Egg quality and age of laying hens: implications for product safety
J. R. Roberts A C , Kapil Chousalkar B andA Animal Science, School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
B School of Animal and Veterinary Sciences, Roseworthy Campus, University of Adelaide, SA 5005, Australia.
C Corresponding author. Email: jrobert2@une.edu.au
Animal Production Science 53(12) 1291-1297 https://doi.org/10.1071/AN12345
Submitted: 4 October 2012 Accepted: 5 May 2013 Published: 6 June 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Eggs were collected from commercial caged layer flocks in early, mid, late and very late lay. Eggs were candled and scored for translucency. Cuticle cover was estimated using MST Cuticle Stain and a Konica Minolta hand-held spectrophotometer. Traditional measures of egg quality were determined using specialised equipment. Shell ultrastructural features were scored following plasma ashing of shell samples and viewing under a benchtop scanning electron microscope. Translucency score was significantly higher in late lay than for all other age groups. Shell quality declined with increasing flock age. However, the extent of cuticle cover on the egg shell was not significantly different among flock age groups. The incidence of shell ultrastructural features associated with good quality shells was lower for older flocks and incidence of ultrastructural features associated with poorer quality shells was higher for older flocks. Translucency score had a low correlation with the ultrastructural features of the mammillary layer.
Additional keywords: cuticle, food safety, shell quality, translucency.
Introduction
Eggs produced in Australia are considered medium to low risk for foodborne illness. However, the egg industry in Australia is periodically implicated in cases of food poisoning (OzFoodNet Working Group 2009). It is difficult for bacteria to move across an intact good quality egg shell. However, small defects in the egg shell may provide means for bacteria on the egg shell to penetrate the shell and move into the egg contents (Messens et al. 2005; De Reu et al. 2006). The importance of the cuticle as a barrier against water loss and bacterial ingress into the egg has been discussed by several authors (Sparks and Board 1984) and it has also been suggested that egg shell translucency may facilitate bacterial penetration (Chousalkar et al. 2010). In the present study, unwashed eggs collected directly from the cage front were scored for translucency and tested for egg quality measurements. Egg shells were stained for estimation of cuticle deposition and the ultrastructural characteristics of the mammillary layer of the eggshell were scored and related to the translucency scores.
Materials and methods
Eggs were collected from commercial flocks in different stages of lay: early (<25–40 weeks – 10 flocks), mid (40–55 weeks – 10 flocks), late (55–65 weeks – 6 flocks) and very late (>65 weeks – 8 flocks).
Ninety eggs were used for measurements of egg quality, which were conducted in the Egg Quality Laboratory at the University of New England, Australia. Thirty eggs were scored for translucency (0 lowest to 5 highest) using an egg candler and analysed for traditional egg shell quality measurements: shell colour by reflectivity, egg weight, egg shell breaking strength by quasi-static compression, shell deformation to breaking point and shell weight (egg quality equipment, Technical Services and Supplies, Dunnington, York, UK). Shell thickness was measured using a custom-made gauge based on a Mitutoyo Dial Comparator gauge Model 2109-10 (Kawasaki, Japan). Percentage shell was calculated from shell weight and egg weight. Egg internal quality was measured as albumen height, Haugh units and yolk colour (Technical Services and Supplies equipment).
Thirty eggs were stained with MST cuticle blue stain and the cuticle colour measured using a Konica Minolta hand-held spectrophotometer (CM-2600d; Ramsey, NJ, USA). Eggs were immersed in cuticle blue dye (MS Technologies, Europe Ltd, Kettering, Northamptonshire, UK), made up according to the manufacturer’s recommendation, for 1 min. They were then rinsed in water for 3 s, placed on a plastic egg filler and allowed to dry.
