The potential for probiotics to prevent reproductive tract lesions in free-range laying hens
S. Shini A C D , A. Shini B and P. J. Blackall CA School of Veterinary Science, The University of Queensland, Gatton, Qld 4343, Australia.
B School of Agriculture and Food Sciences, The University of Queensland, Gatton, Qld 4343, Australia.
C Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, St Lucia, Qld 4072, Australia.
D Corresponding author. Email: s.shini@uq.edu.au
Animal Production Science 53(12) 1298-1308 https://doi.org/10.1071/AN12337
Submitted: 26 September 2012 Accepted: 19 February 2013 Published: 7 May 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
A study was undertaken to investigate the ability of two commercial probiotics applied in free-range laying hens (from 18 to 22 weeks of age) in reducing the occurrence of reproductive tract pathologies, and improving hen health and performance. In all, 630 17-week-old brown layers were transferred to a freshly cleaned free-range laying facility, and randomly divided into three groups, with three replicates of 70 birds each. Both probiotics were administered in the drinking water (Groups 1 and 2) on a daily basis for 4 weeks, while Group 3 was left untreated. At 38 weeks of age, the results demonstrated that treatment with either probiotic significantly reduced the occurrence of reproductive tract pathologies (control vs probiotics, 33% vs 22% and 11%; P < 0.01), mortalities (control vs probiotics; 3.8% vs 1.5 and 1.9%; P < 0.01), and increased the performance of hens, for another 20 weeks post-treatments (hen day production for control vs probiotics 75% vs 90% and 94%; P < 0.01). Birds treated with probiotics maintained their bodyweight and egg weights at standard ranges, while untreated birds did not perform at this level. Although we were unable to show any effect on cloacal bacterial colonisation, the results of the present study provided some initial evidence that reproductive pathologies that often cause drops in egg production and sudden deaths of birds, can be reduced if free range hens are treated with a commercial probiotic before or during the onset of lay. The use of a probiotic benefits the health and performance status of hens, resulting in better hen welfare and significant economic gains to egg producers.
Additional keywords: egg production, good bacteria, laying hens, oviduct pathology.
Introduction
Free range accounts for ~26% of Australia’s commercial egg production (AECL 2010). Although this system allows hens to express full behavioural repertories, hens held under free-range conditions can be exposed to pathogenic bacteria that may disturb the normal flora of their body, leading to a range of diseases. From an early age, the chicken’s intestinal tract is colonised by a large number of microbial species that play an important role in some physiological processes of the host in the gastrointestinal tract and other body systems, such as respiratory and reproductive system (Apajalahti et al. 2004). Several husbandry factors (in earlier stages and during laying period) alter the composition of the intestinal microflora and cause increased susceptibility of hens to pathogens and food safety-related bacteria, especially Clostridium perfringens, avian pathogenic Escherichia coli, Salmonella spp. and Campylobacter spp. (Durant et al. 1999; Yamauchi et al. 2006; Burkholder et al. 2008). Other predisposing conditions such as mucosal damage (of gut or reproductive tract) and immunosuppression caused by stress can contribute to increased morbidity of gastrointestinal and reproductive tract and decreased performance or mortality of birds (Yamauchi et al. 2006; Burkholder et al. 2008).
In laying hens, the oviduct is the site for egg formation, and defence against pathogens in this organ is essential not only for the health of birds, but also for the production of safe eggs. Pathologies such as oophoritis, salpingitis, peritonitis and metritis are frequently encountered at onset and during the laying period, causing losses in egg production (Jordan et al. 2005). Most bacteria commonly associated with reproductive tract infections originate from both the ascending and descending route (Gast and Beard 1990; Shivaprasad et al. 1990; Hoop and Pospischil 1993; Keller et al. 1995). Consequently, before or after eggshell formation, developing eggs can be exposed to bacteria. Eggs from free-range systems were typically more contaminated than those from cage systems (De Reu et al. 2008). New data from extensive surveys in Europe and Australia have shown that reproductive tract infections such as salpingitis and peritonitis are more common in layers in non-cage or litter-based (Tauson et al. 1999) and free-range systems (Nagle and Shini 2008; Fossum et al. 2009; Neubauer et al. 2009), providing a strong link between a contaminated environment and reproductive tract diseases.
One key problem facing the egg industry is that there are virtually no suitable agents to prevent, or treat, reproductive tract infections. In recent years, probiotics or direct-fed microbials have been proposed as a natural and useful choice for providing protection against enteric diseases, improved immunity and performance of broiler chicks and laying hens (Nahashon et al. 1996; Balevi et al. 2001; Willis and Reid 2008). It has been proposed that after oral administration, probiotics can change the bacterial community structure in the avian gastro-intestinal tract (Mountzouris et al. 2007; Willis and Reid 2008).
The presence of lactobacilli in the cloaca and uterus of hens has been seen as an important factor in maintaining the microbial ecosystem and preventing the growth of pathogens, such as Salmonella (Van Coillie et al. 2007). Studies in humans have demonstrated that the use of orally delivered probiotics containing lactobacilli restores commensal vaginal flora. Reid and Bocking (2003) showed that oral or vaginal administration of certain lactobacilli strains can safely colonise the vagina, displace and kill pathogens including Escherichia coli, and modulate the immune responses. Oral formulations of lactobacilli intended for use in genitourinary infections have been shown to be capable of maintaining their structural integrity during passage through the gut, or when delivered to the rectal area can colonise the vaginal tract (Shalev et al. 1996; Reid et al. 2003). As another example, normalisation of the urogenital tract in females was observed 14 days after oral administration of lactobacilli (Morelli et al. 2004).
