Ensiling corn silage with different levels of a multi-species lactic acid bacteria inoculant
H. Mohammadzadeh A C , M. Khorvash B and G. R. Ghorbani BA Department of Animal Science, Faculty of Agriculture, University of Tabriz, 51666-14766, Tabriz, Iran.
B Department of Animal Science, Faculty of Agriculture, Isfahan University of Technology, 84156-83111, Isfahan, Iran.
C Corresponding author. Email: hamidmhz@tabrizu.ac.ir
Animal Production Science 54(2) 165-171 https://doi.org/10.1071/AN12092
Submitted: 14 March 2012 Accepted: 4 March 2013 Published: 19 April 2013
Abstract
A multi-species lactic acid bacterial inoculant (Lactisil maize, LM) was applied to whole-crop corn at different maturities in laboratory silos, to evaluate its effects on biochemical characteristics and aerobic stability. The corn crop was harvested at hard dough (HD, 253.1 g/DM kg), one-third milkline (ML, 293.7 g/DM kg) and one-third milkline with a killing frost (MLF, 297.6 g/DM kg). Crops were chopped to a 2.5-cm theoretical cut length, subsampled and treated with two levels of inoculant (LB1 = 1.5 × 105 cfu/g forage, LB2 = 3 × 105 cfu/g forage) or untreated (WO). The chemical composition of MLF crops was very similar to that of ML crops. However, lower (P < 0.01) numbers of lactic acid bacteria and higher numbers of yeast were enumerated in MLF than in ML crops. Higher percentages of DM and neutral detergent fibre and higher pH, but lower (P < 0.01) concentrations of water soluble carbohydrate and crude protein were measured in ML and MLF crops than in HD crops. Application of the inoculant increased (P < 0.01) concentrations of volatile fatty acids, neutral detergent fibre and acid detergent fibre in silages. Lactic acid concentration increased (P < 0.01) in HD treatments with an increasing level of inoculant. In contrast, the highest (P < 0.01) lactic acid concentration was measured in LB1 treatment compared with WO and LB2 in ML and MLF silages. Silages prepared from ML and MLF crops had higher (P < 0.01) lactic and acetic acid concentrations but lower (P < 0.01) butyric acid concentrations than did those prepared from HD. The pH in LB1 and LB2 silages was higher (P < 0.01) than that measured in WO silages. Aerobic stability was not influenced by inoculant treatment but low-DM silages were more (P < 0.01) resistant to spoilage. Frost-killed corn crops had a good potential to produce well fermented silage. Using LM resulted in silages with slightly higher fermentation products but it failed to improve aerobic stability of silage after 120 days of ensiling. These results indicated that inoculation of corn crops with LM for a short-duration ensilage period cannot enhance aerobic stability of silages due to insufficient acetic acid production from lactic acid conversion.
Additional keywords: aerobic stability, frost-killed corn, heterofermentative fermentation, homofermentative lactic acid bacteria, Lactobacillus buchneri, maturity stage.
Introduction
Ensiling is a common preservative method for forage crops. Anaerobic conditions in silo and fermentation of water soluble carbohydrates (WSC) into lactic acid with lactic acid bacteria (LAB) preserve the nutrients in crops (McDonald et al. 1991). In Iran, corn (Zea mays) fodder is one of the major forages for ensiling and makes up approximately half of the dietary forage on many commercial dairies. However, in Iran, corn silage is usually planted in July as a second crop and is ensiled with low DM content (20–25% DM) due to insufficient time to reach an ideal maturity for ensiling (Khorvash et al. 2006). Moreover, late planting date and early falling in ambient temperature may result in situations where corn is killed by frost. Frosting reduces the level of LAB on the standing plant and elevates numbers of spoilage organisms (Mohammadzadeh et al. 2012) and then may produce silages with restricted fermentation and low aerobic stability.
To improve the nutritive value of silage, various types of additives, including bacterial inoculants, have been developed. Most bacterial inoculants consist of homofermentative LAB (e.g. Lactobacillus plantarum) rather than heterofermentative LAB (e.g. Lactobacillus buchneri). Homofermentative LAB inoculants enhance the rate of acidification and reduce the final pH or protein breakdown in silages (Sheperd et al. 1995; Weinberg and Muck 1996; Driehuis et al. 1997; Aksu et al. 2004). However, homofermentative LAB could induce aerobic deterioration of whole-crop cereal silages due to insufficient production of volatile fatty acids (VFA) to inhibit fungi (Weinberg et al. 1993; Filya et al. 2000). L. buchneri is the main heterofermentative LAB strain that produces higher concentrations of acetic acid than does L. plantarum in silage (Kleinschmit and Kung 2006). Improvement of aerobic stability using L. buchneri has been demonstrated in laboratory (Driehuis et al. 1999; Filya 2001, 2003b; Kung and Ranjit 2001; Weinberg et al. 2002) and field (Mari et al. 2009; Kristensen et al. 2010) studies.
