock was used to supply new clean control sheep to each of the treatment paddocks in order to detect disease transfer (Table 3).

|
In late August 2000, the sheep were transported to Molong for the second trial. To ensure no cross-contamination occurred, each animal was physically carried onto the truck and they were transported in separate straw covered pens according to their ear-tag colour, which was an identifier for the different footrot strains. At Molong, the sheep were individually carried off the truck to keep the footrot strains apart and then placed in their respective separate paddocks.
The paddocks were lush with white clover, subterranean clover (Trifolium subterraneum) and lucerne (Medicago sativa). A wet spring ensured ideal conditions for the development and transmission of footrot.
The sheep were foot scored each week by Footrot Advisory officers from the local RLPB and other nearby Boards who were interested in the trial. This took place from 31 August 2000 until the middle of December. A field day at the Molong trial site was held on 10 November 2000.
The trial was conducted within a paddock on the ‘Wavertree’ cattle property in the Molong RLPB. The pasture had been sown in 1995 with 200 kg/ha of superphosphate and top dressed with a similar rate of superphosphate in both 1997 and 1999. The pasture had been used to successfully finish steers up to mid 2000 but was due for renovation. At the commencement of the experiment (September 2000), the pasture was lush, being dominated by subterranean clover, white clover (cv. Haifa) and lucerne (cv. Aurora); it was subdivided into 1-ha paddocks before introduction of animals infected with each of the footrot strains.
The environment at the start of the trial and through to late November was considered likely to encourage more disease expression than the original Uralla ‘high’ clover plots. However, by late November 2000 and continuing to the completion of the trial on 7 December 2000, the trial experienced considerably drier conditions, which may have contributed to the regression of the lesions observed across footrot strain flocks, even in the virulent flock which had to have their feet pared.
The five strains under observation were run with clean sheep in their separate 1-ha plots at a stocking rate of between 9 and 10 sheep per ha. Precautions to avoid transfer of infection from one plot to another were taken as with the Uralla trial.
The scoring at Molong was done by the local RLPB staff – that is, different operators. Sheep were foot scored using the same system as for the Uralla trial (adapted from Stewart et al. 1984) and data analysed in the same way.
The results of the trials were released promptly to members of the Cicerone Project by publication of summary reports in issues of the Cicerone Newsletter.
Swabs for DNA and gelatin gel testing
For each trial, the six groups of sheep identified with D. nodosus infections were inspected and 10 swabs per group collected before the start of the trial. These were sent to the NSW DPI Regional Veterinary Laboratory at Orange where D. nodosus was cultured and subjected to the gelatin gel test. DNA fingerprinting on the cultured strains was carried out at the University of New England.
DNA fingerprinting
Southern blot analysis of DNA prepared from isolates of D. nodosus from the Cicerone footrot trial was carried out as described previously (Bloomfield et al. 1997).
Statistical analyses
Attempts were made to separate the conformation footscores by treatment and infection and the highest scores by treatment at Weeks 16 and 20 using Bayesian regression as ordered categorical data (Gelman and Hill 2007). The longitudinal high scores data were subsequently analysed over time using a generalised additive model (Wood 2006), which provided a graphical display of changes over time with 95% confidence limits clearly indicating the significant differences.
Results
Uralla field trial
The limited number of replicates and the relatively small number of sheep per replicate, especially after some were removed on animal welfare grounds, combined with the variability in the clover content of the paddocks made it difficult to determine significant differences between treatments using Bayesian regression. Hence, the data have been presented with a fitted generalised additive model and confidence intervals.
The results of the highest footscores showed that the sheep infected with either of the virulent strains became progressively worse over the period of the trial, while the remaining groups of sheep did not show any clinical signs of virulent footrot (Fig. 2). Sheep infected with HVS became progressively worse and clearly caused infection of the clean sheep running with them. Sheep infected with LVS developed more severe lesions over the period and caused some infection of the clean sheep running with them but not to the same extent as those with HVS.
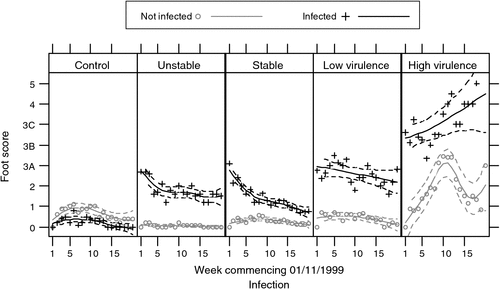
|
Sheep infected with BUS did not develop more severe footscore lesions. Instead, the lesions improved and the clean sheep did not become infected. Sheep with BS1, BS2, BS3, shown collectively as the BS group in Fig. 2, did not show any clinical signs of virulence and did not infect clean sheep. All three BS groups acted in the same way as the BUS group.
For all strains, including the clean sheep running both with the various footrot strains and running separately, a noticeable effect of clover content was observed especially over the first 14 weeks although this effect was not statistically significant (P > 0.05), due perhaps to the variability of the clover levels between plots.