Poultry producers, and especially organic producers, are interested in the use of probiotics in laying hens to improve egg production and health of hens kept in free range. Evidence is required of the efficacy of commercial probiotic use. Randomised controlled trials can provide evidence of probiotic efficacy in the prevention of diseases and improvement of hen liveability and profitability. The objective of the present study was to explore the ability of two commercially available probiotics when applied in drinking water before and during the onset of lay in reducing the occurrence of reproductive tract pathologies and improving general health and performance of hens kept in free range.
Materials and methods
Birds, housing and treatments
In total, 630 17-week-old Hy-Sex Brown layers were transferred to a freshly cleaned free-range laying facility and randomly divided into three groups, with three replicates of 70 birds each. Sheds contained automated chain feeders and drinkers for supplying feed and water, respectively. Birds were kept at a density of two birds/m2 floor space (inside the shed) and had access to an outdoor area (separated area for each replicate) of 15 birds/100 m2. Birds were vaccinated for avian encephalomyelitis, egg drop syndrome, fowl pox, infectious bronchitis, Marek’s disease, Newcastle disease, fowl cholera, infectious coryza, Mycoplasma gallisepticum and M. synoviae and had regular worming. Other bird husbandry and management (feeding regime, lighting, and indoor and outdoor conditions) were as recommended by breeding company and in accordance with industry standards, the Free Range Egg and Poultry Association of Australia, and Australian regulations (Primary Industries Standing Committee 2002).
All groups (two treatments and one control) received the same diet, a corn-based organic diet (containing an average of 11.6 MJ/kg ME, 19% crude protein, 4.3% fibre, 3.82% Ca, and 0.83% P) with no probiotics or other antimicrobial agents. Two commercially available probiotics approved for use in poultry in Australia were employed in the present study. The probiotics were in a powder form and were dissolved in the drinking water on a daily basis for 4 weeks (from 18 to 22 weeks of age). Probiotic 1, a multi-strain probiotic product, contained a source of live viable naturally occurring microorganisms isolated from the crop (Lactobacillus reuteri), jejunum (Enterococcus faecium), ileum (Bifidobacterium animalis), and caecum (Pediococcus acidilactici and Lactobacillus salivarius) of healthy adult chickens and had a total bacterial count of 2 × 1012 colony-forming units (cfu)/kg of product. The fructo-oligosaccharides used in Probiotic 1 are derived from a natural plant source and are selected for their ability to stimulate the growth of beneficial bacteria such as bifidobacteria and lactobacilli in the intestine. Following recommendations of the manufacturer, Probiotic 1 was administered in the drinking water at a level to supply 108 bacteria/hen.day for 4 weeks. Probiotic 2 was a highly concentrated pre-mix containing seven strains of bacteria and two yeasts (Lactobacillus plantarum 1.89 × 1010 cfu/kg, L. delbrueckii subsp. bulgaricus 3.09 × 1010 cfu/kg, L. acidophilus 3.09 × 1010 cfu/kg, L. rhamnosus 3.09 × 1010 cfu/kg, Bifidobacterium bifidum 3.00 × 1010 cfu/kg, Streptococcus salivarius subsp. thermophilus 6.15 × 1010 cfu/kg, Enterococcus faecium 8.85 × 1010 cfu/kg, Aspergillus oryza 7.98 × 109 cfu/kg, Candida pintolopesii 7.98 × 109 cfu/kg), with all microorganisms having been isolated from a wide range of feed, plant, animal, bird and human sources. Probiotic 2 was also administered for 4 weeks at a dose recommended by manufacturer (1 g/L in the drinking water). Control hens received water with no probiotics. All procedures conducted in the study were approved by the Animal Ethics Committee of the University of Queensland (approval number SVS/248/09/POULTRY CRC).
Sampling and data collection
The health status of birds was checked daily and birds found dead were recorded and necropsied to assess for reproductive tract pathologies. Samples (for microbiological and histology tests) were taken before the treatment started (at 18 weeks of age), 4 weeks after first treatment (at 22 weeks of age), 4 weeks after last treatment (at 26 weeks of age) and at the point of lay period (at 38 weeks of age).
Microbiological testing
Microbiological samples from cloaca and oviduct were collected only from live or freshly killed and necropsied birds. At 18, 22, 26, and 38 weeks of age, samples from cloaca of nine control hens and nine hens per each treatment were collected aseptically by swabs, and streaked onto 5% sheep blood agar, MacConkey agar and xylose–lysine decarboxylase agar. The plates were incubated aerobically at 37°C overnight. The following day, bacteria considered significant were single-colony picked onto a fresh sheep-blood agar plate. All primary sheep-blood plates were re-incubated for a further 24 h and re-examined. Normal bacterial growth (e.g. coliforms, Pseudomonas spp., Bacillus spp.) were identified purely on colony morphology. An abbreviated identification scheme based on conventional phenotypic tests was used to identify Escherichia coli (only if present as pure culture with no other coliforms evident on the MacConkey agar) and Gallibacterium anatis biovar haemolytica. The phenotypic tests used were Gram stain, oxidase reaction, catalase reaction, indole reaction and colony type on MacConkey agar. Isolates were presumptively identified as E. coli if they were Gram negative, oxidase negative, catalase and indole positive and produced typical E. coli colonies on MacConkey agar. Isolates were presumptively identified as Gallibacterium anatis biovar haemolytica if they were Gram negative, oxidase and catalase positive and indole negative and produced only weak or no growth on MacConkey agar. The control stains used for the phenotypic tests were E. coli ATCC 25922 and Gallibacterium anatis biovar haemolytica CCUG 15563.
On the same day, the birds used for cloacal swabs were humanely killed and then necropsied. Swab samples were aseptically collected from oviduct (in the region between the magnum and isthmus) of each hen for microbiological testing as above. Birds were subsequently examined to determine the prevalence of the subclinical and chronic forms of the reproductive tract lesions.
