Natural plant extracts and prebiotic compounds as alternatives to antibiotics in broiler chicken diets in a necrotic enteritis challenge model
J. K. Vidanarachchi A B , L. L. Mikkelsen A , C. C. Constantinoiu C , M. Choct D and P. A. Iji A EA School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
B Department of Animal Science, University of Peradeniya, Peradeniya 20400, Sri Lanka.
C School of Veterinary and Biomedical Sciences, James Cook University, Townsville, Qld 4811, Australia.
D Poultry CRC, PO Box U242 UNE, Armidale, NSW 2351, Australia.
E Corresponding author. Email: piji@une.edu.au
Animal Production Science 53(12) 1247-1259 https://doi.org/10.1071/AN12374
Submitted: 30 October 2012 Accepted: 8 July 2013 Published: 1 October 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
An experiment was conducted to determine the effects of two different water-soluble carbohydrate extracts (renga renga lily extract and Acacia extract), and two commercially available prebiotic compounds, Fibregum and Raftifeed-IPE, on the performance of broiler chickens subjected to a necrotic enteritis (NE) challenge model. These treatments were compared with negative control and a positive (Zn-bacitracin) control treatments. An overall 8.8% NE-related mortality was recorded, with mean jejunal and ileal lesion scores in dead birds ranging from 3.03 to 3.90 in all challenged groups except the positive control groups. NE-specific deaths or clinical abnormalities were not observed with unchallenged control and positive control groups. At 7 days post-challenge, the concentration of specific IgY antibodies against the α-toxin of Clostridium perfringens in the serum was lower (P < 0.05) in birds fed the positive control and Fibregum-supplemented diets than in the negative control group. However, birds fed Fibregum had increased (P < 0.05) IgM concentration compared with those fed Acacia extract and lily extract. The Fibregum-fed group also had higher (P < 0.05) IgA concentrations in serum than did the positive-control and lily extract-supplemented groups at 14 days but this effect did not persist to 21 days. The results from the present study demonstrated that supplementation with water-soluble carbohydrates from two plant sources was not effective in controlling NE. However, the prebiotic compound Fibregum was found to be having some immunomodulatory effects. Addition of Zn-bacitracin and monensin was highly effective in counteracting the negative effects of the disease challenge.
Introduction
Necrotic enteritis (NE) is an acute or chronic enterotoxaemia caused by Clostridium perfringens (van der Sluis 2003; Williams 2005). C. perfringens is a Gram-positive, obligate anaerobic, spore-forming bacterium readily found in soil, dust, used poultry litter and as a normal inhabitant of the gut microflora of healthy birds (Dahiya et al. 2006). Most often the only sign of an outbreak of clinical NE in broilers is a sudden increase in mortality which usually occurs between 2 and 5 weeks of age. The NE mortality rate within a flock is usually 2–10% but can be as high as 40–50% (Hofacre 2005). The proliferation of C. perfringens in the intestine and an increase in its toxin are considered the main cause of hemorrhagic necrosis of the intestinal mucosa (Dahiya et al. 2006). A subclinical form has been associated with reduced feed conversion efficiency and retarded growth rate in birds (Kaldhusdal and Hofshagen 1992). This disease has been reported in most areas of the world where broilers are produced under intensive management conditions, and it has a significant economic impact on the poultry industry (van der Sluis 2000).
A variety of in-feed antibiotics (IFAs), including virginiamycin, bacitracin, penicillin and tylosin, have been used in feed to effectively control and prevent NE (Watkins et al. 1997; Collier et al. 2003). However, many countries are moving towards a reduction in the use of IFAs in animal diets because large-scale use of antibiotics can cause resistant bacterial strains to develop (Barton 1998). As a consequence, the prophylactic use of most IFAs in poultry feed in Europe has been banned and there are increasing indications that other regions of the world will follow this trend (DANMAP 2008). However, this has resulted in problems such as increased mortality in poultry flocks, occurrence of ill-defined intestinal dysbacteriosis, a decline in bird welfare, an increase in the use of anticoccidial drugs and the incidence of food-borne human illness (Pattison 2002; Casewell et al. 2003). In the light of the situation where fewer, if any, antibiotics will be allowed in feeds in the future, it is important for the poultry industry to find alternative ways to control NE.
Several products have been investigated as potential alternatives to IFAs. For a product to be effective, it must simulate one or more of the mechanisms by which IFAs function. It is believed that immune stimulation in meat-type chickens is important because these birds have lower antibody responses and non-specific proliferative responses than do layer-type strains (Koenen et al. 2004). McReynolds et al. (2004) reported that immunosuppression increases the severity of NE in broilers. Therefore, increasing the immunomodulating capacity of broilers to respond effectively to the diversity of antigens during early life is important. Guo et al. (2004) observed that mushroom and herb extracts may have significant impacts on the inductive immune responses against Eimeria tenella infection in broilers, by enhancing both cellular and humoral immunity. Prebiotic compounds such as oligosaccharides may also act as immunomodulators at the intestinal level. Kudoh et al. (1999) reported that Immunoglobulin A (IgA) secretion from caecal mucosa was promoted by orally administered highly fermentable, indigestible saccharides. Kleessen et al. (2003) reported that fructans-rich Jerusalem artichoke syrup administered via drinking water resulted not only in a significant reduction in the numbers of C. perfringens in caecal chyme, but also decreased the levels of microbial endotoxins in the blood of broilers. However, there is very little information available in relation to the influence of most of these prebiotic and bioactive substances on immune responses in chickens challenged with C. perfringens or Eimeria spp. In view of the growing interest in investigating potential alternatives for IFAs in poultry feed, testing novel forms of prebiotic and bioactive compounds is of utmost importance.
Previous feeding experiments conducted using caged broilers indicated that inclusion of prebiotic plant extracts beneficially modulated the composition of the microflora in the ileum and caeca, by increasing the number of lactobacilli and reducing harmful bacteria, such as potentially pathogenic coliforms and C. perfringens (Vidanarachchi et al. 2006, 2010b). Hence, it was hypothesised in the present study that dietary supplementation with Acacia extract, renga renga lily extract and commercially available prebiotic compounds with chemical composition similar to the plant extracts, namely, Fibregum and Raftifeed-IPE, would exert prebiotic effects and, thereby, control NE in birds challenged with C. perfringens. The objectives of the present study were to evaluate the effects of these prebiotic compounds on production performance, gut microflora composition, gut morphology and humoral immune responses of broiler chickens subjected to a NE disease challenge model involving oral inoculation with C. perfringens.
Materials and methods
Birds and housing
One thousand and fifty day-old male, commercial broilers (Cobb), vaccinated against Marek’s disease, infectious bronchitis and Newcastle disease, were obtained from a local hatchery (Baiada hatchery, Kootingal, New South Wales (NSW), Australia). The research facility was thoroughly cleaned and disinfected before bird placement. One-day-old chicks were placed in 42 floor pens in a semi-commercial broiler shed located at the University of New England Kirby Research Station, Armidale, NSW, Australia. The pens were randomly assigned to seven treatments, each treatment being replicated six times. Each pen (1.44 m2) was stocked with 25 chicks. The pens were enclosed with metal plates at the sides. A space of at least 4 m was maintained between the challenged and unchallenged bank of pens to avoid cross-contamination. The shed temperature was set at 33−34°C during the first week and gradually decreased by 3°C per week until 24−25°C was reached by the third week. Relative humidity was between 65% and 70%. Chicks were subjected to artificial fluorescent illumination of 23 h from 1 to 21 days of age, and of 18 h from 22 to 35 days of age. Each pen was equipped with a separate feeding trough and water was supplied through nipple drinkers. Water and feed were provided ad libitum.