The aim of the present study was to investigate the effects of a new multi-species LAB additive, Lactisil maize (Medipharm, Kågeröd, Sweden), on chemical composition, fermentation characteristics and aerobic stability of corn silages prepared from crops at different maturities as well as on frost-killed corn crops. This inoculant consists of homofermentative (Enterococcus faecium M74, Lactobacillus plantarum LS1, L. casei and Pediococcus pentosaceus) and heterofermentative (Lactobacillus buchneri) LAB. E. faecium and P. pentosaceus have a high optimum pH and start to produce lactic acid at a high pH of forage crops. This leads to faster falling in pH and provides optimal environmental conditions for L. plantarum and L. casei (low optimum pH). At latter phases of ensilage period, the inhibitory effect of low pH on homofermentative LAB provides a suitable situation for L. buchneri to proliferate and compete with homofermentative LAB (Nishino et al. 2003). As a result, Lactisil maize (LM) may simultaneously enhance both fermentation rate and aerobic stability of corn silage, especially in matured and frost-killed corn crops.
Materials and methods
Experimental silages
The whole-crop corn (hybrid 700; Plant Breeding, Karaj, Iran) was sown on 17 June and was harvested on 28 September at a hard-dough maturity stage (HD, 253.1 g DM/kg), on 13 October at a one-third milkline maturity stage (ML, 293.7 g DM/kg) and on 26 October at a one-third milkline maturity stage after a killing frost (MLF, 297.6 g DM/kg). When the ambient temperature decreased below 0°C (−4°C) for one night, forages in the field were harvested as frost-killed forages. In frost-killed corn crops, leaves were of brown or blackish-green colour. Crops at each maturity stage were cut by a chopper (Model 965, Claas, Omaha, NE, USA) to a average cut length of 2.5 cm and subsampled to be inoculated and ensiled. For inoculant-treated silages, 4 or 8 g of LM powder was suspended in 2 L of distilled water according to manufacturer’s recommendations and the suspension was then sprayed over the 400-kg subsamples of crop using a pressure sprayer, to get the final inoculation rates of 1.5 × 105 colony forming units (cfu)/g (LB1 treatment) and 3 × 105 cfu/g (LB2 treatment). The subsamples were each mixed thoroughly to obtain an even and homogeneous distribution of inoculums over the crops. The same amount of distilled water was applied to the control crops (silages without inoculants, WO). Laboratory PVC silos, 70 cm in height, 10 cm in diameter and equipped with a sink at the bottom to allow seepage outflow, were used for ensiling the crops. Before filling, each laboratory silo was weighed and then filled with ~2.5 kg of fresh forage. On filling, the silos were packed with a manual steel packer and were capped tightly. There were five silo replicates for each treatment. The silos were stored in the dark at room temperature (20–23°C), until opened for sampling after 120 days of ensiling.
Analytical procedures
Five replicated samples of corn crops and silages were analysed. DM of the samples was determined after drying in a forced-air oven for 72 h at 60°C, and corrected for loss of volatiles using the equation of Porter and Murray (2001). The dried samples were ground to pass through a 1-mm screen by using a Wiley mill (Arthur H. Thomas Co., Philadelphia, PA, USA). Organic matter was determined by burning at 550°C for 12 h. The ground samples were analysed for total N using the Kjeldahl method (Kjeltec 1030 Auto Analyzer, Tecator, Höganäs, Sweden). Neutral detergent fibre (NDF) was determined according to Van Soest et al. (1991) using α amylase and reported ash free. Concentrations of acid detergent fibre (ADF) were measured as described by AOAC (1990).
Subsamples of 20 g of corn crop or fresh silage were mixed with 180 mL of distilled water for 30 s in a blender to obtain extracts. Immediately after extracting, the pH was measured using a portable pH meter (HI8314, Hanna Instruments, ClujNapoca, Romania). The WSC concentration of samples was determined using the phenol–sulfuric acid method of DuBois et al. (1956). The silage extracts were filtered through filter paper (Whatman No. 54) for determination of concentration of fermentation products and ammonia-N (NH3-N). VFA were determined using gas chromatography (0.25 × 0.32, id of 0.3 µ WCOT Fused Silica Capillary, CHROMPACK CP 9002, Model CP-9002, Delft, The Netherlands) according to Mohammadzadeh et al. (2012). Lactic acid concentration was determined spectrophotometrically according to Barker and Summerson (1941), as modified by Pennington and Sutherland (1956). Ammonia-N concentration was measured by distillation in a Kjeltec Auto Analyzer (Tecator), without previous digestion step, as described by Filya (2003a).
Aerobic-stability measurement and microbiological analysis after exposure to air
Aerobic stability in air-exposed silage was defined as the number of hours the silage remained stable before temperature rising >2°C above the ambient temperature (Moran et al. 1996). When the silos were opened, ~1200 g of silage in each silo was placed in a plastic open-top container. The silage was loosely packed to fill approximately one-half of the volume of the container. A thermometer was placed in the geometric centre of the silage mass. The containers were covered with two layers of sterile cheesecloth to minimise drying. The containers were kept at room temperature and the temperatures were measured at 2-h intervals (Mohammadzadeh et al. 2012). Water extracts were obtained from silages just before exposing to air, for enumerating microbial population according to Adesogan and Salawu (2004). Yeast and mould populations were enumerated in triplicate by using potato dextrose agar (Merck, Darmstadt, Germany) with 0.15% tartaric acid and incubation at 25°C for 4 days under aerobic conditions (Higginbotham et al. 1998). The LAB population was enumerated in triplicate by using MRS agar (Difco Laboratories, Detroit, MI, USA) and incubation at 35°C for 2 days. Colonies were counted from the plates of appropriate dilutions containing a minimum of 30 colonies. All microbial data were log10 transformed and are presented on a wet-weight basis.