Post-mortem examination
All birds that died during the experimental period (from 18 to 38 weeks of age) and birds that were killed at each sampling point (at 22, 26 and 38 weeks of age) were subjected to a post-mortem examination to identify the cause of death or evaluate the reproductive tract, respectively. The necropsy included the bodyweight (BW) of the bird, an examination of the overall condition, and external and internal observations. Where required, a tentative diagnosis was based on the presence of macroscopic lesions in organs. A scoring system was used for an effective recognition of macroscopic changes observed in dead or killed hens. Birds were inspected for pathological changes, including inflammatory signs such as, for example, hyperaemia and oedema of the mucosal membranes and the presence of serous, fibrinous or caseous exudates in the peritoneal cavity, the ovary, the oviduct or all of them. The area of the vent and cloaca was examined for signs of infections and prolapse.
Histology
Samples for histology evaluation (six from each treatment) were taken from intestinal tract (ileum segments at the mid-point) and the oviducal magnum of killed birds at 38 weeks of age. Oviducal magnum samples were taken from hens that had an egg in the mid-magnum. All samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned and stained with haematoxylin and eosin stain before microscopic examination. For each section, 10 randomly located areas were assessed using light microscopy at ×4, ×10, ×40 and ×100 magnification. Structural integrity of the villus was assessed from the ratio of villus height : crypt depth, which were measured under a light microscope (×100 magnification) by using a calibrated ocular micrometer. The values of the villus height and crypt depth in each bird were obtained from the mean of measurements made from four sections for each segment per bird. All slides were evaluated in a blind manner by two observers.
Performance records
The BW of hens was recorded on a monthly basis (before treatments started at 18 weeks of age and then at 22, 26, 30, 34 and 38 weeks of age). Fourteen hens per replicate (or 20% of hens) were weighed individually at each time and the average BW (expressed as g per bird) was calculated. Feed intake was recorded daily per each replicate (i.e. 70 birds), and the average feed consumption was calculated as g/bird.day. The feed conversion ratio (FCR) was calculated weekly for the whole duration of the experiment. Egg production was recorded daily for each replicate, and the percentage hen-day egg production (%HDP) was expressed on a weekly basis from 18 to 38 weeks of age. Egg weight was recorded on a monthly basis at 22, 26, 30, 34, and 38 weeks of age. In total, 50% of eggs per replicate were individually weighed at each sampling point and the average weight (g/egg) was calculated.
Statistical analyses
Statistical analyses were performed using the general linear modelling procedure of SAS (SAS Institute 1996). Data on performance parameters (BW, %HDP and egg weight) were on a replicate basis. To test for the treatment effect at each sampling point, recorded values were subjected to one-way ANOVA. Statistically significant effects were further analysed, and means were compared using Duncan’s multiple-range test. Statistical significance was determined at P ≤ 0.05. To evaluate whether significant differences existed for pathological findings, an unpaired t-test was used for comparing two means (control and Probiotic 1 or 2 treated) and determine the P-value. A 99% confidence interval for the true difference between the means was set, and in this case, the values were considered significant at P ≤ 0.01
Results
Bacterial evaluation of the cloaca
Table 1 presents data on the type and the frequency (%) of bacteria isolated from cloacal swabs before and after treatments with the respective probiotic. There were no major differences in the colonisation of the cloacal microflora between probiotic-treated and control hens before the treatment started and at each sampling point (4 weeks after the first treatment started, and 8 and 16 weeks after the last treatment with the probiotic). It is to be noted that all isolates of Gallibacterium anatis were the biovar haemolytica form of this species.
Evaluation of reproductive tract (pathological and bacteriological findings)
In total, 81 birds (of ~630 birds) were killed and the reproductive tracts were examined. Table 2 summarises pathological and bacteriological findings of the control and probiotic-treated hens. At 22 weeks of age, treated hens had a significantly (P < 0.01) lower occurrence of the reproductive tract pathologies than did control hens (22% vs 44% of birds necropsied). At 38 weeks of age, the incidence of reproductive tract pathologies in probiotic-treated hens had decreased to 11%, whereas control hens had an incidence of 33% (P < 0.001).
|
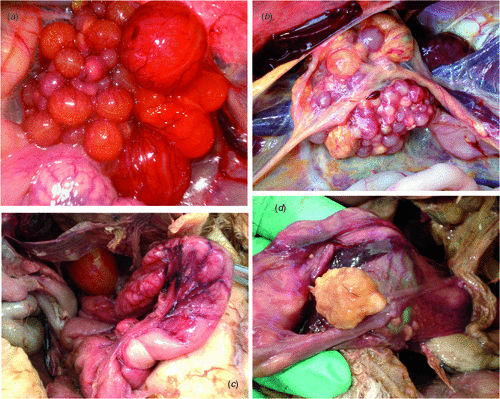
Gross examination of oviducts, ovaries and peritoneum of control and probiotic-treated hens (Table 2) showed the presence of inflammation in some birds. In affected birds, oviducal mucosa was found to be hyperaemic and sometimes covered with greyish-white or fibrinous exudate. The peritoneum was in many cases congested, especially the part covering the ovary having hyperaemic, deformed or atrophied follicles. In the case of chronic infections, the mesentery was also congested or covered with white fibrinous or caseous exudate. In three birds, caseous yolk material was found in the oviduct (between magnum and uterus) mixed with inflammatory debris and affecting the oviduct. There were some cases of abdominal cavity containing egg material, thickened yolk and caseous material associated with inflamed ovary or follicular atresia. In the case of chronic pathologies, birds were commonly in good condition, but the ova and occasionally the oviduct were inactive atrophied and/or covered with thickened exudates). Fig. 1a, b shows pathological reproductive tract lesions that were found in hens after the post-mortem of clinically normal birds killed during sampling, while Fig. 1c, d shows pathologies from control birds that died during the trial.