Experimental diets
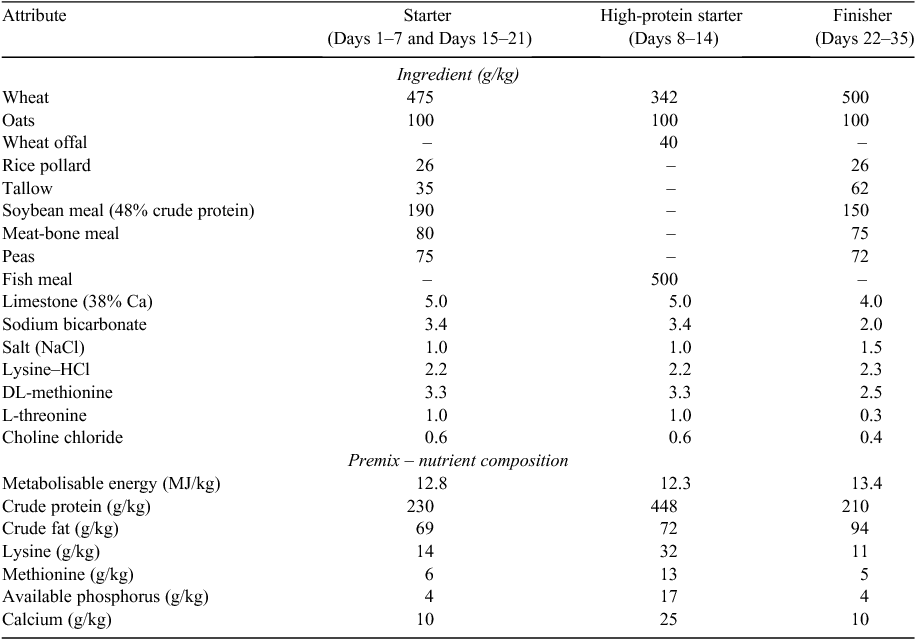
The composition of the basal diets is shown in Table 1. The seven treatment groups were (1) an unchallenged group fed basal diet (UC; unchallenged control), (2) a challenged group fed basal diet (NC; negative control), (3) a challenged group fed basal diet supplemented with antibiotic (PC; positive control; Zn-bacitracin, 45 mg/kg) and coccidiostat (monensin, 100 mg/kg), (4) a challenged group fed basal diet supplemented with 10 g/kg water-soluble carbohydrates (WSCs) from golden wattle (Acacia pycnantha) exudate (Acacia extract, 76% (w/w) arabinogalactans), (5) a challenged group fed basal diet supplemented with 10 g/kg prebiotic arabinogalactan product Fibregum (Fibregum, Colloїdes Naturels International, Rouen Cedex, France), (6) a challenged group fed basal diet supplemented with 10 g/kg water-soluble carbohydrates from renga renga lily (Arthropodium cirratum) rhizomes (lily extract, 65% (w/w) inulins) and (7) a challenged group fed basal diet supplemented with 10 g/kg prebiotic inulin compound, Raftifeed-IPE (Raftifeed, Orafti Active Food Ingredients, Tienen, Belgium). The extraction and characteristics of WSCs from the two plant sources was described previously (Vidanarachchi et al. 2009). The plant extracts and prebiotic products were included as substitutes for wheat, and all supplements were added to the respective diets for the entire experimental period at the inclusion rates indicated above. Raftifeed-IPE contains mainly inulin (>700 g/kg fructo-oligosaccharides) extracted from chicory root and some glucose, fructose and sucrose (100 g/kg). The average chain length (degree of polymerisation) of the inulin in Raftifeed-IPE is ~2–60. Fibregum is a naturally occurring arabinogalactan extracted from the exudate of Acacia senegal. The Fibregum contains >900 g/kg arabinogalactans.
The basal diets were prepared commercially (Ridley Agriproducts, Tamworth, NSW, Australia) with the same batch of ingredients and bulk-shipped to University of New England. On arrival, the various supplements were properly mixed in according to the treatments, and then cold-pelleted (52−63°C).
Necrotic enteritis challenge
From a day after hatching until 7 days of age, the birds were fed with starter diets. From Day 8 to Day 14 before inoculation with C. perfringens, the birds were fed a high-protein diet based on 50% (w/w) fish meal (with the full dose of supplements). After Day 14, the starter diets were returned until Day 21. The starter feed was replaced by the finisher feed on Day 22 and birds were kept until Day 35. On Day 9, all the birds, except those in the UCs, were given, per os, a suspension of 2500 oocysts of Eimeria acervulina, E. maxima and E. tenella in 1 mL of phosphate buffered saline (PBS). Unchallenged birds received sterile PBS. The attenuated Eimeria isolates originated from commercial broiler farms and were obtained from Bioproperties, Glenorie, NSW, Australia. The three species of Eimeria had been purified by serial passages through 3-week-old Eimeria-free chickens, and the sporulated oocysts were stored in 2% (w/v) potassium dichromate at 10°C before inoculation.
A primary poultry isolate of C. perfringens Type A was obtained from the CSIRO laboratory, Geelong, Victoria, Australia, and maintained in thioglycollate broth (USP alternative, Oxoid, Thermo Fisher Scientific Australia Pty Ltd, Adelaide, Australia) with 30% (v/v) glycerol at −20°C. The challenge inocula were prepared fresh by growing the bacterium overnight at 39°C in 1000 mL of thioglycollate broth with added starch (10 g/L) and casitone (5 g/L). The stock culture of C. perfringens had been previously subcultured in cooked meat media (Oxoid, CM81) and thioglycollate broth. On Days 14, 15 and 16, birds in challenged groups were individually inoculated per os with 1 mL of C. perfringens grown in thioglycollate broth at a concentration of 3.5 × 108 colony forming units (CFU)/mL, and birds in unchallenged cages were gavaged with 1 mL of sterile thioglycollate broth. Unchallenged birds were serviced first to lessen the likelihood of cross contamination and feed was withdrawn from all pens for 3 h on all days, before commencement of inoculation.
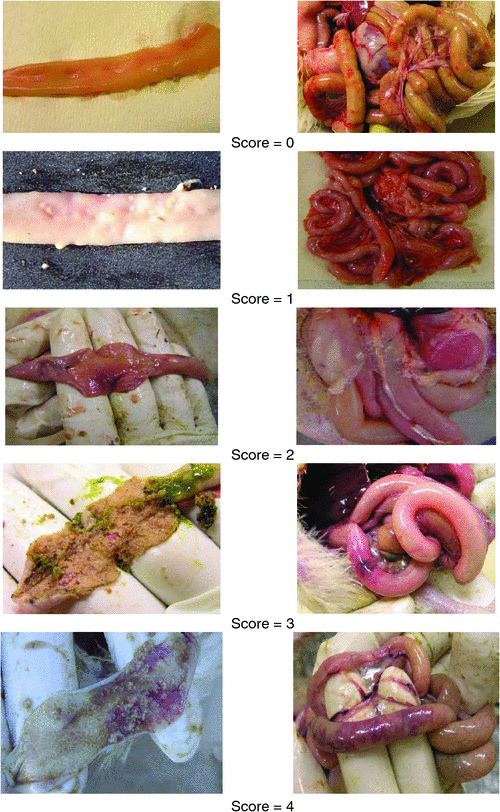
A gross pathologic diagnosis of NE in all dead birds and sampled birds was based on the presence of intestinal lesions typical of naturally occurring and experimentally induced NE, as described by Prescott et al. (1978) and Broussard et al. (1986). The small intestine from each bird was incised longitudinally and examined for evidence of gross necrotic lesions. Small intestinal lesions were scored according to the criteria of Prescott et al. (1978) with slight modifications as illustrated in Fig. 1. All birds were examined twice daily and all dead chickens were immediately collected for postmortem analysis.
|
Growth performance, collection and processing of intestinal samples
Weekly weight gain and feed intake per pen were measured and feed conversion ratio (FCR) was adjusted for mortality, calculated on a pen basis. Birds were observed twice daily for general health. All dead birds and culls (due to unhealthy condition) were weighed and necropsied.