Statistical analyses
This research was conducted using a factorial experiment based on completely randomised design. Main effects of stage of maturity (HD, ML and MLF), inoculation (WO, LB1 and LB2) and their interactions were included in the model. General linear model of SAS (2003) was used for analyses. Data for the composition of crops were analysed using one-way ANOVA. Significant differences among means were identified by Tukey’s studentised range test and P < 0.05 was designated as significant.
Results
Table 1 reports the chemical and microbial composition of whole corn crop at different maturities before ensiling. Proportions of DM, OM and NDF and pH were higher (P < 0.01) in ML and MLF crops than in the HD crop. In contrast, lower (P < 0.01) WSC and crude protein (CP) concentrations were measured in ML and MLF crops than in the HD crop. The ML and MLF crops had larger numbers of epiphytic fungi (P < 0.01) and lower numbers of epiphytic LAB (P < 0.01) than did the HD crop. Chemical composition of MLF crops was similar to that of the ML crop. However, lower (P < 0.01) numbers of LAB and higher (P < 0.01) numbers of yeasts were found in the MLF than in ML crop.
Chemical composition of corn silages is given in Table 2. After 120 days of storage, there was no effect of inoculant application on the percentage of DM in the silages (Table 2). However, inoculation of MLF silage with a higher dosage of LM resulted in a silage with a higher (P < 0.01) percentage of DM. Inoculated silages had a higher (P < 0.01) concentration of NDF than did the control silage. The increase in the concentration of NDF in response to inoculation was more evident in MLF silages. A higher application level of the inoculant further increased (P < 0.01) NDF and ADF fractions in the silages. Although the LB2 treatment increased (P < 0.01) the concentration of CP in the HD and ML silages, a reduction was observed in the MLF silage.
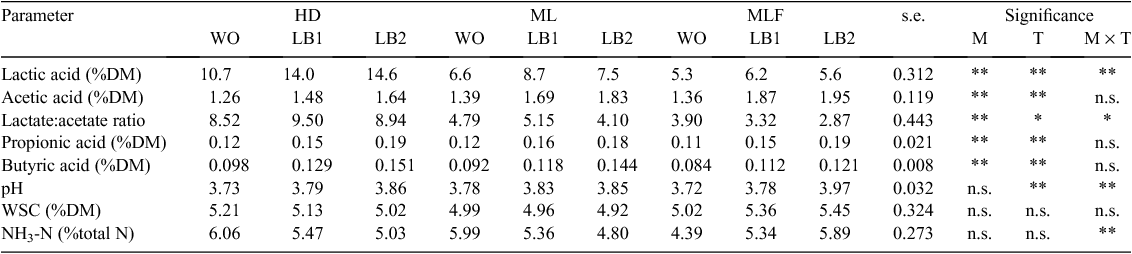
Fermentative characteristics of corn silages are given in Table 3. Concentration of lactic acid increased (P < 0.01) in the HD crop with an increasing application level of the inoculant. A higher (P < 0.01) lactic acid concentration was measured in the LB1 treatment than in the WO or LB2 treatments of ML and MLF silages. Higher (P < 0.01) concentrations of acetic, propionic and butyric acids were measured in treated silages than in the respective control. Moreover, the higher application level of the inoculant increased (P < 0.01) the concentration of VFA in the silages. ML and MLF silages had a higher (P < 0.01) acetic acid concentration than did the HD silage. In contrast, HD silage had a higher (P < 0.01) butyric acid concentration than did the ML and MLF silages. A higher ratio of lactic to acetic acid was measured in LB1 than in LB2 treatments. The pH of the inoculated silages was higher (P < 0.01) than that of untreated silages. Inoculation resulted in an increase (P < 0.01) in NH3-N concentration in the MLF silage, while a decrease (P < 0.01) was observed in the ML and HD silages.
Parameters of aerobic stability of inoculated and untreated corn silages are given in Table 4. Low-DM silages were more (P < 0.01) resistant to spoilage than were silages with a higher DM percentage. Greater (P < 0.01) numbers of yeasts and moulds were found in ML and MLF silages than in the HD silage. Silages prepared from crops at the HD maturity stage had greater (P < 0.01) numbers of LAB than did ML and MLF silages. Silages with a greater resistance to spoilage had a lower (P < 0.01) DM percentage and fewer (P < 0.01) fungi.
Discussion
Percentages of DM and NDF were higher in corn crops at a later maturity stage. These crops also had lower concentrations of WSC and CP and a higher pH. Our findings are in agreement with those of Johnson et al. (2003) who reported a lower concentration of WSC and a higher pH for high-DM corn crops. Frosting did not affect the chemical composition of corn crops except ADF. The increase in the proportion of ADF in response to frosting may have been due to binding of soluble cell contents to the cell wall, thus increasing the concentration of ADF. However, an increase in the numbers of yeasts and a decrease in the numbers of moulds and LAB in corn crops occurred due to frosting. These findings implied that fermentation rate can be limited in frosted or matured crops due to a higher proportion of DM, a lower concentration of WSC and fewer LAB. Furthermore, the greater number of fungi on matured and frosted crops may enhance spoilage of produced silage (Mohammadzadeh et al. 2012).