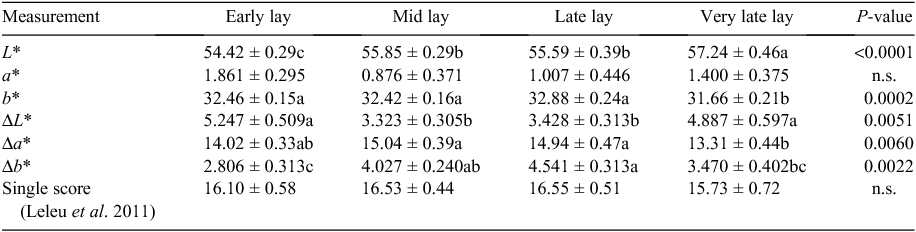
The colour of the egg shell cuticle, stained with MST cuticle blue dye was measured using the ‘L*, a*, b*’ colour space. L* has a maximum of 100 (white) and a minimum of 0 (black). For a*, green is towards the negative end of the scale and red towards the positive end. For b*, blue is towards the negative end and yellow towards the positive end of the scale. The difference between the reading before and after staining was measured for 24 out of the total of 34 flocks (6 early, 7 mid, 4 late and 7 very late lay flocks). A single score was calculated after the method of Leleu et al. (2011) as:
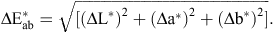
The remaining 30 eggs were scored for the extent of translucency, photographed individually on a candling box, and processed for viewing of the ultrastructure of the mammillary layer. Small pieces of shell (1 cm2) were cut from the equator of the egg shell, soaked overnight in distilled water and the shell membranes removed. The samples were then dried thoroughly and placed in a BioRad PT7 150 Plasma Asher (BioRad, Hertfordshire, UK) for 4 h to remove any remaining shell membrane (Reid 1983). Samples were then mounted on aluminium stubs using conductive silver paint (1005 aqueous conductive silver liquid – SEM adhesive, ProSciTech, Kirwan, Qld, Australia), gold sputter coated in a Jeol MP-19020NCTR Neocoater and viewed under a Jeol JCM-5000 Neoscope desktop scanning electron microscope (JEOL, Tokyo, Japan). Each sample was scored for ultrastructural features as described by Solomon (1991). Mammillary cap size was scored as 1 (similar), 2 (variable), and 3 (highly variable). Mammillary caps were scored according to their quality, which was assessed as both the size of the cap in relation to its cone and the degree of membrane attachment, from best (1) to worst (5). Alignment, changed membrane (membrane not removed by plasma ashing), cubic cone formations, confluence, cuffing, early fusion, late fusion, depression, erosion, hole, type-A bodies, type-B bodies, aragonite and cubics were each ranked for incidence from 1 (none) to 4 (extensive).
To verify the suitability of MST cuticle blue stain as an indicator of the presence and extent of cuticle, 90 eggs (30 from each of 3 flocks aged 33, 50 and 67 weeks) were stained with cuticle blue dye and egg internal contents were removed by making a small hole at the blunt end of the egg using a Dremel High Speed rotary tool, 300 series (Robert Bosch Tool Corporation, Racine, WI, USA). The inner shell walls were then rinsed with tap water to remove the adherent albumen, taking care not to wash off any cuticle stain. Small pieces of shell (1 cm2) were cut out, mounted on aluminium stubs (0.9 cm diameter) using conductive silver paint (1005 aqueous conductive silver liquid – SEM adhesive, ProSciTech) and photographed under a dissecting microscope with attached camera. The same pieces of shell were then gold sputter coated in a Jeol MP-19020NCTR Neocoater and viewed and photographed under a Jeol JCM-5000 Neoscope benchtop scanning electron microscope.
Results
Egg quality
All egg shell quality measurements varied significantly among age categories (Table 1). Translucency score was significantly higher in late lay than for all other age groups. Shell reflectivity increased (shells became lighter in colour) from early to late lay, although late and very late lay were not significantly different from each other. Egg weight increased from early to mid lay, remained relatively constant into late lay and then increased again from late to very late lay. Shell breaking strength decreased with flock age although late and very late lay were not significantly different from each other. Shell deformation to breaking point decreased from early to mid lay but then remained relatively constant. Shell weight increased from early to mid lay before decreasing in late lay and then increasing in very late lay to levels not different from mid lay. Percentage shell was higher at early and mid lay than for late and very late lay. Shell thickness increased from early to mid lay, decreased in late lay to values significantly different lower than all other age groups and then increased in very late lay to values not significantly different from early and mid lay.

|
Egg internal quality, as measured by albumen height and Haugh unit, decreased consistently with increasing flock age (Table 1), with each age group being statistically significantly different from the others. However, there were no significant differences among age categories for yolk colour score.
