Odour, dust and non-methane volatile organic-compound emissions from tunnel-ventilated layer-chicken sheds: a case study of two farms
Mark Dunlop A B D , Zoran D. Ristovski C , Erin Gallagher A , Gavin Parcsi B , Robin L. Modini C , Victoria Agranovski C and Richard M. Stuetz BA Department of Agriculture, Fisheries and Forestry (DAFF), PO Box 102 Toowoomba, Qld 4350, Australia.
B UNSW Water Research Centre, School of Civil and Environmental Engineering, The University of New South Wales, Sydney, NSW 2052, Australia.
C International Laboratory for Air Quality and Health, Queensland University of Technology, 2 George Street, Brisbane, Qld 4000, Australia.
D Corresponding author. Email: mark.dunlop@daff.qld.gov.au
Animal Production Science 53(12) 1309-1318 https://doi.org/10.1071/AN12343
Submitted: 28 September 2012 Accepted: 14 April 2013 Published: 30 May 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
An observational study was undertaken to measure odour and dust (PM10 and PM2.5) emission rates and identify non-methane volatile organic compounds (NMVOCs) and odorants in the exhaust air from two tunnel-ventilated layer-chicken sheds that were configured with multi-tiered cages and manure belts. The study sites were located in south-eastern Queensland and the West Gippsland region of Victoria, Australia. Samples were collected in summer and winter on sequential days across the manure-belt cleaning cycle. Odour emissions ranged from 58 to 512 ou/s per 1000 birds (0.03–0.27 ou/s.kg) and dust emission rates ranged 0.014–0.184 mg/s per 1000 birds for PM10 and 0.001–0.190 mg/s per 1000 birds for PM2.5. Twenty NMVOCs were identified, including three that were also identified as odorants using thermal desorption–gas chromatography–mass spectrometry/olfactometry analysis. Odour emission rates were observed to vary with the amount of manure accumulation on the manure belts, being lowest 2–4 days after removing manure. Odour emission rates were also observed to vary with diurnal and seasonal changes in ventilation rate. Dust emissions were observed to increase with ventilation rate but not with manure accumulation. Some NMVOCs were identified at both farms and in different seasons whereas others were observed only at one farm or in one season, indicating that odorant composition was influenced by farm-specific practices and season.
Additional keywords: aerial dust, emission rate, olfactometry, particulate matter.
Introduction
The Australia egg industry annually produces 357 million eggs, with the majority being produced in caged housing systems (AECL 2011). More layer farms will be required in the future as egg production grows at a rate of ~7–10% per year (AECL 2009, 2010, 2011) to meet consumer demands for fresh eggs.
Odour and dust emissions are a normal part of layer-chicken sheds but may lead to environmental impacts and amenity issues for nearby residents. The potential for odour and dust nuisance has increased in recent years due to larger egg production farms, increasing population densities near layer farms and greater air-quality requirements by the community. The development of new layer farms, or the expansion of existing farms, requires careful consideration and planning to ensure compatibility with adjacent land uses (EPA Qld Government 2004).
Layer hens are housed in specially designed buildings where they lay eggs that are collected and sold for human consumption. Hens enter the egg-laying stage of their life at 18 weeks and lay eggs for 12–15 months. Sheds may be naturally or mechanically ventilated and can be configured and managed for caged, barn or free-range production. The focus of the present paper is the housing configuration with tunnel ventilation and multi-tiered cages with manure belts. Tunnel-ventilated sheds provide a high degree of control of the in-shed environment in terms of temperature, fresh air exchange and manure conditions, to provide optimal conditions for hen comfort and egg production. Manure belts positioned under each tier of cages allow manure to be removed at regular intervals, e.g. weekly or semi-weekly (twice per week).
In layer sheds, there are many interdependent factors that may influence the generation and emission of odour and dust, including ventilation-system design and ventilation rate, shed design, manure properties (composition, moisture content, surface crusting, quantity, age and removal frequency), feed properties, stage of production (bird numbers, age, stocking density), bird activity, bird health and shed microclimate (e.g. temperature, humidity) (Navaratnasamy and Feddes 2004; Nimmermark and Gustafsson 2005; Angel et al. 2008; Modini et al. 2010; Mostafa and Buescher 2011; Xin et al. 2011; Costa et al. 2012; Ni et al. 2012a). The interaction of these many factors creates challenges for measuring ‘typical’ emission rates, and also for comparing odour emission rates from discrete studies.
Unpleasant odours originating from layer sheds are primarily produced from the microbial decomposition of manure (Mackie et al. 1998; Hobbs et al. 2004), although some odour may also be emitted from the birds themselves (Lacey et al. 2004). Where decomposing manure has surfaces exposed to the atmosphere, odorous compounds are released and exhausted from the shed through the fans. Once exhausted from the shed, the odour plume is subjected to dispersion, and is diluted as it travels downwind. When planning for a new or enlarged production facility, odour dispersion modelling may be used to estimate the likelihood of odour nuisance. Prediction of odour impacts with dispersion modelling requires accurate odour emission-rate values. There is therefore a requirement for reliable odour emission rate data.
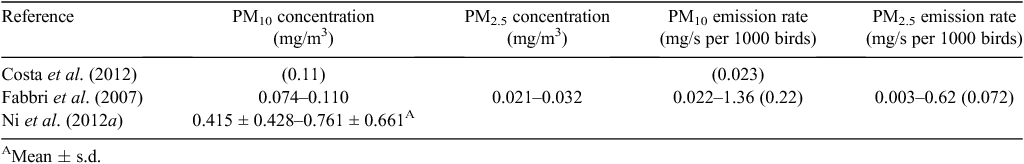
Dust originates from the mechanical breakdown and subsequent entrainment of mineral and organic material from birds, manure, feed and manure into the air, or the conversion of gases to the particle phase (Modini et al. 2010). Only tunnel-ventilated layer sheds with manure belts will be considered in the present paper because, in general, layer sheds with manure belts have been found to have lower emissions than other shed styles (e.g. high-rise barns, deep pit barns, aviary systems) (Fabbri et al. 2007; Costa et al. 2012; Ni et al. 2012a).