|
In general, there was a low contamination of the oviduct with aerobic bacteria in all birds (Table 2). A total of 60% of the oviduct samples (from control and probiotic-treated hens) had no bacterial growth. The most prevalent bacteria recovered from control hens were micrococci, while probiotic-treated birds had a mixed bacterial growth, with Gallibacterium anatis, α-streptococci and coryneforms being common. No bacteria were recovered from the oviducts of hens with pathological mesentery, ovaries and oviducts. Only two samples from oviducts with pathology (one from control hens and one from probiotic-treated) showed bacterial growth. A correlation between clinical symptoms of reproductive pathologies and specific bacteria could not be established. No correlation was also noticed between bacteria isolated from the cloaca and oviduct. At 38 weeks of age, a large number of hens necropsied showed infestation with parasites (roundworms and tapeworms), but surprisingly with a lower frequency in probiotic-treated hens (control vs probiotics, 66% vs 33%).
Histology
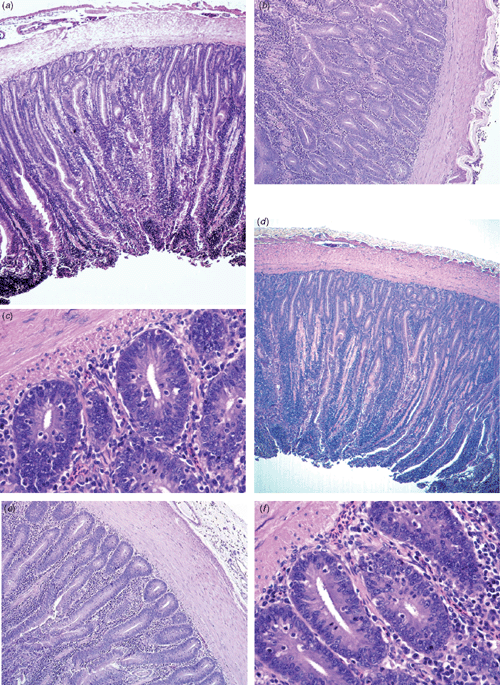
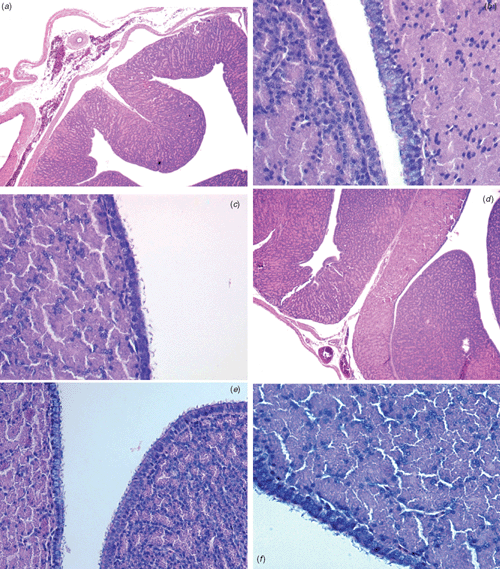
The histological examination of sections from intestine of control and probiotic-treated hens (Fig. 2a–f) did not show any significant changes in the ileal villus height, crypt depth and villus height : crypt depth ratio for any of the treatment and control birds (data not shown). However, birds treated with probiotic showed a thicker (P < 0.05) mucosa layer than did controls (Fig. 2a, d). Further histological examination showed a more uniform epithelial thickness for probiotic-treated hens (Fig. 2b, e) and an increased (P < 0.05) density of intraepithelial leukocytes (Fig. 2c, f). Histological slides from the oviducal magnum of control and probiotic-treated hens (Fig. 3a–f) showed differences (P < 0.01) in the density of mucosal folds, the thickness of epithelia (Fig. 3a, d), and density of hair-like cilia (Fig. 3c, f). Areas of granular cells were more evident between ciliated cells of the magnum in the hens from probiotic-treated groups.
|
|
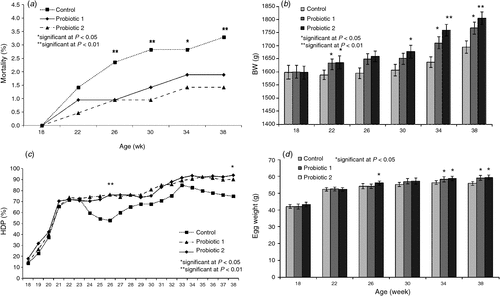
Cumulative mortality
The percentage of cumulative mortality in control and probiotic-treated hens in the period from 18 to 38 weeks of age is presented in Fig. 4a. Starting at 26 weeks of age, cumulative mortality in control hens was significantly (P < 0.01) higher than in both of the probiotic-treated groups (3.5% vs 1.5% and 1.9%, respectively). The necropsy examination of the total of eight chickens that died in the control group indicated that the death in four chickens was caused by acute reproductive pathologies. Two hens had a combination of pericarditis, perihepatitis and airsacculitis (polyserositis) and one of the hens was cannibalised. In one bird, the cause of death could not be identified. In the Probiotic 1 treatment group, only three birds died during the experimental period (two were cannibalised, and one of the hens had salpingoperitonitis). In the Probiotic 2 treatment group, four birds died in total (two were cannibalised, one had acute peritonitis, and one had neck injury).
|
Bodyweight
Hen weight increased as the flock aged (Fig. 4b). At 18 weeks of age, there were no significant differences in BWs among all experimental groups. At all other points of measurement, probiotic-treated hens were heavier (P < 0.01) than non-treated (control) hens. Furthermore, at 30, 34, and 38 weeks of age, the BW of the Probiotic 2-treated hens was significantly higher (P < 0.05) than that of Probiotic 1-treated hens.