The foot conformation results are presented as the average number of feet per sheep classified within each footrot strain group as open, touching or closed (Fig. 3). The number with closed claws was lowest in the CON group and increased somewhat from BS to BUS to LVS with the highest number being the HVS group, which had no open clawed feet.
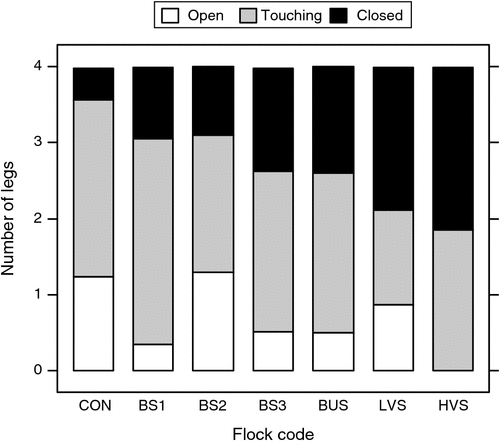
|
DNA tests of isolates taken from the sheep at the beginning and end of the trial showed that both the low and high virulent groups were positive for the intA gene while the remaining groups were negative. DNA from the reference virulent strain A198 and all other virulent strains tested were also positive for the intA gene. It is clear from these results that strains which are gel stable but benign in the field do not show bands in this DNA fingerprint, while strains which behave in a virulent manner show bands.
This trial showed that gel stable, field benign isolates did not cause virulent footrot, even under favourable climatic conditions and that the intA test could distinguish between gel stable, field virulent and gel stable, field benign strains. However, concern was expressed that the results were peculiar to the New England region and that the gel stable, field benign strains may be capable of causing virulent footrot in a different, more ‘expressive’ location. To address this concern, a second trial was conducted, again with the support of a PIRD grant.
Molong field trial
The results of this trial confirmed the previous study as there was a rapid progression of footrot in the group with gel stable, field virulent footrot but little change in the footscores of the remaining four groups (Fig. 4). Footrot in the local field virulent strain progressed rapidly with clean sheep becoming infected. The field benign but gel stable strains showed limited expression of footscore lesions (highest score 3A) with average scores less than the BUS group. There was no spread to the clean sheep in any of the field benign strains.
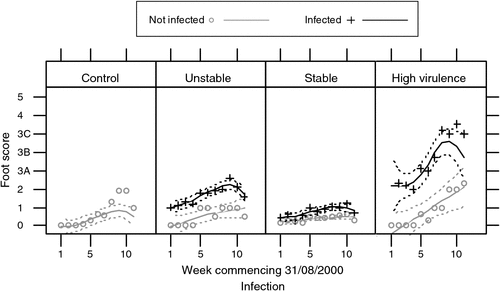
|
DNA testing again confirmed that the intA test could distinguish the gel stable, field virulent strain from the gel stable, field benign strains. These results not only confirm the findings of the Uralla trial but refute the claim that the gel test negates environmental influence and predicts the potential for an isolate to become virulent.
Discussion and conclusions
Both the Uralla and Molong trials studied various strains of footrot under similar expressive environments. The use of an accepted method and the use of skilled and experienced operators from the Armidale and Molong RLPB ensured that the foot scoring and disease diagnosis were carried out objectively and in a repeatable fashion. The sheep used were already infected with known strains of footrot from properties identified with footrot problems and so were likely to provide a reliable indication of how these sheep would react away from their home environment. The consistency of the gelatin gel results from these strains confirms their stability.
The foot conformation results deserve further mention as there were clear differences between the foot characteristics, especially of the HVS and the CON sheep. Closed feet appear to allow soil and faecal bacteria to invade the inter-digital skin and cause dermatitis creating a favourable environment for the footrot bacteria to thrive. The results suggest that livestock producers would be well advised to pay attention to the conformation of feet of any sheep they purchase and it should also be considered when sheep culling is carried out.
The gelatin gel test was found to have produced both ‘false’ positive as well as ‘false’ negative results. Whereas the diagnostician should ensure that all investigations are thorough and consistent with the guidelines of the NSW Footrot Strategic Plan, the gelatin gel test should be used only as an aid to diagnosis, not as a definitive result.
The use of DNA fingerprinting has clarified the situation regarding the potential for false positive diagnoses of footrot. Several genes have been identified that are found in strains of D. nodosus which produce virulent footrot but which are absent from most benign strains. The existence in the New England region of strains which are gel stable but field benign led to the analysis of the distribution of these genes in the atypical strains. It was found that intA was present in all strains classed as field virulent, but absent from the gel stable, field benign strains. This result suggests that these strains are genetically different and not capable of causing virulent footrot despite a gel stable result.