Shell cuticle cover
The spectrophotometric measurements of shells with stained cuticle indicated that the value for L* increased from early to mid lay, remained constant to late lay before increasing again in very late lay (Table 2). The value for a* was not significantly different among age categories and the coefficient of variation was very high for all age categories. The value for b* was similar for early, mid and late lay and then decreased for the very late lay group. The difference in L* values before and after staining was significantly higher for early and very late lay than for mid and late lay. The difference in a* before and after staining was highest in mid and late lay and lowest in very late lay, with early lay intermediate. The difference in b* before and after staining was highest in mid and late lay and lowest in early lay, with very late lay intermediate. There was no significant difference among age categories for the single score value, which was calculated after the method of Leleu et al. (2011).
Egg shell ultrastructure
For the eggs studied for shell mammillary layer ultrastructure, there were no statistically significant differences among age categories for translucency score (measured 7 days after the egg was laid by the hen), as shown in Table 3. However, there were statistically significant effects of age category on some of the ultrastructural scores. Cap size variability was lower in early lay than for all other age categories. The incidence of confluence of mammillary caps was higher in early lay than for all other ages. Cap quality score was lower (i.e. cap quality was higher) for early and mid lay than for late lay although the score for very late lay was similar to that for early and mid lay. The incidence of early fusion was higher for early and mid lay than for late lay, with very late lay intermediate between the two. Late fusion had a lower incidence in early lay than for all other age categories. The incidence of type-B bodies was lower in early and mid lay than in late and very late lay. The incidence of aragonite was lowest in early and mid lay, highest in late lay, with very late lay intermediate. The incidence of cubic cones was lower in mid lay than for all other ages. The incidence of cuffing and changed membrane was higher in early lay than for all other age categories. The incidence of alignment of mammillary cones, type-A bodies, cubics, depression and erosion were not significantly different among age categories. Holes were not recorded for any age category.

|
There were significant positive correlations (higher incidence for higher translucency score) between translucency score and the incidence of mammillary layer variations for alignment, type-A bodies, type-B bodies, aragonite and cubic cones. There were significant negative correlations for cap quality (higher translucency, lower cap quality) and changed membrane (higher translucency score, lower incidence). However, there was no significant correlation with translucency score for the incidence of confluence, early fusion, late fusion, cubics, cuffing, depression, erosion and cap size variability.
Verification of cuticle stain
There was a high correlation between the presence of cuticle blue stain on the egg shells and the amount of cuticle present, as viewed under the scanning electron microscope. Eggs with good quality intact cuticle stained well; eggs with patchy cuticle acquired patchy stain whereas, in the absence of the cuticle, the eggs did not stain at all (Fig. 1). These findings confirmed the suitability of MST cuticle blue dye as an indicator of the amount of cuticle present on the surface of the egg shell.

|
Discussion
Translucency score was higher for the late lay age group than for all other groups. The significance of this finding is not clear and there are no published data against which to compare these results. Results on egg quality obtained in the present study are consistent with previous reports of the changes that occur in egg quality as flocks get older both in Australia (Roberts and Ball 2003, 2006; Roberts 2004) and in other countries (Washburn 1982; Travel et al. 2011). The increased shell reflectivity (indicative of lighter shell colour) with increasing hen age has been reported previously by several authors (Roberts and Ball 2006; Tumova and Ledvinka 2009; Zita et al. 2009). Increased egg weight with increasing hen age may be viewed as positive or negative depending on the market involved. Increased egg weight with increasing hen age has been reported previously by many authors (Guesdon and Faure 2004; Roberts and Ball 2006; Tumova and Ledvinka 2009). The decrease in shell breaking strength as hens age, found in this study, has also been reported previously (Rodriguez-Navarro et al. 2002; Roberts and Ball 2006). Shell deformation to breaking point is an indicator of the degree of elasticity of the eggshell. A higher shell deformation was found in the younger flocks in the present study as has been described by Roberts and Ball (2006) although the results of Zita et al. (2009) for shell deformation varied with the breed of laying hen. Although egg weight increased with increasing hen age, as has been described previously (Nys 1986; Silversides and Budgell 2004; Zita et al. 2009), shell weight did not always increase in proportion. This resulted in percentage shell decreasing with increased hen age as has been described previously (Silversides and Scott 2001; Roberts and Ball 2006). Shell thickness fluctuated among the age groups, being highest in mid lay. Most studies report a decrease in shell thickness with increasing hen age (Roland et al. 1975; Roland 1979). The higher shell weight and shell thickness for the very late lay group, as compared with the late lay group, correlate with a larger egg size (65 g as compared with 62.6 g for the late lay group). However, the percentage shell was not significantly different between the late lay and very late lay groups. In addition, shell breaking strength was similar for late lay and very late lay groups, suggesting that the increases in shell weight and shell thickness in very late lay were commensurate with the increase in egg size. The steady reduction in albumen quality with increased flock age has also been reported by several researchers (Williams 1992; Silversides 1994). The heritability of many egg quality parameters is high (Dunn 2011; Dunn et al. 2012), which means that genetic selection by major breeding companies can assist with improvements in quality, in addition to management improvements.