Egg production and egg weights
From 18 to 23 weeks of age, the HDP% increased at a similar rate in all experimental groups (Fig. 4c). There were two significant (P < 0.01) drops in %HDP of controlled hens (at Weeks 23 and 24, and at Weeks 34 and 35). Overall, the birds in the probiotic-treated groups performed better than those in the control group (control vs probiotics, 75% vs 90% and 94%). Average egg weights from onset until peak of lay (i.e. from 18 to 38 weeks of age) are presented in Fig. 4d. The egg weights increased as the flock aged. However, at 34 and 38 weeks of age, the average egg weights of both probiotic-treated groups were significantly (P < 0.05) higher than that of control birds. At 26 weeks of age, hens in the Probiotic 2 treatment group had a significantly (P < 0.05) higher egg weight than did those in the control and the Probiotic 1 group, respectively.
Feed consumption and FCR
There were no significant (P > 0.05) differences between control and probiotic-treated groups for feed consumption and FCR.
Discussion
The effects of two commercial probiotics administered in the drinking water for 4 weeks (from 18 to 22 weeks of age) on the prevention of the occurrence of reproductive tract pathologies in laying birds were investigated. The experiments performed in the present study demonstrated that treatment with probiotics significantly improved the reproductive tract health, reduced mortality and increased performance (BW, egg production and egg weight) of hens at 4 weeks post-first treatment and in the subsequent period, for further 20 weeks post-treatments. A decrease of reproductive pathologies together with an improvement of overall health (i.e. decreased mortality) and performance of probiotic-treated hens was demonstrated in the present study. To the best of our knowledge, the present study is the first demonstration of the commercial probiotic use for preventing reproductive tract pathologies in free-range laying hens.
Reproductive tract pathologies such as peritonitis and salpingoperitonitis are conditions often referred to in laying hens as egg peritonitis (Jordan et al. 2005). There are many factors that can initiate such pathologies. In particular, in alternative production systems, birds live in an open environment and they can become contaminated from several different sources. Thus, bacterial infections become a major contributory factor that should be taken into consideration if the frequency of reproductive lesions increases in a flock. In some cases, hens may look healthy, but due to chronic pathologies of the ovary or oviduct, they stop laying eggs. Mortalities from reproductive pathologies are rare, and in most of cases, are caused by other complications, such as acute and chronic peritonitis (Jones and Owen 1981; Kinde et al. 2000; Jordan et al. 2005). Various bacteria have been reported to cause primary or secondary reproductive tract infections in free-range birds (Shini et al. 2008; Neubauer et al. 2009). Although the route of infections is not clearly known, contamination of vent, cloaca and oviduct with faecal material has been seen as an important source of such infections (Keller et al. 1995). Many investigators have previously isolated pathogenic bacteria (e.g. Mycoplasma gallisepticum, E. coli, Salmonella spp., Pasteurella multocida and Staphylococcus aureus) from lesions in the peritoneum and reproductive tract of hens (Gross and Siegel 1959; Jones and Owen 1981; Riddell 1996; Trampel et al. 2007). Mirle et al. (1991) examined 496 hens with reproductive tract lesions and isolated Gallibacterium in pure culture from 23% of the diseased organs. Haemolytic G. anatis was associated with infection in birds kept in alternative husbandry systems and suffering from reproductive disorders (Neubauer et al. 2009).
In the current study, the bacterial evaluation of the intestinal and reproductive tract of hens did not demonstrate particular changes of the microbial populations in control or probiotic-treated birds, with or without pathological changes. It should be noted that the microbiological analysis was limited in nature. A more extensive examination, including the use of molecular profiling methods could have helped detect bacterial changes and potentially established correlations between bacteria in the cloaca and oviduct of normal birds and birds with reproductive pathologies, respectively. It has been shown that, in a healthy state, the chicken gut contains a diverse bacterial population. The relative proportions of major groups of bacteria may change, but, are mainly dominated by bacteria classified as lactobacilli, Enterobacteriaceae, clostridia, Bacteroides, and enterococci and many other groupings, both anaerobic and facultative (Ewing 2008). As stated above, most of the bacteria that were isolated in the present study are endemic to hens and are commonly found in the environment of chickens (Reiber et al. 1995). As expected, coliforms were found present in the cloaca of all sampled hens, in all sampling points. While, α-streptococci were detected at all sampling points, the frequency of detection was lower than for the coliforms, averaging 50% of sampled birds. Gallibacterium anatis was consistently found in most cloacal samples. At 38 weeks of age, micrococci were found to be significantly (P < 0.001) higher in the cloaca of control hens than were both probiotic groups (control vs probiotics, 55% vs 0% and 22%, respectively). Surprisingly, micrococci were never found in the oviduct of probiotic-treated hens, while control hens showed some presence of micrococci in the oviduct (11–33% of samples were positive). Another interesting observation from the present study was an increased presence of coryneforms in samples of probiotic-treated hens in both cloaca and oviduct. Coryneforms are a group of Gram-positive rod-shaped bacteria (a common genus being Corynebacterium) that are environmental residents of normal flora often isolated from poultry litter (Schefferle 1966; Chinivasagam et al. 2010). Thus, it is often difficult to determine their significance. There is some evidence on the probiotic activities of coryneform bacteria in fish. It has been reported that strains of Pseudomonas sp., Vibrio sp., Aeromonas sp. and groups of coryneforms isolated from salmonid hatcheries showed antiviral activity against infectious haematopoietic necrosis virus, with more than 50% plaque reduction (Kamei et al. 1988). This observation could be of interest for further identification of coryneform organisms from laying hens, and their potential probiotic properties.