A higher concentration of lactic acid was found in silages at an earlier maturity stage. It has been demonstrated that a lower percentage of DM, low pH and a higher concentration of WSC in immature crops increase the production of organic acids in produced silage (Goodrich et al. 1975; Baron et al. 1986; Bal et al. 1997). Lactic and acetic acid concentrations were higher in inoculated silages than in the respective control silage. This finding agrees with those of Kleinschmit and Kung (2006) and Mohammadzadeh et al. (2011) who reported higher concentrations of lactic acid and acetic acid in response to inoculation of corn crops with a mixture of homo- and heterofermentative LAB. A higher application level of the inoculant resulted in elevated concentrations of lactic and acetic acid in the HD silage. In ML and MLF silages, the LB1 treatment resulted in a higher concentration of lactic acid and a lower concentration of acetic acid than did the LB2 treatment. The latter may have been due to higher numbers of L. buchneri in LB2 treatments and the inability of homofermentative LAB to compete with the heterofermentative LAB. The high DM percentage, low concentration of WSC and low numbers of LAB in ML and MLF crops had an inhibitory effect on the higher concentration of homofermentative LAB in the LB2 treatments. However, the higher concentration of heterofermentative LAB in LB2 treatments and the inhibitory effects on these bacteria resulted in a higher heterolactic activity than in the LB1 treatment, and in an increase in the concentration of acetic acid and a decrease in the concentration of lactic acid. Nishino et al. (2003) suggested that the high acetic acid concentration in silages inoculated with L. buchneri could be attributed mainly to lactic acid degradation and not to heterolactic fermentation. In general, these findings imply that LM causes an increase in numbers and activity of L. buchneri rather than homofermentative LAB in high-DM silages which then results in an increase in acetic acid and a decrease in lactic acid concentrations. However, a greater increase in lactic acid concentration in HD silage, in response to applying the inoculant at a high concentration, than in silages prepared from crops at ML and MLF stages suggested the dominance of homofermentative LAB in silage at an earlier maturity stage. The probable reason for this finding is the greater suspension in activity of homofermentative LAB in more matured crops due to relatively less available WSC, lower water activity and low numbers of epiphytic LAB in respective crops (Bernardes et al. 2005). Suspension in the activity of WSC-fermentative LAB provides an opportunity to heterolactic LAB (e.g. L. buchneri) to grow and develop by lactic acid to acetic acid conversion. Then, acetic acid accumulates faster and earlier in silages prepared from more matured corn forages. Nishino et al. (2003) showed that an increase in acetic acid concentration was more distinct at 120 days than at 60 days of ensiling.
Higher concentrations of propionic and butyric acids were measured in inoculant-treated silages due to heterolactic fermentation of L. buchneri. Krooneman et al. (2002) speculated that certain members of the epiphytic microfloora (L. diolivorans) are involved in the conversion of 1,2-propanediol (produced by L. buchneri) to propionic acid. The pH was higher in inoculant-treated silages due to a higher concentration of acetic acid and a lower ratio of lactic to acetic acid. Silages inoculated with L. buchneri usually have a higher pH than does the control or silages treated with homofermentative LAB (Mari et al. 2009; Kristensen et al. 2010; Mohammadzadeh et al. 2011). LAB use mainly WSC as a substrate during fermentation and produce lactic acid and VFA to gain energy for growth and proliferation (McDonald et al. 1991). Concentrations of residual WSC were substantially reduced in silages compared with those found in fresh corn forages. However, concentrations of WSC in silages were above 3.5% DM, a value considered to be the minimum required for a good fermentation to occur (Wilkinson 1990). The concentration of ammonia-N was lower in treated HD and ML silages. Filya (2003b) and Driehuis et al. (1999) reported that the concentration of ammonia-N was lower in the L. plantarum- and L. buchneri + L. plantarum-inoculated silages than in untreated silages or silages inoculated with L. buchneri. Previously, Zahiroddini et al. (2004) and McDonald et al. (1991) demonstrated the inhibitory effect of a rapid fall in silage pH on proteolytic activity of aerobic microorganisms and plant enzymes. However, in MLF silage, concentrations of CP and NH3-N decreased and increased, respectively, in response to inoculation. This may be due to a delay in pH drop in frost-killed corn silages because of lower concentrations of WSC and epiphytic LAB and a higher pH in parent crops, which limits LAB activity (McDonald et al. 1991). A higher pH in the MLF silage at a higher application level of the inoculant is in agreement with this finding. However, concentrations ammonia-N in all silages were very low (due to low pH in silages), indicating that all silages had a good fermentation.