The use of MST cuticle blue stain for determining the extent of cuticle cover in avian egg shells has been verified by correlating the extent of staining with the appearance of the shell cuticle under the scanning electron microscope. Therefore, MST cuticle blue stain is a reliable indicator of the presence of cuticle on the egg shell surface. However, quantification of the degree of staining using the Konica spectrophotometer proved more complex than had been anticipated. The use of shell colour, as measured by the spectrophotometer, is confounded by the underlying colour of the egg shell. The L* values show the same pattern as shell reflectivity, which is that the shells became paler in colour with increased flock age. The results from the b* spectrum suggest that the shells from the eggs of the very late lay flocks were less yellow than for the other three flocks which may correlate with the paler brown-coloured shells of this group. Even when the extent of shell colour is measured by a range of techniques, including the calculation of a single value to integrate the L*, a*, b* values, there appears to be no clear correlation between average cuticle cover and flock age. However, the higher mean values for Δa* in mid and late lay suggest the cuticle is thickest during these stages of the laying life of a hen. Previous studies report that the cuticle becomes thinner with increasing flock age (Sparks and Board 1984). The significance of the extent of cuticle cover is still uncertain. The most important role of the cuticle in relation to food safety may be the presence of sufficient cuticle to block the outside of most of the pores.
The translucency scores for the eggs used for ultrastructural examination of the mammillary layer were not significantly different among flock age groups. Translucency score was measured at an average of 3 days following lay for the egg quality eggs and an average of 7 days following lay for the eggs used for ultrastructure studies, which may explain the difference. Shell translucency increases with time following oviposition. In general, the incidence of mammillary layer variations, which appear to be associated with better shell quality (confluence, early fusion, cuffing) decreased with increasing flock age and mammillary cap quality decreased. Conversely, the incidence of mammillary layer variations, which appear to be associated with reduced shell quality (cap size variability, late fusion, type-A bodies, type-B bodies, aragonite) increased with increasing flock age. Our data accord with previous studies (Parsons 1982; Nascimento and Solomon 1991; Solomon 1991, 1992a, 1992b, 2009; Nascimento et al. 1992; Roberts and Brackpool 1994). It is not clear why the incidence of ‘changed membrane’ was higher for the early lay flocks. Changed membrane is membrane remaining following the plasma ashing procedure. It is possible that, in the younger flocks, the attachment between the outer shell membrane and the mammillary cones is greater, making it more difficult to remove the membrane. However, this suggestion needs to be confirmed.
The correlations between egg shell translucency scores and the scores obtained from examination of the mammillary layer of the egg shells suggest that the ultrastructure scoring system only partially explains the phenomenon of egg shell translucency. It is still not completely clear what structural features of the eggs cause translucency. Shell translucency appears to be associated with the ultrastructure of the egg shell and is associated with the presence of moisture in the spaces of the mammillary layer and perhaps also the pores. Studies are underway to examine egg shells using a Phoenix v|tome|x dual X-ray system in an attempt to further explain the phenomenon of translucency.