Increased parasitic incidence is an important problem, and an additional risk to free-range flocks. Heavy worm burdens can predispose birds to develop secondary bacterial infections. In some cases, Ascaridia galli eggs may act as mechanical vectors of reproductive tract bacterial infections such as Salmonella (Chadfield et al. 2001). In the present study, at 38 weeks of age, necropsied birds from the control group had higher (P < 0.05) infestation with round- and band-worms (Table 2), potentially contributing to a lower performance and higher incidence of reproductive tract infections and mortality.
In the current study, probiotic-treated hens showed a significant reduction in reproductive tract pathologies, potentially due to an improvement of general immunity and an enhancement of the local (oviduct) immunity. An increased resistance to the diseases, resulting in a significantly decreased mortality and significantly higher performance of probiotic-treated hens was also seen. The mechanism, by which probiotics could have enhanced mucosal immunity and reduced pathologies of the reproductive tract of treated birds is unclear, and was not thoroughly investigated in the present study. However, it is known that cellular and molecular events in the local mucosa can contribute to an improved mucosal defence against pathogens (Patterson and Burkholder 2003). From the small number of histology samples that were analysed in the present study, it was shown that there was an improvement of structural integrity of mucosal ileum and magnum of probiotic treated hens, as evidenced by an increased thickness of epithelial layer and a higher density of the intraepithelial cellular components. Other investigators have shown longer villi in the broiler birds treated with probiotic (Awad et al. 2009; Lee et al. 2010). Longer villi provide an increased surface area that allows greater absorption of available nutrients, consequently promoting growth (Yamauchi et al. 2006). In the current study, some beneficial changes in the architecture of intestinal histology were seen, but these changes were not associated with significant changes of the villus height, and villus height : crypt depth ratio.
Hen performance (egg production, egg weight and BW) increased in all treatment groups as the flock aged. Significant differences were found between probiotic-treated and control birds. Both probiotics significantly improved the performance of birds, with Probiotic 2 showing a greater effect on all production parameters. Previous investigators have revealed that addition of probiotics to the feed of poultry (broilers and laying hens) has beneficial effects on growth performance and egg production. In laying hens, Gallazzi et al. (2008) indicated that egg production and FCR were significantly improved when hens were treated with probiotic strain Lactobacillus acidophilus D2/CSL. Similar results were found by Li et al. (2005) when a Bacillus subtilis culture was used. It has been proposed that these effects are achieved by different mechanisms, including competitive exclusion of pathogens (Morishita et al. 1997; Nisbet 1998) and improved digestion and absorption of nutrients (Thomke and Elwinger 1998). There have been some previous attempts to correlate gut microbiota with higher levels of performance (Apajalahti et al. 2004; Torok et al. 2008). However, because of the varying nature of such investigations, this area would need to be investigated further.
Overall, the results of the present study have provided promising initial data for the use of probiotics as a tool to reduce reproductive pathologies in free-range laying hens.
Acknowledgements
This work was funded by the Australian Poultry CRC. Biomin, GmBH Austria and International Animal Health Products P/L, Australia, are greatly acknowledged for donating products (Probiotic 1: Biomin® Poultry5Star, and Probiotic 2: Protexin®) used in this study.
References
AECL (2010) ‘Egg industry overview – 2010.’ (Australian Egg Corporation Limited: Sydney).Apajalahti J, Kettunen A, Graham H (2004) Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World’s Poultry Science Journal 60, 223–232.
| Characteristics of the gastrointestinal microbial communities, with special reference to the chicken.Crossref | GoogleScholarGoogle Scholar |
Awad WA, Ghareeb K, Abdel-Raheem S, Böhm J (2009) Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poultry Science 88, 49–56.
| Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlamt74%3D&md5=23e2f304d265593501250dc83104aabfCAS | 19096056PubMed |
Balevi T, Ucan US, Coskun B, Kurtoglu V, Cetingul IS (2001) Effect of dietary probiotic on performance and humoral immune response in layer hens. British Poultry Science 42, 456–461.
| Effect of dietary probiotic on performance and humoral immune response in layer hens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnsFSisrg%3D&md5=7d5ac646655178d42648b23b166f7b73CAS | 11572620PubMed |
Burkholder KM, Thompson KL, Einstein ME, Applegate TJ, Patterson JA (2008) Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poultry Science 87, 1734–1741.
| Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFWqu7nE&md5=3f5c7e6fe40cbf60d56c6374e7dca69bCAS | 18753440PubMed |
Chadfield M, Permin A, Nansen P, Bisgaard M (2001) Investigation of the parasitic nematode Ascaridia galli (Shrank 1788) as a potential vector for Salmonella enterica dissemination in poultry. Parasitological Research 87, 317–325.
| Investigation of the parasitic nematode Ascaridia galli (Shrank 1788) as a potential vector for Salmonella enterica dissemination in poultry.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M3mtlensA%3D%3D&md5=a6d700da4862aa656adc199e589ba64fCAS |
Chinivasagam HN, Tran T, Maddock L, Gale A, Blackall PJ (2010) The aerobiology of the environment around mechanically ventilated broiler sheds. Journal of Applied Microbiology 108, 1657–1667.
| The aerobiology of the environment around mechanically ventilated broiler sheds.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3czgs1aguw%3D%3D&md5=5e0b19f6fd07a6cb1b8c1eeb46ec68b6CAS | 19849770PubMed |
De Reu K, Messens W, Heyndrickx M, Rodenburg TB, Uyttendaele M, Herman L (2008) Bacterial contamination of table eggs and the influence of housing systems. World’s Poultry Science Journal 64, 1–19.
| Bacterial contamination of table eggs and the influence of housing systems.Crossref | GoogleScholarGoogle Scholar |
Durant JA, Corrier DE, Byrd JA, Stanker LH, Ricke SC (1999) Feed deprivation affects crop environment and modulates Salmonella enteritidis colonization and invasion of Leghorn hens. Applied and Environmental Microbiology 65, 1919–1923.