In the current study, the inoculant had no significant effect on the aerobic stability of silages, in spite of the higher lactic acid concentration. This is due to the fact that LM increases the concentration of acetic acid in silage simultaneously with an increasing lactic acid concentration. Thus, the ratio of lactic acid to acetic acid can be a better tool to predict the aerobic stability of silage within each maturity stage, instead of the sole lactic acid concentration. Driehuis et al. (1999) and Nishino et al. (2003) demonstrated the inhibitory effects of acetic acid on fungi. The results achieved by Ranjit and Kung (2000), Driehuis et al. (2001) and Filya (2003a) showed that inoculation with L. buchneri alone or in combination with homofermentative LAB impairs the growth of moulds on the surface of silage. Acetic acid acts as a growth inhibitor of spoilage organisms by decreasing the maximum growth rate and, therefore, acetic acid increases the aerobic stability (Holzer et al. 2003). Moreover, L. buchneri produces other metabolites, as yet unidentified, with antifungal activity (Mann and Spoelstra 2001). Oude Elferink et al. (2001) showed that 1,2-propanediol, coupled with acetic acid, is produced during anaerobic degradation of lactic acid by L. buchneri and this enhances aerobic stability. The parameters of aerobic stability implied that the 120 days of ensiling was not long enough for L. buchneri (and consequently for LM) to produce enough acetic acid from lactic acid conversion or to produce high concentrations of propionic acid by heterolactic fermentation to improve the aerobic stability of silages.
Also, a great difference was found in the aerobic stability between high- and low-DM silages and HD silages showed greater aerobic stability. In a previous study, Weinberg et al. (2010) reported that the porosity of the silage and its susceptibility to air ingress depend on the degree of compaction in the silo, which is affected by the DM content of the crop. It was also reported that wet-pack density in the silo tended to decline as the corn plant matured (Harrison et al. 1998). Porosity determines the rate that air can infiltrate the silo and, subsequently, affects the amount of spoilage at the time of feed out (Muck and Holmes 1999).
A major cause of spoilage in silage is undesirable activity of some microorganisms, such as yeasts and moulds (Woolford 1990). The lowest numbers of yeasts and moulds in silages contributed to the highest aerobic stability (Stryszewska and Pys 2006). Silage yeast, but not airborne ones, initiates deterioration of silages on exposure to air (Lindgren et al. 1985; McDonald et al. 1991; Oude Elferink et al. 2001). In our study, within each maturity stage, silages with a low ratio of lactic to acetic acid had low numbers of yeasts and moulds on the day of opening. This finding was confirmed with a higher measured aerobic stability in silages with a higher acetic to lactic acid ratio. The results of Driehuis et al. (2001) showed that inoculation with a combination of L. buchneri and homofermentative LAB impairs the growth of moulds on the surface of silage. Filya (2003a) reported that after 90 days of ensiling, yeasts and moulds were not found in silages inoculated with L. buchneri + L. plantarum, whereas appreciable numbers of yeasts and moulds were detected in the control silages. In a study by Ranjit and Kung (2000), the numbers of yeasts were reduced dramatically in the silage inoculated with L. buchneri compared with the untreated silage.
Conclusions
The maturity stage of corn forage significantly affected anaerobic fermentation characteristics in produced silages. Lower concentrations of fermentation end products were measured in silages prepared from more matured forages due to a higher DM percentage, a higher pH and lower concentrations of WSC and LAB. Homofermentative fermentation was dominant over heterofermentative fermentation in high-moisture crops, but a reverse was found in more matured crops. Frosting of one-third milkline corn crop resulted in silages with good fermentation characteristics without any considerable effects on chemical composition. Frost-killed matured corn crops have a good potential to be ensiled and can be considered as a good feed source for ruminants when frosting occurs before harvesting the crops. During the short ensiling period, using LM as a LAB inoculant (especially in higher doses) accelerated homofermentative fermentation in unmatured corn silages and heterolactic fermentation in silages prepared from more matured crops. However, LM failed to improve aerobic stability of silage after 120 days of ensiling, maybe due to insufficient time to produce a higher concentration of acetic acid by lactic acid conversion. Lactisil maize may enhance aerobic stability and fermentation rate of matured corn crops when ensiled for a long time period.
Acknowledgements
The authors acknowledge the financial support given to this project by Isfahan University of Technology (Isfahan, Iran). Authors specially thank management board of Sana Dam Co. (Isfahan, Iran) for providing Lactisil maize.
References
Adesogan A, Salawu M (2004) Effect of applying formic acid, heterolactic bacteria or homolactic and heterolactic bacteria on the fermentation of bi crops of peas and wheat. Journal of the Science of Food and Agriculture 84, 983–992.| Effect of applying formic acid, heterolactic bacteria or homolactic and heterolactic bacteria on the fermentation of bi crops of peas and wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsFGrsbs%3D&md5=6d2fee0be2d04107f47002a5668f3548CAS |
Aksu T, Baytok E, Bolat D (2004) Effects of a bacterial silage inoculant on corn silage fermentation and nutrient digestibility. Small Ruminant Research 55, 249–252.
| Effects of a bacterial silage inoculant on corn silage fermentation and nutrient digestibility.Crossref | GoogleScholarGoogle Scholar |
Association of Official Analytical Chemists (AOAC) (1990) ‘Official methods of analysis.’ 15th edn. (Association of Official Analytical Chemists: Arlington, VA)
Bal MA, Coors JG, Shaver RD (1997) Impact of the maturity of corn for use as silage in the diets of dairy cows on intake, digestion, and milk production. Journal of Dairy Science 80, 2497–2503.
| Impact of the maturity of corn for use as silage in the diets of dairy cows on intake, digestion, and milk production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXntFemsbg%3D&md5=4b1a231b398e3ac9a3e7793c6e13b4c9CAS | 9361221PubMed |
Barker B, Summerson WH (1941) The colorimetric determination of lactic acid in biological material. The Journal of Biological Chemistry 138, 535–554.