Eggs from the same flocks utilised for the egg quality studies were evaluated for total bacterial counts and Enterobacteriacae on the outside of the shells, in shell crush (to incorporate any bacteria present in the shell pores) and egg internal contents, as described in Musgrove et al. (2005) and results are presented in other publications. There were no effects of flock age on the bacterial counts on the shell surface and in shell crush and no bacteria were isolated from the egg contents (Chousalkar and Roberts 2012b). However, some Salmonella were isolated from the shell surface (Chousalkar and Roberts 2012a). In Australia and most European countries, there is some debate about the benefits of washing eggs. Previous research suggests that washing removes faecal material and reduces microbial load on the egg shell surface and thus reduces the likelihood of horizontal transmission occurring as well as reducing the potential for cross contamination during food handling/preparation (Musgrove et al. 2004). However, research has also shown that wet washing can damage the cuticle layer, (which prevents the entry of bacteria across the egg shell) thereby leaving pores exposed and potentiating bacterial penetration (Sparks and Burgess 1993). Egg washing is widely used in many countries including Australia (Hutchison et al. 2004). It has been demonstrated that the extent of cuticle deposition can influence the egg shell penetration of Salmonella Enteritidis at 20°C (De Reu et al. 2006). However, it is not clear how the measurements of cuticle cover undertaken in the present study correlate with the ease with which bacteria can pass through the egg shell. It is possible that the presence of cuticle in the egg shell pores is the most significant barrier to bacterial entry and that a thick cuticle on the outside of other parts of the egg is of lesser significance.
The fact that cuticle cover, as measured by MST cuticle blue staining, was not significantly different among age categories, coupled with the relatively constant translucency scores from mid to very late lay, and the absence of bacteria inside the eggs tested, suggests that translucency is not a major risk factor for food safety of table eggs. However, this remains to be tested in future experiments using whole eggs and agar-filled eggs exposed to specific serovars of Salmonella. In addition, experiments are ongoing to better characterise the basis of egg shell translucency. Similarly, the extent to which variation in cuticle cover represents a risk factor for food safety is still not fully understood. This topic is also under further investigation using both washed and unwashed eggs with varying degrees of cuticle cover in whole egg and agar-filled egg experiments.
References
Chousalkar KK, Roberts JR (2012a) Recovery of Salmonella from egg shell wash, egg shell crush and egg internal contents of unwashed commercial shell eggs in Australia. Poultry Science 91, 1739–1741.| Recovery of Salmonella from egg shell wash, egg shell crush and egg internal contents of unwashed commercial shell eggs in Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38jhvFKltg%3D%3D&md5=211a7a90df0507939aa962e941c2160eCAS | 22700522PubMed |
Chousalkar KK, Roberts JR (2012b) Recovery of enterobacteriaceae from shell surface and shell in early mid and late lay. In ‘Proceedings of the Australian poultry science symposium. Vol. 23’. (Ed. J. Roberts) pp. 233–236. (The Poultry Research Foundation and World’s Poultry Science Association: Sydney)
Chousalkar KK, Flynn P, Sutherland M, Roberts JR, Cheetham BF (2010) Recovery of Salmonella and Escherichia coli from commercial shell eggs and effect of translucency on bacterial penetration in eggs. International Journal of Food Microbiology 142, 207–213.
| Recovery of Salmonella and Escherichia coli from commercial shell eggs and effect of translucency on bacterial penetration in eggs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cjlslOktA%3D%3D&md5=25e464b2031d59f76261f5bd4c0e0141CAS | 20663580PubMed |
De Reu K, Grijspeerdt K, Messens W, Heyndrickx M, Uyttendaele M, Debevere J, Herman L (2006) Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella Enteritidis. International Journal of Food Microbiology 112, 253–260.
| Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella Enteritidis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28nmt1yruw%3D%3D&md5=e48eb555020c2b723fce3ff919313325CAS | 16822571PubMed |
Dunn I (2011) Poultry breeding for egg quality: traditional and modern genetic approaches. In ‘Improving the safety and quality of eggs and egg products. Volume 1’. (Eds Y Nys, M Bain, F Van Immerseel) pp. 245–260. (Woodhead Publishing Limited: Cambridge, UK)
Dunn IC, Rodriguez-Navarro AB, Mcdade K, Preisinger R, Waddington D, Wilson PW, Bain MM (2012) Genetic variation in eggshell crystal size and orientation is large and these traits are correlated with shell thickness and are associated with eggshell matrix protein markers. Animal Genetics 43, 410–418.