Odour is formed by combinations of hundreds of odorants, some of which are classified as volatile organic compounds (VOCs), while others are gases such as ammonia or hydrogen sulfide (O’Neill and Phillips 1992; Schiffman et al. 2001; Hobbs et al. 2004; Lacey et al. 2004; Cai et al. 2006). VOCs are compounds with a molecular structure that contains at least one carbon and one hydrogen atom, and readily evaporates under normal indoor temperature and pressure conditions (Ni et al. 2012b). When investigating VOCs for odour research, it is useful to exclude methane (which is an odourless gas) because it can dominate the sample analysis. The remaining non-methane VOCs (NMVOCs) have a range of odour detection thresholds (concentration at which they can be smelt), although many are non-odorous or make very little contribution to the whole odour.
There are few examples of studies where NMVOCs in poultry housing have been identified (Cai et al. 2007; Trabue et al. 2010), and even fewer where the odorous NMVOCs have been specifically investigated. Early research into the identification of odorants from poultry housing determined that the ‘poultry’ odour was a mixture of compounds that individually had characters that could be described with the terms such as rotten eggs, cabbage, onion-like, butter-like and garlic (Burnett 1969; Miner 1977). Ongoing research has focussed on identifying and quantifying the abundance of NMVOCs and odorants. Investigation of NMVOCs from poultry farms has been limited due to technical challenges and requirements for a multiplicity of techniques to detect most of the NMVOCs present due to the range of compound reactivity and volatility (Trabue et al. 2010). Despite the challenges, identification, measurement and prioritisation of NMVOCs provides a useful and important dimension to odour research that will lead to improved methods of odour measurement as well as development and assessment of odour abatement strategies.
The aims of the present observational study were to measure odour and dust emission rates and identify NMVOCs in the air emitted from modern tunnel-ventilated layer sheds where manure is regularly removed with a manure-belt system. We anticipate that these data will be used to support and improve planning and assessment of new or expanding layer farms and will contribute to the development of improved odour measurement techniques and odour reduction strategies.
Materials and methods
Farm description and sampling program
Odour, dust and NMVOC samples were collected from two tunnel-ventilated layer sheds (Table 1). Farm A was located in south-eastern Queensland and Farm B was located in the West Gippsland region of Victoria. Both farms had Hyline Brown hens (http://www.hyline.com.au, verified 1 May 2013), with average bird weight 1.85–1.93 kg on the days when samples were collected. Manure belts were used at each of these farms to regularly remove manure from beneath multi-tiered cages. At Farm A, manure accumulating on the belts was dried using normal shed ventilation, whereas at Farm B, a forced-air drying system was used continuously to dry manure on the manure belts. Both sheds were constructed using concrete floors, metal framework and insulated panel walls (foam laminated with a metal skin, similar to materials used in commercial cold rooms).

|
Tunnel-ventilation systems were configured differently at each farm. At Farm A, 11 tunnel-ventilation fans were installed on one of the narrow ends walls of the shed, with air being drawn the full length of the shed in a single direction. At Farm B, 16 tunnel-ventilation fans were installed in the centre of one of the long side walls, with air being drawn from both ends of the shed towards the middle.
At each farm, odour, dust and NMVOC samples were collected during summer and winter over a series of days spanning the manure collection cycle, to assess the influence of manure accumulation on emissions. For Farm A, samples were collected on four sequential days, beginning on the day following removal of manure from the manure belts. For Farm B, samples were collected on Days 1–3, 5 and 6 following removal of manure from the manure belts.
Summer sampling at Farm A was conducted on 10–13 December 2007 and winter sampling at Farm A was conducted on 23–26 July 2007. Summer sampling at Farm B was conducted 25–29 February 2008 and winter sampling at farm B was conducted 16–20 June 2008.
Sample collection and analysis
Methods for the collection and analysis of odour, dust and NMVOC samples have previously been described by Dunlop et al. (2010), Modini et al. (2010), Parcsi et al. (2011) and Murphy et al. (2012). The following is a brief summary of these methods and also describes variations from the previously described methods.
To facilitate sample collection, a temporary polyethylene duct ~14.0 m long and 1.3 m in diameter, similar to the duct previously described by Sohn et al. (2008), was attached to one of the tunnel-ventilation fans, with the other end opened to the atmosphere. This duct was designed to provide a sampling position in accordance with AS 4323.1:1995 (Standards Australia 1995a) and to prevent interference at the sampling position due to cross-winds. Samples were drawn from the duct at a distance of eight duct diameters from the fan face.
Concentration of odour was determined using dilution olfactometry, which involved presenting a series of diluted odorous air samples to a panel of trained human assessors using a standardised process. In Australia, odour concentration is assessed according to the Australian/New Zealand Standard AS/NZS 4323.3:2001 (Standards Australia/Standards New Zealand 2001). Odour samples were collected into customised 120-L drums lined with a specially prepared polyethylene terephthalate bag (PET, Melinex, 15 µm, Dupont Teijin Films, Chester, VA, USA). Bags were filled using negative pressure (lung principle) in an average sampling time of 10 min. Odour samples were transported to the olfactometer and analysed within 8 h of collection. It was necessary to use two different olfactometry laboratories because the two farms were located in different states. On each sampling day, three or four pairs of duplicate samples were collected (six to eight sample bags analysed by the olfactometer). The geometric mean was calculated for each pair of duplicate samples to produce a single value of odour concentration for each set of sampling conditions.
Dust samples were collected isokinetically in accordance with AS4323.2:1995 (Standards Australia 1995b) from within the temporary polyethylene duct that was attached to one of the layer-shed exhaust fans. Isokinetic sampling was used to obtain representative dust samples independently of the dust particle-size distribution. No measurements of shed input air were conducted during the study because background dust concentrations in these rural areas are generally only a small fraction of the expected in-shed and exhaust dust concentration. It was judged more important to use the limited number of sampling instruments to comprehensively characterise the total dust emission from the sheds.