Baron VS, Stevenson KR, Buchanan-Smith JG (1986) Proteolysis and fermentation of grain-corn ensiled at several moisture levels and under several simulated storage methods. Canadian Journal of Animal Science 66, 451–461.
| Proteolysis and fermentation of grain-corn ensiled at several moisture levels and under several simulated storage methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XltFeqs7g%3D&md5=008eed6f5c4badb5e230d45ff7056395CAS |
Bernardes TF, Reis RA, Moreira AL (2005) Fermentative and microbiological profile of marandu-grass ensiled with citrus pulp pellets. Scientia Agricola 62, 214–220.
| Fermentative and microbiological profile of marandu-grass ensiled with citrus pulp pellets.Crossref | GoogleScholarGoogle Scholar |
Driehuis F, Van Wikselaar PG, Van Vuuren AM, Spoelstra SF (1997) Effect of a bacterial inoculant on rate of fermentation and chemical composition of high dry matter grass silages. The Journal of Agricultural Science 128, 323–329.
| Effect of a bacterial inoculant on rate of fermentation and chemical composition of high dry matter grass silages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjs1Cksro%3D&md5=6612e0eb201e4c93984ffe72191f9531CAS |
Driehuis F, Elferink SJ, Spoelstra SF (1999) Anaerobic lactic acid degradation during ensilage of whole crop maize inoculated with Lactobacillus buchneri inhibits yeast growth and improves aerobic stability. Journal of Applied Microbiology 87, 583–594.
| Anaerobic lactic acid degradation during ensilage of whole crop maize inoculated with Lactobacillus buchneri inhibits yeast growth and improves aerobic stability.Crossref | GoogleScholarGoogle Scholar | 10583687PubMed |
Driehuis F, Oude Elferink S, Van Wikselaar P (2001) Fermentation characteristics and aerobic stability of grass silage inoculated with Lactobacillus buchneri, with or without homofermentative lactic acid bacteria. Grass and Forage Science 56, 330–343.
| Fermentation characteristics and aerobic stability of grass silage inoculated with Lactobacillus buchneri, with or without homofermentative lactic acid bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhvF2qtrw%3D&md5=3d2104aef90841f6981829b6cd41d5adCAS |
DuBois M, Gilles KA, Hamilton JK, Rebers A, Smith FF (1956) Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28, 350–356.
| Colorimetric method for determination of sugars and related substances.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG28XjvFynsg%3D%3D&md5=e534fe2cb02c7c27744b4a149b2396a7CAS |
Filya I (2001) Aerobic stability of sorghum and maize silages treated with homofermentative and heterofermentative lactic acid bacteria. In ‘Turkey–Israeli workshop on silage and agricultural by-products for high lactating cows’. (Ed. ZG Weinberg) pp. 24–26. (Agricultural Research Organization, The Volcani Center: Bet Dagan, Israel)
Filya I (2003a) The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation, aerobic stability, and ruminal degradability of low dry matter corn and sorghum silages. Journal of Dairy Science 86, 3575–3581.
| The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation, aerobic stability, and ruminal degradability of low dry matter corn and sorghum silages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptFajurY%3D&md5=1cbee65547322d628da6dbadfbdd7dc3CAS | 14672188PubMed |
Filya I (2003b) The effect of Lactobacillus buchneri, with or without homofermentative lactic acid bacteria, on the fermentation, aerobic stability and ruminal degradability of wheat, sorghum and maize silages. Journal of Applied Microbiology 95, 1080–1086.
| The effect of Lactobacillus buchneri, with or without homofermentative lactic acid bacteria, on the fermentation, aerobic stability and ruminal degradability of wheat, sorghum and maize silages.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3srlsValsg%3D%3D&md5=97e6888f1d86da8b99d6cda156e7afe6CAS | 14633037PubMed |
Filya I, Ashbell G, Hen Y, Weinberg ZG (2000) The effect of bacterial inoculants on the fermentation and aerobic stability of whole crop wheat silage. Animal Feed Science and Technology 88, 39–46.
| The effect of bacterial inoculants on the fermentation and aerobic stability of whole crop wheat silage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXot12lurY%3D&md5=2b4cbfddcc2fdce1d6da0307147fc439CAS |
Goodrich RD, Byers FM, Meiske JC (1975) Influence of moisture content, processing and reconstitution on the fermentation of com grain. Journal of Animal Science 41, 876–881.
Harrison JH, Johnson L, Hunt C, Doggett CD, Rotz CA, Shinners K, Sapienza D (1998) Mechanical processing of corn silage: looking back two years. In ‘Western Washington Horticultural Association, Annual convention, Seattle, WA’. pp. 154–170.