| Genetic variation in eggshell crystal size and orientation is large and these traits are correlated with shell thickness and are associated with eggshell matrix protein markers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38rksV2itw%3D%3D&md5=316c4b9b9652dfe49e12270545006fb4CAS | 22497523PubMed |
Guesdon V, Faure JM (2004) Laying performance and egg quality in hens kept in standard or furnished cages. Animal Research 53, 45–57.
| Laying performance and egg quality in hens kept in standard or furnished cages.Crossref | GoogleScholarGoogle Scholar |
Hutchison ML, Gittins J, Walker A, Spark N, Humphrey TJ, Burton C, Moore A (2004) An assessment of the microbiological risks involved with egg washing under commercial conditions. Journal of Food Protection 67, 4–11.
Leleu S, Messens W, De Reu K, De Preter S, Herman S, Heyndrickx M, de Baerdemaeker J, Michiels CW, Bain M (2011) Effect of egg washing on the cuticle quality of brown and white table eggs. Journal of Food Protection 74, 1649–1654.
| Effect of egg washing on the cuticle quality of brown and white table eggs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbhvFelug%3D%3D&md5=76aeca18c4b903d9258724d745e1fbb2CAS | 22004811PubMed |
Messens W, Grijspeerdt K, Herman L (2005) Eggshell characteristics and penetration by Salmonella enterica serovar Enteritidis through the production period of a layer flock. British Poultry Science 46, 694–700.
| Eggshell characteristics and penetration by Salmonella enterica serovar Enteritidis through the production period of a layer flock.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28%2FjvFSjtA%3D%3D&md5=8ba08aa44fe3d49f8ec4e437661a2efaCAS | 16428112PubMed |
Musgrove MT, Jones DR, Shaw J, Sheppard M, Harrison MA (2004) Identification of Enterobacteriaceae from washed and unwashed commercial shell eggs. Journal of Food Protection 67, 2613–2616.
Musgrove MT, Jones D, Northcutt JK, Harrison MA, Cox NA, Ingram KD, Hinton AJ (2005) Recovery of Salmonella from commercial shell eggs by shell rinse and shell crush Methodologies. Poultry Science 84, 1955–1958.
Nascimento VP, Solomon SE (1991) The transfer of bacteria (Salmonella Enteritidis) across the eggshell wall of eggs classified as ‘poor’ quality. Animal Technology 42, 157–166.
Nascimento VP, Cranstoun S, Solomon SE (1992) Relationship between shell structure and moment of Salmonella Enteritidis across the eggshell wall. British Poultry Science 33, 37–48.
| Relationship between shell structure and moment of Salmonella Enteritidis across the eggshell wall.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK383ktVChtg%3D%3D&md5=b5c391201fc0ab604ed8e8f65314103fCAS | 1571806PubMed |
Nys Y (1986) Relationship between age, shell quality and individual rate and duration of shell formation in domestic hens. British Poultry Science 27, 253–259.
| Relationship between age, shell quality and individual rate and duration of shell formation in domestic hens.Crossref | GoogleScholarGoogle Scholar |
OzFoodNet Working Group (2009) Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: Annual Report of the OzFoodNet network. 2006. Communicable Diseases Intelligence 34, 396–426.
Parsons AH (1982) Structure of the eggshell. Poultry Science 61, 2013–2021.
| Structure of the eggshell.Crossref | GoogleScholarGoogle Scholar |
Reid J (1983) The use of the plasma chemistry unit as an aid to the scanning electron microscope study of avian egg-shell structure. British Poultry Science 24, 233–235.
| The use of the plasma chemistry unit as an aid to the scanning electron microscope study of avian egg-shell structure.Crossref | GoogleScholarGoogle Scholar |
Roberts JR (2004) Factors affecting egg internal quality and egg shell quality in laying hens. International Journal of Poultry Science 41, 166–177.