Ewing WN (2008) Analysing microbial gut population. In ‘The living gut’. 2nd edn. (Eds WN Ewing, with contributions by LA Tucker) pp. 51–57. (Nottingham University Press: Nottingham, UK)
Fossum O, Jansson DS, Etterlin PE, Vågsholm I (2009) Causes of mortality in laying hens in different housing systems in 2001–2004. Acta Veterinaria Scandinavica 15, 51–53.
Gallazzi D, Giardini A, Mangiagalli MG, Marelli S, Ferrazzi V, Orsi C, Cavalchini LG (2008) Effects of Lactobacillus acidophilus D2/CSLon laying hen performance. Italian Journal of Animal Science 7, 27–37.
Gast RK, Beard CW (1990) Isolation of Salmonella enteritidis from internal organs of experimentally infected hens. Avian Diseases 34, 991–993.
| Isolation of Salmonella enteritidis from internal organs of experimentally infected hens.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M7ivVaqsA%3D%3D&md5=82630c7e088599561630d2f515ed55ddCAS | 2282024PubMed |
Gross WB, Siegel PB (1959) Coliform peritonitis of chickens. Avian Diseases 3, 370–373.
| Coliform peritonitis of chickens.Crossref | GoogleScholarGoogle Scholar |
Hoop RK, Pospischil A (1993) Bacteriological, serological, histological and immunohistochemical findings in laying hens with naturally acquired Salmonella enteritidis phage type 4 infection. The Veterinary Record 133, 391–393.
| Bacteriological, serological, histological and immunohistochemical findings in laying hens with naturally acquired Salmonella enteritidis phage type 4 infection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2c7ksVSrsg%3D%3D&md5=60a12d5d4bba901d130890d69f58d012CAS | 8310606PubMed |
Jones HGR, Owen DM (1981) Reproductive tract lesions of the laying fowl with particular reference to bacterial infection. The Veterinary Record 108, 36–37.
| Reproductive tract lesions of the laying fowl with particular reference to bacterial infection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M3gt1ensg%3D%3D&md5=bb8aaa2499665ae99a88b8f91567acbcCAS |
Jordan FT, Williams NJ, Wattret A, Jones T (2005) Observations on salpingitis, peritonitis and salpingoperitonitis in a layer breeder flock. The Veterinary Record 157, 573–577.
Kamei Y, Yoshimizu M, Ezura Y, Kimura T (1988) Screening of bacteria with antiviral activity from fresh water salmonid hatcheries. Microbiology and Immunology 32, 67–73.
Keller LH, Benson CE, Krotec K, Eckroade RJ (1995) Salmonella enteritidis colonization of the reproductive tract and forming and freshly laid eggs of chickens. Infection and Immunity 63, 2443–2449.
Kinde H, Shivaprasad HL, Daft BM, Read DH, Ardans A, Breitmeyer R, Rajashera G, Nagaraja KV, Gardner IA (2000) Pathologic and bacteriologic findings in 27-week-old commercial laying hens experimentally infected with Salmonella enteritidis phage type 4. Avian Diseases 44, 239–248.
| Pathologic and bacteriologic findings in 27-week-old commercial laying hens experimentally infected with Salmonella enteritidis phage type 4.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FlsV2htg%3D%3D&md5=f852ef6aaa510bd3999427b5f3b4532bCAS | 10879902PubMed |
Lee K, Lillehoj HS, Siragusa GR (2010) Direct-fed microbials and their impacts on the intestinal microflora and immune system of chickens. Japanese Poultry Science 47, 106–114.
| Direct-fed microbials and their impacts on the intestinal microflora and immune system of chickens.Crossref | GoogleScholarGoogle Scholar |
Li L, Xu CL, Ji C, Ma Q, Hao K, Jin ZY, Li K (2005) Effects of a dried Bacillus subtilis culture on egg quality. Poultry Science 85, 364–368.
Mirle C, Schongarth M, Meinhart H, Olm U (1991) Studies into incidence of Pasteurella haemolytica infections and their relevance to hens, with particular reference to diseases of the egg-laying apparatus. Monatshefte fur Veterinarmedizin 46, 545–549.
Morelli L, Zonenenschain D, Del Piano M, Cognein P (2004) Utilization of the intestinal tract as a delivery system for urogenital probiotics. Journal of Clinical Gastroenterology 38, S107–S110.
| Utilization of the intestinal tract as a delivery system for urogenital probiotics.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2czjtF2iuw%3D%3D&md5=9e30a208980f4621aaf93c03f0042735CAS | 15220672PubMed |
Morishita TY, Aye PP, Harr BS, Cobb CW, Clifford JR (1997) Evaluation of an avian- specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian Diseases 41, 850–855.
| Evaluation of an avian- specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7hsF2gsA%3D%3D&md5=1f2634f39e4981ccc90b6b16b8d0a05eCAS | 9454918PubMed |
Mountzouris KC, Tsirtsikos P, Kalamara E, Nitsch S, Schatzmayr G, Fegeros K (2007) Evaluation of the efficacy of probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poultry Science 86, 309–317.
Nagle T, Shini S (2008) Pilot trial – mortality in free range flocks. Final report. Poultry CRC, Armidale, NSW.
Nahashon SN, Nakaue HS, Mirosh LW (1996) Performance of single comb white Leghorn fed a diet supplemented with a live microbial during the growth and egg laying phases. Animal Feed Science and Technology 57, 25–38.
| Performance of single comb white Leghorn fed a diet supplemented with a live microbial during the growth and egg laying phases.Crossref | GoogleScholarGoogle Scholar |
Neubauer C, De Souza-Pilz M, Bojesen AM, Bisgaard M, Hess M (2009) Tissue distribution of haemolytic Gallibacterium anatis isolates in laying birds with reproductive disorders. Avian Pathology 38, 1–7.
| Tissue distribution of haemolytic Gallibacterium anatis isolates in laying birds with reproductive disorders.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M%2FnvFCgsg%3D%3D&md5=499bd4ac30dd3307b7170215a9970192CAS | 19089694PubMed |
Nisbet DJ (1998) Use of competitive exclusion in food animals. Journal of the American Veterinary Medical Association 213, 1744–1746.