On Days 14 and 21, 12 chickens per treatment were randomly selected for necropsy. The intestines were removed aseptically. To synchronise the feeding pattern of the birds, light was switched off for 2 h, followed by at least 1 h of light before the chickens were sacrificed. Subsequently, the chest cavity and the abdomen were opened and the small intestine was ligated and removed from the bird. The contents of the ileum and caeca were collected by gently finger-stripping the respective intestinal segments into plastic containers. About 0.2 g of ileal and caecal contents were suspended in 0.8 mL of distilled water, and the pH was measured with a glass pH electrode (EcoScan 5/6 pH meter, Eutech Instruments, Singapore) corrected for temperature. The remaining ileal and caecal digesta samples were frozen immediately after collection so as to measure short-chain fatty acid (SCFA) contents (only on Day 21). For histomorphological analysis, ~2.5 cm of the middle portion of the ileum was excised, flushed with PBS buffer (pH 7.6) and fixed in 10% (v/v) neutral buffered formalin. The bursa of Fabricius and spleen were removed from 12 birds of each treatment at Days 14 and 21 and weighed to the nearest gram. The data were expressed as a percentage of bodyweight.
Enumeration of bacteria and gut histomorphology
Samples in pre-reduced salt medium were homogenised for 2 min in CO2-flushed plastic bags using a MiniMix bag mixer (Interscience, St Nom, France) and serially diluted in 10-fold increments in pre-reduced salt medium according to the technique of Miller and Wolin (1974). An aliquot (100 μL) was plated on the following agar media. Total anaerobic bacteria were enumerated on Wilkins–Chalgren anaerobic agar (Oxoid, CM0619) after incubation at 39°C for 7 days in an anaerobic cabinet (Model SJ-3, Kaltec, Edwardstown, SA, Australia). Lactobacilli were enumerated on Rogosa agar (Oxoid, CM0627) after anaerobic incubation at 39°C for 48 h in anaerobic jars (Oxoid) with an anaerobic environment (<1% O2 and 9–13% CO2), generated using anaerobic AnaeroGen sachets (AN0025A, Oxoid). Coliform bacteria and lactose-negative enterobacteria were counted on MacConkey agar (Oxoid, CM0115) after aerobic incubation at 39°C for 24 h. The population of C. perfringens was determined on tryptose–sulfite–cycloserine and Shahidi–Ferguson perfringens agar base (TSC & SFP; Oxoid, CM0587) mixed with egg yolk emulsion (Oxoid, SR0047) and perfringens-selective (TSC) supplement (Oxoid, SR0088E) according to the pour-plate technique, where plates were overlaid with the same agar after spreading the inoculum. Bacterial numbers were expressed as log10 CFU/g digesta. The morphology of the ileal tissues was determined as described by Vidanarachchi et al. (2010a).
Measurement of short-chain fatty acids and organic acids
To measure the concentration of SCFAs, lactic and succinic acids, ~2.0 g of thawed ileal and caecal sample were suspended in 1.0 mL of 0.02 M-2-ethylbutyric acid and thoroughly mixed by using a vortex mixer, followed by centrifugation at 25 700g at 4°C for 15 min. To a sample of 1 mL supernatant fraction, 0.5 mL of concentrated HCl and 2 mL of diethyl ether were added and thoroughly mixed by using a vortex mixer, followed by centrifugation at 2060g at 4°C for 15 min. Aliquots (360 μL) from the ether phase were recovered and mixed with 40 μL N-methyl-N-tert-butyldimethylsilyltrifluoroacetamide for derivatisation of organic acids. The concentration of derivatised organic acids was quantified using a Varian CP 3400 CX gas chromatograph (Varian Analytical Instruments, Palo Alto, CA, USA).
Enzyme-linked immunosorbent assay (ELISA)
Total serum antibody concentrations
Total antibody concentrations of IgY, IgM and IgA in serum were determined before C. perfringens challenge (14 days), and at 7 days after first challenge (21 days) using a sandwich ELISA (Bethyl Laboratories, Montgomery, TX, USA). Blood samples were collected from the jugular vein into 7-mL serum tubes and clotted at room temperature (RT; 25°C) for 2 h, and serum was separated from the cells by centrifugation at 2300g for 5 min and stored at −20°C.
Specific IgY antibodies against C. perfringens α-toxin
The specific IgY antibodies against the α-toxin of C. perfringens in blood serum were determined as described by Heier et al. (2001), with the following modifications: briefly, the microtitre plates were coated with 1 μg of phospholipase C Type I (7633; Sigma-Aldrich, St Louis, MO, USA) in 100 μL of carbonate buffer, pH 9.6 for 1 h at RT, washed three times with phosphate-buffered saline at pH 7.4 with 0.05% Tween 20 (PBST) and blocked with 200 μL of 1% bovine serum albumin in PBST for 1 h at RT. After washing as above, the plates were incubated with 100 μL of serum diluted to 1 : 250 in PBST, washed and incubated with 100 μL horseradish peroxidase-labelled goat anti-chicken IgY (Bethyl Laboratories, Montgomery, TX, USA) diluted to 1 : 200 in 1% bovine serum albumin in PBST for 1 h at RT. After washing, the colour reaction was developed with 100 μL of 3,3′,5,5′-tetramethylbenzidine. The reactions were stopped by adding 100 μL of 2 M H3PO4 and the absorbance was read at 450 nm using an ELISA microplate reader (Model 680, Bio-Rad Laboratories, Hercules, CA, USA). The samples were analysed in duplicate and the results were expressed as average optical density values.
Statistical analyses
Bacterial counts were transformed to log10 values, and molar proportions of SCFA and organic acids were subjected to arcsine transformation before statistical analysis; SCFA data are presented as natural numbers (Steel and Torrie 1981). Each variable (except mortality data and lesion scores) was analysed as a completely randomised design, with a pen of broilers composing an experimental unit. The experiment consisted of seven dietary treatments and data were analysed according to the following model:
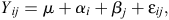
where Yij = observed dependent variable, μ = overall mean, αi = fixed effect of treatment, i = 1, 2, 3, 4, 5, 6, 7, βj = random effect of replicates, j = 1, 2, 3, 4, 5, 6, εij = residual error for Treatment i of Replicate j ~N (0, σε2).
All possible interactions were tested for significance (at P = 0.05) and were eliminated from the models because they were not significant. The errors were assumed to be independently and normally distributed, with a mean of zero and variance of σε2. All data were analysed by using ANOVA option of PROC MIXED procedure (SAS Institute 2000), with treatment as the fixed effect and replicate as the random effect. Treatment least-squares means were compared using predetermined contrasts and considered significant at P = 0.05. Results are reported as least-squares means (n = 6) and pooled standard error (s.e.). The mortality data were compared using a chi-square test.