Dust concentrations were characterised in terms of PM10 and PM2.5 particle mass concentration (mass of dust per m3 of air) and particle number concentration (number of suspended particles in the 0.5–20-µm size range per m3 of air). Particle mass concentration was measured using two TSI Model 8520 DustTrak instruments with appropriate inlets (TSI Inc., http://www.tsi.com, verified 1 May 2013). Particle number concentration was measured using a TSI Model 3320 Aerodynamic Particle Sizer (TSI Inc.). All three instruments were operated in parallel, downstream of the isokinetic sampling probe, and recorded mass concentrations every 30 s and number concentration and size distributions every 20 s.
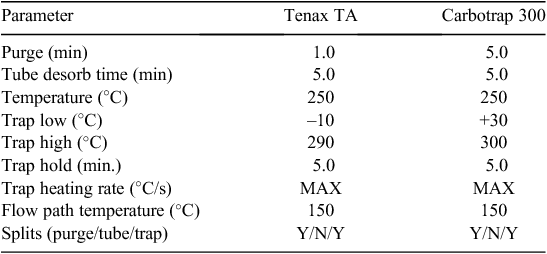
NMVOC samples were collected from within the temporary polyethylene duct that was attached to one of the layer-shed exhaust fans. Odorous air was drawn into sorbent tubes (Markes International Ltd, Pontyclun, UK) that were filled with Tenax TA or Carbotrap 300 sorbent (both types of sorbent tubes were used simultaneously for all sampling). A vacuum pump with low-flow tube holders (SKC Universal Pump 224-PCXR8 and 224-26-01, respectively, SKC Inc., Eighty Four, PA, USA) was used to induce a 100 mL per minute flow rate through each tube for a 30-min sampling period (total sample volume 3.0 L). Flow through individual tubes was set using a flow meter (Model 4143, TSI Inc.). Air drawn into the sorbent tubes was filtered to prevent contamination of the sorbent material by airborne particulate matter. Following sample collection, sorbent tubes were sealed and stored in a refrigerator before analysis.
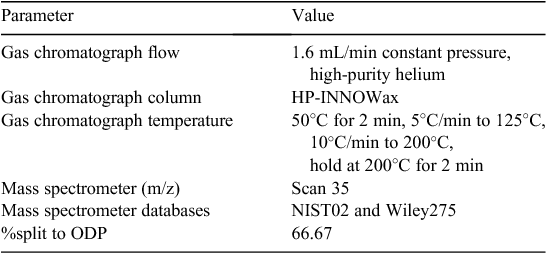
Sorbent tubes were analysed using a thermal desorber (TD), gas chromatograph (GC), mass spectrometer (MS) and odour detection port (ODP), which is a series of instruments commonly referred to as TD–GC–MS/O. The TD was a Markes Unity Thermal Desorber (Markes International) and was connected to the GC using a heated transfer line. The GC and MS were Agilent 6890N gas chromatograph and Agilent 5973N mass spectrometer (Agilent Technologies, Sydney, Australia). The TD method is summarised in Table 2 and GC–MS/O methods are summarised in Table 3.
|
|
Odour, dust and NMVOC samples were collected concurrently from each of the layer sheds. Samples were collected between 0530 hours and 1400 hours on each sampling day. This sampling window was chosen to enable the odour samples to be analysed on the same day as sample collection, to comply with AS/NZS 4323.3:2001 (Standards Australia/Standards New Zealand 2001) and to minimise potential olfactometry error due to sample decay (van Harreveld 2003). In addition, collecting odour and NMVOC samples within this time frame enabled emission rates to be measured over a range of ventilation rates on each sampling day (targeting sample collection at 25%, 50%, 70% and 100% of daily maximum ventilation rate if possible). This was because ventilation rate generally increased with ambient temperature throughout the morning, as determined by the in-shed environmental control system. Dust was measured continuously over this period.
Measuring ventilation rate, manure moisture and environmental conditions
Ventilation rate was recorded during each sampling event to enable calculation of emission rate. Ventilation rate was determined using fan-performance information supplied by the fan manufacturer, the shed static pressure at the time of sampling (measured in the shed ~6 m upwind of the fans) and the number of active fans. Ventilation rate was regularly checked by measuring air-speed through each active fan with a hot-wire anemometer using the traverse method (adapted from AS 4323.1:1995; Standards Australia 1995a) on the inlet side of each fan. Ventilation rate was found to be similar with both methods. Ventilation rates were adjusted for standard conditions (0°C and 101.3 kPa), as required by AS/NZS 4323.3:2001 (Standards Australia/Standards New Zealand 2001). In addition to measuring ventilation rate, fan activity and static pressure at the fans was continuously monitored at Farm A, by using a customised logging system, mercury tilt switches on fan shutters, similar to the method described by Wilhelm et al. (2001), and differential pressure transducer (Setra model 264, range –63 Pa to +63 Pa, part number 2641R25WB11T1C, Setra Systems, Boxborough, MA, USA). Fan-activity monitoring was undertaken to confirm that odour samples were collected across the normal daily range of shed-ventilation rates.
Odour and dust emission rates were calculated by multiplying the concentration of odour (ou/m3) or dust (mg or particles per m3) by the standardised ventilation rate (m3/s). Emission rate values were then normalised in terms of emission rate per 1000 birds placed in the shed at the start of the production cycle (ou/s per 1000 birds for odour and mg/s per 1000 birds or particles/s per 1000 birds for dust) or per kilogram of total liveweight (ou/s.kg for odour).
Manure on the belts was sampled for moisture content analysis, which was determined gravimetrically according to AS 4454-2003 (Standards Australia 2003). Ambient temperature and humidity were measured using a Kestrel Pocket Weather Tracker (Model 4500, Nielsen-Kellerman, Boothway, PA, USA). Production information, including number of birds placed, number of birds present on each sample collection day and average daily liveweight, were supplied by the farm manager.