Following these trials, additional funding from Australian Wool Innovation enabled a large-scale study of the usefulness of the intA test to be carried out. Testing of 595 isolates of D. nodosus from 124 farms showed that for gel stable strains, there was a 90% correlation between the presence of the intA gene and ability to cause virulent footrot and the absence of the intA gene and benign footrot (Cheetham et al. 2006). This study also confirmed that there are a significant number of gel stable isolates of D. nodosus, which do not cause virulent footrot. The intA test is now available as a supplement to the gelatin gel test for use in footrot diagnosis in NSW. Ongoing vigilance and the availability of a range of reliable laboratory tests are vital to maintain the gains made since the introduction of the NSW Footrot Strategic Plan.
The footrot trial clearly demonstrated that a genuine concern by livestock producers that there was an anomaly in the testing regime for virulent footrot was found to be correct. Practical observations from producers, followed by producer support for trial work, which was then undertaken in a scientifically rigorous manner, has shown the value of producer/research partnerships in bringing about outcomes of great benefit to the livestock industry.
Acknowledgements
Support for this work was provided by the University of New England, Australian woolgrowers and the Australian Government through Producer Initiated Research and Development funding, the Woolmark Co. and Australian Wool Innovation Limited. We thank members of the Cicerone Project team in particular the Cicerone Board members, also Mr Les Gallagher and Mr Justin Hoad for looking after the Uralla field facilities and the sheep. The support from staff of NSW DPI, and staff of the Armidale and Molong Rural Lands Protection Boards, is particularly appreciated. We are grateful also to CSIRO Livestock Industries for the use of their land and equipment and their in-kind support. Valuable assistance was given in data handling and analysis by D. Mackay and J. M. Scott and statistical analysis by R. Murison of the University of New England. Mr Henry Thomas of ‘Wavertree’, Toogong kindly allowed us the use of the paddock at Molong for the trial and field day.
References
Bloomfield GA, Whittle G, McDonagh MB, Katz ME, Cheetham BF (1997) Analysis of sequences flanking the vap regions of Dichelobacter nodosus: evidence for multiple integration events, a killer system, and a new genetic element. Microbiology 143, 553–562.| Analysis of sequences flanking the vap regions of Dichelobacter nodosus: evidence for multiple integration events, a killer system, and a new genetic element.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXht1emsrk%3D&md5=5172c00c63a4c4cd9bd0e249adf76d33CAS | 9043132PubMed |
Cheetham BF, Tanjung LR, Sutherland M, Druitt J, Green G, McFarlane J, Bailey GD, Seaman JT, Katz ME (2006) Improved diagnosis of virulent ovine footrot using the intA gene. Veterinary Microbiology 116, 166–174.
| Improved diagnosis of virulent ovine footrot using the intA gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnsFyntb0%3D&md5=7956d6022cd25bc6713eb8dd716b779eCAS | 16716540PubMed |
Depiazzi LJ, Richards RB, Henderson J, Rood JI, Palmer M, Penhale WJ (1991) Characterisation of virulent and benign strains of Bacteroides nodosus. Veterinary Microbiology 26, 151–160.
| Characterisation of virulent and benign strains of Bacteroides nodosus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M3itVKjtw%3D%3D&md5=48da683ebe2428b4d541ddf78faa8aa1CAS | 2024437PubMed |
Gelman A, Hill J (2007) ‘Data analysis using regression and multilevel/hierarchical models.’ (Cambridge University Press: Cambridge)
Palmer MA (1993) A gelatin test to detect activity and stability of proteases produced by Dichelobacter (bacteroides) nodosus. Veterinary Microbiology 36, 113–122.
| A gelatin test to detect activity and stability of proteases produced by Dichelobacter (bacteroides) nodosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXivFamt7o%3D&md5=5397e4bba9639bad12584039eb864362CAS | 8236773PubMed |
Stewart DJ (1979) The role of elastase in the differentiation of Bacteroides nodosus infections in sheep and cattle. Research in Veterinary Science 27, 99–105.
Stewart DJ, Clark BL, Jarrett RG (1984) Differences between strains of Bacteroides nodosus in their effects on the severity of foot-rot, bodyweight and wool growth in Merino sheep. Australian Veterinary Journal 61, 348–352.
| Differences between strains of Bacteroides nodosus in their effects on the severity of foot-rot, bodyweight and wool growth in Merino sheep.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2M7kvFSgtg%3D%3D&md5=bf34fce3652ee78064fd92f080122796CAS | 6529394PubMed |
Sutherland H, Scott JM, Gray GD, Woolaston RR (2013) Creating the Cicerone Project: seeking closer engagement between livestock producers, research and extension. Animal Production Science 53, 593–601.
| Creating the Cicerone Project: seeking closer engagement between livestock producers, research and extension.Crossref | GoogleScholarGoogle Scholar |
Whittington RJ, Nicholls PJ (1995) Grading the lesions of ovine footrot. Research in Veterinary Science 58, 26–34.
| Grading the lesions of ovine footrot.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M3isVektA%3D%3D&md5=06d785e20b00102af8c1583fe77cfc07CAS | 7709056PubMed |
Wood SN (2006) ‘Generalized additive models: an introduction with R.’ (CRC Press: Boca Raton, FL)