Animal ethics
All experimental procedures were approved by the University of New England Animal Care and Ethics Committee and throughout the experiments, health and husbandry practices complied with the Code of Practice for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council 2004), for the Commonwealth of Australia and the Australian Model Code of Practice for the Welfare of Animals: Domestic Poultry (Primary Industries Standing Committee 2002).
Results
Bird performance
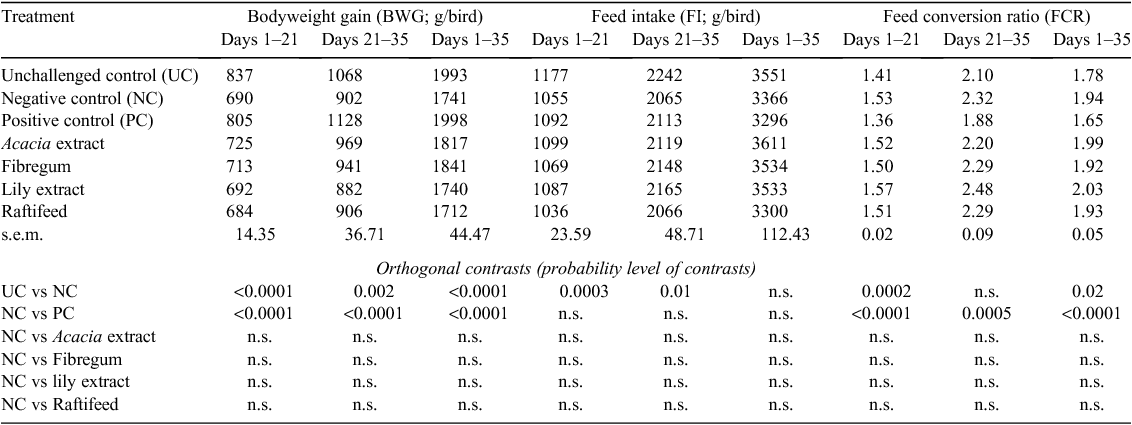
The mean bodyweight gain (BWG), feed intake (FI) and FCR of chickens fed the experimental diets are shown in Table 2. There were no differences in growth performance between plant extract- or prebiotic-supplemented groups challenged with C. perfringens and the NC group during the entire experimental period. The BWG of challenged chickens on the control diet and plant extract (prebiotic) diets was markedly reduced compared with the UC group. This effect was most pronounced in the first 3 weeks. At the end of the 5-week period, BWG was decreased by 14% (P < 0.0001) in the NC group compared with the UC group. Over the same period, FCR was impaired by 16 points due to C. perfringens challenge in the NC group. The FCR of the UC group was similar to that of broilers fed the PC diet during the 4th week of experiment. Over the whole experimental period, there were no treatment effects on cumulative feed intake of birds. However, FI of challenged birds during the first 3 weeks was reduced (P < 0.0003) compared with that of the UC group. The addition of Zn-bacitracin and monensin increased (P < 0.05) average BWG and improved FCR throughout the experimental period. At 21 and 35 days, broilers on the PC diet were 17% and 15%, respectively, heavier than chickens from the NC group.
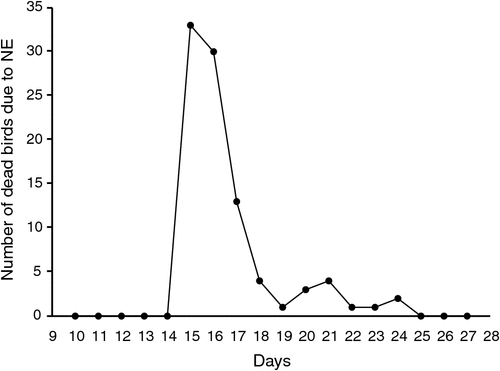
Mortality resulting from NE started at 1 day after birds were first challenged with C. perfringens (Fig. 2). Total NE-associated mortalities of 8.8% were observed for the whole experimental period. NE-associated mortalities occurred during the first 4 days and continued for a maximum of 7 days after the first challenge. NE-specific deaths or clinical abnormalities were not observed with UC and PC groups. The mean lesion scores in the jejunum in the challenged NC, Acacia extract, Fibregum, lily extract and Raftifeed groups were 3.18, 3.30, 3.34, 3.03 and 3.31, respectively. Corresponding values for the ileum were 3.18, 3.90, 3.36, 3.24 and 3.06, while mortality was 13.8%, 18.2%, 7.5%, 12.3% and 10.8%. Lesion scores were absent when the birds were sampled 7 days post-challenge.
|
Gut histomorphology
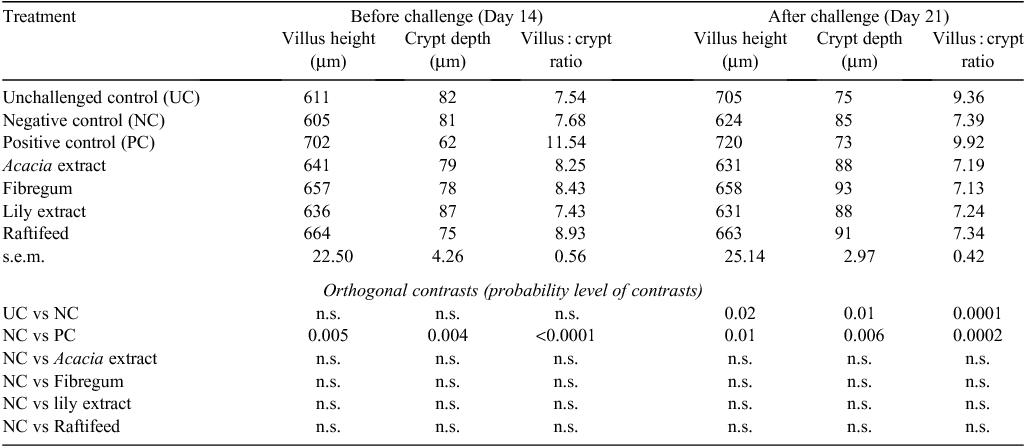
Supplementation of the diets with plant extracts or prebiotic compounds had no effect on villus height or crypt depth of the ileum compared with the NC group on either sampling day (Table 3). At 14 and 21 days, the birds fed the PC diet had longer villi, shallower crypts and greater villus : crypt ratio in the ileum than did those in the NC group. However, there was no difference (P > 0.05) in villus height of all prebiotic- and plant extract-supplemented groups compared with the PC group before C. perfringens challenge. In the current study, the villus height and crypt depth of UC birds and NC birds were different (P < 0.05) on Day 21 (after challenge). The histomorphological analysis of ileal tissues on Day 21 also revealed that detachment and disruption of the normal apical microvilli of enterocytes, which is characteristic of NE, was observed in two regions of the gut.
Ileal and caecal microflora
The results of the bacterial counts in ileal and caecal contents are shown in Tables 4 and 5. In general, coliforms, lactose-negative enterobacteria and C. perfringens counts were higher in both ileal and caecal contents at 14 days (before challenge) than at 21 days. Total anaerobic counts in caecal digesta were decreased (P < 0.05) by ~0.5 log units in birds fed PC versus NC, on Day 14. Compared with the NC group, no difference (P > 0.05) was detected among various dietary treatment groups with respect to total anaerobic bacterial numbers in ileal and caecal contents on Day 21 (Table 5).