Results
Odour
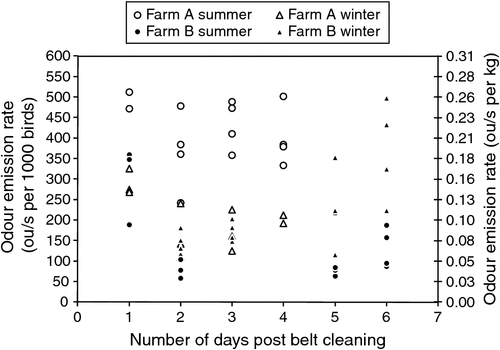
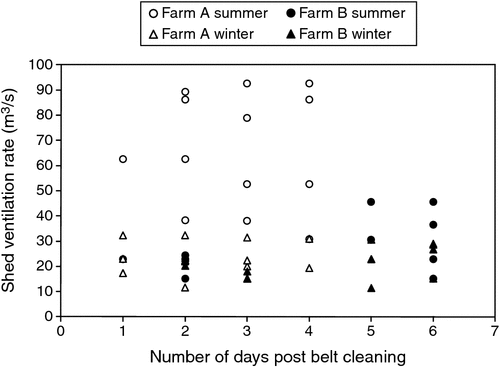
Odour emission rates varied during each sampling day and on each day of additional manure accumulation on the manure belts. Odour emission rates (OER) ranged from 58 to 512 ou/s per 1000 birds (0.03–0.27 ou/s.kg, see Fig. 1). Daily average odour emission rates were not calculated because odour emission rates vary throughout each day due to a variety of influences, and each measurement is representative of different conditions.
|
Ventilation rates varied with season (Fig. 2), with ventilation rates at Farm A tending to be greater during summer (as would be expected), while at Farm B, seasonal variation of ventilation rate was less obvious. One explanation for this was unseasonably cooler weather during the summer sampling period (mean daily maximum temperature for February 2008 was 22.9°C, compared with the long-term value of 25.2°C) and unseasonably warm weather during the winter sampling period (mean daily minimum temperature for June 2008 was 6.5°C compared with the long-term value of 4.0°C). These seasonal effects (and arguably unseasonal effects at Farm B) on ventilation rate had a similar influence on shed odour emission rate, with odour emissions from Farm A being greater in summer than in winter, while at Farm B, emission rates were similar for both seasons, with slightly higher emission rates being measured during winter. To complicate matters, the higher odour emission rates measured at Farm B in winter may have also been related to higher moisture content of the manure.
|
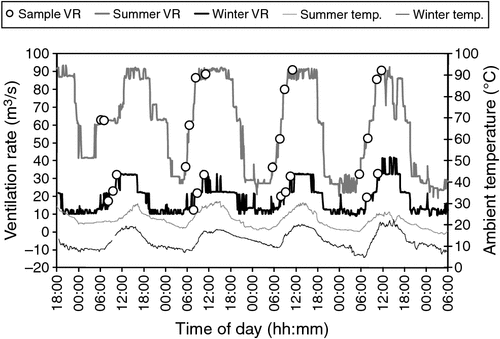
Odour samples were collected over a range of ventilation rates on each sampling day, in an attempt to measure daily variations of odour emission rate. Fig. 3 displays the shed-ventilation rate on each of the sampling days at Farm A, with the ventilation rate at the collection time of each odour sample highlighted. It can be seen that, on most days, odour samples were collected at a range of ventilation rates spanning the normal range of ventilation rate for that day. This figure also shows that odour samples were collected during the earlier parts of the day when the rate of ventilation was increasing.
|
Dust
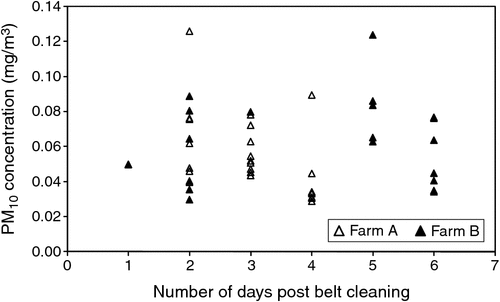
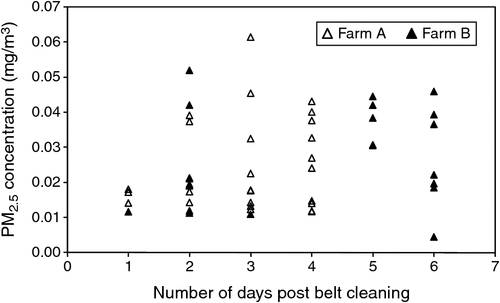
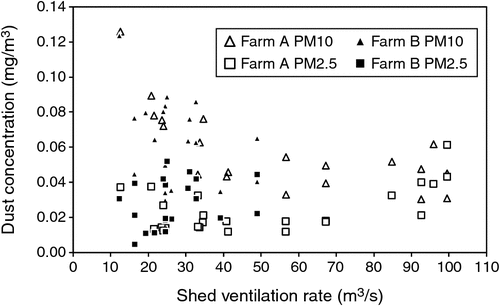
PM10 mass concentrations ranged from 0.029 to 0.124 mg/m3 (Fig. 4), PM2.5 concentrations ranged from 0.005 to 0.061 mg/m3 (Fig. 5) and particle-number concentrations ranged from 1.45 × 106 to 1.95 × 108 particles/m3, although the higher value corresponded with a short-term spike, with the majority of the number concentrations being less than 2.00 × 107 particles/m3.