Higginbotham G, Mueller S, Bolsen K, DePeters E (1998) Effects of inoculants containing propionic acid bacteria on fermentation and aerobic stability of corn silage. Journal of Dairy Science 81, 2185–2192.
| Effects of inoculants containing propionic acid bacteria on fermentation and aerobic stability of corn silage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlvVCmt7g%3D&md5=4dd26c005fc2b7d54a4f8d912d39b42cCAS |
Holzer M, Mayrhuber E, Danner H, Braun R (2003) The role of Lactobacillus buchneri in forage preservation. Trends in Biotechnology 21, 282–287.
| The role of Lactobacillus buchneri in forage preservation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkt1aru7w%3D&md5=cea96a425f576c7e34b02cc0c44d51e8CAS | 12788549PubMed |
Johnson L, Harrison J, Davidson D, Mahanna W, Shinners K (2003) Corn silage management: effects of hybrid, maturity, inoculation, and mechanical processing on fermentation characteristics. Journal of Dairy Science 86, 287–308.
| Corn silage management: effects of hybrid, maturity, inoculation, and mechanical processing on fermentation characteristics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtlCktr8%3D&md5=87351c6c919eeac7a9858575919a2a8aCAS | 12613872PubMed |
Khorvash M, Colombatto D, Beauchemin KA, Ghorbani GR, Samei A (2006) Use of absorbants and inoculants to enhance the quality of corn silage. Canadian Journal of Animal Science 86, 97–107.
Kleinschmit DH, Kung L (2006) A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain silages. Journal of Dairy Science 89, 4005–4013.
| A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain silages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVWgsbzI&md5=e053a8c2b1c31a141ea1da2cf404f639CAS | 16960077PubMed |
Kristensen NB, Sloth KH, Hojberg O, Spliid NH, Jensen C, Thogersen R (2010) Effects of microbial inoculants on corn silage fermentation, microbial contents, aerobic stability, and milk production under field conditions. Journal of Dairy Science 93, 3764–3774.
| Effects of microbial inoculants on corn silage fermentation, microbial contents, aerobic stability, and milk production under field conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Ggur%2FM&md5=15f1c570a49969b9d3502437dbd53a67CAS | 20655446PubMed |
Krooneman J, Faber F, Alderkamp A, Elferink SO, Driehuis F, Cleenwerck I, Swings J, Gottschal J, Vancanneyt M (2002) Lactobacillus diolivorans sp. nov., a 1,2-propanediol-degrading bacterium isolated from aerobically stable maize silage. International Journal of Systematic and Evolutionary Microbiology 52, 639–646.
Kung L, Ranjit N (2001) The effect of Lactobacillus buchneri and other additives on the fermentation and aerobic stability of barley silage. Journal of Dairy Science 84, 1149–1155.
| The effect of Lactobacillus buchneri and other additives on the fermentation and aerobic stability of barley silage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjslGju78%3D&md5=93151d5da5472f458d6ee18b26f3b744CAS | 11384041PubMed |
Lindgren S, Pettersson K, Kaspersson A, Jonsson A, Lingvall P (1985) Microbial dynamics during aerobic deterioration of silages. Journal of the Science of Food and Agriculture 36, 765–774.
| Microbial dynamics during aerobic deterioration of silages.Crossref | GoogleScholarGoogle Scholar |
Mann S, Spoelstra S (2001) Microorganisms and their use in treating animal feed and silage. Google Patents. Available at http://www.google.com/patents/US6326037 [verified 5 April 2013].
Mari LJ, Schmidt RJ, Nussio LG, Hallada CM, Kung L (2009) An evaluation of the effectiveness of Lactobacillus buchneri 40788 to alter fermentation and improve the aerobic stability of corn silage in farm silos. Journal of Dairy Science 92, 1174–1176.
| An evaluation of the effectiveness of Lactobacillus buchneri 40788 to alter fermentation and improve the aerobic stability of corn silage in farm silos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXivVaktbk%3D&md5=9ddbc3329e395dbd5baf7b769a6850cbCAS | 19233810PubMed |
McDonald P, Henderson A, Heron S (1991) ‘The biochemistry of silage.’ 2nd edn. (Chalcombe Publications: Bucks, UK)
Mohammadzadeh H, Khorvash M, Ghorbani G, Yang W (2011) Effects of a dual-purpose bacterial inoculant on the fermentation characteristics of high-moisture maize silage and dairy cattle performance. South African Journal of Animal Science 41, 368–376.
| Effects of a dual-purpose bacterial inoculant on the fermentation characteristics of high-moisture maize silage and dairy cattle performance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xlt1Wnsb8%3D&md5=1184e7b29ccb24fab514fa9b1de71603CAS |
Mohammadzadeh H, Khorvash M, Ghorbani G, Yang W (2012) Frosted corn silage with or without bacterial inoculants in dairy cattle ration. Livestock Science 145, 153–159.