The Lactobacillus counts in ileal and caecal digesta on Day 14 tended to be higher in Acacia extract-, lily extract- and Raftifeed-supplemented treatment groups than in the NC group. Birds fed with the same supplements had higher (P < 0.05) Lactobacillus counts in ileal digesta than those of the NC group on Day 21. Furthermore, birds supplemented with Acacia extract had higher (P < 0.05) counts of lactobacilli in caecal digesta on Day 21 than did the birds in the NC group. The counts of lactobacilli decreased by 9.5- and 8.3-fold in the ileal digesta of PC versus NC groups at 14 days and 21 days, respectively.
Compared with the NC group, ileal and caecal lactose-negative enterobacteria numbers were not different between dietary treatment groups. Both Acacia- and Fibregum-fed groups had lower (P < 0.05) coliform numbers in ileal and caecal digesta on Day 14. Neither the ileal nor caecal coliform counts at 21 days were affected by dietary supplementation of plant extracts or prebiotic compounds.
Clostridium perfringens counts in ileal and caecal digesta in all treatment groups, except in the PC group, before C. perfringens-challenge were higher than those after C. perfringens challenge. The mean C. perfringens count in the ileum decreased from 8.96 to 6.56 log10 cfu/g of digesta in all treatment groups 7 days after the first C. perfringens challenge. Corresponding values for caecal contents decreased from 8.02 to 6.46 log10 cfu/g of digesta. Fibregum decreased (P < 0.05) the C. perfringens numbers in caecal contents before C. perfringens challenge but this effect was not significant after C. perfringens challenge, although the values tended to be lower in that group than in the NC group. Raftifeed and the two plant extracts did not significantly (P > 0.05) affect C. perfringens populations before and after C. perfringens challenge. The C. perfringens counts in luminal contents from the ileum and caeca of birds fed the PC diet were significantly (P < 0.001) lower than those from the NC groups at both sampling days.
Organic acids and pH
There were no major effects of treatments on ileal and caecal pH, concentration of total organic acids and molar proportions of acetic and lactic acids assessed at 21 days of age (data not shown). However, the pH (P < 0.002) and molar proportion of acetic acid (P < 0.008) in the ileal digesta of birds on the PC diet were higher than those on the NC diet. Conversely, birds on the NC diet had a higher (P < 0.01) content of lactic acid in the ileal digesta than those on the PC diet. Similarly, the pH of the caecal digesta of birds on the NC diet was higher than that of birds on the Acacia extract (P < 0.0001) and Raftifeed (P < 0.007).
Lymphoid organ weights and humoral immune responses
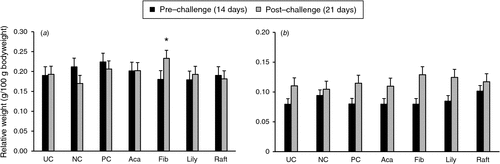
There were no differences in relative weights of spleen or bursa as a result of supplementation by either of the two plant extracts or of Raftifeed on either sampling day (before and after challenge; Fig. 3). However, the relative weight of bursa of birds supplemented with Fibregum was higher (P < 0.05) than that of the NC group on Day 21.
|
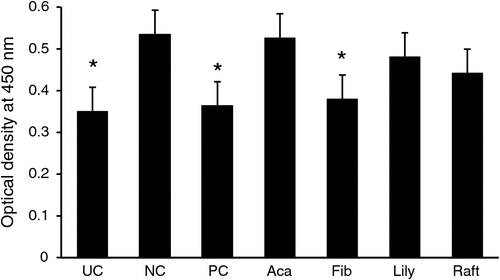
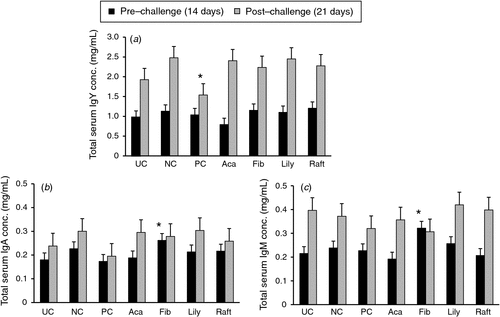
Figure 4 shows the serum optical-density values of the specific serum IgY antibodies against α-toxin (Phospholipase C) analysed by ELISA technique. At 7 days post-challenge with C. perfringens, the concentration of the specific IgY antibodies against the α-toxin of C. perfringens in the serum, as represented in optical-density values (Fig. 4), was significantly (P < 0.05) lower in birds fed with UC, PC and Fibregum diets than was the concentration from the NC group. The total antibody responses to C. perfringens before and after challenge are shown in Fig. 5. The serum IgY response was not different among treatments before challenge. However, birds fed Fibregum had a higher IgM response than did birds in the Acacia extract-supplemented (P < 0.05) and lily extract-supplemented (P < 0.05) groups. The Fibregum-fed group also had higher (P < 0.05) concentrations of IgA in serum than did the PC and lily extract-supplemented groups at 14 days, but this effect did not persist at 21 days. The concentration of IgY antibody titres in sera from the PC group was lower (P < 0.05) than that in the NC group on Day 21.
|
|
Discussion
Bird performance
In the current study, except in the PC group, there was a significant decline in the growth performance of birds challenged with C. perfringens in all treatment groups compared with unchallenged birds. The impaired FCR observed with all C. perfringens-challenged groups can be explained by decreased FI and BWG in the first 3 weeks of the period. Hofacre et al. (1998) and Dahiya et al. (2005) also reported decreased BWG and impaired feed conversion in broiler chickens challenged with C. perfringens. None of the test plant extracts or prebiotic products prevented the outbreak of NE or improved growth in a NE challenge model. These results are in agreement with the findings of Hofacre et al. (2003) who found no effect of fructo-oligosaccharide on growth performance or reduction in lesion scores in C. perfringens-challenged boilers. In contrast, Butel et al. (2001) reported that C. perfringens-induced necrotising enterocolitis in gnotobioic quails could be controlled by dietary supplementation with oligofructose.
The Zn-bacitracin and monensin supplementation successfully prevented an outbreak of NE, while mortality due to NE occurred in spite of all the other supplements. Birds on Fibregum showed lower mortality from NE than those fed the Acacia extract, which had the highest mortality due to NE. High mortality of birds at 24 h post-challenge suggested that the experimental disease had a rapid onset in challenged animals and continued for a maximum of 7 days after the first challenge began. The lack of confluent necrosis and sloughing of the epithelial lining of the jejunum and ileum among the treatment groups was most likely influenced by the very low mortality among the challenged treatment groups 7 days post-challenge.
Gut histomorphology
Our initial hypothesis was that plant extracts and prebiotic compounds in broiler diets challenged with C. perfringens would preserve the villus structure and villus length. However, the results showed that villus length was not affected by the plant extracts or prebiotic compounds. Dietary supplements such as prebiotic and bioactive compounds have been shown to have a trophic effect on the gut mucosal morphology in chickens (Iji et al. 2001; Yamauchi et al. 2006). It should be noted that these prior studies assessed compounds under hygienic husbandry practices and not in a disease-challenged condition as was the case of the present study. However, fructo-oligosaccharides have been observed to alleviate salmonella-induced necrosis of the caecal mucosa and enhance the ileal micro-villus length of broilers (Choi et al. 1994).