|
|
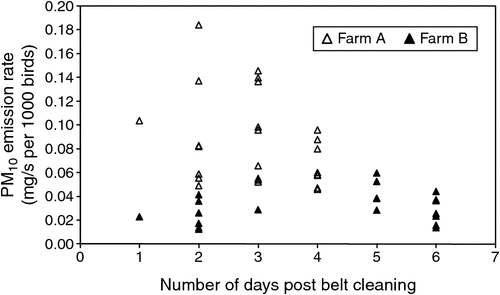
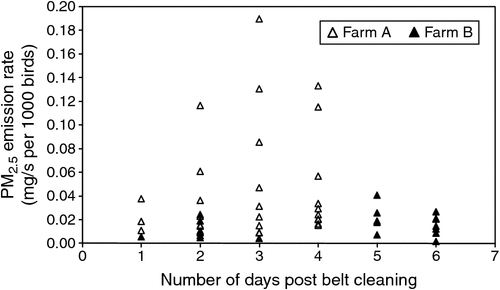
PM10 emission rates ranged from 0.014 to 0.184 mg/s per 1000 birds (Fig. 6), PM2.5 emission rates from 0.001 to 0.190 mg/s per 1000 birds (Fig. 7) and particle-number emission rates ranged from 7.90 × 105 to 5.93 × 108 particles/s per 1000 birds, although the majority of particle-number emission rates were below 3.00 × 107 particles/s per 1000 birds.
|
|
At Farm B, a single dust measurement was performed while farm staff used a petrol-powered blower (similar to a garden blower) to blow dust out of the shed, which is an operation performed approximately once weekly on this farm. While staff used the blower, the ventilation rate in the shed was increased to ~62.5% of the maximum tunnel-ventilation rate of the shed. During this cleaning operation, PM10 concentration was 0.192 mg/m3, PM2.5 concentration was 0.030 mg/m3 and particle number concentration was 1.01 × 107 particles/m3. Emission rates were 0.293 mg/s per 1000 birds for PM10, 0.046 mg/s per 1000 birds for PM2.5, and the particle number emission rate was 1.53 × 107 particles/s per 1000 birds. The research team did not consider these values to be representative of normal emission rates due to manual over-riding of the ventilation system and re-entrainment of settled dust caused by the blower. These values were not included in Figs 4–7).
Non-methane volatile organic compounds (NMVOCs)
Twenty NMVOCs were identified in the emissions from the layer sheds (listed in Table 4). Some NMVOCs were present at both farms such as 1-butanol, 2-butanone, 2-ethyl-1-hexanol, acetic acid, benzaldehyde and toluene, while others were found at only one of the farms, such as dimethyl sulfide, 3-hydroxy-2-butanone, 2,3-butanedione and cyclohexanone.

|
Many of the NMVOCs were measured in such low abundance that they did not elicit an olfactory response at the odour port during TD–GC–MS/O analysis. The only odorants identified during analysis were 2,3-butanedione, 2-butoxy-ethanol and cyclohexanone.
Manure moisture content
At Farm A, manure moisture content during the winter sampling period was 59.8%. Data from the summer sampling period at Farm A were unfortunately lost. At Farm B, manure moisture content during the summer sampling period was 54.6% and during winter it was 66.9%.
Discussion
Odour and dust emissions need to be individually considered, along with environmental and in-shed conditions at the time of measurement. Comparison of the odour and dust emission rates and identified NMVOCs reported in the literature with those measured in the present study need to be undertaken cautiously due to differences in building design, ventilation system, shed management practices, manure management practises, bird breed and feed.
OERs measured in the present project ranged from 58 to 512 ou/s per 1000 birds or from 0.03 to 0.27 ou/s/kg (Fig. 1) and are generally within the range of previously reported OERs from layer sheds; however, the minimum values were lower than those reported in the literature (refer to Table 5).

|
OERs were observed to be higher on the day following removal of manure from the manure belts and again after 4–6 days of manure accumulation, and tended to be lower on Days 2–4 following removal of manure from the manure belts. This may have occurred because manure-belt scrapers freshly smeared a thin layer of manure over the surface of each manure belt, increasing emission surface area and specific emission rate, which resulted in increased emissions during the first 24 h. Drying and crusting of the manure film during the first 24 h may have then reduced emission rates for a couple of days, until accumulation of manure after 4 days led to increased odour emissions. OERs reported in the literature were measured in sheds with a range of manure-removal intervals and manure drying methods, but it is unclear when in the manure cycle those OERs were measured and whether active manure drying had any influence. Further studies should focus on quantifying the effect of the manure-removal interval and active manure-drying systems on OERs. On the basis of the limited observations in the present study, removing manure from the belts every 4 days may be an effective odour reduction strategy, but this requires further investigation.
Seasonal variability in odour emission rates was observed at Farm A, where high OERs coincided with higher summer ventilation rates and, conversely, low OERs coincided with lower winter ventilation rates. Seasonal variability was less obvious at Farm B, presumably due to unseasonable ambient temperatures during the winter and summer sampling campaigns that resulted in uncharacteristic ventilation rates and OERs in both summer and winter. Seasonal effects on OERs from intensive animal housing, similar to those seen at Farm A, have previously been reported, with higher odour emissions being measured during summer (Hayes et al. 2006; Cai et al. 2010). In contrast, findings by Sun et al. (2010) supported the OERs measured at Farm B because odour emission rates did not correlate with season and were high and low throughout the year due to a variety of reasons. In contrast to OERs, they found that odour concentration within an intensive animal house increased during winter due to low ventilation rates and decreased in summer due to high ventilation rates. Emission rates should be measured throughout the year to reduce uncertainty when the data are to be used in odour dispersion modelling.
Odour emission measurements on each day were spread fairly zevenly across the normal range of ventilation rate for that day (as shown in Fig. 3 for Farm A). The sampling procedure was applied equally at Farm B, where ventilation rate was not continuously monitored outside the odour-sample collection period, but it is assumed that measuring OERs over the normal daily range of ventilation rate was achieved with equal success. The importance of measuring the emission rates over the full daily spread of ventilation rates cannot be understated, especially when quantifying daily emission rates at mechanically ventilated poultry sheds where there can be significant diurnal variation in ventilation requirements.