Roberts J, Ball W (2003) ‘Egg quality guidelines for the Australian egg industry.’ (University of New England and Australian Egg Corporation Limited: Armidale, NSW)
Roberts JR, Ball W (2006) Egg and eggshell quality guidelines for the Australian egg industry. In ‘The amazing egg: nature’s perfect functional food for health promotion’. (Eds By JS Sim, HH Sunwoo) pp. 499–508. (Department of Agricultural, Food and Nutritional Science, University of Alberta: Edmonton)
Roberts JR, Brackpool CE (1994) The ultrastructure of avian egg shells. Poultry Science Reviews 5, 245–272.
Rodriguez-Navarro A, Kalin O, Nys Y, Garcia-Ruiz JM (2002) Influence of the microstructure on the shell strength of eggs laid by hens of different ages. British Poultry Science 43, 395–403.
| Influence of the microstructure on the shell strength of eggs laid by hens of different ages.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38vjvVersg%3D%3D&md5=d9b4b82b7991b4d4a54a492078a5a9b9CAS | 12195799PubMed |
Roland DA (1979) Factors influencing shell quality of aging hens. Poultry Science 58, 774–777.
| Factors influencing shell quality of aging hens.Crossref | GoogleScholarGoogle Scholar |
Roland DA, Sloan DR, Harms RH (1975) The ability of hens to maintain calcium deposition in the eggshell and egg yolk as the hen ages. Poultry Science 54, 1720–1723.
| The ability of hens to maintain calcium deposition in the eggshell and egg yolk as the hen ages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XivVaqsQ%3D%3D&md5=adc871058dd385a92c627d58828c54c9CAS |
Silversides FG (1994) The Haugh unit correction for egg weight is not adequate for comparing eggs from chickens of different lines and ages. Journal of Applied Poultry Research 3, 120–126.
Silversides FG, Budgell K (2004) The relationships among measures of egg albumen height, pH, and whipping volume. Poultry Science 83, 1619–1623.
Silversides FG, Scott TA (2001) Effect of storage and layer age on quality of eggs from two lines of hens. Poultry Science 80, 1240–1245.
Solomon SE (1991) ‘Egg and eggshell quality.’ (Wolfe Publishing Limited: London)
Solomon SE (1992a) Can eggshell structure measure quality? Poultry International 31, 24–27.
Solomon SE (1992b) Eggshell quality and microbial penetration. Poultry International 31, 20–22.
Solomon SE (2009) Foundation is key for eggshell quality. World Poultry 25, 16–18.
Sparks NH, Board RG (1984) Cuticle, shell porosity and water uptake through hen’s eggshells. British Poultry Science 25, 267–276.
| Cuticle, shell porosity and water uptake through hen’s eggshells.Crossref | GoogleScholarGoogle Scholar |
Sparks NH, Burgess AD (1993) Effect of spray sanitising on hatching egg cuticle efficacy and hatchability. British Poultry Science 34, 655–662.
| Effect of spray sanitising on hatching egg cuticle efficacy and hatchability.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2c%2FmtFGqsg%3D%3D&md5=8b89d3d7e72d66847c13954d29d86305CAS | 8242405PubMed |
Travel A, Nys Y, Bain M (2011) Effect of hen age, moult, laying environment and egg storage on egg quality. In ‘Improving the safety and quality of eggs and egg products. Volume 1’. (Eds Y Nys, M Bain, F Van Immerseel) pp. 300–329. (Woodhead Publishing Limited: Cambridge, UK)
Tumova E, Ledvinka Z (2009) The effect of time of oviposition and age on egg weight, egg components weight and eggshell quality. Archiv fur Geflugelkunde 73, 110–115.
Washburn KW (1982) Incidence, cause, and prevention of egg shell breakage in commercial production. Poultry Science 61, 2005–2012.
| Incidence, cause, and prevention of egg shell breakage in commercial production.Crossref | GoogleScholarGoogle Scholar |
Williams KC (1992) Some factors affecting albumen quality with particular reference to Haugh unit score. World’s Poultry Science Journal 48, 5–16.
| Some factors affecting albumen quality with particular reference to Haugh unit score.Crossref | GoogleScholarGoogle Scholar |
Zita L, Tumova E, Stolc L (2009) Effects of genotype, age and their interaction on egg quality in brown-egg laying hens. Acta Veterinaria 78, 85–91.
| Effects of genotype, age and their interaction on egg quality in brown-egg laying hens.Crossref | GoogleScholarGoogle Scholar |