In the current study, birds fed with the PC diet had longer villi and shallower crypts in the ileum, thus increasing the villus : crypt depth ratio, both before and after challenge. Supplementation of broiler diets with Zn-bacitracin and monensin effectively reduced the numbers of C. perfringens in the ileal and caecal contents during the experimental period. Hence, the damage that would have been caused to the intestinal mucosa by NE may have been prevented. Such morphological changes in Zn-bacitracin- and monensin-fed birds may also be responsible for the improved growth performance as seen in the current study in the disease-challenged groups. Antibiotic-treated farm animals are known to have longer villi and villus : crypt depth ratios in the small intestine than are their antibiotic-free counterparts (Nousiainen 1991; Ao 2004).
Ileal and caecal microflora
Although high C. perfringens counts (6.5–7.0 log10 cfu/g) were observed in ileal and caecal digesta, focal necrotic lesions in the jejunum and ileum were not observed 7 days post-challenge. This demonstrated that a high population count of C. perfringens may not always be associated with subclinical or clinical NE due to the fact that it is a complex, multifactorial disease with many unknown factors (Kaldhusdal et al. 1999; van Immerseel et al. 2004). Another explanation could be the TSC & SFP agar medium used in the present study may also have supported the growth of physiologically similar clostridia species resembling C. perfringens, as suggested by Adams and Mead (1980).
In the present study, dietary supplementation with two plant extracts (Acacia extract and lily extract) and the prebiotic product Raftifeed significantly increased or tended to increase the number of lactobacilli in the ileum at both sampling periods. These results are consistent with other work performed with plant extracts (Vidanarachchi et al. 2010b). Several mechanisms may account for the enhanced growth of lactobacilli in response to dietary supplementation with plant extracts. Certain dietary carbohydrates such as fructo-oligosaccharides and arabinogalactans are not digested in the small intestine, since the chicken lacks the enzymes to hydrolyse them (Xu et al. 2003; Saeed et al. 2011). Therefore, these carbohydrates are more likely to be degraded by the gastrointestinal microflora, which they selectively stimulate. Al-Tamimi et al. (2006) found that fermentation of arabino-oligosaccharides in a batch-culture fermentation system increased the lactobacilli and bifidobacteria counts, while decreasing C. perfringens numbers.
In the present study, feeding of Acacia extract or prebiotic compound Raftifeed caused reduction in coliform counts in the ileal and caecal digesta on Day 14, before C. perfringens challenge. The Lactobacillus counts in the same groups were significantly higher or tended to be higher than in the NC group. Many studies have demonstrated a relationship between an increase in numbers of lactobacilli and decline in numbers of coliforms in chickens (Watkins et al. 1982; Jamroz et al. 2005).
As expected, the present study demonstrated that supplementation of broiler diets with Zn-bacitracin and monensin significantly reduced the C. perfringens counts in ileal and caecal digesta, with a consequent significant improvement in growth performance and pathophysiological indices of NE. The counts of C. perfringens in luminal contents from the ileum and caeca of birds fed the PC diet were as low as 3–4 log10 cfu/g digesta on both sampling days, indicating that the IFAs used in the present study were highly effective in controlling C. perfringens proliferation in the gut of broilers. These findings are consistent with previous studies of the culture-based and molecular studies, which demonstrated that dietary supplementation with antibiotics such as Zn-bacitracin, bacitracin methylene disalicylate and tylosin causes a significant reduction in lesion scores and mortality caused by NE (Brennan et al. 2003; Collier et al. 2003).
Organic acids and pH
The supplementation of broiler diets with Zn-bacitracin and monensin in the present study significantly increased or tended to increase pH of ileal and caecal digesta. Correspondingly, the same treatment group had the lowest total organic acids and molar proportion of lactate in the ileal digesta. Since the PC group had significantly lower Lactobacillus counts, compared with the challenged control group (NC), it can be speculated that low microbial fermentation activities in the ileal digesta resulted in less volatile fatty acid production. Engberg et al. (2000) also observed higher pH and lower lactate concentrations in ileal digesta of broilers fed diets supplemented with salinomycin alone or salinomycin and Zn-bacitracin. The present results also confirmed those of Engberg et al. (2002) who found that lactate and acetate are the predominant organic acids produced due to bacterial fermentation in the ileum.
Lymphoid organ weights and humoral immune responses
The increase in the relative weight of the bursa by Fibregum treatment could be associated with an improvement in immune responses in broilers after C. perfringens challenge. The results of the present study are in agreement with the findings of Kleessen et al. (2003) who observed an increase in the relative weight of bursa of broilers in response to the consumption of Jerusalem artichoke fructans syrup (0.5%) administered via drinking water. Chen et al. (2003) noted a significantly larger bursa and greater immunomodulatory effect in broilers fed diets supplemented with two Chinese herbal polysaccharides, astragalan and achyranthan.
Birds from the UC, PC and Fibregum-supplemented treatment groups had lower serum concentrations of IgY antibody against C. perfringens α-toxin and less mortality due to C. perfringens-associated NE. This indicated that antigenic stimulation was lower in these three groups. In agreement with this, C. perfringens counts in ileal and caecal digesta from the same three groups were either significantly lower or tended to be lower than those of the other C. perfringens-challenged treatment groups. These immunological findings are in accordance with those of Løvland et al. (2003), who observed a higher level of IgY antibody against C. perfringens α-toxin in the serum of broilers with a subclinical form of NE or C. perfringens-associated hepatitis. Detection of IgY antibody against C. perfringens α-toxin in birds in the UC group may be due to a natural exposure of these birds to C. perfringens from the pen environment. The same group also had C. perfringens counts between 6.00 and 6.44 log CFU/g digesta in both ileal and caecal contents 7 days post-challenge. Løvland et al. (2003) also suggested that birds may become seropositive without exhibiting any C. perfringens-associated enteric gut lesions.
Results from the current study showed that Fibregum elevates serum IgM and IgA concentrations and enhances systemic immune capacity in chickens. The exact reason for this observation is not clear and further work on the determination of cell-mediated immune responses such as the proportions of different subpopulations of B and T lymphocytes is warranted. Elevated concentrations of serum IgA have been shown to correlate well with higher secretory IgA in the intestine (Brito et al. 1993) and may explain the mechanism of C. perfringens reduction from the intestinal lumen in the Fibregum-fed group. In conclusion, the results of the study suggested that neither the plant extracts nor the commercial prebiotic product were effective in controlling NE. The present study also showed no effect of dietary plant extracts on humoral immunomodulation in broiler chickens challenged with C. perfringens. Although some beneficial effects were observed with Fibregum, the product did not improve performance parameters, as BWG and FCR were similar to those for the NC. Under the conditions of the present study, Fibregum was effective in reducing NE-associated mortalities and in improving some immune response to the NE infection in broiler chickens. Dietary supplementation with Zn-bacitracin and monensin was highly effective in counteracting the negative effects of the disease challenge.
Acknowledgement
This work was conducted within the Poultry CRC, established and supported under the Australian Government’s Cooperative Research Centres Program.
References
Adams BW, Mead GC (1980) Comparison of media and methods for counting Clostridium perfringens in poultry meat and further-processed products. The Journal of Hygiene 84, 151–158.| Comparison of media and methods for counting Clostridium perfringens in poultry meat and further-processed products.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3c%2FpsFCjsQ%3D%3D&md5=86b7fc1d1ac5981f670fcca8c87819a3CAS | 6243326PubMed |
Al-Tamimi MAHM, Palframan RJ, Cooper JM, Gibson GR, Rastall RA (2006) In vitro fermentation of sugar beet arabinan and arabino-oligosaccharides by the human gut microflora. Journal of Applied Microbiology 100, 407–414.
| In vitro fermentation of sugar beet arabinan and arabino-oligosaccharides by the human gut microflora.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsFaqsro%3D&md5=b6d112889f4a6ed3dce146f2acb0fe6eCAS |
Ao Z (2004) Diet and early nutrition: their effects on immunity and gut development in broiler chickens. PhD Thesis, University of New England, Armidale, NSW.