In the present study, odour emission rates were measured only in the mornings as daily ventilation requirements were increasing. In future studies, OER measurements using olfactometry or instrumental techniques should be undertaken to quantify emission rates throughout the entire day, especially targeting times that may be more likely to correspond with odour impacts. Sun et al. (2008) also recommended monitoring emissions from intensive animal housing throughout the entire day when the intention is to use the data in odour dispersion modelling. This will reduce uncertainty in the prediction of odour impacts that may be associated with using randomly measured data.
Dust concentration and emission rates (PM10 and PM2.5) measured in the present study ranged from 0.029 to 0.124 mg/m3 for PM10, and from 0.005 to 0.061 mg/m3 for PM2.5. PM10 emission rates were 0.014–0.184 mg/s per 1000 birds and PM2.5 emission rates were 0.001–0.190 mg/s per 1000 birds. These measured concentration and emission rates were similar or lower than values reported in the literature for caged layer sheds with manure belts (refer to Table 6). It is likely that some of the observed variability in dust concentrations can be attributed to in-shed activities that increase bird activity such as feeding, lighting changes, egg collection and farm staff activity (Mostafa and Buescher 2011; Costa et al. 2012).
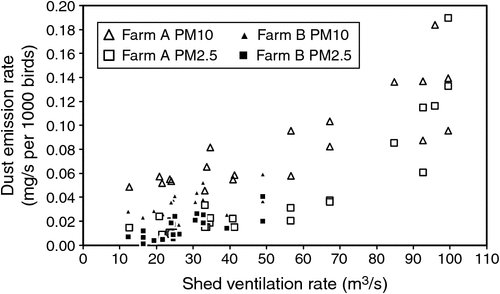
There were no obvious relationships between PM10 and PM2.5 dust concentrations and manure accumulation on the manure belts (Fig. 4, 5). Shed-ventilation rate also appeared to have little influence on dust concentration, although slightly higher dust concentrations were measured at low ventilation rates (Fig. 8), presumably due to reduced dilution with fresh air. Because PM10 and PM2.5 dust concentrations were insensitive to ventilation rate, dust emission rates tended to increase with ventilation rate (Fig. 9). A short spike in dust concentrations and emission rates was observed while the farm staff were using a petrol-powered blower to blow dust out of the shed. Activities such as this should be scheduled for times when they are less likely to cause impacts.
|
|
NMVOCs identified in the present study that have been previously identified in layer sheds included 1-butanol, acetic acid, butanoic acid and phenol (Hobbs et al. 1995; Mårtensson et al. 1999; Cai et al. 2007). NMVOCs identified in the present study that have not previously been reported included 2,3-butanedione, 2-butoxy-ethanol, cyclohexanone, 2-butanone, dimethyl sulfide, 2-ethyl-1-hexanol, toluene, styrene, benzaldehyde, acetophenone, 3-methyl-butanal, 3-hydroxy-2-butanone, o-xylene, p-xylene and nonanal. Many of these NMVOCs were measured in such low abundance that identification of odorants was challenging. The only odorants identified using the odour port on the TD–GC–MS/O were 2,3-butanedione, 2-butoxy-ethanol and cyclohexanone. Future NMVOC studies on layer-shed emissions may benefit from a refinement of sampling methods such as collecting a larger sample volume in the sorbent tubes. Additional research to identify NMVOCs and odorants on poultry farms should be undertaken to improve understanding of the relationships between primary odorants and odour concentration and to enable the development of instrumental odour/odorant assessment techniques. Such techniques will complement or be able to replace dilution olfactometry, improving odour measurement, enabling continuous odour measurement, especially at times when dilution olfactometry is impractical and will improve confidence in the assessment of odour-reduction strategies (as previously demonstrated by Cai et al. (2007)).
Odour and dust emission rates measured in the present study were observed to vary with diurnal and seasonal ventilation requirements. Odour emission rate also varied with accumulation of manure on the manure belts. Ventilation rate appeared to have a strong influence on emission rates and, therefore, requires close attention in future emission rate studies in mechanically ventilated poultry sheds. The expected range of ventilation rates at the time of sampling should be understood before sampling, to enable planning of emission rate measurements across the full, normal range of ventilation rates for that time of day and season. Future studies in layer sheds with manure belts should also consider the manure-removal cycle as well as manure moisture content, and measure emission rates throughout the cycle.
Acknowledgements
This research was undertaken as part of Project 04-45 ‘Dust and odour emissions from poultry sheds’, completed through the Australian Poultry Cooperative Research Centre in close collaboration with researchers at the Department of Agriculture, Fisheries and Forestry, Queensland (DAFF); The University of New South Wales; Queensland University of Technology; and the Department of Primary Industries, Victoria. The researchers also acknowledge and thank the management and staff of the layer farms for their cooperation with this research.
References
AECL (2009) 2009 annual report. Australian Egg Corporation Limited, Sydney. Available at http://www.aecl.org/system/attachments/211/original/Annual_Report_web_2009.pdf?1255483139 [Accessed 31 July 2012].AECL (2010) 2010 annual report. Australian Egg Corporation Limited, Sydney. Available at http://www.aecl.org/system/attachments/347/original/Annual_Report_2010.pdf?1289462015 [Accessed 31 July 2012].
AECL (2011) 2010/2011 annual report. Australian Egg Corporation Limited, Sydney. Available at http://www.aecl.org/system/attachments/484/original/Annual%20Report%202010.2011.pdf?1321843838 [Accessed 31 July 2012].
Angel R, Powers W, Applegate T (2008) Diet impacts for mitigating air emissions from poultry. In ‘Livestock environment VIII’, Iguazu Falls, Brazil’. (American Society of Agricultural and Biological Engineers: St Joseph, MI)
Burnett WE (1969) Odor transport by particulate matter in high density poultry houses. Poultry Science 48, 182–185.