| Frosted corn silage with or without bacterial inoculants in dairy cattle ration.Crossref | GoogleScholarGoogle Scholar |
Moran JP, Weinberg Z, Ashbell Y, Hen Y, Owen T (1996) In ‘Proceedings of the XI International silage conference’. (Eds DIH Jones, R Jones, R Dewhurst, R Merry, PH Haigh) pp. 162–163. (University of Wales: Aberystwyth, UK)
Muck RE, Holmes BJ (1999) Factors affecting bunker silo densities. In ‘Proceedings of the XII international silage conference’. (Ed. T Pauly) pp. 278–278. (Swedish University of Agricultural Sciences: Uppsala, Sweden)
Nishino N, Yoshida M, Shiota H, Sakaguchi E (2003) Accumulation of 1,2 propanediol and enhancement of aerobic stability in whole crop maize silage inoculated with Lactobacillus buchneri. Journal of Applied Microbiology 94, 800–807.
| Accumulation of 1,2 propanediol and enhancement of aerobic stability in whole crop maize silage inoculated with Lactobacillus buchneri.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktVSksLk%3D&md5=d1af8feaee36ed0b775b30a5ef2ca546CAS | 12694444PubMed |
Oude Elferink S, Krooneman J, Gottschal J, Spoelstra S, Faber F, Driehuis F (2001) Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediol by Lactobacillus buchneri. Applied and Environmental Microbiology 67, 125–132.
| Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediol by Lactobacillus buchneri.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M3ls1GmtA%3D%3D&md5=3b96883e8d760402be1c358df4b4db20CAS | 11133436PubMed |
Pennington R, Sutherland T (1956) Ketone-body production from various substrates by sheep-rumen epithelium. Biochemical Journal 63, 353–361.
Porter MG, Murray PG (2001) The volatility of components of grass silage on oven drying and the inter-relationship between drymatter content estimated by different analytical methods. Grass and Forage Science 56, 405–411.
| The volatility of components of grass silage on oven drying and the inter-relationship between drymatter content estimated by different analytical methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhvF2qtro%3D&md5=d859253a384ae3ef62cc3c1a63daa848CAS |
Ranjit NK, Kung L (2000) The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. Journal of Dairy Science 83, 526–535.
| The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXit1ymsLk%3D&md5=b637f970e2659b702125a260db956cbeCAS | 10750111PubMed |
SAS (2003) ‘SAS/STAT user’s guide. (SAS Institute: Cary, NC)
Sheperd AC, Maslanka M, Quinn D, Kung L (1995) Additives containing bacteria and enzymes for alfalfa silage. Journal of Dairy Science 78, 565–572.
| Additives containing bacteria and enzymes for alfalfa silage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXkslSmt7Y%3D&md5=92b1a80a963bfc524d6df42c67a72747CAS | 7540187PubMed |
Stryszewska K, Pys J (2006) Effects of different silage additives on the microbial population and aerobic stability of maize silage. Journal of Animal and Feed Sciences 15, 121–124.
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fibre, neutral detergent fibre, and nonstarch poly-saccharides in relation to animal nutrition. Journal of Dairy Science 74, 3583–3597.
| Methods for dietary fibre, neutral detergent fibre, and nonstarch poly-saccharides in relation to animal nutrition.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38%2FnvVCltA%3D%3D&md5=83605a987aed1cab5024d20cbdabf9b5CAS | 1660498PubMed |
Weinberg ZG, Muck RE (1996) New trends and opportunities in the development and use ofoculants for silage. FEMS Microbiology Reviews 19, 53–68.
| New trends and opportunities in the development and use ofoculants for silage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xmtlehsb8%3D&md5=15b09b50bca983937378eeeb22ec5b93CAS |
Weinberg ZG, Ashbell G, Hen Y, Azrieli A (1993) The effect of applying lactic acid bacteria at ensiling on the aerobic stability of silages. Journal of Applied Microbiology 75, 512–518.
| The effect of applying lactic acid bacteria at ensiling on the aerobic stability of silages.Crossref | GoogleScholarGoogle Scholar |
Weinberg ZG, Ashbell G, Hen Y, Azrieli A, Szakacs G, Filya I (2002) Ensiling whole-crop wheat and corn in large containers with Lactobacillus plantarum and Lactobacillus buchneri. Journal of Industrial Microbiology Biotechnology 28, 7–11.
Weinberg ZG, Khanala P, Yildiz C, Chena Y, Arieli A (2010) Effects of stage of maturity at harvest, wilting and LAB inoculant on aerobic stability of wheat silages. Animal Feed Science and Technology 158, 29–35.
| Effects of stage of maturity at harvest, wilting and LAB inoculant on aerobic stability of wheat silages.Crossref | GoogleScholarGoogle Scholar |
Wilkinson J (1990) ‘Silage.’ (Chalcombe Publications: Marlow, UK)
Woolford MK (1990) The detrimental effects of air in silage. The Journal of Applied Bacteriology 68, 101–116.
| The detrimental effects of air in silage.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3c3gt1GmtA%3D%3D&md5=d37f4da5fd6ed5a7d894a0c73f269a0fCAS | 2180886PubMed |
Zahiroddini H, Baah J, Absalom W, McAllister TA (2004) Effect of an inoculant and hydrolytic enzymes on fermentation and nutritive value of whole crop barley silage. Animal Feed Science and Technology 117, 317–330.
| Effect of an inoculant and hydrolytic enzymes on fermentation and nutritive value of whole crop barley silage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXpsFSjtrY%3D&md5=917a2d3642576e2fd6e265bcb17f2a12CAS |