Barton MD (1998) Does the use of antibiotics in animals affect human health? Australian Veterinary Journal 76, 177–180.
| Does the use of antibiotics in animals affect human health?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3jvF2jsw%3D%3D&md5=da9bb79f00adafa256ed1496b4c77de7CAS | 9578753PubMed |
Brennan J, Skinner J, Barnum DA, Wilson J (2003) The efficacy of bacitracin methylene disalicylate when fed in combination with narasin in the management of necrotic enteritis in broiler chickens. Poultry Science 82, 360–363.
Brito JRF, Hinton M, Stokes CR, Pearson GR (1993) The humoral and cell mediated immune response of young chicks to Salmonella typhimurium and S. kendougou. The British Veterinary Journal 149, 225–234.
| The humoral and cell mediated immune response of young chicks to Salmonella typhimurium and S. kendougou.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3szislWmtw%3D%3D&md5=06969b0da1e0d082c7d5667a87c81cf8CAS |
Broussard CT, Hofacre CL, Page RK, Fletcher OJ (1986) Necrotic enteritis in cage-reared commercial layer pullets. Avian Diseases 30, 617–619.
| Necrotic enteritis in cage-reared commercial layer pullets.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s%2FisFClsw%3D%3D&md5=7d4ede9270d17378f08919f544f7a77fCAS | 2876698PubMed |
Butel MJ, Catala I, Waligora-Dupriet AJ, Taper H, Tessedre AC, Durao J, Szylit O (2001) Protective effect of dietary oligofructose against cecitis induced by clostridia in gnotobiotic quails. Microbial Ecology in Health and Disease 13, 166–172.
| Protective effect of dietary oligofructose against cecitis induced by clostridia in gnotobiotic quails.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXns1GktLc%3D&md5=5723c069f65d69b943a93acc5333e1c3CAS |
Casewell M, Friis C, Granell EM, Mcmullin P, Phillips I (2003) The European ban on growth-promoting antibiotics and its consequences for human and animal health. The Journal of Antimicrobial Chemotherapy 52, 159–161.
| The European ban on growth-promoting antibiotics and its consequences for human and animal health.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmtFKku7Y%3D&md5=d2c5c81142f696f778846051906848e0CAS | 12837737PubMed |
Chen HL, Li DF, Chang BY, Gong LM, Dai JG, Yi GF (2003) Effects of Chinese herbal polysaccharides on the immunity and growth performance of young broilers. Poultry Science 82, 364–370.
Choi KH, Namkung H, Paik IK (1994) Effects of dietary fructooligosaccharides on the suppression of intestinal colonization of Salmonella typhimurium in broiler chickens. Korean Journal of Animal Science 36, 271–284.
Collier CT, van der Klis JD, Deplancke B, Anderson DB, Gaskins HR (2003) Effects of tylosin on bacterial mucolysis, Clostridium perfringens colonization, and intestinal barrier function in a chick model of necrotic enteritis. Antimicrobial Agents and Chemotherapy 47, 3311–3317.
| Effects of tylosin on bacterial mucolysis, Clostridium perfringens colonization, and intestinal barrier function in a chick model of necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnvVWmtL8%3D&md5=88c79d0e72aeb3ca6ab74bac733719f2CAS | 14506046PubMed |
Dahiya JP, Hoehler D, Wilkie DC, van Kessel A, Drew MD (2005) Dietary glycine concentration affects intestinal Clostridium perfringens and lactobcilli populations in broiler chickens. Poultry Science 84, 1875–1885.
Dahiya JP, Wilkie DC, van Kessel AG, Drew MD (2006) Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Animal Feed Science and Technology 129, 60–88.
| Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era.Crossref | GoogleScholarGoogle Scholar |
DANMAP (2008) Danish Integrated Antimicrobial Resistance Monitoring and Research Programme: use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark. Available at www.danmap.org [Accessed September 2010]
Engberg RM, Hedemann MS, Leser TD, Jensen BB (2000) Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poultry Science 79, 1311–1319.
Engberg RM, Hedemann MS, Jensen BB (2002) The influence of grinding and pelleting of feed on the microbial composition and activity in the digestive tract of broiler chickens. British Poultry Science 43, 569–579.
| The influence of grinding and pelleting of feed on the microbial composition and activity in the digestive tract of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38ngvFeisw%3D%3D&md5=4a33cc66adaeeca07fb70cd04de92447CAS | 12365514PubMed |
Guo FC, Kwakkel RP, Williams BA, Parmentier HK, Li WK, Yang ZQ, Verstegen MWA (2004) Effects of mushroom and herb polysaccharides on cellular and humoral immune responses of Eimeria tenella-infected chickens. Poultry Science 83, 1124–1132.
Heier BT, Lovland A, Soleim KB, Kaldhusdal M, Jarp J (2001) A field study of naturally occurring specific antibodies against Clostridium perfringens alpha toxin in Norwegian broiler flocks. Avian Diseases 45, 724–732.
| A field study of naturally occurring specific antibodies against Clostridium perfringens alpha toxin in Norwegian broiler flocks.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MritFenuw%3D%3D&md5=78f47d78ff49ed1d2479a5cdb7512906CAS | 11569751PubMed |
Hofacre CL (2005) Natural alternatives to prevent necrotic enteritis. International Poultry Production 13, 7–9.
Hofacre CL, Froyman R, George B, Goodwin MA, Brown J (1998) Use of aviguard, virginiamycin, or bacitracin MD against Clostridium perfringens-associated necrotizing enteritis. Journal of Applied Poultry Research 7, 412–418.
Hofacre CL, Beacorn T, Collett S, Mathis G (2003) Using competitive exclusion, mannan-oligosaccharide and other intestinal products to control necrotic enteritis. Journal of Applied Poultry Research 12, 60–64.
Iji PA, Saki AA, Tivey DR (2001) Intestinal structure and function of broiler chickens on diets supplemented with a mannan oligosaccharide. Journal of the Science of Food and Agriculture 81, 1186–1192.
| Intestinal structure and function of broiler chickens on diets supplemented with a mannan oligosaccharide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXms1SksLs%3D&md5=704ef3f91201ff3c61a7b46bd6404c60CAS |
Jamroz D, Wiliczkiewicz A, Wertelecki T, Orda J, Skorupinska J (2005) Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. British Poultry Science 46, 485–493.
| Use of active substances of plant origin in chicken diets based on maize and locally grown cereals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2MrovFeqtA%3D%3D&md5=2a9fe77cc5f3641bdd856df5150afa0aCAS | 16268107PubMed |
Kaldhusdal M, Hofshagen M (1992) Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poultry Science 71, 1145–1153.
| Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38zltVOgtQ%3D%3D&md5=dbc3ceb6f252b82826e8b6416ddb6065CAS | 1641378PubMed |
Kaldhusdal M, Hofshagen M, Lovland A, Langstrand H, Redhead K (1999) Necrotic enteritis challenge models with broiler chickens raised on litter: evaluation of preconditions, Clostridium perfringens strains and outcome variables. FEMS Immunology and Medical Microbiology 24, 337–343.
| Necrotic enteritis challenge models with broiler chickens raised on litter: evaluation of preconditions, Clostridium perfringens strains and outcome variables.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjs1SitLg%3D&md5=dace8c59c483b4084167a0a468fed257CAS | 10397320PubMed |
Kleessen B, Elsayed NAAE, Loehren U, Schroedl W, Krueger M (2003) Jerusalen artichokes stimulate growth of broiler chickens and protect them against endotoxins and potential cecal pathogens. Journal of Food Protection 11, 2171–2175.