Cai L, Koziel JA, Lo Y-C, Hoff SJ (2006) Characterisation of volatile organic compounds and odorants associated with swine barn particulate matter using solid-phase microextraction and gas chromatography–mass spectrometry–olfactometry. Journal of Chromatography. A 1102, 60–72.
| Characterisation of volatile organic compounds and odorants associated with swine barn particulate matter using solid-phase microextraction and gas chromatography–mass spectrometry–olfactometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlagsrvP&md5=30e1e7bd71f7f661cb6a675b4b7fc001CAS | 16297922PubMed |
Cai L, Koziel JA, Liang Y, Nguyen AT, Xin H (2007) Evaluation of zeolite for control of odorants emissions from simulated poultry manure storage. Journal of Environmental Quality 36, 184–193.
| Evaluation of zeolite for control of odorants emissions from simulated poultry manure storage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnvFaksA%3D%3D&md5=e29e79a75ac337216f95982c86ef88c6CAS | 17215226PubMed |
Cai L, Zhang S, Koziel JA, Sun G, Heathcote KY, Hoff SJ, Parker DB, Caraway EA, Jacobson LD, Akdeniz N, Hetchler BP, Cortus EL, Bereznicki SD, Heber AJ (2010) Odor and odorous chemical emissions from animal buildings: Part 3 – chemical emissions. In ‘International symposium on air quality and manure management for agriculture, Dallas, Texas, 13–16 September’. (American Society of Agricultural and Biological Engineers: St Joseph, MI)
Costa A, Ferrari S, Guarino M (2012) Yearly emission factors of ammonia and particulate matter from three laying-hen housing systems. Animal Production Science 52, 1089–1098.
Dunlop M, Gallagher E, Sohn JH (2010) Odour emissions from tunnel-ventilated broiler sheds: case study of nine Queensland farms. Animal Production Science 50, 546–551.
| Odour emissions from tunnel-ventilated broiler sheds: case study of nine Queensland farms.Crossref | GoogleScholarGoogle Scholar |
EPA Qld Government (2004) Guideline: odour impact assessment from developments. Environmental Protection Agency, Brisbane, Queensland. Available at http://www.derm.qld.gov.au/register/p01344aa.pdf [Accessed 11 November 2008].
Fabbri C, Valli L, Guarino M, Costa A, Mazzotta V (2007) Ammonia, methane, nitrous oxide and particulate matter emissions from two different buildings for laying hens. Biosystems Engineering 97, 441–455.
| Ammonia, methane, nitrous oxide and particulate matter emissions from two different buildings for laying hens.Crossref | GoogleScholarGoogle Scholar |
Fournel S, Pelletier F, Godbout S, Lagacé R, Feddes JJR (2012) Odour emissions, hedonic tones and ammonia emissions from three cage layer housing systems. Biosystems Engineering 112, 181–191.
| Odour emissions, hedonic tones and ammonia emissions from three cage layer housing systems.Crossref | GoogleScholarGoogle Scholar |
Hayes ET, Curran TP, Dodd VA (2006) Odour and ammonia emissions from intensive poultry units in Ireland. Bioresource Technology 97, 933–939.
| Odour and ammonia emissions from intensive poultry units in Ireland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xoslymsw%3D%3D&md5=942e7a554d3f07178548043d7c1f1891CAS | 15964188PubMed |
Hobbs PJ, Misselbrook TH, Pain BF (1995) Assessment of odours from livestock wastes by a photoionization detector, an electronic nose, olfactometry and gas chromatography–mass spectrometry. Journal of Agricultural Engineering Research 60, 137–144.
| Assessment of odours from livestock wastes by a photoionization detector, an electronic nose, olfactometry and gas chromatography–mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
Hobbs PJ, Webb J, Mottram TT, Grant B, Misselbrook TM (2004) Emissions of volatile organic compounds originating from UK livestock agriculture. Journal of the Science of Food and Agriculture 84, 1414–1420.
| Emissions of volatile organic compounds originating from UK livestock agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmvVaht7k%3D&md5=c0ad6d83e02f3478651255be4181fa2fCAS |
Lacey RE, Mukhtar S, Carey JB, Ullman JL (2004) A review of literature concerning odors, ammonia, and dust from broiler production facilities: 1. Odor concentrations and emissions. Journal of Applied Poultry Research 13, 500–508.
Mackie RI, Stroot PG, Varel VH (1998) Biochemical identification and biological origin in key odor components in livestock waste. Journal of Animal Science 76, 1331–1342.
Mårtensson L, Magnusson M, Shen Y, Jönsson JÅ (1999) Air concentrations of volatile organic acids in confined animal buildings-determination with ion chromatography. Agriculture, Ecosystems & Environment 75, 101–108.
| Air concentrations of volatile organic acids in confined animal buildings-determination with ion chromatography.Crossref | GoogleScholarGoogle Scholar |
Miner JR (1977) Characterization of odors and other volatile emissions. Agriculture and Environment 3, 129–137.
| Characterization of odors and other volatile emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXht1Gmsbk%3D&md5=05747e845a201195947f391f76424a10CAS |
Modini RL, Agranovski V, Meyer NK, Gallagher E, Dunlop M, Ristovski ZD (2010) Dust emissions from a tunnel-ventilated broiler poultry shed with fresh and partially reused litter. Animal Production Science 50, 552–556.
| Dust emissions from a tunnel-ventilated broiler poultry shed with fresh and partially reused litter.Crossref | GoogleScholarGoogle Scholar |
Mostafa E, Buescher W (2011) Indoor air quality improvement from particle matters for laying hen poultry houses. Biosystems Engineering 109, 22–36.
| Indoor air quality improvement from particle matters for laying hen poultry houses.Crossref | GoogleScholarGoogle Scholar |
Murphy KR, Wenig P, Parcsi G, Skov T, Stuetz RM (2012) Characterizing odorous emissions using new software for identifying peaks in chemometric models of gas chromatography–mass spectrometry datasets. Chemometrics and Intelligent Laboratory Systems 118, 41–50.