Patterson JA, Burkholder KM (2003) Application of prebiotics and probiotics in poultry production. Poultry Science 82, 627–631.
Primary Industries Standing Committee (2002) ‘Model codes of practice for the welfare of animals: domestic poultry.’ 4th edn. (CSIRO Publishing: Melbourne)
Reiber MA, McInroy JA, Conner DE (1995) Enumeration and identification of bacteria in chicken semen. Poultry Science 74, 795–799.
| Enumeration and identification of bacteria in chicken semen.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2MzislGqug%3D%3D&md5=fbd20d742d91ce43c151175fa15d18efCAS | 7603955PubMed |
Reid G, Bocking A (2003) The potential for probiotics to prevent bacterial vaginosis and preterm labor. American Journal of Obstetrics and Gynecology 189, 1202–1208.
| The potential for probiotics to prevent bacterial vaginosis and preterm labor.Crossref | GoogleScholarGoogle Scholar | 14586379PubMed |
Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, Bruce AW (2003) Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunology and Medical Microbiology 35, 131–134.
| Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhs1aht7k%3D&md5=cb5527dcf7f8f89b1fb9b9304e371dc1CAS | 12628548PubMed |
Riddell C (1996) Endocrine and reproductive systems. In ‘Avian histopathology’. 2nd edn. (Ed. C Riddell) pp. 211–217. (The American Association of Avian Pathologists: Kennett Square, PA)
SAS Institute (1996) ‘SAS/STAT® software. SAS system for Microsoft Windows release 6.12.’ (SAS Institute: Cary, NC)
Schefferle HE (1966) Coryneform bacteria in poultry deep litter. The Journal of Applied Bacteriology 29, 147–160.
| Coryneform bacteria in poultry deep litter.Crossref | GoogleScholarGoogle Scholar |
Shalev E, Battino S, Weiner E, Colodner R, Keness Y (1996) Ingestion of yoghurt containing Lactobacillus acidophilus compared to pasteurized yoghurt as prophylaxis for recurrent candidal vaginitis and/or bacterial vaginosis. Archives of Family Medicine 5, 593–596.
| Ingestion of yoghurt containing Lactobacillus acidophilus compared to pasteurized yoghurt as prophylaxis for recurrent candidal vaginitis and/or bacterial vaginosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2Fotlyruw%3D%3D&md5=b3ff6af4debb3e5f9213772a9284e4bfCAS | 8930233PubMed |
Shini S, Shini A, Nagle T (2008) Reproductive tract lesions in free range hens with particular reference to bacterial infections. World’s Poultry Science Journal 64, 321
Shivaprasad HL, Timoney JF, Morales S, Lucio B, Baker RC (1990) Pathogenesis of Salmonella enteritidis infection in laying chickens. I. Studies on egg transmission, clinical signs, fecal shedding and serologic responses. Avian Diseases 34, 548–557.
| Pathogenesis of Salmonella enteritidis infection in laying chickens. I. Studies on egg transmission, clinical signs, fecal shedding and serologic responses.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M%2FlsVSrtA%3D%3D&md5=7c1de613597160a58683956c8d409ea4CAS | 2241680PubMed |
Tauson R, Wahlström A, Abrahamsson P (1999) Effect of two floor housing systems and cages on health, production, and fear response in layers. Journal of Applied Poultry Research 8, 152–159.
Thomke S, Elwinger K (1998) Growth promotants in feeding pigs and poultry. III. Alternatives to antibiotic growth promotants. Annales de Zootechnie 47, 245–271.
| Growth promotants in feeding pigs and poultry. III. Alternatives to antibiotic growth promotants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXnt1Whu7c%3D&md5=d4fe9f27a6aceda8aba3d3d4e8cb9c65CAS |
Torok VA, Ophel-Keller K, Loo M, Hughes RJ (2008) Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism. Applied and Environmental Microbiology 74, 783–791.
| Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhvVWjsr0%3D&md5=9ad23b085c83cf2927e07355256cfe35CAS | 18065621PubMed |
Trampel DW, Wannemuehler Y, Nolan LK (2007) Characterization of Escherichia coli isolates from peritonitis lesions in commercial laying hens. Avian Diseases 51, 840–844.
| Characterization of Escherichia coli isolates from peritonitis lesions in commercial laying hens.Crossref | GoogleScholarGoogle Scholar | 18251391PubMed |
Van Coillie E, Goris J, Cleenwerck I, Grijspeerdt K, Botteldoorn N, Van Immerseel F, De Buck J, Vancanneyt M, Swings J, Herman L, Heyndrickx M (2007) Identification of lactobacilli isolated from the cloaca and vagina of laying hens and characterization for potential use as probiotics to control Salmonella enteritidis. Journal of Applied Microbiology 102, 1095–1106.
Willis WL, Reid L (2008) Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poultry Science 87, 606–611.
| Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c7otVylug%3D%3D&md5=2b524852a7dd1a7cad3715681a99949eCAS | 18339979PubMed |
Yamauchi K, Buwjoom T, Koge K, Ebashi T (2006) Histological alterations of the intestinal villi and epithelial cells in chickens fed dietary sugar cane extract. British Poultry Science 47, 544–553.
| Histological alterations of the intestinal villi and epithelial cells in chickens fed dietary sugar cane extract.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28nhs1GksQ%3D%3D&md5=70a921369c256931a4d50d3fcfb5d4f1CAS | 17050097PubMed |