Koenen ME, Kramer J, Van Der Hulst R, Heres L, Jeurissen SHM, Boersma WJA (2004) Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens. British Poultry Science 45, 355–366.
| Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2cvitFKkug%3D%3D&md5=de9867d55a70a777666480306a7eb229CAS | 15327122PubMed |
Kudoh K, Shimizu J, Ishiyama A, Wada M, Takita T, Kanke Y, Innami S (1999) Secretion and excretion of Immunoglobulin A to cecum and feces differ with type of indigestible saccharides. Journal of Nutritional Science and Vitaminology 45, 173–181.
| Secretion and excretion of Immunoglobulin A to cecum and feces differ with type of indigestible saccharides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjsVOhtbc%3D&md5=a800635111ccdbf7c5bcfda7c8ce703cCAS | 10450558PubMed |
Løvland A, Kaldhusdal M, Reitan LJ (2003) Diagnosing Clostridium perfringens-associated necrotic enteritis in broiler flocks by an Immunoglobulin G anti-alpha-toxin enzyme-linked immunosorbent assay. Avian Pathology 32, 527–534.
| Diagnosing Clostridium perfringens-associated necrotic enteritis in broiler flocks by an Immunoglobulin G anti-alpha-toxin enzyme-linked immunosorbent assay.Crossref | GoogleScholarGoogle Scholar | 14522709PubMed |
McReynolds JL, Byrd JA, Anderson RC, Moore RW, Edrington TS, Genovese KJ, Poole TL, Kubena LF, Nisbet DJ (2004) Evaluation of immunosuppresants and dietary mechanisms in an experimental disease model for necrotic enteritis. Poultry Science 83, 1948–1952.
Miller TL, Wolin MJ (1974) A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Applied Microbiology 27, 985–987.
National Health and Medical Research Council (2004) ‘Australian code of practice for the care and use of animals for scientific purposes.’ 7th edn. (Animal Welfare Committee, National Health and Medical Research Council: Canberra)
Nousiainen J (1991) Comparative observations on selected probiotics and olaquindox used as feed additives for piglets around weaning. 2. Effect on villus length and crypt depth in the jejunum, ileum, caecum and colon. Journal of Animal Physiology and Animal Nutrition 66, 224–230.
| Comparative observations on selected probiotics and olaquindox used as feed additives for piglets around weaning. 2. Effect on villus length and crypt depth in the jejunum, ileum, caecum and colon.Crossref | GoogleScholarGoogle Scholar |
Pattison M (2002) Some clinical and pathological features of enteritis in boilers observations on treatment in the UK. In ‘The Elanco global enteritis symposium, Cambridge, UK’. pp. C1–C10. Available at http://www.poultry-health.com/fora/inthelth/pdfs/pattison02.pdf [Verified 20 August 2013]
Prescott JF, Sivendra R, Barnum DA (1978) The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. The Canadian Veterinary Journal. La Revue Veterinaire Canadienne 19, 181–183.
Primary Industries Standing Committee (2002) ‘Model code of practice for the welfare of animals: somestic poultry.’ 4th edn. SCAM Report 83. (CSIRO Publishing: Melbourne)
Saeed F, Pasha I, Anjum FM, Sultan MT (2011) Arabinoxylans and arabinogalactans: a comprehensive treatise. Critical Reviews in Food Science and Nutrition 51, 467–476.
| Arabinoxylans and arabinogalactans: a comprehensive treatise.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFKgtr4%3D&md5=b86385c541b5dab0debe06fe286582b9CAS | 21491271PubMed |
SAS Institute (2000) ‘SAS user’s guide: version 8.’ (SAS Institute: Cary, NC)
Steel RGD, Torrie JH (1981) ‘Principles and procedures of statistics: a biometrical approach.’ 2nd edn. (McGraw-Hill International Book Company: Sydney)
van der Sluis W (2000) Clostridial enteritis is an often underestimated problem. World Politics 16, 42–43.
van der Sluis W (2003) Selected and specific probiotics may reduce Salmonella and Clostridium infections in broiler. World Politics 19, 16–17.
van Immerseel F, Buck JD, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R (2004) Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathology 33, 537–549.
| Clostridium perfringens in poultry: an emerging threat for animal and public health.Crossref | GoogleScholarGoogle Scholar | 15763720PubMed |
Vidanarachchi JK, Mikkelsen LL, Sims IM, Iji PA, Choct M (2006) Selected plant extracts modulate the gut microflora in broilers. In ‘Proceedings of the Australian Poultry Science Symposium, University of Sydney, Sydney, NSW, Australia’. (Ed. TA Scott) pp. 145–148.
Vidanarachchi JK, Iji PA, Mikkelsen LL, Sims I, Choct M (2009) Isolation and characterisation of water-soluble prebiotic compounds from Australian and New Zealand Plants. Carbohydrate Polymers 77, 670–676.
| Isolation and characterisation of water-soluble prebiotic compounds from Australian and New Zealand Plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtVeqtL4%3D&md5=5caf90524f14c3732a78f1a38548b744CAS |
Vidanarachchi JK, Iji PA, Mikkelsen LL, Choct M (2010a) Fructans from rengarenga lily (Arthropodium cirratum) extract and Frutafit® as prebiotics for broilers: their effects on growth performance, digestive organ size, gut morphology and nutrient digestibility. Asian-Australasian Journal of Animal Sciences 23, 580–587.
Vidanarachchi JK, Elangovan AV, Mikkelsen LL, Choct M, Iji PA (2010b) Effect of some plant extracts on growth performance, intestinal morphology, microflora composition and activity in broiler chickens. Animal Production Science 50, 880–889.
| Effect of some plant extracts on growth performance, intestinal morphology, microflora composition and activity in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Watkins BA, Miller BF, Neil DH (1982) In vivo inhibitory effects of Lactobacillus acidophilus against pathogenic Escherichia coli in gnotobiotic chicks. Poultry Science 61, 1298–1308.
| In vivo inhibitory effects of Lactobacillus acidophilus against pathogenic Escherichia coli in gnotobiotic chicks.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3s%2FjvFWmtQ%3D%3D&md5=ecc68ace81c7962690d4b896078f7e8dCAS | 6813835PubMed |
Watkins KL, Shryock TR, Dearth RN, Saif YM (1997) In vitro antimicrobial susceptibility of Clostridium perfringens from commercial turkey and broiler chicken origin. Veterinary Microbiology 54, 195–200.
| In vitro antimicrobial susceptibility of Clostridium perfringens from commercial turkey and broiler chicken origin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhsFKhtbs%3D&md5=e6bee423490f0dab1293f6f6fa875f55CAS | 9057262PubMed |
Williams RB (2005) Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathology 34, 159–180.
| Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2MrhvVSksQ%3D%3D&md5=2d4fbbaf97307d0a10e68ae80290c461CAS | 16191699PubMed |
Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ (2003) Effects of dietary fructooligosaccharides on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poultry Science 82, 1030–1036.
Yamauchi K, Buwjoom T, Koge K, Ebashi T (2006) Histological intestinal recovery in chickens refed dietary sugar cane extract. Poultry Science 85, 645–651.