Navaratnasamy M, Feddes JJR (2004) Odour emissions from poultry manure/litter and barns. PIC Project No. 155. Available at www.cpc-ccp.com/documents/Feddes_LEI_Poultry_Emissions_Final.pdf [Verified 1 May 2013]
Ni J-Q, Chai L, Chen L, Bogan BW, Wang K, Cortus EL, Heber AJ, Lim T-T, Diehl CA (2012a) Characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter concentrations in high-rise and manure-belt layer hen houses. Atmospheric Environment 57, 165–174.
| Characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter concentrations in high-rise and manure-belt layer hen houses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovFynsbs%3D&md5=67de9cff2e7f2766e0fc4901399dc386CAS |
Ni J-Q, Robarge WP, Xiao C, Heber AJ (2012b) Volatile organic compounds at swine facilities: a critical review. Chemosphere 89, 769–788.
| Volatile organic compounds at swine facilities: a critical review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xot12mtLs%3D&md5=5c3d714544876d60bb497c5bc7dbd312CAS | 22682363PubMed |
Nimmermark S, Gustafsson G (2005) Influence of temperature, humidity and ventilation rate on the release of odour and ammonia in a floor housing system for laying hens. Agricultural Engineering International VII, 1–14.
O’Neill DH, Phillips VR (1992) A review of the control of odour nuisance from livestock buildings: Part 3. Properties of the odorous substances which have been identified in livestock wastes or in the air around them. Journal of Agricultural Engineering Research 53, 23–50.
| A review of the control of odour nuisance from livestock buildings: Part 3. Properties of the odorous substances which have been identified in livestock wastes or in the air around them.Crossref | GoogleScholarGoogle Scholar |
Ogink NWM, Groot Koerkamp PWG (2001) Comparison of odour emissions from animal housing systems with low ammonia emission. Water Science and Technology 44, 245–252.
Parcsi G, Pillai SM, Sohn JH, Gallagher E, Dunlop M, Atzeni M, Lobsey C, Stuetz RM (2011) Optimising non-specific sensor arrays for poultry emission monitoring using GC-MS/O, In ‘ISSNIP2011 – Seventh international conference on intelligent sensors, sensor networks and information processing, Adelaide, 6–9 December 2011’. pp. 205–210. (Institute of Electrical and Electronics Engineers: New York)
Schiffman SS, Bennett JL, Raymer JH (2001) Quantification of odors and odorants from swine operations in North Carolina. Agricultural and Forest Meteorology 108, 213–240.
| Quantification of odors and odorants from swine operations in North Carolina.Crossref | GoogleScholarGoogle Scholar |
Sohn JH, Hudson N, Gallagher E, Dunlop M, Zeller L, Atzeni M (2008) Implementation of an electronic nose for continuous odour monitoring in a poultry shed. Sensors and Actuators. B, Chemical 133, 60–69.
| Implementation of an electronic nose for continuous odour monitoring in a poultry shed.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXosVOqsr8%3D&md5=74b33943c10a7dbca819caf8c85e9f24CAS |
Standards Australia (1995a) ‘Australian standard: stationary source emissions: selection of sampling positions (AS4323.1-1995).’ (Standards Australia International: Sydney)
Standards Australia (1995b) ‘Australian/New Zealand standard: stationary source emissions: seterminaton of total particulate matter – Isokinetic sampling – Gravimetric method (AS4323.2).’ (Standards Australia International: Sydney)
Standards Australia (2003) ‘Australian Standard: composts, soil conditioners and mulches (AS 4454-2003).’ (Standards Australia International: Sydney)
Standards Australia/Standards New Zealand (2001) ‘Stationary source emissions part 3: determination of odour concentration by dynamic olfactometry (AS/NZS 4323.3-2001).’ (Standards Australia/Standards New Zealand: Sydney)
Sun G, Guo H, Lague C (2008) Diurnal odor, ammonia, hydrogen sulfide, and carbon dioxide emission profiles of confined swine grower/finisher rooms. Journal of the Air & Waste Management Association 58, 1434–1448.
| Diurnal odor, ammonia, hydrogen sulfide, and carbon dioxide emission profiles of confined swine grower/finisher rooms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmslygtw%3D%3D&md5=b36fd93c9c6e91423d4846b67dcfa255CAS |
Sun G, Guo H, Peterson J (2010) Seasonal odor, ammonia, hydrogen sulfide, and carbon dioxide concentrations and emissions from swine grower-finisher rooms. Journal of the Air & Waste Management Association 60, 471–480.
| Seasonal odor, ammonia, hydrogen sulfide, and carbon dioxide concentrations and emissions from swine grower-finisher rooms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXls12nsro%3D&md5=1ba9371ffc0a871cd94029d8427f9f51CAS |
Trabue S, Scoggin K, Li H, Burns R, Xin H, Hatfield J (2010) Speciation of volatile organic compounds from poultry production. Atmospheric Environment 44, 3538–3546.
| Speciation of volatile organic compounds from poultry production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpvVWhtLg%3D&md5=e034402d8ea19e57b84c0b69c78cc6cfCAS |
van Harreveld APT (2003) Odor concentration decay and stability in gas sampling bags. Journal of the Air & Waste Management Association 53, 51–60.
| Odor concentration decay and stability in gas sampling bags.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXht1yksLY%3D&md5=9020aa7365898939787338068e2f7b91CAS |
Wilhelm LR, Milner JM, Snyder SD, McKinney DB (2001) An instrumentation system for environmental measurements in broiler and swine housing. Applied Engineering in Agriculture 17, 677–681.
Xin H, Gates RS, Green AR, Mitloehner FM, Moore PA, Wathes CM (2011) Environmental impacts and sustainability of egg production systems. Poultry Science 90, 263–277.
| Environmental impacts and sustainability of egg production systems.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M%2FmtFWlsw%3D%3D&md5=1a8dc35afb4485ba1d597590e9d7ee5eCAS | 21177468PubMed |